Abstract
Aims:
Small randomized controlled trials (RCTs) of GLP1 receptor agonists in HFrEF suggested detrimental effects, but analyses had limited power. Our aim was to perform a post-hoc analysis of the FIGHT trial, evaluating the effect of liraglutide (vs. placebo) on the totality of events in patients with HFrEF.
Materials and methods:
FIGHT was a double-blind RCT that studied liraglutide vs. placebo in 300 recently hospitalized patients with HFrEF followed for 180 days. The main outcome of the present analysis was total events of HF hospitalizations or all-cause death. Secondary outcomes included total arrhythmic events and prespecified total events of interest (arrhythmias, sudden cardiac death, acute coronary syndrome, worsening heart failure, cerebrovascular event, venous thromboembolism, lightheadedness, presyncope/syncope, or worsening renal function). Treatment effect was evaluated with negative binomial regression.
Results:
Compared to placebo, there was a trend towards increased risk with liraglutide of total HF hospitalizations or all-cause deaths (96 vs. 143 events, IRR 1.41, 95%CI 0.98–2.04, P=0.064) and total arrhythmias (21 vs. 39, IRR 1.76, 95%CI 0.92–3.37, P=0.088). Total prespecified events of interest were increased with liraglutide compared to placebo (196 vs. 295, IRR 1.43, 95%CI 1.06–1.92, P=0.018). The risk of HF hospitalizations or all-cause deaths with liraglutide was higher among patients in NYHA class III-IV (IRR 1.86, 95%CI 1.21–2.85) than in those in NYHA class I-II (IRR 0.62, 95%CI 0.31–1.23, interaction-P=0.008), and among patients with diabetes (interaction-P=0.051). The risk of arrhythmic events was higher among those without an implanted cardiac device (interaction-P=0.047).
Conclusions:
In patients with HFrEF, liraglutide might increase the risk of cardiovascular adverse effects, an effect possibly driven by excess risk of arrhythmias and worsening HF events. As a post-hoc analysis, these results should be interpreted as exploratory and hypothesis-generating. Further RCTs must be conducted before drawing definitive conclusions.
Keywords: GLP1 receptor agonists, liraglutide, heart failure with reduced ejection fraction, heart failure hospitalizations, arrhythmia, adverse events
Introduction
Glucagon-like peptide-1 receptor agonists (GLP1-RA) are a drug class that promote glycemic control and weight loss, lower blood pressure and improve the lipid profile.1 In randomized controlled trials (RCTs), GLP1-RA decreased the risk of major adverse cardiovascular events (MACE) in patients with type 2 diabetes (T2D) with a high cardiovascular risk,2 and current guidelines for treatment of T2D propose GLP1-RA as a preferential class for patients with T2D at high risk of atherosclerotic cardiovascular events.3,4
Despite these beneficial effects, GLP1-RA are known for increasing heart rate and activating cyclic adenosine monophosphate (cAMP)-dependent pathways,5 which have been associated with detrimental effects in patients with heart failure with reduced ejection fraction (HFrEF).6
Despite the reduction in MACE in RCTs with patients with T2D, these trials included only a small fraction of HF patients without measuring ejection fraction or natriuretic peptides;7 thus, the efficacy and safety of GLP-1RA among patients with HF, particularly those with HFrEF, is not well established.
In the two small RCTs with the GLP1-RA liraglutide in HFrEF (the Heart Failure Network Functional Impact of GLP-1 for Heart Failure Treatment [FIGHT]8 and the Effect of Liraglutide on Left Ventricular Function in Stable Chronic Heart Failure Patients with and without Diabetes [LIVE]9), treatment with liraglutide was not associated with beneficial effects and there was a higher number of hospitalizations for worsening HF and arrhythmia-related events in the group treated with liraglutide compared to placebo, albeit not reaching statistical significance. The analysis of both trials focused on the evaluation of first events which may have limited their power to detect potential adverse effects of GLP1-RA. The effect of the GLP1-RA albiglutide was also evaluated in patients with stable HFrEF.10 In this small RCT (82 participants, 12 weeks follow-up) albiglutide did not improve cardiac function or myocardial glucose use. Although albiglutide was well tolerated in this RCT, the number of participants and the short follow-up may have limited the detection of potential adverse effects (no hospitalizations for worsening HF were reported during this period).
In the present analysis, we aim to evaluate the effect of the GLP1-RA liraglutide (vs. placebo) on the totality of events of HF hospitalisation or all-cause death in patients with HFrEF enrolled in the FIGHT trial.
Materials and methods
FIGHT trial
FIGHT was a multicenter, double-blind, RCT designed to determine if the use of the GLP1-RA liraglutide improved clinical stability in recently hospitalized patients with HFrEF.11 The trial was conducted between August 2013 and March 2015 at 24 sites in the United States and participants were identified based on hospital admission records. Participants, aged 18 years or older, were required to have an established diagnosis of HF and a LVEF of 40% or lower during the preceding 3 months. Additional inclusion criteria included: 1) a recent (within 14 days) hospitalization for acute HF and 2) a preadmission oral diuretic dose of at least 40 mg of furosemide or an equivalent per day. Key exclusion criteria were 1) a recent acute coronary syndrome or coronary intervention, 2) known intolerance of GLP1-RA therapy, and 3) severe renal, hepatic, or pulmonary disease. Subjects with and without T2D were enrolled in the trial. In total, 300 patients were randomized. A permuted block randomization scheme stratified by clinical site and type 2 diabetes status was performed. Of these, 154 were randomized to liraglutide and 146 to placebo. Liraglutide and placebo were packaged identically to maintain blinding to patients and investigators. Study drug dosage was uptitrated as tolerated every 14 days from 0.6 mg/d to 1.2 mg/d to 1.8 mg/d during the first 30 days of the trial. In the primary analysis, the main outcome was a global rank score in which patients were ranked across 3 hierarchical tiers (higher values indicate better health): time to death, time to rehospitalization for heart failure, and time-averaged proportional change in N-terminal pro-B-type natriuretic peptide level from baseline to 180 days. There was no significant between-group difference in the global rank scores (146 for the liraglutide group vs 156 for the placebo group, P = 0.31).
The FIGHT trial was sponsored by the National Heart, Lung, and Blood Institute (NHLBI). The study protocol was approved by the institutional review board and ethics committee at each participating center. The access to the FIGHT database was provided through NHLBI/BioLINCC (https://biolincc.nhlbi.nih.gov/studies/hfn_fight/) with ethical approval from the Centro Hospitalar Universitário São João / Faculdade de Medicina da Universidade do Porto (process number #432/21).
