Abstract
Importance
Sodium-glucose cotransporter-2 inhibitors (SGLT-2i) have demonstrated many cardiovascular and renal benefits for patients with type 2 diabetes (T2D). However, the results of SGLT-2i use on primary prevention of atrial fibrillation (AF) were inconsistent in clinical trials. Additionally, incident AF was not a pre-specified endpoint.
Objective
To examine the association of incident AF with initiation of an SGLT-2i compared to initiation of a dipeptidyl peptidase 4 inhibitor (DPP-4i) or a glucagon-like peptide 1 receptor agonist (GLP-1RA) amongst older adults (≥66 years of age) with T2D in routine practice.
Design, Setting and Participants
Population-based new-user cohort study including older adults with T2D who had no history of AF and were enrolled in Medicare fee-for-service from April 2013 to December 2018. Data analysis was performed in 2021.
Exposures
To control for potential confounding, new users of SGLT-2i were 1:1 propensity score (PS) matched to new users of DPP-4i or GLP-1RA, in two pairwise comparisons, based on 138 baseline covariates.
Main outcomes and measures
The primary outcome was incident AF, defined as an inpatient diagnosis code for AF. Hazard ratios (HRs) and rate differences (RDs) per 1000 person-years, with their 95% confidence intervals (CIs), were estimated in the PS-matched groups.
Results
A total of 74,868 and 80,475 new users of SGLT-2i were 1:1 PS-matched to new users of DPP-4i or GLP-1RA, respectively. Overall, the mean age of study participants was 72 years and nearly half of them were male. The risk of incident AF was lower in the SGLT-2i group than the matched DPP-4i group [HR, 0.82 (95% CI, 0.76–0.89); RD, −3.7 (95% CI, −5.2 to −2.2) per 1000 person-years] or the matched GLP-1RA group [HR, 0.90 (95% CI, 0.83–0.98); RD, −1.8 (95% CI, −3.2 to −0.3) per 1000 person-years]. Results were consistent across several sensitivity and subgroup analyses.
Conclusions and Relevance
In this cohort study of Medicare beneficiaries with T2D, we found that the initiation of an SGLT-2i was associated with a reduced risk of incident AF compared to a DPP-4i or GLP-1RA. Our results may be helpful when weighing the potential risks and benefits of various glucose-lowering agents in older adults with T2D.
INTRODUCTION
More than 30 million (10%) Americans have type 2 diabetes (T2D), with a higher prevalence—more than one in four—among adults aged 65 years or greater.1 T2D is associated with a 35% to 60% relative increase in the risk of developing atrial fibrillation (AF) or atrial flutter (collectively referred to as AF).2–4 Compared with T2D alone, the presence of comorbid T2D and new-onset AF carries a 3.8-fold increased risk for heart failure (HF) and a 2.7-fold increased risk for all-cause mortality.5 Among patients older than 65 years, 27% of them develop AF in 10 years.6 Due to the high risk of developing AF, preventing this condition in older patients is an important goal.
Large randomized controlled trials (RCTs) have proven the efficacy of sodium-glucose cotransporter-2 inhibitors (SGLT-2i) in reducing major cardiovascular events (MACE), hospitalization for heart failure (HHF), and kidney disease progression.7–9 However, the role of SGLT-2i in incident AF remains controversial. In the DECLARE-TIMI 58 trial, compared with placebo, dapagliflozin reduced the incidence of AF by 19% in patients with T2D.10 However, there was no consistent reduction in incident AF with SGLT-2i in other RCTs.7,9,11,12,13 Meta-analyses suggested either no effect or possible protective effects of SGLT-2i against AF.13–18However, incident AF was not a pre-specified endpoint and was typically reported as an adverse event in RCTs. This reporting generally depends on investigators identifying events and reporting them in a relatively unstructured way.19 In addition, incident AF was not a common event in RCTs, further limiting the ability to assess this outcome robustly. Finally, head-to-head trials comparing SGLT-2i with other anti-diabetes drugs were lacking. Thus, we sought to quantify the association of SGLT-2i initiation with incident AF compared to two active comparators in a nationwide cohort of older adults with T2D.
METHODS
Study Design and Data Sources
We conducted a population-based cohort study using Medicare fee-for-service data. Medicare is a federally funded health insurer for eligible individuals primarily aged ≥65 years. We leveraged Medicare claims data from inpatient services (Part A), outpatient services (Part B), and prescription medications (Part D).
The initiators of SGLT-2i were compared to the initiators of dipeptidyl peptidase-4 inhibitors (DPP-4i) or glucagon-like peptide-1 receptor agonists (GLP-1RA) in two pair-wise comparisons. Any of these three anti-diabetes drug classes may have been selected as a second-line treatment for T2D, per clinical guidelines available during the study period.20,21 This study was approved by the Mass General Brigham Institutional Review Board, and an appropriate data use agreement was in place. Informed consent was not obtained because the study used claims data with anonymous identifiers. Data analysis was performed in 2021. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for observational studies.
Study Population
We identified patients who newly filled a prescription for an SGLT-2i or a comparator between April 1, 2013 (date after the first SGLT-2i approval in the U.S.) and December 31, 2018 (end date for available data). For each pair-wise comparison, the cohort entry date was the date of the first prescription of an SGLT-2i or the specific comparator during the study period. Eligible patients had at least 365 days of continuous Medicare Part A, B, and D enrollment before cohort entry. We excluded patients with prior use of SGLT-2i or the specific comparator in the 365-day baseline period. We restricted the study population to patients with T2D aged ≥ 66 years. Patients with missing demographic information (age, gender, or race) were excluded, because these data may influence outcome. Race data in Medicare are derived from source data from the Social Security Administration and the results of an algorithm that applies to the source data. We also excluded those who had a diagnosis of any of the following during the baseline period: type 1 diabetes, secondary diabetes, malignancy, chronic kidney disease (CKD) stage 5 or dialysis, organ transplant, nursing home admission, prior AF or factors suggestive of AF22. Codes for inclusion and exclusion criteria are shown in eTable 1.
Follow-up and Outcomes
Follow-up began on the day after cohort entry and continued in an as-treated scheme until the first occurrence of any of the following: an outcome event, death, switching to a comparator class, discontinuation of index therapy, end of the study period, or end of healthcare or pharmacy enrollment. Medication use was evaluated by prescription refill date and supply. Treatment discontinuation was defined as not refilling a prescription within 60 days after the most recent filled prescription’s days supply ran out.
The primary outcome was incident AF, defined as an inpatient diagnosis code for AF (henceforth called “AF hospitalization”), using a previously validated algorithm that has 95% sensitivity and 99% specificity.23 Secondary outcomes included “AF diagnosis” (based on at least one inpatient or two outpatient diagnosis codes for AF),24,25 any AF diagnosis code combined with dispensing of any AF medication within 30 days (henceforth “AF treated with medication”),26 “hospitalization for AF” (defined as AF discharge diagnosis codes in the primary position). Other secondary outcomes included stroke or transient ischemic attack (stroke/TIA), HHF (HF discharge diagnosis codes in the primary position), AF hospitalization censored for HF, and hospitalization for AF and HF (AF and HF discharge diagnosis codes in any position). Codes for outcomes are available in eTable 2.
Statistical Analyses
To account for the non-random allocation of patients to the treatment groups, we used 1:1 propensity score (PS) matching. Propensity scores were calculated using a multivariable logistic regression that modeled the probability of initiating an SGLT-2i versus a comparator as a function of 138 predefined baseline covariates. Covariate selection was based on the best of our knowledge that these covariates were either confounders or risk factors for the outcome. These covariates were assessed during a 365-day period before the cohort entry date and included demographic (such as age, gender, or race), year of cohort entry, comorbid conditions (e.g., CKD, hypertension, or HF), drugs (e.g., anticoagulants or beta blockers), and health care utilization (e.g., electrocardiogram, hospitalization, or cardiologist visit). To further quantify the burden of comorbidities, we calculated a claims-based frailty index27 and a combined comorbidity score28. For each of the two pair-wise comparisons, we created a 1:1 PS-matched cohort using a nearest-neighbor matching without replacement approach within a maximum caliper width of 0.01.29 We assessed covariate balance among the matched cohorts by using standardized mean differences, with values less than 0.1 suggesting an adequate balance between matched groups.30
For all outcomes, in each PS-matched cohort we calculated the incidence rates (IRs) as well as hazard ratios (HRs) using Cox proportional hazards models and rate differences (RDs) using a weighted least-squares regression approach,31 each with 95% CIs. For the primary outcome, we produced Kaplan-Meier plots of cumulative incidence and compared IRs between treatment groups with log-rank tests.
