Abstract
Recombinant zoster vaccine (RZV) (Shingrix; GlaxoSmithKline, Brentford, United Kingdom) is an adjuvanted glycoprotein vaccine that was licensed in 2017 to prevent herpes zoster (shingles) and its complications in older adults. In this prospective, postlicensure Vaccine Safety Datalink study using electronic health records, we sequentially monitored a real-world population of adults aged ≥50 years who received care in multiple US Vaccine Safety Datalink health systems to identify potentially increased risks of 10 prespecified health outcomes, including stroke, anaphylaxis, and Guillain-Barré syndrome (GBS).Among 647,833 RZV doses administered from January 2018 through December 2019, we did not detect a sustained increased risk of any monitored outcome for RZV recipients relative to either historical (2013–2017) recipients of zoster vaccine live, a live attenuated virus vaccine (Zostavax; Merck & Co., Inc., Kenilworth, New Jersey), or contemporary non-RZV vaccine recipients who had an annual well-person visit during the 2018–2019 study period. We confirmed prelicensure trial findings of increased risks of systemic and local reactions following RZV. Our study provides additional reassurance about the overall safety of RZV. Despite a large sample, uncertainty remains regarding potential associations with GBS due to the limited number of confirmed GBS cases that were observed.
Keywords: herpes zoster, managed-care programs, population surveillance, sequential analysis, shingles, vaccine safety, vaccines, combined, zoster vaccine
Recombinant zoster vaccine (RZV) (Shingrix; GlaxoSmithKline, Brentford, United Kingdom) was licensed in October 2017 by the Food and Drug Administration for use in immunocompetent US adults aged ≥50 years for prevention of herpes zoster (i.e., shingles) and its complications (1). It is given as a 2-dose series at least 2 months apart and contains recombinant varicella-zoster virus glycoprotein E and a novel adjuvant (AS01B) (1). In 2018, the Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices recommended (2) that RZV be preferentially given over the live attenuated virus vaccine (zoster vaccine live (ZVL); Zostavax; Merck & Co., Inc., Kenilworth, New Jersey) licensed in 2006 (3), given RZV’s high initial efficacy against herpes zoster (>90%) in comparison with ZVL (51.3%) and ZVL’s rapid waning (4–8). RZV has since replaced the use of ZVL for herpes zoster prevention in the United States, since ZVL is no longer sold in the United States (9).
Initial safety data for RZV have generally not raised concerns, but these data are limited. Data have been derived primarily from prelicensure trials (4, 5, 10) and postlicensure passive reports submitted by health-care providers, vaccinees, and others to GlaxoSmithKline (11) and to the CDC and the Food and Drug Administration (12) via the Vaccine Adverse Event Reporting System (13). Pooled placebo-controlled trial data identified increased risks of local and systemic reactions following RZV administration and found no differences in risks of other adverse events but lacked statistical power (4, 5, 10). Early data-mining analyses of passive reports produced similar findings: Serious adverse events were rare, and no disproportionate reporting of any serious adverse event associated with RZV was observed (11, 12). However, known data-quality issues, like underreporting, limit the strength of conclusions that can be drawn from such sources (13). More recently, the Food and Drug Administration reported a potential increase in the risk of Guillain-Barré syndrome (GBS) based on results of a self-controlled case-series analysis in a Medicare population of persons aged ≥65 years (14), but the estimated attributable risk was small (about 3 per million RZV doses).
We conducted active postlicensure surveillance, involving proactive capture and rapid analysis of data from large health-care systems, to provide a timely, targeted assessment of RZV safety during its initial uptake period that addressed gaps in safety evidence from prelicensure randomized clinical trials and early passive adverse event reporting. We did so by leveraging the CDC Vaccine Safety Datalink’s (VSD) electronic health record (EHR) data infrastructure and methods framework for real-world observational data monitoring (15). Specifically, we used these structures to conduct near real-time sequential monitoring of the short-term risks of 10 prespecified priority health outcomes potentially associated with receipt of RZV.
METHODS
Study design
Using comprehensive and weekly-updated data on immunizations, medical-care utilization, and demographic factors, we conducted a prospective cohort study among persons aged ≥50 years enrolled in 7 VSD-data–contributing integrated health-care systems (Kaiser Permanente (Colorado, Northern California, Northwest, Southern California, and Washington), the Marshfield Clinic (Wisconsin), and HealthPartners (Minnesota)). This population was evaluated from January 2018, when RZV was first used among VSD enrollees, through December 2019, when an adequate number of RZV doses had been administered in the cohort to achieve sufficient statistical power for prespecified high-priority safety outcomes (Table 1). Each site’s institutional review board approved this study; informed consent was not required.
Table 1.
Definitions of Primary and Secondary Adverse Events for a Vaccine Safety Datalink Recombinant Zoster Vaccine Safety Studya
| Adverse Event Group | ICD-9-CM Code(s)b,c | ICD-10-CM Code(s)b | Medical Setting | Postvaccination At-Risk Interval, daysd |
|---|---|---|---|---|
| Primary adverse events | ||||
| Acute myocardial infarction | 410.x | I21.* | Inpatient | 1–42 |
| Stroke (both hemorrhagic and nonhemorrhagic) | 431 (hemorrhagic) 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91 | I61.9 (hemorrhagic) I63.* | Inpatient | 1–42 |
| Supraventricular tachycardia | 427.0 | I47.1 | ED, inpatient | 1–42 |
| Polymyalgia rheumatica | 725 | M35.3 | Outpatient, ED, inpatient | 1–42 |
| Convulsion-associated terms excluding epilepsy | 780.3x | R56.0*, R56.9 | ED, inpatient | 1–42 |
| Bell’s palsy | 351.0 | G51.0 | Outpatient, ED, inpatient | 1–42 |
| Optic ischemic neuropathy | 377.41 | H47.01* | Outpatient, ED, inpatient | 1–42 |
| Giant cell arteritis | 446.5 | M31.6 | Outpatient, ED, inpatient | 1–42 |
| Anaphylaxis | 995.0, 999.42 | T78.2*, T80.52XD | Outpatient, ED, inpatient | 0–1 |
| GBS | 357.0 | G61.0 | Outpatient, ED, inpatient | 1–42 |
| Secondary adverse events | ||||
| Gout | 274.01, 274.00, 274.9 | M10.* | Outpatient, ED | 1–42 |
| Diagnoses consistent with systemic reactions | 780.60, 780.63, 729.1, 780.64, 787.0x, 780.7, 784.0, 339.89 | R50.9, R50.83, M79.1, R68.83, R11.*, R53.*, R51, G44.* | Outpatient, ED | 1–7 |
| Diagnoses consistent with local reactions | 682.3, 683, 729.5 | L03.113, L03.114, L03.119, L04.9, L04.2, M79.62* | Outpatient, ED | 1–7 |
| Any adverse reaction diagnosis, including systemic, local and nonspecific reactions | Any systemic or local reaction code (defined above) plus nonspecific codes 995.29, 979.6, 979.9, E949.6, E949.9 | Any systemic or local reaction code (defined above) plus nonspecific code T50.B9* | Outpatient, ED | 1–7 |
| Pneumonia | 052.1, 480.x, 487.0, 481, 482.x, 483.x, 485, 486 | J18.*, B01.2, J11.0*, J12.*, J13, J14, J15.*, J16.* | Inpatient | 1–42 |
| Keratitis | 370.0x, 370.2x, 370.40, 370.35, 370.31, 370.4x, 370.5x, 370.8, 370.9 | H16.0*, H16.1*, H16.20*, H16.23*, H16.25*, H16.29*, H16.3*, H16.8, H16.9 | Outpatient, ED | 1–42 |
| Uveitis and retinitis | 364.0x, 364.3, 363.0x, 363.1x, 363.2x, 362.84, 362.82, 362.85, 362.89 | H20.0*, H20.9, H30*, H35.82, H35.89 | Outpatient, ED | 1–42 |
| Zoster ocular disease | 053.2x, 052.7 | B02.3*, B01.81 | Outpatient, ED | 1–42 |
| Pericarditis | 420.9x | B33.23, I30* | Outpatient, ED, inpatient | 1–42 |
| Myocarditis | 422.9.x | B33.22, I40* | Outpatient, ED, inpatient | 1–42 |
| Urgent or emergency health-care visit for any reason | N/A | N/A | Urgent care, ED | 1–7 |
Abbreviations: ED, emergency department; GBS, Guillain-Barré syndrome; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10-CM, International Classification of Diseases,Tenth Revision, Clinical Modification; N/A, not applicable; RZV, recombinant zoster vaccine; VSD, Vaccine Safety Datalink; ZVL, zoster vaccine live.