Study Outcomes
The main outcome of this analysis was total events (first and recurrent) of HF hospitalizations or all-cause death. As secondary outcomes we evaluated 1) total HF hospitalizations, 2) all-cause death, 3) total events of HF hospitalizations, urgent HF visits requiring intravenous (IV) diuretic treatment or all-cause death, 4) total events of urgent HF visit requiring intravenous treatment; 5) total arrhythmic events (investigator reported); and 6) prespecified total events of interest (predefined by the study investigators as any of the following: arrhythmias, sudden cardiac death, acute coronary syndrome, worsening heart failure, cerebrovascular event, venous thromboembolism, lightheadedness, presyncope or syncope, or worsening renal function).
Statistical analysis
The main analyses of this study were conducted using the intention-to-treat principle and included all randomized participants during the study follow-up of approximately 180 days. The main outcome and the secondary outcomes were analyzed by a negative binomial regression model with the count of events as outcome and the treatment group as independent variable. The results of the negative binomial test for the treatment effect of liraglutide vs. placebo are described as incidence rate ratios and respective 95% confidence intervals (IRRs with 95%CIs). Andersen-Gill models for recurrent events were also performed for internal consistency assessment. The introduction of anti-arrhythmic drugs during the follow-up period was assessed by mixed effects logistic regression as exploratory analysis.
Subgroup analyses were performed according to age (≥65 years vs. <65 years), sex (women vs. men), diagnosis of T2D (yes vs. no), LVEF (≤25% vs. >25%), BMI (≥30 kg/m2 vs. <30 kg/m2), NT-proBNP levels (≥2000 pg/mL vs. <2000 pg/mL), NYHA class (class III-IV vs. I-II), HF etiology (ischemic vs. non-ischemic), use of cardiac resynchronization therapy or implantable cardioverter-defibrillator (ICD: yes vs. no), use of digoxin (yes vs. no), and use of amiodarone or other anti-arrhythmic drugs (yes vs. no), with differences in the effect of liraglutide vs. placebo assessed using subgroup analyses with interaction terms.
A two-sided P-value of <0.05 was considered statistically significant. No correction for multiple testing was performed due to the exploratory nature of this work. All analyses were performed using Stata® (StataCorp. 2021. Stata Statistical Software: Release 17. College Station, TX: StataCorp LLC).
Results
Baseline patient’s characteristics by number of total events
The median (percentile25–75) age of the patients was 61 (52–68) years, the median LVEF was 25 (19–33) %, and 64 (21%) were women. Compared to patients alive and without HF hospitalizations during the 180-day follow-up (N =156), those who had only one (N =56) and particularly those who had two or more HF hospitalizations or a fatal event (N =88) presented more frequently with severely symptomatic HF (NYHA class III or IV: 63.8% vs. 69.1% vs. 79.1%, P =0.050), had lower LVEF (median: 23% vs. 25% vs. 20%, P =0.016), had more frequent previous HF hospitalizations (two or more: 40.4% vs. 60.7% vs. 69.3%, P <0.001), used cardiac devices more often (78.2% vs. 73.2% vs. 88.6%, P =0.048), had lower hemoglobin levels (median: 13 g/dL vs. 12 g/dL vs. 12 g/dL, P =0.041), and used ACEi/ARBs less frequently (76.9% vs. 75.0% vs. 61.4%, P =0.029). Table 1. The distribution of total HF hospitalizations or death is shown in Figure 1A. The distribution of total investigator-reported events of interest is shown in Figure 1B.
Table 1.
Baseline characteristics of the patients by the number of events of heart failure hospitalization or all-cause mortality
| Number of events of heart failure hospitalization or all-cause death | ||||
|---|---|---|---|---|
| None | 1 event | 2 or more events | P-value* | |