We performed several pre-specified sensitivity analyses. We changed the grace period and risk period from 60 days to 30 days and from 60 days to 90 days. In addition to the primary as-treated analysis, we carried the index exposure forward to 365 days without considering treatment discontinuation or switching to mimic an intention-to-treat (ITT) approach. Finally, to assess the presence of potential unmeasured confounding, we evaluated the association of SGLT-2i with the risk for herpes zoster shown previously to be unrelated to this drug.32
We also quantified the association of SGLT-2i and AF in several relevant subgroups: (1) age ≤ 70 versus >70 years; (2) female versus male; (3) no history of HF versus a history of HF; (4) no history of atherosclerotic cardiovascular disease (ASCVD) versus a history of ASCVD.
All analyses were performed using Aetion Evidence Platform® (2020) version R4.34, software33 for real-world data analysis.34,35
RESULTS
Study Cohort and Patient Characteristics
A total of 408,294 patients met study criteria for the SGLT-2i versus DPP-4i cohort (82,430 SGLT-2i users and 325,864 DPP-4i users) and 234,530 met criteria for the SGLT-2i versus GLP-1RA cohort (121,371 SGLT-2i users and 113,159 GLP-1RA users) (eFigures 1 and 2). Before PS-matching, patients initiating an SGLT-2i had lower frailty and combined comorbidity scores28(eTables 3 and 4).
After 1:1 PS-matching (c-statistic of 0.5 for both models), we identified 149,736 patients (74,868 pairs) initiating either an SGLT-2i or DPP-4i and 160,950 patients (80,475 pairs) initiating either an SGLT-2i or GLP-1RA. The mean age was 72 years old and nearly half of them were male (selected list of variables in Table 1). In both matched cohorts, approximately 55% of SGLT-2i patients initiated canagliflozin, followed by empagliflozin (27%) and dapagliflozin (18%). After PS-matching, the median (interquartile range, IQR) duration of follow-up was 191 (90–401) days among SGLT-2i users and 214 (116–438) days among DPP-4i users in the SGLT-2i versus DPP-4i cohort, and 188 (90–395) days among SGLT-2i users and 173 (88–374) days among GLP-1RA users in the SGLT-2i versus GLP-1RA cohort.
Table 1.
Selected baseline characteristics of SGLT-2i versus DPP-4i and SGLT-2i versus GLP-1RA cohorts after 1:1 propensity score matching.
| Characteristics | SGLT-2i versus DPP-4i (n=74,868 pairs) | SGLT-2i versus GLP-1RA (n=80,475 pairs) | ||||
|---|---|---|---|---|---|---|
| SGLT-2i Patients, No. (%) | DPP-4i Patients, No. (%) | St. D | SGLT-2i Patients, No. (%) | GLP-1RA Patients, No. (%) | St. D | |
| Age, mean (SD), years | 71.8 (5.0) | 71.7 (5.1) | 0.02 | 71.8 (5.1) | 71.8 (5.1) | 0.00 |
| Male | 36,302 (48.5) | 36,398 (48.6) | 0.00 | 35,977 (44.7) | 36,025 (44.8) | 0.00 |
| Race | ||||||
| Black | 5,904 (7.9) | 5,985 (8.0) | 0.00 | 6,581 (8.2) | 6,616 (8.2) | 0.00 |
| White | 61,904 (82.7) | 61,794 (82.5) | 0.01 | 66,883 (83.1) | 66,583 (82.7) | 0.01 |
| Othera | 7,060 (9.4) | 7,089 (9.5) | 0.00 | 7,011 (8.7) | 7,276 (9.0) | −0.01 |
| Diabetes-related comorbidities | ||||||
| Diabetic nephropathy | 8,293 (11.1) | 8,333 (11.1) | 0.00 | 10,960 (13.6) | 11,044 (13.7) | 0.00 |
| Diabetic neuropathy | 18,495 (24.7) | 18,420 (24.6) | 0.00 | 21,951 (27.3) | 21,972 (27.3) | 0.00 |
| Diabetic retinopathy | 9,930 (13.3) | 9,816 (13.1) | 0.01 | 11,586 (14.4) | 11,656 (14.5) | 0.00 |
| Hyperglycemia | 25,879 (34.6) | 26,023 (34.8) | −0.01 | 30,568 (38.0) | 30,289 (37.6) | 0.01 |
| Hypoglycemia | 6,251 (8.3) | 6,126 (8.2) | 0.00 | 7,230 (9.0) | 7,212 (9.0) | 0.00 |
| Other comorbid conditions | ||||||
| Hyperthyroidism | 778 (1.0) | 803 (1.1) | −0.01 | 905 (1.1) | 916 (1.1) | 0.00 |
| Chronic kidney disease stages 3–4 | 5,896 (7.9) | 5,763 (7.7) | 0.01 | 8,636 (10.7) | 8,780 (10.9) | −0.01 |
| Hypertension | 68,604 (91.6) | 68,639 (91.7) | 0.00 | 74,383 (92.4) | 74,397 (92.4) | 0.00 |
| Coronary atherosclerosis | 21,324 (28.5) | 21,302 (28.5) | 0.00 | 22,927 (28.5) | 22,827 (28.4) | 0.00 |
| Cardiac conduction disordersb | 3,157 (4.2) | 3,173 (4.2) | 0.00 | 3,494 (4.3) | 3,537 (4.4) | 0.00 |
| Other cardiac dysrhythmiac | 7,227 (9.7) | 7,229 (9.7) | 0.00 | 7,904 (9.8) | 7,887 (9.8) | 0.00 |
| Cardiomyopathy | 1,764 (2.4) | 1,732 (2.3) | 0.01 | 1,943 (2.4) | 1,922 (2.4) | 0.00 |
| Congestive heart failure | 5,907 (7.9) | 5,865 (7.8) | 0.00 | 6,883 (8.6) | 6,864 (8.5) | 0.00 |
| Valve disorders | 7,508 (10.0) | 7,449 (9.9) | 0.00 | 8,240 (10.2) | 8,240 (10.2) | 0.00 |
| Other cardiovascular diseased | 8,292 (11.1) | 8,237 (11.0) | 0.00 | 9,262 (11.5) | 9,295 (11.6) | 0.00 |
| Transient ischemic attack | 1,710 (2.3) | 1,689 (2.3) | 0.00 | 1,928 (2.4) | 1,917 (2.4) | 0.00 |
| Ischemic stroke | 7,730 (10.3) | 7,758 (10.4) | 0.00 | 8,509 (10.6) | 8,496 (10.6) | 0.00 |
| Peripheral artery disease | 8,505 (11.4) | 8,531 (11.4) | 0.00 | 9,841 (12.2) | 9,853 (12.2) | 0.00 |
| Alcohol abuse or dependence | 860 (1.1) | 854 (1.1) | 0.00 | 832 (1.0) | 815 (1.0) | 0.00 |
| Overweight | 6,133 (8.2) | 6,311 (8.4) | 0.00 | 6,522 (8.1) | 6,483 (8.1) | 0.00 |
| Obesity | 24,452 (32.7) | 24,383 (32.6) | 0.00 | 29,408 (36.5) | 29,319 (36.4) | 0.00 |
| Claims-based frailty index | ||||||
| Non-frail | 25,196 (33.7) | 25,506 (34.1) | −0.01 | 23,802 (29.6) | 23,779 (29.5) | 0.00 |
| Pre-frail | 43,438 (58.0) | 43,094 (57.6) | 0.01 | 48,716 (60.5) | 48,669 (60.5) | 0.00 |
| Frail | 6,234 (8.3) | 6,268 (8.4) | 0.00 | 7,957 (9.9) | 8,027 (10.0) | 0.00 |
| Combined comorbidity index (CCI), mean (SD) | 1.0 (1.8) | 1.0 (1.8) | 0.00 | 1.2 (1.9) | 1.2 (1.9) | 0.00 |
| Use of diabetes drugs | ||||||
| Metformin | 59,258 (79.1) | 59,503 (79.5) | −0.01 | 61,810 (76.8) | 61,816 (76.8) | 0.00 |
| Sulfonylureas | 34,628 (46.3) | 34,814 (46.5) | 0.00 | 39,270 (48.8) | 39,280 (48.8) | 0.00 |
| Insulin | 20,174 (26.9) | 20,112 (26.9) | 0.00 | 25,194 (31.3) | 25,180 (31.3) | 0.00 |
