Abstract
Initial reports of SARS-CoV-2 caused COVID-19 suggested that patients with malignant diseases were at increased risk for infection and its severe consequences. In order to provide early United States population-based assessments of SARS-CoV-2 primary infections in unvaccinated patients with hematologic malignancies or cancer, and SARS-CoV-2 breakthrough infections in vaccinated patients with hematologic malignancies or cancer, we conducted retrospective studies using two, unique nationwide electronic health records (EHR) databases. Using these massive databases to provide highly statistically significant data, our studies demonstrated that, compared to patients without malignancies, risk for COVID-19 was increased in patients with all cancers and with all hematologic malignancies. Risks varied with specific types of malignancy. Patients with hematologic malignancies or cancer were at greatest risk for COVID-19 during the first year after diagnosis. Risk for infection was increased for patients 65 years and older, compared to younger patients and among Black patients compared to white patients.
When patients with hematologic malignancies or cancer were vaccinated against SARS-CoV-2, their risk for breakthrough infections was decreased relative to primary infections but remained elevated relative to vaccinated patients without malignancies. Compared to vaccinated patients without malignancies, vaccinated patients with hematologic malignancy or cancer showed increased risk for infection at earlier post vaccination time points. As with primary infections, risk for breakthrough infections was greatest in patients during their first year of hematologic malignancy or cancer. There were no signs of racial disparities among vaccinated patients with hematologic malignancies or cancer. These results provide the population basis to understand the significance of subsequent immunologic studies showing relative defective and delayed immunoresponsiveness to SARS-CoV-2 vaccines among patients with hematologic malignancies and cancers. These studies further provide the basis for recommendations regarding COVID-19 vaccination, vigilance and maintaining mitigation strategies in patients with hematologic malignancies and cancers.
Keywords: SARS-CoV-2, COVID-19, Hematologic malignancies, Cancer
1. SARS-CoV-2 early warnings
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was detected in 2019 in China as the cause of a previously unknown severe pneumonic disease ultimately named Coronavirus Disease 2019 (COVID-19) [1,2]. Worldwide spread of the virus rapidly ensued and by early 2020, cases of COVID-19 leading to hospitalization and death were clearly identified in the U.S [3,4]. On March 11, 2020, with dire health outcomes occurring on a worldwide basis, the World Health Organization (WHO) declared COVID-19 a pandemic [5]. By the end of 2020, over 20 million cases of COVID-19 with more than 340,000 deaths were reported in the U.S [5,6]. In addition to wreaking havoc on patients’ lives and on medical institutions [7–9], the COVID-19 pandemic caused profound social and economic disruptions and uncovered significant healthcare disparities in the U.S., where African Americans showed disproportionately higher rates of COVID-19 infection and death [10–12]. In December 2020, federal drug authorities issued an Emergency Use Authorization (EUA) for two, newly developed, COVID-19 mRNA vaccines [5]. Although uptake and utilization of these vaccines were initially slow, they ultimately were shown to effectively protect people against severe consequences of COVID-19 infection, including death [13,14].
Initial reports from Wuhan and from other Hubei hospitals in China, from Memorial Sloan Kettering Cancer Center in New York, and from the COVID-19 and Cancer Consortium, including medical centers in the U.S., Canada and Spain, indicated that patients with cancer or hematologic malignancies were at increased risk for SARS-CoV-2 infection and its severe consequences, including death rates as high as 41% [15–18]. However, most of these early reports came from specialty hospitals and/or hospital systems in geographically focused areas, with limited patient numbers.
2. Seeking population based data
In order to provide a U.S. population based assessment of primary and breakthrough SARS-CoV-2 infections and consequences, including hospitalizations and death, across the spectrum of cancers and hematologic malignancies, we conducted retrospective, case control, nationwide studies of de-identified, unique electronic health record (EHR) databases, derived from both inpatient and outpatient facilities, distributed across all 50 states, and representing diverse geographic locations, age groups, racial and ethnic groups, income levels and insurance types [19–23]. Availability of these extensive EHR databases allowed us to rapidly establish real time, broad nationwide insights with high statistical power regarding SARS-CoV-2 infection, incidence, associated patient characteristics, co-morbidities and adverse outcomes including hospitalization and death in patients with cancer and hematologic malignancies as well as to document related age and ethnic disparities [19–23].
All the disease terms used in these studies were based on EHR recorded Systematized Nomenclature of Medicine-Clinical Terms. (SNOMED-CT) For studies of primary infection, we used the IBM Watson Health Explorys Cohort Discovery Informatic System covering 366 hospitals and 317,000 clinicians across 50 states in the U.S. At the time of our studies for primary infections, August 14, 2020, for patients with all cancers, and September 1, 2020 for patients with hematologic malignancies, the IBM Watson Explorys database contained more than 73 million unique patient EHR’s including more than 25 million diagnosed with at least one of 13 common cancers and more than 500,000 with hematologic malignancies [19,20].
For studies of breakthrough infections in vaccinated patients, we used the TriNetX Analytics Network Platform which provides access to the unique, de-identified EHR’s for 66 healthcare systems, both inpatient and outpatient facilities, across 50 states in the U.S. At the time of our breakthrough infection studies on hematologic malignancies, December 2020 to October 2021, the TriNetX Platform provided access to 84.5 million unique patients, including 513,413 who were vaccinated for COVID-19, of whom 5956 had hematologic malignancies [21,22]. At the time of our studies on all cancers, December 2020 to November 2021, the TriNetX Analytics database contained more than 90 million unique patient EHRs, including 636,465 who were vaccinated for COVID-19 and 45,253 who were vaccinated patients with one of 12 common cancer types [23].
Our population based results provided early indication of the consequences of COVID-19 in patients with cancer and hematologic malignancies as well as on their relative resistance to COVID-19 vaccination [19–23]. These data highlight the need for vigilance, vaccination and maintaining COVID-19 mitigation strategies in patients with cancer and hematologic malignancies. They also provide the clinical basis to better understand the subsequent results of serologic studies demonstrating relative resistance to SARS-CoV-2 infection and/or vaccination in patients with cancer and hematologic malignancies [24–27].
