Abstract
Background:
Spinal cord damage is a hallmark of Friedreich ataxia (FRDA), but its progression and clinical correlates remain unclear.
Objective:
To perform a characterization of cervical spinal cord structural damage in a large multisite FRDA cohort.
Methods:
We performed a cross-sectional analysis of cervical spinal cord (C1 to C4) cross-sectional area (CSA) and eccentricity using MRI data from eight sites within the ENIGMA-Ataxia initiative, including 256 individuals with FRDA and 223 age- and sex-matched controls. Correlations and subgroup analyses within the FRDA cohort were undertaken based on disease duration, ataxia severity, and onset age.
Results:
Individuals with FRDA, relative to controls, had significantly reduced CSA at all examined levels, with large effect sizes (d>2.1) and significant correlations with disease severity (r<−0.4). Similarly, we found significantly increased eccentricity (d>1.2), but without significant clinical correlations. Subgroup analyses showed that CSA and eccentricity are abnormal at all disease stages. However, while CSA appears to decrease progressively, eccentricity remains stable over time.
Conclusion:
Previous research has shown that increased eccentricity reflects dorsal column (DC) damage, while decreased CSA reflects either DC or corticospinal tract (CST) damage or both. Hence, our data support the hypothesis that damage to DC and CST follow distinct courses in FRDA: developmental abnormalities likely define the DC, whereas CST alterations may be both developmental and degenerative. These results provide new insights about FRDA pathogenesis and indicate that CSA of the cervical spinal cord should be investigated further as a potential biomarker of disease progression.
Keywords: Friedreich Ataxia, MRI, Spinal Cord, Enigma-Ataxia, SCT
Introduction
Friedreich ataxia (FRDA) is a neurogenetic disease caused by GAA expansions or point mutations in the first intron of the FXN gene1, leading to lower levels of the protein frataxin and resulting in mitochondrial dysfunction and neurodegeneration2. FRDA is the most common autosomal recessive ataxia worldwide2. The first symptoms typically begin in late childhood or adolescence and are characterized by slowly progressive ataxia and sensory abnormalities2,3. A smaller subset of individuals manifest symptoms after the age of 25 years and are known as people with Late-Onset Friedreich Ataxia (LOFA)4,5. These individuals are clinically characterized by slower disease progression and milder non-neurological symptoms6.
Pathology studies in FRDA show that structural damage affects both the central and peripheral nervous system7,8. In fact, the spinal cord, dorsal root ganglia and dentate nucleus of the cerebellum are the main targets of damage in the disease7. MRI-based studies have confirmed such findings and, beyond that, have shown structural damage in the cerebellum, brainstem, cerebellar peduncles and motor cortex9.
In recent years, there has been renewed interest in assessing spinal cord damage using non-invasive MRI in FRDA10-14. Quantitative structural neuroimaging studies have revealed atrophy and antero-posterior flattening in affected subjects, particularly at cervical and thoracic levels10,11,14. Using diffusion tensor imaging (DTI), Hernandez et al (2021) and Joers et al (2022) also reported microstructural changes in the corticospinal tracts and dorsal columns of the cervical spinal cord in individuals with FRDA. Using magnetic resonance spectroscopy, Joers et al (2022) reported large neurochemical changes in the spinal cord in FRDA. In all these studies, the authors were able to find significant correlations between spinal cord MRI metrics and disease severity. Thus, in vivo imaging is well-aligned with histological evidence that spinal cord compromise plays a major role in the pathophysiology of FRDA.
Several aspects of spinal cord changes in people with FRDA remain unclear. It is not yet established how spinal cord morphometric abnormalities – atrophy and flattening – change along the disease course. Moreover, differences may exist in the magnitude, progression, and association with clinical variables of spinal cord damage in pediatric vs adult patients, and in individuals with early vs late symptom onset. These are relevant issues, not only to understand the underlying biology of the disorder, but also to uncover potential imaging-based biomarkers.
Prior neuroimaging studies have generally relied on modest sample sizes from single sites, limiting opportunities to provide robust disease characterization, reliable effect size estimates, and subgroup analyses. The ENIGMA-Ataxia working group is an international collaboration that aggregates MRI data from individuals with ataxias. This consortium offers a unique opportunity to enlarge cohort sizes and to accomplish more detailed analyses in rare diseases, such as FRDA15. Hence, the main goal of the present study was to perform a comprehensive evaluation of cervical spinal cord damage in FRDA using a large dataset collected within the ENIGMA-Ataxia group. We sought to characterize the pattern of damage and how it evolves across disease subgroups, stratified according to the time from onset and the magnitude of disease severity.
Methods
Participants and Data
We performed a retrospective cross-sectional analysis of data from eight sites in the ENIGMA-Ataxia working group, totaling 256 patients with molecular confirmation of FRDA and 223 age- and sex-matched non-ataxic controls (Table 1, Supplementary Table 1). Disease duration and age at symptom onset were recorded for all participants with FRDA, and disease severity was quantified using one of the following validated clinical scales: the Friedreich Ataxia Rating Scale (FARS)16,17, the modified FARS (mFARS)18 or the Scale for Assessment and Rating of Ataxia (SARA)19. All diagnoses of FRDA were genetically confirmed at all sites, but individual-level GAA repeat information was not consistently available due to local reporting procedures or data privacy considerations. To assess the cervical spinal cord, we used high-resolution T1-weighted MRIs covering the brain and upper cervical vertebrae acquired on 3T clinical scanners with spatial resolution not inferior to 1-mm isotropic (Supplementary Table 2). Individuals with FRDA and controls from each site underwent MRI scans using the same scanner and protocol.