| GLP-1RA | 8,686 (11.6) | 8,114 (10.8) | 0.03 | |||
| DPP-4i | 29,216 (36.3) | 29,545 (36.7) | −0.01 | |||
| Number of antidiabetic medications at index date, mean (SD) | 2.3 (0.9) | 2.3 (0.8) | 0.00 | 2.5 (1.0) | 2.5 (1.0) | 0.00 |
| Other drugs | ||||||
| Antiarrhythmics | 91 (0.1) | 96 (0.1) | 0.00 | 97 (0.1) | 106 (0.1) | 0.00 |
| Anticoagulants | 796 (1.1) | 802 (1.1) | 0.00 | 879 (1.1) | 875 (1.1) | 0.00 |
| Angiotensin converting enzyme inhibitors | 34,519 (46.1) | 34,580 (46.2) | 0.00 | 37,156 (46.2) | 37,080 (46.1) | 0.00 |
| Angiotensin II receptor blockers | 26,959 (36.0) | 26,801 (35.8) | 0.00 | 30,279 (37.6) | 30,455 (37.8) | 0.00 |
| Beta blockers | 31,674 (42.3) | 31,645 (42.3) | 0.00 | 35,054 (43.6) | 34,988 (43.5) | 0.00 |
| Calcium channel blockers | 24,164 (32.3) | 23,959 (32.0) | 0.01 | 26,754 (33.2) | 26,710 (33.2) | 0.00 |
| Antiplatelet agents | 9,711 (13.0) | 9,777 (13.1) | 0.00 | 10,632 (13.2) | 10,646 (13.2) | 0.00 |
| Statins | 57,809 (77.2) | 57,683 (77.0) | 0.00 | 63,174 (78.5) | 63,169 (78.5) | 0.00 |
| Number of total medications, mean (SD) | 12.6 (5.7) | 12.6 (5.8) | 0.00 | 13.6 (6.1) | 13.6 (5.8) | 0.00 |
| Health care utilization | ||||||
| Electrocardiogram | 33,390 (44.6) | 33,344 (44.5) | 0.00 | 36,342 (45.2) | 36,337 (45.2) | 0.00 |
| Cardiac imaging | 17,791 (23.8) | 17,702 (23.6) | 0.00 | 19,578 (24.3) | 19,620 (24.4) | 0.00 |
| HbA1c test order | 72,440 (96.8) | 72,426 (96.7) | 0.01 | 78,054 (97.0) | 78,042 (97.0) | 0.00 |
| Metabolic panel test | 71,881 (96.0) | 71,923 (96.1) | −0.01 | 77,204 (95.9) | 77,246 (96.0) | −0.01 |
| Emergency department visit | 18,739 (25.0) | 18,740 (25.0) | 0.00 | 21,157 (26.3) | 21,214 (26.4) | 0.00 |
| Hospitalization | 7,113 (9.5) | 7,148 (9.5) | 0.00 | 7,880 (9.8) | 7,888 (9.8) | 0.00 |
| Internal medicine visit | 66,533 (88.9) | 66,620 (89.0) | 0.00 | 71,508 (88.9) | 71,537 (88.9) | 0.00 |
| Cardiologist visit | 29,731 (39.7) | 29,597 (39.5) | 0.00 | 32,550 (40.4) | 32,512 (40.4) | 0.00 |
| Endocrinologist visit | 13,832 (18.5) | 13,600 (18.2) | 0.01 | 17,389 (21.6) | 17,450 (21.7) | 0.00 |
| Nephrologist visit | 2,927 (3.9) | 2,958 (4.0) | −0.01 | 4,199 (5.2) | 4,376 (5.4) | −0.01 |
| Number of office visits, mean (SD) | 10.3 (7.1) | 10.3 (7.3) | 0.00 | 11.0 (7.5) | 11.0 (7.4) | 0.00 |
Values are numbers (percentages) unless stated otherwise.
Abbreviations: SGLT-2i, sodium-glucose cotransporter-2 inhibitors; DPP-4i, dipeptidyl peptidase-4 inhibitors; GLP-1RA, glucagon-like peptide-1 receptor agonist; St. D, standardized difference; SD, standard deviation; HbA1c, hemoglobin A1c
Other: Asian, North American Native, Hispanic, and other (plus an unknown category).
Cardiac conduction disorders: atrioventricular block, left bundle branch block, right bundle branch block, et al.
Other cardiac arrhythmia: ventricular tachycardia, premature beats, et al.
Other cardiovascular disease: rheumatic heart disease, pericarditis, myocarditis, et al.
Primary Outcome
Reasons for censoring in the matched cohorts are shown in eTable 5. After PS-matching, there were 1,082 AF hospitalization events amongst SGLT-2i users and 1,410 events amongst DPP-4i users in the SGLT-2i versus DPP-4i cohort (IR 16.8 vs 20.5 per 1000 person-years; HR, 0.82 [95% CI, 0.76–0.89]; RD, −3.7 [95% CI, −5.2 to −2.2] per 1000 person-years), and 1,175 events amongst SGLT-2i users and 1,235 events amongst GLP-1RA users in the SGLT-2i versus GLP-1RA cohort (17.0 vs 18.7 per 1000 person-years; HR, 0.90 [95% CI, 0.83–0.98]; RD, −1.8 [95% CI, −3.2 to −0.3] per 1000 person-years), as shown in Table 2.
Table 2.
Number of events, incidence rate, hazard ratios, and rate differences for primary and secondary outcomes in 1:1 propensity score matched initiators of SGLT-2i versus DPP-4i or GLP-1RA.
| Outcomes | SGLT-2i | DPP-4i | SGLT-2i vs DPP-4i (n=74,868 pairs) | SGLT-2i | GLP-1RA | SGLT-2i vs GLP-1RA (n=80,475 pairs) | ||
|---|---|---|---|---|---|---|---|---|
| Primary Outcome | N events (IR) | N events (IR) | HR (95% CI) | RD (95% CI) | N events (IR) | N events (IR) | HR (95% CI) | RD (95% CI) |
| AF hospitalization | 1,082 (16.8) | 1,410 (20.5) | 0.82 (0.76, 0.89) | −3.7 (−5.2 to −2.2) | 1,175 (17.0) | 1,235 (18.7) | 0.90 (0.83, 0.98) | −1.8 (−3.2 to −0.3) |
| Secondary Outcomes | ||||||||
| AF diagnosis | 1,354 (21.1) | 1,707 (24.9) | 0.85 (0.79, 0.91) | −3.8 (−5.5 to −2.2) | 1,438 (20.8) | 1,562 (23.8) | 0.87 (0.81, 0.94) | −3.0 (−4.6 to −1.4) |
| AF treated with medication | 1,098 (17.1) | 1,339 (19.5) | 0.88 (0.81, 0.95) | −2.4 (−3.9 to −1.0) | 1,143 (16.5) | 1,281 (19.5) | 0.85 (0.78, 0.92) | −3.0 (−4.4 to −1.5) |
| Hospitalization for AF | 212 (3.3) | 251 (3.6) | 0.91 (0.75, 1.09) | −0.4 (−1.0 to 0.3) | 211 (3.0) | 275 (4.1) | 0.73 (0.61, 0.87) | −1.3 (−1.9 to −0.8) |
| Stroke or transient ischemic attack (stroke/TIA) | 536 (8.3) | 662 (9.5) | 0.86 (0.77, 0.96) | −1.3 (−2.3 to −0.3) | 587 (8.4) | 552 (8.3) | 1.01 (0.90, 1.14) | 0.1 (0.9 to 1.1) |
| Hospitalization for heart failure (HHF) | 312 (4.8) | 684 (9.9) | 0.49 (0.43, 0.56) | −5.1 (−6.0 to −4.1) | 388 (5.6) | 519 (7.8) | 0.71 (0.62, 0.81) | −2.3 (−3.1 to −1.4) |
| AF hospitalization censored for HF hospitalization | 988 (13.2) | 1,215 (16.2) | 0.86 (0.79, 0.93) | −2.6 (−4.0 to −1.2) | 1,089 (13.5) | 1,122 (13.9) | 0.92 (0.84, 1.00) | −3.1 (−4.2 to −2.0) |
| Hospitalization for AF and heart failure (AF + HF) | 376 (5.8) | 574 (8.3) | 0.70 (0.62, 0.80) | −2.5 (−3.4 to −1.6) | 464 (6.7) | 525 (7.9) | 0.92 (0.83, 1.01) | −1.3 (−2.2 to −0.4) |
Abbreviations: SGLT-2i, sodium-glucose cotransporter-2 inhibitors; DPP-4i, dipeptidyl peptidase-4 inhibitors; GLP-1RA, glucagon-like peptide-1 receptor agonist; N events, number of events; IR, incidence rate in 1000 patient years; HR, hazard ratio; CI, confidence interval; RD, rate difference in 1000 patient years; AF, atrial fibrillation; HF, heart failure.