This report reprises the results of our population based, nationwide EHR studies of SARS-CoV-2 primary and breakthrough infections and their consequences [19–23] and summarizes their implications for patient care. These studies show how utilization of EHR databases can provide early and significant insights and data-driven indications with statistical power to evaluate emerging health problems to orient both research and patient care strategies.
3. COVID-19 infections in unvaccinated patients with hematologic malignancies or cancer
Our studies showed that patients with any of 12 common cancer types or 8 hematologic malignancies, had higher risks of COVID-19 infection when compared to patients without cancer [19,20]. Risk for COVID-19 was greatest for patients who were recently diagnosed with cancer within the past year compared to all patients with the same tumor, presumably reflecting more active disease, greater suppression of body defenses and/or consequences of anti-cancer therapies. Fig. 1 shows that after adjustment for potential confounders, COVID-19 infection risk varied by tumor type ranging from adjusted odds ratio (aOR) for all leukemias (aOR, 12.16, 95% CI, 11.03–13.40), and for all cancers (aOR, 7.14, 95% CI, 6.91–7.39). There were significant variations within each group ranging from liver cancer (aOR, 6.49, 95% CI, 5.71–7.38), and lung cancer (aOR, 7.66, 95% CI, 7.07–8.29) to thyroid cancer (aOR, 3.10, 95% CI, 2.47–3.87).
Fig. 1. Comparison of risk of primary or breakthrough SARS-CoV-2 infections in patients with all cancer and hematologic malignancy.
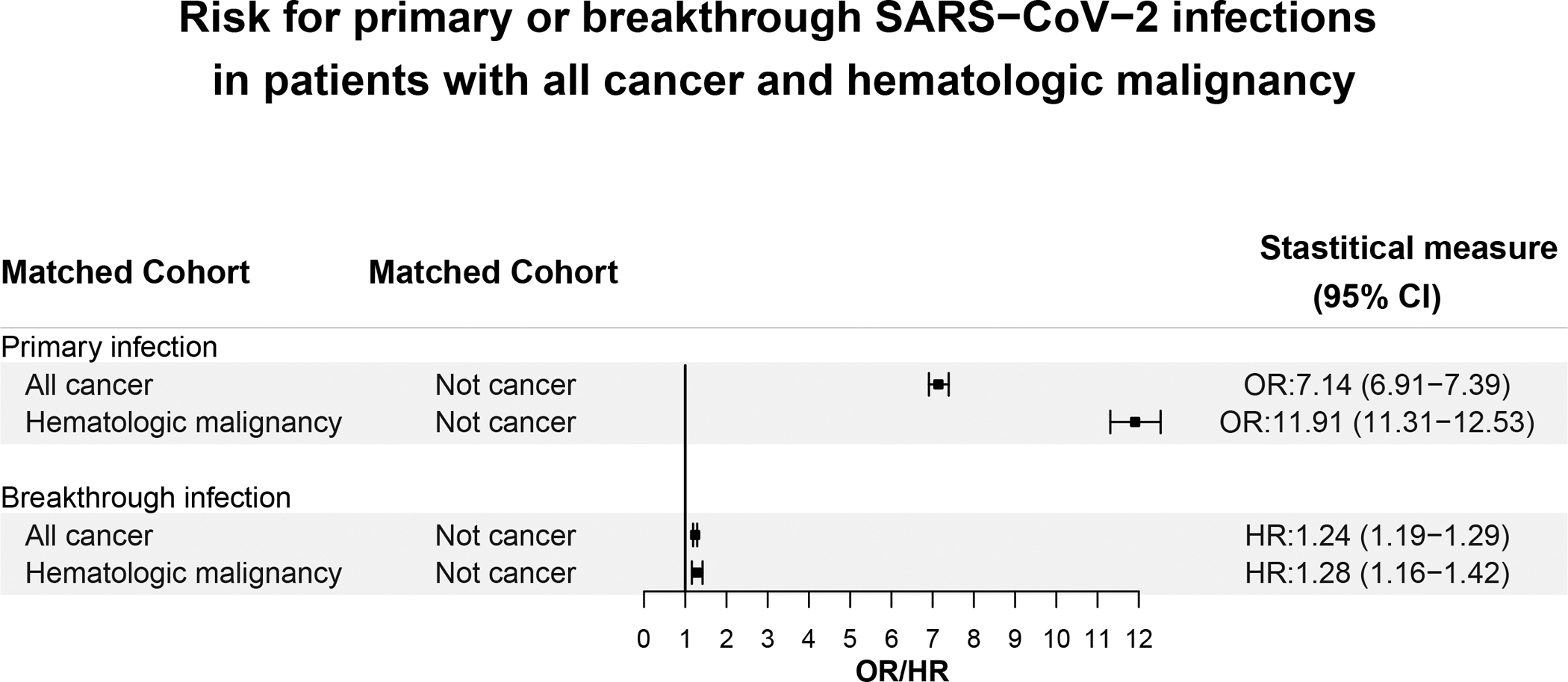
The outcome “Primary infection” during February–August 2020 was compared between the “All-cancer” cohort that comprised patients who had a diagnosis of cancer but no prior SARS-CoV-2 infections and the “Not cancer” cohort that comprised patients who did not have diagnosis of cancer nor prior SARS-CoV-2 infections. Similar comparision was performed between the “Hematologic malignancy” cohort that comprised patients who had a diagnosis of a hematological malignancy but no prior SARS-CoV-2 infections and the “Not cancer cohort” described above.The outcome “Breaktrhough infection” between December 2020 and November 2021 was compared between the “All-cancer” cohort that comprised fully vaccinated patients who had a diagnosis of cancer but no prior SARS-CoV-2 infections and the “Not cancer” cohort that comprised patients who did not have diagnosis of cancer nor prior SARS-CoV-2 infections. Similar comparision was performed between the “Hematologic malignancy cohort” that comprised fully vaccinated patients who had a diagnosis of a hematological malignancy but no prior SARS-CoV-2 infections and the “Not cancer cohort” described above.