January 2018–December 2019 for the RZV and well-person visit groups and January 2013–December 2017 for the historical ZVL group.
Three-digit codes (e.g., 345) included those that started with those 3 digits and contained any additional fourth or fifth digit (e.g., 345.11); 4-digit codes included those that started with those 4 digits and had any fifth digit.
ICD-9-CM–coded definitions were needed to identify events among historical ZVL vaccinees 365 days prior to vaccination, which overlapped with the ICD-9-CM era for some ZVL recipients.
Outcomes were excluded if any of the ICD-9-CM or ICD-10-CM codes specifying that outcome were recorded during the 365 days prior to the index date, except for the anaphylaxis outcome. The anaphylaxis outcome was excluded if any of the codes defining that outcome were recorded in the 60 days prior to the index date.
Exposure and comparator groups
VSD health systems capture immunization data from EHRs and medical and pharmacy claims, and in some cases bidirectional communication with regional or state immunization information systems. For primary analyses, to assess whether safety risks were relatively higher following receipt of RZV, we used a historical ZVL comparator group and conducted routine analyses over time. Outcome risk in a postvaccination interval among RZV recipients aged ≥50 years was compared with that estimated in a postvaccination risk interval among those aged ≥60 years who received ZVL from January 2013 through December 2017 (since ZVL was only recommended for use among persons aged ≥60 years). A historical ZVL comparator group was designated as primary because recipients of RZV were expected to be similar to past recipients of ZVL, particularly with respect to preventative health-care–seeking behaviors, a known source of bias in vaccine studies of older adults (16).
In secondary end-of-surveillance analyses, we used concurrent “well-visit comparators.” We compared the postvaccination outcome risk for RZV vaccinees with the risk for non–RZV-vaccinated persons aged ≥50 years who: 1) had an annual well-person health-care visit (identified by International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes Z00.00 and Z00.01) during the 2018–2019 RZV uptake period, 2) had not received RZV on or before their well visit, and 3) had received influenza vaccine during the year prior to their well visit. We identified outcomes for this group as those occurring in a risk interval after their well-visit date. Use of well-visit comparators complements the historical ZVL comparator analysis by 1) providing a contemporaneous comparison to avoid temporal bias, 2) increasing age comparability, 3) minimizing differences in health-care–seeking behavior by requiring prior receipt of an influenza vaccine, and 4) serving as a comparator that cannot cause adverse outcomes via boosting of immunity to herpes zoster, as occurs with ZVL.
Eligibility was assessed on a rolling basis throughout the study period. Enrollees were considered eligible if, at the time of their RZV, ZVL, or well-visit date, they 1) were aged 50 years or older and 2) had continuous health insurance enrollment in their site’s health plan for 365 days prior to assessment of baseline characteristics (see “Covariates”).
Safety outcomes
On the basis of 1) imbalances in safety data from prelicensure studies of RZV (4, 5, 10), 2) inclusion of outcomes in prior vaccine safety studies (17–29), and 3) theoretical concerns about biologically plausible vaccine-associated outcomes, we prespecified 10 primary health outcomes and 11 secondary health outcomes with preset risk intervals (Table 1). Primary outcomes were acute myocardial infarction, stroke, supraventricular tachycardia, polymyalgia rheumatica, convulsions, Bell’s palsy, optic ischemic neuropathy, giant cell arteritis, anaphylaxis, and GBS. Secondary outcomes included systemic and local reactions occurring within 1–7 days, as well as gout, myocarditis, pericarditis, and several eye-related diseases, diagnosed within 1–42 days. Investigators at each study site updated data weekly to capture these outcomes, which were defined using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) or ICD-10-CM diagnosis codes assigned during outpatient, emergency department, or inpatient encounters. Each outcome was assessed during a postvaccination risk interval ranging from day 1 (the day after the RZV vaccination, ZVL vaccination, or well visit) through day 42, except for anaphylaxis, which was assessed from day 0 (the day of vaccination) through day 1.
Covariates
We required continuous enrollment in the site’s health insurance plan for 365 days prior to the date of RZV, ZVL, or the well visit so that baseline characteristics could be assessed. Selected covariates captured during this period included age, site, sex, indicators of comorbidity (diabetes; hypertension; hyperlipidemia; ischemic conditions, including ischemic heart disease, transient ischemic attack, or prior stroke; gastroesophageal reflux disease; osteoarthritis; atrial fibrillation; herpes zoster; dementia; congestive heart failure; and chronic obstructive pulmonary disease), and indicators of health-care utilization that may reflect healthy-user behaviors (e.g., a dermatology visit, an optometry or ophthalmology visit) (30). We also captured receipt of concomitant vaccinations (i.e., those given on the same day as the RZV or ZVL vaccine) and prior receipt of ZVL vaccine at any time before the RZV or well-visit date.
Statistical analysis
Monthly sequential testing using historical ZVL comparators.
Every month, for each primary outcome, we estimated an adjusted relative risk (RR) and conducted an exact sequential Poisson-based likelihood ratio test of the 1-sided hypothesis that outcome risk was elevated for RZV vaccinees versus historical ZVL recipients (H0: RR = 1 vs. HA: RR > 1) using unifying family group sequential methods (31). Covariate adjustment involved computing historical event rates by site, age group, and sex. For acute myocardial infarction and stroke, adjustment also included baseline diabetes and hypertension status. Covariate-stratified historical rates were then used to compute stratum-specific expected counts based on the observed distribution of covariates among RZV recipients. Expected counts were then summed across strata and compared with the total number of observed counts among RZV vaccinees (17). Since analytical databases are dynamic and updated weekly to add previously missing data, in order to maximize data integrity we did not permit subjects to enter the monthly analyses until 12 weeks after their RZV vaccination date, in order to allow sufficient time for more complete capture of events during the 42-day postvaccination risk window.
We conducted 19 monthly analyses, starting 6 months into surveillance (while RZV uptake remained relatively low). We used a flat (on the log likelihood ratio test scale) stopping threshold over time computed via simulation methods to ensure that the overall Type 1 error level across all tests performed for a given outcome over time was 0.05 (31). P values that adjusted for the multiple tests conducted over time were computed (17). We did not adjust statistically for multiple testing across the 10 outcomes, to conservatively avoid missing potential safety concerns.
