Abstract
Background:
Autopsy studies of former contact sports athletes, including soccer and rugby players, frequently report chronic traumatic encephalopathy; a neurodegenerative pathology associated with traumatic brain injury. Nevertheless, little is known about risk of neurodegenerative disease in these populations. We hypothesised that neurodegenerative disease risk would be higher among former elite rugby union players than the general population.
Methods:
We conducted a retrospective cohort study accessing national electronic records on death certification, hospital admissions and dispensed prescriptions for a cohort of 412 male Scottish former international rugby union players and 1,236 members of the general population, matched to former players by age, sex and area socioeconomic status. Mortality and incident neurodegenerative disease diagnoses among former rugby players were then compared to the matched comparison group.
Results:
Over a median 32 years follow-up from study entry at age 30 years, 121 (29.4%) former rugby players and 381 (30.8%) of the matched comparison group died. All-cause mortality was lower among former rugby players until 70 years of age with no difference thereafter. During follow-up, 47 (11.4%) former rugby players and 67 (5.4%) of the comparison group were diagnosed with incident neurodegenerative disease (hazard ratio 2.67; 95% confidence interval 1.67 to 4.27; p<0.001).
Conclusions:
This study adds to understanding of the association between contact sports participation and risk of neurodegenerative disease. While further research exploring this interaction is required, in the meantime strategies to reduce exposure to head impacts and injuries in sport should be promoted.
Keywords: neurodegenerative disease, dementia, motor neuron disease, chronic traumatic encephalopathy, traumatic brain injury, concussion
INTRODUCTION
Traumatic brain injury (TBI) is recognised as a major risk factor for neurodegenerative disease and is estimated to be responsible for 3% of dementia in the general population.1 This association has attracted particular attention in recent years through autopsy reports of a neuropathology uniquely associated with prior history of TBI or repetitive head impact exposure, chronic traumatic encephalopathy neuropathologic change (CTE-NC), in former athletes from a range of sports, including American football,2 soccer3–5 and rugby union.5,6 To date, however, although CTE-NC has been documented in numerous studies of former athletes and others exposed to TBI and repetitive head impacts, there is only limited understanding of risk of neurodegenerative disease among these populations.
Studies have reported higher neurodegenerative disease mortality among former National Football League professional American football players7–9 and former professional soccer players.10–15 For example, among former professional soccer players overall neurodegenerative disease mortality is approximately three and a half fold higher than among a general population comparison group matched by age and area socioeconomic status, with the risk ranging from a doubling of deaths with Parkinson’s disease to a fivefold increase in deaths with Alzheimer’s disease.14 Furthermore, evidence from professional soccer and American football suggests the risk of neurodegenerative disease reflects cumulative exposure to risk factors within the sport, with risk of neurodegenerative disease higher with longer career lengths9,15 and in player positions associated with greater exposure to head impacts.15 Nevertheless, although thorough, these few studies report neurodegenerative disease outcomes in exclusively professional athletes and from limited sports.
Played in over 120 countries internationally, with just under 10million active participants,16 rugby union (hereafter ‘rugby’) is acknowledged as a contact sport in which there is relatively high risk of concussion/mild TBI, with current data suggesting injury rates ranging 2.08 concussions/1000 player hours at community level17 to 19.8 concussion/1000 player hours in professional rugby.18 Although first played in the 19th century, rugby remained an amateur sport until 1995 when professionalism was permitted, and professional league competitions were instituted. To date, multiple autopsy proven cases of CTE-NC have been reported among former rugby players, all from athletes participating in the amateur era.5,6 In parallel, data on cognitive outcomes from cohorts of former rugby players using various methodologies report measurable, if perhaps clinically insignificant, cognitive deficits.19–23 Nevertheless, risk of neurodegenerative disease among former rugby players remains unknown.
Reflecting experience in former professional athlete populations where CTE-NC is documented, we hypothesised that, despite being an amateur sport until relatively recently, risk of neurodegenerative disease among former elite rugby players would be higher than among a matched general population comparison group. This retrospective cohort study is designed to test this hypothesis by accessing national electronic health records to explore risk of neurodegenerative disease among male former international rugby players.
METHODS
Approvals
Ethical approval was provided by the University of Glasgow College of Medical, Veterinary and Life Sciences Ethics Committee (Project Number 200200034), with protocol and data governance procedures reviewed and approved by National Health Service (NHS) Scotland’s Public Benefit and Privacy Panel for Health and Social Care (Reference 1920–0262). As all data from health record were anonymised to researchers, participant-level consent was not required. The analysis and reporting of this study are consistent with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines.24
Cohort identification and inclusion criteria
Former international rugby players were identified from the book ‘Scottish Rugby: Game by Game’ by Kenneth Bogle, which lists all Scottish men’s international representative team players since 1890.25 This was cross-referenced and supplemented with publicly available information on Scottish international rugby players available via the website ESPNscrum.com.26 Available data included full name and date of birth of players and their field position. Study inclusion was restricted to individuals aged 30 years or over at 31/12/2020. Individuals with missing or incomplete date of birth were excluded. All subjects included in this study were male.