Table 1:
Demographics data for all sites. For the healthy controls demographics data, please see Supplementary Table 1.
| Sites | Age (years) | Sex | GAA1 | GAA2 | Onset Age (years) |
Disease Duration (years) |
Disease Severity | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Average [Range] |
Male | Female | Average | Average | Average | Average | Scale | Average | Normalized Scale | |
| Aachen (N=32) | 36±12 [19-59] | 16 | 16 | 497±224 | 819±218 | 17±8 | 20±9 | SARA | 20±9 | 0.486±0.227 |
| Campinas (N=83) | 30±13 [7-66] | 31 | 52 | 1026±267 | 869±215 | 18±9 | 12±10 | FARS | 55±22 | 0.440±0.178 |
| Conegliano (N=46) | 24±12 [8-51] | 18 | 21 | 671±179 | - | 12±7 | 13±9 | SARA | 18±8 | 0.441±0.199 |
| Essen (N=15) | 44±11 [26-60] | 6 | 9 | 415±292 | 648±306 | 17±10 | 23±9 | SARA | 24±4 | 0.598±0.107 |
| Melbourne1 (N=22) | 39±14 [22-63] | 11 | 11 | 532±234 | 908±222 | 21±9 | 18±10 | FARS | 84±29 | 0.667±0.226 |
| Melbourne2 (N=14) | 30±9 [18-49] | 8 | 6 | 604±206 | 870±186 | 15±4 | 14±7 | mFARS | 47±22 | 0.526±0.164 |
| Minnesota (N=26) | 19±7 [10-35] | 14 | 12 | 598±184 | 960±213 | 14±5 | 6±4 | FARS* | 43±14 | 0.341±0.111 |
| Tubingen (N=14) | 32±11 [18-53] | 10 | 4 | - | - | 18±9 | 14±7 | SARA | 18±8 | 0.447±0.208 |
| Total (N=252) | 30±13 [7-66] | 114 | 131 | 639±286 | 640±421 | 17±9 | 14±10 | - | - | 0.473±0.201 |
Maximum score 117.
This study was approved by the Institutional Review Board of each site and all participants provided written informed consent.
Image Processing
Data processing was undertaken using harmonized protocols developed by the ENIGMA-Ataxia consortium (http://enigma.ini.usc.edu/ongoing/enigma-ataxia/), based on publicly available and well-validated software toolboxes20.
To measure the cross-sectional area (CSA) and eccentricity, we employed the Spinal Cord Toolbox (SCT) version 4.2.2, an open-source software package specifically designed to process spinal cord multimodal MRI data20. In brief, automatic segmentation of the cervical spinal cord was conducted using a deep-learning algorithm21 and, if deemed necessary after visual inspection, the segmentations were manually corrected. Next, the C2 and C3 vertebral levels were manually marked at the posterior tip of the vertebral discs, which enabled the registration of subject images to a standardized template of the spinal cord and brainstem (the PAM50 template)22-24. Lastly, the mean CSA and eccentricity were computed at each of the C1 to C4 vertebrae after correcting for the curvature of the spine. The CSA is quantified by the number of pixels in the set of axial slices defining each vertebral level of the segmented spinal cord, reported in millimeters squared. Eccentricity is computed by fitting an ellipse to each axial spinal slice and determining the deviation (i.e., flattening) of the ellipse relative to a perfect circle. Mathematically, such a measure characterizes the shape of the spinal cord cross-section defined as the square root of 1 - (d/D)2, where d and D are respectively defined as the smallest and largest diameter of the ellipse. Values closer to 1 indicate an antero-posterior flattening of the spinal cord. We only assessed the upper cervical spinal cord, since we used MR images centered on the brain with limited spinal cord coverage (Figure 1). Since the spinal cord coverage was slightly different across individuals due to head size variability or field-of-view placement during data acquisition, different sample sizes were available for each vertebral level we examined (Controls: C1=223, C2=223, C3=215 and C4=170; Patients: C1=252, C2=252, C3=237 and C4=170).
Figure 1:
Study design and imaging processing pipeline. For healthy controls numbers, please see Supplementary Table 1. N is the number of FRDA patients enrolled by each site and C1, C2, C3 and C4 the sample size available for each vertebral levels assessed.
Statistical Analysis
Overall FRDA vs. Control Comparison
We compared CSA and eccentricity at each vertebral level from C1 to C4 in all individuals with FRDA relative to the age- and sex-matched control cohort using ANCOVAs with age, sex and site as covariates of no interest. There is no systematic relationship between spinal cord CSA or eccentricity and brain/head size25, therefore brain volume was not included as a covariate. We corrected for multiple comparisons using Bonferroni adjustment of statistical significance thresholds. Effect sizes (ES) of statistically significant results were computed as follows (Cohen’s d):
| (1) |
| (2) |
where, μ1 and μ2 are the mean values for the control and FRDA groups respectively, spooled is the pooled standard deviation, n1 and n2 are the number of subjects in each group, and s1 and s2 are the respective group standard deviations. We considered effect size values of 0.2 as small, 0.5 as moderate, 0.8 as large, and > 1.2 as very large, according to established convention26.
Clinical Correlation Analyses
To assess correlations between spinal cord morphometric data (CSA and eccentricity) and clinical parameters (disease duration and disease severity), we used the Pearson correlation coefficient. Before performing the analyses, we first adjusted the data to account for site, age and sex effects using a linear model. Multiple clinical rating scales were used to assess disease severity across the sites (FARS, mFARS and SARA). There was a high correlation between SARA and FARS total neurological scores (r=0.860 and p<0.0001) in our participants for whom both scales were collected at the same time (Conegliano: N=28, age=23.7±11.4 years, 19M/23F, SARA=17.5±8.0, FARS=57.1±20.4; Melbourne1: N=31, age=36.6±13.0 years, 17M/14F, SARA=19.3±8.6, FARS=81.1±28.4; Minnesota: N=11, age=18.1±7.7 years, 6M/5F, SARA=9.4±2.7, FARS=40.7±9.7), which is in agreement with comparable previous work from Bürk and colleagues (2009; r=0.953 and p<0.0001)27. To accomplish a direct pooled analysis, we therefore created a normalized disease severity variable by dividing the disease severity scores by the respective maximum value of the respective scale, e.g., disease severity measures quantified using FARS were divided by 125 (except by Minnesota site, max score 117), mFARS were divided by 93 and SARA measures were divided by 40. We note that these scales likely have slightly different psychometric properties (e.g., differing ceiling and floor effects)18,28, and thus while this normalization approach is strongly supported by the very high inter-scale correlations, we acknowledge that there is not a precise 1-to-1 correspondence in their absolute or relative scores. However, our goal is not to establish absolute harmonization across scales or investigate detailed symptom expression and progression, but rather to test for general trends between overall ataxia severity and spinal cord structure. To corroborate the results obtained using this normalization and data pooling approach, we also performed secondary correlation analyses separately for subjects only with FARS and only with SARA.
