Abstract
Mutationally-activated BRAF is detected in ~7% of human lung adenocarcinomas, with BRAFT1799A serving as a predictive biomarker for treatment of patients with FDA-approved inhibitors of BRAFV600E oncoprotein signaling. In genetically engineered mouse (GEM) models, expression of BRAFV600E in the lung epithelium initiates growth of benign lung tumors that, without additional genetic alterations, rarely progress to malignant lung adenocarcinoma. To identify genes that cooperate with BRAFV600E for malignant progression, we employed Sleeping Beauty-mediated transposon mutagenesis, which dramatically accelerated the emergence of lethal lung cancers. Amongst the genes identified was Rbms3, which encodes an RNA-binding protein previously implicated as a putative tumor suppressor. Silencing of RBMS3 via CRISPR/Cas9 gene editing promoted growth of BRAFV600E lung organoids and promoted development of malignant lung cancers with a distinct micropapillary architecture in BRAFV600E and EGFRL858R GEM models. BRAFV600E/RBMS3Null lung tumors displayed elevated expression Ctnnb1, Ccnd1, Axin2, Lgr5, and c-Myc mRNAs, suggesting that RBMS3 silencing elevates signaling through the WNT/β-catenin signaling axis. Although RBMS3 silencing rendered BRAFV600E-driven lung tumors resistant to the effects of dabrafenib plus trametinib, the tumors were sensitive to inhibition of Porcupine, an acyltransferase of WNT ligands necessary for their secretion. Analysis of TCGA patient samples revealed that chromosome 3P24, which harbors RBMS3, is frequently lost in NSCLC and correlates with poor prognosis. Collectively, these data reveal the role of RBMS3 as a lung cancer suppressor and suggest RBMS3 silencing may contribute to malignant progression.
Keywords: BRAF, lung cancer, transposon, progression
INTRODUCTION
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death globally, with adenocarcinoma (LUAD) being the largest sub-type (1). Over the past decade, treatment outcomes have improved for lung cancer patients whose tumors are driven by actionable oncogenic mutations in genes such as Epidermal Growth Factor Receptor (EGFR), Anaplastic Lymphoma Kinase (ALK), Neurotrophin Tyrosine Kinase Receptor Type 1 (NTRK1) or the v-Raf murine sarcoma viral oncogene homolog B (BRAF). Indeed, these mutations serve as predictive biomarkers for the use of FDA-approved agents: osimertinib; alectinib, larotrectinib or dabrafenib plus trametinib, respectively (2–4). However, our understanding of how such oncoproteins promote the initiation, progression and maintenance of lung adenocarcinoma remains incomplete. Moreover, since lung cancers result from the accrual of multiple genetic/epigenetic alterations that cooperate in the conversion of normal lung cells into malignant lung cancer cells, we need a deeper mechanistic understanding of how such cooperation operates at the molecular and cellular levels and how it may influence lung cancer therapeutic strategies.
BRAFT1799A is detected in ~2% of NSCLC patients, translating to ~3,300 patients/year in the US (5,6). As a member of the RAF family of protein kinases, BRAF plays an important role in the activation of the RAS-regulated RAF>MEK>ERK Mitogen Activated Protein Kinase (MAPK) signal transduction pathway. This pathway plays a critical role in normal development and tissue homeostasis, and is frequently dysregulated in human tumorigenesis (7). BRAFT1799A encodes BRAFV600E, a constitutively active oncoprotein kinase, mutated in numerous malignancies including melanoma, hairy cell leukemia, colorectal, pancreatic and thyroid cancers (8). The importance of BRAFV600E in cancer maintenance is emphasized through the FDA approval of three pairwise targeted therapeutic combinations that target BRAFV600E>MEK>ERK signaling: 1. Vemurafenib plus cobimetinib; 2. Dabrafenib plus trametinib and; 3. Encorafenib plus binimetinib (9–11). However, although responses to vertical inhibition of BRAFV600E signaling often elicit striking responses, many patients develop lethal drug resistant disease, emphasizing the need for improved therapeutic approaches for these diseases.
We have previously described genetically engineered mice carrying conditional alleles of Braf engineered to express normal BRAF prior to CRE-mediated recombination after which BRAFV637E (analogous to human BRAFV600E, nomenclature used hereafter) is expressed at normal physiological, levels in cells in a temporally and spatially restricted manner (12). BrafCAT mice were further developed to express both BRAFV600E plus the tdTomato fluorescent reporter from a single bicistronic mRNA upon CRE-mediated recombination (13). Expression of BRAFV600E in alveolar type 2 (AT2) pneumocytes of the mouse lung elicits the development of clonally-derived, benign lung adenomas (12), the malignant progression of which such is constrained by a senescence-like growth arrest triggered by an insufficiency in WNT>β-catenin>c-MYC signaling (14). However, mutationally activated BRAFV600E cooperates with numerous alterations including silencing of TP53 or INK4A-ARF expression, as well as the expression of mutationally-activated PI3-kinase-α (PIK3CAH1047R) or β-catenin (CTNNB1Δex3) for malignant lung carcinogenesis (12,15). Sleeping Beauty-mediated transposon insertional mutagenesis (SB-TIM) has facilitated identification of genes that participate in various aspects of tumorigenesis in GEM models, and was previously used to identify genes that cooperate with BRAFV600E in melanomagenesis (16–19). Here, we employed SB-TIM to identify numerous candidate genes that cooperate with BRAFV600E in lung carcinogenesis. Indeed, CRISPR/CAS9 gene editing of one of these genes, Rbms3, allowed us to validate it as a novel suppressor of both BRAFV600E-and EGFRL858R- driven lung cancers. Importantly, data from our GEM models are consistent with the observation that loss of some or all of chromosome 3p, where human RBMS3 is located, is a common event in numerous human lung cancers. These data suggest that RBMS3 is a previously underappreciated, but frequently silenced lung cancer suppressor that cooperates with multiple oncogenic events to promote the malignant conversion of normal AT2 cells into lung cancer cells.
MATERIALS AND METHODS
SB tumor sequencing, informatics and statistical analyses
Illumina sequencing
Tumor DNA was extracted from formalin-fixed paraffin-embedded (FFPE) tissues using the Gentra PureGene cell kit (Qiagen; 158767) according to the manufacturer’s instructions, and barcoded genomic fragments containing transposon-genome junctions sequences were amplified using linker-mediated PCR (LM-PCR) as previously described (20). These products were sequenced on the Illumina 454 platform, from which unique sequencing reads were generated.
Raw processing of sequence data
28 tumors were taken from 10 mice and pair-end sequencing was performed using customized baits and aligned to the mouse genome reference assembly GRcm38 using BWA (version 1.16). The GATK ‘indel realigner’ was used to realign reads near indels from the Mouse Genome Programme to improve indel/SNP identification. The BAM files were re-sorted to recalibrate quality scores with the GATK ‘TableRecalibration” tool. SAMtools ‘calmd” was utilized to recalculate the MD/NM tags within the BAM files. Every lane from the same library were merged into a single BAM files using Picard tools (version 1.72) and PCR duplicates were marked using Picard ‘MarkDuplicates’.
Merging and Filtering
The BAM files were processed using RetroSeq 9version 1.41) to identify pair reads where one read aligned to the reference mouse genome and the other read to the Sleeping Beauty (SB) transposon sequence (Retroseq was operated in “Discovery” mode using the default parameters: Min anchor quality; 20; Min percent identity: 80; Min length for hit: 36). This generated a total of 72,981 individual putative transposon insertion regions (70 across all 28 tumor samples). The sequence and analysis methodologies do not allow the exact SB insertion sites to be identified to the resolution of genomic base pairs hence the location of transposons are referred to as regions as opposed to sites.
Overlapping, individual inverted repeats (IRs) within each sample were merged using bedtools to generate a set of 41,152 IRs. Chromosome four is the donor chromosome for the 6070 transposon line. To reduce the effects of local-hopping that can skew the downstream statistical analysis, all IRs that were located on chromosome four (4,609) were thereby excluded from the analysis. Insertions on the other secondary scaffolds e.x. GL45693, were also excluded. This left a total of 36,510 IRs. Further filtering of the IRs were performed by removing IRs within the regions of two known genes into which the SB concatemer preferentially inserts (on GRCm38:En2; chr5;2816569628173612 and Foxf2; chr13;31625816–31631403.) Nineteen genomic regions reported into which the SB transposon inserts under no selection pressure were also used to exclude IRs that are likely not to be cancer drivers. Following these final filtering step resulted in 36,426 IRs.
DNA sequences corresponding to mouse genomic regions flanking T2/Onc2 insertions sites were mapped using these complimentary standard bioinformatics approaches including the locus-centric Gaussian kernel convolution, as well as gene-centric common insertion site (gCIS) analysis (16,21). These methods uniformly help identify genomic regions with a higher density of transposon insertions, and strongly suggest these regions contain a potential cancer-relevant gene.
Common insertion sites and trunk driver analysis
Common insertion sites (CIS) were as described previously (22). Briefly, to detect CIS, a GKC method was employed using 15,000, 30,000, 50,000, 75,000, 120, 000, and 240,0900 kernel widths. When CISs were detected over several kernel widths, the CISs were merged and the smallest window size is reported. Gene-centric CISs (gCISs) were analyzed as previously described (16). In brief, a gene-centric statistical method was used to identify CIS genes such that genes that had 5 or more read counts, and had insertions in three or more tumors were selected as trunk driver genes. A Bonferroni correction was added to help eliminate false positives, and adjust the p-values (21). This initial list of candidate genes was further analyzed by bioinformatics tools such as Ingenuity Pathway Analysis, as well as STRING and DAVID tools to assess biological relevance, followed by cross-referencing of human tumors analyzed by TCGA.