Patients with hematologic malignancies, likewise, showed a broad range of disease dependent risks for COVID-19 infection, ranging from acute lymphoid leukemia, (aOR, 31.08, 95% CI, 35.84–37.27), to myelodysplastic syndrome (aOR, 6.59, 95% CI, 5.31–8.18), and polycythemia vera (aOR, 4.89, 95% CI, 3.91–6.11). As with all cancer patients, those with recent diagnosis of hematologic malignancies showed significantly higher risks for COVID-19 infection (aOR, 11.91, 95% CI, 11.31–12.53) compared to patients with all-time diagnoses of hematologic malignancies (aOR, 2.27, 95% CI, 2.17–2.36). Nonetheless, all patients with cancer and all patients with hematologic malignancies of any duration remained at elevated risk for COVID-19 infection [19,20].
When compared to patients without cancer, infected by COVID-19, those with cancer infected by COVID-19 had more severe consequences, as shown by increased rates of hospitalization (Fig. 2) and death (Fig. 3) [19]. Fig. 2 shows that in adult patients, (>18 years old) with cancer who contracted COVID-19, 47.6% were hospitalized, compared to 25.2% of adult patients without cancer who contracted COVID-19. The hospitalization rate of Black patients with COVID-19 and cancer was even higher at 55.56% compared to white patients, 43.2%. Overall, the 47.6% hospitalization rate for patents with COVID-19 and cancer was higher than for COVID-19 patients with no cancer, 24.26%, as well as for patients with cancer, but no COVID-19, 12.39%. Thus, the combination of COVID-19 plus cancer had a synergistic effect on severity requiring patient hospitalization. Fig. 3 shows that similar synergistic effects were seen in death rates among patients with cancer and COVID-19 who had death rates of 14.9% compared to patients with COVID-19 alone or cancer alone, where the death rates were respectively 5.26 and 4.03%.
Fig. 2. Comparison risk of hospitalization among patients with primary or breakthrough SARS-CoV-2 infections between All cancer, Not Cancer and Hematologic maligancy cohorts.
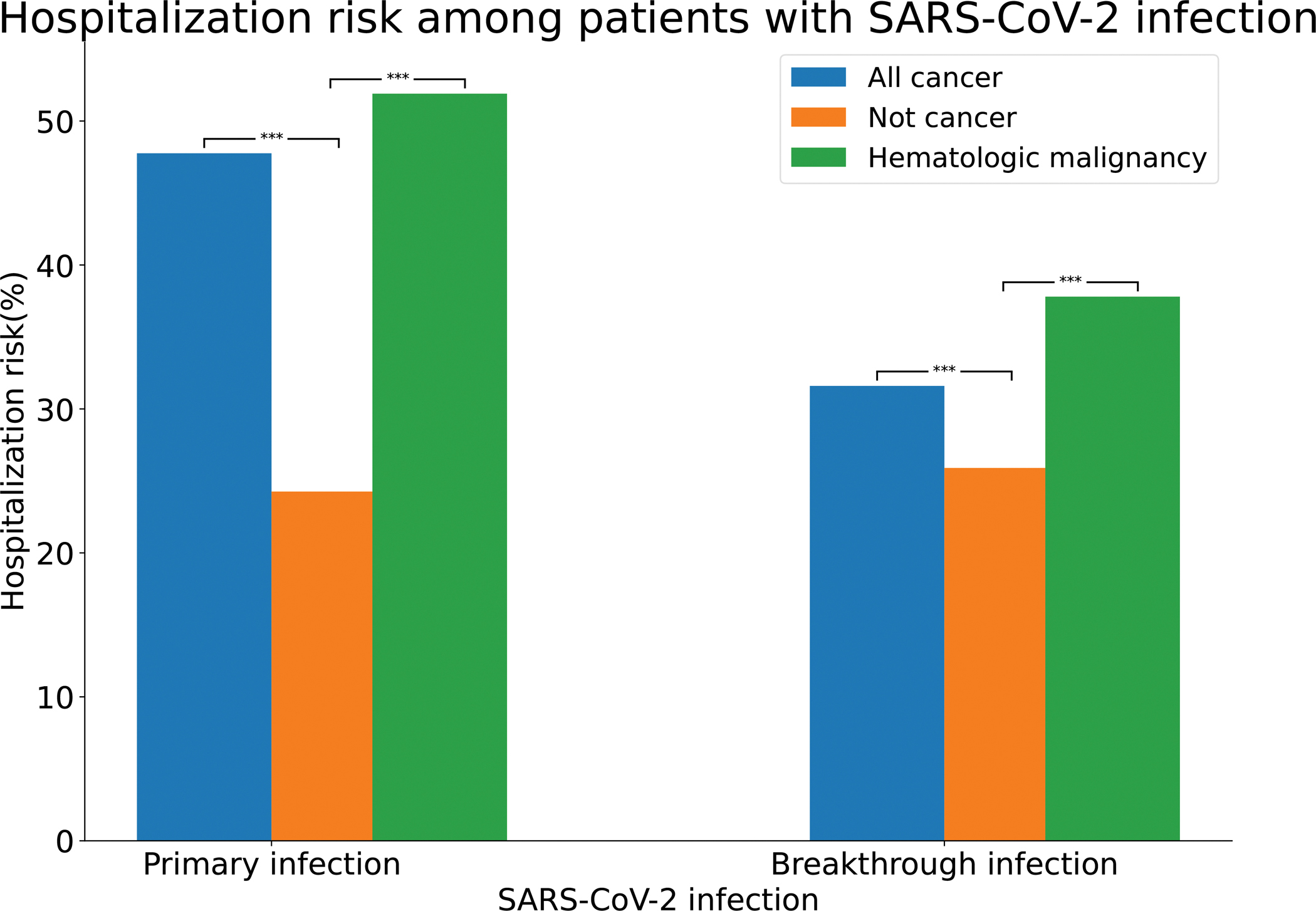
All cancer – patients who had a preexisting diagnosis of cancer and a SARS-CoV-2 infection between February–August 2020 (“Primary infection”) or fully vaccinated patients who had a preexisting diagnosis of cancer and a breakthrough SARS-CoV-2 infection between December 2020 and November 2021 (“Breakthrough infection”). All cancer – patients who had no preexisting diagnosis of cancer but had a SARS-CoV-2 infection between February–August 2020 (“Primary infection”) or fully vaccinated patients who had no preexisting diagnosis of cancer but a breakthrough SARS-CoV-2 infection between December 2020 and November 2021 (“Breakthroug infection”). Hematologic malignancy – patients who had a preexisting diagnosis of hematologic malignancy and a SARS-CoV-2 infection between February–August 2020 (“Primary infection”) or fully vaccinated patients who had a preexisting diagnosis of hematologic malignancy and a breakthrough SARS-CoV-2 infection between December 2020 and November 2021 (“Breakthrough infection”).