| (N=156) | (N=56) | (N=88) | ||
| Age (years) | 61.0 (53.0, 68.5) | 62.5 (50.5, 68.0) | 61.0 (51.0, 66.0) | 0.57 |
| Women | 27 (17.3%) | 12 (21.4%) | 25 (28.4%) | 0.13 |
| Race, n. (%) | 0.42 | |||
| White | 94 (60.3%) | 30 (53.6%) | 48 (54.5%) | |
| Black | 53 (34.0%) | 25 (44.6%) | 37 (42.0%) | |
| Other | 9 (5.8%) | 1 (1.8%) | 3 (3.4%) | |
| BMI (kg/m2) | 31.6 (26.1, 35.6) | 32.1 (25.5, 36.6) | 30.8 (26.0, 36.9) | 0.96 |
| NYHA class III-IV | 97 (63.8%) | 38 (69.1%) | 68 (79.1%) | 0.050 |
| Systolic BP (mmHg) | 107.0 (98.0, 120.0) | 110.0 (96.0, 120.5) | 108.0 (100.0, 117.0) | 0.97 |
| Diastolic BP (mmHg) | 66.0 (59.0, 75.0) | 67.0 (59.5, 79.0) | 66.0 (59.0, 74.0) | 0.67 |
| Heart rate (bpm) | 76.0 (68.0, 86.0) | 76.0 (66.5, 89.5) | 75.5 (69.0, 86.0) | 0.61 |
| LVEF (%) | 23.0 (18.0, 25.0) | 25.0 (17.8, 30.0) | 20.0 (15.0, 25.5) | 0.016 |
| HF of ischemic etiology | 125 (80.1%) | 47 (83.9%) | 74 (84.1%) | 0.68 |
| Time from HF diagnosis (years) | 6.5 (3.3, 11.1) | 5.1 (3.0, 9.6) | 7.3 (3.3, 12.5) | 0.63 |
| HHF within past year | <0.001 | |||
| 0 | 22 (14.1%) | 6 (10.7%) | 10 (11.4%) | |
| 1 | 71 (45.5%) | 16 (28.6%) | 17 (19.3%) | |
| 2+ | 63 (40.4%) | 34 (60.7%) | 61 (69.3%) | |
| Type 2 Diabetes | 93 (59.6%) | 29 (51.8%) | 56 (63.6%) | 0.37 |
| Hypertension | 122 (78.2%) | 45 (81.8%) | 68 (77.3%) | 0.80 |
| Atrial fibrillation / flutter | 78 (50.0%) | 34 (60.7%) | 42 (47.7%) | 0.28 |
| History of sustained VT, VF or resuscitated cardiac arrest | 23 (14.7%) | 6 (10.7%) | 19 (21.6%) | 0.18 |
| Pacemaker or ICD | 122 (78.2%) | 41 (73.2%) | 78 (88.6%) | 0.048 |
| Stroke of TIA | 23 (14.8%) | 7 (12.5%) | 11 (12.8%) | 0.86 |
| Chronic kidney disease | 54 (34.6%) | 22 (40.0%) | 42 (48.3%) | 0.11 |
| Sodium (mEq/L) | 137.0 (135.0, 140.0) | 136.5 (134.0, 139.0) | 136.0 (134.0, 139.0) | 0.080 |
| Potassium (mEq/L) | 4.1 (3.8, 4.5) | 4.0 (3.7, 4.3) | 4.2 (3.8, 4.4) | 0.11 |
| eGFR (ml/min/1.73m2) | 51.1 (38.8, 69.4) | 55.5 (38.4, 72.2) | 49.4 (34.1, 65.9) | 0.38 |
| Cystatin C (mg/L) | 1.3 (1.1, 1.7) | 1.5 (1.2, 1.8) | 1.4 (1.1, 1.9) | 0.23 |
| Glucose (mg/dL) | 108.0 (95.0, 143.0) | 109.0 (94.5, 137.5) | 115.0 (96.0, 148.0) | 0.38 |
| HbA1c (%) | 6.6 (6.0, 7.6) | 6.6 (5.8, 7.9) | 6.8 (6.0, 8.0) | 0.42 |
| Hemoglobin (g/dL) | 13.0 (11.5, 14.5) | 12.0 (11.2, 13.8) | 12.3 (11.0, 13.5) | 0.041 |
| NT-proBNP (pg/mL) | 2150.5 (1016.5, 4271.5) | 1908.5 (1153.0, 3944.0) | 1982.5 (1141.0, 4754.0) | 0.80 |
| ACEi or ARB | 120 (76.9%) | 42 (75.0%) | 54 (61.4%) | 0.029 |
| Beta-blocker | 149 (95.5%) | 52 (92.9%) | 81 (92.0%) | 0.51 |
| Aldosterone antagonist | 87 (56.5%) | 37 (66.1%) | 53 (60.2%) | 0.45 |
| Loop diuretic | 156 (100.0%) | 54 (96.4%) | 87 (98.9%) | 0.070 |
| Digoxin | 52 (33.3%) | 17 (30.4%) | 33 (37.5%) | 0.66 |
| Amiodarone or other antiarrhythmic drug | 38 (24.4%) | 18 (32.1%) | 26 (29.9%) | 0.44 |
Results are presented as median (IQR) or n. (%). Legend: ACEi: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker; BMI, body mass index; BP: blood pressure; bpm: beats per minute; eGFR: estimated glomerular filtration rate (CKD-EPI creatinine formula); HF: heart failure; HHF: heart failure hospitalizations; ICD: implantable cardioverter-defibrillator; IQR: interquartile range; LVEF: left ventricle ejection fraction; NYHA New York Heart Association; TIA: Transient ischemic attack, VT: ventricular tachycardia; VF: ventricular fibrillation.
P-value for trend.
Figure 1.
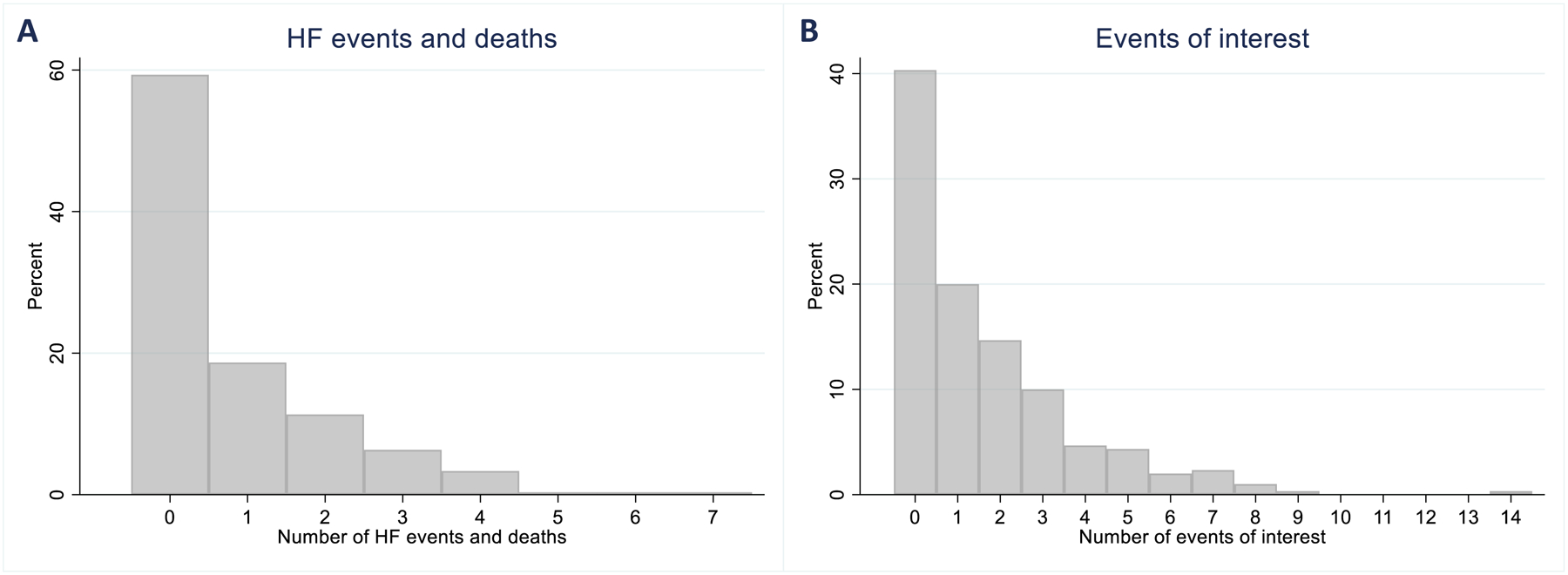
Total number of events of HF hospitalization or all-cause mortality (panel A). Total number of events of interest - predefined by the study investigators as any of the following: arrhythmias, sudden cardiac death, acute coronary syndrome, worsening heart failure, cerebrovascular event, venous thromboembolism, lightheadedness, presyncope or syncope, or worsening renal function (panel B).