Vertebrate Animals: Breeding and Experimental Manipulation
All animal care and experimental procedures were approved by Institutional Animal Care and Use Committees (IACUC) at both UCSF and HCI. All mice, whether at UCSF or HCI, were housed in environmentally controlled rooms. BrafCA (RRID:IMSR_JAX:017837), BrafCAT, H11LSL-CAS9 (provided by Dr. Monte Winslow, Stanford University; RRID:IMSR_JAX:026816 ) and T2/Onc2 (Strain 6070 [B6;C3-TgTn(sb-T2/Onc2)6070Njen] (MGI: 3613048) mice were bred as appropriate and genotyped as previously described (13,23). Mouse health was assessed using the Ullmann-Cullere Body Conditioning Score (BCS) to determine if euthanasia endpoints were met (24), at which point mouse lungs were inflated using either PBS or 10% neutral buffered formalin for perfusion through the larynx, followed by an additional cardiac perfusion of the lung though the right ventricle of the heart until the lungs turned white. Lungs were fixed for 24 hours in formalin prior to transfer to ethanol for paraffin-embedding and the generation of 4μm sections.
Recombinant adeno- or lentiviruses were administered to mice in a BSL2+ room per IACUC protocol and Institutional Biosafety Committee guidelines. Adenoviruses encoding CRE recombinase (Viraquest or the University of Iowa Viral Vector Core) were delivered through intranasal instillation, whereas the lentivirus encoding CRE (described below) was delivered through intratracheal instillation under isoflurane anesthesia (25). Tumor initiation was performed blinded to genotype. All mice were randomized equally among experimental groups based on gender, age, and the correct genotype. All mice used in these experiments had never undergone other experimental procedures. Mice were on a mixed background of C57BL/6, 129, and FVB.
Generation of Rosa-LSL-CAGGS-SB11 mice
The Rosa26-CAGGS-loxP-STOP-loxP (64)::SB mouse was created by taking a Rosa26 targeting vector (26,27) and engineered as follows: A construct with an EcoRV restriction site followed by 521bp of homology to the Rosa26 locus, intron 1 and exon 2 of the mouse Engrailed 2 gene, the CAGGS promoter, and loxP-flanked EGFP, 2xSV40 polyA sequences, and a bovine Growth Hormone (BGH) polyadenylation sequence was modified by inserting a linker containing NotI, XhoI, and SphI restriction sites into a SalI restriction site downstream of the 3’ loxP site. pCMV-SB11 (Addgene plasmid # 26552, Dr. Perry Hackett, University of Minnesota; RRID:SCR_002037) was modified to include a NotI site downstream of the SB11 coding sequence. This vector was digested with EagI and NotI and ligated into the NotI site of the modified Rosa26 targeting vector. A sequence containing SV40 polyA, a flippase-recognition target (FRT), PGK promoter, neomycin resistance cassette and BGH poly A signal, a second FRT, and 601bp of homology to the Rosa26 locus was isolated from the initial Rosa26 targeting vector and ligated into the XhoI site downstream of SB11. This shorter targeting vector was then recombined into a larger Rosa26 targeting construct containing 3.5kb and 2.9kb of Rosa26 homology on the 5’ and 3’ ends respectively. This plasmid was linearized and transfected into E14 mouse embryonic stem (ES) cells. DNA was isolated from selected ES cell clones, digested with ApaI, and screened by Southern blot using a probe (US1) outside of the targeting construct to identify clones with restriction fragment length polymorphisms indicating correct integration of the CAGGS-LSL-SB11 cassette into the Rosa26 locus. One clone (A3) was injected into blastocysts to generate 13 chimeric mice. Chimeric males were mated to 129/SvJ females; one chimera was able to propagate the targeted allele through the germline.
Southern blot probe sequence:
US1:
5’ctgggaaggttccttaagaagttatgttctgagaccattctcagtggctcaacaacacttggtcaaaaattttaattctcccctcagagaaatggagtagttactccactttcaagttccttataagcttaccatcaaccttatagtacactctagatgtcctgaaatatttctatcagaacaaggtagtataaagctggtaggtatacaaaacgctagactagtttctatccctgacccttaatctgctagtatatccgtaggaagttgcttaagtgccactagtacca3’
Cell lines, 2D and 3D culture conditions, and imaging
HEK-293T cells (ATCC; CRL-3216) were maintained in DMEM media supplemented with 10%(v/v) fetal bovine serum and 1% penicillin plus streptomycin. All established human cell lines used for these studies have been authenticated by STR profiling and mycoplasma testing is done quarterly using PlasmoTest (InvivoGen; rep-pt1).
Organoids were established by dissociating lung tissues minced with a razor and scissors in digestive media comprised of collagenase (400U/ml)(Life Tech #17100–017), dispase (5U/ml)(Corning # 354235), elastase (4U/ml) (Worthington 2279), and DNaseI (0.25mg/ml)(Sigma DN25–100 mg) in advanced DMEM:F12 HAM media in a 37°C shaker for 30 minutes. The resulting single cell suspension was strained using 100, 70 and 40-micron filters. Red blood cell (RBC) lysis was performed at room temperature by incubating each sample with 1x RBC Lysis Buffer (eBioscience; 00–4333-57). Finally, cells were seeded at 50,000 cells/well in matrigel (Corning; #356327 or #354230) in a 24-well plate. Organoids were initially (and experimentally) grown in organoid culture media containing Advanced DMEM/F12 (Gibco), 1x B-27 (Thermofisher; #17504001), N-2 (Thermofisher; #17502001), 1% Penicillin/Streptomycin, 1.25mM N-Acetylcysteine (Sigma-Aldrich; #A0737), 10 nM Gastrin (Sigma-Aldrich; G9020), 10μM Nicotinamide (Sigma-Aldrich; #47865-U), 100 ng/ml EGF (Peprotech; #AF-100–15), 100 ng/ml FGF10 (Peprotech; #100–26), 100ng/mL R-Spondin-1 (Peprotech; #315–32), and 100ng/mL Noggin (Peprotech; #25038) (28,29). To enrich for cells expressing BRAFV600E at organoid initiation, growth factors that activate ERK1/2 signaling (EGF or FGF) were obviated from the organoid media. Following organoid initiation experiments and prior to qRT-PCR, organoids were maintained and expanded in LWRN media (30).
Immunohistochemistry and immunofluorescence of lung sections
Immunohistochemistry and immunofluorescence was performed as previously described (31), with the following reagents: Xylene (Fisher Scientific; UN1307), Antigen Retrieval: Citrate Buffer pH6 (Sigma-Aldrich; #C9999), Peroxide Block: BLOXALL Blocking Solution (Vector Laboratories; SP-6000–100), Protein Block: Normal Horse Serum Blocking Solution 2.5 (Vector Laboratories; S2012–50); and primary antibodies: c-MYC (Santa Cruz; sc-764; 1:150) (32), pro-SPC (Millipore; AB3786; 1:2000), NKX2.1/TTF-1(Abcam; 76013-EP1584Y; 1:250; RRID:AB_1310784), EGFR-pY1068 (clone D7A5) XP (CST; 3777; 1:200), pERK T202/Y204 D13.14.4E XP (CST; 4370; 1:600; RRID: AB_10694057), Beta-Catenin D10A8 XP (CST; 8480; 1:50). Secondary antibody: ImmPRESS horse anti-rabbit IgG polymer kit; Peroxidase (Vector Laboratories; MP7401), DAB: ImmPACT DAB Eqv Peroxidase (HRP) Substrate (Vector Laboratories; SK-4103–400), Harris Hematoxylin Solution: (sigma; HHS32), Acid Alcohol (Fisher Scientific; 6769008), Bluing solution: Scott’s Tap Water 26070–07 (VWR; 100504–452) and mounted with Permount Mounting Medium (Fisher Scientific; SP15–500). Similarly, immunofluorescence staining of the Sleeping Beauty SB11 transposase was performed by fixing mouse lungs in zinc-buffered formalin, processed and embedded in paraffin, cut into 5-micron sections, and mounted on glass slides. Citrate mediated antigen retrieval was performed, followed by staining with the indicated primary antibody (R&D Systems; #AF2798).
Slide scanning, imaging, and histological analyses and quantification
Hematoxylin and eosin (H&E) stained slides, and immunofluorescence slides from the SB screen shown in Figure 1 were scanned using an Aperio Scanscope Scanner. Following H&E staining of sectioned lungs from remaining figures, as well as immunohistochemistry, or immunofluorescence analysis, slides of sectioned mouse lungs from each indicated genotype were loaded and scanned automatically using a 3D Histech Pannoramic MIDI scanner (ThermoFisher). Slides were imaged and analyzed using CaseViewer Software or the QuantCenter analytical center provided by 3D Histech, and experimental identifiers were blinded for all histological and immunohistochemical analyses. Tumor burden was manually calculated on each lung lobe and total tumor area was compared to total lung area. Tumor diameters were measured using QuantCenter software from 3D Histech. Cellprofiler was used to quantitate median fluorescence intensity with a previously described pipeline following immunofluorescence analysis of mouse tumor bearing lungs (13).
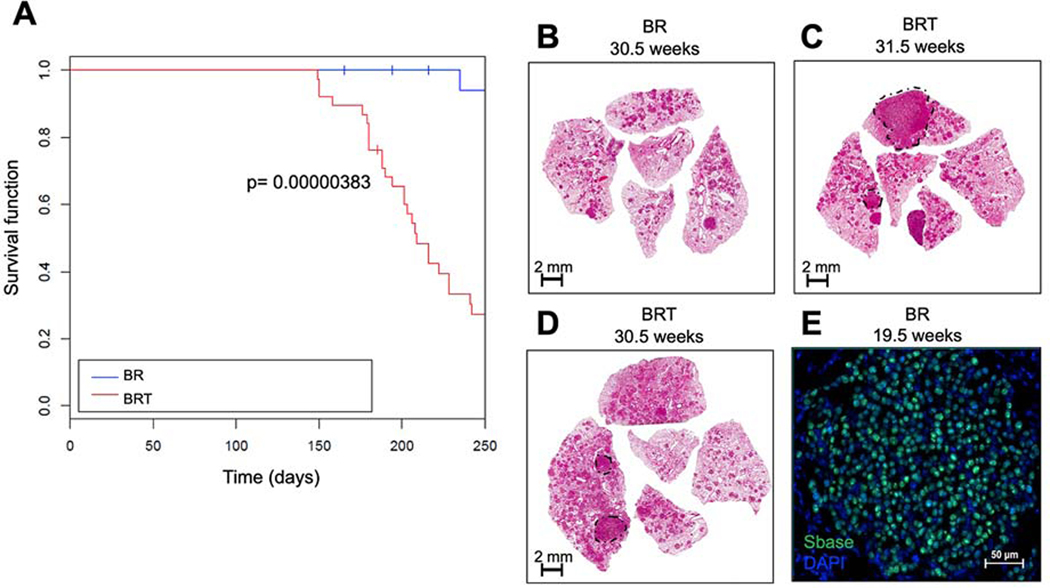
Figure 1. The Sleeping Beauty (SB) transposon system promotes lethal malignant progression of BRAFV600E-driven lung tumors in a GEM model.
