Abstract
The ten-eleven translocation (TET) family of dioxygenases consists of three members, TET1, TET2, and TET3. All three TET enzymes have Fe+2 and α-ketoglutarate (α-KG)-dependent dioxygenase activities, catalyzing the 1st step of DNA demethylation by converting 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), and further oxidize 5hmC to 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC). Gene knockout studies demonstrated that all three TET proteins are involved in the regulation of fetal organ generation during embryonic development and normal tissue generation postnatal. TET proteins play such roles by regulating the expression of key differentiation and fate-determine genes via 1) enzymatic activity-dependent DNA methylation of the promoters and enhancers of target genes; and 2) enzymatic activity-independent regulation of histone modification. Interacting partner proteins and posttranslational regulatory mechanisms regulate the activities of TET proteins. Mutations and dysregulation of TET proteins are involved in the pathogenesis of human diseases, specifically cancers. Here, we summarize the research on the interaction partners and posttranslational modifications of TET proteins. We also discuss the molecular mechanisms by which these partner proteins and modifications regulate TET functioning and target gene expression. Such information will help in the design of medications useful for targeted therapy of TET-mutant-related diseases.
Keywords: TETs, mutations, interaction partners, posttranslational modifications, gene expression
Introduction
Lineage commitment and differentiation of tissue stem/progenitor cells are tightly controlled by transcriptional programing1,2 and are delicately regulated by an ordered, stepwise reconfiguration of the DNA methylome and histone modifications.3–8 Dysregulation of either transcriptional programing or the epigenetic machinery will cause diseases such as cancers by disrupting cell fate determination and differentiation. Thus, a more complete understanding of how transcriptional programing and epigenetic functioning collaboratively regulate lineage fate and differentiation of stem/progenitor cells will provide information that will improve our understanding of disease pathogenesis and can point the way toward the development of novel medications for the treatment of diseases.
Transcription factors (TFs) regulate target gene expression by binding to specific consensus motifs in their enhancers and promoters.9 The binding motifs of most TFs contain CpG dinucleotides. Such TFs have different sensitivities to methyl-CpG (mCpG) motifs for DNA binding. Many genes have CpG-rich (CpG islands or CGIs) promoters. Methylation of these promoters is associated with target gene repression due to the condensation of local chromatin.8,10,11 Removing methyl groups from these promoters is required for TF binding and gene expression. In genes with non-CGI promoters and enhancers, TF-regulated expression of such genes is determined by the methylation status of CpG within the binding motifs.12–14
The dynamic methylation of DNA is regulated by a balance of DNA methyltransferases (including DNMT1, DNMT3a, and DNMT3b) and the Ten-eleven translocation (TET) family of dioxygenases (including TET1, TET2, and TET3).15,16 The methylation state of DNA sequences regulates the accessibility of key TFs to genetic regulatory elements including promoters and enhancers of target genes, which in turn determines cell fate.8,17 Disruption of the dynamic methylation programming of DNA has been observed in almost all types of hematopoietic malignancies and has emerged as a hallmark of various types of hematological cancers, including myelodysplastic syndromes (MDS), acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), diffuse large B cell lymphomas (DLBCLs) and peripheral T-cell lymphoma (PTCL).18–42 Consistently, somatic mutations of several key regulators of DNA methylation including DNMT3A, isocitrate dehydrogenase (IDH1), IDH2, and TET2 have been detected in almost all types of hematopoietic cancers.43 Detailed studies demonstrated that somatic mutations of DNMT3A and TET2 are also frequently detected in small clones in the hematopoietic tissue of healthy people, specifically those >50 years old. The frequency of such mutations is increased during aging and has been called age-related clonal hematopoiesis (ARCH).44–46 The selective acquisition and expansion of DNMT3A- or TET2-mutant clones during aging suggest that ARCH might be a consequence of compensatory hematopoiesis against the pressure of aging. In support of such a concept, it was found that hematopoietic stem and progenitor cells (HSPCs) showing either DNMT3A or TET2 mutations display growth advantages in response to treatment with interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α), respectively.47,48 Nevertheless, people with ARCH showed a 10–12 fold increased risk for developing hematopoietic malignancies than age-matched ARCH-negative populations.49–51 Thus, as is the case with somatic DNMT3A mutations, somatic TET2 mutations are founder mutations for almost all types of hematopoietic malignancies, and occur in hematopoietic stem cells (HSCs) during aging and are selected under the pressure of aging-associated inflammation. Additional genetic mutations are required for the full malignant transformation of TET2-mutant HSCs, which drive the abnormal proliferation, lineage commitment, differentiation, and survival of HSPCs. In addition, TET1 is frequently mutated in B-cell malignancies and TET3 is down-regulated in HSPCs during aging as well as in the malignant cells of many types of hematopoietic cancers.52 Thus, all three members of the TET family are involved in the pathogenesis of hematopoietic cancers. In this review, we summarize the research on TET protein interaction partners and translational modifications of three TETs in the regulation of TET function. We also discuss the molecular mechanism by which TET proteins regulate target gene expression.
1. The three TET genes and their isoforms
The human TET1 gene is located on chromosome 10q21.3. It expresses two transcriptional isoforms owing to the use of alternate promoters (Figure 1a).53 Transcription starting from promoter 1a or 1b (distal) produces a 2,136 a.a. full-length TET1 protein (2039 a.a. for the mouse), while transcription starting from promoter 2 (proximal) in front of exon 2 gives rise to a 1465 a.a. short isoform of TET1 (TET1s, 1386 a.a. for the mouse).53,54 TET1s lacks a large portion of the TET1 N-terminus, including the CXXC (CXXC5) domain. Both TET1 and TET1s have enzymatic activity. In mice, TET1 is primarily expressed in the embryo and is replaced by TET1s in adult tissues53.
Figure 1. TET genes and TET proteins.
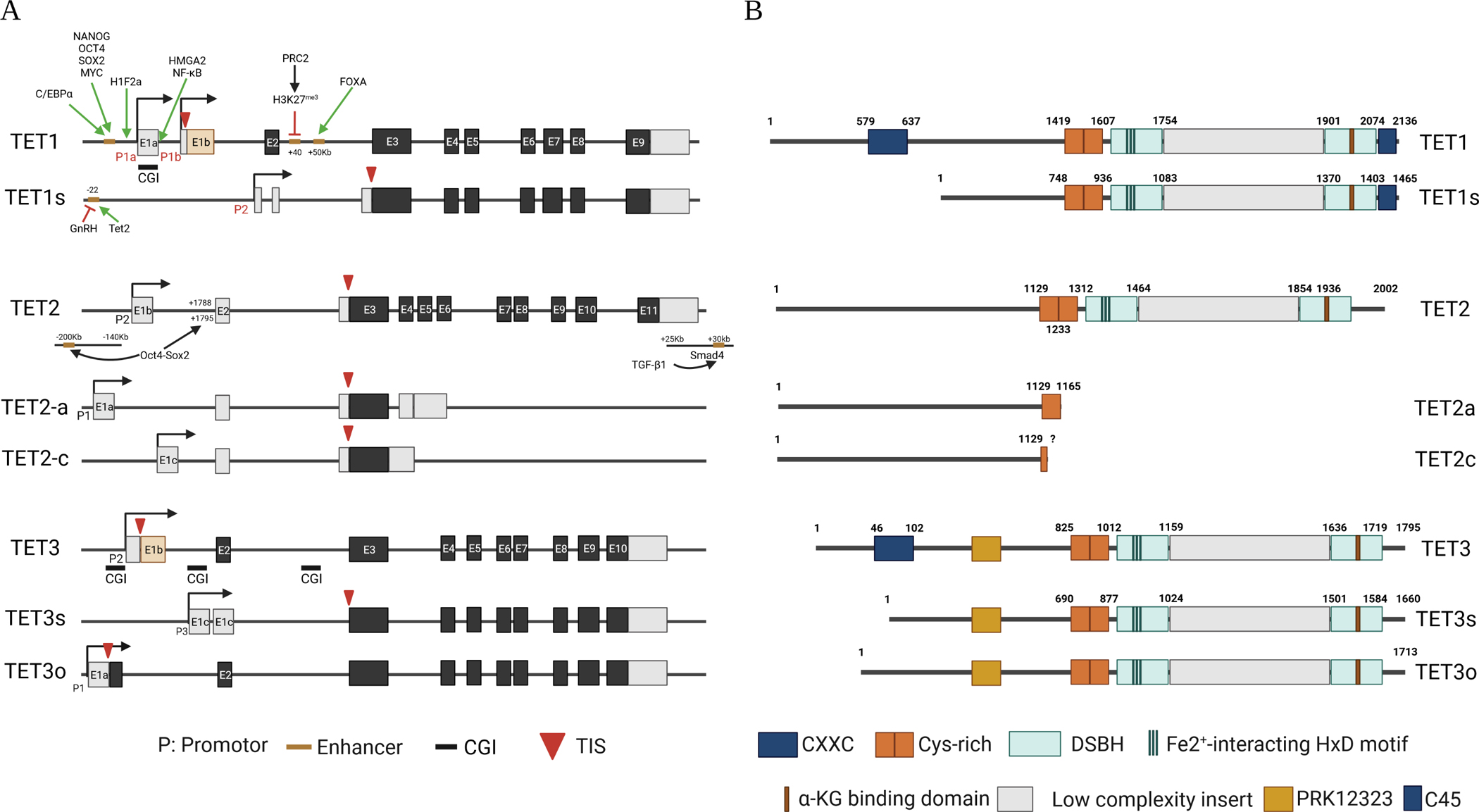
A. TET1, TET2, and TET3 have 2, 3, and 3 transcriptional products respectively due to the alternative use of promoters, which are regulated by the alternative activation of enhancers. The green arrow indicates induction of expression; the red cross depicts inhibition of expression. B. The corresponding protein isoforms of TET1, TET2, and TET3. The structural domains of the proteins are indicated.