Effect of liraglutide vs. placebo on total events
During the 6-month follow-up period, in participants treated with liraglutide, compared to placebo, there was a trend towards increased number of total HF hospitalizations or all-cause deaths: 96 vs. 143 events, IRR 1.41, 95%CI 0.98–2.04, P =0.064; total HF hospitalizations: 80 vs. 124 events, IRR 1.47, 98%CI 0.98–2.20, P =0.061; IV diuretic therapy, HF hospitalizations or all-cause deaths: 102 vs. 153 events, IRR 1.48, 95%CI 1.00–2.19, P =0.056; and total investigator-reported arrhythmias: 21 vs. 39, IRR 1.76, 95%CI 0.92–3.37, P =0.088. Total prespecified investigator-reported events of interest were increased with liraglutide compared to placebo: 196 vs. 295, IRR 1.43, 95%CI 1.06–1.92, P =0.018. Table 2. The Andersen-Gill model provided similar results: HR 1.53, 95%CI 1.02–2.31, P =0.040 for total HF hospitalizations or all-cause deaths and HR 1.41, 95%CI 1.01–1.97, P =0.043 for total prespecified investigator-reported events of interest. Figure 2.
Table 2.
Study outcomes (results are displayed as total events)
| Outcome | Events Placebo (N =146) | Events Liraglutide (N =154) | Incidence rate ratio (95%CI) | P-value |
|---|---|---|---|---|
| Total HFH or death | 96 | 143 | 1.41 (0.98–2.04) | 0.064 |
| Total HFH | 80 | 124 | 1.47 (0.98–2.20) | 0.061 |
| Death | 16 | 19 | 1.13 (0.58–2.19) | 0.72 |
| Total HFH, urgent HF visit or death | 102 | 153 | 1.48 (1.00–2.19) | 0.056 |
| Urgent HF visits | 6 | 10 | 1.58 (0.52–4.81) | 0.42 |
| Total arrhythmic events# | 21 | 39 | 1.76 (0.92–3.37) | 0.088 |
| Total events of interest#* | 196 | 295 | 1.43 (1.06–1.92) | 0.018 |
Legend: HF, heart failure; HHF, heart failure hospitalizations.
investigator-reported;
events of interest, predefined by the study investigators as any of the following: arrhythmias, sudden cardiac death, acute coronary syndrome, worsening heart failure, cerebrovascular event, venous thromboembolism, lightheadedness, presyncope or syncope, or worsening renal function.
Figure 2.
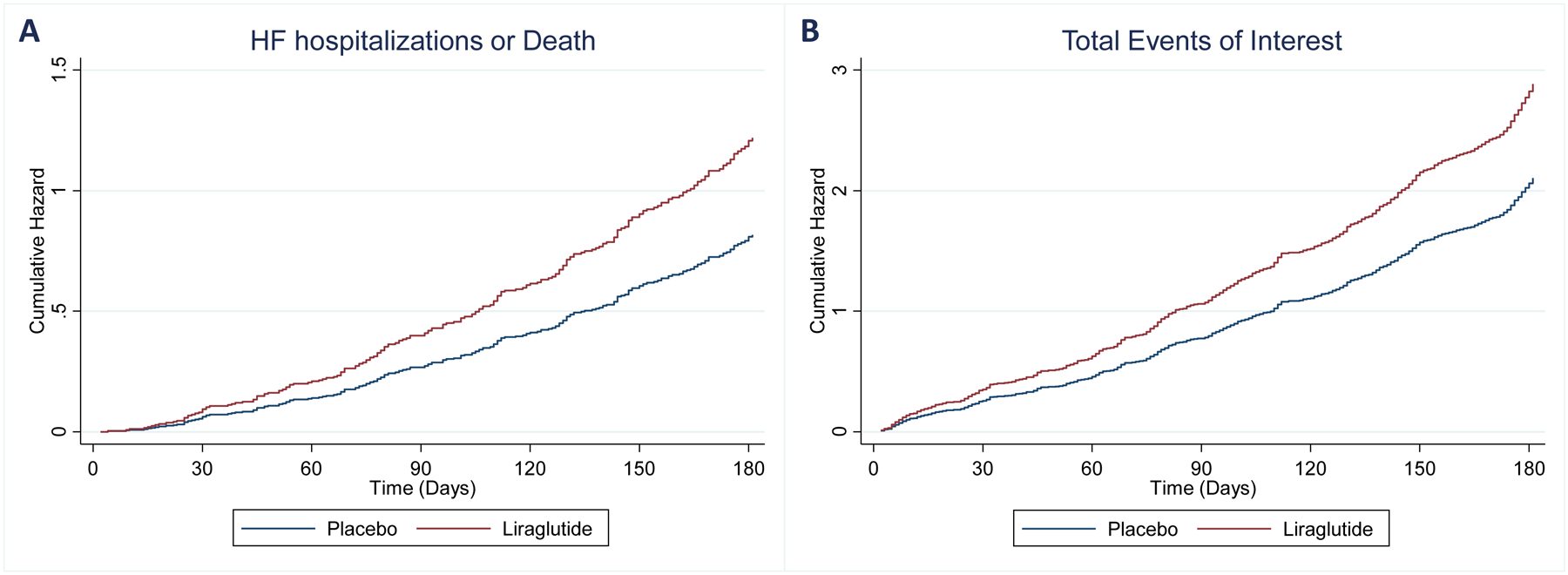
Cumulative hazard (Andersen-Gill model) of total events of HF hospitalization of all-cause mortality - HR 1.53, 95%CI 1.02–2.31, P =0.040 (panel A); and total events of interest - HR 1.41, 95%CI 1.01–1.97, P =0.043 (panel B). HF, heart failure; CV cardiovascular.
Liraglutide increased the new use of anti-arrhythmic drugs (amiodarone, propafenone, digoxin): 75 vs. 115, OR 4.01, 95%CI 0.78–20.6, P =0.097.
Subgroup analyses
The risk of total HF hospitalizations or all-cause deaths with liraglutide was higher among patients in NYHA class III or IV (IRR 1.86, 95%CI 1.21–2.85) than in those in NYHA class I or II (IRR 0.62, 95%CI 0.31–1.23), interaction-P =0.008; and among patients with T2D (IRR 1.91, 95%CI 1.19–3.08) than in those without diabetes (IRR 0.92, 95%CI 0.52–1.61), interaction-P =0.051. Figure 3. The risk of total arrhythmic events with liraglutide was higher among those without an ICD (IRR 12.57, 95%CI 1.42–111.54) than in those with an ICD (IRR 1.23, 95%CI 0.61–2.48), interaction P =0.047; and in those not using digoxin (IRR 3.11, 95%CI 1.26–7.68) than in those using digoxin (IRR 0.92, 95%CI 0.34–2.50), interaction P =0.076. Supplementary Figure 1. A similar pattern was found for total investigator-reported events of interest. Supplementary Figure 2.