Clinical Subtype Comparison
We also analyzed spinal cord damage in two subgroups that merit special attention in FRDA. First, we examined pediatric patients (age <18 years; N=40, mean age=13.3±2.5 years, 18M/22F, mean disease duration=4.8±2.3 years, normalized disease severity=0.379±0.124) relative to an age/sex-matched control group (N=26, mean age=14.0±2.4 years, 12M/14F; Supplementary Table 3), and to a disease duration matched adult FRDA group (N=30, mean age=26.7±9.5 years, 17M/13F, mean disease duration=5.6±2.7 years, normalized disease severity=0. 0.295±0.134). Next, we examined individuals with LOFA (first onset of symptoms at age >25 years; N=45, mean age=45.6±8.8 years, 25M/20F, mean disease duration=14.6±8.8 years, normalized disease severity=0.394±0.189) first relative to an age/sex-matched control group (N=42, mean age=45.4±9.3 years, 22M/20F; Supplementary Table 3), second to an age/sex-matched ‘classical onset’ FRDA group (N=43, mean age=39.2±9.3 years, 11M/32F, mean disease duration=24.3±9.2 years, normalized disease severity=0.666±0.172), and finally to a disease duration matched ‘classical onset’ FRDA group (N=35, mean age=28.7±10.4 years, 15M/20F, mean disease duration=14.2±8.9 years, normalized disease severity=0.546±0.215).
In both analyses, between-group comparisons of spinal cord measures (CSA and eccentricity) and correlations with clinical parameters (disease duration and severity) were undertaken as described above.
Disease Staging
To supplement the clinical correlations described above, provide a clearer picture of disease staging and progression, and allow for direct quantitative and qualitative comparisons between disease subgroups and healthy control data, we also undertook a categorical division of the data. Five subgroups (DD1 – DD5) were defined according to disease duration (time since first symptom expression) at the time of each participant’s scan: <5 years, 5-10 years, 11-15 years, 16-20 years, and >20 years, respectively. Four subgroups (DS1 – DS4) were also defined according to the normalized disease severity at the time of each participant’s scan: <0.25, 0.26-0.50, 0.51-0.75 and >0.75, respectively. These divisions do not represent clinically-determined cut-offs, but rather provide an intuitive means of quantitatively assessing and reporting changes in effect sizes with disease progression.
Each subgroup was first compared with a non-ataxic control cohort matched by age, sex and site. Subsequently, we compared each subgroup with the earliest (DD1) or least severe (DS1) subgroup to assess evidence for progressive degeneration independent of early/pre-symptomatic effects. Similar to the statistical approach used in the general comparison, we used ANCOVA to assess each group's differences, using age, sex and site as covariates and used Bonferroni correction to adjust for multiple comparisons.
Results
Overall FRDA vs Control Comparison
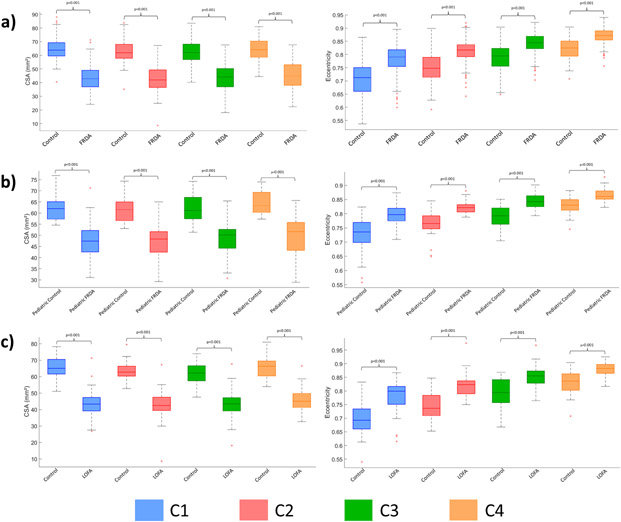
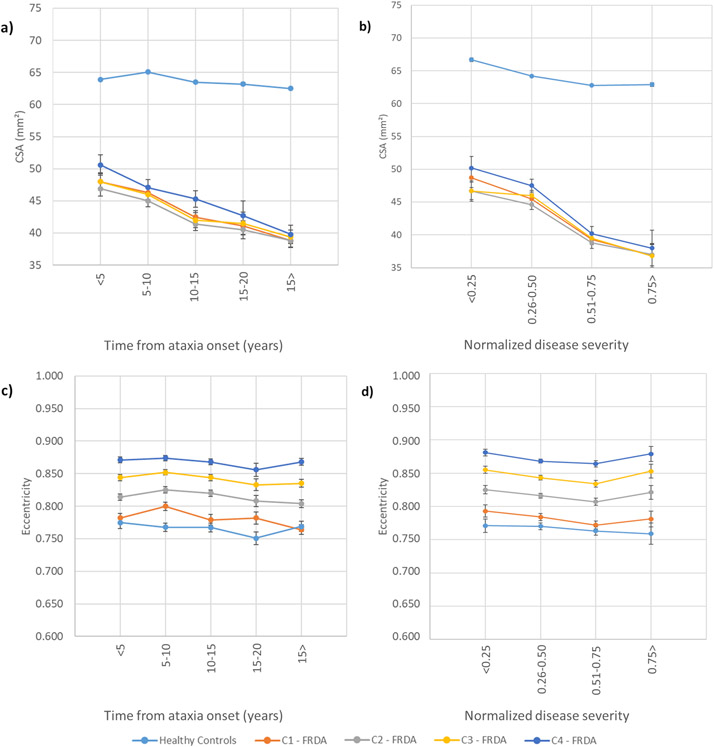
Individuals with FRDA relative to controls had significantly reduced CSA at all vertebral levels (Figure 2a) with very large effect sizes (C1 ES=2.6, C2 ES=2.6, C3 ES=2.3, C4 ES=2.1). Similarly, we found significantly increased eccentricity at all vertebral levels (Figure 2a), also with very large effect sizes (C1 ES=1.2, C2 ES=1.4, C3 ES=1.3, C4 ES=1.4), although substantially smaller in comparison to CSA. In addition, the spinal cord growth curve, i.e., the plot of spinal cord CSA vs age, revealed distinct patterns in the control group (C1: r=−0.050, p=0.999; C2: r=−0.045, p=0.999; C3: r=−0.068, p=0.999; C4: r=−0.039, p=0.999), CSA remains stable over the entire lifespan, whereas in individuals with FRDA (C1: r=−0.247, p<0.001; C2: r=−0.216, p=0.003; C3: r=−0.227, p=0.002; C4: r=−0.244, p=0.006), CSA appears to show a progressive decline with age (Figure 3a).
Figure 2:
Box plots displaying group differences at each spinal cord segment, C1-C4, for the total cohort. a) all FRDA patients vs all matched controls; b) children (age <18 years) with FRDA vs matched controls; and c) individuals with late-onset Friedreich ataxia (LOFA) vs matched controls.