Kaplan-Meier plots comparing the cumulative incidence of AF over time in the matched groups were shown in Figures 1 and 2.
Figure 1. Kaplan-Meier plots for cumulative incidence of AF in propensity score matched SGLT-2i versus DPP-4i cohort.
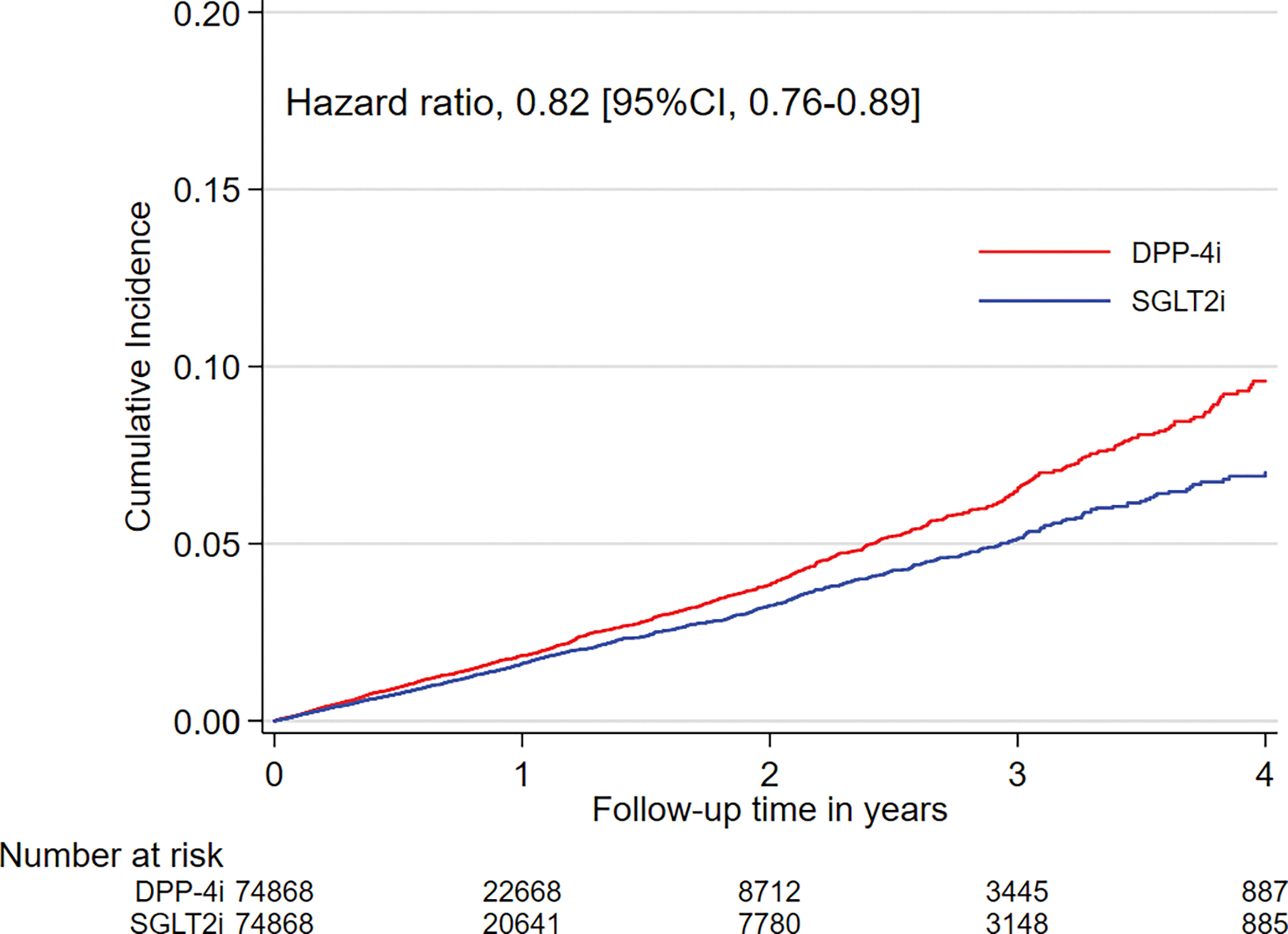
Abbreviations: AF, atrial fibrillation; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; DPP-4i, dipeptidyl peptidase-4 inhibitors; CI, confidence interval. The graphs were truncated at four years of follow-up.
Figure 2. Kaplan-Meier plots for cumulative incidence of AF in propensity score matched SGLT-2i versus GLP-1RA cohort.
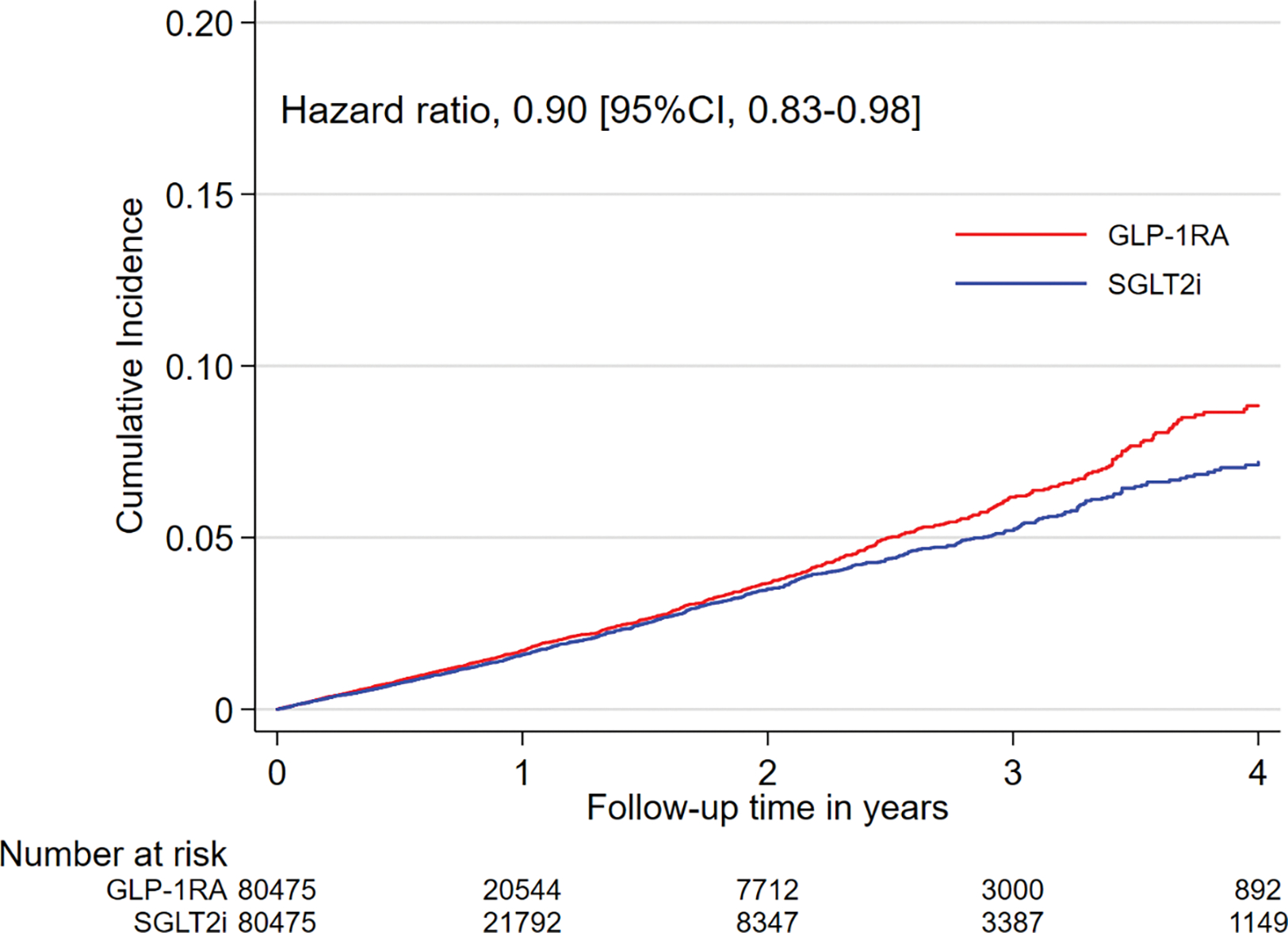
Abbreviations: AF, atrial fibrillation; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; GLP-1RA, glucagon-like peptide-1 receptor agonist; CI, confidence interval. The graphs were truncated at four years of follow-up.