A preliminary safety signal for an outcome was considered to occur if the log likelihood ratio test statistic exceeded the predefined threshold at any test. Signals were followed up for further evidence to either support or refute the initial finding. This included data quality assessments, examination of the distribution of events in the postvaccination risk window, and physician review of the medical chart to confirm whether the International Classification of Diseases code–based presumptive outcomes were true incident events. If a preliminary signal occurred, formal sequential analyses were stopped for that outcome only, and descriptive statistics were monitored going forward until the end of the surveillance.
End-of-surveillance analyses using concurrent well-visit comparators.
At the end of surveillance, we assessed the robustness of the monthly historical ZVL comparator sequential results for the primary outcomes by using a complementary well-visit concurrent comparator group in a one-time set of analyses, adjusting for additional confounders and exploring associations in predefined subgroups by dose, age, and site. We also examined secondary outcomes. We made comparisons between the RZV and well-visit cohorts that adjusted for confounding using propensity scores in order to incorporate more confounders via dimensionality reduction, which is especially useful in settings with rare events.
Overall and in subgroups, we computed the marginal RR of each outcome for RZV recipients compared with well-visit comparators, using 4 steps (32). First, we used logistic regression to estimate the probability of receiving RZV (i.e., the propensity score) using age, sex, site, an optometry visit, a dermatology visit, and prior receipt of ZVL. For cardiovascular outcomes, we also included hypertension, hyperlipidemia, diabetes, and the presence of at least 1 ischemic condition: ischemic heart disease, transient ischemic attack, or prior stroke. Second, we used logistic regression to model the association between each outcome and receipt of RZV vaccine and adjusted flexibly for the propensity score using cubic splines and selecting 3–5 knots via cross-validation (33). Third, we applied standardization to obtain marginal, population-level risk estimates for RZV and well-visit groups from the logistic regression model in step 2. Standardization is a causal inference technique that predicts a pair of potential outcomes for each participant (regardless of actual RZV exposure status) by first assuming that they are in the RZV group, then assuming they are in the well-visit group, and then computing the empirical average prediction (i.e., marginal estimate) from the potential outcomes across all participants under each assumption. Fourth, we computed the marginal RR as the ratio of these 2 marginal averages, including 95% confidence intervals (CIs) that we obtained through the estimated influence functions (32).
RESULTS
Figure 1 shows RZV uptake by 10-year age group and site during the study period. Overall, 647,833 doses of RZV were administered (403,522 first doses, 243,785 second doses, 496 third doses, and 30 fourth doses) with 16%, 40%, 32%, and 12% of doses being received by persons aged 50–59, 60–69, 70–79, and ≥80 years, respectively. On average, RZV recipients were older than historical ZVL and concurrent well-visit comparators and were more likely to have sought preventive health care in the prior year. The distributions of sex and most comorbid conditions were similar across groups, although RZV recipients were somewhat more likely to have had some conditions, like hypertension (Table 2).
Figure 1.
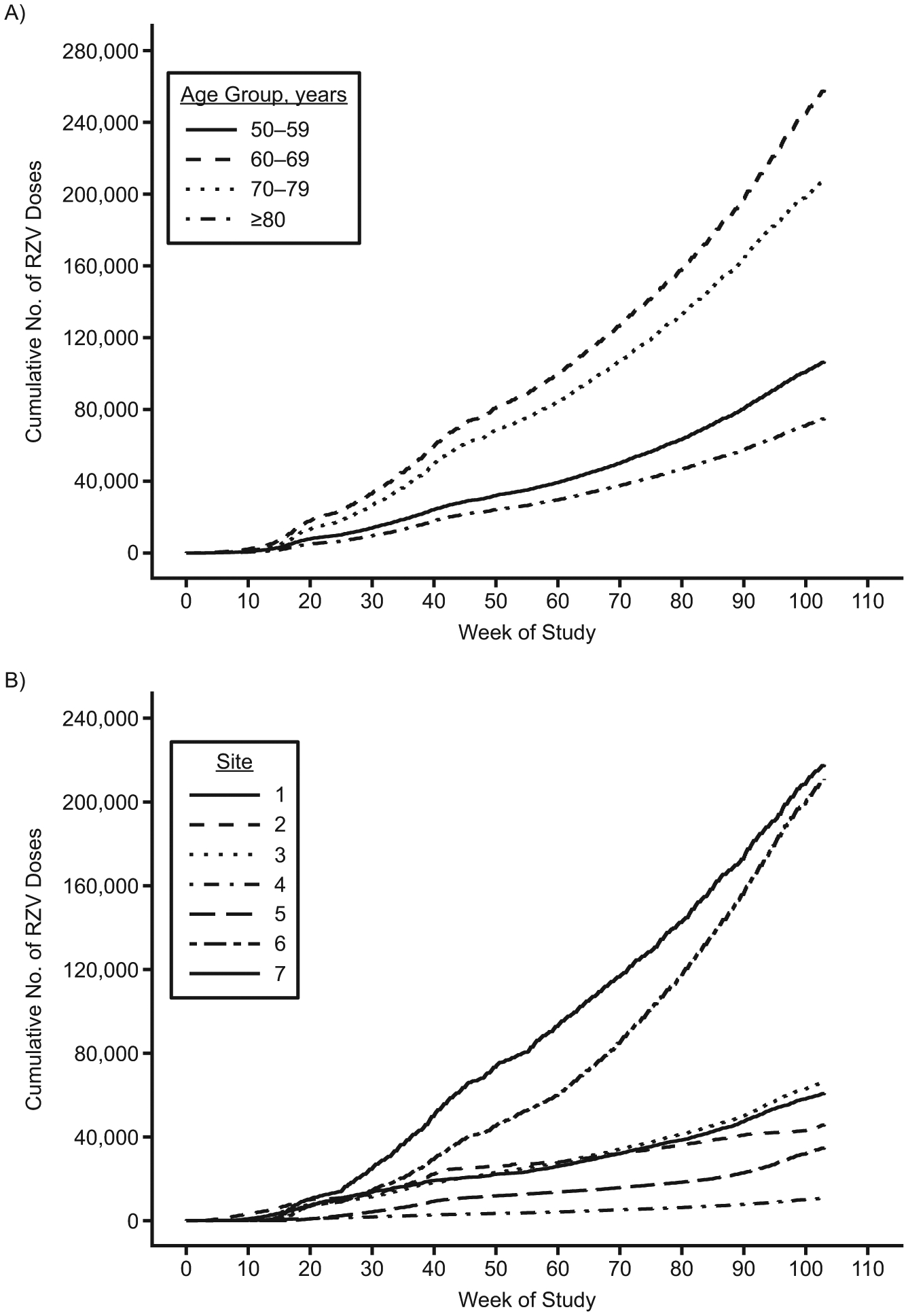
Cumulative numbers of recombinant zoster vaccine (RZV) doses administered in a Vaccine Safety Datalink study cohort, by 10-year age group (A) and study site (B), January 2018–December 2019. (Study sites 1–7 cannot be identified because of data privacy concerns.)
Table 2.