The human TET2 gene is located on chromosome 4q24. In contrast to the TET1 and TET3 genes, the ancient TET2 gene was split during evolution into two genes, IDAX (also called CXXC4) and TET2. The IDAX gene is located 700 kb upstream of TET2 and is transcribed in the opposite direction; it encodes the CXXC domain-containing IDAX protein.55 The TET2 gene produces three protein isoforms, TET2-a, TET2-b, and TET2-c, because of the alternating use of 3 promoters and associated transcriptional initiation sites (TIS) (Figure 1a). TET2-b utilizes the second promoter in front of exon 1b and produces a 2002 a.a. full-length TET2 protein (TET2 hereafter, 1912 a.a. for the mouse). TET2-a uses the first promoter, which is localized upstream of the second promoter and produces a truncated 1165 a.a. protein terminating at a poly-A site in the fourth intron, while TET2-c utilizes the 3rd promoter in front of exon 1c and produces a much shorter truncated protein terminating within the 3rd exon. Both TET2-a and TET2-c lack enzymatic activity and might function as dominant-negative forms of TET2. TET2 is most abundant in normal human tissues, while TET2-a is primarily expressed in the human spleen, and TET2-c is weakly expressed in most tissues, with the highest levels observed in human spleen, bone marrow, fetal brain, and embryoid bodies. The dynamic switching of active promoters and enhancers regulates TET2-a, TET2, and TET2-c expression during cell state transitions between pluripotency and differentiation.56
The human TET3 gene is located on chromosome 2p13.1. The CXXC10–1 ORF is about 13 kb upstream of the annotated TSS of TET3 with the same orientation as the TET3 ORF. TET3 gene encodes three isoforms owing to the alternative use of promoters and alternative splicing (Figure 1a). A 1795 a.a. full-length TET3 protein (TET3 hereafter; 1803 a.a. for the mouse) is transcribed starting from promoter 2 in front of exon 1b, and a 1660 a.a. TET3 short isoform (TET-3s; 1668 a.a. for the mouse) is transcribed starting from promoter 3 in front of exon 2. A 1713 a.a. oocyte-specific isoform of TET3 (TET-3o) has been identified in the mouse, which is transcribed starting from promoter 1 in front of exon 1a approximately 5 kb upstream of the start codon with skipping of exon 1b.57 Human TET-3o has not been reported.
TET1, TET2, and TET3 share a conserved dioxygenase domain at their C-termini (Figure 1b).58–60 The dioxygenase domain is composed of a cysteine (Cys)-rich domain and a double-stranded β-helix fold (DSβH) domain that is compactly arranged to mediate catalytic activity. The DSβH domain consists of 3 Fe2+ binding sites and one α-ketoglutarate (α-KG) binding site. In addition, full-length TET1 and TET3 proteins contain N-terminal CXXC-type zinc finger domains. CXXC regulates the recruitment and binding of TET1 and TET3 to DNA sequences and provides a unique regulation of methylation signature for genes associated with embryogenesis, gametogenesis, and neuronal development.53,61–63 TET2 protein lacks a DNA recognition domain and depends on other DNA binding proteins for interaction with DNA. In addition, the short forms of TET1 and TET3, including TET1s, TET-3s, and TET-3o (an oocyte-specific isoform), all lack CXXC domains.54,63 Therefore, TET1s, TET-3o, and TET-3s are primarily dependent on the interaction of other DNA binding proteins for DNA binding.
2. The mutations and expression of TET genes in the pathogenesis of cancer
The expression of TET genes in cancers.
Compared to non-cancerous surrounding tissues, reduced levels of 5-hydroxymethylcytosine (5hmC) have been reported in multiple types of human cancers, such as hematopoietic malignancies, melanoma, lung cancers, pancreatic cancers, hormone-receptor-positive breast cancers, colon cancers, liver cancers, and glioblastoma multiforme, which are all associated with loss of functional TET mutations or decreased levels of TET proteins.64–74 The reduction of 5hmC results in the aberrant methylation of the tumor suppressor genes that leads to tumor formation, progression, and invasion. Studies suggested that low 5hmC is an important marker for early diagnosis and predicts poor prognosis in some cancer types.73–80 However, in some other cancer types, including gastric cancers, lung cancers, triple-negative breast cancer, human epidermal growth factor receptor-enriched breast cancers, ovarian cancers, and glioma, levels of TET proteins and 5-hmC are increased.81–84 TET proteins in such cancers function as oncoproteins, which promote cell proliferation and tumor progression. Thus, the roles of TET proteins in cancer pathogenesis might be tissue- and cell type-specific.85
The mutations of TET genes in cancers.
Loss-of-function TET2 mutations are frequently detected in blood cells from healthy individuals over 50 years old; it is referred to as ARCH. TET2 mutations in ARCH lead to a premalignant condition in hematopoietic tissue, which predisposes to leukemia/lymphoma transformation. TET2 mutations are commonly detected in almost all types of hematopoietic malignancies including MDS, myeloproliferative neoplasms, AML, PTCL, and DLBCL.30–42,86 Loss-of-function TET1 and TET3 mutations are detected in non-Hodgkin B-cell lymphoma, including DLBCL, and follicular lymphoma.87–91 In addition, TET1 is also mutated in 12–15% of T-ALL and 1–5% of AML patients.92,93 TET3 mutations are very rarely identified in PTCL37,94 and chronic lymphocytic leukemia.95 However, mutations of the TET1/2/3 genes are infrequent in solid cancers and their significance in such cases is unknown.66 In prostate cancers, TET2 mutations are detected in 6% of primary tumors and 20% of metastatic lesions.96 Whether TET2 mutations contribute to the metastatic advantage of prostate cancer needs to be determined experimentally.
Transcriptional regulation of TET1 gene in cancers (Figure 1a).