Figure 3.
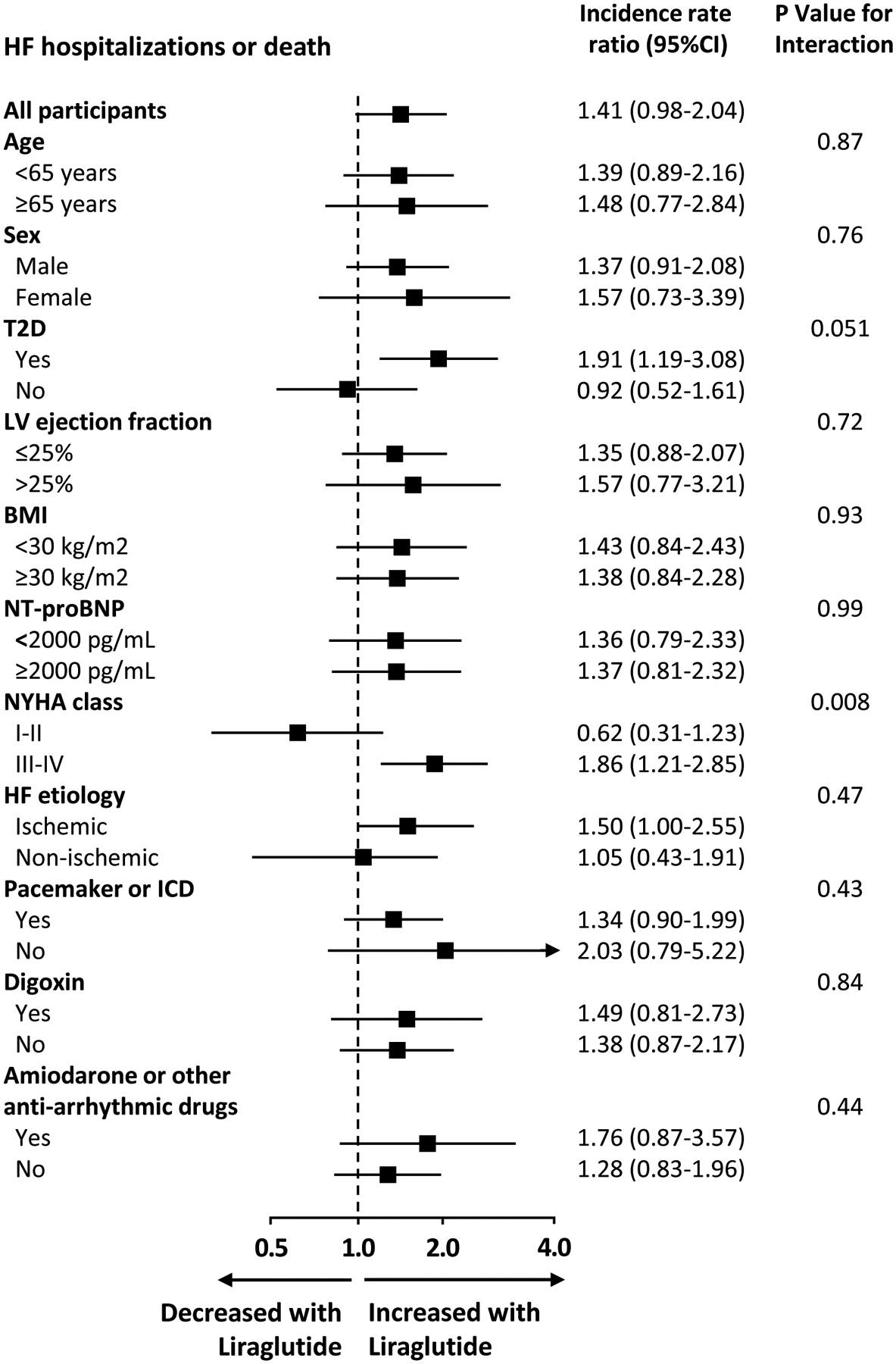
Subgroup analyses for the total events of HF hospitalization or all-cause mortality. HF, heart failure; CI, confidence interval; T2D, type 2 diabetes; LV, left ventricle; BMI, body mass index; NYHA New York Heart Association; ICD, implantable cardioverter-defibrillator.
Discussion
Our re-analysis of the FIGHT trial found a consistent pattern of increased risk of adverse events with liraglutide compared to placebo. Albeit not reaching the statistical significance threshold of 0.05 for many of the studied endpoints, the consistency of the pattern across all outcomes strongly suggests that there is a potential risk associated with the use of liraglutide (and likely other GLP1-RA) in patients with HFrEF. This risk is possibly driven by an excess of arrhythmias and worsening HF events with liraglutide use. A potentially increased risk of arrhythmic and worsening HF events with the use of GLP1-RA in HFrEF is biologically plausible and has been externally replicated. In the small LIVE trial that enrolled 241 stable HFrEF patients randomized to either liraglutide or placebo (a lower risk population compared to FIGHT), an excess risk of serious cardiac events was seen in the liraglutide group, driven by ventricular tachycardias (including one death) and atrial fibrillation (10% vs. 3%, P =0.04).9
Despite its modest size and short follow-up (300 patients followed for 180 days), the FIGHT trial included a remarkably high-risk population due to the requirement of high natriuretic peptide levels and of a recent HF hospitalization prior to inclusion. Thus, the FIGHT trial was able to provide many “hard” events to assess the effect of liraglutide among patients with advanced HFrEF. Contrary to the original analysis, we evaluated not only time-to-first event, but the total number of events, which may more fully capture the total burden of disease in HF.12 Potentially harmful effects of liraglutide could already be seen in the primary report of the FIGHT trial with a time-to-first event analysis, where the risk of hospitalization for cardiovascular reasons (HR 1.33, 95%CI 0.95–1.85, P =0.09); emergency department visit, hospitalization for cardiovascular reasons, or all-cause death (HR 1.34, 95%CI 1.00–1.80, P =0.05); and emergency department visit, HF hospitalization, or all-cause death (HR 1.36, 95%CI 0.99–1.85, P =0.05), all were increased with liraglutide compared to placebo.8 These findings are reinforced and expanded by the present total event analysis, highlighting the risk of HF re-hospitalizations, arrhythmias (supported by the introduction of anti-arrhythmic agents), and total events of interest (including arrhythmias, sudden cardiac death, acute coronary syndrome, worsening heart failure, cerebrovascular event, venous thromboembolism, lightheadedness, presyncope or syncope, or worsening renal function). The heightened risk among patients with severe symptoms (NYHA III-IV), and those not treated with cardiac devices or anti-arrhythmic agents such as digoxin, suggests that liraglutide may be particularly harmful among unstable HFrEF patients who are not protected against arrhythmias, including potentially fatal ventricular arrhythmias.
