Abstract
Azithromycin enhances the response to root planing and produces anti-inflammatory effects in treating chronic lung disease. This led us to hypothesize that azithromycin inhibits inflammatory mediator production in gingiva, leading to decreased gingival crevicular fluid (GCF) volume. To test this hypothesis, ten periodontally healthy volunteers received azithromycin every 24 hours for 48 hours. GCF samples were collected from twelve maxillary interproximal sites prior to azithromycin (baseline) and 2, 4, 7 and 14 days later. Samples were assayed for IL-1β, IL-8, TNF-α, VEGF, IL-6 and IL-10. With azithromycin treatment, GCF volume decreased significantly on days 2 through 7 (P < 0.05), but increased toward baseline levels on day 14. This was accompanied by a transient decrease in the content of IL-1β, IL-8, TNF-α and VEGF (P < 0.05). IL-6 and IL-10 were not detected. Since plaque was absent throughout the study, the findings suggest that azithromycin produces anti-inflammatory effects in gingiva.
Keywords: macrolide, inflammation, interleukin-1β, interleukin-8, tumor necrosis factor
INTRODUCTION
Scaling and root planing is commonly used in the treatment of inflammatory periodontitis, but is not always effective in eliminating disease-associated bacteria (Slots and Ting, 1999). This is due, in part, to the tissue-invasive properties of certain subgingival bacterial pathogens. Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans, which can invade epithelial cells that line periodontal pockets (Christersson et al., 1987; Lamont et al., 1995), are very difficult to eliminate by scaling and root planing alone. Adjunctive systemic antimicrobial chemotherapy has been used in conjunction with mechanical debridement to enhance the elimination of these pathogens (van Winkelhoff et al., 1996).
Azithromycin is a good choice for eliminating periodontal pathogens due to its bioavailability and spectrum of action. Azithromycin produces high concentrations in tissues and is highly concentrated within polymorphonuclear leukocytes (Amsden, 2001). Clarithromycin, a closely-related macrolide, is actively transported by gingival fibroblasts and oral epithelial cells (Chou and Walters, 2008). By increasing intracellular levels of these macrolides, active transport may enhance their effectiveness against invasive periodontal pathogens and sustain their therapeutic levels in the gingiva. Azithromycin is detectable in inflamed periodontal tissues for at least 14 days after systemic administration (Gomi et al., 2007). Systemic administration of clarithromycin yields higher levels at gingivitis sites than in healthy gingiva (Burrell and Walters, 2008). Azithromycin produces potent inhibition of Aggregatibacter actinomycetemcomitans and Porphyromonas gingivalis (Pajukanta et al., 1992; Pajukanta, 1993; Goldstein et al., 1999) and has a low incidence of gastrointestinal toxicity. It is administered in a once-daily regimen that promotes patient compliance. Previous studies have shown that azithromycin improves clinical treatment outcomes in patients with chronic and aggressive periodontitis (Smith et al., 2002; Mascarenhas et al., 2005; Haas et al., 2008).
Macrolide antimicrobials are effective for the treatment of bronchiectasis in cystic fibrosis and chronic bronchitis. In addition to their antimicrobial effects, macrolides appear to inhibit pro-inflammatory cytokine production when used to treat chronic inflammatory airway diseases (Giamarellos-Bourboulis, 2008). Macrolides antagonize production of interleukin-8 (IL-8), tumor necrosis factor-α (TNF-α), and leukotriene B4 (LTB4), which may enhance its clinical effectiveness (Tamaoki et al., 2004; Vanaudenaerde et al., 2007). However, there have been no previous studies to determine whether macrolides inhibit the production of inflammatory mediators in gingival tissue.
Gingival crevicular fluid (GCF) is a complex mixture which includes bacterial byproducts, inflammatory mediators, host derived enzymes and tissue breakdown products. The rate of GCF flow is strongly correlated with histological signs of gingival inflammation (Griffiths, 2003). In the presence of gingivitis, GCF is expressed at a relatively high rate of flow and exhibits characteristics of an inflammatory exudate. In healthy gingiva, GCF is a transudate of gingival tissue interstitial fluid and its flow rate is relatively low. Clinically healthy gingiva exhibits histological signs of low-level inflammation (Schroeder et al., 1973; Bosshardt and Lang, 2005). Thus, it is feasible that drugs that produce anti-inflammatory effects in the gingiva could induce a reduction in GCF volume even at clinically healthy sites. A previous study provided indirect evidence that systemic administration of clarithromycin reduces the rate of GCF flow (Burrell and Walters, 2008). It is reasonable to hypothesize that azithromycin decreases production of IL-1β, TNF-α, IL-6, IL-8 and VEGF in clinically healthy gingiva, triggering a reduction in GCF volume. It is also possible that azithromycin could increase the production of the anti-inflammatory cytokine IL-10. These hypotheses were tested in the present prospective human clinical study.