Sensitivity and Subgroup Analyses
We performed several sensitivity analyses to assess the robustness of our primary study findings, which produced consistent results. The expected null association with SGLT-2i and the risk for herpes zoster was correctly estimated (eTable 6). There was no evidence of effect heterogeneity in the association between SGLT-2i and incident AF by age, gender, history of HF, or history of ASCVD (Figure 3).
Figure 3. Subgroup analyses for incident AF in matched SGLT-2i versus DPP-4i cohort (A) and SGLT-2i versus GLP-1RA cohort (B).
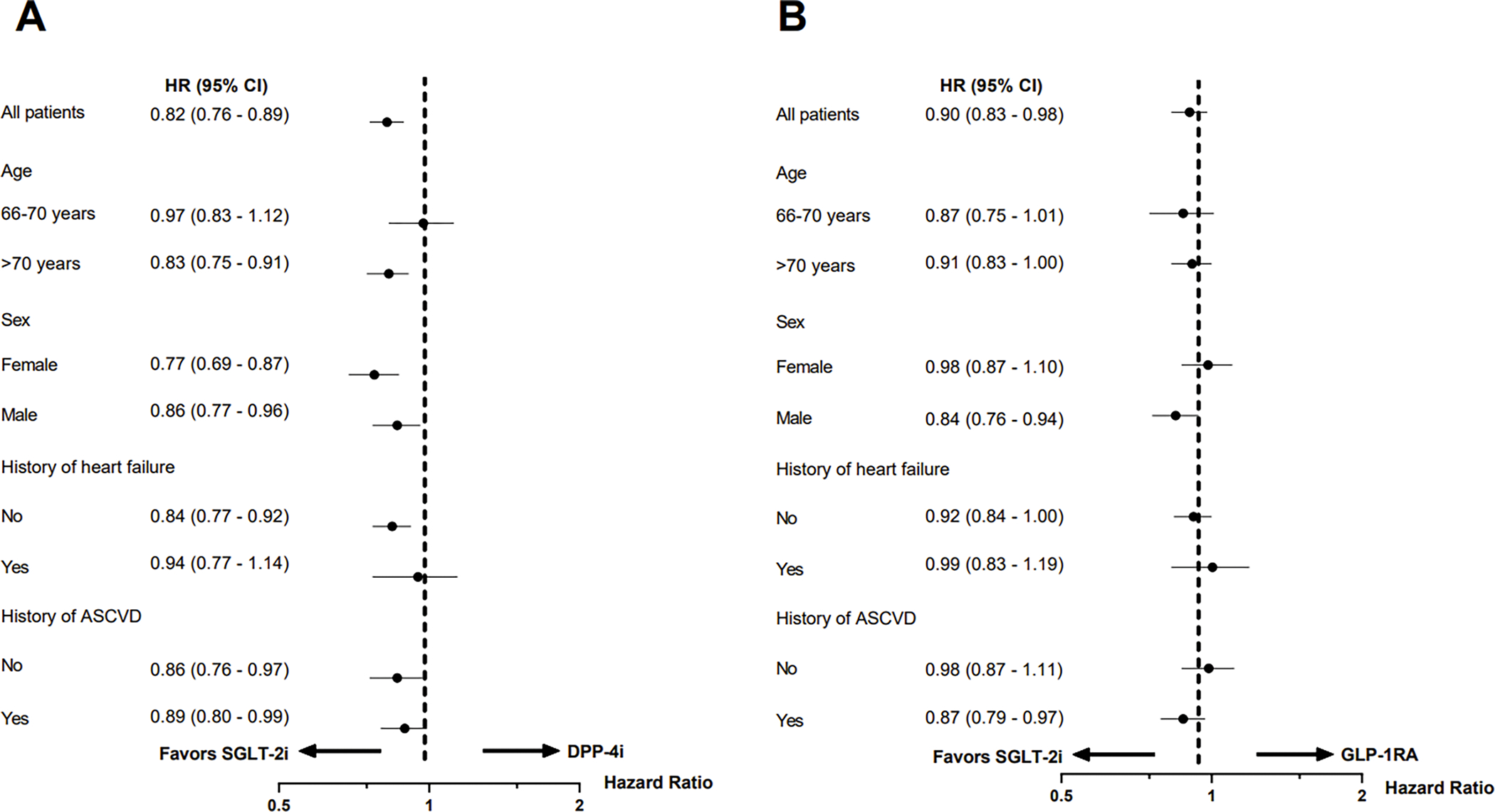
Abbreviations: AF, atrial fibrillation; SGLT-2i, sodium-glucose cotransporter-2 inhibitors; DPP-4i, dipeptidyl peptidase-4 inhibitors; GLP-1RA, glucagon-like peptide-1 receptor agonist; HR, hazard ratio; CI, confidence interval; ASCVD, atherosclerotic cardiovascular disease.
Secondary Outcomes
Risks for AF diagnosis, AF treated with medication, and hospitalization for AF were also consistently lower for SGLT-2i users compared to matched DPP-4i users (HR, 0.85 [95% CI, 0.79–0.91]; HR, 0.88 [95% CI, 0.81–0.95]; HR, 0.91 [95% CI, 0.75–1.09], respectively) and GLP-1RA users (HR, 0.87 [95% CI, 0.81–0.94]; HR, 0.85 [95% CI, 0.78–0.92]; HR, 0.73 [95% CI, 0.61–0.87], respectively) (Table 2). SGLT-2i were associated with a lower risk of stroke/TIA (HR, 0.86 [95% CI, 0.77–0.96]), HHF (HR, 0.49 [95% CI, 0.43–0.56]), AF hospitalization censored for HF (HR, 0.86 [95% CI, 0.79–0.93]), and AF + HF (HR, 0.70 [95% CI, 0.62–0.80]) compared to DPP-4i. Compared to GLP-1RA, SGLT-2i were associated with a similar risk of stroke/TIA (HR, 1.01 [95% CI, 0.90–1.14]), a reduced risk of HHF (HR, 0.71 [95% CI, 0.62–0.81]), AF hospitalization censored for HF (HR, 0.92 [95% CI, 0.84–1.00]), and AF + HF (HR, 0.70 [95% CI, 0.62–0.80].
DISCUSSION
In this nationwide cohort study including more than 200,000 routine-care older patients with T2D, after PS-matching, we found that the initiation of SGLT-2i was associated with an 18% reduction in the risk of incident AF compared to DPP-4i and a 10% reduction compared to GLP-1RA. Study findings were robust to a range of predefined sensitivity analyses and did not appear to differ substantially across subgroups. SGLT-2i were associated with a lower risk of stroke/TIA compared to DPP-4i. Patients initiating SGLT-2i also experienced a decreased risk of HHF, AF hospitalization censored for HF, and AF + HF compared to DPP-4i or GLP-1RA initiation.