Matched population comparison group
Probabilistic matching was applied to the full name and date of birth of former rugby players to link them to their unique Community Health Index (CHI) number. Using this process, multiple demographic variables (such as date of birth or full name) are compared between the two datasets and a score reflecting the level of agreement allocated to each. The scores are then summated to provide the overall likelihood of those two records belong to the same individual, with a cut-off applied to determine which matches are accepted or rejected. Thereafter, using an automated linkage agent computer programme, CHI numbers for individual former rugby players were then used to randomly identify a matched population comparison group defined as men matched by year of birth and quintile of area-based socioeconomic status to the former rugby players on a 3:1 ratio; in other words, each rugby player was individually matched to 3 general population comparators. The NHS Information Services Division records last known postcode of residence for all individuals, from which area-based socioeconomic deprivation is calculated using the Scottish Index of Multiple Deprivation (SIMD) 2020; derived from information on income, employment, health, education, housing and crime.27 The SIMD is categorised into general population quintiles ranging from 1 (most deprived) to 5 (most affluent).
Lifelong health outcomes
Outcomes for all former rugby players and the matched population comparison group, whether living or deceased at time of data capture, were obtained by individual-level record linkage to: hospitalisations, ascertained from Scottish Morbidity Record (SMR) 01 (General/Acute Inpatient and Day Case) and SMR04 (Mental Health Inpatient and Day Case) datasets; dispensed prescriptions ascertained from the national Prescribing Information System; and death certificates. SMR01, SMR04 and death certification datasets were accessed for each individual by matching to their unique CHI number, with all outcomes coded using the International Classification of Diseases ICD9/10. Supplementary Table 1 lists the ICD9/10 codes used to define the outcomes: neurodegenerative disease (all neurodegenerative diseases; dementia, not otherwise specified, Alzheimer’s disease, non-Alzheimer’s dementias, motor neuron disease (MND)/ amyotrophic lateral sclerosis (ALS) and Parkinson’s disease in addition to the remaining most common causes of death among adult Scottish males (diseases of the circulatory system [classified as all diseases of the circulatory system, ischemic heart disease, and stroke or cerebrovascular disease], diseases of the respiratory system, or cancer [classified as any cancer or lung cancer]). The Prescribing Information System records every prescription dispensed in the community, with medications coded using the British National Formulary (BNF).28 The medications relevant to this study were coded under Section 4.9 (drugs used in parkinsonism and related disorders, including drugs used in MND/ALS) and Section 4.11 (drugs for dementia) of the BNF (Supplementary Table 2). For all former players and the matched comparison group, neurodegenerative disease events were ascertained from the earliest coding of neurodegenerative disease captured from either hospitalisation or death certification (listed as the primary or contributing cause of death) or relevant medication captured from the prescribing database. SMR01, SMR04 and death certification data were available from January 1, 1981 and prescribing data were available from January 1, 2009. All analyses included data up to 31st December 2020, with database interrogation performed on the 30th November 2021.
Statistical analyses
Cox proportional hazards models were used to model time to death and time to incident neurodegenerative disease, with results reported as hazard ratios (HRs) and 95% confidence intervals. Schoenfeld residuals were used to test the assumption of proportional hazards. Where the proportional hazards assumption did not hold, a time-varying model was used to derive hazard ratios over different periods of follow up. In the analysis of motor neuron disease/amyotrophic lateral sclerosis zero events were recorded among the general population comparison group. To accommodate this, one general population observation was reallocated from no event to event, with results reported as a Baptista-Pike mid-p odds ratio (OR) and 95% confidence interval.29 All statistical analyses were undertaken using Stata v16, with statistical significance set at two-sided p<0·05.30
RESULTS
Study Cohort
A total of 654 former Scottish international rugby players were identified from available records. Of these, 414 were successfully matched to their CHI. The remaining 240 rugby players could not be matched to their CHI due to incomplete or inaccurate demographic information. Following CHI matching, a further 2 former rugby players were excluded because their last known postcode of residence could not be ascertained, precluding identification of appropriately matched general population individuals for comparison. The final cohort for analysis, therefore, comprised 412 former international rugby players and a comparison group of 1,236 sex, age and area socioeconomic status matched members of the general population (Table 1) (Supplementary Figure 1).
