Abstract
Background
Many colorectal cancer (CRC)-related procedures were suspended during the COVID-19 pandemic. In this study, we predict the impact of resulting delays in screening (colonoscopy, FIT, sigmoidoscopy) and diagnosis on CRC outcomes, and compare different recovery scenarios.
Methods
Using the MISCAN-Colon model, we simulated the US population and evaluated different impact and recovery scenarios. Scenarios were defined by the duration and severity of the disruption (% of eligible adults affected), the length of delays, and the duration of the recovery. During recovery (6, 12 or 24 months), capacity was increased to catch up missed procedures. Primary outcomes were excess CRC cases and deaths, and additional colonoscopies required during recovery.
Results
With a 24-month recovery, the model predicted that the US population would develop 7,210 (0.18%) excess CRC cases during 2020–2040, and 6,950 (0.65%) excess CRC deaths, and require 108,500 (8.6%) additional colonoscopies per recovery month, compared to a no-disruption scenario. Shorter recovery periods of 6 and 12 months, respectively, decreased excess CRC deaths to 4,190 (0.39%) and 4,580 (0.43%), at the expense of 260,200–590,100 (20.7–47.0%) additional colonoscopies per month.
Conclusions
The COVID-19 pandemic will likely cause more than 4,000 excess CRC deaths in the US, which could increase to more than 7,000 if recovery periods are longer.
Impact
Our results highlight that catching-up CRC services within 12 months provides a good balance between required resources and mitigation of the impact of the disruption on CRC deaths.
Keywords: SarsCoV2, Cancer epidemiology, Computer simulation, Care delays, Excess mortality
Introduction
During the first wave of the COVID-19 pandemic, stay-at-home orders were announced worldwide to reduce the risk of transmission. Many healthcare providers delayed or cancelled non-urgent and elective procedures to preserve capacity for COVID-19 patients (1). In March 2020, the American Cancer Society recommended that routine (non-diagnostic) cancer screenings should be halted. During the months of March and April 2020, the percentage of people screened for colorectal cancer (CRC) decreased by 75% to 90% of rates during the same period in previous 3 years (2). Although stay-at-home orders were lifted in June 2020, the number of cancer related services was still behind numbers of previous years. Fear of contracting the coronavirus in health care settings has dissuaded people from cancer screening and subsequent diagnosis and treatment (3).
Furthermore, new CRC diagnoses decreased by 30% by mid-April 2020, and the number of colorectal cancer surgeries fell by 37% compared with the previous year (4). Postponing cancer screening and other delays in cancer care may negatively impact cancer outcomes, as rates of later-stage initial encounters increase (5, 6). Modeling studies have predicted that delayed access to CRC services due to the pandemic will likely result in excess CRC-related deaths over the next years (7, 8). It is therefore important to explore catch-up approaches that can minimize excess CRC deaths.
As the daily number of COVID-19 cases decrease and individuals are getting vaccinated, there is opportunity to catch up on missed screens and maintain regular CRC related services. However, to clear this backlog additional capacity is required for a certain amount of time. It is unclear how the resource requirements needed to efficiently clear this backlog balance out against health benefits.
The aim of this work is to estimate the impact of the disruptions in CRC services on CRC outcomes during the next 20 years. This work extends previous analyses (9) with updated scenarios for the severity of disruption, based on published data. We also investigate capacity increases required to clear the backlog during recovery periods of different lengths.
Materials and Methods
MISCAN-Colon
To assess how delays in CRC screening and diagnosis affect CRC incidence and mortality, we used the Microsimulation Screening Analysis-Colon (MISCAN-Colon) model. This model was developed by the Department of Public Health within Erasmus Medical Center (Rotterdam, the Netherlands) and is part of the US National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network (CISNET).
In short, the MISCAN-Colon model simulates the life histories of a large population similar to the US population in terms of life expectancy and CRC risk. Each simulated person ages and can develop one or more adenomas, which can progress in size and can develop into preclinical cancer (stages I to IV). In each stage, CRC may be clinically detected because of symptoms. Screening can alter the life histories, as CRC can be prevented by detecting and removing adenomas or CRC can be detected at an earlier stage. However, screening can also result in complications, over-diagnosis and over-treatment. A detailed description of the model structure and underlying assumptions can be found in other publications (10, 11).
Study population and background screening
Birth cohorts from 1900 through 2000 were simulated reflecting the age distribution of the 2020 US population, with mortality based on generational lifetables from the Berkeley mortality database (12). Simulated screening consisted of fecal immunochemical testing (FIT), sigmoidoscopy, or primary colonoscopy. Simulated background CRC screening and surveillance were based on age and test-specific trends in National Health Interview Survey (NHIS) data for 1987–2015 (13) (Supplementary Figure 1). When extrapolating these data, it was estimated that by early 2020, 69.6% of US adults who were 50 years old or older had ever been screened with any test, 5.5% had undergone FIT within the last year, 48.1% had undergone endoscopy within the last 5 years, 55.1% had undergone endoscopy in the past 10 years. Of the endoscopies, 96.2% were colonoscopies
Colonoscopy surveillance was assumed for patients with detected adenomas, in accordance with U.S. guidelines (14). In addition to preventive tests and examinations, we simulated diagnostic colonoscopy examination including follow-up after a positive FIT or sigmoidoscopy result, or after development of CRC symptoms.