Characteristics of the Cohort Included in a Vaccine Safety Datalink Recombinant Zoster Vaccine Safety Studya
| Study Cohort Characteristic | RZV Recipients (n = 647,307) | Historical ZVL Comparators (n = 732,152) | Well-Visit Comparators (n = 1,086,260) | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Age group, years | ||||||
| 50–59 | 106,621 | 16 | 60,971b | 8 | 270,963 | 25 |
| 60–69 | 258,030 | 40 | 475,516 | 65 | 373,996 | 34 |
| 70–79 | 207,812 | 32 | 144,377 | 20 | 299,177 | 28 |
| ≥80 | 74,844 | 12 | 51,288 | 7 | 142,124 | 13 |
| Female sex | 377,048 | 58 | 393,061 | 54 | 598,883 | 55 |
| Health-care–seeking behavior | ||||||
| Dermatology visit during prior year | 149,711 | 23 | 99,401 | 14 | 167,639 | 15 |
| Optometry or ophthalmology visit during prior year | 317,631 | 49 | 291,564 | 40 | 457,229 | 42 |
| Receipt of ZVL vaccine at any time prior to RZV receipt or well-person visit | 372,053 | 57 | N/A | N/A | 562,597 | 52 |
| Comorbidity during prior year | ||||||
| Diabetes | 116,226 | 18 | 141,905 | 19 | 185,698 | 17 |
| Hypertension | 281,370 | 43 | 301,720 | 41 | 401,758 | 37 |
| Hyperlipidemia | 313,644 | 48 | 293,134 | 40 | 410,616 | 38 |
| Ischemic conditionc | 52,305 | 8 | 55,836 | 8 | 74,396 | 7 |
| Gastroesophageal reflux disease | 130,149 | 20 | 106,904 | 15 | 175,640 | 16 |
| Osteoarthritis | 115,837 | 18 | 99,325 | 14 | 141,672 | 13 |
| Atrial fibrillation | 37,615 | 6 | 32,072 | 4 | 51,237 | 5 |
| Herpes zoster | 16,727 | 3 | 13,773 | 2 | 11,245 | 1 |
| Dementia | 13,257 | 2 | 6,962 | 1 | 25,429 | 2 |
| Congestive heart failure | 19,286 | 3 | 21,612 | 3 | 30,202 | 3 |
| Chronic obstructive pulmonary disease | 27,985 | 4 | 27,733 | 4 | 43,231 | 4 |
| No. of concomitant vaccines | ||||||
| 0 | 505,129 | 78 | 491,865 | 67 | N/A | N/A |
| 1 | 127,192 | 20 | 204,020 | 28 | N/A | N/A |
| ≥2 | 14,986 | 2 | 36,267 | 5 | N/A | N/A |
Abbreviations: N/A, not applicable; RZV, recombinant zoster vaccine; ZVL, zoster vaccine live.
January 2018–December 2019 for the RZV and well-person visit groups and January 2013–December 2017 for the historical ZVL group.
This group is shown here for descriptive purposes but was not included in subsequent analyses, since ZVL is only recommended for use among persons aged ≥60 years.
Includes ischemic heart disease, transient ischemic attack, and prior stroke.
Monthly testing using historical ZVL comparators
All 647,833 vaccination doses were included in these analyses. During the surveillance period, a preliminary safety signal indicating a potentially elevated risk for RZV recipients as compared with historical ZVL recipients was observed for 2 outcomes: GBS and Bell’s palsy. Figure 2 shows the trajectory of estimated RRs for both outcomes over time. The GBS signal occurred at the second analysis time point after 3 presumptive cases were observed as compared with only 0.6 cases expected (RR = 5.25, P = 0.02). The Bell’s palsy signal occurred at the fifth analysis time point based on 36 observed cases as compared with 24 expected (RR = 1.51, P = 0.03). No preliminary signals were observed for any other primary outcomes.
Figure 2.
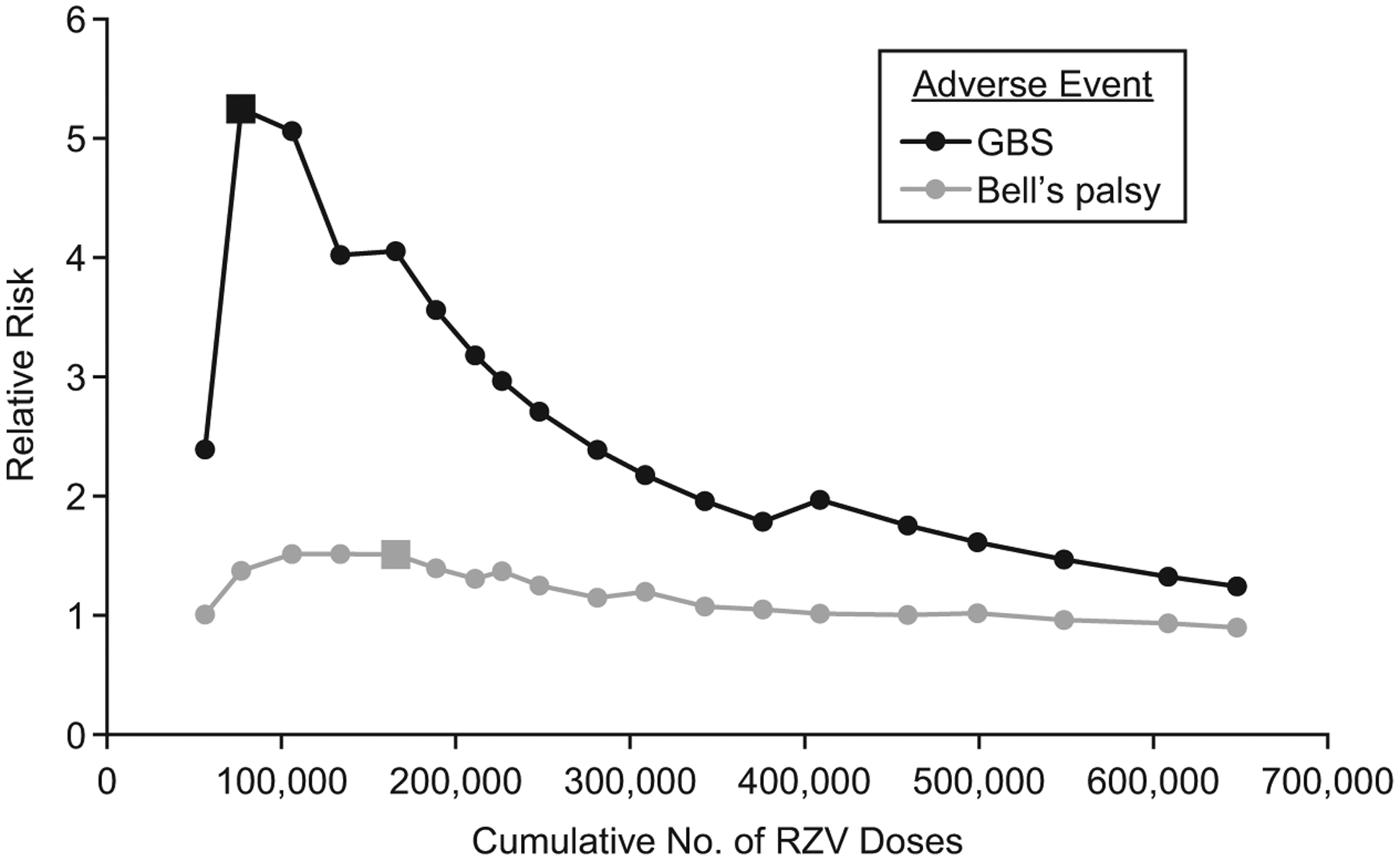
Monthly estimated relative risk of Guillain-Barré syndrome (GBS) and Bell’s palsy over time as recombinant zoster vaccine (RZV) was administered in a Vaccine Safety Datalink study cohort, January 2018–December 2019. The large square indicates where the preliminary signal occurred.