Figure 3:
a) Plot of spinal cord cross-sectional (CSA) versus age in patients and controls for all assessed vertebral level, b) Significant correlations between normalized disease severity and cross-sectional area in individuals with FRDA at vertebral level C1, C2, C3 and C4.
In the Supplementary Figure 1, we also present the site-specific between-group effects, demonstrating largely consistent results irrespective of scan site and protocol.
Clinical Correlation Analysis
We found significant correlations between the normalized disease severity or ataxia duration and CSA at all vertebral levels assessed (C1-C4) (Figure 3b) after Bonferroni adjustment for multiple comparisons (Normalized disease severity - C1: r=−0.424, p<0.001; C2: r=−0.395, p<0.001; C3: r=−0.399, p<0.001; C4: r=−0.435, p<0.001. Ataxia Duration- C1: r=−0.174, p=0.006; C2: r=−0.146, p=0.044; C3: r=−0.164, p=0.026; C4: r=−0.237, p=0.004). In contrast, we did not find any significant correlations between eccentricity and normalized disease severity or ataxia duration. Secondary analysis of clinical correlations separately for subjects with FARS and again for those subjects with SARA produced comparable results (Supplementary Table 4).
Comparison of Clinical Subtypes
Children with FRDA showed abnormal CSA and eccentricity when compared to matched non-ataxic controls (Figure 2b) with very large effect sizes (CSA: C1 ES=1.7, C2 ES=2.1, C3 ES=2.0, C4 ES=2.1; eccentricity: C1 ES=1.3, C2 ES=1.8, C3 ES=1.8, C4 ES=1.5). Differences relative to adults with FRDA (with ‘classical’ onset age) did not reach statistical significance (Supplementary Table 5). We did not find significant correlations between spinal cord measures and clinical variables in the pediatric cohort (Supplementary Table 6).
Individuals with LOFA also had very large effect sizes when compared to matched non-ataxic controls (Figure 2c) (CSA: C1 ES=3.0, C2 ES=2.9, C3 ES=2.6, C4 ES=2.3; eccentricity: C1 ES=1.4, C2 ES=1.7, C3 ES=1.4, C4 ES=1.2). Differences relative to adults with classical FRDA did not show statistical significance. Significant correlations were evident between CSA and normalized disease severity for all vertebral levels assessed, except for C4, after Bonferroni correction (C1: r=−0.385, p<0.010; C2: r=−0.371, p=0.026; C3: r=−0.357, p=0.035).
Disease Staging
Disease Duration
The subgroup analyses based on disease duration showed that CSA and eccentricity are already abnormal in the earliest stages of the disease, with significant differences relative to controls in all subgroups (Figures 4, Supplementary Table 7). In addition, we found significantly reduced CSA when DD3 (10-15yrs post-symptom duration), DD4 (15-20yrs duration) and DD5 (20+ years duration) were compared to DD1 (<5 years duration) at all vertebral levels (Supplementary Table 8). In contrast, eccentricity remained stable across the subgroups.
Figure 4:
Results showing the progressive atrophy of the cervical spinal cord area (CSA) (a, b) and eccentricity (c, d) in participants with FRDA and healthy controls. Panels a) and c) depict subgroups based on disease duration (DD); b) and d) show subgroups based on disease severity (DS). To the healthy controls, the measures represent the mean cervical spinal cord area or eccentricity; error bars = standard error of the mean. Subgroups based on disease duration, DD1: Time from ataxia onset <5 years, DD2: Time from ataxia onset between 5-10 years, DD3: Time from ataxia onset between 10-15 years, DD4: Time from ataxia onset between 15-20 years, DD5: Time from ataxia onset >20 years. Subgroups based on disease severity, DS1: Normalized disease severity <0.25, DS2: Normalized disease severity between 0.26-0.50, DS3: Normalized disease severity between 0.51-0.75, DS4: Normalized disease severity >0.75.
Disease Severity
The subgroup analyses based on disease severity showed similar results. Abnormalities in CSA and eccentricity are observable in patients with normalized disease severity <0.25, with significant effects relative to controls in all subgroups (Figure 4, Supplementary Table 9). We also found significantly reduced CSA when DS3 (normalized disease severity 0.51-0.75) and DS4 (severity >0.75) were compared to DS1 (severity <0.25) at all vertebral levels. Meanwhile, DS2 (severity 0.26-0.50) showed reduced CSA, relative to DS1, only for C1 and C2; eccentricity remained stable across the subgroups (Supplementary Table 10).
Discussion
Spinal cord damage has been recognized as a hallmark of FRDA since Nikolaus Friedreich’s first reports and confirmed in more recent histology and neuroimaging studies7,10-14,29. In this study, we performed a harmonized and reliable retrospective cross-sectional analysis of cervical spinal cord structure using MRI data from a large multisite cohort. We report significant and substantial CSA reduction in individuals with FRDA at all vertebral levels examined, relative to non-ataxic individuals, and significant correlations with disease severity scores. Eccentricity differences were also pronounced in this cohort relative to controls, but effect sizes were smaller than for CSA and no significant clinical correlations were observed. Subgroup analyses based on disease duration and severity showed that CSA and eccentricity are already abnormal in the early stages of the disease and that CSA likely declines with disease progression, whereas eccentricity remains stable. Taken together, CSA emerges as a potential MRI biomarker candidate for clinical tracking in FRDA.
Our results are consistent with previous MRI-based studies that found cervical spinal cord atrophy and anteroposterior flattening in FRDA10-14,30. Post-mortem studies indicate that the pathological correlates of these findings are severe depletion of myelinated fibers in the dorsal columns, dorsal spinocerebellar and lateral corticospinal tracts29. These findings are also consistent with a single-site prospective study that showed a decrease in CSA over time in individuals with FRDA in an early-stage cohort, with no decrease over time in eccentricity14.