MATERIALS AND METHODS
Subjects
Ten healthy adult volunteers in good periodontal health were recruited from the student population of the Ohio State University College of Dentistry. Aside from localized minor facial gingival recession, these subjects exhibited no signs of clinical periodontal attachment loss. Informed consent was obtained under a protocol approved by the Institutional Review Board. Care was taken to exclude subjects with systemic disease, a history of a drug allergy or a history of use of any medications in the two weeks prior to the study. Pregnant or lactating females were also excluded. The sample size was estimated from data obtained in a previous clinical study with clarithromycin (Burrell and Walters, 2008). Assuming a mean difference in GCF volume of 0.10 μl per 30 second sample and an expected pooled standard deviation of 0.05, a sample size of nine provides a power of 0.800.
Study Design
In this prospective longitudinal study, each subject received a prophylaxis and oral hygiene instructions (including interproximal flossing and tooth brushing) one week before the first dose of azithromycin. Subjects were administered a regimen of azithromycin (500 mg on day 0, then 250 mg per day for the following two days) to establish steady-state antibiotic levels in gingiva. Subjects were asked to document the times they took the medication. The Plaque Index (PI) (Silness and Loe, 1964) and the Gingival Index (GI) (Loe and Silness, 1963) were determined for each subject on study day 0 (baseline) and every subsequent appointment. GCF samples were obtained on day 0 (before the first dose of azithromycin) and on days 2, 4, 7 and 14 after the first dose. GI and PI were recorded from 12 maxillary posterior interproximal sites.
Gingival crevicular fluid samples were obtained from the same 12 maxillary interproximal sites, using filter paper strips (Oraflow, Inc., Smithtown, NY) as previously described (Conway et al., 2000). Briefly, the site was isolated and the teeth and gingiva were gently air dried to avoid contamination with saliva. GCF samples were collected by positioning paper strips at the orifice of the crevice for 30 seconds. GCF sample volume was measured with a calibrated Periotron 6000 (IDE Interstate, Amityville, NY). The paper strips from each subject then were pooled and stored in sealed vials in liquid nitrogen until time for cytokine analysis. GCF was collected only from maxillary posterior sites because they were relatively easy to protect from salivary contamination.
Sample preparation and analysis
Paper strips containing the pooled GCF samples were placed in a 0.5 ml microtube which had been modified with a small diameter perforation at its tip. The tube was placed inside a 1.5 ml microtube and 200 μl of Hanks balanced salt solution was added. GCF was eluted from each pool of sample strips by incubation on ice for 20 minutes. The samples were eluted into the bottom of the larger tube by centrifugation at 6,000 × g for 1 minute. A 40 μl aliquot of each sample was used for assays of cytokine content. A commercially available multiplex bead-based cytokine immunoassay (BioPlex, BioRad Laboratories Inc, Hercules, CA, USA) was used to measure the content of interleukin-1β (IL-1β), interleukin-6 (IL-6), interleukin-8 (IL-8), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α) and vascular endothelial growth factor (VEGF) according to the manufacturer’s directions. Results were expressed as the total amount (in picograms) recovered from each pool of twelve paper strips. The limits of detection were 0.5 pg/ml for IL-8 and VEGF, 0.8 pg/ml for IL-1β, 0.9 pg/ml for IL-10, 1.1 pg/ml for IL-6 and 3.0 pg/ml for TNF-α.
To assess the presence of periodontal pathogens, a 2 μl aliquot of each GCF sample pool was subjected to the BANA test for trypsin-like enzymatic activity produced by Porphyromonas gingivalis, Treponema denticola and Tannerella forsythensis. BANA-Zyme reagent strips were obtained from OraTec Corporation (Manassas, VA) and developed at 55° C for 5 minutes, following the manufacturer’s directions.