Table 3 shows results from the final monthly sequential analysis conducted at the end of surveillance for all primary outcomes, comparing the risk among all RZV recipients (647,833 doses received during 2018–2019) with that expected on the basis of historical ZVL recipients (671,181 doses administered during 2013–2017). RRs for both GBS and Bell’s palsy had attenuated considerably (RR = 1.24 for GBS; RR = 0.90 for Bell’s palsy) (Figure 2). In total, 6 presumptive GBS cases were observed following RZV as compared with 4.83 expected (based on 5 events among ZVL recipients), and fewer presumptive Bell’s palsy events were observed post-RZV (n = 86) compared with the number expected (n = 95.72). RRs for all other outcomes were not statistically greater than 1 at any time during surveillance (P > 0.05).
Table 3.
Age-, Sex-, and Site-Comparable Event Rates and Risk of Adverse Events for Recipients of Recombinant Zoster Vaccine Versus Recipients of Zoster Vaccine Live in a Vaccine Safety Datalink Study Cohort (Final Primary Sequential Analysis Results)a
| Adverse Event Group | Observed No. of Events | Observed Rate per 10,000 Doses of RZV | Expected No. of Eventsb | Relative Risk |
|---|---|---|---|---|
| Acute myocardial infarctionc | 320 | 4.94 | 379.83 | 0.84 |
| Strokec | 287 | 4.43 | 376.19 | 0.76 |
| Nonhemorrhagic | 267 | 4.12 | 321.04 | 0.83 |
| Hemorrhagic | 41 | 0.63 | 77.21 | 0.53 |
| Supraventricular tachycardia | 151 | 2.33 | 125.45 | 1.20 |
| Polymyalgia rheumatica | 134 | 2.07 | 152.68 | 0.88 |
| Convulsion-associated terms excluding epilepsy | 112 | 1.73 | 123.56 | 0.91 |
| Bell’s palsyd | 86 | 1.33 | 95.72 | 0.90 |
| Optic ischemic neuropathye | 37 | 0.57 | 52.61 | 0.70 |
| Giant cell arteritise | 35 | 0.54 | 49.20 | 0.71 |
| Anaphylaxise | 20 | 0.31 | 15.15 | 1.32 |
| GBSd,f | 6 | 0.09 | 4.83 | 1.24 |
Abbreviations: GBS, Guillain-Barré syndrome; RZV, recombinant zoster vaccine; ZVL, zoster vaccine live.
January 2018–December 2019 for the RZV group and January 2013–December 2017 for the historical ZVL group.
The expected number of events following receipt of RZV was estimated from historical data on ZVL recipients, adjusting for site, age group, and sex.
Results for acute myocardial infarction and stroke were also adjusted for diabetes and hypertension during the year prior to RZV or ZVL vaccination.
In analysis 5, a preliminary safety signal was observed for Bell’s palsy (36 cases vs. 24 expected; RR = 1.51, adjusted P = 0.03), indicating a potentially elevated risk for RZV recipients versus historical ZVL recipients. In analysis 2, a preliminary safety signal was observed for GBS (3 cases vs. 0.6 expected; RR = 5.25, adjusted P = 0.02).
Results for optic ischemic neuropathy, giant cell arteritis, and anaphylaxis were adjusted for site and age group (60–64, 65–74, or ≥75 years) only.
GBS results were unadjusted.
Due to the magnitude of the preliminary GBS signal (RR = 5.25) and the potential severity of the outcome, all post-RZV/ZVL GBS cases presumptively identified using ICD-10-CM codes underwent manual medical record review by a physician to confirm whether each was a true incident case. Among the 6 potential GBS cases following RZV, 3 were confirmed as incident and 3 were reclassified as involving symptoms that appeared prior to vaccination. Among the 5 potential GBS cases following ZVL, 2 were confirmed as incident, 2 were ruled out, and 1 did not have chart data available for review. Based on chart validation, if we conservatively assume that the missing historical event was not a true case, the RR for confirmed GBS among RZV recipients as compared with historical ZVL recipients was 1.56 (95% CI: 0.18, 18.62). If the missing event was assumed to be a true case, the RR estimate was 1.04 (95% CI: 0.14, 7.74).
End-of-surveillance analyses using concurrent well-visit comparators
All 647,307 first and second RZV doses received during the surveillance period were included in these analyses. Consistent with the final monthly sequential analyses using historical ZVL comparators, we found no statistically significantly elevated risks of any primary outcome for RZV recipients in propensity-score–adjusted analyses using concurrent well-visit comparators (Table 4, upper section). RRs were all less than 1 except those for polymyalgia rheumatica and giant cell arteritis, which were not statistically different from 1 (P > 0.05). Estimated risks of myocardial infarction (RR = 0.82, 95% CI: 0.72, 0.94), Bell’s palsy (RR = 0.75, 95% CI: 0.57, 0.98), and anaphylaxis (RR = 0.53, 95% CI: 0.31, 0.91) were significantly lower for RZV vaccinees than for well-visit comparators.
Table 4.
Propensity-Score–Adjusted Results From End-of-Surveillance Analyses of a Vaccine Safety Datalink Study Population Comparing Primary and Secondary Outcome Risks Between Recombinant Zoster Vaccine Vaccinees and Well-Visit Comparatorsa
| Adverse Event Group | RZV Recipients (n = 647,307) | Well-Visit Comparators (n = 1,086,260) | Comparative Results | |||
|---|---|---|---|---|---|---|
| No. of Events | Adjusted Rate per 10,000 Dosesb | No. of Events | Adjusted Rate per 10,000 Dosesb | Adjusted Relative Riskb | 95% Confidence Interval | |
| Primary outcomes | ||||||
| Acute myocardial infarctionc | 320 | 4.72 | 607 | 5.81 | 0.82 | 0.72, 0.94 |
| Strokec | 285 | 4.30 | 501 | 4.71 | 0.92 | 0.79, 1.07 |
| Supraventricular tachycardia | 151 | 2.29 | 285 | 2.64 | 0.86 | 0.71, 1.05 |
| Polymyalgia rheumatica | 134 | 1.96 | 187 | 1.76 | 1.10 | 0.87, 1.38 |
| Convulsion-associated terms excluding epilepsy | 111 | 1.73 | 219 | 2.01 | 0.87 | 0.68, 1.10 |
| Bell’s palsy | 86 | 1.30 | 187 | 1.74 | 0.75 | 0.57, 0.98 |
| Optic ischemic neuropathy | 37 | 0.56 | 76 | 0.71 | 0.80 | 0.54, 1.18 |
| Giant cell arteritis | 35 | 0.52 | 41 | 0.38 | 1.36 | 0.85, 2.17 |
| Anaphylaxis | 20 | 0.27 | 51 | 0.52 | 0.53 | 0.31, 0.91 |
| GBS | 6 | 0.09 | 10 | 0.09 | 0.92 | 0.34, 2.52 |
| Secondary outcomes | ||||||
| Gout | 1,890 | 28.95 | 2,905 | 26.88 | 1.08 | 1.01, 1.14 |
| Diagnoses consistent with systemic reactions | 2,202 | 31.49 | 2,795 | 27.02 | 1.17 | 1.10, 1.24 |
| Diagnoses consistent with local reactions | 202 | 2.69 | 96 | 0.98 | 2.75 | 2.14, 3.54 |
| Any adverse reaction diagnosis | 2,497 | 35.63 | 2,896 | 28.04 | 1.27 | 1.20, 1.34 |
| Pneumonia | 512 | 8.04 | 1,060 | 9.67 | 0.83 | 0.75, 0.93 |
| Keratitis | 400 | 5.55 | 623 | 6.15 | 0.90 | 0.79, 1.03 |
| Uveitis and retinitis | 445 | 6.36 | 650 | 6.29 | 1.01 | 0.89, 1.15 |
| Zoster ocular disease | 90 | 1.33 | 141 | 1.34 | 0.99 | 0.75, 1.31 |
| Pericarditis | 21 | 0.31 | 21 | 0.20 | 1.60 | 0.87, 2.92 |
| Myocarditis | 4 | 0.06 | 1 | 0.01 | 7.18 | 0.79, 65.63 |
| Urgent or emergency health-care visit for any reason | 2,541 | 36.93 | 3,812 | 36.46 | 1.01 | 0.96, 1.07 |
Abbreviations: GBS, Guillain-Barré syndrome; RZV, recombinant zoster vaccine.