A: Kaplan Meier survival curve tracking survival of 50 BrafCA and SB (CAGG/R26LSL-SB11) or (BR) mice, either with or without a T2/Onc2 transposon or (BRT) mice donor on chromosome 4 (C4T2/Onc2) for 250 days. Mice were initiated through intranasal instillation with 106 pfu of Ad5.CMV-CRE. Statistical analysis was performed using a log-rank Mantel-Cox test where p = 0.00000383.B-D: Histological analyses of formalin fixed paraffin embedded (FFPE) tumor bearing lung sections stained with hematoxylin and eosin (H&E). E: Expression of SB transposase in BR mouse lung tumors at 19.5 weeks post-initiation assessed by immunofluorescence analysis of FFPE sections of mouse lungs. DAPI stained DNA is blue and SB11 Transposase is in green.
Plasmid cloning, lentivirus production, cell transduction
The CAG-HA-RBMS3-PCDH cDNA expression plasmid was cloned using a cDNA template made from RNA from the lungs of a wild-type mouse using the Q5 polymerase (NEB) and restriction endonuclease cloning with the following primers: 5’: tttttGAATTCCCACCATGTACCCCTATGATGTGCCAGACTACGCCGGCAAACGCCTGGATCAGCCACAA. 3’: TttttgcggccgcCTATGGTTTGGACTGTTGGAAGGA. The cDNA was digested with EcoRI and NotI and then inserted into the pCDH mammalian expression vector. Lenti-sgNT/CRE and Lenti-sgLkb1/CRE was a gift from Monte Winslow (Addgene plasmid # 66894 and #66895) (23). Three sgRNAs designed against Rbms3 were cloned into the pLL3.3 sgRNA-CRE vector by modifying the original sgLkb1 plasmid with the Q5 Site-Directed Mutagenesis kit (NEB; #E0554S). The following sgRNAs against Rbms3 were used and pooled to make 2 lentiviruses.
Pool 1:
GTACACGTACTACTGTCCTC.
GAGCACGTCATGGACGCCAC.
ATGCAGCCAACTAACATCGT.
Pool 2:
ATGCAGCCAACTAACATCGT.
TTGGACACGTGATATCCACC.
ATCAAGCTATGTCAACCGTA.
Successful clones were verified by Sanger sequencing. Lentiviral supernatants were generated by co-transfection of HEK-293T cells using Transit-X2 (Mirus; #MIR 6004) with a 3-vector lentiviral system: using either the non-targeting sgRNA expression vector or sgRBMS3 sgRNA pool 1 or pool 2 combined with the lentiviral packaging and envelope plasmids pCMV-Δ8.9 or psPAX2 and pCMV-VSVG. pCMV-VSV-G was from Robert Weinberg (Addgene plasmid # 8454), psPAX2 is from Dider Trono (Addgene plasmid #12260) and pCMV-Δ8.9 is from PMID: 19561589 (25). Virus was collected 36, 48, 60, and 72 hours-post-transfection, and filtered using a 45-micron filter. Viral supernatants were concentrated by centrifugation at 25,000 rpm for 105 minutes at 4°C. Viral pellets were resuspended in PBS, and stored at −80C. Viral titering was performed using KP1 cells, and flow cytometric analysis of RFP+ cells as previously described (33). 5 × 104 – 1 × 105 pfu of lentivirus was administered in 75 μl volume per mouse during intratracheal administration of lentiviruses.
DNA isolation and the Surveyor assay
Lung tumor tissue was micro-dissected and isolated from FFPE tumor blocks and DNA was purified using the QIAamp kit (Qiagen; #56404). Alternatively, DNA was also isolated from cell lines using the DNeasy Blood and Tissue kit (Qiagen; #69504). The Surveyor assay mutation detection kit was used according to the manufacturer’s instructions (IDT; # 706025). PCR amplicons of Rbms3 for the Surveyor assay were generated sing the following primers: 5’ CTGGATCAGCCACAAATGTACCCCC. 3’ TGCTCTGGACCTGGTATGT. The following PCR conditions were utilized with the Q5 polymerase according to the manufacturer’s instructions for 25 or 50 μl reactions (NEB): 98° for 30s, 32 cycles of (98° for 10s, 53° for 20s, 72° for 40s), 72° for 2 m, and then stored at 4°C for short periods or −20 C for long-term storage.
RNA isolation and qRT-PCR
Lung tumors were isolated by laser-capture microdissection of formalin-fixed paraffin embedded (FFPE) blocks with RNA purified using the RNeasy FFPE Kit (Qiagen; #73504). RNA was purified from cultured organoids following dissociation with TrypLE (ThermoFisher; #12604013), pelleting, and resuspending the organoid cell suspension in Trizol (Invitrogen; #15596026). One-fifth volume of chloroform was added, and the tube was shaken vigorously, followed by centrifugation for 15 minutes at 12,000 x g at 4°C. The aqueous phase was transferred to a new tube, and 10μg of glycogen (ThermoFisher; #R0551) was added. RNA was precipitated with 1/10 volume pH 5.2 3M sodium Acetate (pH 5.2; Thermofisher; catalog #R1181) and 0.5 mL of isopropanol. After mixing the tube was incubated at −80°C for 30 minutes. The mixture was then centrifuged for 10 minutes at 12,000 x g at 4°C, the supernatant removed, and the pellet was washed with 1 mL of cold 75% ethanol. After vortexing, the samples were centrifuged again, before the pellets were air dried, and resuspended in RNase-free water.
cDNA was synthesized using 250ng of template RNA using iSCRIPT reverse transcription supermix (Bio-Rad; # 1708841) according to the manufacturer’s recommended protocol. SSOAdvanced Universal Probes Supermix (Bio-Rad; catalog # 1725280) was used also according to the manufacturer’s protocol. qRT-PCR was performed using Taqman Gene Expression assays (Applied Biosystems; Thermofisher) and the following 20x probes: Ppia (Mm03302254_g1 and Mm02342429_g1) as a housekeeping gene for normalization, Rbms3 (mm01350499_m1; mm00618362_m1; mm01350496_m1), Axin2 (Mm00443610_m1), Lgr5 (Mm00438890_m1), Ccnd1 (Mm00432359_m1), C-Myc (Mm00487804_m1), and Ctnnb1 (Mm00483029_g1).
cBioPortal analysis of human lung cancer databases
Point mutations were defined as single base-pair alterations and copy number alterations were defined with copy number values less than or equal to −1, consistent with TCGA standards. Search criteria involved listing chromosome arm 3p as a separate field that was queried and automatically aggregated. Individual gene copy number abnormality analysis was conducted through downloading individual patient .cnv files and aggregating manually. Point mutations for individual genes were queried directly within the site interface. Copy number variation (CNV) data were collected via cBioPortal for TCGA-LuSC (n=487) and TCGA-LuAD (n=500) projects from the PanCancer Atlas. From these data, deletions were defined as a copy number equal to −1 and gains defined as copy number equal to 1. Kaplan-Meier curves were generated between cohorts that incurred a deletion of the 3p arm against those that did not incur a deletion.
Data availability
All data and additional resources presented in this manuscript are available upon request to the corresponding author, if access is not already readily available through the indicated, established resources
RESULTS
The BrafCA|SB|Lung (BSL) Insertional Mutagenesis Screen
To conduct the insertional mutagenesis screen, we utilized the following genetic elements: 1. BrafCA, a CRE-activated allele of Braf (12); 2. RCL::SB, Rosa26-CAGGS-LSL-SB11, a CRE-activated SB11 transgene in the Rosa26 locus and; 3. C4T2/Onc2, a T2/Onc2 transposon donor located on chromosome 4. Two cohorts of mice (n=50/cohort) were generated: 1. BrafCA; RCL::SB; C4T2/Onc2 (BRT mice) and; 2. BrafCA; RCL::SB (BR mice), the latter lacking the T2/Onc2 transposon. Lung tumorigenesis was initiated by intranasal instillation of 106 pfu of adenovirus encoding CRE recombinase (Ad5.CMV-CRE). In control BR mice, the action of CRE recombinase delivered to the lung results in co-expression of BRAFV600E plus SB11 without the T2/Onc2 transposon, which is only present in the BRT mice. Initiated mice were monitored for signs of lethal lung tumorigenesis for 250 days (Fig. 1A). As anticipated, control BR mice did not develop disease over the monitoring period, whereas ~70% of BRT mice developed end-stage pulmonary disease as evidenced by labored breathing and/or loss of body weight requiring euthanasia. Median survival of initiated BRT mice was 205 days compared to 338 days for the BR mice (Fig. 1A, p=0.00000383). These data suggested that SB-mediated mobilization of the T2/Onc2 transposon dramatically accelerated malignant progression of BRAFV600E-driven lung tumorigenesis in BRT mice.
At euthanasia, mice were subjected to necropsy revealing that the lungs of BRT mice displayed histological evidence of malignant lung cancer in situ whereas the lungs of BR mice exclusively contained benign adenomas (Fig. 1B). BRT mice developed a wide range of tumor grades from benign adenomas to adenocarcinomas (Figs. 1B–D). To identify sites of T2/Onc2 insertion in the mouse genome, formalin-fixed, paraffin-embedded (FFPE) tissue from 28 individual lung cancers (from 10 mice) were micro-dissected from H&E-stained sections. Tumors from within the same mouse were selected for microdissection based on higher tumor grade (adenocarcinoma), larger tumor size, and detection of the SB transposase (by immunofluorescence; Fig. 1E). T2/Onc2 chromosomal insertion sites in genomic DNA of these tumors were identified by splinkerette PCR as described previously (34).