Prior studies undertaken in other spinal cord diseases help us understand the pathological underpinnings of changes in CSA and eccentricity31-34. Indeed, different patterns emerge when one compares diseases characterized by predominant/exclusive lateral column involvement (e.g., amyotrophic lateral sclerosis, pure subtypes of hereditary spastic paraplegia) vs diseases with predominant/exclusive dorsal column involvement (e.g., acquired sensory neuronopathies)31-33. CSA reduction is evident in both groups, but eccentricity increase is only reported in the latter31. Therefore, eccentricity can be considered a surrogate MRI marker for dorsal column damage, whereas CSA may be related to abnormal integrity in both lateral and dorsal columns. Using this conceptual framework, relevant insights can be inferred from our results. The stability of eccentricity alongside decreasing CSA across FRDA stages (based on duration or severity) suggests that the corticospinal tract and dorsal columns follow distinct mechanisms and time courses of damage in the disease. Corticospinal tract damage is most consistent with a combination of abnormal developmental and progressive degenerative processes, as shown by both early (already seen in the pediatric subgroup) and progressive CSA abnormalities. In contrast, dorsal column abnormalities, assessed by eccentricity, may be related to early maldevelopment but remain stable along the entire disease course, at least from the point of first symptom expression. However, extrapolation of these results beyond the cervical spinal cord must be approached with caution. Indeed, a whole-spine study in a small FRDA cohort reported similar findings in the cervical cord, but also observed significant correlations between disease duration and eccentricity in thoracic regions11.
Our assumption that dorsal column damage is largely neurodevelopmental is in line with neuropathological reports from Koeppen et al (2017). These authors suggest that the developmental failure of the dorsal root ganglia (DRG) leads to the secondary hypoplasia of dorsal columns, since DRG are the source of myelinated fibers in the dorsal columns. Indeed, the autopsy of two young patients with FRDA showed that the neurons in the dorsal nuclei were severely reduced or absent, probably due to the lack of innervation from the dorsal root collaterals that occurs during the gestational period34, arguing in favor of a developmental failure. Furthermore, experiments using animal models provide evidence that frataxin plays a role in embryonic development35,36. Our imaging data suggest that CSA of individuals with FRDA, on average, reaches its maximum before 10 years of age and then starts to decrease, whereas healthy controls have higher CSA values relative to FRDA patients even at the earliest disease stages, and keep stable over time. A preceding MRI-based study performed by Rezende and colleagues (2019)12 found a very similar result.
Progressive neurodegeneration in the corticospinal tract is consistent with the hypothesis that pyramidal tract damage in FRDA arises from a ‘dying back’ process. Neuropathological studies have found that the corticospinal tract is more affected in the spinal cord than in the brain, with the exception of the lack of Betz cells in the motor cortex37,38. Koeppen and Mazurkiewicz (2013) also showed that spinal cord damage is more severe in thoracic levels compared to cervical regions. Previous neuroimaging studies also support such a concept. Indeed, a diffusion MRI-based study showed that microstructural abnormalities were more robust in caudal levels, although clinical correlations were stronger at the upper levels of the corticospinal tract (Hernandez et al, 2021). Rezende and colleagues (2019) also reported motor cortex thinning only in adults, but not in children with FRDA, alongside progressive damage in the cerebral corticospinal tract. This is in agreement with Harding and colleagues (2021) who proposed a disease staging schema for brain damage in FRDA. These authors highlight the progressive pattern of damage in the disease that begins in infratentorial structures and spreads to cortical structures in later disease stages15.
From a clinical perspective, our data indicate that CSA at C1 level is a potential biomarker candidate as it showed the highest correlation coefficient with disease severity and the highest effect size compared to controls. CSA at C1 level also had the highest effect size in a recent single-site longitudinal study14. However, this may not be the case for all FRDA stages or sub-phenotypes. For the pediatric cohort (age <18 years), we did not find any significant correlations between CSA and normalized disease severity, whereas such associations were evident in the adult cohort. Although this observation may reflect statistical power as there were fewer pediatric individuals (n=40) relative to adults (n=159), a similar result was previously reported by Rezende and colleagues (2019). This relative decoupling of structure and function may reflect partial neural reserve, or the influence of parallel developmental and degenerative processes obscuring clear clinical associations in pediatric patients, relative to a pure degenerative profile in adults. Different neuroimaging biomarkers may also vary in their sensitivity to change at different disease stages, similar to what has been suggested for SCA339. This hypothesis is supported by the proposed mechanism of corticospinal degeneration in FRDA, which seems to follow a dying-back motor axonopathy. Lower levels of the spinal cord may therefore already be extensively impacted very early in the disease course and reach an early floor effect. Ongoing damage to the spinal corticospinal tract may therefore be more easily captured by MRI metrics at upper levels. At this point, prospective studies with pediatric and adult cohorts must be undertaken to confirm such hypotheses.
Notwithstanding the original contributions of this study, several limitations must be acknowledged. This is a cross-sectional study and many of the findings presented here must be confirmed by prospective longitudinal neuroimaging studies, particularly those enriched with a pediatric cohort. Our analyses were performed using T1-weighted brain MRI, which is the most common and widely used MRI sequence for research and clinical practice. However, this confines our assessment to the upper portions of the cervical spinal cord, and prevents investigation of whether different patterns of degeneration characterize different levels of the spinal cord. More targeted spinal cord imaging acquisitions would enable more detailed analyses, such as tract-specific microstructural evaluation and individual assessment of white matter and grey matter regions. The use of different clinical scales across sites, and general availability only of total scores (instead of individual subitems) also limits more extensive investigation of correlations between spinal cord damage and both overall disease severity and different symptom domains. Here, we employ a relatively blunt normalization approach to pool scores across different clinical scales. Future work modeling the relationship between different clinical scales (i.e., SARA and FARS) would be beneficial to establish more specific conversion scores. Lastly, the small sample size of the pediatric and LOFA cohorts did not allow us to split them in subgroups in order to assess their disease evolution. Prospective natural history imaging studies (e.g., TRACK-FA; https://www.monash.edu/medicine/trackfa) will be key to addressing many of these limitations.
To conclude, our data support the hypothesis that damage to spinal dorsal column and corticospinal tract follow distinct courses in the disease: developmental damage likely defines the former, whereas alterations in the latter may be both developmental and degenerative in origin. These results provide new insights about FRDA pathogenesis and indicate that spinal cord MRI may be a useful biomarker to track disease progression.
Supplementary Material
Supplementary Figure 1: Site-specific effect sizes for a) cross-sectional area (CSA) and b) eccentricity for each vertebral level assessed.
Supplementary Table1: Demographics data for non-ataxic individuals for each site.
Supplementary Table2: Scanner and imaging acquisition details for each site.
Supplementary Table 3: Demographics data of pediatric and LOFA patients.
Supplementary Table 4: Separated clinical correlations analysis for subjects with FARS only and SARA only. N is the sample size used for each clinical scale.
Supplementary Table 5: Group differences between pediatric vs adult patients with FRDA at each spinal cord level, C1-C4.