Modeled impact and recovery scenarios
The impact of COVID was defined in terms of the duration of the disruption period, the severity of disruption in CRC services, the distribution of the time delays in services, and the duration of the recovery period. The disruption period was defined as the period during which CRC screening and diagnostic procedures were severely limited compared to previous years. This was modeled by cancelling and delaying part of preventive and diagnostic procedures. Delays in screening could result in excess CRC cases, as the opportunity to remove precursor lesions may be missed. Due to the delays in screening and diagnosis, it may also take longer before cancers are being (screen) detected, which may subsequently result in progression of the cancer to a later stage. The increase in overall and advanced-stage CRC can both increase the number of CRC deaths. We distinguish disruption in preventive (primary screening, surveillance) and diagnostic procedures (colonoscopy follow-up for positive stool test or symptoms).
The recovery period is defined as the period immediately following the disruption. During this recovery period, we assumed that colonoscopy capacity was temporarily increased to catch up missed preventive and diagnostic procedures. After the recovery period, we assumed screening and diagnostic testing proceeded as usual.
In the base-case analysis, we evaluated several recovery scenarios. Assumptions on length and severity of disruption were varied in additional scenario analyses to reflect uncertainties.
Base-case scenarios
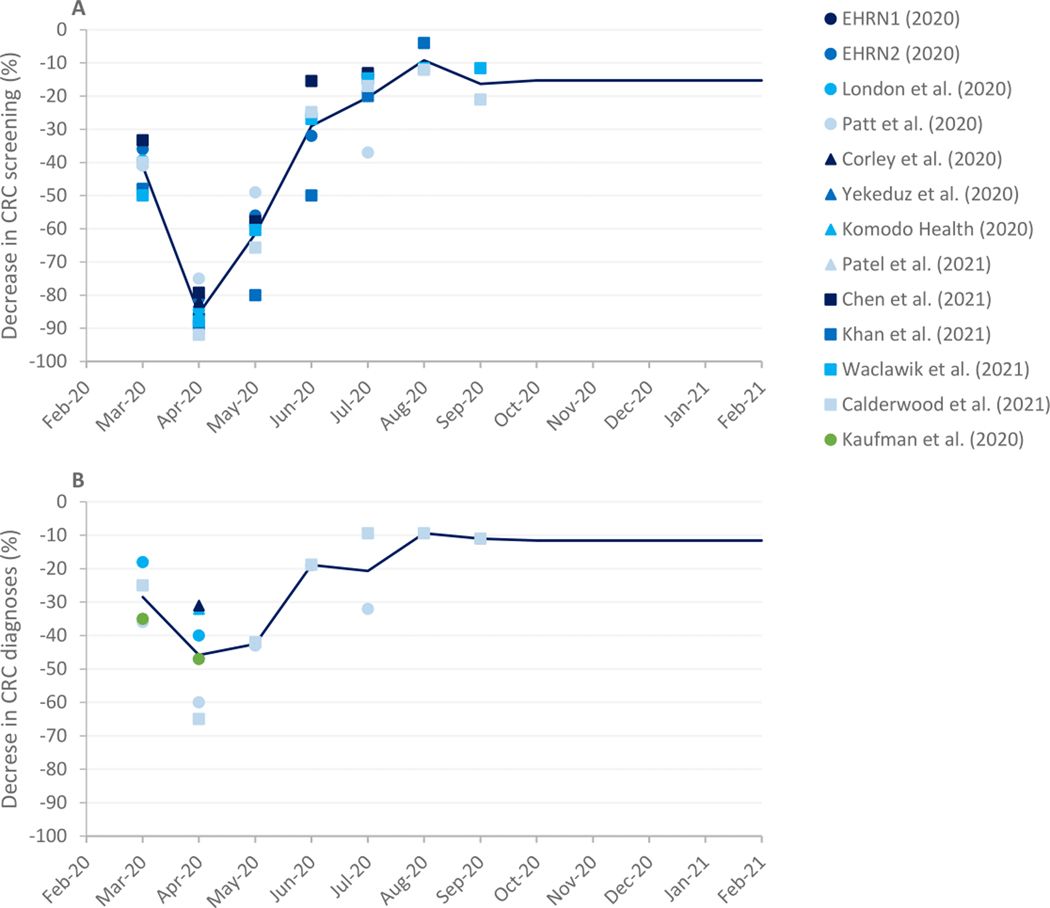
In the base-case analysis, we considered a 12-month disruption period, starting in March 2020. The severity of disruption, or the percent decrease in preventive and diagnostic procedures, by month was based on a review of published literature. We searched Embase and Ovid Medline (December 21, 2020), and identified 2,553 relevant articles. Only articles quantifying the impact of COVID-19 on CRC screening or diagnosis rates in the US were included. Our initial search identified 12 articles for CRC screening/surveillance, and 6 for CRC diagnosis. Available information was limited to the first COVID-19 wave (February to July 2020) compared to previous years. We therefore updated our literature search on October 13, 2021 and identified 5 new articles for screening rates and 1 for diagnostic rates, which provided additional data for the second half of 2020. An overview is provided in Figure 1 and Supplementary Table 1 and 2.
Figure 1.
Published estimates of the decrease in CRC screening (panel A) and diagnosis (panel B) as a result of the COVID-19 pandemic identified by our literature search (2, 4, 26–36). The severity of disruption was determined by taking the average across all studies (solid line). From September 2020, the average decrease of the last three months with available data was used as severity of disruption for preventive services. The average ratio between preventive and diagnostic procedures from March 2020-September 2020 was used to set the severity of disruption for diagnostic procedures for the remaining months of the disruption period. EHRN = Epic Health Research Network
This literature was used to determine the severity of disruption for preventive and diagnostic procedures separately for the period March 2020-December 2020 in our simulations. For preventive services we took the average of the decrease in screening rates across studies for each month (Figure 2). For months for which no data was available, we assumed a fixed severity of disruption equal to the average of the last three months with available data (−15%). During disruption, we assumed that the same mix of screening tests was used as in earlier years.