In embryonic stem (ES) cells, pluripotent genes OCT4, NANOG, MYC, and SOX2 are strongly enriched in a super-enhancer upstream of promoter 1 of the TET1 gene, stimulating the expression of TET1 but not TET1s.53 During differentiation, TET1 is down-modulated by PRC2 binding of the super-enhancer, localized +40 kb downstream of the TET1 TIS.97 HIF-2α binds −158 to −91 bp upstream of the TIS of TET1 and induces TET1 expression in response to conditions of hypoxia.98 In lung epithelial cells, p53 binds to −192bp/+29 bp of the promoter and represses TET1 expression.84 FOXA1 occupies the TET1 enhancer at +50 kb downstream of TIS and induces TET1 gene expression.99 During the prepubertal period, gonadotropin-releasing hormone (GnRH) stimulates luteinizing hormone-β polypeptide expression and differentiation of gonadotropic cells by repressing TET1s expression. GnRH plays such a role by inactivating a distal enhancer located −20 to 22 kb upstream of the TIS.100 TET2 binds to this enhancer to maintain TET1s expression.100 TET1 is downregulated in many types of cancers such as breast cancer, pancreas cancer, rectal cancer, oral squamous cell carcinoma, lymphoma, multiple myeloma, bladder cancer, liver cancer, and non-small-cell lung cancer, implying a tumor repressive activity for TET1.78,85,87,101,102 A CGI has been identified in the TET1 promoter and exon 1 region. In many types of cancers, downregulation of TET1 might be mediated by HMGA2 and PRC2 via epigenetic methylation of the CGI promoter.97,103 C/EBPα directly binds to the TET1 promoter and regulates TET1 expression104. In lung cancer and glioblastoma multiforme, epidermal growth factor receptor and MAPK activation silence TET1 expression by down-regulating C/EBPα. In basal-like breast cancer, thyroid carcinoma, skin cutaneous melanoma, and lung adenocarcinoma, TNFα stimulates NF-κB activation, which represses TET1 expression by binding to the TET1 promoter.105 In both cellular and animal models, inhibition of EGFR signaling restores TET1 expression.104 In colon cancers, BRAFV600E downregulates TET1 and TET2 expression which results in a hypermethylation phenotype in the cancer cells.106 TET1 downregulation is involved in disease initiation and cancer invasiveness/metastasis and is associated with a poor prognosis. In breast cancers, down-regulation of TET1 results in HOXA9/HOXA7 repression, which leads to breast cancer growth and metastasis.103 In prostate cancers, TET1 suppresses cancer invasiveness by activating the tissue inhibitors of metalloproteinases.107 In rectal cancers, TET1 inhibits the WNT signaling pathway by upregulating WNT inhibitors DKK3 and DKK4. Downregulation of TET1 promotes cancer development due to the activation of WNT signaling. Interestingly, a study suggests that TET1 is overexpressed in 40% of triple-negative breast cancer patients. In these types of cancers, TET1 expression is involved in cancer activation pathways including EGFR, PI3K, and PDGF, and is correlated with cell migration, cancer stemness, tumorigenicity, and poor survival.108–110 It suggests that TET1 might function as an oncoprotein and a therapeutic target in these types of cancers.85 Furthermore, TET1s is aberrantly expressed in multiple cancer types including breast, uterine, and glioblastoma. The predominant TET1s activation in cancer cells results in dynamic site-specific demethylation outside of CGIs, which is associated with worse overall survival in breast, uterine and ovarian cancers.54
Transcriptional regulation of TET2 and TET3 genes in cancer.
Compared to TET1, the transcriptional regulation of TET2 and TET3 genes has been studied much less (Figure 1a). In ES cells, OCT4 binds to the promoter at +1,788/+1,795 bp (relative to the TSS) of the TET2 gene and promotes TET2 expression.111 In addition, OCT4-SOX2 binding elements are identified at ∼−140 kb and −200 kb of the TET2 TSS.112 In response to hypoxia, HIF1α was found to repress TET2 expression in melanoma cells.113 In pancreatic cells, TGF1β induces the expression of TET2 by stimulating SMAD4 binding of an enhancer proximal to the distal 3’ region of the TET2 gene.70 Decreased TET2 and 5-hmC were found in ovarian carcinoma tissues and colorectal cancer patients, which was associated with high tumor grade, pathologic stage, lymph node metastasis, and vascular thrombosis as well as chemoresistance and poor clinical outcomes.114–116 GATA6 is a key TF for the differentiation of pancreatic progenitors. In aggressive squamous-like PDAC subtypes, TET2 is downregulated due to the loss of SMAD4, which is correlated, with a reduction of 5hmC and GATA6. Metformin and Vitamin C restore 5hmC and GATA6 levels by enhancing TET2 stability, reverting squamous-like tumor phenotypes, and WNT-dependence both in vitro and in vivo.70
CGIs have also been identified at the promoter, intron 1, and intron 2 of the TET3 gene. TET3 is epigenetically repressed in gliomas due to the methylation of these CGIs.117 Loss of TET3 expression was identified in 32 % of GCs and 28 % of CRCs.118 TET3 was downregulated in ovarian cancer cells during TGF-β1-induced epithelial-mesenchymal transition (EMT) and was correlated with pathological grade. TET3 over-expression was found to suppress ovarian cancer by up-regulating miR-30d, which then blocks TGF-β1-induced EMT.119 However, a study suggested that increased TET3 levels in ovarian carcinoma are associated with poor clinical-pathological status and poor prognosis.120
MicroRNAs regulate the expression of TET genes.
The expression of TET proteins is also regulated by microRNA (miR)-mediated posttranscriptional repression.121 Approximately 30 miRNAs have been identified that repress TET2 expression, including miR-7, miR-125b, miR-29b/c, miR-26, miR-101, miR142, and Let-7.122 TET1 expression is regulated by miR-29 family members including miR-26a, miR-767, miR-494, and miR-520b.123–126 In hematopoietic tissues, miR-22 promotes HSC self-renewal and leukemic transformation by repressing TET2.127 In inflamed mouse epithelial cells, inflammatory cytokines such as IL-1β and TNF-α repress the expression of TET proteins by inducing NF-κB signaling-mediated miR20a, miR26b, and miR29c expression.128 In gastric carcinogenesis, miR-26 represses TET1/2/3 expression.81 In hepatocellular carcinomas, miR29a promotes SOCS1–MMP9 signaling axis-mediated tumor metastasis by repressing TET proteins.72 In models of type 1 diabetes, miR142–3p targets TET2 and impairs Treg differentiation and stability.129 In macrophages, Let-7 promotes IL-6 by repressing Tet2 expression.130
3. TETs-TDG-BER system regulates DNA demethylation.
TET1, TET2 and TET3 are Fe2+ and α-KG-dependent dioxygenases. TETs catalyze the 1st step of demethylation by the hydroxylation of 5-methylcytosine (5mC) to 5hmC, and further oxidize 5hmC to 5-formylcytosine (5fC) and 5-carboxylcytosine (5caC).16 To fully complete the demethylation process, 5fC and 5caC, the products of TETs, can be replaced by cysteine via either replication-dependent dilution/passive DNA demethylation or thymine DNA glycosylase (TDG) and base excision repair (BER)-mediated active DNA demethylation.59,131 Both 5fC and 5caC are substrates for TDG. TDG catalyzes the excision of 5fC and 5caC to generate an apyrimidinic site (AP site). By coordinating with BER enzymes, TDG mediates the replacement of 5fC and 5caC with cysteine. Studies suggest that TDG is essential in protecting CpG-rich promoters from hypermethylation and collaborating with key TFs by actively removing methyl groups from enhancers and promoters of target genes.132 Thus, active dynamic DNA demethylation is primarily mediated by an IDHs-TETs-TDG-BER-driven cytosine modification system. In addition, it was found that activation-induced cytidine deaminase (AID)/APOBEC mediates an alternative oxidative deamination-demethylation pathway. AID/APOBEC is required for DNA demethylation during reprogramming of somatic cells and B cell maturation.133,134 AID catalyzes cytidine deaminases primarily at 5hmC sites to generate 5-hydroxymethyluracil (5hmU). 5hmU is subsequently cleaved by TDG, single-strand-selective monofunctional uracil-DNA glycosylase 1 (SMUG1), Nei-like DNA Glycosylase 1 (NEIL1), or methyl-CpG binding protein 4 (MBD4) and can be replaced by cytosine, as mediated by BER enzymes.135 Thus, AID mediates TET-dependent DNA demethylation.133,136,137 Furthermore, it was reported that both growth arrest and DNA damage-inducible protein 45a (GADD45a)138,139 and GADD45b play critical roles in the demethylation of specific promoters,138–140, and BER plays essential roles in genome-wide active DNA demethylation in primordial germ cells (PGCs).141 Further study demonstrated that TDG, AID, and GADD45a form a ternary complex in regulating the methylation state of promoters and enhancers within the genome. Thus, it is most likely that GADD45a/b-TDG-AID-BER altogether mediate active DNA demethylation.132
AID is a key enzyme that mediates DNA methylation dynamics in germinal center B- cells.142–144 AID initiates the somatic hypermutation process through deamination of cytidine to uridine in the recombined variable region, followed by removal of the uracil base by uridine DNA glycosylase and DNA repair by several error-prone BER and mismatch-repair enzymes.136 AID further induces the second step of antibody diversification, class-switch recombination, through deamination of bases in the switch region, causing double-strand breaks and recombination.137 AID is a key regulator of myeloid and erythroid differentiation and DNA methylation in HSPCs.134,145 The demethylation activity of AID is severely impaired in the absence of TET2, without impairment of AID mutability, suggesting that AID is dependent on TET2 for its demethylating capacity. This explains an AID-dependent hyper-mutagenesis feature and tumor development in TET2-deficient animals.