The mechanisms by which liraglutide, and other GLP1-RA, may increase the risk of adverse cardiovascular events in patients with HFrEF are not fully understood, but might relate to altered intracellular cAMP dynamics. GLP-1 receptors are expressed in cardiomyocytes and sinoatrial node cells, signaling through a cAMP-dependent pathway.13 Preclinical data have already shown an increase in cardiac intracellular cAMP levels with GLP1-RA treatment.14–16 Furthermore, extensive mechanistic data have shown that increased intracellular cAMP raises the risk of arrhythmia and leads to myocardial dysfunction in HF.17–19 Importantly, the macromolecular complexes responsible for restricting cAMP action have been shown to be disrupted in HF,20 extending cAMP-dependent signaling activation in time and space within the cardiomyocyte, causing calcium overload and predisposing to myocardial dysfunction and fatal arrhythmic events.17,21 This is in accordance with data from clinical trials, showing that drugs that increase cAMP levels (e.g., milrinone) increase the risk of arrhythmic events and mortality in HFrEF,22 while drugs that decrease cAMP levels (e.g. beta-blockers) are associated with decreased risk.23 GLP1-RA increase cAMP levels by pathways independent of beta-adrenergic receptors.24–26 The increased risk of cardiovascular adverse effects in the FIGHT trial, despite more than 90% of participants being treated with beta-blockers, suggests that beta-blockers do not protect from arrhythmic events and worsening HF events with GLP1-RA in HFrEF.
While in populations without myocardial dysfunction and with low risk of arrhythmias, the myocardial effects of GLP1-RA may not significantly increase the risk of HF events or arrhythmias,27 in patients with HFrEF, the lower cardiac reserve and the higher pro-arrhythmogenic potential may make this population particularly susceptible for adverse cardiac effects with GLP1-RA. In agreement with this hypothesis, some RCTs with GLP1-RA in T2D showed significant interactions of the treatment arm with baseline HF status on study endpoints, with event rate reductions seen only in patients without HF at baseline. In the EXSCEL trial with exenatide, all-cause mortality was not reduced in the subgroup with HF (HR 1.05, 95% CI, 0.85–1.29) but was significantly reduced in those without HF at baseline (HR 0.79, 95%CI, 0.68–0.92; interaction P =0.03).28 Also, in a combined analysis of the SUSTAIN-6 and PIONEER-6, semaglutide reduced MACE among participants without HF (HR, 0.79, 95%CI, 0.68–0.92), but not in those with HF at baseline (HR 1.06, 95%CI, 0.72–1.57; interaction P=0.03).29 In the LEADER trial, liraglutide reduced the composite of HF hospitalization or cardiovascular death in the subgroup without baseline HF history (HR 0.77, 95%CI, 0.65 to 0.91) but not in the subgroup with baseline HF (HR 0.92, 95% CI, 0.74 to 1.15), even though the interaction was not significant (interaction P=0.19).30 In all these trials, HF subgroup included patients with HFrEF and patients with heart failure with preserved ejection fraction (HFpEF) which may explain the differences compared to the FIGHT trial. From the RCTs with GLP1-RA in T2D, only the EXSCEL reported left ventricular ejection fraction and among participants with baseline HF and documented ejection fraction only 22% had reduced ejection fraction.28 Furthermore, some trials with GLP1-RA vs. placebo in patients with T2D have reported an excess of risk of ventricular fibrillation/tachycardia or cardiovascular conduction disorders with GLP1-RA (EXCSEL: 41/7356 vs. 26/7396; LEADER 18/4668 vs 8/4672; REWIND: 216/4949 vs. 192/4952).31–33
The findings here reported have important clinical consequences and further trials are needed to confirm the potential excess risk with GLP1-RA in patients with HFrEF. Until such trials are conducted, GLP1-RA should be avoided in patients with HFrEF.
Whether this increased risk is also observed in patients with HFpEF is uncertain; no RCTs have specifically evaluated GLP1-RA in this population. Given the contribution of metabolic dysfunction and obesity to the pathophysiology of HFpEF,34 the systemic effects of GLP1-RA may counterweight, at least partially, the potential direct increased risk of arrhythmogenic effects. The Research Study to Investigate How Well Semaglutide Works in People Living With Heart Failure and Obesity (STEP-HFpEF; clinicaltrials.gov identifier: NCT04788511) and the Research Study to Look at How Well Semaglutide Works in People Living With Heart Failure, Obesity and Type 2 Diabetes (STEP HFpEF DM; clinicaltrials.gov identifier: NCT04916470) are evaluating the effect of the GLP1-RA semaglutide on the function and symptoms of patients with HFpEF.
It is important to distinguish the effects of GLP1-RA in patients with T2D without HF from the effects in patients with established HF. While our results suggest that, in those with established HFrEF treatment with GLP1-RA may increase the risk of adverse effects; in those without HF, GLP1-RA may prevent the development of HF. A recent meta-analysis of RCTs in T2D showed that treatment with GLP1-RA significantly reduced hospitalizations for HF.35 Observational data also suggest that GLP1-RA may decrease HF events in primary prevention.36 The improvement of metabolic control and the prevention of coronary atherosclerotic disease with GLP1-RA may explain the primary prevention of HF development in T2D. Furthermore, GLP1-RA decrease epicardium fat37 which likely plays a role in the pathogenesis of HFpEF.38 It is plausible that heart failure events prevented by GLP1-RA in diabetes are predominantly HFpEF events.