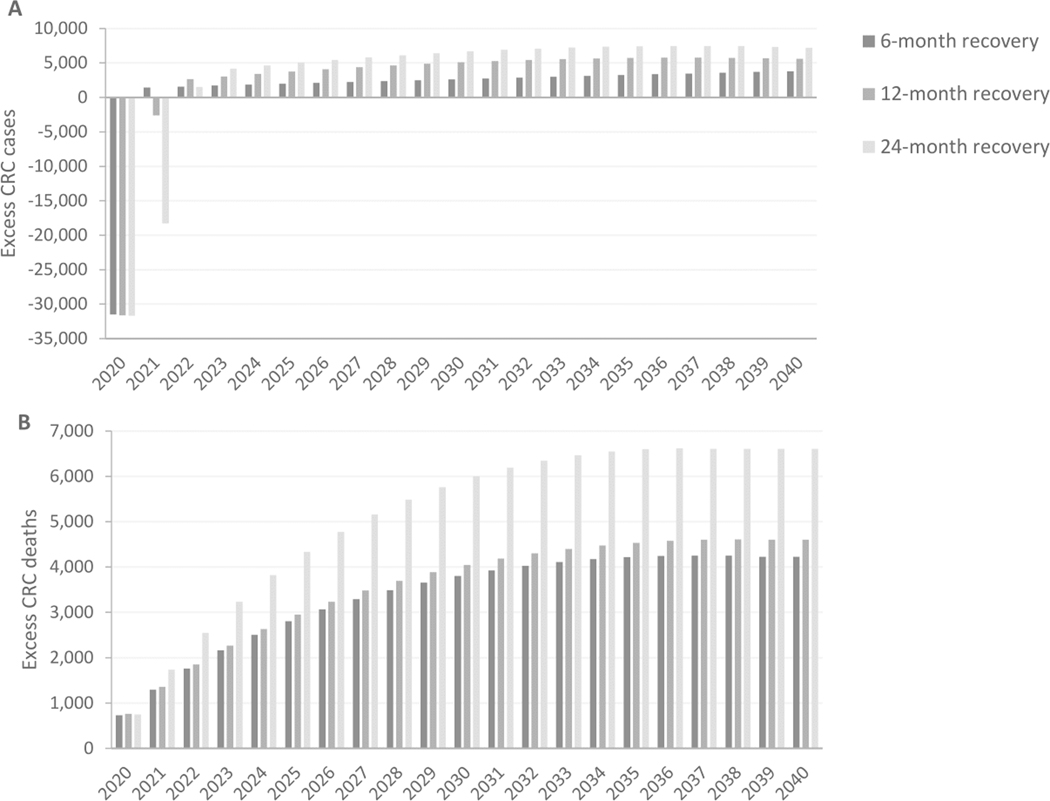
Figure 2.
Cumulative excess CRC cases (A) and deaths (B) compared to a scenario without pandemic-induced delays over time for different recovery scenarios.
The severity of disruption per month for diagnostic procedures was determined by taking the average of the decrease in CRC diagnoses across studies. However, for diagnostic services, data was only available through September 2020. The average ratio between preventive and diagnostic procedures from March 2020-September 2020 was used to set the severity of disruption for diagnostic procedures for the remaining months of the disruption period. The simulated number of diagnosed CRC cases in 2020 was validated afterwards in our analysis.
For the extent of the delay, we considered a delay of 3 months for preventive procedures and 1 month for diagnostic procedures. For each scheduled preventive or diagnostic procedure, a random probability was drawn to determine whether it is delayed or not based on the severity of disruption at the time of the procedure. Procedures could therefore be delayed multiple times. For the last 3 months of the disruption period, the delays for preventive procedures were drawn from a distribution to distribute peaks in backlog more evenly (Weibull, with heterogeneity in means).
Following the disruption, we simulated recovery periods of length 6, 12 and 24 months starting in March 2021. For each of these three scenarios, we estimated the required number of additional preventive and diagnostic procedures compared to a situation without delays to clear the backlog of procedures within that period. First, we estimated the backlog by counting the number of individuals with delayed preventive or diagnostic procedures at the end of the disruption period. The required number of additional colonoscopies was then calculated such that with a similar assumed capacity over the entire recovery period, the backlog decreased linearly from peak at the beginning of the period to zero at the end of the recovery period. Despite the additional assumed capacity for procedures, some individuals could still be delayed during the recovery period if the number due for screening exceeded the capacity.
Outcomes
The impact of delays was assessed by comparing predicted resource use and CRC outcomes across scenarios. All outcomes were calculated in individuals aged 40–85.
We estimated model-predicted CRC incidence and mortality rates, and absolute number of CRC cases and deaths by year over the period 2020–2040. In the post-processing, mortality and incidence rates were age-standardized using the US 2000 population, and then scaled up/down such that the predicted rates in 2018 matched observed age-standardized rates in Surveillance Epidemiology and End Results Program (SEER) data for 2018. To obtain estimates of the absolute number of cases and deaths, model-predicted incidence and mortality rates were first smoothed using a logistic regression model (Supplementary Figure 2). Smoothed rates were then multiplied with US population projections from 2020–2040 to obtain estimates of absolute number of cases and deaths. The cumulative excess CRC cases and CRC-related deaths compared to the no disruption strategy were reported.