Fig. 3. Comparison overall mortality rate among patients with primary or breakthrough SARS-CoV-2 infections between All cancer, Not Cancer and Hematologic maligancy cohorts.
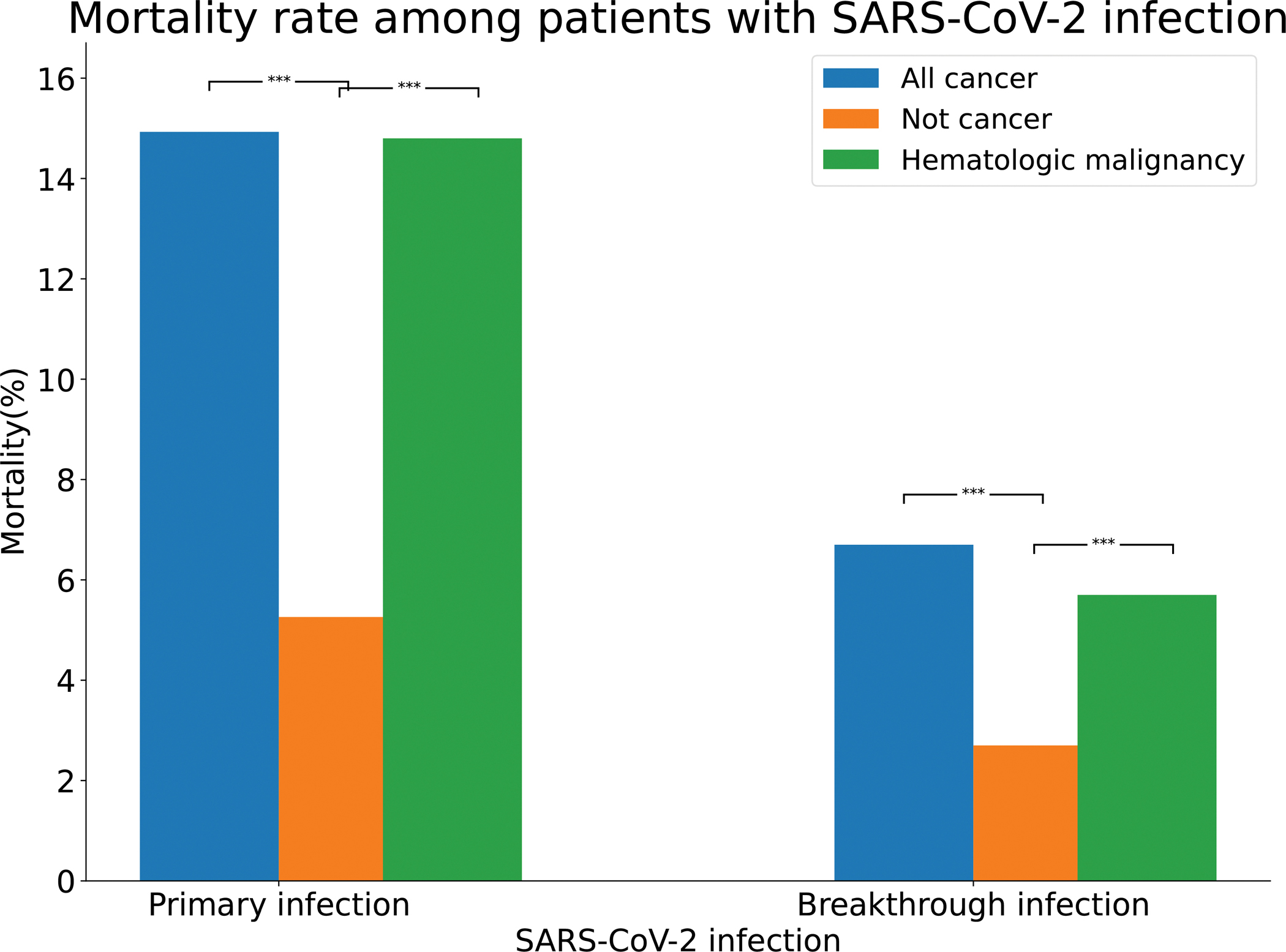
All cancer – patients who had a preexisting diagnosis of cancer and a SARS-CoV-2 infection between February–August 2020 (“Primary infection”) or fully vaccinated patients who had a preexisting diagnosis of cancer and a breakthrough SARS-CoV-2 infection between December 2020 and November 2021 (“Breakthrough infection”). All cancer – patients who had no preexisting diagnosis of cancer but had a SARS-CoV-2 infection between February–August 2020 (“Primary infection”) or fully vaccinated patients who had no preexisting diagnosis of cancer but a breakthrough SARS-CoV-2 infection between December 2020 and November 2021 (“Breakthroug infection”). Hematologic malignancy – patients who had a preexisting diagnosis of hematologic malignancy and a SARS-CoV-2 infection between February–August 2020 (“Primary infection”) or fully vaccinated patients who had a preexisting diagnosis of hematologic malignancy and a breakthrough SARS-CoV-2 infection between December 2020 and November 2021 (“Breakthrough infection”).
Among patients with recent onset hematologic malignancies and COVID-19 infection, Fig. 2 shows that the hospitalization rate was 51.9% compared to 23.5% for patients with COVID-19 without hematologic malignancies and 15.0% for patients with hematologic malignancies without COVID-19, again indicating synergistic effects between COVID-19 infection and recent hematologic malignancies leading to patient hospitalization. Fig. 3 shows that similar results, indicating synergistic effects were noted for death rates with 14.8% mortality for patients with recently diagnosed hematologic malignancy plus COVID-19 infection, compared to 5.1% mortality for COVID-19 infections alone, and 4.1% mortality for patients with recently diagnosed hematologic malignancies without COVID-19.