Table 1:
Cohort demographic information
| Former international rugby players (n=412) | Matched population comparison group (n=1,236) | |
|---|---|---|
| Number (percent) | ||
| SIMD quintile | ||
| 1 | 11 (2.7) | 33 (2.7) |
| 2 | 41 (10.0) | 123 (10.0) |
| 3 | 66 (16.0) | 198 (16.0) |
| 4 | 127 (30.8) | 381 (30.8) |
| 5 | 167 (40.5) | 501 (40.5) |
| Player position | ||
| Forward | 222 (53.9) | N/A |
| Front row | 96 (23.3) | N/A |
| Lock | 40 (9.7) | N/A |
| Back row | 86 (20.9) | N/A |
| Backs | 190 (46.1) | N/A |
| Half Backs | 58 (14.1) | N/A |
| Three quarters | 111 (26.9) | N/A |
| Fullback | 21 (5.1) | N/A |
| Year of birth | ||
| 1900–1919 | 47 (11.5) | 141 (11.5) |
| 1920–1939 | 91 (22.1) | 273 (22.1) |
| 1940–1959 | 89 (21.6) | 267 (21.6) |
| 1960–1979 | 109 (26.4) | 327 (26.4) |
| 1980–1990 | 76 (18.5) | 228 (18.5) |
All-cause and neurodegenerative disease mortality among former international rugby players
Over a median of 32 years follow-up from study entry at the age of 30 years, 121 (29.4%) of 412 former rugby players and 381 (30.8%) of 1,236 individuals in the comparison group died, with age at death higher among former rugby players than the comparison group (78.9±10.2 (mean±SD) years versus 76.4±11.4 years; p=0.03) (Figure 1). Overall, there was no difference in all-cause mortality between former international rugby players and the matched comparison group using a Cox proportional hazard model (hazard ratio [HR], 0.86; 95% confidence interval [CI] 0.68–1.08; p=0.19) (Table 2). However, the proportional hazards assumption was not met. All-cause mortality among former international rugby players was lower to age 70 years, thereafter, it was no different from that of the matched population comparison group (Figure 2). All other analyses fulfilled the proportional hazards assumption. No differences in mortality or age at death were observed between former rugby players and the general population comparison group for the most common primary causes of death for Scottish adult males, including cardiovascular disease, respiratory disease, cancer, and neurodegenerative disease (Table 2; Figure 1). However, when the analyses were repeated including both primary and secondary causes of death, risk of death with respiratory disease was lower (HR 0.61; 95% CI 0.37–0.99; p=0.045), while risk of death with neurodegenerative disease was higher (HR 2.60; 95% CI 1.44–4.70; p=0.002) among former rugby players than the comparison group. No differences in mortality were observed for the remaining causes of death using the composite outcome of primary and contributory cause of death.
Figure 1:
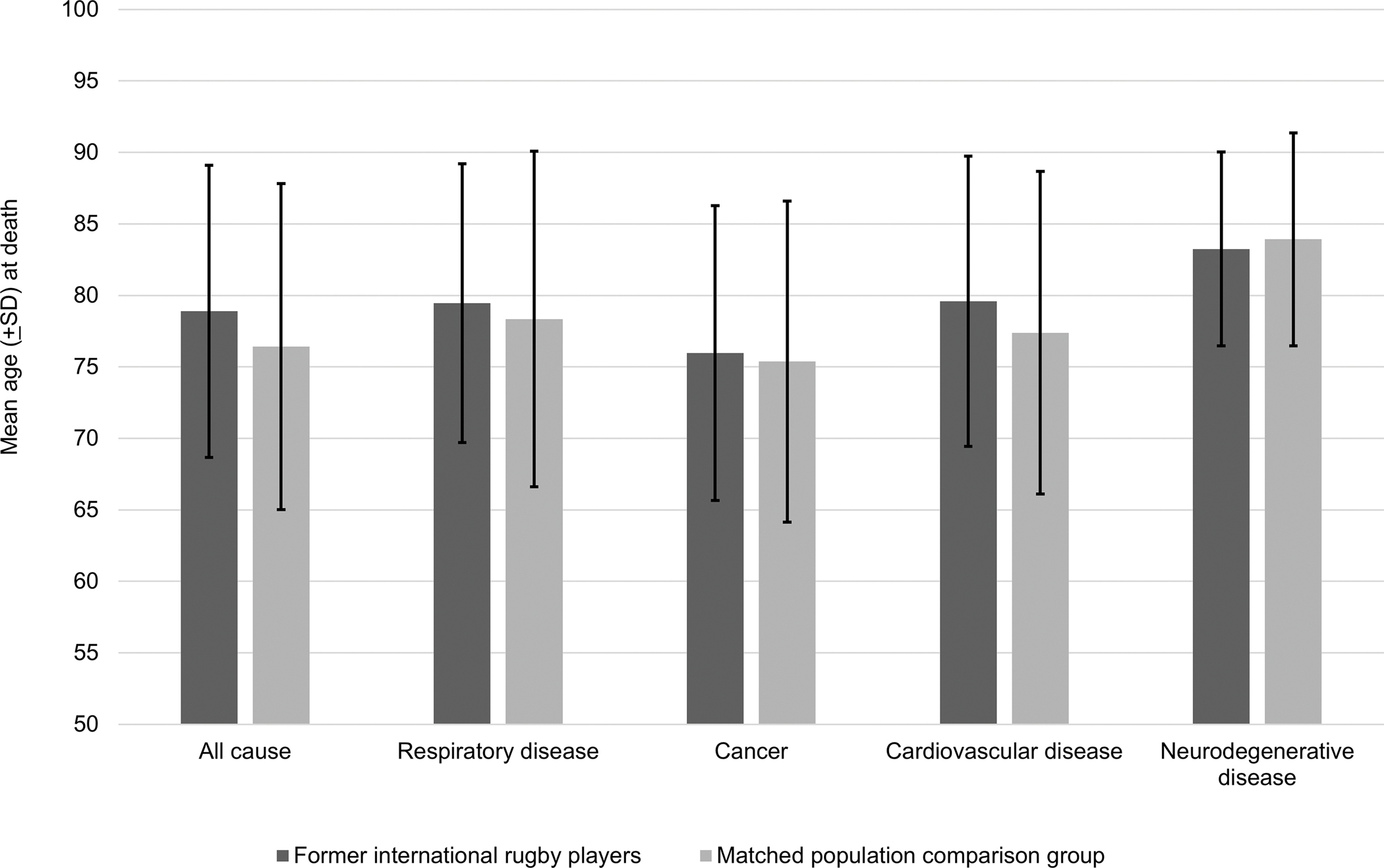
Mean age at death for common causes of death among former international rugby players compared with a matched population comparison group. Overall age at death was higher in former international rugby players than a matched population comparison group (mean ± SD) (78.9 ± 10.2 years vs 76.4 ± 11.4 years respectively; p=0.003). There was no difference in age at death for all mortality subtypes assessed.