4. DNA 5-hmC is an epigenetic mark of gene activation
It should be clarified that 5hmC, 5fC, and 5caC are not only intermediates of passive and active DNA demethylation but also serve as stable epigenetic marks146,147 and have distinct epigenetic regulatory functions because they are distributed genome-wide and can be recognized by specific reader proteins.148,149 For example, several selective 5-hmC readers have been identified, such as MeCP2, the MBD3/NURD complex, E3 ubiquitin-protein ligases (UHRF1 and UHRF2), DNA glycosylases (MPG and NEIL3), SALL1/SALL4, Thy28, PRMT1 (CHTOP)-methylome complex, Recql helicase, RBM14, PRP8, RPL26, MSH6, PNKP, and WDR76.149–153 Only three of them, NP95/UHRF1, MeCP2, and MBD3, have been confirmed in more than one study.150 These proteins bind to 5hmC-DNA and regulate gene expression by recruiting co-activators or co-repressors. 5hmC is present in high amounts at active enhancers and the gene bodies of highly transcribed genes.154 5-hmC is associated with the activating histone marks H3K4me1, H3K4me3, and H3K27ac154–158. This explains, in many cases, that gene expression is closely related to the 5hmC/5mC ratio of enhancers and/or promoters.159–161 In addition, some of these 5-hmC reader proteins bind to 5hmC-DNA and recruit TETs, which further recruit TDG-BER complexes for completing the remaining steps of DNA demethylation.
5. The selective DNA binding of TET proteins.
Although all 3 TET family members and their isoforms have similar catalytic activity as demonstrated by certain levels of functional redundancy,162,163 the distinct phenotypes of Tet1, Tet2, and Tet3 knockout mice, as well as the distinct 5hmC/5mC patterns of Tet1, Tet2, and Tet3-deficient cells, suggest significant non-redundant functions for the Tet proteins.164–169 Such distinct roles of the three Tets are partially explained by their distinct expression profiles within developmental tissues. For example, Tet1 and Tet2 mRNA levels are abundant in ES cells and PGCs,170,171 while Tet3 is the only Tet gene expressed at substantial levels in oocytes and zygotes.172,173 Tet1 is expressed in fetal heart, lung, and brain, and adult skeletal muscle, thymus, and ovary, but not in adult heart, lung, or brain. Tet2 is primarily expressed in hematopoietic tissues.101,174 Tet3 is highly expressed in neural progenitor cells where it preferentially binds to TSSs and regulates cellular identity and genes associated with the lysosomes, autophagy, and base excision repair pathways.57,175 The non-redundant functions of the three TETs and their isoforms are also determined by their selective binding to genomic DNA regions. TET1 has a high affinity for a high density of CpG promoters, while TET2 is more commonly located at low CpG density promoters.169,175 In mouse ES cells, Tet1 primarily regulates 5hmC levels at gene promoters and TSSs, whereas Tet2 mainly regulates 5hmC levels in gene bodies and exon boundaries of highly expressed genes and exons, respectively.166,176 In induced pluripotent stem cells (iPSCs), TET1 and TET2 appear to target different genomic regions and promote opposing functions in reprogramming-mediated erasure of imprints and naïve pluripotent state transitions. TET1 promotes a primed state of pluripotency, while TET2 regulates a naïve state of pluripotency.166,177,178
DNA binding by TET1 and TET3 is primarily mediated by their CXXC domains, while the DNA binding of TET2 and the short isoforms of TET1 and TET3 is mediated by interactions with partner proteins. Both TET1 and TET3 regulate DNA methylation specifically at CpG sites within and around CGIs, appearing to show more flexible substrate specificity.179,180 The TET1-CXXC domain binds CpG-rich DNA irrespective of methylation status, while the TET3-CXXC domain binds methylated CpG sites with relatively low affinity compared to a non-methylated CpG dinucleotide, with the highest affinity toward 5caC sequences.61,181,182 In addition, TET1-CXXC also binds to TFs FOXA1 and HIF2α, selectively mediating active epigenetic modifications at FOXA1 and HIF2α-dependent enhancers, respectively.99
TET1 binds CGI chromatin globally via its CXXC to protect CpG sites within and around CGIs from gaining aberrant methylation,179 while TET1s preferentially binds to CpG sites at non-CGIs and some targeted CGI chromatin.54 Due to the selective binding of DNA regulatory regions, the roles of TET1 and TET3 are not always the same as their short isoforms and in many cases are the opposite. For example, compared to neurons, TET1 is highly expressed in glial cells, while TET1s is downregulated. TET1 and TET1s expression have opposing effects on synaptic transmission and hippocampal-dependent memory.183 In mice, Tet1 is restricted to early embryos, ES cells, and PGCs, whereas Tet1s is preferentially expressed in somatic cells. The expression of Tet1 and Tet1s switches during development and regulates epigenetic memory erasure.53 TET1s is overexpressed in multiple cancer types including breast, uterine, and glioblastoma, which is associated with worse overall survival.54
6. The interaction partner proteins of TETs.
Many partner proteins of TET1, TET2, and TET3 have been identified; however, the interaction regions have been defined only for some of them (Figure 2). Based on available information, most of the partner proteins bind to the C-terminal fragment including the DSβH enzymatic domain of TETs; only a few of them bind to the N-terminal fragment. However, the details of the interaction sites are only well known for Sin3A on TET1 and TET3, and O-linked GlcNAc transferase (OGT) on TET1. Sin3A interacts with the Sin3-interaction domain (SID) on TET1 (a.a. 889–903) and TET3 (a.a. 257–271). Although SID is absent from TET2 and its dimeric partner, CXXC4 might mediate the Sin3A-TET2 interaction.184,185 All three TETs interact with OGT through its C-terminal fragment.186–188 Detailed analysis demonstrated that the last 45 a.a. at the C-terminus (C45) of TET1 mediates the OGT binding.189 However, the detailed binding sites of OGT on TET2 and TET3 have not been determined.
Figure 2. Interaction partner proteins of TET proteins.