African Americans with COVID-19 plus all cancers had a higher death rate of 18.52% compared to white patients, 13.51%, however this difference did not reach statistical significance. Similarly, African American patients with COVID-19 plus hematologic malignancies had higher death rates, 20.0% compared to white patients 13.8% without reaching statistical significance.
4. COVID-19 breakthrough infections in vaccinated patients with hematologic malignancies or cancer
By the 2020–21 winter, within one year of the World Health Organization declaration of COVID-19 as a global pandemic, three vaccines became available in the U.S. including two mRNA vaccines, mRNA-1273 from Moderna and BNT162b2 from Pfizer-BioNTech as well as a modified viral vector vaccine from Jansen/Johnson and Johnson. All three vaccines were noted to activate T cells, B cells, and plasma cells and to induce cellular and humoral immunity [28]. Initial priority for emergency use of COVID-19 vaccination in the U.S. was provided for healthcare workers, older patients and those with comorbidities, including cancer which put them at higher risk for COVID-19 infection [5]. However, early studies indicated that some patients with hematologic malignancies either failed to undergo serologic conversion or were weak antibody responders [25,29]. It therefore became important to demonstrate whether cancer patients that were fully vaccinated were protected against COVID-19 infection and/or the serious outcomes associated with infection.
We initially evaluated patients with multiple myeloma (MM) who were fully vaccinated against SARS-CoV-2, for COVID-19 breakthrough infection and compared them to breakthrough infections in patients without myeloma or any other malignancy [21]. At the time of this study, full vaccination was identified as two doses of the Pfizer-BioNTech or Moderna mRNA vaccine or a single dose of the Jansen/Johnson and Johnson viral vector vaccine. The overall COVID-19 infection breakthrough rate among fully vaccinated patients with MM was 15.4% compared to 3.4% in patients without cancer [21]. When patients with MM were propensity matched with patients without cancer for demographics, adverse socioeconomic determinants of health, comorbidities, medications and vaccine types, MM patients still showed increased risk for breakthrough infection Hazard Ratio (HR) 1.34 (95% CI 1.06–1.69). Severe outcomes as measured by hospitalization rates, revealed 30% for vaccinated patients with MM and breakthrough infections compared to 4.5% for matched MM patients without breakthrough infections. Thus, our initial study of vaccinated patients with a specific hematologic malignancy, MM, showed an increased infection breakthrough rate compared to patients with no malignancy. We furthermore showed that patients with MM with breakthrough infections were at increased risk for hospitalization compared to patients with breakthrough, but no malignancy or no breakthrough infection. These studies showed the value of the EHR massive database in providing early warnings of the increased risk and severity for COVID-19 infection in patients with a hematologic malignancy, even though they had completed the vaccination schedule recommended at that time. Such information might have been useful to maintain enhanced mitigation strategies for patients like Colin Powell who had MM and was vaccinated but succumbed to COVID-19 induced mortality [30].
We subsequently extended our investigation to examine breakthrough infections in vaccinated patients with all cancers combined, and with each of 12 common cancers individually, including breast, prostate, colorectal, skin, lung, thyroid, bladder, kidney, endometrial, liver, and pancreatic cancers. We also examined breakthrough infections in vaccinated patients with all hematologic malignancies as a group and with 7 specific hematologic malignancies, including MM, acute myelogenous leukemia, chronic myelocytic leukemia, chronic lymphocytic leukemia, Hodgkin lymphoma, and non-Hodgkin lymphoma. These results were compared to breakthrough infections in propensity matched vaccine patients without cancer [22]. Overall, the breakthrough rates for vaccinated patients with all cancer was 13.6% while the breakthrough rate for vaccinated patients with all hematologic malignancies was 13.4% compared to control vaccinated patients without malignancy which ranged from 4.5% to 4.9% in two separate studies [22,23].
Within each group, cancers and hematologic malignancies, there was a range of breakthrough infections associated with different tumor types. Among vaccinated patients with cancers, breakthrough rates ranged from 24.7% for patients with pancreatic cancer, 22.8% for liver cancer, 17.5% for colorectal cancer, 11.9% for endometrial cancer, 11.9% for breast cancer and 10.3% for thyroid cancer, all higher than the 4.5–4.9% breakthrough infection rate in vaccinated patients without cancers. Variations in breakthrough rates were also noted in vaccinated patients with specific hematologic malignancies, ranging from 17.4% for patients with Chronic Myeloid Leukemia, 17.2% for MM, to 11% for Acute Lymphocytic Leukemia [22]. These elevated breakthrough infection rates in vaccinated patients with cancer, who had medical encounters during the past year, presumably reflected active disease and elevated risk for breakthrough infections compared to cancer patients without medical encounters during the past year.
Patients with cancer or hematologic malignancies with breakthrough infections had increased risk for hospitalization and mortality of 30.6% and 3.9% respectively, compared to 6.7% and 1.3% in patients with cancer without breakthrough infections. Similarly, patients with hematologic malignancies and breakthrough infections had hospitalization and mortality rates of 37.8% and 5.7% respectively, compared to 2.2% hospitalization and 0.8% mortality for patients with hematologic malignancies without COVID-19 breakthrough infections. Interestingly, among vaccinated patients with all cancers as well as for those with hematologic malignancies, there were no significant differences in breakthrough infections, hospitalizations or death based on race or ethnicity [22,23].
Results summarized in Fig. 1 show that risks for primary COVID-19 infection in patients with all cancers or with all hematologic malignancies are considerably increased relative to those in patients without cancers. Fig. 1 also shows, that while risks for breakthrough infections in vaccinated patients with all cancers or vaccinated patients with hematologic malignancies are higher than in vaccinated patients without cancers, the results for breakthrough infections are considerably less in vaccinated patients compared to risk for primary infections in non-vaccinated patients. Thus, vaccination reduces but does not eliminate the increased risk for infection in patients with cancers and with hematologic malignancies [19–23].
Fig. 2 shows that hospitalization rates for patients with SARS-CoV-2 infection are significantly higher in patients with all cancers or with all hematologic malignancies compared to effects of primary infections in patients without cancer. Among vaccinated patients with breakthrough infections, those with all cancers and those with all hematologic malignancies had higher mortality rates than those without cancer, however overall mortality rates were reduced compared to those that occurred with primary infections [19–23] (see Fig. 3).