Blinding
To enhance objectivity and reduce delays that could potentially contribute to salivary contamination or sample evaporation, GCF samples were collected by a team that included a clinical operator, a Periotron operator and a data recorder. The clinical operator assessed the plaque and gingival index measurements, isolated the maxillary GCF collection sites from salivary contamination and collected GCF samples. The samples were quickly passed to the Periotron operator, who measured sample volume, confirmed that the instrument was always properly zeroed, and transferred the samples to a cooled microtube for storage. The Periotron readout was not visible to the clinical operator during sample collection and the site of origin of the GCF sample was not visible to the Periotron operator. Only the data recorder had direct access to this information. Immunoassay of the GCF samples was conducted by a separate team. The samples were coded to blind the analysis team to sample identities and collection dates.
Statistical analysis
Differences in PI and GI were analyzed with the Friedman repeated measures ANOVA on ranks. GCF IL-1ß and VEGF contents, which were normally distributed, were analyzed by one way repeated-measures ANOVA. The Holm-Sidak test was used for post-hoc comparisons. GCF volume and GCF IL-8 and TNF-α contents, which were not normally distributed, were analyzed using Friedman repeated measures ANOVA on ranks. Dunn’s test was used for post-hoc comparisons.
RESULTS
Subject population
The subject population included 6 males and 4 females with a mean age of 30 years. None of the subjects exhibited clinical signs of inflammation. All study subjects were compliant with the azithromycin regimen.
Effect of azithromycin on GCF volume
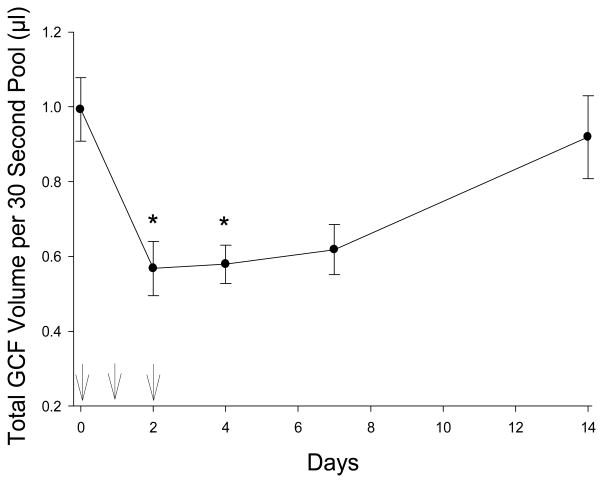
Azithromycin had a significant treatment effect on pooled GCF volume (Figure 1 and Table 1, P < 0.001). After initiation of azithromycin therapy, a significant decrease in pooled GCF volume from baseline was observed on days 2, 4 and 7 after the first dose (P < 0.05). Over days 7–14 after initiation of azithromycin, pooled GCF volume increased to 90% of baseline levels (difference not statistically significant from baseline). The median Gingival Index and Plaque Index values were 0 throughout the study period (Table 1).
Figure 1.
Pooled GCF sample volumes before, during and after administration of azithromycin. Arrows indicate the times azithromycin was administered. The data represent the mean (± SEM) pooled GCF volume collected from twelve different maxillary interproximal sites. Significant differences from baseline are denoted by * (P < 0.05, Dunn’s test).
Table 1.
GCF Volume, Plaque Index and Gingival Index Observed at Study Visits*
| Day | Pooled GCF Volume (μl)# | Plaque Index | Gingival Index |
|---|---|---|---|
| −7 | ND | 0 (0 to 1) | 0 (0 to 0) |
| 0 (baseline) | 1.0 (0.6 to 1.4) | 0 (0 to 1) | 0 (0 to 0) |
| 2 | 0.5 (0.2 to 1.0)§ | 0 (0 to 0) | 0 (0 to 0) |
| 4 | 0.5 (0.4 to 0.9)§ | 0 (0 to 0) | 0 (0 to 0) |
| 7 | 0.6 (0.3 to 1.0)§ | 0 (0 to 0) | 0 (0 to 0) |
| 14 | 0.9 (0.5 to 1.8) | 0 (0 to 0) | 0 (0 to 0) |
Data are presented as median and range observed in 10 subjects.
In this column, differences in median values among the different dates are statistically significant (P < 0.001, Friedman Repeated Measures ANOVA on Ranks).