SGLT-2i have led to a paradigm shift in the management of T2D. However, the effects of SGLT-2i on incident AF in patients with T2D remained unclear from large RCTs,7,8,11 and meta-analyses suggested either no effect or possible protective effects of SGLT-2i against AF.13–18 Notably, patients with baseline AF were not excluded from RCTs and AF events were documented as serious adverse events, rather than being a pre-specified endpoint. In addition, patients older than 65 years with multiple co-morbidities, who are the patients at the greatest risk for AF, were not meaningfully represented in these RCTs. Similarly, the association between SGLT-2i use and the risk for AF in routine practice has been primarily evaluated among patients younger than 65 years. In an observational study including patients with T2D in Nordic countries, among 1:3 PS-matched new users (mean age 61 years) of dapagliflozin (n = 10,227) or DPP-4i, dapagliflozin was associated with a similar risk of AF as DPP-4i (HR, 0.92 [95% CI, 0.76–1.12]).36 A cohort study from Taiwan found that patients with T2D who were prescribed an SGLT-2i (72% aged <60 years) had a similar risk of AF compared with 1:1 PS-matched patients not taking an SGLT-2i.37 Another cohort study showed that SGLT-2i (n = 15,606) were associated with a decreased risk of incident AF (HR, 0.61 [95% CI, 0.50–0.73]) compared with PS-weighted DPP-4i users (mean age 60 years).38 Large population-based studies, which specifically focus on older adults with multiple co-morbidities, are lacking. In order to fill these knowledge gaps, we used a large nationwide sample drawn from 100% U.S. Medicare data, which include health care information on the vast majority of legal U.S. residents aged 65 years and older leading to high precision of the estimates and generalizability. The absolute rate reduction in incident AF we observed among SGLT-2i users in the SGLT-2i vs. DPP-4i cohort corresponds to a number needed to treat39 for preventing an additional AF event of 435 at six months and 250 at 12 months after treatment initiation. In the SGLT-2i vs GLP-1RA group, the number needed to treat39 for preventing an additional AF event at six months and 12 months after treatment initiation was 588 and 263, respectively.
Our study has important clinical implications. The latest clinical guidelines for the treatment of T2D recommend SGLT-2i, DPP-4i, and GLP-1RA as second-line therapies, and suggest the choice among these medications should be based on patient-specific characteristics, e.g., history of cardiovascular disease.40 Notably, T2D affects 27% of U.S. adults aged 65 years or older.1 T2D per se is a risk factor for AF,3 as is older age, with each decade of advancing age increasing the risk of AF by more than two-fold.3 All treatments for AF are associated with risk, and a number are also associated with high cost.41 Therefore, a glucose-lowering medication preventing AF would be advantageous for older adults with T2D. To our knowledge, our study is the first real-world investigation to describe the risk of AF in older patients (mean age 72 years) with T2D who were started on an SGLT-2i. Our results are consistent with a previous study using the Food and Drug Administration adverse event reporting system (FAERS), which supports a protective role of SGLT-2i against the occurrence of AF.42
To date, the mechanisms through which SGLT-2i could reduce the risk of AF are still under investigation. In addition to potential AF protection through the reduction in risk of HF and atrial stretch, experimental and clinical data have suggested several explanations. It has been postulated that SGLT-2i reduce electrical and structural remodeling of the atrium,43–45 as well as attenuate the late sodium current-induced calcium overload and arrhythmogenesis.46 Furthermore, SGLT-2i improve mitochondrial function,47 reverse diabetes-induced sodium/hydrogen exchanger hyperactivity and oxidative stress, stimulate adenosine monophosphate-activated protein kinase activation, and enhance myocardial energetics.46,48,49 Lastly, SGLT-2i ameliorate many risk factors associated with AF development, such as obesity, hypertension, and hyperglycemia.16 Further research is needed to better elucidate the mechanisms of protection against incident AF associated with SGLT-2i.
Our study has limitations. First, residual confounding by unmeasured factors cannot be ruled out. For example, socioeconomic status might affect the choice of diabetes treatments due to the Medicare reimbursement system,50,51 and lower socioeconomic status is associated with higher risk of AF.52 Despite extensive through propensity score adjustment for many measured confounders and confounder proxies, including previous use of generic and brand medications, it is possible that residual imbalances in socioeconomic status across treatment groups may still exist. Our Medicare dataset lacked laboratory results, which limited our ability to match for baseline glucose control and renal function. However, a previous study has suggested PS-matching on claims-based data could achieve balance in unmeasured characteristics, such as hemoglobin A1c.53 In addition, along with the changing guidelines, patients with HF were more likely to receive SGLT-2i in more recent years.54,55 Although our PS model included the year of cohort entry, cardiac and renal comorbidities, we do not have data on ejection fraction or New York Heart Association (NYHA) functional class. Such potential residual imbalances in HF severity, however, would have disfavored SGLT-2i, resulting in conservative estimates. Second, we chose two commonly used second-line novel antidiabetic drug classes, DPP-4i and GLP-1RA, as active comparators. However, the association between DPP-4i or GLP-1RA and the risk of incident AF remains inconclusive.18,56–59 Beyond anti-metabolic effects, GLP-1RA might prevent AF through anti-inflammatory effect, inhibition of vascular smooth muscle cell proliferation, and reducing cardiovascular events.60–62 However, GLP-1RA may have a direct effect at the sinus node, increase heart rate, and raise the possibility of an increased risk of AF.63 Third, our study had a median follow-up time of less than one year. The long-term effect of SGLT-2i use on AF remains undetermined. Nevertheless, the large size of our study population allowed us to generate results with high precision despite the relatively short follow-up duration compared to RCTs. Fourth, the magnitude of the observed absolute risk reduction in AF associated with SGLT-2i was not large. Health care professionals need to consider the absolute effect when making comparative therapeutic decisions. Finally, our findings may not be generalizable to younger adults with T2D.
CONCLUSION
In this large population-based cohort including more than 200,000 PS-matched older adults with T2D, initiating treatment with SGLT-2i, as compared with DPP-4i and GLP-1RA, was associated with an 18% and 10% reduction in the risk of incident AF, respectively. Our data suggest the initiation of SGLT-2i may be beneficial in older adults with T2D who are at risk of AF in clinical practice. Besides proven cardiovascular and renal benefits, the potential prevention of SGLT-2i in incident AF among older adults with T2D might be considered.
Supplementary Material
eTable 1. Definition of inclusion and exclusion criteria
eTable 2. Outcome definitions
eFigure 1. Flowchart of patients included in SGLT-2i versus DPP-4i cohort.
eFigure 2. Flowchart of patients included in SGLT-2i versus GLP-1RA cohort.
eTable 3. Baseline characteristics of SGLT-2i versus DPP-4i cohort before and after 1:1 propensity score matching.
eTable 4. Baseline characteristics of SGLT-2i versus GLP-1RA cohort before and after 1:1 propensity score matching.
eTable 5. Reasons for censoring in 1:1 propensity score matched cohorts
eTable 6. Number of events, incidence rate, hazard ratios for sensitivity analyses in 1:1 propensity score matched cohorts
Key points.
Question: Do sodium-glucose cotransporter-2 inhibitors (SGLT-2i) reduce the risk of atrial fibrillation (AF) in older patients with type 2 diabetes (T2D)?
Findings: In this nationwide cohort study, we assessed the risk of AF among Medicare beneficiaries with T2D. After propensity score matching, the initiation of SGLT-2i was associated with an 18% and 10% decrease in the risk of AF compared with dipeptidyl peptidase 4 inhibitor (DPP-4i) or glucagon-like peptide 1 receptor agonist (GLP-1RA), respectively.
Meaning: The use of SGLT-2i was associated with a reduced risk of AF in older adults with type 2 diabetes.
Acknowledgments:
Sources of Funding: This study was funded by the Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA. MZ was supported by a NIH NIDDK T32 award DK007199. EP was supported by grants from the National Institute on Aging (K08AG055670), the Patient Centered Outcomes Research Institute (DB-2020C2–20326), and the Food and Drug Administration (5U01FD007213). These funders had no role in study design, data collection, analysis, reporting, or the decision to submit for publication.