Given the potential adverse effects of GLP1-RA in HFrEF, in patients with T2D or obesity with suggestive symptoms or clinical suspicion of HF, an echocardiogram and natriuretic peptides may be considered before starting the treatment. On the other hand, in those without HFrEF, our results should not discourage clinicians from using GLP1-RA given their well-established atherosclerotic cardiovascular benefit in T2D2 and their potential cardiovascular benefits in obesity.39
Limitations
Our study has some limitations. This is a post-hoc analysis of a RCT and some studied endpoints did not reach the statistical significance threshold of 0.05; however, the consistent trend for increased risk of adverse events across different outcomes, the biological plausibility, and the finding of similar results in the LIVE trial provide robustness to our analyses.9 This RCT had a short follow-up (6 months) and a modest size sample (300 participants); however, the remarkably high-risk population included led to a high number of “hard” outcomes during the trial. The increased risk observed in this high-risk population with a recent hospitalization may not apply to other groups of patients with HFrEF. The FIGHT trial was conducted between August 2013 and March 2015, and new therapeutic interventions have been introduced since then in the management of HFrEF (including ARNI and SGLT2 inhibitors); although the potential detrimental effects of GLP1-RA are not expected to be modified by the current management of HFrEF, we cannot exclude this possibility. We could not determine the cause of HF decompensation that led to hospitalization, but it is possible that arrythmias could have contributed to some of these HF events. No correction for multiplicity of tests was made, which may increase the risk of chance findings and type I error.
Despite these limitations, this analysis is an important addition to what was already known from the original analysis. In the original analysis, the global rank score did not differ between groups, and the study was interpreted as neutral. Although there was already a trend towards worse outcomes with liraglutide in the original analysis (supplementary table 1), the use of the totality of events allowed a clearer assessment of the effects of liraglutide in advanced HFrEF. The identification that the risk of HF hospitalizations or death with liraglutide was higher in patients with NYHA III-IV is clinically relevant. Furthermore, the higher risk of HF hospitalizations or death with liraglutide in patients with T2D is also relevant as this is the population most commonly treated with GLP1-RA. Arrhythmic events were not specifically evaluated in the original analysis and our analysis showed a trend towards an increased risk of arrhythmias, particularly among those without an implanted cardiac device. As any post-hoc analysis, our study must be interpreted as exploratory and hypothesis-generating, and further RCTs must be conducted before drawing definitive conclusions.
Conclusions
In patients with HFrEF, treatment with liraglutide might increase the risk of cardiovascular adverse effects, an effect possibly driven by an excess risk of arrhythmias and worsening HF events. Further trials with GLP1-RA should be performed to better assess the risk-benefit of GLP1-RA in patients with HFrEF; until then, the use of liraglutide, and possibly other GLP1-RA, should be avoided in patients with advanced HFrEF.
Supplementary Material
Acknowledgements
Sources of Funding:
This study was supported by national funds through FCT Fundação para a Ciência e Tecnologia, I.P., under the scope of the Cardiovascular R&D Center - UnIC (UIDB/00051/2020 and UIDP/00051/2020).
Disclosures:
Dr Neves has received consulting or speaker fees from AstraZeneca, BIAL, Boehringer Ingelheim, Lilly, Merck, and Novo Nordisk. Dr Sharma is supported by the McGill University Health Centre (MUHC) Foundation, Montreal General Hospital (MGH) Foundation, Sarah Louise King Award, Marjorie Cadham Award, Inez and Willena Beaton award, Fonds de Recherche Santé Quebec (FRSQ) Junior 1 clinician scholars’ program, Canada Institute for Health Research grant - 175095. Dr Sharma reports receiving support from the European Society of Cardiology young investigator grant, Roche Diagnostics, Boehringer-Ingelheim, Novartis, and Takeda. Dr. Carvalho: received consultancy fees from Novo-Nordisk and Eli-Lilly, and has held lectures Novo-Nordisk, Eli-Lilly and Astra-Zeneca. Dr Packer has received personal fees from Abbvie, Actavis, Amarin, Amgen, AstraZeneca, Boehringer Ingelheim, Caladrius, Casana, CSL Behring, Cytokinetics, Imara, Lilly, Moderna, Novartis, Reata, Relypsa, and Salamandras. Dr Zannad reports personal fees from Boehringer Ingelheim, Janssen, Novartis, Boston Scientific, Amgen, CVRx, AstraZeneca, Vifor Fresenius, Cardior, Cereno pharmacuetical, Applied Therapeutics, Merck, Bayer, and Cellprothera outside the submitted work; and other support from CVCT and Cardiorenal, outside the submitted work. Dr Ferreira is a consultant for Boehringer-Ingelheim, and receives research support from AstraZeneca and Novartis. All other authors have no potential conflicts of interest to disclose.
Data availability statement:
The FIGHT database can be fully available from NHLBI/BioLINCC upon reasonable request.