Table 2:
Primary cause of death among former international rugby players and a matched population comparison group.
| Primary cause of death | Former international rugby players (n=412) | Matched population comparison group (n=1,236) | Hazard Ratio (95%CI) | p* |
|---|---|---|---|---|
| Any cause ** | 121 (29.4%) | 381 (30.8%) | 0.86 (0.68–1.08) | 0.19 |
| Cardiovascular disease | 54 (13.1%) | 155 (12.5%) | 0.89 (0.63–1.28) | 0.534 |
| Cancer | 30 (7.3%) | 108 (8.7%) | 0.77 (0.50–1.19) | 0.242 |
| Neurodegenerative disease | 11 (2.7%) | 18 (1.5%) | 2.43 (0.92–6.42) | 0.073 |
| Respiratory disease | 11 (2.7%) | 43 (3.5%) | 0.62 (0.28–1.39) | 0.247 |
Cox proportional hazards regression.
All-cause mortality analysis did not fulfil the proportional-hazards assumption and showed time-dependent variability
Figure 2:
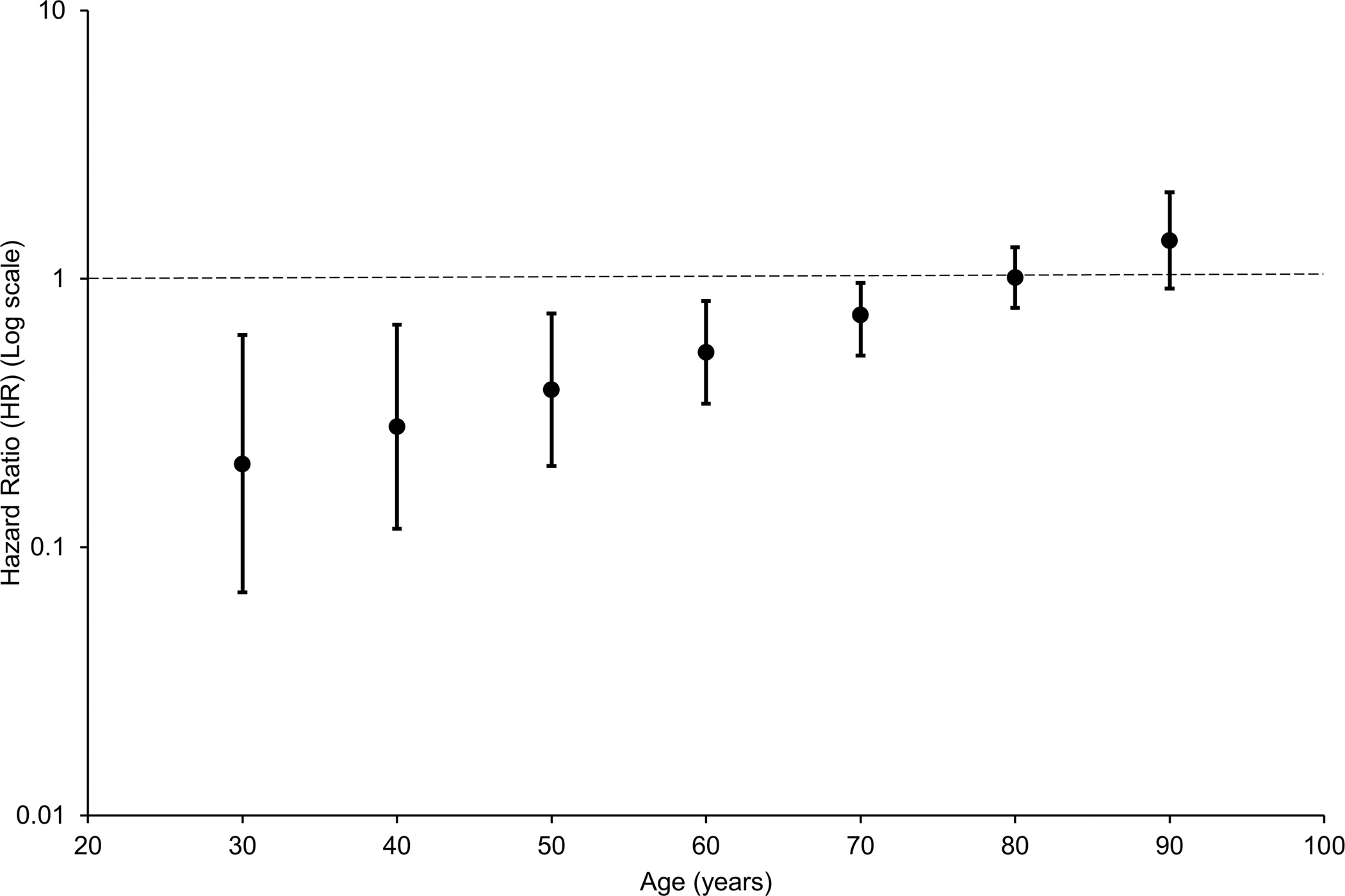
Time-varying hazard ratios for all-cause mortality among former international rugby players compared with a matched population comparison group. The proportional-hazards assumption did not hold for all-cause mortality, so a time-dependent analysis was performed. This showed all-cause mortality was lower among former international rugby players up until the age of 70 years, thereafter, it was no different from that of a matched population comparison group.