Collectively, a pool of bioinformatics analyses, tailored strategically towards SB mutagenesis screens, identified a stratified list of genes that cooperated with BRAFV600E to promote lung tumor progression in a statistically robust manner (Fig. 2 and Table 1). Bioinformatic analysis of the insertion sites suggested that most of the identified genes were likely inactivated by the T2/Onc2 transposon insertions consistent with our previous SB|Braf screen in melanoma (p < 0.005 ; Table 1) (16). Ultimately, this SB-TIM screen identified numerous candidates that might cooperate with BRAFV600E in driving malignant lung carcinogenesis.
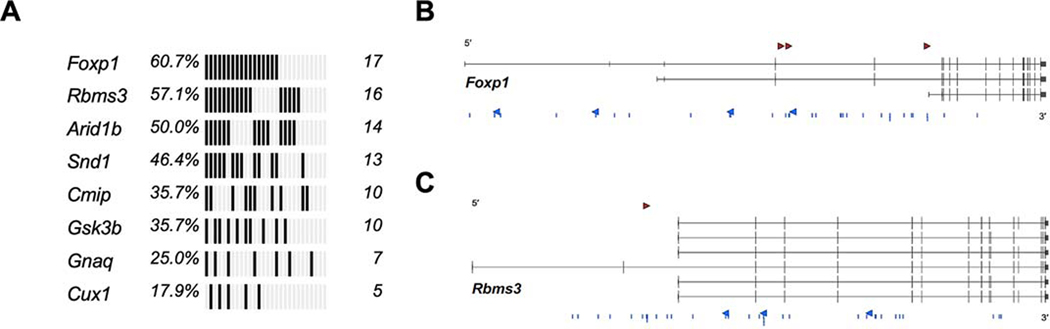
Figure 2. Genomic landscape of SB|Braf lung drivers.
A: Oncoprint of statistically significant drivers in BRAFV600E-driven lung tumors detected using GKC analysis, using SB common integration regions (CIRs), and Truncal SB Driver Analysis, using unique, directional SB insertions at TA-dinucleotides.B: SB insertions at TA-dinucleotides with sense (red arrowhead) and anti-sense (blue arrowheads) and within CIRs (blue lines) for Foxp1 (3 transcripts of the genes are shown).C: SB insertions at TA-dinucleotides with sense (red arrowhead) and anti-sense (blue arrowheads) and within CIRs (blue lines) for Rbms3 (6 transcripts of the candidate gene are shown).
Table 1.
Trunk CIS genes involved in lung adenocarcinoma progression of BRAFV600E-initiated tumors. CIS genes containing 5 or more sequence read counts per tumor from 3 or more tumors and have corrected p < 0.05 by gCIS analysis.
| Gene | p value (adj) |
|---|---|
| Cux1 | 3.25E-99 |
| Wapal | 1.24E-79 |
| Top1 | 1.50E-71 |
| Cmip | 5.90E-50 |
| Gnaq | 5.74E-23 |
| Snd1 | 3.75E-14 |
| Foxp1 | 1.73E-11 |
| Rbms3 | 4.99E-03 |
Rbms3 is a tumor suppressor that cooperates with BRAFV600E in lung carcinogenesis
The two most significantly enriched common insertion sites (CIS) identified in this screen were Foxp1 and Rbms3, which carried 17 and 16 SB insertions respectively (Figs. 2A–C). RBMS3 is a single-stranded RNA binding protein that has been implicated as a potential tumor suppressor in a number of malignancies, including squamous cell lung cancer (35–42). Furthermore, RBMS3 is implicated as a regulator of WNT signaling, a pathway shown to play critical roles in normal lung development and homeostasis, as well as progression of KRASG12D- or BRAFV600E-driven lung cancer (14,43–46).
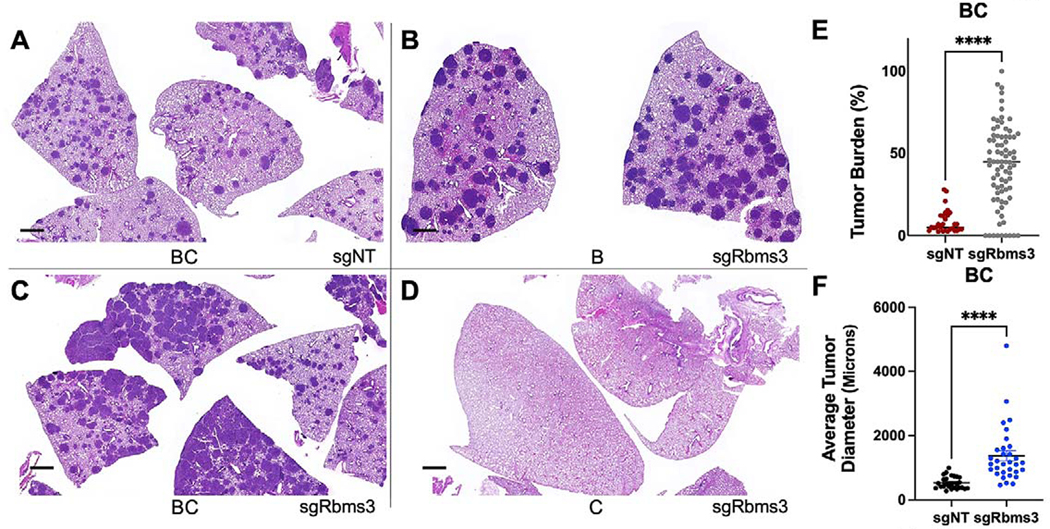
To assess the potential tumor suppressor activity of RBMS3 in BRAFV600E-driven lung carcinogenesis, lentiviral vectors expressing CRE recombinase and either a non-targeting control sgRNA (sgNT) or sgRNAs targeting Rbms3 were generated (23). Lentiviral supernatants were introduced by intratracheal intubation into the lungs of mice that carried the BrafCAT allele, or an H11LSL-CAS9 allele that allows for CRE-mediated CAS9 expression from the Hipp11 (H11) “safe-harbor” locus, either alone or in combination (23). These mice were euthanized at 11 weeks post-initiation for assessment of lung tumorigenesis and evaluated for differences. Infection of BrafCAT (B) mice with sgRbms3-CRE virus or of BrafCAT; H11LSL-CAS9 (BC) mice with sgNT-CRE virus led to the development of benign BRAFV600E-driven lung tumors, as expected (Figs. 3A & B). Strikingly, infection of BC mice with sgRbms3-CRE virus resulted in a significant increase in overall lung tumor burden as well as a significant increase in tumor diameter compared to relevant controls (p<0.0001; T-Test) (Figs. 3C–F). Importantly, no lung tumorigenesis was observed in the H11LSL-CAS9 (C) mice infected with sgRbms3-CRE virus control group (Fig. 3D). These data suggest that RBMS3 silencing was sufficient to bypass the senescence-like growth arrest observed in benign BRAFV600E-induced lung tumors (12,14). Analysis of genomic DNA isolated from large tumors confirmed CAS9-mediated editing of the Rbms3 gene (Supp. Fig. 1A). Moreover, unique, micro-dissected tumors also displayed a significant decrease of Rbms3 mRNA expression compared to controls (Supp. Fig. 1B). Collectively, these data suggest that: 1. Rbms3 was appropriately edited in vivo models using CRISPR/CAS9 editing technology; 2. CRISPR/CAS9-mediated Rbms3 gene editing resulted in substantially reduced Rbms3 mRNA expression and; 3. RBMS3 silencing promoted the progression of BRAFV600E-driven lung tumors.
Figure 3. CRISPR/Cas9 editing of Rbms3 cooperates with BRAFV600E in a mouse model of lung cancer.
A-D: Representative images of different genotypes of harvested mouse lung sections following necropsy analyses stained with hematoxylin and eosin (H&E) 13 weeks post initiation with 5 × 104 pfu lenti-CRE. CRISPR/CAS9-mediated genome editing was used in panels A, C, D to edit Rbms3 in vivo. Genotype and average tumor burden calculation of each experimental group was: A: sgNT-CRE virus in BrafCAT/+; H11LSL-CAS9 (BC) mice: 8.5%. B: sgRbms3-CRE virus in BrafCAT/+ (B) mice: 7.7%. C: sgRbms3-CRE virus in BC mice: 38.8%. D: sgRbms3-CRE virus in H11LSL-CAS9/+ (C) mice: 0%. Black bar in bottom left of each panel represents a 1000-micron scale bar. E: Quantification of individual tumor burden from genotypes in panel A compared to panel C. Tumor bearing lungs from panel B were identical to panel A. A paired T-test was used to determine statistical significance; p < 0.01.F: Quantification of tumor diameter was performed in microns using 25 individual tumors from genotypes in panel A compared to panel C using the 3D Histech MIDI Slide Scanner QuantCenter. Comprehensive analyses was conducted with over 200 lung tumors. N=50 mice individual or (biological replicates). N = 2 experimental replicates were performed comparing the indicated genotypes in A and C. Individual values are graphed, the black bar represents the mean, and the error bars represent SEM. A paired T-test was used to determine statistical significance; p < 0.0001.
Histological examination of BRAFV600E-driven lung tumors in this study was consistent with previous observations that such tumors are benign adenomas displaying characteristic cytomorphological features with a discreet papillary structure, a central fibrovascular core and with well-circumscribed borders (Supp. Figs. 2A–B) (12,14). Interestingly, the larger BRAFV600E-driven lung tumors (>1mm) that emerged in the context of reduced Rbms3 expression displayed clear evidence of cancer progression including poorly circumscribed borders, increased nuclear:cytoplasmic ratio, and avascular neoplastic nests free floating in air spaces (Supp. Figs. 2C–F). Importantly, we observed a distinct micropapillary architecture previously shown to be indicative of malignant adenocarcinoma of the lung in patients whose cancers are driven by EGFR, KRAS or BRAF oncoproteins (Supp. Figs. 2E–F) (47). This distinctive phenotype suggests that expression of BRAFV600E in combination with RBMS3 silencing in the mouse lungs mimics key features of human BRAFV600E-driven lung adenocarcinomas. These data are consistent with the hypothesis that RBMS3 is a tumor suppressor, such that its reduced/silenced expression promotes lung cancer progression.