January 2018–December 2019 for the RZV and well-person visit groups.
Rates and relative risks were propensity-score–adjusted for age, sex, study site, a dermatology visit, an optometry visit, and prior zoster vaccine live vaccination.
Rates and relative risks for cardiovascular outcomes were additionally adjusted for hypertension, diabetes, hyperlipidemia, and ischemic conditions.
For most outcomes, estimated RRs using well-visit comparators were relatively consistent with those estimated using historical ZVL comparators in that the 95% CI for the well-visit RR contained the historical ZVL RR point estimate. For instance, the estimated RR of presumptive GBS using well-visit comparators (Table 4) was 0.92 (95% CI: 0.34, 2.52), and the estimated RR of 1.24 using historical ZVL recipients (Table 3) fell within those confidence limits. The RR of GBS using well-visit comparators was also similar when based on chart-confirmed outcomes (unadjusted RR = 0.84, 95% CI: 0.14, 3.93). Three outcomes (supraventricular tachycardia, anaphylaxis, and giant cell arteritis) had less consistent RR trends when RZV recipients were compared with well-visit versus historical ZVL comparators, though none of these RRs were statistically significantly different from a null association for either comparator (Tables 3 and 4).
For secondary outcomes (Table 4, lower section), significantly elevated risks were identified for 4 outcomes: gout, systemic reactions, local reactions, and a combined “any reaction” group. RRs ranged from 1.08 (gout) to 2.75 (local reactions). The risk of pneumonia was significantly lower for RZV vaccinees than for well-visit comparators (RR = 0.83, 95% CI: 0.75, 0.93).
End-of-surveillance subgroup analyses
Web Figure 1 (available at https://doi.org/10.1093/aje/kwac170) presents a forest plot with propensity-score–adjusted RRs and 95% CIs comparing risks for RZV recipients with those for well-visit comparators for the 6 highest-prevalence primary outcomes for which subgroup exploration was possible. RRs are depicted overall and by dose (1 dose vs. 2 doses), age group (age 50–64 years vs. ≥65 years), and site. Web Figure 2 provides similar information for the most common secondary outcomes. RRs were largely consistent across subgroups or, because of sparsity, too imprecise to draw conclusions from. Exceptions included 1) experiencing convulsions or making an urgent or emergency health-care visit for any reason, where the RRs trended above 1 for persons aged 50–64 years and below 1 for those aged ≥65 years, and 2) polymyalgia rheumatica, for which the RR was above 1 for dose 2 but not dose 1. In addition, more variability in RRs was generally observed across sites as compared with other subgroups, with wider uncertainty at several smaller sites for many outcomes.
DISCUSSION
Among 647,833 RZV doses received from January 2018 through December 2019, we did not detect a sustained increased risk of any sequentially monitored primary outcome for RZV recipients relative to either historical ZVL recipients or non–RZV-vaccinated well-visit comparators. Two preliminary safety signals, one for Bell’s palsy and one for GBS, were observed early during surveillance. However, the Bell’s palsy RR became attenuated to less than 1 with accumulation of more data by the end of surveillance, and the initially higher RR was not replicated when using a well-visit concurrent comparator. For GBS, the RR was also considerably attenuated over time. In addition, signal follow-up involving physician chart review of outcomes determined that half of the presumptive cases among RZV recipients were not confirmed upon validation, yielding too few (n = 3) truly incident cases to draw meaningful conclusions. In addition, the direction of the estimated RR for GBS was mixed when using ZVL historical (RR > 1) versus well-visit (RR < 1) comparators, for both the presumptive and chart-validated event definitions, reflecting further uncertainty.
We also compared RZV recipients with well-visit comparators for a set of secondary, relatively more common events, several of which were explored previously in prelicensure randomized efficacy trials for RZV (4, 5). Consistent with the 2 placebo-controlled pivotal phase 3 trials (4, 5), we found significantly elevated risks of local, systemic, or nonspecific adverse reactions postvaccination among RZV recipients. Despite intrinsic design and methodological differences, our estimated RR of any postvaccination reaction (RR = 1.27) was of a comparable order of magnitude to the pooled trial RR estimate of unsolicited adverse event risk (RR = 1.58) (10) and was similarly driven by both local and systemic symptoms. The lack of significant difference between RZV and well-visit groups with respect to urgent or emergency department health-care visits 1–7 days after vaccination also suggests that, as in prelicensure trials, most adverse reactions were not severe. This information is important, as it shows health providers that although such presentations are common after RZV, patients may not need elaborate workup. This is helpful information that can be used to counsel potential recipients of RZV. Last, our study identified a slight increase in risk of gout for RZV vaccinees (RR = 1.08) that parallels the numerical imbalance previously noted for gout as an exploratory prelicensure finding (reporting ratio = 3.38, 95% CI: 1.49, 8.60) (34) and discussed by Didierlaurent et al. (35).
Although our study was designed to detect potential adverse safety risks associated with RZV, we also observed that risks of several outcomes (myocardial infarction, Bell’s palsy, anaphylaxis, pneumonia) were statistically significantly lower among RZV vaccinees than among well-visit comparators. These potentially protective associations should be interpreted with caution, since the upper limits of the 95% CIs only excluded the null value of no association (RR = 1) by a small amount. If we had formally adjusted for multiple comparisons across the 21 outcomes we evaluated, these associations may not have achieved statistical significance, so they could have been due to chance. Additionally, although we adjusted for some measured confounders that attempted to capture healthy-user behaviors, we cannot exclude the possibility that unmeasured bias may have been influencing these results, especially since 1) healthy-user bias is known to be large when estimating associations of other vaccines (like influenza) with outcomes (like pneumonia) in seniors, and 2) healthy-user behaviors are known to be difficult to measure accurately using EHR data (16). This issue warrants additional evaluation in future studies.