Neurodegenerative disease risk among former international rugby players
Over the period of follow-up, 47 (11.4%) former international rugby players and 67 (5.4%) of the matched, general population comparison group had an incident neurodegenerative disease diagnosis, based on death certification, hospital admission or prescribing information (HR 2.67; 96% CI 1.67–4.27; p<0.001) (Table 3). In addition to higher deaths with neurodegenerative disease, hospitalisation for neurodegenerative disease (HR 2.29; 95% CI 1.19–4.41; p=0.013) and prescriptions for medications used in neurodegenerative disease (HR 4.59; 95% CI 2.14–9.82; p<0.001) were higher among former rugby players than the comparison group. Furthermore, risk of incident neurodegenerative disease diagnosis varied by disease subtype (Table 4).
Table 3:
Neurodegenerative disease outcomes among former international rugby players compared to that of a matched population comparison group.
| Former international rugby players (n=412) | Matched population comparison group (n=1,236) | Hazard ratio (95%CI) | p* | |
|---|---|---|---|---|
| All NDD | 47 (11.4%) | 67 (5.4%) | 2.67 (1.67–4.27) | <0.001 |
| Deaths | 32 (7.8%) | 39 (3.2%) | 2.60 (1.44–4.70) | 0.002 |
| Hospitalisation | 21 (5.1%) | 37 (3.0%) | 2.29 (1.19–4.41) | 0.013 |
| Prescribing NDD | 24 (5.8%) | 28 (2.3%) | 4.59 (2.14–9.82) | <0.001 |
Cox proportional hazards regression
Table 4:
Neurodegenerative disease subtypes among former international rugby players compared to a matched population comparison group.
| Neurodegenerative disease | Hazard*/Odds ratio** (95% CI) | p |
|---|---|---|
| Dementia (NOS) | 2.17* (1.26 – 3.72) | 0.005 |
| Parkinson’s disease | 3.04* (1.51 – 6.10) | 0.002 |
| MND/ALS † | 15.17** (2.10 – 178.96) | 0.009 |
MND/ALS adjusted to accommodate zero events by transferring one general population observation from no event to event.
Cox proportional hazards regression
Baptista-Pike mid-p odds ratio and confidence interval
Player position and risk of neurodegenerative disease
In a sub-group analysis of risk of incident neurodegenerative disease among former international rugby players, there was no difference in risk between forwards and backs (HR 1.00; 95% CI 0.56–1.78; p=0.991) (Table 5).
Table 5:
Player position and risk of neurodegenerative disease among former international rugby players compared with matched population controls.
| Forwards (n=222) | Backs (n=190) | HR (95%CI) | p | |
|---|---|---|---|---|
| All NDD | 26 (11.7%) | 21 (11.1%) | 1.00 (0.56–1.78) | 0.991 |
DISCUSSION
Our findings demonstrate that, up to 70 years of age, all-cause mortality was lower among male, former Scottish international rugby union players than the general population. However, neurodegenerative disease was more likely to cause or contribute to deaths among former rugby players than the general population, in contrast to respiratory disease mortality which was less among former rugby players. This finding of higher neurodegenerative disease mortality was corroborated by a higher risk of incident neurodegenerative disease among rugby players. Finally, while risk of incident neurodegenerative disease varied by subtype among former rugby players, no difference in risk was observed between player positions when sub-grouped as either forwards or backs.
Our finding of an age-dependent relationship between former rugby players and all-cause mortality is consistent with our previous finding of a similar relationship among former soccer players.14 However, in contrast to former international soccer players, we did not observe reduced risk of deaths due to cardiovascular disease or cancer among former international rugby players, suggesting the possibility of sport-specific influences on lifelong outcomes. In this context, similar between sport differences in all-cause and cause-specific mortality have been reported elsewhere.8 There is, therefore, a continued need to consider broad, lifelong health outcomes among such populations with robust methodologies to allow meaningful cross sport comparisons.
The mortality outcomes observed in this retrospective cohort study of former international rugby players might appear at odds with previous data from a previous cross-sectional study of former international rugby players that reported lower prevalence of cardiovascular disease than in a comparison group of similar age.20 Further, that previous study and other, similar cross-sectional studies in former rugby players demonstrated only limited evidence of minor, sub-clinical reductions in cognitive performance among former players.19–23 The differences are likely to reflect the methodological limitations of cross-sectional studies such as being prone to survival bias. Since our study demonstrated higher mortality from neurodegenerative disease among former rugby players, former players with neurodegenerative disease are likely to be under-represented in a cross-sectional study. Previous cross-sectional studies may also have been subject to volunteer, or self-selection, bias31,32 which was obviated in our retrospective cohort study using secondary data.