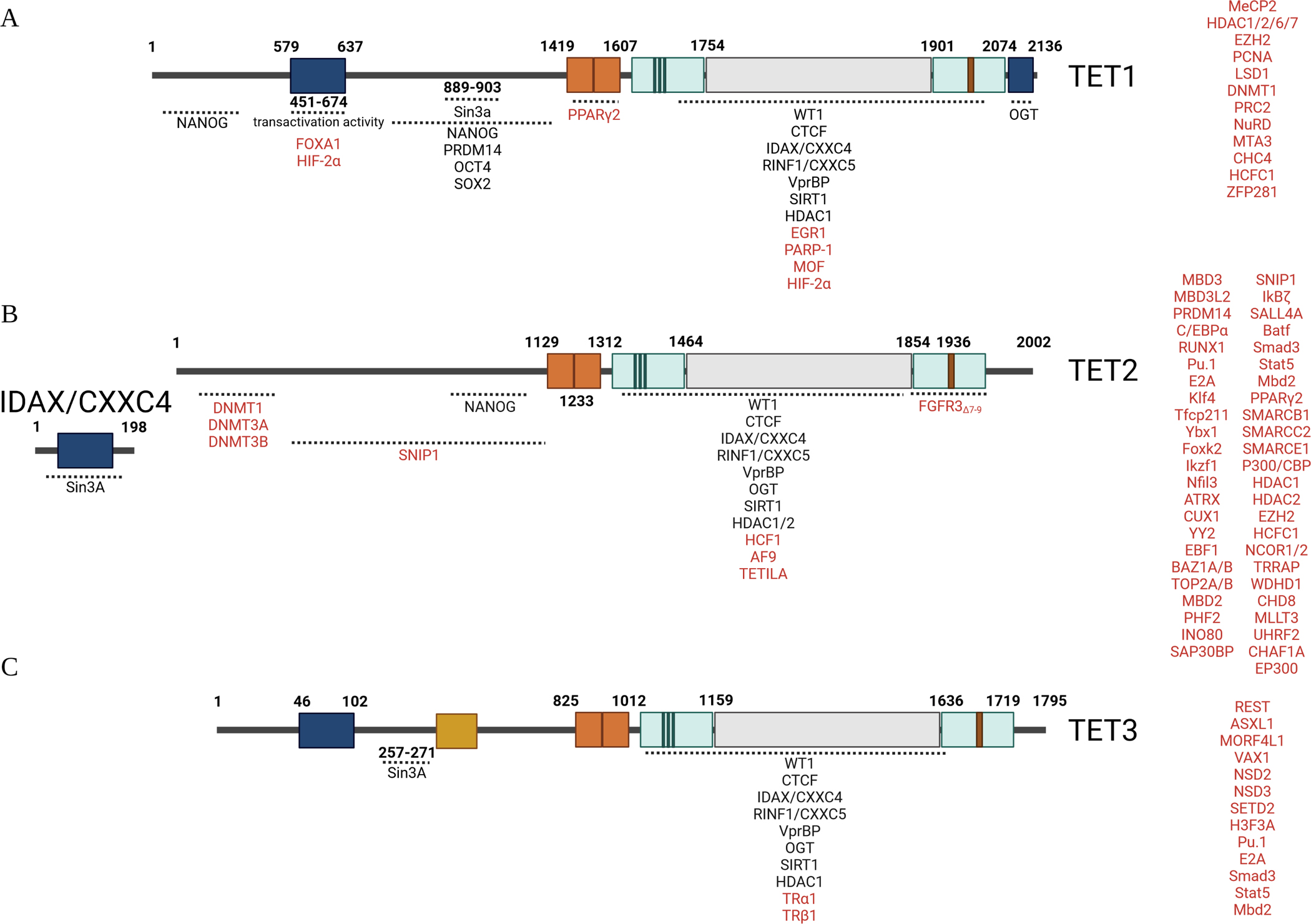
The interacting partner proteins of TET1, TET2, and TET3 are listed in a, b, and c, respectively. The partner proteins for which the interaction regions have been identified are listed under each TET at the corresponding regions. The partner proteins for which the interaction regions have not yet been defined are listed on the right side of each TET. The partners that are shared by all three TETs are listed in black font, while the partners that are specific for one or 2 TETs are listed in red font.
Partner proteins for all 3 TETs.
Among all partner proteins, some of them can interact with all three TETs. For example, CTCF can interact with all three TETs and recruit them to the CTCF-binding sites outside of CGIs, regulating DNA methylation and gene expression.190–193 CXXC4 and CXXC5 interact with the catalytic domain of TET2 as well as short isoforms of TET1 and TET3, recruiting TETs to DNA.194 As is true for the CXXC domain of TET1 and TET3, the CXXC domain in CXXC4 and CXXC5 proteins preferentially bind to unmethylated CGIs in gene promoter regions to maintain hypomethylation of CGIs.194,195 CXXC5 forms a complex with NANOG, OCT4, TET1, and TET2 and positively regulates the transcription of pluripotency genes and TET enzymes.196 Interestingly, CXXC4 negatively regulates TET2 activity by promoting caspase-mediated degradation of TET2 protein.194 WT1 physically interacts with TET2 and selectively regulates TET2-dependent expression of target genes such as RUNX1.197,198 WT1 also interacts with TET1 and TET3 for target gene expression.199 In addition, some histone modifiers such as SIRT1, histone deacetylases (HDACs) 1/2, and OGT as well as a variety of factors of the BER-DNA glycosylase pathway, including PARP1, MBD4, NEIL1, NEIL2, NEIL3, TDG, SMUG1, PARP1, LIG3, and XRCC1, also interact with all three TETs.200–202 All these shared interaction partners might partially explain the overlapping and compensatory functions of the three TET molecules.
Partner proteins that have been identified for certain TET proteins.
Many of the partner proteins selectively bind to one or two of the TETs and their isoforms. Several partner proteins for TET1 have been identified; these include MeCP2, EZH2, LSD1, hMOF, and PCNA.152,157,203–206 TET3 interacts with TFs including REST, ASXL1, MORF4L1, VAX1, and thyroid hormone nuclear receptor (TR), as well as the H3K36 methyltransferases NSD2, NSD3, and SETD2, as determined by immunoprecipitation and LC-MS/MS.193 Significantly more TET2-interacting partners have been identified, including TFs (C/EBPα, PU.1, Klf4, Tfcp2l1, MBD3, MBD3L2, YBX1, FOXK2, IKZF1, NFIL3, ATRX, CUX1, YY2, WT1, EBF1, SNIP1, PML and IκBζ159,198,207–212), histone modifiers (SMARCB1, SMARCC2, SMARCE1, P300/CBP, HDAC1, HDAC2, SIN3A EZH2, HCFC1, NCOR1/2, BAZ1A/B, TOP2A/B, MBD2, PHF2, INO80, SAP30BP, TRRAP, WDHD1, CHD8, MLLT3, UHRF2 and CHAF1A149,159,208,213–217), and signaling regulators (AMPK, JAK2, 14–3-3Z/D and 14–3-3E proteins203). These selective interacting partners of TETs determine the TETs’ functional specificity. For example, Mbd3/NURD recruits TET1 to genomic sites to regulate the expression of 5-hmC-marked genes in ES cells.152 Lin28A binds to active promoters and recruits TET1 to regulate gene expression.218 EGR1 recruits TET1s to target genes and selectively regulates the expression of EGR1 target genes by DNA demethylation.219 In iPSCs, ZFP281 drives TET1 to the promoter of target genes including TET2 to promote primed pluripotency. SNIP1 selectively interacts with TET2 (but not TET1 nor 3), bridging TET2 to TFs, including C-MYC, CDC5L, and BCLAF1.209 SNIP1 recruits TET2 to C-MYC target genes and regulates C-MYC target gene expression. TET2-SNIP1-cMYC ternary complex regulates target gene expression, playing a crucial role in DNA damage response and cellular apoptosis.209 REST recruits neuronal TET3 to mediate 5hmC formation and transcriptional activation.193 TET-3s also interacts with NSD2, NSD3, and SETD2 to regulate gene expression by mediating H3K36 trimethylation. In addition, TET3 interacts with TR to stabilize it and enhance its function independent of TET3 catalytic activity.220 TET3 also interacts with histone variants H3F3A, regulating chromatin modification.193
Functional subgroups of the partner proteins.