Supplementary Table 6: Correlations between time from ataxia onset or normalized disease severity and CSA or eccentricity for the pediatric cohort.
Supplementary Table 7: Results of ROI-based analyses to assess CSA damage at each stage of FRDA (DD1: Time from ataxia onset <5 years, DD2: Time from ataxia onset between 5-10 years, DD3: Time from ataxia onset between 10-15 years, DD4: Time from ataxia onset between 15-20 years, DD5: Time from ataxia onset > 20 years). All comparisons are significant, i.e., p<0.001 after Bonferroni correction.
Supplementary Table 8: Results of ROI-based analyses to assess progressive CSA damage at each stage of FRDA relative to the subgroup small time from ataxia onset (DD1: Time from ataxia onset <5 years, DD2: Time from ataxia onset between 5-10 years, DD3: Time from ataxia onset between 10-15 years, DD4: Time from ataxia onset between 15-20 years, DD5: Time from ataxia onset > 20 years). All comparisons are significant, i.e., p<0.001 after Bonferroni correction.
Supplementary Table 9: Results of ROI-based analyses to assess CSA damage at each stage of FRDA (DS1: Normalized disease severity <0.25, DS2: Normalized disease severity between 0.26-0.50, DS3: Normalized disease severity between 0.51-0.75, DS4: Normalized disease severity >0.75). All comparisons are significant, i.e., p<0.001 after Bonferroni correction.
Supplementary Table 10: Results of ROI-based analyses to assess progressive CSA damage at each stage of FRDA relative to the subgroup small normalized disease severity (DS1: Normalized disease severity <0.25, DS2: Normalized disease severity between 0.26-0.50, DS3: Normalized disease severity between 0.51-0.75, DS4: Normalized disease severity >0.75). All comparisons are significant, i.e., p<0.001 after Bonferroni correction.
Acknowledgments:
The methods of harmonization and multi-site data analysis elements of this work were supported by NIH Big Data to Knowledge (BD2K) program grant number U54 EB020403, and grants from the Australian National Health and Medical Research Council (Fellowship 1106533, Grant 1184403). FARA (Friedreich’s Ataxia Research Alliance, grant 92133) and FAPESP (São Paulo Research Foundation) also supported this study through CEPID/BRAINN (grant 2013/07559-3), the German Research Foundation (DFG, DE 2516/1-1 and TI 239/17-1) and by the European Joint Programme on Rare Diseases (EJPRD), under the EJP RD COFUND-EJP N° 825575 as part of the PROSPAX consortium (to M.S. and D.T. via DFG, German Research Foundation). CMRR at the University of Minnesota is supported by grants NIH P41 EB027061 and P30 NS076408. PGH and CL also acknowledge support by grants from the FARA, GoFAR, Ataxia UK and the Bob Allison Ataxia Research Center.
Funding:
NIH Big Data to Knowledge (BD2K) program grant number U54 EB020403, and grants from the Australian National Health and Medical Research Council (Fellowship 1106533, Grant 1184403). FARA (Friedreich’s Ataxia Research Alliance, grant 92133) and FAPESP (São Paulo Research Foundation) also supported this study through CEPID/BRAINN (grant 2013/07559-3). The funding agencies did not interfere with the design of the study, collection of data or drafting of the manuscript.
Footnotes
Financial Disclosures:
The authors report no conflict of interest regarding this research. This work was supported by NIH Big Data to Knowledge (BD2K) program grant number U54 EB020403, and grants from the Australian National Health and Medical Research Council (Fellowship 1106533, Grant 1184403). FARA (Friedreich’s Ataxia Research Alliance, grant 92133) and FAPESP (São Paulo Research Foundation) also supported this study through CEPID/BRAINN (grant 2013/07559-3)
Data Sharing:
All code and data processing instructions are available at https://github.com/Harding-Lab/enigma-ataxia.
References
- 1.Campuzano V, Montermini L, Molto MD, et al. Friedreich’s ataxia: autosomal recessive disease caused by anintronic GAA triplet repeat expansion. Science. 1996;271:1423 – 7. [DOI] [PubMed] [Google Scholar]
- 2.Pandolfo M. Friedreich ataxia. Arch Neurol 2008;65:1296–1303. [DOI] [PubMed] [Google Scholar]
- 3.Reetz K, Dogan I, Hilgers RD, Giunti P, Parkinson MH, Mariotti C, Nanetti L, Durr A, Ewenczyk C, Boesch S, Nachbauer W, Klopstock T, Stendel C, Rodríguez de Rivera Garrido FJ, Rummey C, Schöls L, Hayer SN, Klockgether T, Giordano I, Didszun C, Rai M, Pandolfo M, Schulz JB; EFACTS study group. Progression characteristics of the European Friedreich's Ataxia Consortium for Translational Studies (EFACTS): a 4-year cohort study. Lancet Neurol. 2021;20:362–372. [DOI] [PubMed] [Google Scholar]
- 4.De Michele G, Filla A, Cavalcanti F, Di Maio L, Pianese L, Castaldo I, Calabrese O, Monticelli A, Varrone S, Campanella G, et al. Late onset Friedreich's disease: clinical features and mapping of mutation to the FRDA locus. J Neurol Neurosurg Psychiatry. 1994;57:977–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez AR, Moro A, Abrahao A, Faber I, Borges CR, Rezende TJ, Martins CR Jr, Moscovich M, Munhoz RP, Segal SL, Arruda WO, Saraiva-Pereira ML, Karuta S, Pedroso JL, D'Abreu A, Jardim LB, Lopes-Cendes Í, Barsottini OG, Teive HA, França MC Jr. Nonneurological Involvement in Late-Onset Friedreich Ataxia (LOFA): Exploring the Phenotypes. Cerebellum. 2017;16:253–256. [DOI] [PubMed] [Google Scholar]
- 6.Bhidayasiri R, Perlman SL, Pulst SM, Geschwind DH. Late-onset Friedreich ataxia: phenotypic analysis, magnetic resonance imaging findings, and review of the literature. Arch Neurol. 2005;62:1865–9. [DOI] [PubMed] [Google Scholar]