Footnotes
Disclosures: ED and JP have nothing to disclose. MZ is a full-time employee of Visterra since May 2022. DW reports serving on Data Monitoring Committees for Novo Nordisk (oral and subcutaneous semaglutide), not directly related to the topic of the submitted work. BE reports consulting income from Eli Lilly & Company, Gilead Sciences, Inc., Johnson and Johnson, Provention Bio, and a research award from Novo Nordisk, not related to the topic of the submitted work. RJG reports grants from Amarin, Kowa, Novartis, and Pfizer outside the submitted work. SK has received research support to the Brigham and Women’s Hospital from Pfizer, AbbVie, Roche and Bristol-Myers Squibb for unrelated studies. EP is co-investigator of a research grant to the Brigham and Women’s Hospital from Boehringer-Ingelheim, outside the submitted work.
Access to data and data analysis: MZ and EP had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References:
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2020. Atlanta, GA; 2020. [Google Scholar]
- 2.Huxley RR, Alonso A, Lopez FL, et al. Type 2 diabetes, glucose homeostasis and incident atrial fibrillation: the Atherosclerosis Risk in Communities study. Heart. 2012;98(2):133–138. doi: 10.1136/heartjnl-2011-300503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271(11):840–844. [PubMed] [Google Scholar]
- 4.Aksnes TA, Schmieder RE, Kjeldsen SE, Ghani S, Hua TA, Julius S. Impact of new-onset diabetes mellitus on development of atrial fibrillation and heart failure in high-risk hypertension (from the VALUE Trial). Am J Cardiol. 2008;101(5):634–638. doi: 10.1016/j.amjcard.2007.10.025 [DOI] [PubMed] [Google Scholar]
- 5.Fatemi O, Yuriditsky E, Tsioufis C, et al. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study). Am J Cardiol. 2014;114(8):1217–1222. doi: 10.1016/j.amjcard.2014.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet (London, England). 2009;373(9665):739–745. doi: 10.1016/S0140-6736(09)60443-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):1–12. doi: 10.1056/NEJMoa1504720 [DOI] [PubMed] [Google Scholar]
- 8.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017;377(7):644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 9.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):NEJMoa1811744. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 10.Zelniker TA, Bonaca MP, Furtado R, et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients with Type 2 Diabetes Mellitus: Insights from the DECLARE-TIMI 58 Trial. Circulation. 2020;(617). doi: 10.1161/circulationaha.119.044183 [DOI] [PubMed] [Google Scholar]
- 11.McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 2019:1–13. doi: 10.1056/nejmoa1911303 [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z, Lindley RI, Rådholm K, et al. Canagliflozin and Stroke in Type 2 Diabetes Mellitus. Stroke. 2019;50(2):396–404. doi: 10.1161/STROKEAHA.118.023009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z, Jardine MJ, Li Q, et al. Effect of SGLT2 Inhibitors on Stroke and Atrial Fibrillation in Diabetic Kidney Disease: Results From the CREDENCE Trial and Meta-Analysis. Stroke. 2021;52(5):1545–1556. doi: 10.1161/STROKEAHA.120.031623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Usman MS, Siddiqi TJ, Memon MM, et al. Sodium-glucose co-transporter 2 inhibitors and cardiovascular outcomes: A systematic review and meta-analysis. Eur J Prev Cardiol. 2018;25(5):495–502. doi: 10.1177/2047487318755531 [DOI] [PubMed] [Google Scholar]
- 15.Li W-J, Chen X-Q, Xu L-L, Li Y-Q, Luo B-H. SGLT2 inhibitors and atrial fibrillation in type 2 diabetes: a systematic review with meta-analysis of 16 randomized controlled trials. Cardiovasc Diabetol 2020;19(1):130. doi: 10.1186/s12933-020-01105-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okunrintemi V, Mishriky BM, Powell JR, Cummings DM. Sodium-glucose co-transporter-2 inhibitors and atrial fibrillation in the cardiovascular and renal outcome trials. Diabetes Obes Metab. 2021;23(1):276–280. doi: 10.1111/dom.14211 [DOI] [PubMed] [Google Scholar]
- 17.Pandey AK, Okaj I, Kaur H, et al. Sodium-Glucose Co-Transporter Inhibitors and Atrial Fibrillation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2021;10(17):e022222. doi: 10.1161/JAHA.121.022222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Yu X, Zheng Y, et al. Association of glucose-lowering medications with cardiovascular outcomes: an umbrella review and evidence map. lancet Diabetes Endocrinol. 2020;8(3):192–205. doi: 10.1016/S2213-8587(19)30422-X [DOI] [PubMed] [Google Scholar]
- 19.Granger CB, Mahaffey KW. Preventing Atrial Fibrillation With Treatments for Diabetes Mellitus. Circulation. 2020;141(15):1235–1237. doi: 10.1161/CIRCULATIONAHA.120.045864 [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association. ADA Standards of Diabetes Care 2015. Diabetes Care. 2015;38(January):S1–S2. doi: 10.2337/dc15-S001 [DOI] [Google Scholar]
- 21.Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American association of clinical endocrinologists and american college of endocrinology - clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2015;21 Suppl 1(Suppl 1):1–87. doi: 10.4158/EP15672.GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Silva KM, Cromer SJ, Yu EW, Fischer M, Kim SC. Risk of Incident Atrial Fibrillation With Zoledronic Acid Versus Denosumab: A Propensity Score-Matched Cohort Study. J bone Miner Res Off J Am Soc Bone Miner Res. 2021;36(1):52–60. doi: 10.1002/jbmr.4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glazer NL, Dublin S, Smith NL, et al. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med. 2007;167(3):246–252. doi: 10.1001/archinte.167.3.246 [DOI] [PubMed] [Google Scholar]
- 24.Lee MP, Desai RJ, Jin Y, Brill G, Ogdie A, Kim SC. Association of Ustekinumab vs TNF Inhibitor Therapy With Risk of Atrial Fibrillation and Cardiovascular Events in Patients With Psoriasis or Psoriatic Arthritis. JAMA dermatology. 2019;155(6):700–707. doi: 10.1001/jamadermatol.2019.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949–955.e3. doi: 10.1016/j.ahj.2013.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim SC, Liu J, Solomon DH. Risk of incident atrial fibrillation in gout: a cohort study. Ann Rheum Dis. 2016;75(8):1473–1478. doi: 10.1136/annrheumdis-2015-208161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim DH, Schneeweiss S, Glynn RJ, Lipsitz LA, Rockwood K, Avorn J. Measuring Frailty in Medicare Data: Development and Validation of a Claims-Based Frailty Index. J Gerontol A Biol Sci Med Sci. 2018;73(7):980–987. doi: 10.1093/gerona/glx229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–759. doi: 10.1016/j.jclinepi.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ripollone JE, Huybrechts KF, Rothman KJ, Ferguson RE, Franklin JM. Implications of the Propensity Score Matching Paradox in Pharmacoepidemiology. Am J Epidemiol. 2018;187(9):1951–1961. doi: 10.1093/aje/kwy078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franklin JM, Rassen JA, Ackermann D, Bartels DB, Schneeweiss S. Metrics for covariate balance in cohort studies of causal effects. Stat Med. 2014;33(10):1685–1699. doi: 10.1002/sim.6058 [DOI] [PubMed] [Google Scholar]
- 31.Xu Y, Cheung YB, Lam KF, Tan SH, Milligan P. A simple approach to the estimation of incidence rate difference. Am J Epidemiol. 2010;172(3):334–343. doi: 10.1093/aje/kwq099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patorno E, Htoo PT, Glynn RJ, et al. Sodium-Glucose Cotransporter-2 Inhibitors Versus Glucagon-like Peptide-1 Receptor Agonists and the Risk for Cardiovascular Outcomes in Routine Care Patients With Diabetes Across Categories of Cardiovascular Disease. Ann Intern Med. 2021;174(11):1528–1541. doi: 10.7326/M21-0893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aetion Evidence Platform (2020). Software for real-world data analysis. http://aetion.com.