In summary, our studies showed on a population basis in the U.S., that patients with cancer and patients with hematologic malignancies were at increased risk for primary COVID-19 infections and that infections among these groups of patients were associated with increased mortality compared to infections in patients without malignancies. Our studies also showed that vaccination reduced risk of breakthrough infections in patients with cancer and patients with hematologic malignancies, however these patients were still at increased risk for breakthrough infections compared to patients without cancer. Moreover, patients with cancers and patients with hematologic malignancies were at increased risk for mortality associated with COVID-19 infections. Vaccination reduced but did not eliminate the increased mortality rate associated with the combination of COVID-19 breakthrough infection plus cancer or hematologic malignancy.
While individual cancer types and hematologic malignancies are varied in their relative risk for primary and breakthrough infections and associated severity, they all showed similar patterns. In all cases, risks increased with medical encounters during the most recent year, suggesting risk increased with more active disease, disease severity, and/or disease specific therapeutic interventions.
We and others have reported significant disparities for primary SARS-CoV-2 infection among African American individuals compared to white individuals and among African American patients with cancer compared to white patients with cancer and/or hematologic malignancies [8,9,12]. We also reported that African Americans with cancer and/or hematologic malignancies and primary COVID-19 infection have more severe disease measured by increased hospitalization and mortality rates. These disparities have been attributed to a variety of factors including access to quality care, adverse social determinants of health and structural racism. Interestingly, however, we found no significant differences in breakthrough infection rates and/or severity of outcomes between vaccinated African Americans and white patients with cancer and/or hematologic malignancies. These observations suggest that vaccination itself and/or associated medical care mitigates against factors leading to racial/ethnic disparities. Alternatively, it is possible that patients with cancer and/or hematologic malignancies who are vaccinated against SARS-CoV-2 are also more cautious to practice all other mitigation strategies.
In more recent studies of patients who were treated with Paxlovid (niratrelvir/ritonavir) or Molnupiravir antiviral therapy for COVID-19, who then developed COVID-19 rebound within 30 days of treatment, greater than 55% of those rebounds occurred in patients with cancer [31]. These results again demonstrate the increased susceptibility of patients with cancer to SARS-CoV-2 and indicate the need for continued surveillance of patients with COVID-19 and cancer, even after completion of antiviral therapy with Paxlovid or Molnupiravir.
In summary, our nationwide, real-time population based studies using unique de-identified EHRs provided early indication of the multiple aspects of the impact of SARS-CoV-2 infection in patients with cancer and hematologic malignancies.
Patients with cancer and hematologic malignancies are at increased risk for SARS-CoV-2 infection.
Risk of Infection with SARS-CoV-2 among patients with cancer and with hematologic malignancies is greatest among those with medical encounters during the most recent year relative to those without recent encounters. These observations suggest that patients with newly diagnosed disease, active disease and/or those undergoing therapy are at greatest risk for infection.
Risk for infection with SARS-CoV-2 varies significantly among patients with different malignant disease types, however, all patients with cancer and all patients with hematologic malignancies showed increased risk for infection. These observations suggest that cancer itself has an impact on susceptibility to SARS-CoV-2 infection that is further modulated by effects of cancer on different organ systems, health status and overall resistance mechanisms.
Patients with cancer or hematologic malignancies, who are infected with SARS-CoV-2 show greater disease severity as measured by increased hospitalization and increased death rates. The combination of cancer or hematologic malignancies with SARS-CoV-2 infections are synergistic leading to hospitalization and death.
In unvaccinated patients with hematologic malignancies or cancer, there were racial/ethnic and age dependent disparities in SARS-CoV-2 infection and severity. However, disparities for breakthrough infections were not present in vaccinated patients.
SARS-CoV-2 vaccinated patients with hematologic malignancies or cancer showed lower risk for breakthrough infections compared to risk for primary infections in unvaccinated patients. However, SARS-CoV-2 breakthrough infections among patients with hematologic malignancies or cancer occurred at a higher rate and at earlier time points compared to breakthrough infections in vaccinated patients without cancer or hematologic malignancies.
Risk for breakthrough infections, varied with different malignancies and was greatest in patients with medical encounters during most recent years.
Mortality rates for vaccinated patients with hematologic malignancies or cancer who developed SARS-CoV-2 breakthrough infections were decreased relative to mortality rates associated with primary infections in hematologic malignancies or cancer patients, however, they were still greater than mortality rates in patients with breakthrough infections without underlying malignancies.
Our recent studies indicate that patients with hematologic malignancies or cancer who are treated with Paxlovid or Molnupiravir represent a large percentage of those patients who developed SARS-CoV-2 rebound within 30 days of completing anti-viral therapy. Information on mortality of these rebound infections in patients with hematologic malignancies or cancer is not yet available.
5. Clinical implications for pre and post SARS-CoV-2 exposure in patients with hematologic malignancies or cancer
Our studies outlined above have been substantiated by subsequent reports [32,33]. While we did not specifically study mechanisms of susceptibility and/or resistance to SARS-CoV-2 infection or prognosis, our population-based observations were predictive and are supported at a mechanistic level by multiple subsequent immunologic studies. The complete and protective adaptive immune response to SARS-CoV-2 infection and/or immunization requires a complex and coordinated interaction between antibody producing B cells, cellular responses in CD4+T helper cells and CD8+ T killer cells [28,34]. As anticipated by our studies, patients with hematologic malignancies or cancer are capable of mounting those protective responses against SARS-CoV-2 virus and vaccine, however, compared to normal patients without hematologic malignancies or cancer, these responses may be delayed, take longer to develop, and frequently require more encounters with the immunogen i.e., multiple vaccinations. In addition, patients with hematologic malignancies or cancer when compared to normal patients, frequently do not reach equivalent virus neutralization titers. Moreover, immunity wains more rapidly providing less sustained protection [25,29,35–39]. Furthermore, the immune response to SARS-CoV-2 varies with different hematologic malignancies or cancer and is particularly compromised in those with hematologic malignancies compared to solid tumors, especially those with B cell malignancies and those receiving anti B-cell therapies with agents such as Rituximab and BTK inhibitors as well as other immunosuppressants [18,27,38,40,41]. These studies indicate the need for all patients with hematologic malignancies or cancer to receive early and regularly scheduled SARS-CoV-2 vaccinations, including regular boosters, to coordinate vaccination with immune suppressive therapy so as to optimize effectiveness. Moreover, the compromised immune status and defective immunoresponsiveness of patients with hematologic malignancies or cancer stresses the importance for patients, their families, friends and caregivers, to be fully immunized and even when fully immunized, to maintain vigilance and full observance of social and behavioral mitigation strategies [42].