That former international rugby players have higher mortality with neurodegenerative disease than the matched comparison group is consistent with similar observations from studies of former National Football League (NFL)7–0 and professional soccer players.10–15 Notably, in contrast to data from NFL and soccer, our cohort of rugby players largely comprises amateur athletes, albeit participating at an elite, international level. In this respect, it is the first demonstration that high neurodegenerative disease risk is not a phenomenon exclusive to professional athletes. This is in line with data from autopsy studies on former athletes with dementia, where CTE-NC has been reported in a majority of both former footballers and rugby players, the latter largely comprising amateur era athletes.5 With the advent of professional rugby in 1995, it is likely that exposure to repetitive head impacts in training and match play will have increased, while data from elite level participants confirm that concussion/mild TBI incidence has increased over the past 20 years or so.18 As such, it will be important to monitor brain health in current and former athletes emerging from the professional era and to consider interventions to mitigate any potential adverse brain health outcomes in former rugby players.
There are no ICD9/10 codes for CTE or dementia pugilistica, nor are we aware of a surrogate code which is used consistently and widely in clinical practice to capture putative diagnoses. As such, we have no information on this diagnosis. Nevertheless, the mixed neurodegenerative disease diagnoses captured in this study would be in line with similar observations in previous similar studies of former athletes,7,14 and with autopsy findings reporting mixed neurodegenerative pathologies among former rugby players with dementia.5 Further, while neurodegenerative disease risk overall was higher among former rugby players than the comparison group, our data suggest risk varied by neurodegenerative disease subtype, similar to observations in NFL7 and soccer.14
Perhaps most notable in this regard, while the absolute number of former rugby players diagnosed with motor neuron disease (MND) was low in the present study, the high risk of MND observed relative to the comparison group is at least consistent with, if not higher than, that observed in previous athlete studies.7,9–14 Furthermore, since we had to allocate an MND event to the comparison group, to obviate the problem of a zero count, the reported effect size is likely to be an underestimate of the true strength of association. Limited data suggest exposure to TBI, including sports concussion, might serve as a risk factor for MND.33–36 However, the risk observed in this study and similar athlete studies appears several-fold higher than reported from research looking at non-sport associated TBI.36 As such, the possibility that head impacts and/or TBI in sport lead to greater risk of MND than non-sports TBI, or that an additional, unknown risk factor contributes to the remarkably high risk observed among these populations, might be considered. Certainly, there is an immediate need for further, robust studies considering the specific association between sport, traumatic brain injury and risk of this rare, but uniformly fatal outcome.
Concussion risk in rugby union is recognised to be among the highest for contact sports,37,38 with data demonstrating increasing concussion incidence over the past decade at professional level.39 Around half of concussions occur during the tackle; the majority of those experienced by the player making the tackle.40 In an effort to address concerns over concussion in the sport, rugby authorities have introduced initiatives directed towards improved injury detection41 and risk reduction in match play.42 However, head impact exposures and concussion risk are not isolated to match play. As such, measures to reduce exposures in training might also be considered a priority.43 In addition to these primary prevention measures, interventions targeted towards risk mitigation among former rugby players with already accumulated head impact exposures might also be considered, including the development of specialist brain health clinics.44
A strength of our study is the inclusion of a relatively large number of former rugby players and, importantly, a matched general population comparison group, with outcomes derived from comprehensive electronic health records. As such, potential biases were minimised. However, we must recognise that around 37% of our potential cohort of former international rugby players could not be matched to their health records. We cannot, therefore, exclude the possibility that there may be differences in outcomes between our matched and unmatched populations, although inaccurate and incomplete recording of names and dates of birth is likely to introduce random error, rather than bias. Further, as SMR datasets are used extensively to inform Scottish healthcare policy planning and in research, their quality is maintained through a set of validation rules. Nevertheless, previous work has shown a propensity to under-reporting of neurodegenerative disease case numbers when these and other such existing datasets are interrogated.45 Again, however, we have no reason to believe any error would systematically favor one study population over the other. Our observations included only adult male athletes who, although playing largely in an amateur era, nevertheless, were participating at elite level. As such, the applicability of our findings to youth, female and amateur athletes remains uncertain. Finally, we have no information in the available datasets regarding total career length in rugby or history of head impact and traumatic brain injury exposure or of exposure to wider dementia risk factors, including alcohol consumption. Further studies specifically designed to explore these questions should be pursued.
In summary, our data demonstrate neurodegenerative disease risk is higher among Scottish male former international rugby union players than the general population after matching for sex, age and area socioeconomic status. These data add to our understanding of the association between contact sports and lifelong health outcomes, specifically risk of adverse brain health outcomes. There remains a need for further research exploring the relationship between contact sports and risk of neurodegenerative disease. In the meantime, strategies to reduce exposure to head impacts and injuries across all sports should continue to be developed and promoted, while measures to mitigate risk of adverse brain health in former athletes should be considered.
Supplementary Material
Key Messages.
What is already known on this topic
High neurodegenerative disease mortality has been reported among former professional American footballers and soccer players. The risk of neurodegenerative disease among former rugby players, however, remains unknown.