References
- 1.Rangaswami J, Bhalla V, de Boer IH, et al. Cardiorenal Protection With the Newer Antidiabetic Agents in Patients With Diabetes and Chronic Kidney Disease: A Scientific Statement From the American Heart Association. Circulation. 2020;142(17):e265–e286. [DOI] [PubMed] [Google Scholar]
- 2.Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653–662. [DOI] [PubMed] [Google Scholar]
- 3.Draznin B, Aroda VR, Bakris G, et al. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S125–s143. [DOI] [PubMed] [Google Scholar]
- 4.Das SR, Everett BM, Birtcher KK, et al. 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients With Type 2 Diabetes: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2020;76(9):1117–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drucker DJ. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab. 2016;24(1):15–30. [DOI] [PubMed] [Google Scholar]
- 6.Packer M Will long-acting glucagon-like peptide-1 analogues recapitulate our agonizing experience with cyclic AMP-dependent positive inotropic agents in heart failure? Eur J Heart Fail. 2018;20(4):627–629. [DOI] [PubMed] [Google Scholar]
- 7.Draznin B, Aroda VR, Bakris G, et al. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S144–s174. [DOI] [PubMed] [Google Scholar]
- 8.Margulies KB, Hernandez AF, Redfield MM, et al. Effects of Liraglutide on Clinical Stability Among Patients With Advanced Heart Failure and Reduced Ejection Fraction: A Randomized Clinical Trial. Jama. 2016;316(5):500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jorsal A, Kistorp C, Holmager P, et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE)-a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19(1):69–77. [DOI] [PubMed] [Google Scholar]
- 10.Lepore JJ, Olson E, Demopoulos L, et al. Effects of the Novel Long-Acting GLP-1 Agonist, Albiglutide, on Cardiac Function, Cardiac Metabolism, and Exercise Capacity in Patients With Chronic Heart Failure and Reduced Ejection Fraction. JACC Heart failure. 2016;4(7):559–566. [DOI] [PubMed] [Google Scholar]
- 11.Margulies KB, Anstrom KJ, Hernandez AF, et al. GLP-1 agonist therapy for advanced heart failure with reduced ejection fraction: design and rationale for the functional impact of GLP-1 for heart failure treatment study. Circ Heart Fail. 2014;7(4):673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Claggett B, Pocock S, Wei LJ, Pfeffer MA, McMurray JJV, Solomon SD. Comparison of Time-to-First Event and Recurrent-Event Methods in Randomized Clinical Trials. Circulation. 2018;138(6):570–577. [DOI] [PubMed] [Google Scholar]
- 13.Pyke C, Heller RS, Kirk RK, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155(4):1280–1290. [DOI] [PubMed] [Google Scholar]
- 14.Vila Petroff MG, Egan JM, Wang X, Sollott SJ. Glucagon-like peptide-1 increases cAMP but fails to augment contraction in adult rat cardiac myocytes. Circ Res. 2001;89(5):445–452. [DOI] [PubMed] [Google Scholar]
- 15.Noyan-Ashraf MH, Momen MA, Ban K, et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58(4):975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao YF, Nikolskaya A, Jaye DA, Sigg DC. Glucagon-like peptide-1 enhances cardiac L-type Ca2+ currents via activation of the cAMP-dependent protein kinase A pathway. Cardiovasc Diabetol. 2011;10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antos CL, Frey N, Marx SO, et al. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a. Circ Res. 2001;89(11):997–1004. [DOI] [PubMed] [Google Scholar]
- 18.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006;103(3):511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guellich A, Mehel H, Fischmeister R. Cyclic AMP synthesis and hydrolysis in the normal and failing heart. Pflugers Archiv : European journal of physiology. 2014;466(6):1163–1175. [DOI] [PubMed] [Google Scholar]
- 20.Wehrens XH, Lehnart SE, Huang F, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113(7):829–840. [DOI] [PubMed] [Google Scholar]
- 21.Khan MS, Fonarow GC, McGuire DK, et al. Glucagon-Like Peptide 1 Receptor Agonists and Heart Failure: The Need for Further Evidence Generation and Practice Guidelines Optimization. Circulation. 2020;142(12):1205–1218. [DOI] [PubMed] [Google Scholar]
- 22.Lechat P, Packer M, Chalon S, Cucherat M, Arab T, Boissel JP. Clinical effects of beta-adrenergic blockade in chronic heart failure: a meta-analysis of double-blind, placebo-controlled, randomized trials. Circulation. 1998;98(12):1184–1191. [DOI] [PubMed] [Google Scholar]
- 23.Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. N Engl J Med. 1991;325(21):1468–1475. [DOI] [PubMed] [Google Scholar]
- 24.Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferdinand KC, White WB, Calhoun DA, et al. Effects of the once-weekly glucagon-like peptide-1 receptor agonist dulaglutide on ambulatory blood pressure and heart rate in patients with type 2 diabetes mellitus. Hypertension (Dallas, Tex : 1979). 2014;64(4):731–737. [DOI] [PubMed] [Google Scholar]
- 26.Vukotic R, Raimondi F, Brodosi L, et al. The Effect of Liraglutide on β-Blockade for Preventing Variceal Bleeding: A Case Series. Annals of internal medicine. 2020;173(5):404–405. [DOI] [PubMed] [Google Scholar]
- 27.Boulmpou A, Patoulias D, Teperikidis E, et al. Meta-analysis of cardiovascular outcome trials assessing the impact of glucagon-like peptide-1 receptor agonists on major cardiac arrhythmias. European Heart Journal. 2021;42(Supplement_1). [DOI] [PubMed] [Google Scholar]
- 28.Fudim M, White J, Pagidipati NJ, et al. Effect of Once-Weekly Exenatide in Patients With Type 2 Diabetes Mellitus With and Without Heart Failure and Heart Failure-Related Outcomes: Insights From the EXSCEL Trial. Circulation. 2019;140(20):1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Husain M, Bain SC, Jeppesen OK, et al. Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes Obes Metab. 2020;22(3):442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marso SP, Baeres FMM, Bain SC, et al. Effects of Liraglutide on Cardiovascular Outcomes in Patients With Diabetes With or Without Heart Failure. Journal of the American College of Cardiology. 2020;75(10):1128–1141. [DOI] [PubMed] [Google Scholar]
- 31.Holman RR, Bethel MA, Mentz RJ, et al. Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2017;377(13):1228–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet (London, England). 2019;394(10193):121–130. [DOI] [PubMed] [Google Scholar]
- 33.Husain M, Bain SC, Mann JFE, et al. Arrythmias and heart rate increase in the LEADER trial and relation to risk of cardiovascular events. European heart journal. 2018;39(suppl_1). [Google Scholar]
- 34.Kitzman DW, Lam CSP. Obese Heart Failure With Preserved Ejection Fraction Phenotype: From Pariah to Central Player. Circulation. 2017;136(1):20–23. [DOI] [PubMed] [Google Scholar]
- 35.Giugliano D, Scappaticcio L, Longo M, et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20(1):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright AK, Carr MJ, Kontopantelis E, et al. Primary Prevention of Cardiovascular and Heart Failure Events With SGLT2 Inhibitors, GLP-1 Receptor Agonists, and Their Combination in Type 2 Diabetes. Diabetes Care. 2022;45(4):909–918. [DOI] [PubMed] [Google Scholar]
- 37.Iacobellis G, Baroni MG. Cardiovascular risk reduction throughout GLP-1 receptor agonist and SGLT2 inhibitor modulation of epicardial fat. J Endocrinol Invest. 2022;45(3):489–495. [DOI] [PubMed] [Google Scholar]
- 38.Ayton SL, Gulsin GS, McCann GP, Moss AJ. Epicardial adipose tissue in obesity-related cardiac dysfunction. Heart. 2022;108(5):339–344. [DOI] [PubMed] [Google Scholar]
- 39.Leite AR, Angélico-Gonçalves A, Vasques-Nóvoa F, et al. Effect of glucagon-like peptide-1 receptor agonists on cardiovascular events in overweight or obese adults without diabetes: A meta-analysis of placebo-controlled randomized trials. Diabetes, obesity & metabolism. 2022;24(8):1676–1680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The FIGHT database can be fully available from NHLBI/BioLINCC upon reasonable request.