This study shares the many well-documented strengths of other safety surveillance studies that have been conducted within the VSD (17–29). Its nimble data infrastructure with real-time data updating, combined with use of a sequential design that involves frequent testing over time, facilitates early detection of potential safety signals. The large, well-defined, and geographically diverse population enables the study of rare adverse events. The presence of an interdisciplinary collaborative team, which includes clinicians who are directly embedded in the health systems that capture the EHR data, permits rapid investigation of preliminary findings via real-time chart validation and bolsters the integrity of results. Further, although it was not implemented in the current study, the VSD can conduct real-time monitoring of chart-confirmed outcomes (e.g., for GBS) rather than reserving chart confirmation as a later follow-up step to presumptive International Classification of Diseases code–based outcome monitoring, as demonstrated in the recent coronavirus disease 2019 mRNA vaccine surveillance study (36). An additional novel analytical strength of the current study is the use of a smooth propensity score adjustment method (32) to obtain adjusted causal estimates, which would not have otherwise been possible for rare events like GBS.
This study shares the known limitations of health-care database studies that rely on EHR data, which are not collected for research purposes (17–29, 36): variable accuracy of diagnostic-code–based adverse event definitions and onset timing (37–42), potentially incomplete capture of vaccination status, and the possibility of missing or incomplete data due to delays in claims by some health-care systems (29). To minimize the latter challenge, we did not include subjects in analyses until 12 weeks had elapsed since their vaccination date. In addition, at the time of each analysis, we cumulatively refreshed all data that had been collected since the start of surveillance in order to include the most up-to-date information. Additionally, as in other safety surveillance studies (17–29, 36) where early detection is the highest priority, this sequential design was purposefully conservative, with relatively low signaling thresholds in early analyses, to facilitate rapid identification of true safety concerns and prevent potential harm among healthy vaccinees. The inherent trade-off is that false-positive signals can occur early in the surveillance period (when the number of events is relatively few and variability is higher) that are not ultimately chart-confirmed.
There were limitations to using a historical ZVL comparator group. It is possible that vaccination against herpes zoster, be it with RZV or ZVL, may provoke similar immune responses (43). If ZVL increases the risk of an adverse outcome due to an immune mechanism, then RZV may also increase risk via the same mechanism, which would not allow detection of a true increased risk due to RZV. In addition, use of a concurrent instead of historical comparison group would have been ideal to inherently control for any potential temporal bias, but it was difficult to identify a well-suited concurrent comparator group in advance due to uncertainty about who might receive RZV and whether ZVL use would continue. After observing the characteristics of the RZV cohort by the end of the surveillance period, however, we were able to find and use a comparable concurrent well-visit group in secondary end-of-study analyses, with results largely similar to those seen using ZVL comparators.
Overall, this study provides additional reassurance regarding the overall safety of RZV in real-world practice, but there is additional research worth pursuing. Future studies that build on this initial surveillance effort are critical and could include an evaluation of nonacute events (e.g., from 43 days to 1 year postvaccination), data mining for outcomes that are not prespecified in advance, deeper exploration to better understand reasons for differences in some findings by site, and more focused follow-up investigations using chart-validated outcomes to further strengthen the level of evidence generated. Last, given the insidious nature of healthy-user bias and its impacts on estimation of associations in vaccine studies of older adults (16, 30), it will be useful to leverage novel and more sophisticated methods to address unmeasured confounding, such as double-negative control adjustment (44), to more fully assess the validity of the apparent protective associations we observed.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by contract 200-2012-53421 with the Centers for Disease Control and Prevention.
Access to Vaccine Safety Datalink (VSD) data can be requested through the VSD Data Sharing Program at the National Center for Health Statistics (https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/data-sharing-guidelines.html).
We thank Erika Kiniry and Rachael Burganowski for their technical efforts related to study project and data management.
The findings and conclusions presented in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
J.C.N. has received research funding from Moderna, Inc. (Cambridge, Massachusetts). M.L.J. has received research funding from Sanofi-Pasteur S.A. (Lyon, France). N.P.K. has received research support from GlaxoSmithKline plc (London, United Kingdom), Merck & Co., Inc. (Kenilworth, New Jersey), Pfizer, Inc. (New York, New York), Sanofi Pasteur, and Protein Sciences Corporation (Meriden, Connecticut; now part of Sanofi Pasteur). A.L.N. has received research support from Pfizer and Vir Biotechnology, Inc. (San Francisco, California). H.-F.T. has received funding from Moderna, GlaxoSmithKline, and Sequirus (Maidenhead, United Kingdom) and has served on advisory boards for Janssen Pharmaceuticals, Inc. (Titusville, New Jersey) and Pfizer. W.K.Y. has received research funding from GlaxoSmithKline and Pfizer.
Abbreviations:
- CDC
Centers for Disease Control and Prevention
- CI
confidence interval
- EHR
electronic health record
- GBS
Guillain-Barré syndrome
- ICD-10-CM
International Classification of Diseases, Tenth Revision, Clinical Modification
- RR
relative risk
- RZV
recombinant zoster vaccine
- VSD
Vaccine Safety Datalink
- ZVL
zoster vaccine live
REFERENCES
- 1.Food and Drug Administration, US Department of Health and Human Services. SHINGRIX (Zoster Vaccine Recombinant, Adjuvanted) [package insert]. Silver Spring, MD: Food and Drug Administration; 2017. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM581605.pdf. Accessed June 29, 2022. [Google Scholar]
- 2.Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67(3):103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Food and Drug Administration, US Department of Health and Human Services. ZOSTAVAX (Zoster Vaccine Live) [package insert]. Silver Spring, MD: Food and Drug Administration; 2006. https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM132831.pdf. Accessed June 29, 2022. [Google Scholar]
- 4.Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–1032. [DOI] [PubMed] [Google Scholar]
- 5.Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med. 2015;372(22):2087–2096. [DOI] [PubMed] [Google Scholar]
- 6.Oxman MN, Levin MJ, Johnson GR, et al. Shingles prevention study group. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352(22):2271–2284. [DOI] [PubMed] [Google Scholar]
- 7.Tseng H, Harpaz R, Luo Y, et al. Declining effectiveness of herpes zoster vaccine in adults aged ≥60 years. J Infect Dis. 2016;213(12):1872–1875. [DOI] [PubMed] [Google Scholar]