We further tallied the number of individuals participating in screening, and the total colonoscopy demand over the impact and recovery period (2020–2023). Next, the required additional number of preventive and diagnostic procedures during recovery was determined. For screening participation and preventive procedures, we focused on clinical resources (primary colonoscopy, sigmoidoscopy, and surveillance colonoscopy).
Finally, we considered the additional number of colonoscopies needed (ACN) per month of recovery to prevent one excess CRC death, to represent the trade-off between colonoscopy demand and excess CRC-related deaths. Specifically, we compared additional colonoscopies required and deaths prevented for 6 and 12-month recovery periods, vs. a longer recovery of 24 months.
Additional scenarios
In scenario analyses, we evaluated additional scenarios with a shorter/longer disruption period (6/18 months), or a lower/higher severity of disruption in preventive and diagnostic procedures (Supplementary Figure 3). Further, we evaluated a scenario in which 10% of patients whose procedures were delayed would never return to screening. All three recovery scenarios were re-evaluated for these additional disruption scenarios.
Sensitivity analysis
In sensitivity analysis, we re-evaluated the base-case scenarios with 5% higher or 5% lower background screening and surveillance rates, to assess uncertainty in the extended screening trends. Further, we re-evaluated scenarios assuming no increases in background CRC incidence over time, in contrast to the base-case analysis, which assumed increased background incidence based on estimated age-adjusted trends for persons aged 20–44 in 2012–2016 vs. 1975–1979 SEER data (15).
Data Availability Statement
The data generated in this study are available within the article and its supplementary data files.
Results
Excess CRC incidence and mortality
Without disruption in CRC services due to the COVID-19 pandemic, the model predicted that there would have been a total of 3.9 million CRC cases and 1.1 million CRC-related deaths in the US during 2020–2040.
For all three recovery scenarios fewer cancers would be diagnosed in 2020 due to the disruption in preventive and diagnostic CRC services (Figure 2, Supplementary Figure 4). The predicted number of diagnosed CRC cases in 2020 decreased by 31,500 (19%) compared to no disruption, which is consistent with our average assumed severity of disruption for diagnostic services in 2020. The number of diagnosed CRC cases increased immediately after the end of the disruption when recovery started. In the case of a 6-month recovery period, the model predicted 3,810 excess CRC cases in 2020–2040, or a small relative increase of 0.10% in overall CRC cases. With 12 and 24-month recovery periods the predicted number of excess CRC-cases increased to 5,590 (0.14%) and 7,210 (0.18%), respectively. For all three recovery scenarios, the impact of the disruption on the number of excess CRC cases was largest in the first 5 years after the disruption, but quickly attenuated after that.
In contrast, relative increases in CRC mortality were greater. Depending on the length of the recovery period, the disruption in preventive and diagnostic CRC services was estimated to cause 4,190 (0.39%; 6 months recovery), 4,580 (0.43%; 12 months recovery) or 6,950 (0.65%; 24 months recovery) excess deaths. The largest part of the excess deaths was estimated to occur during the first 10 years after the disruption period, after which the effect of the disruption diminished. From 2035–2040, there are hardly any additional deaths.
Impact on screening participation and colonoscopy demand
Without disruption in services due to the COVID-19 pandemic, an estimated 15.1 million preventive colonoscopy and sigmoidoscopy procedures would have been performed in 2020, and 0.6 million diagnostic procedures. The COVID-19 pandemic was estimated to have caused an average reduction of 380,600 (−30.3%) in preventive procedures and 400,900 (−30.6%) in total endoscopy demand per disruption month in 2020, regardless of the recovery scenarios evaluated.
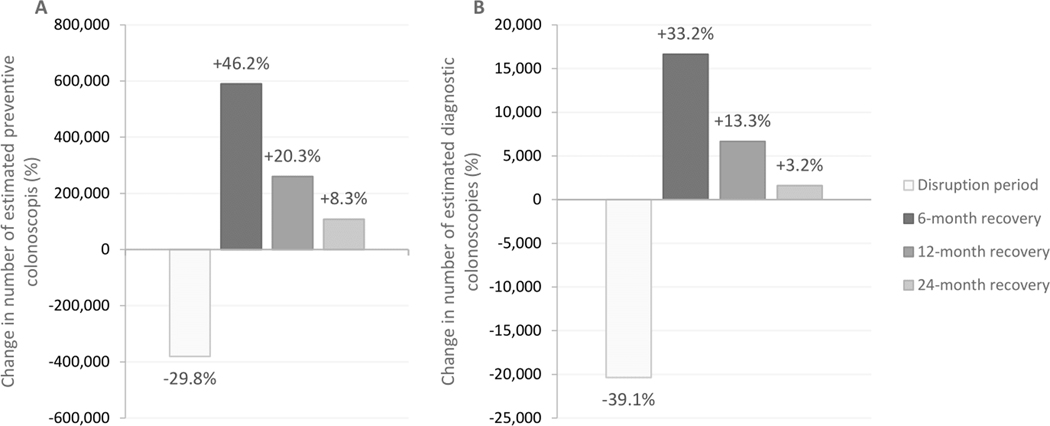
The required number of additional colonoscopy procedures to catch up delayed preventive services decreased proportionally to the length of the recovery period with an additional 590,100 per month, 260,200 per month and 108,500 per month, respectively, for a 6, 12, and 24-month recovery (Table 1, Figure 3). The required number of additional diagnostic colonoscopies was 16,600 per month, 6,700 per month and 1,600 per month. The total colonoscopy demand over 2020–2040 over time did not increase, however.
Table 1.