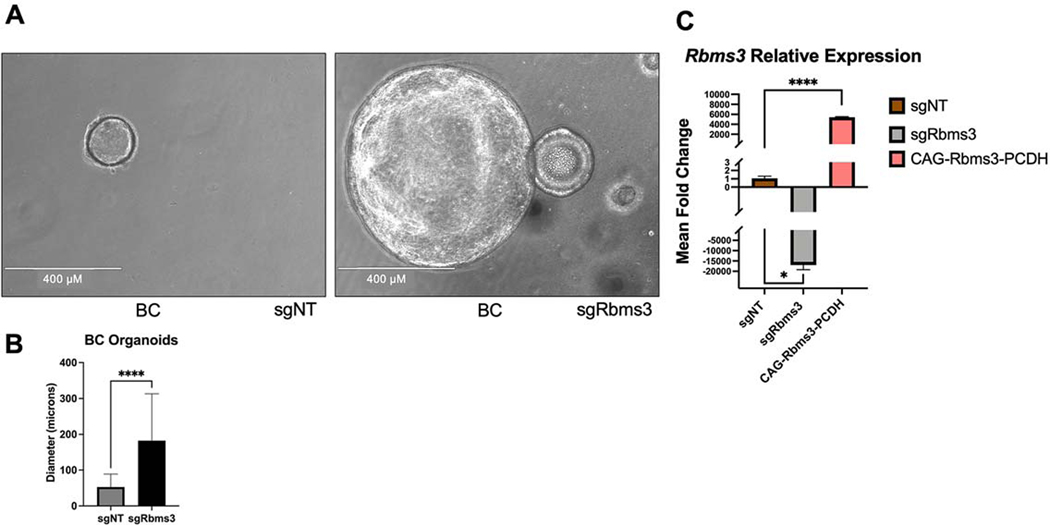
As an additional approach to test oncogenic cooperation between BRAFV600E expression and RBMS3 silencing we developed a mouse lung organotypic model system as a useful and relevant complement to our in vivo studies (28,48,49). When single cell suspensions of normal mouse lung are seeded in matrigel in the presence of: R-Spondin-1; Noggin; EGF and FGF10, organoids will develop over the course of approximately 7 days. Consequently, we generated single-cell suspensions from lungs of BC mice infected with either sgNT-CRE or sgRbms3-CRE viruses. After one week of culture, organoids established from BC mouse lungs in which BRAFV600E expression is combined with RBMS3 silencing were significantly larger than those observed with BRAFV600E expression alone (p<0.0001; T test) (Figs. 4A & 4B). Furthermore, as anticipated, organoids derived from BC mice infected with sgRbms3-CRE demonstrated lower Rbms3 mRNA expression compared to either control or to cells with ectopic expression of Rbms3 (Fig. 4C). These data provide additional evidence that RBMS3 silencing cooperates with BRAFV600E to promote lung organoid growth in vitro consistent with the in vivo experiments described above.
Figure 4. Rbms3 silencing cooperates with BRAFV600E to promote the growth of lung organoids.
A: Representative images are shown of qualitative analyses of phase contrast images of organoids established following tumor dissociation from BC mice at 7 days post-initiation of organoids. White scale bar indicates 400 microns, and was taken with 10x magnification. N=12 technical replicates with 3 biological replicates leveraging pooled lung lobes from N=8 mice. B: Quantification of organoid diameters at 7 days post-initiation of organoids from lungs of the indicated mouse genotypes described in (A). C: qRT-PCR analysis of Rbms3 mRNA expression in organoids derived from BC mice labeled by the lentivirus they were initiated with. Transient over-expression of wild-type Rbms3 was used as a positive control for gene expression. Mean is graphed and error bars represent SEM. Statistical analysis was conducted using a paired T test; * = p < 0.05; **** = p < 0.0001.
Rbms3 cooperates with EGFRL858R in lung carcinogenesis
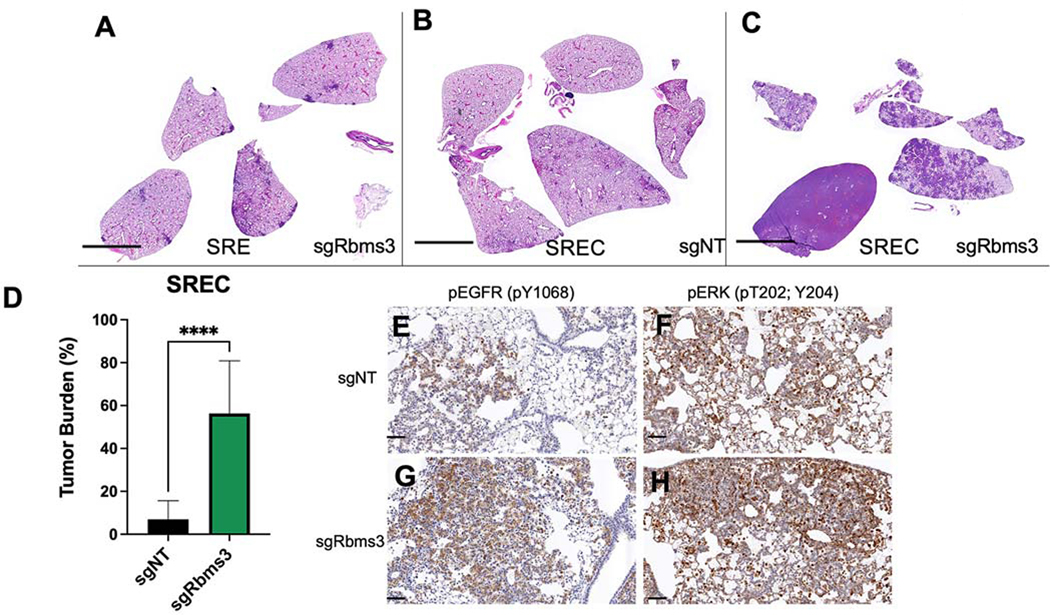
To determine if the cooperation observed between BRAFV600E and RBMS3 silencing was specific to BRAFV600E, we employed a GEM model of EGFRL858R-driven lung tumorigenesis (50). To that end we used mice carrying SPC::CRE-ERT2/+; Rosa26CAGs-LSL-rtTa3; TetO::EGFRL858R that either did (SREC) or did not (SRE) carry the H11LSL-CAS9/+ allele. In this model, EGFRL858R-driven lung tumorigenesis can be initiated by the activation of CRE-ERT2 in AT2 cells leading to induced expression of the reverse tetracycline transactivator (rtTa3) from the Rosa26 locus, or Cre. As previously described, subsequent addition of doxycycline to initiated mice leads to induced expression of EGFRL858R in AT2 cells and lung tumorigenesis (50). The lung epithelium of initiated SRE or SREC mice was infected with lentiviruses expressing either a control sgRNA (sgNT) or one targeted against Rbms3 as described above (Fig. 3). Here, tamoxifen was not administered to induce SPC specific delivery of CRE Recombinase, but minor leakiness may have occurred. Indeed, RBMS3 silencing cooperated significantly to accelerate EGFRL858R-driven tumorigenesis, compared to relevant controls where Rbms3 was not altered (Figs. 5A–D) (T-test; p < 0.0001). Combined expression of EGFRL858R with RBMS3 silencing led to diffuse replacement of the lung parenchyma with adenocarcinoma, and neoplastic cells arranged in disorganized clusters that replaced alveolar air spaces that displayed evidence of incited microhemorrhages (Supp. Figs. 3A–F). Cells in these tumors displayed expression of NKX2.1 and pro-Surfactant Protein C, markers of AT2 cell identity and well-differentiated lung adenocarcinoma (Supp. Figs. 3G–J). Interestingly, lung tumors with combined EGFRL858R expression and RBMS3 silencing displayed higher levels of phosphorylated EGFR and phosphorylated ERK1/2 compared to the relevant sgNT controls (Fig. 5E–H). We also observed that SREC mice initiated with sgRbms3 compared to sgNT controls (Supplementary Fig. 3K&L) harbored higher expression levels of β-Catenin protein by immunohistochemistry. Taken together, these data indicate that RBMS3 silencing cooperates with multiple drivers of lung tumorigenesis, expanding its relevance from just BRAFV600E to EGFRL858R.
Figure 5. Rbms3 loss cooperates with EGFRL858R to accelerate malignant lung adenocarcinoma.
A-C: Images of harvested mouse lung sections following necropsy analyses stained with hematoxylin and eosin (H&E) 11 weeks post initiation with 1 × 105 pfu lenti-CRE, followed by continuous administration of doxycycline chow to induce EGFRL858R expression. CRISPR/CAS9-mediated genome editing was used in panels B and C to edit Rbms3 in vivo. Genotype of each experimental group was: A: sgRbms3-CRE virus in SPC::CRE-ERT2/+; Rosa26CAGs-LSL-rTTa3; EGFRL858R (SRE) mice. B: sgNT-CRE virus in or SPC::CRE-ERT2/+; Rosa26CAGs-LSL-rTTa3; EGFRL858R; H11LSL-CAS9/+ (SREC) mice. C: sgRbms3-CRE virus in SREC mice. Black bar in bottom left of each panel represents a 1000-micron scale bar. D: Quantification of tumor burden from genotypes in panel B compared to panel C. N=5 mice per group. The mean is graphed, and error bars represent SEM. Statistical analysis was conducted using a paired T test; **** = p < 0.0001. E: Representative images of immunohistochemistry on FFPE lung tissue sections from SREC mice initiated with either sgNT- or sgRbms3-CRE and stained with pEGFR (pY1068) or pERK (pT202; Y204) shown at 20x magnification. Scale bar shown in black at the bottom left corner of each image represent 50 microns.