- 7.Koeppen AH, Mazurkiewicz JE. Friedreich ataxia: neuropathology revised. J Neuropathol Exp Neurol 2013; 72: 78–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harding IH, Lynch DR, Koeppen AH, Pandolfo M. Central Nervous System Therapeutic Targets in Friedreich Ataxia. Hum Gene Ther. 2020. Dec;31(23-24):1226–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Öz G, Harding IH, Krahe J, Reetz K. MR imaging and spectroscopy in degenerative ataxias: toward multimodal, multisite, multistage monitoring of neurodegeneration. Curr Opin Neurol. 2020;33:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chevis CF, da Silva CB, D'Abreu A, Lopes-Cendes I, Cendes F, Bergo FP, França MC Jr. Spinal cord atrophy correlates with disability in Friedreich's ataxia. Cerebellum. 2013;12:43–7. [DOI] [PubMed] [Google Scholar]
- 11.Dogan I, Romanzetti S, Didszun C, Mirzazade S, Timmann D, Saft C, Schöls L, Synofzik M, Giordano IA, Klockgether T, Schulz JB, Reetz K. Structural characteristics of the central nervous system in Friedreich ataxia: an in vivo spinal cord and brain MRI study. J Neurol Neurosurg Psychiatry. 2019;90:615–617. [DOI] [PubMed] [Google Scholar]
- 12.Rezende TJR, Martinez ARM, Faber I, Girotto Takazaki KA, Martins MP, de Lima FD, Lopes-Cendes I, Cendes F, França MC Jr. Developmental and neurodegenerative damage in Friedreich's ataxia. Eur J Neurol. 2019;26:483–489. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez ALCC, Rezende TJR, Martinez ARM, de Brito MR, França MC Jr. Tract-Specific Spinal Cord Diffusion Tensor Imaging in Friedreich's Ataxia. Mov Disord. 2022;37:354–364. [DOI] [PubMed] [Google Scholar]
- 14.Joers JM, Adanyeguh IM, Deelchand DK, Hutter DH, Eberly LE, Iltis I, Bushara KO, Lenglet C, Henry PG. Spinal cord MRI and MRS Detect Early-stage Alterations and Disease Progression in Friedreich Ataxia. MedRxiv. 2022. doi: 10.1101/2022.01.28.22270048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harding IH, Chopra S, Arrigoni F, Boesch S, Brunetti A, Cocozza S, Corben LA, Deistung A, Delatycki M, Diciotti S, Dogan I, Evangelisti S, França MC Jr, Göricke SL, Georgiou-Karistianis N, Gramegna LL, Henry PG, Hernandez-Castillo CR, Hutter D, Jahanshad N, Joers JM, Lenglet C, Lodi R, Manners DN, Martinez ARM, Martinuzzi A, Marzi C, Mascalchi M, Nachbauer W, Pane C, Peruzzo D, Pisharady PK, Pontillo G, Reetz K, Rezende TJR, Romanzetti S, Saccà F, Scherfler C, Schulz JB, Stefani A, Testa C, Thomopoulos SI, Timmann D, Tirelli S, Tonon C, Vavla M, Egan GF, Thompson PM. Brain Structure and Degeneration Staging in Friedreich Ataxia: Magnetic Resonance Imaging Volumetrics from the ENIGMA-Ataxia Working Group. Ann Neurol. 2021;90:570–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramony SH, May W, Lynch D, Gomez C, Fischbeck K, Hallett M, Taylor P, Wilson R, Ashizawa T; Cooperative Ataxia Group. Measuring Friedreich ataxia: Interrater reliability of a neurologic rating scale. Neurology. 2005.12;64:1261–2. [DOI] [PubMed] [Google Scholar]
- 17.Lynch DR, Farmer JM, Tsou AY, Perlman S, Subramony SH, Gomez CM, Ashizawa T, Wilmot GR, Wilson RB, Balcer LJ. Measuring Friedreich ataxia: complementary features of examination and performance measures. Neurology. 2006. Jun 13;66(11):1711–6. [DOI] [PubMed] [Google Scholar]
- 18.Rummey C, Corben LA, Delatycki MB, Subramony SH, Bushara K, Gomez CM, Hoyle JC, Yoon G, Ravina B, Mathews KD, Wilmot G, Zesiewicz T, Perlman S, Farmer JM, Lynch DR. Psychometric properties of the Friedreich Ataxia Rating Scale. Neurol Genet. 2019. 29;5:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, Giunti P, Globas C, Infante J, Kang JS, Kremer B, Mariotti C, Melegh B, Pandolfo M, Rakowicz M, Ribai P, Rola R, Schöls L, Szymanski S, van de Warrenburg BP, Dürr A, Klockgether T, Fancellu R. Scale for the assessment and rating of ataxia: development of a new clinical scale. Neurology. 2006. Jun 13;66(11):1717–20. doi: 10.1212/01.wnl.0000219042.60538.92. Erratum in: Neurology. 2006 Jul 25;67(2):299. Fancellu, Roberto [added]. [DOI] [PubMed] [Google Scholar]
- 20.De Leener B, Lévy S, Dupont SM, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 2017;145:24–43. [DOI] [PubMed] [Google Scholar]
- 21.Gros C, De Leener B, Badji A, et al. Automatic segmentation of the spinal cord and intramedullary multiple sclerosis lesions with convolutional neural networks. Neuroimage. 2019;184:901–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ullmann E, Pelletier Paquette JF, Thong WE, Cohen-Adad J. Automatic labeling of vertebral levels using a robust template-based approach. Int J Biomed Imaging. 2014;2014:719520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Leener B, Taso M, Fonov V, et al. Fully-integrated T1, T2, T2*, white and gray matter atlases of the spinal cord. In: Proceedings of the International Society for Magnetic Resonance in Medicine 24th Annual Meeting and Exhibition, Singapore. May 7–8, 2016. [Google Scholar]
- 24.De Leener B, Fonov VS, Collins DL, et al. PAM50: Unbiased multimodal template of the brainstem and spinal cord aligned with the ICBM152 space. Neuroimage. 2018;165:170–179. [DOI] [PubMed] [Google Scholar]
- 25.Papinutto N, Asteggiano C, Bischof A, Gundel TJ, Caverzasi E, Stern WA, Bastianello S, Hauser SL, Henry RG. Intersubject Variability and Normalization Strategies for Spinal Cord Total Cross-Sectional and Gray Matter Areas. J Neuroimaging. 2020. Jan;30(1):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cohen J. (1988). Statistical Power Analysis for the Behavioral Sciences. New York, NY: Routledge Academic. [Google Scholar]