What this study adds
While overall, all-cause mortality was similar among Scottish male former international rugby players and their matched general population comparison group, mean age at death was slightly higher while risk of neurodegenerative disease was just over two and a half times higher among former rugby players.
How this study might affect research, practice, policy
These data provide further insight into the association between contact sports and higher neurodegenerative disease risk and add to evidence in support of the need for measures to reduce risk of head impacts and head injuries in sport, while also exploring strategies to mitigate risk of adverse brain health in former athletes.
Acknowledgements
This work was supported by funding from: The Football Association and The Professional Footballers Association; National Institutes of Neurological Disorders and Stroke, US (U54NS115322; WS); and NHS Research Scotland (WS).
Role of the funding source
The funders of this work had no role in study design, data collection, analyses, and interpretation, writing of the manuscript, or in the decision to submit the work for publication.
Footnotes
Competing interests
The authors declare no competing interests beyond the funding declared above.
Data sharing statement
All data are stored on the NHS Scotland National Safe Haven and cannot be made publicly available due to confidentiality reasons.
REFERENCES
- 1.Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020; 396:413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mez J, Daneshvar DH, Kiernan PT, et al. Clinicopathological Evaluation of Chronic Traumatic Encephalopathy in Players of American Football. JAMA. 2017;318:360–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hales C, Neill S, Gearing M, Cooper D, Glass J, Lah J (2014). Late-stage CTE pathology in a retired soccer player with dementia. Neurology. 2013;83:2307–09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ling H, Morris HR, Neal JW, Lees AJ, Hardy J, Holton JL, Revesz T, Williams DDR. Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol. 2017;133: 337–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee EB, Kinch K, Johnson VE, Trojanowski JQ, Smith DH, Stewart W Chronic traumatic encephalopathy is a common co-morbidity, but less frequent primary dementia in former soccer and rugby players. Acta Neuropathol. 2019;138: 389–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart W, McNamara PH, Lawlor B, Hutchinson S, Farrell M Chronic traumatic encephalopathy: a potential late and under recognized consequence of rugby union? QJM. 2016;109: 11–15. [DOI] [PubMed] [Google Scholar]
- 7.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012; 79:1970–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen VT, Zafonte RD, Chen JT et al. Mortality Among Professional American-Style Football Players and Professional American Baseball Players. JAMA Network Open. 2019; 2: e194223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daneshvar DH, Mez J, Alosco ML et al. Incidence of and Mortality From Amyotrophic Lateral Sclerosis in National Football League Athletes. JAMA Netw Open. 2021;4:e2138801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belli S, Vanacore N Proportionate mortality of Italian soccer players: is amyotrophic lateral sclerosis an occupational disease? Eur J Epidemiol. 2005; 20: 237–42. [DOI] [PubMed] [Google Scholar]
- 11.Chiò A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain. 2005; 128: 472–76. [DOI] [PubMed] [Google Scholar]
- 12.Taioli E All causes mortality in male professional soccer players. Eur J Public Health. 2007; 17: 600–04. [DOI] [PubMed] [Google Scholar]
- 13.Pupillo E, Bianchi E, Vanacore N, et al. Increased risk and early onset of ALS in professional players from Italian Soccer Teams. Amyotroph Lateral Scler Frontotemporal Degener. 2020; 21: 403–09. [DOI] [PubMed] [Google Scholar]
- 14.Mackay DF, Russell ER, Stewart K, MacLean JA, Pell JP, Stewart W. Neurodegenerative disease mortality among former professional soccer players. N Engl J Med. 2019; 381:1801–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell ER, Mackay DF, Stewart K, MacLean JA, Pell JP, Stewart W. Association of Field Position and Career Length With Risk of Neurodegenerative Disease in Male Former Professional Soccer Players. JAMA Neurol. 2021;78:1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rugby World. Global rugby participation. 2018. https://resources.world.rugby/worldrugby/document/2020/07/28/212ed9cf-cd61-4fa3-b9d4-9f0d5fb61116/P56-57-Participation-Map_v3.pdf accessed 23rd May 2022
- 17.Gardner AJ, Iverson GL, Williams WH, Baker S, Stanwell P. A systematic review and meta-analysis of concussion in rugby union. Sports Med. 2014;44:1717–31. [DOI] [PubMed] [Google Scholar]
- 18.England Professional Rugby Injury Surveillance Project Steering Group. England professional rugby injury surveillance project: Season report 2019–2020. https://www.englandrugby.com/dxdam/ab/ab1ea449-5915-4c5c-ab27-9f90ed076bd8/PRISP%20report%2019-20%20Final.pdf accessed on 23rd May 2022
- 19.Decq P, Gault N, Blandeau M et al. Long-term consequences of recurrent sports concussion. Acta Neurochir (Wien). 2016;158:289–300. [DOI] [PubMed] [Google Scholar]
- 20.McMillan TM, McConnachie A, Hay J, Wainman-Lefley J, Maclean LM, McSkimming P, Stewart W. Long Term Health Outcomes after Exposure to Repeated Concussion in Elite Level Rugby Union Players. J Neurol Neurosurg Psychiatry. 2017;88:505–11 [DOI] [PubMed] [Google Scholar]
- 21.Hume PA, Theadom A, Lewis GN et al. A Comparison of Cognitive Function in Former Rugby Union Players Compared with Former Non-Contact-Sport Players and the Impact of Concussion History. Sports Med. 2017;47:1209–20 [DOI] [PubMed] [Google Scholar]
- 22.Cunningham J, Broglio S, Wilson F Influence of playing rugby on long-term brain health following retirement: a systematic review and narrative synthesis BMJ Open Sport & Exercise Medicine 2018;4:e000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallo V, McElvenny DM, Seghezzo G, et al. Concussion and long-term cognitive function among rugby players—The BRAIN Study. Alzheimer’s Dement. 2021; 1–13. 10.1002/alz.12455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007; 370: 1453–57. [DOI] [PubMed] [Google Scholar]
- 25.Bogle KR. Scottish Rugby: Game by Game. Luath Press Limited, Edinburgh: 2013 [Google Scholar]
- 26.ESPNscrum. http://en.espn.co.uk/statsguru/rugby/page/89754.html. accessed February 24, 2021.