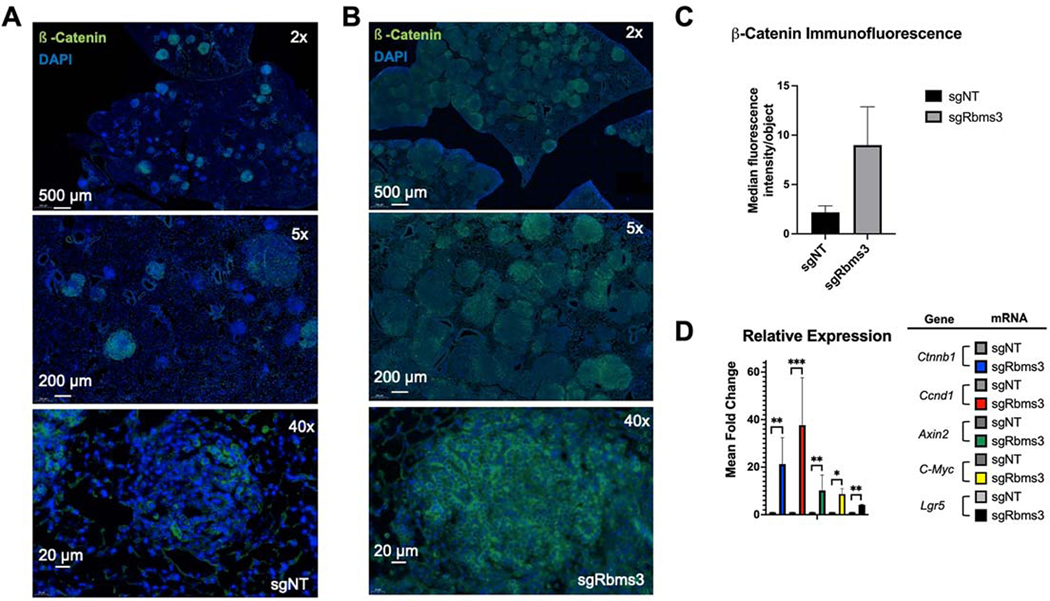
To elucidate the mechanism(s) by which RBMS3 silencing promotes the progression of BRAFV600E-driven lung tumors, we considered previously reported mechanisms of RBMS3 action. It has been reported that RBMS3 binds to the 3’ UTR of c-MYC mRNA such that RBMS3 silencing might lead to elevated expression of c-MYC, or β-Catenin, a key effector of the WNT signaling pathway (14,35,37–40,42–46). When tissue sections were analyzed by immunofluorescence we observed a robust increase in β-Catenin (CTNNB1) expression in BRAFV600E-driven lung tumors without RBMS3 (Figs. 6A–C). To expand on this, we evaluated organoids from BC mouse lungs carrying either sgNT or sgRbms3 for differences in mRNA expression of components and transcriptional targets of the WNT signaling pathway, or specifically β-Catenin/TCF/LEF. Here, we identified a significant increase in relative gene expression of Ctnnb1, as well as known target genes: Ccnd1, Axin2, c-Myc, and Lgr5 (Fig. 6D) in sgRbms3 BC organoids, suggesting that RBMS3 silencing promotes WNT pathway signaling, a pathway known to be rate-limiting for progression of BRAFV600E-driven lung tumors (14).
Figure 6. WNT signaling components are expressed at higher levels in BC lung tumors without Rbms3.
A&B: Representative images of β-catenin expression as assessed by indirect immunofluorescent in tumor bearing FFPE BC mouse lung sections (shown at 2x, 5x, and 40 magnifications) initiated with either A: sgNT-CRE or B: sgRbms3-CRE. Scale bars are shown in white in the bottom left corner of each image as indicated. C: Median fluorescence intensity quantitation using cellprofiler software. D: qRT-PCR analysis of BC organoids from the indicated viral initiation groups using probes to detect Ctnnb1, Ccnd1, Axin2, Lgr5, or c-Myc mRNAs. Mean is graphed with error bars as SEM. Statistical analysis was conducted using a paired T test; * = p < 0.05; **= p < 0.01; ***= p <0.001; **** = p < 0.0001.
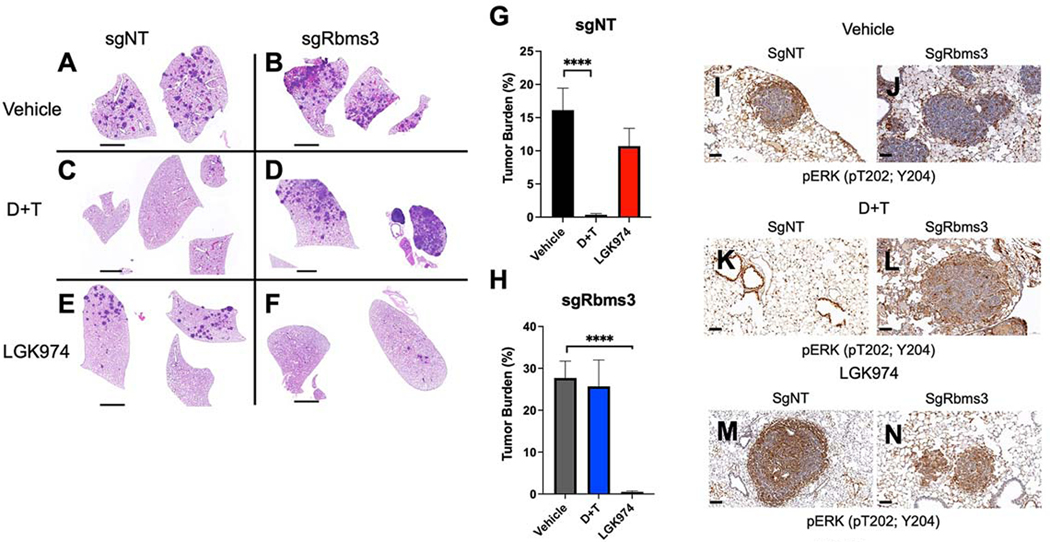
Vertical inhibition of BRAFV600E signaling with dabrafenib + trametinib (D+T) is the standard of care for this molecularly defined subset of lung cancer patients, so we sought to explore the consequences of RBMS3 silencing on pathway targeted therapies against BRAFV600E (D+T), or with LGK974 (a Porcupine/WNT pathway inhibitor). To that end, BC mice were initiated with lenti-CRE vectors that carried either sgNT or sgRbms3 and, six weeks post-initiation, such mice were randomized for treatment with: 1. Vehicle control; 2. D+T or; 3. LGK974 for a fixed period of 5 weeks at which time mice were euthanized and lung tumor burden was quantified. This revealed that while sgNT BC tumor-bearing mice were sensitive to D+T as expected, whereas LGK974 had only a modest effect on tumor burden, but importantly, lung tumors initiated in BC mice with sgRbms3-CRE demonstrated resistance to the anti-tumor effects of D+T, but displayed striking sensitivity to the anti-tumor effects of LGK974 (Figs. 7A–H). Immunohistochemical analysis of pERK1/2 in tumors of drug treated mice revealed potent MAPK pathway suppression with D+T compared to vehicle controls, but maintained positive pERK staining when treated with LGK974 (Fig. 7I–N). Moreover, immunohistochemistry revealed that BC lung tumors initiated with Rbms3-CRE displayed readily detectable expression of SPC and NKX2.1, suggesting that they maintained a well-differentiated state (Supp. Figs. 4A–L). Importantly, SREC mice that are RBMS3Null compared to NT controls (Supplementary Fig. 3K&L) revealed higher levels of β-Catenin protein by immunohistochemistry. Similarly, BC mice treated with vehicle also revealed higher β-Catenin protein expression, further supporting upregulated expression of β-Catenin where RBMS3 expression is silenced (Supp. Fig. 4M–R; compare panels N to M, P to O, and R to Q). Collectively, this data reveals that RBMS3 silencing was sufficient to promote resistance to D+T, and it appeared to enhance the sensitivity of BRAFV600E-driven lung tumors to Porcupine inhibition with LGK974.
Figure 7: Rbms3 silencing drives resistance to pathway-targeted inhibition of BRAFV600E while adapting sensitivity to inhibition of Porcupine.
A: Representative images of H&E stained lung sections harvested 11 weeks post initiation from BC mice following treatment with the indicated pharmacological agents starting at 6 weeks post initiation with 5 × 104 pfu lenti-CRE. BC mice were dosed once daily for 5 weeks with: 1. Vehicle control; 2. dabrafenib (75 mg/kg ) plus trametinib (1 mg/kg) or; 3. LGK974 (5mg/kg). B&C: Quantification of lung tumor burden in BC mice initiated with sgNT-CRE or sgRbms3-CRE and dosed with the indicated pharmacological agents as indicated. Mean tumor burden is graphed, and error bars represent SEM. N= 5–7 mice per dosing arm. Statistical analysis was performed using a one-way ANOVA (**** = p<0.0001.)
Finally, to assess the possible prevalence of alterations in RBMS3 in human lung cancer, we evaluated existing patient data from The Cancer Genome Atlas (TCGA) available through the cBio portal for cancer genomics (51,52). Initial analysis indicated that point mutations in the RBMS3 gene are rare in this collection of human lung tumors. Analysis of changes in the region of chromosome 3p24 where RBMS3 is located in lung cancer patients gave a striking pattern of changes (Supp. Fig 5A). We documented that >45% of lung adenocarcinomas and >89% of lung squamous carcinomas displayed loss of copy number of RBMS3 on chromosome 3p24 (Supp. Figs. 5B–D). However, it must be noted that the majority of these cases showed loss of the entire 3p arm of chromosome 3, not focal deletions of RBMS3. Specifically, 77.8% of squamous cell carcinoma patients (87% of the patients who lost RBMS3) and 38.7% of adenocarcinoma patients (86% of patients who lost RBMS3), showed loss of the entire 3p arm (Supp Fig. 5). Interestingly, these copy number alterations in RBMS3 frequently co-occurred with both EGFR or BRAF oncogenic mutations in these patients (Supp. Fig. 5F). Furthermore, there was a correlation between loss of chromosome 3p in patients and poorer prognosis for such lung cancer patients compared to those whose lung tumors retained chromosome 3p24 (Supp Fig. 5E).
DISCUSSION
Transposon-mediated mutagenesis has played a prominent role in the genetic analysis of normal development, physiology and of various diseases, especially in models of human cancer (53,54). Indeed, the initial identification of c-MYC and WNT1 as oncogenes came from analysis of lymphoma in chickens and mammary neoplasias in mice respectively (55–57). More recently, the resurrection of the TC1/Mariner-based Sleeping Beauty transposase in conjunction with engineered T2/Onc transposable elements in both mice or zebrafish have served to identify numerous tumor suppressors and/or oncogenes involved in cancer initiation, progression or maintenance (16,21,22,26,27,58–64).