- 8.Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015; 60(6):900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee G Zoster Vaccines Session: Introduction. (Slide presentation to the Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention, February 25, 2021). Atlanta, GA: Centers for Disease Control and Prevention; 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/24-25/01-Zoster-Vaccines-Lee.pdf. Accessed July 5, 2015. [Google Scholar]
- 10.López-Fauqued M, Campora L, Delannois F, et al. Safety profile of adjuvanted recombinant zoster vaccine: pooled analysis of two large randomized phase 3 trials. Vaccine. 2019;37(18):2482–2493. [DOI] [PubMed] [Google Scholar]
- 11.Tavares-Da-Silva F, Co MM, Dessart C, et al. Review of the initial post-marketing safety surveillance for the recombinant zoster vaccine. Vaccine. 2020;38(18):3489–3500. [DOI] [PubMed] [Google Scholar]
- 12.Hesse EM, Shimabukuro TT, Su JR, et al. Postlicensure safety surveillance of recombinant zoster vaccine (Shingrix)—United States, October 2017–June 2018. MMWR Morb Mortal Wkly Rep. 2019;68(4):91–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33(36):4398–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goud R, Lufkin B, Duffy J, et al. Risk of Guillain-Barré syndrome following recombinant zoster vaccine in Medicare beneficiaries. JAMA Intern Med. 2021;181(12):1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baggs J, Gee J, Lewis E, et al. The Vaccine Safety Datalink: a model for monitoring immunization safety. Pediatrics. 2011; 127(suppl 1):S45–S53. [DOI] [PubMed] [Google Scholar]
- 16.Jackson LA, Jackson M, Nelson JC, et al. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. Int J Epidemiol. 2006;35(2):337–344. [DOI] [PubMed] [Google Scholar]
- 17.Nelson JC, Yu O, Dominguez-Islas C, et al. Adapting group sequential methods to observational post-licensure safety surveillance: results of a pentavalent combination DTaP-IPV-Hib (Pentacel) vaccine safety study. Am J Epidemiol. 2013;177(2):131–141. [DOI] [PubMed] [Google Scholar]
- 18.Lieu TA, Kulldorff M, Davis RL, et al. Real-time vaccine safety surveillance for the early detection of adverse events. Med Care. 2007;45(10 suppl 2):S89–S95. [DOI] [PubMed] [Google Scholar]
- 19.Yih WK, Nordin JD, Kulldorff M, et al. An assessment of the safety of adolescent and adult tetanus-diphtheria-acellular pertussis (Tdap) vaccine, using active surveillance for adverse events in the Vaccine Safety Datalink. Vaccine. 2009;27(32): 4257–4262. [DOI] [PubMed] [Google Scholar]
- 20.Klein NP, Fireman B, Yih W, et al. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics. 2010;126(1):e1–e8. [DOI] [PubMed] [Google Scholar]
- 21.Belongia EA, Irving S, Shui IM, et al. Real-time surveillance to assess risk of intussusception and other adverse events after pentavalent, bovine-derived rotavirus vaccine. Pediatr Infect Dis J. 2010;29(1):1–5. [DOI] [PubMed] [Google Scholar]
- 22.Gee J, Naleway A, Shui I, et al. Monitoring the safety of quadrivalent human papillomavirus vaccine: findings from the Vaccine Safety Datalink. Vaccine. 2011;29(46):8279–8284. [DOI] [PubMed] [Google Scholar]
- 23.Lee GM, Greene SK, Weintraub ES, et al. H1N1 and seasonal influenza vaccine safety in the Vaccine Safety Datalink Project. Am J Prev Med. 2011;41(2):121–128. [DOI] [PubMed] [Google Scholar]
- 24.Tseng HF, Sy LS, Liu IL, et al. Postlicensure surveillance for pre-specified adverse events following the 13-valent pneumococcal conjugate vaccine in children. Vaccine. 2013; 31(22):2578–2583. [DOI] [PubMed] [Google Scholar]
- 25.Daley MF, Yih WK, Glanz JM, et al. Safety of diphtheria, tetanus, acellular pertussis and inactivated poliovirus (DTaP-IPV) vaccine. Vaccine. 2014;32(25):3019–3024. [DOI] [PubMed] [Google Scholar]
- 26.Li R, Stewart B, McNeil MM, et al. Post licensure surveillance of influenza vaccines in the Vaccine Safety Datalink in the 2013–2014 and 2014–2015 seasons. Pharmacoepidemiol Drug Saf. 2016;25(8):928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donahue JG, Kieke BA, Lewis EM, et al. Near real-time surveillance to assess the safety of the 9-valent human papillomavirus vaccine. Pediatrics. 2019;144(6):e20191808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davis RL, Kolczak M, Lewis E, et al. Active surveillance of vaccine safety: a system to detect early signs of adverse events. Epidemiology. 2005;16(3):336–341. [DOI] [PubMed] [Google Scholar]
- 29.Greene SK, Kulldorff M, Yin R, et al. Near real-time vaccine safety surveillance with partially accrued data. Pharmacoepidemiol Drug Saf. 2011;20(6):583–590. [DOI] [PubMed] [Google Scholar]
- 30.Patrick AR, Shrank WH, Glynn RJ, et al. The association between statin use and outcomes potentially attributable to an unhealthy lifestyle in older adults. Value Health. 2011;14(4): 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kittleson JM, Emerson SS. A unifying family of group sequential test designs. Biometrics. 1999;55(3):874–882. [DOI] [PubMed] [Google Scholar]
- 32.Shi X, Wellman R, Heagerty P, et al. Safety surveillance and the estimation of risk in select populations: flexible methods to control for confounding while targeting marginal comparisons. Stat Med. 2020;39(4):369–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hastie TJ, Tibshirani RJ. Generalized Additive Models. London, United Kingdom: Chapman & Hall Ltd.; 1990. [Google Scholar]
- 34.Vaccines and Related Biological Products Advisory Committee, Food and Drug Administration, US Department of Health and Human Services. SHINGRIX* (Zoster Vaccine Recombinant, Adjuvanted). (Briefing document). Silver Spring, MD: Food and Drug Administration; 2017. https://www.fda.gov/media/107553/download. Accessed June 29, 2022. [Google Scholar]
- 35.Didierlaurent AM, Desssart C, Cunningham AL. Clarification regarding the statement of the association between the recombinant zoster vaccine (RZV) and gout flares. Ann Rheum Dis. 2021;80(12):e200. [DOI] [PubMed] [Google Scholar]
- 36.Klein NP, Lewis N, Goddard K, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA. 2021;326(14):1390–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cutrona SL, Toh S, Iyer A, et al. Validation of acute myocardial infarction in the Food and Drug Administration’s Mini-Sentinel Program. Pharmacoepidemiol Drug Saf. 2013; 22(1):40–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee CD, Carnahan RM, McPheeters ML. A systematic review of validated methods for identifying Bell’s palsy using administrative or claims data. Vaccine. 2013;31(suppl 10): K7–K11. [DOI] [PubMed] [Google Scholar]
- 39.Bann MA, Carrell DS, Gruber S, et al. Identification and validation of anaphylaxis using electronic health data in a population-based setting. Epidemiology. 2021;32(3):439–443. [DOI] [PubMed] [Google Scholar]
- 40.Walsh KE, Cutrona SL, Foy S, et al. Validation of anaphylaxis in the Food and Drug Administration’s Mini-Sentinel. Pharmacoepidemiol Drug Saf. 2013;22(11):1205–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guevara RE, Butler JC, Marston BJ, et al. Accuracy of ICD-9-CM codes in detecting community-acquired pneumococcal pneumonia for incidence and vaccine efficacy studies. Am J Epidemiol. 1999;149(3):282–289. [DOI] [PubMed] [Google Scholar]
- 42.Williams DJ, Shah SS, Myers A, et al. Identifying pediatric community-acquired pneumonia hospitalizations: accuracy of administrative billing codes. JAMA Pediatr. 2013;167(9): 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunningham AL, Heineman TC, Lal H, et al. Immune responses to a recombinant glycoprotein E herpes zoster vaccine in adults aged 50 years or older. J Infect Dis. 2018; 217(11):1750–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi X, Miao W, Nelson JC, et al. Multiply robust causal inference with double negative control adjustment for categorical unmeasured confounding. J R Stat Soc Ser B Stat Methodol. 2020;82(2):521–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