Based on their functions, the partner proteins of TETs can be divided into four groups: TFs, histone modifiers, signaling molecules, and factors of the BER-DNA glycosylase pathway. Most TFs such as NANOG, RUNX1, PU.1, and PPARγ bind to regulatory regions of the target genes and recruit TETs to regulate target gene expression.204,216,217,221 The binding motifs of ~66% TFs contain CpG dinucleotides. The binding of these TFs may be affected by CpG methylation.7 Based on the binding affinity of methylated CpG (mCpG) motifs, TFs can be divided into 4 types: MethylPlus TFs (TF1, preferred to bind to mCpG), 5hmC DNA readers (TF2, preferred to bind to 5hmCpG), Methylminus TFs (TF3, preferred to bind to CpG) and methylation insensitive TFs (TF4, little affected by methylation)7,8 (Figure 3). The TF1 (such as CEBPB, MBD1, MBD3, MeCP2, MBD3L2, GATA3, GATA5. WT1, PRDM14, Nanog, ZFP57/KAP1, OCT4, SOX2, HOXB13, KLF4, FOXA1, EBF1, EGR2) preferentially bind to 5mCpG motifs and function as pioneer factors to recruit TETs for converting 5mC into 5hmC.9,198,222–230 The TF2 (such as MeCP2, MBD3/NURD complex, UHRF1, UHRF2, MPG, NEIL3, and SALL1/SALL4) preferentially bind to 5hmC DNA sequences to either recruit TET-BER-DNA glycosylase complexes for fully demethylating DNA or recruit co-activators for activation of gene expression,202 while TF3 (such as AP-1, C-MYC /MAX, N-MYC, ETS-2, C-MYB, NF-κB, PAX5, RUNX1/2/3, NRF1, CTCF, CEBPα, CREB and PU.1) bind to CpG motifs to regulate gene expression by recruiting TET-histone modification complexes.231,232 On the unmethylated DNA sequences, TETs might also play a role in maintaining the unmethylated state. Interestingly, the IDAX protein binds to unmethylated CpGs and inhibits TET2 binding to the demethylated regions through activation of caspase-mediated degradation, which might help to stop the demethylation process.194 Thus, it is most likely that the TFs form a hierarchy, which sequentially binds to DNA sequences and cooperates with TET proteins and histone modifiers to regulate target gene expression. Consequently, cell-type-specific TFs mediate a cell-type-specific binding pattern of TET proteins (Figure 3). For example, in mouse ES cells, Tet1 uses its CXXC domain to bind to enhancers with 5mCpG islands and converts 5mC into 5hmC. Sall4a binds to 5hmC at enhancers and facilitates further oxidation of 5hmC at its binding site by recruiting Tet2.233 MBD3/NURD binds to 5hmC and recruits TET1 to genomic sites to regulate the expression of 5-hmC-marked genes.152 Such TET protein-associated sequential binding of TFs to DNA sequences is observed in almost all cellular processes by regulating the epigenetic landscape and inducing the expression of fate-determining genes.
Figure 3. Subgroups of TET-interacting TFs.
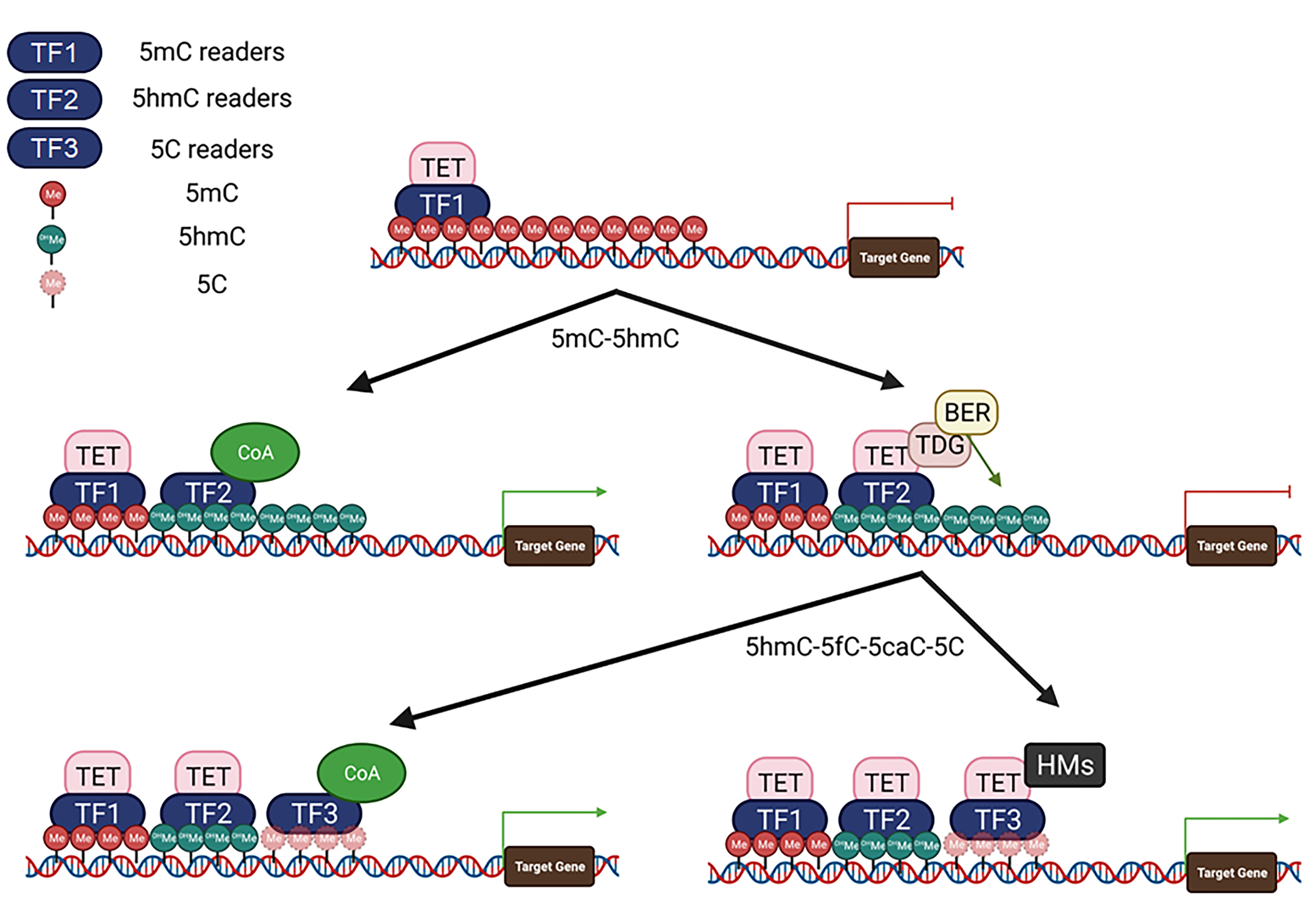
The TET-interacting TFs can be divided into TF1, TF2, and TF3 based on their binding affinity for 5mC, 5hmC, and 5C, respectively. TF1 can bind 5mC DNA and recruits TET to initiate the first step of DNA demethylation by converting 5mC to 5hmC. TF2 can bind to 5hmC promoters/enhancers to turn on gene expression by recruiting coactivators (CoA) or to further complete the DNA demethylation elements by recruiting TET-TDG-BER complexes. TF3 binds 5C promoters/enhancers to promote gene expression by recruiting CoA or regulating gene expression by recruiting TET-histone modifiers (HMs).
Methylation serves as a barrier to reprogramming and differentiation.234,235 During induced reprograming of epiblast-like cells to PSCs, PRDM14 induces TET1/2-demethylation-mediated recruitment of OCT3/4 to the enhancers of pluripotent genes such as Klf2.236 During the specification of PSCs to primordial germ cells, PRDM14, Nanog, and OCT4 are capable of binding to 5mCpG sites to initiate the stepwise epigenetic modification by recruiting TET1/2 proteins and other epigenetic modifiers.224 During induced reprograming of B cells or embryonic fibroblasts to generate PSCs, Tet2 is recruited by Klf4 and Tfcp2lƒ1 respectively to drive active enhancer demethylation of chromatin and induce pluripotency-related genes.210 Thus, most TF1s are fate-instructive pioneer factors that initiate the cellular processes such as lineage commitment and differentiation by establishing epigenetic configurations,237–241 specifically when they collaborate with non-pioneer TFs.242–244
However, such mGpC binding affinity-based sub-classification of TFs is not always accurate because the binding affinity can be influenced by the surrounding sequence context. In addition, many TFs have more than one consensus-binding motif, while methylation only influences the binding of TFs to certain motifs. Thus, many TF3s can also function as pioneer factors, especially in collaboration with other TFs. For example, during the differentiation of fibroblasts to adipocytes, C/EBPα and CREB heterodimerize and bind half-CRE (CGTCA) and half-C/EBP (CGCAA) sequences of the tissue-specific methylated promoters to initiate DNA demethylation by recruiting TET2.245 This allows the binding of other TF3s (such as CEBPα/β, c-Jun, JunD, ATF2 or PU.1) for transcriptional activation.7 During differentiation of pro-B progenitors to pre-B progenitors, PU.1 and E2A bind to the 5mC enhancers of target genes and recruit TET2 and TET3 for stepwise DNA methylation. This is followed by the binding of other key B-cell-specific TFs to turn on the B-cell differentiation process.215 During induced pre-B-cell-to-macrophage trans-differentiation, CEBPα collaborates with PU.1 to induce the myeloid cell fate by regulating two types of enhancers on myeloid genes, pre-existing ones, and de novo ones. The pre-existing enhancers are primed by PU.1, which maintains chromatin accessibility for the binding of CEBPα. In de novo promoters, CEBPα acts as a pioneer factor to initiate TET2-mediated de-methylation followed by PU.1 recruitment.210,246 Therefore, the functional identification of pioneer factor(s) for different cellular processes is needed to elucidate transcriptional-epigenetic regulatory mechanisms for each cellular process.