Outcomes for the three base case recovery scenarios and sensitivity analyses compared to a scenario without disruption.
| Length of disruption | Severity of disruption* | Length of recovery | Additional colonoscopies per month of recovery | Excess cases | Excess deaths | Additional colonoscopies needed per month to prevent one death c | ||
|---|---|---|---|---|---|---|---|---|
| Preventivea | Diagnosticb | |||||||
| No disruption** | 0 months | 0 months | None | |||||
| Base case analysis | ||||||||
| 12 months | Base-case§ | 6 months 12 months 24 months |
590,100 (46.1%) 260,200 (20.3%) 108,500 (8.3%) |
16,600 (33.2%) 6,700 (13.3%) 1,600 (3.2%) |
3,810 (0.10%) 5,590 (0.14%) 7,210 (0.18%) |
4,190 (0.39%) 4,580 (0.43%) 6,950 (0.65%) |
180 66 Ref. |
|
| Scenario analysis | ||||||||
| 6 month disruption | 6 months | Base-case§ | 6 months 12 months 24 months |
478,700 (38.0%) 230,300 (18.3%) 100,000 (7.8%) |
8,700 (16.2%) 2,500 (4.5%) −300 (−1.4%) |
1.310 (0.03%) 2,930 (0.08%) 6,290 (0.16%) |
1,780 (0.17%) 2,850 (0.27%) 4,430 (0.42%) |
146 84 Ref. |
| 18 month disruption | 18 months | Base-case§ | 6 months 12 months 24 months |
653,000 (51.4%) 313,400 (24.7%) 128,100 (10.0%) |
13,500 (27.8%) 3,900 (8.5%) −100 (0.2%) |
7,360 (0.19%) 11,050 (0.28%) 11,490 (0.29%) |
6,040 (0.57%) 8,090 (0.76%) 12,270 (1.16%) |
86 45 Ref. |
| Lower severity of disruption | 12 months | Lower | 6 months 12 months 24 months |
379,100 (37.9%) 165,000 (16.6%) 67,500 (6.7%) |
9,800 (28.5%) 3,500 (11.2%) 600 (2.5%) |
3,310 (0.09%) 3,970 (0.10%) 5,820 (0.15%) |
3,290 (0.31%)± 3,270 (0.31%)± 5,330 (0.50%) |
157 49 Ref. |
| Higher severity of disruption | 12 months | Higher | 6 months 12 months 24 months |
909,200 (70.7%) 399,500 (31.1%) 170,000 (13.1%) |
23,500 (45.6%) 9,700 (18.7%) 2,400 (4.5%) |
5,300 (0.14%) 7,560 (0.19%) 10,300 (0.26%) |
5,070 (0.48%) 5,800 (0.55%) 9,530 (0.90%) |
170 63 Ref. |
| 10% no catch-up | 12 months | Base-case§ | 6 months 12 months 24 months |
561,600 (44.7%) 238,800 (19.0%) 91,500 (7.3%) |
16,700 (30.1%) 6,700 (12.4%) 1,700 (3.1%) |
10,290 (0.26%) 13,780 (0.35%) 19,330 (0.50%) |
8,580 (0.81%) 9,290 (0.88%) 14,440 (1.36%) |
82 30 Ref. |
| Sensitivity analysis | ||||||||
| 5% lower screening rates# | 12 months | Base-case§ | 6 months 12 months 24 months |
560,700 (47.0%) 247,300 (20.7%) 103,300 (8.7%) |
16,200 (31.0%) 6,600 (12.6%) 1,700 (3.2%) |
2,770 (0.07%) 5,220 (0.13%) 7,160 (0.18%) |
4,300 (0.38%) 5,030 (0.45%) 7,660 (0.68%) |
140 56 Ref. |
| 5% higher screening rates# | 12 months | Base-case§ | 6 months 12 months 24 months |
612,000 (47.0%) 237,500 (20.7%) 113,900 (8.6%) |
17,100 (30.1%) 6,800 (12.2%) 1,600 (2.9%) |
2,780 (0.07%) 5,670 (0.15%) 7,240 (0.19%) |
3,190 (0.32%) 3,590 (0.36%) 6,410 (0.64%) |
162 26 Ref. |
| Lower CRC riskǁ | 12 months | Base-case§ | 6 months 12 months 24 months |
595,600 (46.4%) 258,600 (20.1%) 106,100 (8.3%) |
15,800 (30.1%) 5,300 (10.1%) 1,400 (2.7%) |
3,960 (0.12%) 5,460 (0.17%) 8,830 (0.27%) |
3,830 (0.43%) 5,230 (0.59%) 7,720 (0.87%) |
130 63 Ref. |
See supplementary Table 1 for the severity of disruption levels.
Required capacity for the scenario without pandemic-induced delays was 15.1 million for preventive services and 0.6 million for diagnostic serviced. The model predicted 3.9 million CRC cases and 1.1 million CRC-related deaths.
The base-case severity of disruption was calculated from literature.
Compared to a no disruption scenario with 5% lower/higher background screening rates.
Compared to a no disruption scenario with a lower CRC risk.
The number of excess deaths for the 6 and 12-month recovery period are probably similar, but due to random variation the model estimated more deaths in case of a 6-month recovery period.
Screening or surveillance procedures. Screening includes sigmoidoscopies and primary colonoscopies. Colonoscopy surveillance for patients with detected adenomas was based on US guidelines.
Diagnostic colonoscopies following a positive FIT or CRC symptoms.
Ratios were subject to more (random) variation than the number or denominator alone. Caution in interpretation.
Figure 3.