Here, we describe the use of transposon mutagenesis to identify genes that cooperate to promote progression of BRAFV600E-driven benign adenomas to malignant lung cancers. While this screen was not saturating, since known suppressors of BRAFV600E-driven lung cancer such as Trp53, Pten or Cdkn2a were not identified, it was advantageous in that we identified a number of new cooperating genes including: Rbms3, Foxp1, Arid1b, Snd1, Gnaq and Cux1. Some of these have previously been shown to play a role in cancer: 1. SB-mediated Cux1 inactivation was reported to promote progression of myeloid malignancies (65); 2. GNAQ, the human ortholog of Gnaq, is a noted human oncogene mutated in uveal melanoma (66); 3. Foxp1 encodes a transcriptional regulator of lung endoderm development, but is reported to contribute to various cancers (67,68); and 4. SND1 has been found fused to BRAF to form an oncogenic fusion gene in never smoker lung adenocarcinoma (69). Hence, our SB screen collectively identified a number of genes implicated in neoplastic transformation.
The mechanistic role of RBMS3 in normal development, physiology or cancer remains largely obscure, but previous work has shed light on select aspects of its potential biological roles (70). The protein contains two pairs of RNA binding motifs and is related to members of the c-MYC single-strand binding proteins (MSSPs) that are thought to regulate DNA replication, transcription, apoptosis and cell cycle progression by interacting with c-MYC (35). Here, we shown that RBMS3 silencing, in combination with either the BRAFV600E or the EGFRL858R oncoproteins, can promote lung carcinogenesis. The precise mechanism of cooperation remains uncertain but given its reported ability to regulate signaling through the WNT>β-catenin>c-MYC, a pathway essential for KRASG12D- or BRAFV600E-driven lung cancer, this seems like a likely mechanism (35–37,41). Moreover, other studies have suggested a role for RBMS3 as a suppressor of breast, esophageal, ovarian, gastric cancer, and lung squamous cell carcinoma (36–42). Interestingly, RBMS3 is located on chromosome 3 in a region that undergoes copy number loss in a substantial number of lung cancer patients. Taken together, this data provides a compelling rationale for developing a deeper mechanistic understanding of the biochemical mechanisms of RBMS3 tumor suppression, and how loss of such cooperates with common lung cancer oncoprotein kinases such as BRAFV600E and EGFRL858R.
Numerous and diverse approaches have demonstrated that WNT signaling is critical in normal lung development, physiology, and in the progression and/or maintenance of lung cancer (14,43–46). These data not only emphasize the importance of this pathway in lung cancer, but brings about a critical question, begging whether all roads eventually lead to WNT signaling as a major player regulating lung cancer progression? We have previously shown that WNT>β-catenin signaling is essential for BRAFV600E-induced benign lung tumorigenesis. Moreover, diminished WNT signaling serves as a barrier to the malignant progression of BRAFV600E-induced benign adenomas (14). Mechanistically, BRAFV600E plus WNT>β-catenin signaling cooperatively converge to promote expression of c-MYC. Moreover, WNT signaling is also reported to be essential for growth of KRASG12D/TP53Null GEM lung cancers (45). Here, RBMS3 silencing led to elevated expression of β-Catenin, Axin2, Lgr5, c-Myc and Cyclin D1 mRNAs in lung tumors, indicating that silencing of the pathway is promoting transcription of relevant WNT target genes. Interestingly, BRAFV600E/RBMS3Null lung tumors remained sensitive to LGK974, a potent and specific inhibitor of Porcupine (PRCN), the enzyme essential for post-translational acylation and secretion of most WNT ligands (71,72). Our work also revealed that RBMS3 silencing led to resistance of BRAFV600E-driven lung tumors to pathway-targeted inhibition of BRAFV600E signaling. RBMS3 has been implicated in the regulation of TWIST1 expression in metastatic breast cancer, by directly binding to the 3’-UTR of Twist1 mRNA (42). Finally, given the ability of RBMS3 silencing to elicit drug resistance in BRAFV600E-driven lung tumors, it would be interesting in the future to test whether the same is true in lung cancers driven by mutationally-activated EGFR, which could have clinical implications for the treatment of this set of patients.
In summary, using a variety of technique starting with SB-mediated transposon mutagenesis, we revealed RBMS3 to be a suppressor of lung tumorigenesis driven by the BRAFV600E or EGFRL858R oncoprotein kinases. Interestingly, RBMS3 is located on a region of chromosome 3 that is subject to copy number loss in a significant number of lung cancer patients.
Supplementary Material
SIGNIFICANCE.
Loss of RBMS3 cooperates with BRAFV600E to induce lung tumorigenesis, providing a deeper understanding of the molecular mechanisms underlying mutant BRAF-driven lung cancer and potential strategies to more effectively target this disease.
ACKNOWLEDGEMENTS
The authors have made a good-faith effort to appropriately acknowledge everyone who contributed to this project over the past 12 years. The authors thank Dr. Shin-Heng Chiou from Dr. Monte Winslow’s lab at Stanford University for technical advice and assistance in preparing reagents and executing experiments for these studies, and Dr. Winslow himself for graciously sharing the H11LSL-CAS9 mice with us. The authors thank Dr. Rachelle Olsen and Dr. Gurkan Mollaoglu from Dr. Trudy Oliver’s lab at the Huntsman Cancer Institute for their assistance in providing control samples and troubleshooting for certain experiments. The authors thank Dr. Eric Snyder and the members of his lab, especially Dr. Soledad Camolotto and Katy Gillis, for technical expertise and assistance supporting these studies. We particularly acknowledge and thank Dr. Harold Varmus for graciously providing key mouse strains from his laboratory in advance of their publication. The authors thank the staff of the following University of Utah or HCI Shared Resources for advice, guidance and technical support: 1. High Throughput Genomics; 2. Flow Cytometry and; 3. Biorepository and Molecular Pathology Shared Resources (Supported by CA042014). Financial support for this publication was supported by the NCI R01_CA131261 to MM, F32CA228267 and K99CA246084 to AV, Cancer Research UK 21717 to DA, as well as a Genentech Foundation Graduate Fellowship Award to JJ.
Footnotes
Conflicts of interest
Dr. McMahon has served on advisory boards to Genentech Discovery Oncology, Pfizer Oncology, ARO Biotherapeutics, Revolution Medicine.
All additional authors declare no potential conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30 [DOI] [PubMed] [Google Scholar]
- 2.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113–25 [DOI] [PubMed] [Google Scholar]
- 4.Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829–38 [DOI] [PubMed] [Google Scholar]
- 5.Vultur A, Herlyn M. SnapShot: melanoma. Cancer Cell 2013;23:706–e1 [DOI] [PubMed] [Google Scholar]
- 6.Heist RS, Engelman JA. SnapShot: non-small cell lung cancer. Cancer Cell 2012;21:448 e2. [DOI] [PubMed] [Google Scholar]
- 7.Holderfield M, Deuker MM, McCormick F, McMahon M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nature reviews Cancer 2014;14:455–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Planchard D, Smit EF, Groen HJM, Mazieres J, Besse B, Helland A, et al. Dabrafenib plus trametinib in patients with previously untreated BRAF(V600E)-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307–16 [DOI] [PubMed] [Google Scholar]
- 10.Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2018;19:603–15 [DOI] [PubMed] [Google Scholar]
- 11.Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867–76 [DOI] [PubMed] [Google Scholar]
- 12.Dankort D, Filenova E, Collado M, Serrano M, Jones K, McMahon M. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev 2007;21:379–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Veen JE, Scherzer M, Boshuizen J, Chu M, Liu A, Landman A, et al. Mutationally-activated PI3’-kinase-alpha promotes de-differentiation of lung tumors initiated by the BRAF(V600E) oncoprotein kinase. Elife 2019;8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Juan J, Muraguchi T, Iezza G, Sears RC, McMahon M. Diminished WNT -> beta-catenin -> c-MYC signaling is a barrier for malignant progression of BRAFV600E-induced lung tumors. Genes Dev 2014;28:561–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trejo CL, Green S, Marsh V, Collisson EA, Iezza G, Phillips WA, et al. Mutationally activated PIK3CA(H1047R) cooperates with BRAF(V600E) to promote lung cancer progression. Cancer Res 2013;73:6448–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann MB, Black MA, Jones DJ, Ward JM, Yew CC, Newberg JY, et al. Transposon mutagenesis identifies genetic drivers of Braf(V600E) melanoma. Nat Genet 2015;47:486–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montero-Conde C, Leandro-Garcia LJ, Chen X, Oler G, Ruiz-Llorente S, Ryder M, et al. Transposon mutagenesis identifies chromatin modifiers cooperating with Ras in thyroid tumorigenesis and detects ATXN7 as a cancer gene. Proc Natl Acad Sci U S A 2017;114:E4951–E60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber J, Braun CJ, Saur D, Rad R. In vivo functional screening for systems-level integrative cancer genomics. Nature reviews Cancer 2020;20:573–93 [DOI] [PubMed] [Google Scholar]
- 19.de la Rosa J, Weber J, Friedrich MJ, Li Y, Rad L, Ponstingl H, et al. A single-copy Sleeping Beauty transposon mutagenesis screen identifies new PTEN-cooperating tumor suppressor genes. Nature genetics 2017;49:730–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uren AG, Mikkers H, Kool J, van der Weyden L, Lund AH, Wilson CH, et al. A high-throughput splinkerette-PCR method for the isolation and sequencing of retroviral insertion sites. Nat Protoc 2009;4:789–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brett BT, Berquam-Vrieze KE, Nannapaneni K, Huang J, Scheetz TE, Dupuy AJ. Novel molecular and computational methods improve the accuracy of insertion site analysis in Sleeping Beauty-induced tumors. PLoS One 2011;6:e24668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda H, Rust AG, Ward JM, Yew CC, Jenkins NA, Copeland NG. Sleeping Beauty transposon mutagenesis identifies genes that cooperate with mutant Smad4 in gastric cancer development. Proc Natl Acad Sci U S A 2016;113:E2057–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiou SH, Winters IP, Wang J, Naranjo S, Dudgeon C, Tamburini FB, et al. Pancreatic cancer modeling using retrograde viral vector delivery and in vivo CRISPR/Cas9-mediated somatic genome editing. Genes Dev 2015;29:1576–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ullman-Cullere MH, Foltz CJ. Body condition scoring: a rapid and accurate method for assessing health status in mice. Lab Anim Sci 1999;49:319–23 [PubMed] [Google Scholar]