- 27.Scottish Index of Multiple Deprivation 2016. Edinburgh: Scottish Government, August 31, 2016. [Google Scholar]
- 28.Joint Formulary Committee. British National Formulary (online) London. BMJ Group and Pharmaceutical Press; http://www.medicinescomplete.com. accessed 11th June, 2021. [Google Scholar]
- 29.Möller S; Ahrenfeldt LJ Estimating Relative Risk When Observing Zero Events—Frequentist Inference and Bayesian Credibility Intervals. Int. J. Environ. Res. Public Health. 2021;18:5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC. [Google Scholar]
- 31.Fry A, Littlejohns TJ, Sudlow C, et al. Comparison of Sociodemographic and 6 Health-Related Characteristics of UK Biobank Participants With Those of the General 7 Population. Am J Epidemiol. 2017;186:1026–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lyall DM, Quinn T, Lyall LM et al. Quantifying bias in psychological and physical health in the UK Biobank imaging sub-sample, Brain Communications, 2022;, fcac119, 10.1093/braincomms/fcac119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner MR, Abisgold J, Yeates DG, Talbot K, Goldacre MJ. Head and other physical trauma requiring hospitalisation is not a significant risk factor in the development of ALS. J Neurol Sci. 2010;288:45–8. [DOI] [PubMed] [Google Scholar]
- 34.Seals RM, Hansen J, Gredal O, Weisskopf MG. Physical Trauma and Amyotrophic Lateral Sclerosis: A Population-Based Study Using Danish National Registries. Am J Epidemiol. 2016;183:294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu G, Ou S, Cui H, Li X, Yin Z, Gu D, Wang Z. Head Injury and Amyotrophic Lateral Sclerosis: A Meta-Analysis. Neuroepidemiology. 2021;55:11–19. [DOI] [PubMed] [Google Scholar]
- 36.Chen GX, Douwes J, van den Berg LH, Glass B, McLean D, ‘t Mannetje AM. Sports and trauma as risk factors for Motor Neurone Disease: New Zealand case-control study. Acta Neurol Scand. 2022;145:770–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theadom A, Mahon S, Hume P, et al. Incidence of sports-related traumatic brain injury of all severities: a systematic review. Neuroepidemiology. 2020;54:192–199. [DOI] [PubMed] [Google Scholar]
- 38.Van Pelt KL, Puetz T, Swallow J et al. Data-driven risk classification of concussion rates: a systematic review and meta-analysis. Sports Med 2021; 51:1227–1244. [DOI] [PubMed] [Google Scholar]
- 39.West SW, Cross M, Trewartha G, et al. Trends in match concussion incidence and return-to-play time in male professional Rugby Union: A 16-season prospective cohort study, Brain Injury 2021;35:1235–1244 [DOI] [PubMed] [Google Scholar]
- 40.Tucker R, Raftery M, Kemp S, et al. Risk factors for head injury events in professional rugby union: a video analysis of 464 head injury events to inform proposed injury prevention strategies. Br J Sports Med. 2017;51:1152–1157. [DOI] [PubMed] [Google Scholar]
- 41.Fuller CW, Fuller GW, Kemp SP, Raftery M. Evaluation of World Rugby’s concussion management process: results from Rugby World Cup 2015. Br J Sports Med. 2017;51:64–69. [DOI] [PubMed] [Google Scholar]
- 42.Raftery M, Tucker R, Falvey ÉC. Getting tough on concussion: how welfare-driven law change may improve player safety—a Rugby Union experience. Br J Sports Med. 2021;55:527–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCrea MA, Shah A, Suma S et al. Opportunities for prevention of concussion and repetitive head impact exposure in college football players a Concussion Assessment, Research, and Education (CARE) Consortium Study JAMA Neurol. 2021;78:346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stewart W Sport associated dementia. BMJ 2021; 372: n168. [DOI] [PubMed] [Google Scholar]
- 45.Sibbett RA, Russ TC, Deary IJ, Starr JM. Dementia ascertainment using existing data in UK longitudinal and cohort studies: a systematic review of methodology. BMC Psychiatry. 2017;17:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are stored on the NHS Scotland National Safe Haven and cannot be made publicly available due to confidentiality reasons.