Average change in preventive (A) and diagnostic (B) procedures by month during the disruption and recovery period compared to the scenario without pandemic-induced delays. Preventive procedures include primary colonoscopy, sigmoidoscopy and surveillance colonoscopy.
Additional Colonoscopies Needed to Prevent One Death
While the required number of additional colonoscopies due to COVID-19 decreased proportionally to the assumed length of the recovery period, the total number of excess deaths increased relatively more strongly. Compared to the 24-month recovery scenario, a shorter recovery period of 6 or 12 months therefore, resulted in 180 and 66 additional colonoscopies per month of recovery for each excess death prevented, respectively.
Additional scenarios
For all three recovery scenarios, the excess CRC cases and deaths, and the required additional preventive and diagnostic procedures decreased if the length or severity of disruption were reduced, and conversely, they increased with greater length or severity of disruption (Table 1). The number of excess CRC cases and deaths more than doubled if 10% of patients whose procedure were delayed would never go back to screening.
The number of excess CRC cases was lowest for the 6-month disruption with a 6-month recovery period and highest for the no catch-up scenario with a 24-month recovery period. Predicted excess CRC cases ranged from 1,310–19,330 (0.03–0.50%), and predicted excess deaths ranged from 1,780 −14,440 (0.17–1.36%) depending on the assumptions.
The required additional colonoscopy procedures varied from 8.0% when there was a 6-month disruption followed by a 24-month recovery period, to 72.4% with a more severe disruption of 12 months a 6-month recovery. The ACN for all scenarios was similar to the base case scenarios, with the highest ACN for the 6-month recovery periods.
Sensitivity analysis
For all three recovery scenarios, the number of excess CRC cases and deaths and required additional procedures were similar with different background screening rates vs. the base case. Excess CRC cases and deaths were somewhat higher in case of a lower CRC risk. The ACN was robust to both sensitivity analyses.
Discussion
In this study, we estimated the impact of disruptions in preventive and diagnostic procedures for CRC due to the COVID-19 pandemic. We evaluated 6, 12, and 24-month recovery periods for clearing the backlog to compare the balance in associated colonoscopy requirements and CRC outcomes. The length of the recovery period was directly related to the required number of additional colonoscopies needed per month. The ACN to prevent one death increased disproportionally if the recovery period was shortened, from 66 to 180 for a recovery of 12 to 6 months vs. 24 months. Even if all missed procedures were caught up within 6 months, 4,190 excess CRC-related deaths were estimated to occur, due in part to the excess of CRC cases. These results underscore the importance of resuming and catching-up CRC screening and diagnosis services as quickly as possible.
Our work extends a previous CISNET analysis that highlighted how COVID-19 delays may increase avoidable CRC deaths in the US (9), by examining more realistic scenarios for the severity of disruption based on observed declines in screening, alternative scenarios for the subsequent recovery period to clear the backlog in procedures, and a longer impact projection horizon. As we know today, the 6-month disruption assumed in our previous analysis is no longer realistic. Although the overall magnitude of effect was similar, these new estimates of impact are either smaller or larger, depending on the assumptions regarding the length and severity of the disruption, and the recovery scenarios. The impact with a disruption of 6 months or with lower severity of disruption was in all cases smaller than in our previous work. Conversely, the impact with a disruption of 18 months was in all cases larger. In other scenarios, the impact depended on the duration of the recovery period.
Several other studies have been published, which estimated the impact of disruptions in CRC-related services due to the COVID-19 pandemic. A study from Canada estimated 1,100–2,200 excess cases and +0.48–2.0% excess deaths due to delays in CRC screening (16), which is similar order of magnitude as our estimates. Larger impact estimates were found by a modeling study from the UK (+15.3–16.6% deaths), in which screening was completely suspended for 12 months and only urgent referral pathways to diagnosis were possible (7). Excess deaths were only defined relative to a 5-year period and among patients diagnosed with CRC, hence resulting in a smaller denominator and larger impact estimates. Besides the increase in avoidable CRC cases and deaths, modeling studies showed that delays in CRC-related services might also lead to a stage shift, with an increase in advanced CRC cases (8, 17).
Our work can inform strategies to mitigate the impact of the COVID-19 pandemic or future crises and consider the need to invest in capacity for CRC-related services. Although the 6-month recovery period resulted in the fewest excess deaths, it may not be feasible to increase capacity by the required 46%, due to local capacity constraints. A study reported that primary screening colonoscopy volume could be increased to 25.5 million, which would suggest sufficient capacity nationally (13). Another study reported that colonoscopy capacity could be increased by 57%. However, increasing sigmoidoscopy capacity is not likely in the US given low reimbursement for this procedure and declining availability (18, 19). It should be noted that these are pre-pandemic estimates, with unclear geographic distribution. Where a 6-month recovery period is not feasible, a recovery period of 12 months should be aimed for. While the difference in excess CRC-related deaths between 6 and 12-month recovery periods was relatively small, a recovery period of 24 months almost doubled the number of excess deaths. This suggests that the impact of suspending procedures is nonlinear and longer delays might be even more harmful.
The balance between required resources and health impact of delays should be weighed against those for other services. For some conditions, delays in diagnostic services may have greater impact than for other services, depending on the natural history of the disease. For example, for breast cancer screening, which has no clear precursor stage such as CRC, follow-up of positive mammograms may deserve prioritization (9).