Significant differences from baseline (P < 0.05, Dunn’s test)
Effect of azithromycin on GCF cytokine and VEGF content
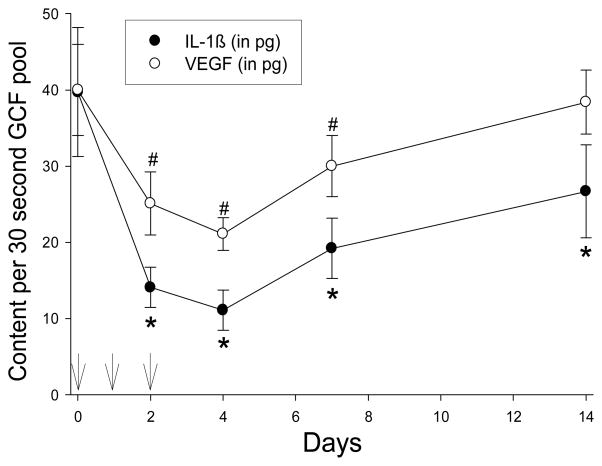
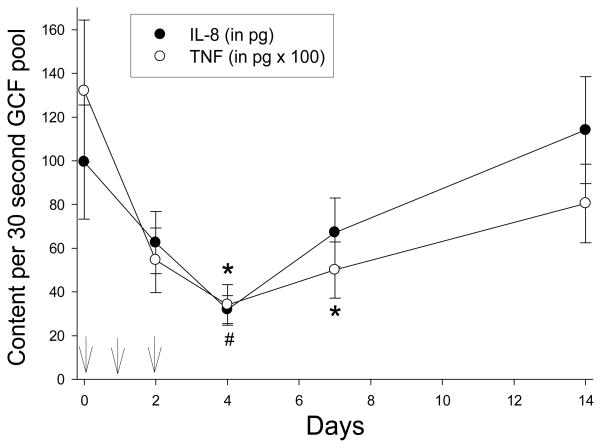
IL-6 and IL-10 were not detected in GCF samples from any of the subjects (data not shown). However, azithromycin treatment significantly reduced the amount of IL-1β, IL-8, TNF-α and VEGF recovered from pooled GCF samples (P < 0.005). Overall, the content of all four mediators reached their lowest levels on day 4 and increased toward baseline levels on days 7 and 14 (Figures 2 and 3). In comparison to baseline levels, a significant decrease in GCF IL-1β content was observed on days 2, 4, 7 and 14 (Figure 2). VEGF content was significantly lower than baseline on days 2, 4 and 7 (Figure 2), while TNF-α content was significantly lower on days 4 and 7 (Figure 3). IL-8 content decreased from baseline levels on days 2, 4 and 7, but the difference was statistically significant only on day 4 (Figure 3).
Figure 2.
Effect of azithromycin on GCF IL-1β and VEGF content. Arrows indicate the times azithromycin was administered. Data represent the mean (± SEM) recovery from samples obtained from twelve separate maxillary interproximal sites. The treatment effects on IL-1β and VEGF content were significant (P < 0.001, repeated measures ANOVA). Significant differences from baseline IL-1β is denoted by * and significant differences from baseline VEGF content is denoted by # (P < 0.05, Holm-Sidak test).
Figure 3.
Effect of azithromycin in GCF IL-8 and TNF-α content. Arrows indicate the times azithromycin was administered. Data represent the mean (± SEM) recovery in samples obtained from twelve separate maxillary interproximal sites. The treatment effects on IL-8 and TNF-α content were significant (P < 0.005, Friedman repeated measures ANOVA on ranks). Significant differences from baseline IL-8 content is denoted by # and significant differences from baseline TNF- α content is denoted by * (P < 0.05, Dunn’s test).
DISCUSSION
The results of this study suggest that azithromycin produces anti-inflammatory effects in gingival tissues that appear clinically healthy. The correlation of GCF volume with severity of gingival inflammation is well documented in the literature (Griffiths, 2003). Since azithromycin significantly decreased GCF flow rate at sites that were essentially plaque-free, it is likely that this effect is not strongly dependent on its antimicrobial activity. The effects occurred rapidly within 2 days of treatment, and GCF flow increased toward baseline levels as azithromycin was eliminated by the subjects. Treatment with azithromycin significantly decreased the amount of IL-1β, IL-8, TNF-α and VEGF in GCF in a manner that paralleled its effects on GCF flow rate. The relationship between the decreases in GCF flow rate and GCF pro-inflammatory cytokine content suggests that azithromycin effects on GCF volume could be mediated by inhibiting the production of one or more of these biological mediators.