- 34.Fralick M, Kesselheim AS, Avorn J, Schneeweiss S. Use of Health Care Databases to Support Supplemental Indications of Approved Medications. JAMA Intern Med. 2018;178(1):55–63. doi: 10.1001/jamainternmed.2017.3919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patorno E, Pawar A, Franklin JM, et al. Empagliflozin and the Risk of Heart Failure Hospitalization in Routine Clinical Care: A First Analysis from the EMPRISE Study. Circulation. 2019;139(25):2822–2830. doi: 10.1161/CIRCULATIONAHA.118.039177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Persson F, Nyström T, Jørgensen ME, et al. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: A multinational observational study. Diabetes Obes Metab. 2018;20(2):344–351. doi: 10.1111/dom.13077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen HY, Huang JY, Siao WZ, Jong GP. The association between SGLT2 inhibitors and new-onset arrhythmias: A nationwide population-based longitudinal cohort study. Cardiovasc Diabetol. 2020;19(1):1–8. doi: 10.1186/s12933-020-01048-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ling AW-C, Chan C-C, Chen S-W, et al. The risk of new-onset atrial fibrillation in patients with type 2 diabetes mellitus treated with sodium glucose cotransporter 2 inhibitors versus dipeptidyl peptidase-4 inhibitors. Cardiovasc Diabetol. 2020;19(1):188. doi: 10.1186/s12933-020-01162-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ. 1999;319(7223):1492–1495. doi: 10.1136/bmj.319.7223.1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Diabetes Association. ADA Standards of Diabetes Care 2021. Diabetes Care. 2021;44(Supplement1). [Google Scholar]
- 41.O’Keefe EL, Sturgess JE, O’Keefe JH, Gupta S, Lavie CJ. Prevention and Treatment of Atrial Fibrillation via Risk Factor Modification. Am J Cardiol. September 2021. doi: 10.1016/j.amjcard.2021.08.042 [DOI] [PubMed] [Google Scholar]
- 42.Frias JP, Bonora E, Ruiz LN, et al. Efficacy and safety of dulaglutide 3.0 mg and 4.5 mg versus dulaglutide 1.5 mg in metformin-treated patients with type 2 diabetes in a randomized controlled trial (award-11). Diabetes Care. 2021;44(3):765–773. doi: 10.2337/dc20-1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shao Q, Meng L, Lee S, et al. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc Diabetol. 2019;18(1):165. doi: 10.1186/s12933-019-0964-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verma S, Mazer CD, Yan AT, et al. Effect of Empagliflozin on Left Ventricular Mass in Patients With Type 2 Diabetes Mellitus and Coronary Artery Disease: The EMPA-HEART CardioLink-6 Randomized Clinical Trial. Circulation. 2019;140(21):1693–1702. doi: 10.1161/CIRCULATIONAHA.119.042375 [DOI] [PubMed] [Google Scholar]
- 45.Sato T, Aizawa Y, Yuasa S, et al. The effect of dapagliflozin treatment on epicardial adipose tissue volume. Cardiovasc Diabetol. 2018;17(1):6. doi: 10.1186/s12933-017-0658-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee T-I, Chen Y-C, Lin Y-K, et al. Empagliflozin Attenuates Myocardial Sodium and Calcium Dysregulation and Reverses Cardiac Remodeling in Streptozotocin-Induced Diabetic Rats. Int J Mol Sci. 2019;20(7). doi: 10.3390/ijms20071680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yurista SR, Silljé HHW, Rienstra M, de Boer RA, Westenbrink BD. Sodium-glucose co-transporter 2 inhibition as a mitochondrial therapy for atrial fibrillation in patients with diabetes? Cardiovasc Diabetol. 2020;19(1):5. doi: 10.1186/s12933-019-0984-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santos-Gallego CG, Requena-Ibanez JA, San Antonio R, et al. Empagliflozin Ameliorates Adverse Left Ventricular Remodeling in Nondiabetic Heart Failure by Enhancing Myocardial Energetics. J Am Coll Cardiol. 2019;73(15):1931–1944. doi: 10.1016/j.jacc.2019.01.056 [DOI] [PubMed] [Google Scholar]
- 49.Lee T-W, Lee T-I, Lin Y-K, Chen Y-C, Kao Y-H, Chen Y-J. Effect of antidiabetic drugs on the risk of atrial fibrillation: mechanistic insights from clinical evidence and translational studies. Cell Mol Life Sci. 2021;78(3):923–934. doi: 10.1007/s00018-020-03648-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luo J, Feldman R, Rothenberger SD, Hernandez I, Gellad WF. Coverage, Formulary Restrictions, and Out-of-Pocket Costs for Sodium-Glucose Cotransporter 2 Inhibitors and Glucagon-Like Peptide 1 Receptor Agonists in the Medicare Part D Program. JAMA Netw open. 2020;3(10):e2020969. doi: 10.1001/jamanetworkopen.2020.20969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gokhale M, Dusetzina SB, Pate V, et al. Decreased Antihyperglycemic Drug Use Driven by High Out-of-Pocket Costs Despite Medicare Coverage Gap Closure. Diabetes Care. 2020;43(9):2121–2127. doi: 10.2337/dc19-1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramkumar S, Ochi A, Yang H, et al. Association between socioeconomic status and incident atrial fibrillation. Intern Med J. 2019;49(10):1244–1251. doi: 10.1111/imj.14214 [DOI] [PubMed] [Google Scholar]
- 53.Patorno E, Gopalakrishnan C, Franklin JM, et al. Claims-based studies of oral glucose-lowering medications can achieve balance in critical clinical variables only observed in electronic health records. Diabetes, Obes Metab. 2018. doi: 10.1111/dom.13184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.American Diabetes Association. Standards of Medical Care in Diabetes—2013. Diabetes Care. 2012;36(Supplement_1):S11–S66. doi: 10.2337/dc13-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(December):dci180033. doi: 10.2337/DCI18-0033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nreu B, Dicembrini I, Tinti F, Sesti G, Mannucci E, Monami M. Major cardiovascular events, heart failure, and atrial fibrillation in patients treated with glucagon-like peptide-1 receptor agonists: An updated meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2020;30(7):1106–1114. doi: 10.1016/j.numecd.2020.03.013 [DOI] [PubMed] [Google Scholar]
- 57.Shi W, Zhang W, Zhang D, et al. Comparison of the effect of glucose-lowering agents on the risk of atrial fibrillation: A network meta-analysis. Hear Rhythm. 2021;18(7):1090–1096. doi: 10.1016/j.hrthm.2021.03.007 [DOI] [PubMed] [Google Scholar]
- 58.Liou Y-S, Yang F-Y, Chen H-Y, Jong G-P. Antihyperglycemic drugs use and new-onset atrial fibrillation: A population-based nested case control study. PLoS One. 2018;13(8):e0197245. doi: 10.1371/journal.pone.0197245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fauchier G, Bisson A, Bodin A, et al. Glucose-lowering drug use and new-onset atrial fibrillation in patients with diabetes mellitus. Diabetologia. 2021;64(11):2602–2605. doi: 10.1007/s00125-021-05551-y [DOI] [PubMed] [Google Scholar]
- 60.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet. 2019;6736(19):1–8. doi: 10.1016/S0140-6736(19)31150-X [DOI] [PubMed] [Google Scholar]
- 61.Scheen AJ, Esser N, Paquot N. Antidiabetic agents: Potential anti-inflammatory activity beyond glucose control. Diabetes Metab. 2015;41(3):183–194. doi: 10.1016/j.diabet.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 62.Nagayama K, Kyotani Y, Zhao J, et al. Exendin-4 Prevents Vascular Smooth Muscle Cell Proliferation and Migration by Angiotensin II via the Inhibition of ERK1/2 and JNK Signaling Pathways. PLoS One. 2015;10(9):e0137960. doi: 10.1371/journal.pone.0137960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakatani Y, Kawabe A, Matsumura M, et al. Effects of GLP-1 Receptor Agonists on Heart Rate and the Autonomic Nervous System Using Holter Electrocardiography and Power Spectrum Analysis of Heart Rate Variability. Diabetes Care. 2016;39(2):e22–3. doi: 10.2337/dc15-1437 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Definition of inclusion and exclusion criteria
eTable 2. Outcome definitions
eFigure 1. Flowchart of patients included in SGLT-2i versus DPP-4i cohort.
eFigure 2. Flowchart of patients included in SGLT-2i versus GLP-1RA cohort.
eTable 3. Baseline characteristics of SGLT-2i versus DPP-4i cohort before and after 1:1 propensity score matching.
eTable 4. Baseline characteristics of SGLT-2i versus GLP-1RA cohort before and after 1:1 propensity score matching.
eTable 5. Reasons for censoring in 1:1 propensity score matched cohorts
eTable 6. Number of events, incidence rate, hazard ratios for sensitivity analyses in 1:1 propensity score matched cohorts