- 27.Bürk K, Mälzig U, Wolf S, Heck S, Dimitriadis K, Schmitz-Hübsch T, Hering S, Lindig TM, Haug V, Timmann D, Degen I, Kruse B, Dörr JM, Ratzka S, Ivo A, Schöls L, Boesch S, Klockgether T, Klopstock T, Schulz JB. Comparison of three clinical rating scales in Friedreich ataxia (FRDA). Mov Disord. 2009;24:1779–84. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Lloret S, van de Warrenburg B, Rossi M, Rodríguez-Blázquez C, Zesiewicz T, Saute JAM, Durr A, Nishizawa M, Martinez-Martin P, Stebbins GT, Schrag A, Skorvanek; members of the MDS Rating Scales Review Committee. Assessment of Ataxia Rating Scales and Cerebellar Functional Tests: Critique and Recommendations. Mov Disord. 2021;36:283–297. [DOI] [PubMed] [Google Scholar]
- 29.Koeppen AH, Becker AB, Qian J, Feustel PJ. Friedreich Ataxia: Hypoplasia of Spinal Cord and Dorsal Root Ganglia. J Neuropathol Exp Neurol. 2017;76:101–108. [DOI] [PubMed] [Google Scholar]
- 30.Mascalchi M, Bianchi A, Ciulli S, Ginestroni A, Aiello M, Dotti MT, Salvi F, Nicolai E, Soricelli A, Diciotti S. Lower medulla hypoplasia in Friedreich ataxia: MR Imaging confirmation 140 years later. J Neurol. 2017;264:1526–1528. [DOI] [PubMed] [Google Scholar]
- 31.França MC Jr, D'Abreu A, Zanardi VA, Faria AV, Lopes-Cendes I, Nucci A, Cendes F. MRI shows dorsal lesions and spinal cord atrophy in chronic sensory neuronopathies. J Neuroimaging. 2008;18:168–72. [DOI] [PubMed] [Google Scholar]
- 32.Rezende TJ, de Albuquerque M, Lamas GM, Martinez AR, Campos BM, Casseb RF, Silva CB, Branco LM, D'Abreu A, Lopes-Cendes I, Cendes F, França MC Jr. Multimodal MRI-based study in patients with SPG4 mutations. PLoS One. 2015;10:e0117666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Querin G, Bede P, El Mendili MM, Li M, Pélégrini-Issac M, Rinaldi D, Catala M, Saracino D, Salachas F, Camuzat A, Marchand-Pauvert V, Cohen-Adad J, Colliot O, Le Ber I, Pradat PF; Predict to Prevent Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis Study Group. Presymptomatic spinal cord pathology in c9orf72 mutation carriers: A longitudinal neuroimaging study. Ann Neurol. 2019;86:158–167. [DOI] [PubMed] [Google Scholar]
- 34.Pisharady PK, Eberly LE, Cheong I, Manousakis G, Guliani G, Clark HB, Bathe M, Walk D, Lenglet C. Tract-specific analysis improves sensitivity of spinal cord diffusion MRI to cross-sectional and longitudinal changes in amyotrophic lateral sclerosis. Commun Biol. 2020. Jul 10;3(1):370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altman J, Bayer SA. Development of the Human Spinal Cord. An Interpretation Based on Experimental Studies in Animals. Oxford: Oxford University Press; 2001. [Google Scholar]
- 36.Jiralerspong S, Liu Y, Montermini L, Stifani S, Pandolfo M. Frataxin shows developmentally regulated tissue-specific expression in the mouse embryo. Neurobiol Dis. 1997;4:103–13. [DOI] [PubMed] [Google Scholar]
- 37.Oppenheimer DR. Brain lesions in Friedreich's ataxia. Can J Neurol Sci. 1979;6:173–6. [DOI] [PubMed] [Google Scholar]
- 38.Urich H, Norman RM, Lloyd OC. Suprasegmental lesions in Friedreich's ataxia. Confin Neurol. 1957;17:360–71. [DOI] [PubMed] [Google Scholar]
- 39.Rezende TJR, de Paiva JLR, Martinez ARM, Lopes-Cendes I, Pedroso JL, Barsottini OGP, Cendes F, França MC Jr. Structural signature of SCA3: From presymptomatic to late disease stages. Ann Neurol. 2018;84:401–408. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Site-specific effect sizes for a) cross-sectional area (CSA) and b) eccentricity for each vertebral level assessed.
Supplementary Table1: Demographics data for non-ataxic individuals for each site.
Supplementary Table2: Scanner and imaging acquisition details for each site.
Supplementary Table 3: Demographics data of pediatric and LOFA patients.
Supplementary Table 4: Separated clinical correlations analysis for subjects with FARS only and SARA only. N is the sample size used for each clinical scale.
Supplementary Table 5: Group differences between pediatric vs adult patients with FRDA at each spinal cord level, C1-C4.
Supplementary Table 6: Correlations between time from ataxia onset or normalized disease severity and CSA or eccentricity for the pediatric cohort.
Supplementary Table 7: Results of ROI-based analyses to assess CSA damage at each stage of FRDA (DD1: Time from ataxia onset <5 years, DD2: Time from ataxia onset between 5-10 years, DD3: Time from ataxia onset between 10-15 years, DD4: Time from ataxia onset between 15-20 years, DD5: Time from ataxia onset > 20 years). All comparisons are significant, i.e., p<0.001 after Bonferroni correction.
Supplementary Table 8: Results of ROI-based analyses to assess progressive CSA damage at each stage of FRDA relative to the subgroup small time from ataxia onset (DD1: Time from ataxia onset <5 years, DD2: Time from ataxia onset between 5-10 years, DD3: Time from ataxia onset between 10-15 years, DD4: Time from ataxia onset between 15-20 years, DD5: Time from ataxia onset > 20 years). All comparisons are significant, i.e., p<0.001 after Bonferroni correction.
Supplementary Table 9: Results of ROI-based analyses to assess CSA damage at each stage of FRDA (DS1: Normalized disease severity <0.25, DS2: Normalized disease severity between 0.26-0.50, DS3: Normalized disease severity between 0.51-0.75, DS4: Normalized disease severity >0.75). All comparisons are significant, i.e., p<0.001 after Bonferroni correction.
Supplementary Table 10: Results of ROI-based analyses to assess progressive CSA damage at each stage of FRDA relative to the subgroup small normalized disease severity (DS1: Normalized disease severity <0.25, DS2: Normalized disease severity between 0.26-0.50, DS3: Normalized disease severity between 0.51-0.75, DS4: Normalized disease severity >0.75). All comparisons are significant, i.e., p<0.001 after Bonferroni correction.
Data Availability Statement
All code and data processing instructions are available at https://github.com/Harding-Lab/enigma-ataxia.