Among the histone modifiers, most of them are negative transcriptional regulators such as the SIN3A complex, the NuRD complex, HDAC1, HDAC2, and EZH2, which mediate target gene repression,157,208,247 while some others are positive transcriptional regulators, including CBP, hMOF, NSD2, NSD3 and SETD2 that promote gene expression via modulating H3K27ac, H4K16ac and H3K36Me on the promoters.248 Such TET-related histone modification is independent of the catalytic activity of TETs. Furthermore, some of the partner proteins such as OGT, PARP1, and VprBP regulate the functions of TETs through post-transcriptional modifications (see the following section).
7. Post-translational regulation of TET proteins
The N-terminal sequence of the TET proteins plays a critical role in regulating TET activity by interacting with their catalytic domains. Mammalian TETs undergo a plethora of post-translational modifications (PTMs). However, the functional significance of some of these modifications is not yet well understood. Some of the well-known PTMs that are commonly found on TETs are GlcNAc, phosphorylation, ubiquitylation, acetylation, and proteolysis213,249–253 (Figure 4).
Figure 4. Post-translational modifications of TET proteins.
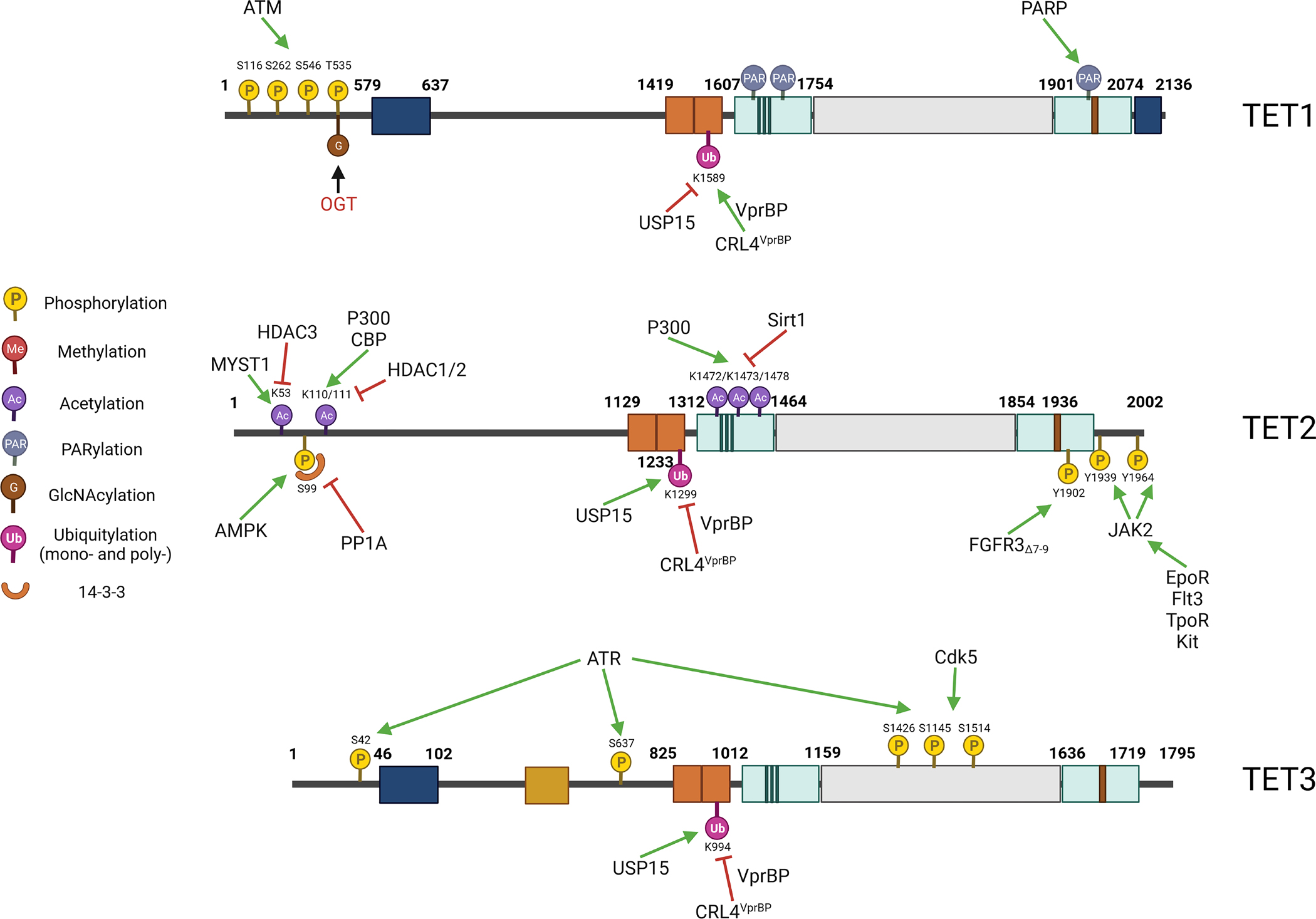
The post-translational modifications of TET1, TET2, and TET3 are listed in a, b, and c, respectively. The green arrow depicts the addition of modifications. The Red Cross indicates removal modifications.
Phosphorylation regulates the activities of TET proteins.
Mass spectrometric analysis identified over 10–20 residues that can be phosphorylated in each of the TET proteins.254 However, the role of phosphorylation has only been functionally studied for a few of these residues. During DNA damage repair, ATM phosphorylates TET1 on S116, S262, and S546, regulating DNA repair.255 The energy sensor, AMPK (AMP-activated protein kinase), phosphorylates human TET2 on Ser99 (murine Tet2, Ser97), protecting TET2 protein from calpain-mediated degradation. Thus, active AMPK promotes TET2 stability and facilitates its tumor-suppressive function.252,256,257 Several members of the 14–3-3 group of adaptor proteins bind to Ser99 phosphorylated TET2 and protect it from phosphatase 2A (PP2A)-mediated dephosphorylation.258,259 The association of 14–3-3 proteins is impaired in some leukemia-related TET2-mutants (around residue Ser99), explaining the reduced protein stability of these mutant TET2 proteins.258 In diabetic mice, high glucose levels impede the tumor-suppressive activity of TET2 and accelerate tumor development by blocking AMPK-mediated phosphorylation of TET2 and reducing TET2 protein levels as demonstrated in xenograft tumor models.252 This explains why diabetic patients have an increased risk for cancer and cancer patients with diabetes have a poor prognosis as observed by epidemiological studies.260,261 The anti-diabetic drug metformin and other AMPK activators such as A769662 display antitumor activity by activating AMPK-mediated phosphorylation of TET2 Ser99 and increasing 5hmC levels. Diabetes risk reduction diets improve the survival of cancer patients.262 In addition, in erythroid progenitor cells, hematopoietic cytokines such as erythropoietin (EPO) stimulate JAK2-mediated phosphorylation of TET2 on Tyr1939 and Tyr1964 residues, which enhances TET2 binding of the TF KLF1 and increases TET2 activity for the proliferation and differentiation of erythroid progenitor cells.263 Consistently, in primary samples from patients with myeloproliferative neoplasms, JAK2V617F increases TET2 activity and 5-hmC with genome-wide loss of cytosine methylation, leading to increased expression of several oncogenic transcripts, such as MEIS1 and HOXA9.263 In hepatocellular carcinoma patients, FGFR3Δ7–9, a splicing mutant of FGFR3, directly interacts with TET2 and phosphorylates TET2 on its Y1902 site, leading to the ubiquitination and proteasome-mediated degradation of TET2.264 Such phosphorylation-related downregulation of TET2 enhances cancer cell proliferation through repression of PTEN and upregulation of AKT signaling. Interestingly, in CML cell lines, the BCR-ABL fusion protein interacts with TET2 and sequesters the latter by cytoplasmic compartmentalization in a complex tethered by FOXO3a.265 Imatinib treatment releases TET2 from the complex and imports TET2 into the nucleus together with FOXO3a to activate BIM expression by binding to the BIM promoter.265 Whether TET2 is phosphorylated by BCR-ABL kinase needs to be determined.