The compromised immune status of patients with hematologic malignancies and cancer, further strongly support the use of prophylactic preexposure passive immune strategies with anti SARS-CoV-2 monoclonal antibodies Tixagevinab and Cilgavimab (Evusheld) [43–45] or intravenous immunoglobulin, which has been shown to carry protective antibodies and has been suggested for patients receiving CAR-T therapy [46,47].
Since both primary and breakthrough SARS-CoV-2 infection in patients with hematologic malignancies or cancer have an increased incidence as well as increased severe consequences including death, the early use of antivirals, Paxlovid and Molnopurivir, to control signs and symptoms in particular with hematologic malignancies or cancer who test positive for SARS-CoV-2 antigens is encouraged to potentially reduce severe outcomes [48].
These results are supportive of the recommendations of the National Comprehensive Cancer Network (NCCN) for COVID-19 vaccination and for preexposure prophylaxis which are briefly outlined below in Practice Points and available in more detail at the NCCN website [49,50].
6. Research agenda
To provide U.S. population-based data for the following questions regarding SARS-CoV-2 infections and breakthrough infections in patients with cancers or hematologic malignancies.
Are patients with cancer or hematologic malignancies at increased risk for SARS-CoV-2 infections and severe outcomes?
In patients with cancer or hematologic malignancies, does SARS-CoV-2 vaccination reduce risk of breakthrough infections?
Are vaccinated patients with cancer or hematologic malignancies at increased risk for SARS-CoV-2 breakthrough infections or severe outcomes?
Do patients with cancer or hematologic malignancies show disparities in risk or outcomes of SARS-CoV-2 primary or breakthrough infections?
7. Practice Points
Patients with cancer should get vaccinated with three vaccine doses followed by two booster shots as soon as they can.
Patients receiving stem cell transplant and/or CAR-T therapy should wait at least three months after treatment to get vaccinated.
Caregivers, family and contacts should get all recommended vaccines and boosters.
Patients with cancer and their contacts and caregivers should practice social distancing, wearing masks, and other mitigation strategies.
In addition to vaccination, preexposure prophylaxis using Tixagevimab plus Cilgavimab (Evusheld) is recommended for patients undergoing active therapy.
In addition, we suggest the prompt initiation of antiviral therapy for patients with hematologic malignancies or cancer who develop symptomatic primary and/or breakthrough SARS-CoV-2 infection and become antigen positive.
Funding
This research was supported by National Institutes of Health Grant Numbers, R25 CA221718, P30 CA43703, AG 057557, AG 061388, AG 062272, UL1T R002548, and American Cancer Society Research Scholar Grant, RSG-16-049-01-MPC.
Role of the funding source
The funders had no role in the design and conduct of this study, collection, analysis or interpretation of data; in the writing of the report; nor in the decision to submit the article for publication.
Footnotes
Declaration of competing interest
All authors declare no conflict of interest.
References
- [1].Lu H, Stratton CW, Tang YW. The Wuhan SARS-CoV-2-What’s next for China. J Med Virol 2020;92(6):546–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395(10223):507–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of hospitalized adults with COVID-19 in an integrated health care system in California. JAMA 2020; 323(21):2195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 2020;323(20):2052–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].CDC Museum COVID-19 Timeline [Internet]. U.S. Department of health & human services [cited 17 August 2022]. Available from: https://www.cdc.gov/museum/timeline/covid19.html.
- [6].Taylor K The U.S. reaches 20 million cases. New York Times [Internet]. 2020. 8/17/2022. Available from: https://www.nytimes.com/2020/12/31/world/the-us-reaches-20-million-cases.html?smid=url-share.
- [7].Kaye AD, Okeagu CN, Pham AD, Silva RA, Hurley JJ, Arron BL, et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: international perspectives. Best Pract Res Clin Anaesthesiol 2021;35(3):293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rubin R The COVID-19 pandemic rages on for people who are immunocompromised. JAMA 2022;327(19):1853–5. [DOI] [PubMed] [Google Scholar]
- [9].Unger JM. Cancer care during COVID-19-A shock to the system. JAMA Netw Open 2022;5(4):e228864. [DOI] [PubMed] [Google Scholar]
- [10].Yancy CW. Academic medicine and Black lives matter: time for deep listening. JAMA 2020;324(5):435–6. [DOI] [PubMed] [Google Scholar]
- [11].Mahajan UV, Larkins-Pettigrew M. Racial demographics and COVID-19 confirmed cases and deaths: a correlational analysis of 2886 US counties. J Public Health 2020;42(3):445–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Karaca-Mandic P, Georgiou A, Sen S. Assessment of COVID-19 hospitalizations by race/ethnicity in 12 states. JAMA Intern Med 2021;181(1):131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].CDC. Benefits of getting A COVID-19 vaccine. U.S. Department of Health and Human Services; 2022. [updated 11 August 2022. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html.