Compared to colonoscopy screening, FIT screening may be a resource-efficient and effective strategy to reduce colonoscopy backlog and its recovery time (20). A modeling study demonstrated that offering FIT to part of the population that could not be screened with colonoscopy due to the pandemic, resulted in a higher screening participation and more CRC diagnoses in an earlier stage (17). The potential benefits might be even greater if FIT uptake could be increased beyond this, although follow-up with colonoscopy of positive results would still have to be ensured in that case. Data also illustrated that FIT rates during the COVID-19 pandemic remained high (4). This suggests that recovery scenarios using FIT may provide a more feasible strategy, than the scenarios evaluated in our study, especially in settings with limited colonoscopy capacity and high competing demands on clinical resources due to new COVID-19 waves. However, FIT-based screening is just one way of triaging patients for colonoscopy. There are other ways to prioritize patients based on their risk. For example, colonoscopies performed could potentially be shifted away from low-yield colonoscopy indications, such as diarrhea, toward CRC-related indications, making recovery efforts more feasible.
Our work has some limitations. First, in our modeling we did not prioritize individuals in order of original date of invitation, resulting in long delays for some and shorter or no delays for others, although intermediate delays of 2–6 months were most common. Next, we considered primary screening and surveillance colonoscopy as one outcome and did not stratify based on surveillance for high vs. low risk adenomas. It is therefore unclear how backlog is distributed between the two and what their contribution is to the increased CRC incidence and mortality. In our modeling we did not adjust for a higher other-cause mortality risk as a result of COVID-19. This might influence our CRC incidence and mortality estimates. Furthermore, there are other delays in the CRC care continuum not considered here, most notably delay in treatment after diagnosis. Although we assumed no such delays, the modeled impact should be similar to that of delay in CRC diagnosis.
While the assumed screening patterns in our study were based on detailed NHIS data, these data stem from before the pandemic. We assumed that individuals utilized the same mix of tests, and that only the overall rate of test utilization decreased temporarily, to rebound after the recovery period. Adherence rates could remain lower, e.g. due to high unemployment rates as a consequence of the pandemic (21). Some individuals may have changed from endoscopy-based to FIT-based screening to avoid coming to a medical facility. Finally, assumptions regarding the length of delay in services or the severity of disruption beyond September 2020 could not be directly informed by real-world data. We therefore evaluated broad ranges for assumptions regarding the length and severity of disruption to quantify uncertainties.
Recent data from the Epic Health Research Network (EHRN) suggest that CRC screenings from March 2020 to March 2021 were 25% down compared to historical baselines (22), consistent with our average assumed base-case severity of disruption (26%). Diagnostic rates in 2020 were also consistent with literature (20% vs. 21%) (23). However, a smaller decrease in CRC diagnosis of 8.7% was found in a cohort study in Kaiser Permanente Northern California patients (24), probably due to their high reliance on FIT screening, for which utilization rates remained relatively high during the pandemic. More recent data also show that screening rates were still lagging behind in June 2021 (25). Rates may have also been affected by other COVID-19 surges (omicron and delta) later in the year. This could mean that the disruption period was longer and more impactful than we estimated.
In conclusion, this study illustrated that the COVID-19 related disruption in CRC screening and diagnosis will likely cause thousands of excess CRC cases and deaths in US alone in years to come. Although the severity of disruption varied substantially across different health care providers and across states (26), these results point to the importance of catching up the CRC screening backlog as soon as possible.
Supplementary Material
Financial support:
R. van den Puttelaar, I. Lansdorp-Vogelaar, A.I. Hahn, C.M. Rutter, A.G. Zauber, and R.G.S. Meester received financial support through grants U01-CA199335 and U01-CA253913 from the National Cancer Institute (NCI) as part of the Cancer Intervention and Surveillance Modeling Network (CISNET). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest: The authors declare no potential conflicts of interest. Dr. Meester became the Director of Health Economics and Outcomes Research at Freenome Holdings Inc. as of May 2022, after this work was completed.
References
- 1.Centers for Medicare & Medicaid Services. CMS adult elective surgery and procedures recommendations. 2020. [Google Scholar]
- 2.Epic Health Research Network. Delayed Cancer Screenings - A Second Look. 2020. [Google Scholar]
- 3.Elflein L. Delayed or cancelled routine cancer screening tests due to COVID-19 in the U.S. 2020. [Google Scholar]
- 4.Corley DA, Sedki M, Ritzwoller DP, Greenlee RT, Neslund-Dudas C, Rendle KA, et al. Cancer Screening during COVID-19: A Perspective from NCI’s PROSPR consortium. Elsevier; 2020. [Google Scholar]
- 5.Meester RGS, Zauber AG, Doubeni CA, Jensen CD, Quinn VP, Helfand M, et al. Consequences of increasing time to colonoscopy examination after positive result from fecal colorectal cancer screening test. Clinical Gastroenterology and Hepatology. 2016;14(10):1445–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rutter CM, Kim JJ, Meester RGS, Sprague BL, Burger EA, Zauber AG, et al. Effect of time to diagnostic testing for breast, cervical, and colorectal cancer screening abnormalities on screening efficacy: a modeling study. Cancer Epidemiology and Prevention Biomarkers. 2018;27(2):158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. The lancet oncology. 2020;21(8):1023–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricciardiello L, Ferrari C, Cameletti M, Gaianill F, Buttitta F, Bazzoli F, et al. Impact of SARS-CoV-2 pandemic on colorectal cancer screening delay: effect on stage shift and increased mortality. Clinical Gastroenterology and Hepatology. 2021;19(7):1410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharpless NE. COVID-19 and cancer. American Association for the Advancement of Science; 2020. [Google Scholar]
- 10.Loeve F, Boer R, van Oortmarssen GJ, van Ballegooijen M, Habbema JDF. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Computers and Biomedical Research. 1999;32(1):13–33. [DOI] [PubMed] [Google Scholar]
- 11.van Hees F, Habbema JDF, Meester RG, Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG. Should colorectal cancer screening be considered in elderly persons without previous screening? A cost-effectiveness analysis. Annals of internal medicine. 2014;160(11):750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkeley Mortality Database. Lifetables by Year of Birth 1900–2000 2015. Available from: http://www.demog.berkeley.edu/~bmd/states.html. [Accessed 01-01-2015].