- 25.DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc 2009;4:1064–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collier LS, Carlson CM, Ravimohan S, Dupuy AJ, Largaespada DA. Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature 2005;436:272–6 [DOI] [PubMed] [Google Scholar]
- 27.Dupuy AJ, Akagi K, Largaespada DA, Copeland NG, Jenkins NA. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature 2005;436:221–6 [DOI] [PubMed] [Google Scholar]
- 28.Boj SF, Hwang CI, Baker LA, Chio II, Engle DD, Corbo V, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 2015;160:324–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zewdu R, Mehrabad EM, Ingram K, Fang P, Gillis KL, Camolotto SA, et al. An NKX2–1/ERK/WNT feedback loop modulates gastric identity and response to targeted therapy in lung adenocarcinoma. Elife 2021;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyoshi H, Stappenbeck TS. In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat Protoc 2013;8:2471–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaishnavi A, Schubert L, Rix U, Marek LA, Le AT, Keysar SB, et al. EGFR Mediates Responses to Small-Molecule Drugs Targeting Oncogenic Fusion Kinases. Cancer Res 2017;77:3551–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mollaoglu G, Guthrie MR, Bohm S, Bragelmann J, Can I, Ballieu PM, et al. MYC Drives Progression of Small Cell Lung Cancer to a Variant Neuroendocrine Subtype with Vulnerability to Aurora Kinase Inhibition. Cancer Cell 2017;31:270–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camolotto SA, Pattabiraman S, Mosbruger TL, Jones A, Belova VK, Orstad G, et al. FoxA1 and FoxA2 drive gastric differentiation and suppress squamous identity in NKX2–1-negative lung cancer. Elife 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su Q, Prosser HM, Campos LS, Ortiz M, Nakamura T, Warren M, et al. A DNA transposon-based approach to validate oncogenic mutations in the mouse. Proc Natl Acad Sci U S A 2008;105:19904–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penkov D, Ni R, Else C, Pinol-Roma S, Ramirez F, Tanaka S. Cloning of a human gene closely related to the genes coding for the c-myc single-strand binding proteins. Gene 2000;243:27–36 [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Yun D, Zhao Y, Wang Y, Sun R, Yan Q, et al. Down regulation of RNA binding motif, single-stranded interacting protein 3, along with up regulation of nuclear HIF1A correlates with poor prognosis in patients with gastric cancer. Oncotarget 2017;8:1262–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Chen L, Nie CJ, Zeng TT, Liu H, Mao X, et al. Downregulation of RBMS3 is associated with poor prognosis in esophageal squamous cell carcinoma. Cancer Res 2011;71:6106–15 [DOI] [PubMed] [Google Scholar]
- 38.Wu G, Cao L, Zhu J, Tan Z, Tang M, Li Z, et al. Loss of RBMS3 Confers Platinum Resistance in Epithelial Ovarian Cancer via Activation of miR-126–5p/beta-catenin/CBP signaling. Clin Cancer Res 2019;25:1022–35 [DOI] [PubMed] [Google Scholar]
- 39.Chen J, Kwong DL, Zhu CL, Chen LL, Dong SS, Zhang LY, et al. RBMS3 at 3p24 inhibits nasopharyngeal carcinoma development via inhibiting cell proliferation, angiogenesis, and inducing apoptosis. PLoS One 2012;7:e44636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y, Quan L, Ling Y. RBMS3 Inhibits the Proliferation and Metastasis of Breast Cancer Cells. Oncol Res 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang YN, Liu Y, Meng Q, Li X, Wang F, Yao G, et al. RBMS3 is a tumor suppressor gene that acts as a favorable prognostic marker in lung squamous cell carcinoma. Med Oncol 2015;32:459. [DOI] [PubMed] [Google Scholar]
- 42.Zhu L, Xi PW, Li XX, Sun X, Zhou WB, Xia TS, et al. The RNA binding protein RBMS3 inhibits the metastasis of breast cancer by regulating Twist1 expression. J Exp Clin Cancer Res 2019;38:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nabhan AN, Brownfield DG, Harbury PB, Krasnow MA, Desai TJ. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science 2018;359:1118–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadzik RS, Cohen ED, Morley MP, Stewart KM, Lu MM, Morrisey EE. Wnt ligand/Frizzled 2 receptor signaling regulates tube shape and branch-point formation in the lung through control of epithelial cell shape. Proc Natl Acad Sci U S A 2014;111:12444–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tammela T, Sanchez-Rivera FJ, Cetinbas NM, Wu K, Joshi NS, Helenius K, et al. A Wnt-producing niche drives proliferative potential and progression in lung adenocarcinoma. Nature 2017;545:355–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 2014;507:190–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Oliveira Duarte Achcar R, Nikiforova MN, Yousem SA. Micropapillary lung adenocarcinoma: EGFR, K-ras, and BRAF mutational profile. American journal of clinical pathology 2009;131:694–700 [DOI] [PubMed] [Google Scholar]
- 48.Fatehullah A, Tan SH, Barker N. Organoids as an in vitro model of human development and disease. Nat Cell Biol 2016;18:246–54 [DOI] [PubMed] [Google Scholar]
- 49.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A 2009;106:12771–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev 2006;20:1496–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wieschaus E, Nusslein-Volhard C. The Heidelberg Screen for Pattern Mutants of Drosophila: A Personal Account. Annu Rev Cell Dev Biol 2016;32:1–46 [DOI] [PubMed] [Google Scholar]
- 54.Cain AK, Barquist L, Goodman AL, Paulsen IT, Parkhill J, van Opijnen T. A decade of advances in transposon-insertion sequencing. Nat Rev Genet 2020;21:526–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hayward WS, Neel BG, Astrin SM. Activation of a cellular onc gene by promoter insertion in ALV-induced lymphoid leukosis. Nature 1981;290:475–80 [DOI] [PubMed] [Google Scholar]
- 56.Neel BG, Hayward WS, Robinson HL, Fang J, Astrin SM. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell 1981;23:323–34 [DOI] [PubMed] [Google Scholar]
- 57.Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell 1982;31:99–109 [DOI] [PubMed] [Google Scholar]
- 58.Copeland NG, Jenkins NA. Harnessing transposons for cancer gene discovery. Nature reviews Cancer 2010;10:696–706 [DOI] [PubMed] [Google Scholar]
- 59.Mann KM, Ward JM, Yew CC, Kovochich A, Dawson DW, Black MA, et al. Sleeping Beauty mutagenesis reveals cooperating mutations and pathways in pancreatic adenocarcinoma. Proc Natl Acad Sci U S A 2012;109:5934–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Collier LS, Adams DJ, Hackett CS, Bendzick LE, Akagi K, Davies MN, et al. Whole-body sleeping beauty mutagenesis can cause penetrant leukemia/lymphoma and rare high-grade glioma without associated embryonic lethality. Cancer Res 2009;69:8429–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.To JC, Chiu AP, Tschida BR, Lo LH, Chiu CH, Li XX, et al. ZBTB20 regulates WNT/CTNNB1 signalling pathway by suppressing PPARG during hepatocellular carcinoma tumourigenesis. JHEP Rep 2021;3:100223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beckmann PJ, Larson JD, Larsson AT, Ostergaard JP, Wagner S, Rahrmann EP, et al. Sleeping Beauty Insertional Mutagenesis Reveals Important Genetic Drivers of Central Nervous System Embryonal Tumors. Cancer Res 2019;79:905–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahrmann EP, Wolf NK, Otto GM, Heltemes-Harris L, Ramsey LB, Shu J, et al. Sleeping Beauty Screen Identifies RREB1 and Other Genetic Drivers in Human B-cell Lymphoma. Molecular cancer research : MCR 2019;17:567–82 [DOI] [PubMed] [Google Scholar]
- 64.McGrail M, Hatler JM, Kuang X, Liao HK, Nannapaneni K, Watt KE, et al. Somatic mutagenesis with a Sleeping Beauty transposon system leads to solid tumor formation in zebrafish. PLoS One 2011;6:e18826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wong CC, Martincorena I, Rust AG, Rashid M, Alifrangis C, Alexandrov LB, et al. Inactivating CUX1 mutations promote tumorigenesis. Nature genetics 2014;46:33–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Truong A, Yoo JH, Scherzer MT, Sanchez JMS, Dale KJ, Kinsey CG, et al. Chloroquine Sensitizes GNAQ/11-mutated Melanoma to MEK1/2 Inhibition. Clin Cancer Res 2020;26:6374–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li S, Morley M, Lu M, Zhou S, Stewart K, French CA, et al. Foxp transcription factors suppress a non-pulmonary gene expression program to permit proper lung development. Dev Biol 2016;416:338–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koon HB, Ippolito GC, Banham AH, Tucker PW. FOXP1: a potential therapeutic target in cancer. Expert Opin Ther Targets 2007;11:955–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jang JS, Lee A, Li J, Liyanage H, Yang Y, Guo L, et al. Common Oncogene Mutations and Novel SND1-BRAF Transcript Fusion in Lung Adenocarcinoma from Never Smokers. Sci Rep 2015;5:9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jayasena CS, Bronner ME. Rbms3 functions in craniofacial development by posttranscriptionally modulating TGF-beta signaling. The Journal of cell biology 2012;199:453–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang X, Hao HX, Growney JD, Woolfenden S, Bottiglio C, Ng N, et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A 2013;110:12649–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lum L, Clevers H. Cell biology. The unusual case of Porcupine. Science 2012;337:922–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and additional resources presented in this manuscript are available upon request to the corresponding author, if access is not already readily available through the indicated, established resources