The baseline GCF volume per strip observed in the present study (0.08 μl per 30 seconds) was similar to that reported for clinically healthy subjects in a previous study (Darany et al., 1992). Baseline levels of the pro-inflammatory cytokines IL-1β, IL-8, and TNF-α were similar to those reported in other studies of healthy human subjects (Zhang et al., 2002; Ren et al., 2007). IL-1β, IL-8 and TNF-α are involved in recruitment of inflammatory cells (Kornman et al., 2003). GCF content of these cytokines is related to the severity of gingival inflammation. The IL-1β content in GCF from severe periodontitis sites is up to 2-fold higher than in samples from sites with mild to moderate disease, and it decreases significantly after scaling and root planing (Engebretson et al., 2002). VEGF plays an important role in angiogenesis and maintenance of periodontal health (Unlu et al., 2003; Booth et al., 1998), and it can enhance the inflammatory response through its effects on vascular permeability. GCF VEGF content at periodontally diseased sites is greater than at healthy sites, and these levels decrease in response to periodontal therapy (Prapulla et al., 2007).
Although anti-inflammatory effects appear to contribute to the effectiveness of macrolides in treating chronic inflammatory airway diseases, (Giamarellos-Bourboulis, 2008) the mechanisms by which macrolides inhibit production of inflammatory mediators are not fully understood. Macrolides inhibit the activation of nuclear factor-κB (NF-κB) and activator protein-1 (AP-1), which are known to regulate the expression of IL-8, IL-6, TNF-α and IL-1β and other pro-inflammatory cytokines (Desaki et al., 2000; Kikuchi et al., 2002). Macrolides may also inhibit mitogen-activated protein kinase (MAPK) and extracellular-regulated kinase (ERK), resulting in a decrease in IL-8 production (Shinkai et al., 2006). It is conceivable that azithromycin works through similar mechanisms to decrease the GCF content of IL-1β, IL-8, and TNF-α in GCF, but further study is needed to elucidate its effects on host cells in the gingiva.
Despite use of a highly sensitive immunoassay, the pro-inflammatory cytokine IL-6 and anti-inflammatory cytokine IL-10 were not detected in any GCF samples during this study. Considering that the subjects had healthy gingival tissues, this is not unexpected. IL-6, which induces B-cell maturation, regulates bone resorption and stimulates acute-phase protein production, is involved in the later stages of the inflammatory response when B-cells increase in number (Okada and Murakami, 1998). IL-10 stimulates B-cell proliferation and inhibits production of many cytokines. It is likely that the levels of IL-6 and IL-10 are extremely low in the healthy gingival crevice.
These results are consistent with the hypothesis that azithromycin produces anti-inflammatory effects in gingiva. This characteristic of azithromycin, along with its antimicrobial and pharmacokinetic properties, is well suited for treatment of inflammatory periodontal diseases. Azithromycin is highly active against invasive periodontal pathogens and is used in a once-daily regimen that encourages patient compliance. It appears to localize in inflamed gingival tissues at levels that are significantly higher than in healthy gingiva (Burrell and Walters, 2008). For these reasons, it would be worthwhile to consider large-scale clinical trials to evaluate the adjunctive effects of azithromycin in the treatment of aggressive, recurrent and chronic forms of periodontitis. It may also be possible to modify the structure of azithromycin to enhance its effectiveness as an anti-inflammatory agent, analogous to modified tetracyclines.
Acknowledgments
This investigation was supported by USPHS research grant R21 DE018804 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892, USA. The authors are grateful for the assistance of Dr. Dimitris Tatakis with study design and Dr. Pin-Chuang Lai with data and GCF sample collection.