- 13.Joseph DA, Meester RGS, Zauber AG, Manninen DL, Winges L, Dong FB, et al. Colorectal cancer screening: estimated future colonoscopy need and current volume and capacity. Cancer. 2016;122(16):2479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–57. [DOI] [PubMed] [Google Scholar]
- 15.Knudsen AB, Rutter CM, Peterse EFP, Lietz AP, Seguin CL, Meester RGS, et al. Colorectal Cancer Screening: An Updated Decision Analysis for the US Preventive Services Task Force. 2021. [PubMed] [Google Scholar]
- 16.Yong JHE, Mainprize JG, Yaffe MJ, Ruan Y, Poirier AE, Coldman A, et al. The impact of episodic screening interruption: COVID-19 and population-based cancer screening in Canada. Journal of medical screening. 2021;28(2):100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Issaka RB, Taylor P, Baxi A, Inadomi JM, Ramsey SD, Roth J. Model-Based Estimation of Colorectal Cancer Screening and Outcomes During the COVID-19 Pandemic. JAMA Network Open. 2021;4(4):e216454-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis JD, Asch DA. Barriers to office-based screening sigmoidoscopy: does reimbursement cover costs? Annals of internal medicine. 1999;130(6):525–30. [DOI] [PubMed] [Google Scholar]
- 19.Levin TR, Corley DA, Jensen CD, Schottinger JE, Quinn VP, Zauber AG, et al. Effects of organized colorectal cancer screening on cancer incidence and mortality in a large community-based population. Gastroenterology. 2018;155(5):1383–91. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tinmouth J, Dong S, Stogios C, Rabeneck L, Rey M, Dubé C, et al. Estimating the backlog of colonoscopy due to coronavirus disease 2019 and comparing strategies to recover in Ontario, Canada. Gastroenterology. 2021;160(4):1400–2. e1. [DOI] [PubMed] [Google Scholar]
- 21.Printz C. Cancer screenings decline significantly during pandemic. Cancer. 2020;126(17):3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Epic Health Research Network. Cancer Screenings Are Still Lagging. 2021. [Google Scholar]
- 23.Englum BR, Prasad NK, Lake RE, Mayorga-Carlin M, Turner DJ, Siddiqui T, et al. Impact of the COVID-19 pandemic on diagnosis of new cancers: A national multicenter study of the Veterans Affairs Healthcare System. Cancer. 2022;128(5):1048–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JK, Lam AY, Jensen CD, Marks AR, Badalov J, Layefsky E, et al. Impact of the COVID-19 pandemic on fecal immunochemical testing, colonoscopy services, and colorectal neoplasia detection in a large United States community-based population. Gastroenterology. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ong MBH. Epic EHR data: Cancer screenings nosedive in 2021 despite easing of COVID restrictions. Cancer Letter. 2021;47(34). [Google Scholar]
- 26.Chen RC, Haynes K, Du S, Barron J, Katz AJ. Association of Cancer Screening Deficit in the United States With the COVID-19 Pandemic. JAMA oncology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epic Health Research Network. Delays in preventive cancer screenings during covid-19 pandemic. 2020. [Google Scholar]
- 28.London JW, Fazio-Eynullayeva E, Palchuk MB, Sankey P, McNair C. Effects of the COVID-19 pandemic on cancer-related patient encounters. JCO Clinical Cancer Informatics. 2020;4:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patt D, Gordan L, Diaz M, Okon T, Grady L, Harmison M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO clinical cancer informatics. 2020;4:1059–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yekedüz E, Karcıoğlu AM, Utkan G, Ürün Y. A clinical dilemma amid COVID-19 pandemic: missed or encountered diagnosis of cancer? : Future Medicine; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Health Komodo. New colorectal cancer diagnoses fall by one-third as colonoscopy screenings and biopsies grind to a halt during height of COVID-19. 2020. [Google Scholar]
- 32.Patel S, Issaka RB, Chen E, Somsouk M. Colorectal cancer screening and COVID-19. The American journal of gastroenterology. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan M, Wright M, Watson K, Jain S. Trends in cancer screening volumes at an urban health center during the COVID-19 pandemic. Wolters Kluwer Health; 2021. [Google Scholar]
- 34.Waclawik G, Benson M, Pfau P, Weiss J. Impact of COVID-19 pandemic on colorectal cancer screening when colonoscopy is the dominant screening modality. Gastrointestinal Endoscopy. 2021;93(6):AB96–AB7. [Google Scholar]
- 35.Calderwood AH, Calderwood MS, Williams JL, Dominitz JA. Impact of the COVID-19 pandemic on utilization of EGD and colonoscopy in the United States: an analysis of the GIQuIC registry. Techniques and innovations in gastrointestinal endoscopy. 2021;23(4):313–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaufman HW, Chen Z, Niles J, Fesko Y. Changes in the number of US patients with newly identified cancer before and during the coronavirus disease 2019 (COVID-19) pandemic. JAMA network open. 2020;3(8):e2017267-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available within the article and its supplementary data files.