- [14].Ssentongo P, Ssentongo AE, Voleti N, Groff D, Sun A, Ba DM, et al. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis. BMC Infect Dis 2022;22(1):439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Chua MLK, Yu J, Xie C. Risk of COVID-19 in patients with cancer-reply. JAMA Oncol 2020;6(9):1472–3. [DOI] [PubMed] [Google Scholar]
- [16].Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol 2020;21(7):904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Robilotti EV, Babady NE, Mead PA, Rolling T, Perez-Johnston R, Bernardes M, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med 2020;26(8):1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020;395(10241):1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol 2021;7(2): 220–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang Q, Berger NA, Xu R. When hematologic malignancies meet COVID-19 in the United States: infections, death and disparities. Blood Rev 2021;47:100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang L, Berger NA, Xu R. Risks of SARS-CoV-2 breakthrough infection and hospitalization in fully vaccinated patients with multiple myeloma. JAMA Netw Open 2021;4(11):e2137575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wang L, Kaelber DC, Xu R, Berger NA. COVID-19 breakthrough infections, hospitalizations and mortality in fully vaccinated patients with hematologic malignancies: a clarion call for maintaining mitigation and ramping-up research. Blood Rev 2022;54:100931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wang W, Kaelber DC, Xu R, Berger NA. Breakthrough SARS-CoV-2 infections, hospitalizations, and mortality in vaccinated patients with cancer in the US between December 2020 and November 2021. JAMA Oncol 2022;8(7):1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Botton J, Semenzato L, Jabagi MJ, Baricault B, Weill A, Dray-Spira R, et al. Effectiveness of Ad26.COV2.S vaccine vs BNT162b2 vaccine for COVID-19 hospitalizations. JAMA Netw Open 2022;5(3):e220868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Grinshpun A, Rottenberg Y, Ben-Dov IZ, Djian E, Wolf DG, Kadouri L. Serologic response to COVID-19 infection and/or vaccine in cancer patients on active treatment. ESMO Open 2021;6(6):100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Harrington P, Doores KJ, Radia D, O’Reilly A, Lam HPJ, Seow J, et al. Single dose of BNT162b2 mRNA vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces neutralising antibody and polyfunctional T-cell responses in patients with chronic myeloid leukaemia. Br J Haematol 2021; 194(6):999–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sakuraba A, Luna A, Micic D. Serologic response following SARS-COV2 vaccination in patients with cancer: a systematic review and meta-analysis. J Hematol Oncol 2022;15(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021;184(4):861–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Parry H, McIlroy G, Bruton R, Ali M, Stephens C, Damery S, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J 2021;11(7):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Stampfer SD, Goldwater MS, Jew S, Bujarski S, Regidor B, Daniely D, et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia 2021;35(12):3534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wang L, Berger NA, Davis PB, Kaelber DC, Volkow ND, Xu R. COVID-19 rebound after Paxlovid and Molnupiravir during January-June 2022. medRxiv. 2022. [Google Scholar]
- [32].Khoury E, Nevitt S, Madsen WR, Turtle L, Davies G, Palmieri C. Differences in outcomes and factors associated with mortality among patients with SARS-CoV-2 infection and cancer compared with those without cancer: a systematic review and meta-analysis. JAMA Netw Open 2022;5(5):e2210880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Pinaña JL, López-Corral L, Martino R, Vazquez L, Pérez A, Martin-Martin G, et al. SARS-CoV-2 vaccine response and rate of breakthrough infection in patients with hematological disorders. J Hematol Oncol 2022;15(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Dörner T, Radbruch A. Antibodies and B cell memory in viral immunity. Immunity 2007;27(3):384–92. [DOI] [PubMed] [Google Scholar]
- [35].Addeo A, Cortellini A, Banna GL. One more piece to SOLIDify our knowledge on the impact of SARS-CoV-2 in patients with cancer. Transl Lung Cancer Res 2022; 11(2):132–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell 2021;39(8):1031–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Haggenburg S, Hofsink Q, Lissenberg-Witte BI, Broers AEC, van Doesum JA, van Binnendijk RS, et al. Antibody response in immunocompromised patients with hematologic cancers who received a 3-dose mRNA-1273 vaccination schedule for COVID-19. JAMA Oncol 2022. 10.1001/jamaoncol.2022.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kuderer NM, Lyman GH. COVID-19 vaccine effectiveness in patients with cancer: remaining vulnerabilities and uncertainties. Lancet Oncol 2022;23(6):693–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Thompson MA, Hallmeyer S, Fitzpatrick VE, Liao Y, Mullane MP, Medlin SC, et al. Real-world third COVID-19 vaccine dosing and antibody response in patients with hematologic malignancies. J Patient Cent Res Rev 2022;9(3):149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jyssum I, Kared H, Tran TT, Tveter AT, Provan SA, Sexton J, et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol 2022;4(3):e177–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fendler A, de Vries EGE, GeurtsvanKessel CH, Haanen JB, Wörmann B, Turajlic S, et al. COVID-19 vaccines in patients with cancer: immunogenicity, efficacy and safety. Nat Rev Clin Oncol 2022;19(6):385–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lee LYW, Starkey T, Ionescu MC, Little M, Tilby M, Tripathy AR, et al. Vaccine effectiveness against COVID-19 breakthrough infections in patients with cancer (UKCCEP): a population-based test-negative case-control study. Lancet Oncol 2022;23(6):748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Stuver R, Shah GL, Korde NS, Roeker LE, Mato AR, Batlevi CL, et al. Activity of AZD7442 (tixagevimab-cilgavimab) against Omicron SARS-CoV-2 in patients with hematologic malignancies. Cancer Cell 2022;40(6):590–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Malani AG, Malani AN. Preventive medication for COVID-19 infection. JAMA 2022. 10.1001/jama.2022.13214. [DOI] [PubMed] [Google Scholar]
- [45].Levin MJ, Ustianowski A, De Wit S, Launay O, Avila M, Templeton A, et al. Intramuscular AZD7442 (Tixagevimab-Cilgavimab) for prevention of covid-19. N Engl J Med 2022;386(23):2188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Romero C, Díez JM, Gajardo R. Anti-SARS-CoV-2 antibodies in healthy donor plasma pools and IVIG products. Lancet Infect Dis 2021;21(6):765–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hill JA, Giralt S, Torgerson TR, Lazarus HM. CAR-T - and a side order of IgG, to go? - immunoglobulin replacement in patients receiving CAR-T cell therapy. Blood Rev 2019;38:100596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Song Q, Bates B, Shao YR, Hsu FC, Liu F, Madhira V, et al. Risk and outcome of breakthrough COVID-19 infections in vaccinated patients with cancer: real-world evidence from the national COVID cohort collaborative. J Clin Oncol 2022;40(13):1414–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Network NCC. Recommendations of the national comprehensive cancer Network (NCCN) advisory committee on COVID-19 vaccination and pre-exposure prophylaxis. 2022. [Google Scholar]
- [50].Network NCC. NCCN COVID-19 vaccination guide for people with cancer. 2022. [Google Scholar]