References
- Amsden GW. Advanced-generation macrolides: tissue-directed antibiotics. Int J Antimicrob Agents. 2001;18(Suppl 1):S11–15. doi: 10.1016/s0924-8579(01)00410-1. [DOI] [PubMed] [Google Scholar]
- Booth V, Young S, Cruchley A, Taichman NS, Paleolog E. Vascular endothelial growth factor in human periodontal disease. J Periodontal Res. 1998;33:491–499. doi: 10.1111/j.1600-0765.1998.tb02349.x. [DOI] [PubMed] [Google Scholar]
- Bosshardt DD, Lang NP. The junctional epithelium: from health to disease. J Dent Res. 2005;84:9–20. doi: 10.1177/154405910508400102. [DOI] [PubMed] [Google Scholar]
- Burrell RC, Walters JD. Distribution of systemic clarithromycin to gingiva. J Periodontol. 2008;79:1712–1718. doi: 10.1902/jop.2008.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou C-H, Walters JD. Clarithromycin transport by gingival fibroblasts and epithelial cells. J Dent Res. 2008;87:777–781. doi: 10.1177/154405910808700812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christersson LA, Albini B, Zambon JJ, Wikesjo UM, Genco RJ. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. I. Light, immunofluroescence and electron microscopic studies. J Periodontol. 1987;58:529–539. doi: 10.1902/jop.1987.58.8.529. [DOI] [PubMed] [Google Scholar]
- Conway TB, Beck FM, Walters JD. Gingival fluid ciprofloxacin levels at healthy and inflamed human periodontal sites. J Periodontol. 2000;71:1448–1452. doi: 10.1902/jop.2000.71.9.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darany DG, Beck FM, Walters JD. The relationship of gingival fluid leukocyte elastase activity to gingival fluid flow rate. J Periodontol. 1992;63:743–747. doi: 10.1902/jop.1992.63.9.743. [DOI] [PubMed] [Google Scholar]
- Desaki M, Takizawa H, Ohtoshi T, Kasama T, Kobayashi K, Sunazuka T, et al. Erythromycin suppresses nuclear factor-kappaB and activator protein-1 activation in human bronchial epithelial cells. Biochem Biophys Res Commun. 2000;267:124–128. doi: 10.1006/bbrc.1999.1917. [DOI] [PubMed] [Google Scholar]
- Engebretson SP, Grbic JT, Singer R, Lamster IB. GCF IL-1β profiles in periodontal disease. J Clin Periodontol. 2002;29:48–53. doi: 10.1034/j.1600-051x.2002.290108.x. [DOI] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis EJ. Macrolides beyond the conventional antimicrobials: a class of potent immunomodulators. Int J Antimicrob Agents. 2008;31:12–20. doi: 10.1016/j.ijantimicag.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Goldstein EJ, Citron DM, Merriam CV, Warren Y, Tyrrell K. Activities of telithromycin (HMR 3647, RU 66647) compared to those of erythromycin, azithromycin, clarithromycin, roxithromycin, and other antimicrobial agents against unusual anaerobes. Antimicrob Agents Chemother. 1999;43:2801–2805. doi: 10.1128/aac.43.11.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomi K, Yashima A, Iino F, Kanazashi M, Nagano T, Shibukawa N, et al. Drug concentration in inflamed periodontal tissues after systemically administered azithromycin. J Periodontol. 2007;78:918–923. doi: 10.1902/jop.2007.060246. [DOI] [PubMed] [Google Scholar]
- Griffiths GS. Formation, collection and significance of gingival crevice fluid. Periodontol 2000. 2003;31:32–42. doi: 10.1034/j.1600-0757.2003.03103.x. [DOI] [PubMed] [Google Scholar]
- Haas AN, de Castro GD, Moreno T, Susin C, Albandar JM, Oppermann RV, et al. Azithromycin as an adjunctive treatment of aggressive periodontitis: 12-months randomized clinical trial. J Clin Periodontol. 2008;35:696–704. doi: 10.1111/j.1600-051X.2008.01254.x. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Hagiwara K, Honda Y, Gomi K, Kobayashi T, Takahashi H, et al. Clarithromycin suppresses lipopolysaccharide-induced interleukin-8 production by human monocytes through AP-1 and NF-kappa B transcription factors. J Antimicrob Chemother. 2002;49:745–755. doi: 10.1093/jac/dkf008. [DOI] [PubMed] [Google Scholar]
- Kornman KS, Page RC, Tonetti MS. The host response to the microbial challenge in periodontitis: assembling the players. Periodontol 2000. 1997;14:1433–1453. doi: 10.1111/j.1600-0757.1997.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Lamont RJ, Chan A, Belton CM, Izutsu KT, Vasel D, Weinberg A. Porphyromonas gingivalis invasion of gingival epithelial cells. Infect Immun. 1995;63:3878–3885. doi: 10.1128/iai.63.10.3878-3885.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loe H, Silness J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- Mascarenhas P, Gapski R, Al-Shammari K, Hill R, Soehren S, Fenno JC, et al. Clinical response of azithromycin as an adjunct to non-surgical periodontal therapy in smokers. J Periodontol. 2005;76:426–436. doi: 10.1902/jop.2005.76.3.426. [DOI] [PubMed] [Google Scholar]
- Okada H, Murakami S. Cytokine expression in periodontal health and disease. Crit Rev Oral Biol Med. 1998;9:248–266. doi: 10.1177/10454411980090030101. [DOI] [PubMed] [Google Scholar]
- Pajukanta R. In vitro antimicrobial susceptibility of Porphyromonas gingivalis to azithromycin, a novel macrolide. Oral Microbiol Immunol. 1993;8:325–326. doi: 10.1111/j.1399-302x.1993.tb00583.x. [DOI] [PubMed] [Google Scholar]
- Pajukanta R, Asikainen S, Saarela M, Alaluusua S, Jousimies-Somer H. In vitro activity of azithromycin compared with that of erythromycin against Actinobacillus actinomycetemcomitans. Antimicrob Agents Chemother. 1992;36:1241–1243. doi: 10.1128/aac.36.6.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prapulla DV, Sujatha PB, Pradeep AR. Gingival crevicular fluid VEGF levels in periodontal health and disease. J Periodontol. 2007;78:1783–1787. doi: 10.1902/jop.2007.070009. [DOI] [PubMed] [Google Scholar]
- Ren Y, Hazemeijer H, de Haan B, Qu N, de Vos P. Cytokine profiles in crevicular fluid during orthodontic tooth movement of short and long durations. J Periodontol. 2007;78:453–458. doi: 10.1902/jop.2007.060261. [DOI] [PubMed] [Google Scholar]
- Schroeder HE, Munzel-Pedrazzoli S, Page R. Correlated morphometric and biochemical analysis of gingival tissue in early chronic gingivitis in man. Arch Oral Biol. 1973;18:899–923. doi: 10.1016/0003-9969(73)90060-5. [DOI] [PubMed] [Google Scholar]
- Shinkai M, Foster GH, Rubin BK. Macrolide antibiotics modulate ERK phosphorylation and IL-8 and GM-CSF production by human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L75–L85. doi: 10.1152/ajplung.00093.2005. [DOI] [PubMed] [Google Scholar]
- Silness J, Loe H. Periodontal Disease in Pregnancy. I. Correlation between Oral Hygiene and Periodontal Condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Slots J, Ting M. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in human periodontal disease: occurrence and treatment. Periodontol 2000. 1999;20:82–121. doi: 10.1111/j.1600-0757.1999.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Smith SR, Foyle DM, Daniels J, Joyston-Bechal S, Smales FC, Sefton A, et al. A double-blind placebo-controlled trial of azithromycin as an adjunct to non-surgical treatment of periodontitis in adults: clinical results. J Clin Periodontol. 2002;29:54–61. doi: 10.1034/j.1600-051x.2002.290109.x. [DOI] [PubMed] [Google Scholar]
- Tamaoki J, Kadota J, Takizawa H. Clinical implications of the immunomodulatory effects of macrolides. Am J Med. 2004;117(Suppl 9A):5S–11S. doi: 10.1016/j.amjmed.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Unlu F, Guneri PG, Hekimgil M, Yesilbek B, Boyacioglu H. Expression of vascular endothelial growth factor in human periodontal tissues: comparison of healthy and diabetic patients. J Periodontol. 2003;74:181–187. doi: 10.1902/jop.2003.74.2.181. [DOI] [PubMed] [Google Scholar]
- van Winkelhoff AJ, Rams TE, Slots J. Systemic antibiotic therapy in periodontics. Periodontol 2000. 1996;10:45–78. doi: 10.1111/j.1600-0757.1996.tb00068.x. [DOI] [PubMed] [Google Scholar]
- Vanaudenaerde BM, Wuyts WA, Geudens N, Dupont LJ, Schoofs K, Smeets S, et al. Macrolides inhibit IL-17-induced IL-8 and 8-isoprostane release from human airway smooth muscle cells. Am J Transplant. 2007;7:76–82. doi: 10.1111/j.1600-6143.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Kashket S, et al. Evidence for the early onset of gingival inflammation following short-term plaque accumulation. J Clin Periodontol. 2002;29:1082–1085. doi: 10.1034/j.1600-051x.2002.291206.x. [DOI] [PubMed] [Google Scholar]