During neuronal differentiation, CDK5 phosphorylates TET3 on residues Ser1310 and Ser1379 (Ser1318 and Ser1387 for the mouse) within its catalytic domain, changing its dioxygenase activity.253 Phosphorylated TET3 promotes the expression of the neuron-specific TF BRN2, as well as neuronal differentiation, through enhancing the enrichment of 5hmC and H2A.Z occupancy at the promoter of the BRN2 gene. Non-phosphorylated TET3 promotes the expression of genes that are linked to metabolic processes.253 In response to DNA damage, ataxia-telangiectasia and Rad3-related kinase (ATR) phosphorylates TET3 on residues Ser42, 637, and 1426. TET3 phosphorylated in this way mediates DNA oxidation which promotes the ATR-dependent DNA damage response.266
GlcNAc regulates the activity of TET proteins.
All three TETs interact with OGT.186–188 OGT regulates their stability and their activities by catalyzing GlcNAc and thereby regulating the phosphorylation of the proteins at their N-termini and low-complexity insert regions.254,267,268 OGT is also involved in the regulation of the binding of TETs to some genomic sites.269 At least 8 GlcNAc sites have been reported for TET1 and up to 20 such have been identified for both TET2 and TET3.186,254,267,269 Many of these GlcNAc sites, such as Ser97 and Ser374 of TET2, Ser362 and Ser557 of TET3, and Ser950 and Ser2016 of TET1, could also be phosphorylated. Thus, GlcNAc represses the phosphorylation of the corresponding sites and regulates the binding of TETs with other partners.254 In addition, the GlcNAc site Thr535 on TET1 enhances this protein’s stability,269 while GlcNAc of TET3 promotes its cytoplasmic relocation,268 and GlcNAc of TET2 reduces its enzymatic activity by enhancing its nuclear export.188 Furthermore, OGT regulates the expression of TET target genes by GlcNAc and several other epigenetic modifiers and histones (see the following section).
Ubiquitination regulates the activities of the TET proteins.
VprBP binds the cysteine-rich, dioxygenase domain of all three proteins, exerting a critical regulatory function on TET dioxygenases in normal tissue development and tumor suppression. VprBP induces CRL4VprBP (VprBP-DDB1-CUL4-ROC1) E3 ubiquitin ligase-mediated monoubiquitylation of TET1 on Lys1589 (Lys1537 in the mouse), of TET2 on Lys1299 (Lys1212 in the mouse), and of TET3 on Lys994 (Lys983 in the mouse). Such monoubiquitylation facilitates TET binding to chromatin and enhances 5hmC in corresponding genomic regions.213 TET2 mutations in leukemic cells on either Lys1299 or residues essential for VprBP binding result in reduced chromatin binding and activity of TET2.213 In addition, mutation of Lys983 of TET3, but of neither TET1 nor TET2, also alters the enzyme’s subcellular localization from almost exclusively nuclear to mostly cytoplasmic. Whether monoubiquitylation selectively regulates the subcellular localization of TET3 needs to be determined. Interestingly, during HIV infection, the viral protein Vpr induces CRL4VprBP–mediated poly-ubiquitination of TET2 Lys1299, inducing the degradation of TET2 to sustain IL-6 expression and enhance HIV-1 replication.213,270
Acetylation regulates the activity of TET proteins.
During oxidative stress, transcriptional co-activator p300 acetylates TET2 on Lys110/111 residues to enhance the enzymatic activity of TET2 and to protect the protein against proteasomal degradation by the inhibition of TET2 ubiquitination on certain residues in the C-terminal DSBH domain.211 TET2 acetylation enhances DNMT1 binding to promote protein stability. Consequently, TET2, along with TDG, is recruited to chromatin by DNMT1 to prevent abnormal DNA methylation. TET2 Lys110/111 deacetylation is mediated by HDAC1/2.208 TET1 and TET3 are also acetylated by p300; however, the detailed sites for such modifications have not been determined.211 In MDS, SIRT1 interacts with the TET2 C-terminal domain (a.a. CD1129–2002) and deacetylates it on Lys1472, 1473, and 1478 in CD34+ HSPCs, regulating the stability and function of TET2 protein. SIRT1-deficient MDS HSPCs exhibit enhanced cell growth and self-renewal due to the reduction of TET2 levels.271 The SIRT1 activator SRT1720 inhibits colony formation in MDS HSPCs and in vivo engraftment in NSGS mice by enhancing the tumor repressive activity of TET2.
Other post-translational modifications of TET proteins.
The stability of TET proteins is regulated by calpains.272 TET1 and TET2 are degraded by calpain 1 in mouse ES cells; whereas TET3 is degraded by calpain 2 during ES cell differentiation.272 TET1 interacts with PARP1/ARTD1 and is targeted by both noncovalent and covalent PARylation in TET1’s catalytic domain. The noncovalent binding of ADP-ribose polymers decreases TET1’s hydroxylase activity, while covalent PARylation stabilizes the TET1 enzyme and enhances its activity.273–275 In addition, PARP1 also promotes TET1 gene expression by regulating DNA and histone modifications on the TET1 promoter.274
Metabolic regulation of TET protein activity.
Fe2+ and α-KG, together with O−2 and vitamin C, function as TET co-factors and are required for their dioxygenase activity.276–279 Both Fe2+ and α-KG bind to the catalytic domain of TETs facilitating the insertion of 5mC into their catalytic pocket and providing accommodation to the oxidized derivatives of 5mC including 5hmC, 5fC, and 5caC.180,280,281 Thus, the dioxygenase activity of TET proteins is dependent on the availability of α-KG, Fe+2, and O2. α-KG is a product of IDHs, a family of metabolic enzymes. The IDHs, IDH1, IDH2, and IDH3, catalyze the oxidative decarboxylation of isocitrate to α-KG, which is an essential step in the tricarboxylic acid cycle. IDH1/2 mutations are commonly detected in gliomas and hematopoietic malignancies which lead to the production of the oncometabolite 2-hydroxyglutarate (2HG). Mutations in other genes encoding for the metabolic enzymes succinate dehydrogenase (SDH) and fumarate hydratase (FH) are prevalent in gliomas, cholangiocarcinomas, renal cell carcinomas, and acute myeloid leukemias, among others. SDH and FH mutations lead to the production of the oncometabolite succinate and fumarate, respectively.282–284 An overabundance of these oncometabolites influences the catalytic activities of TET1/2/3 by competitive inhibition of their α-KG binding site. In activated macrophages, itaconic acid, a metabolic product of the IRG1 enzyme, also inhibits the catalytic activity of TET2 via inhibition of TET2/α-KG binding.285 Thus TET-mediated DNA demethylation is tightly regulated by glucose metabolism. Reactive oxygen species (ROS) and metal chelators can impede TETs’ oxidizing activities by reducing the Fe+2 availability.286 Vitamin C convents inactive Fe+3 to active Fe+2 which promotes TET1/2/3 enzymatic activity.287 Thus, in addition to its antioxidant properties, Vitamin C regulates gene expression and genomic stability by increasing TET-mediated 5hmC formation and promoting DNA demethylation.277,288–290
8. TETs regulate gene expression by both enzyme-dependent and -independent mechanisms.
TETs play critical roles in organ generation during embryonic development and tissue regeneration during postnatal life.162,176 TETs play such roles by regulating the timed expression of the key genes that determine cell identity and control cell differentiation. TETs and lineage-specific TFs cooperate to influence chromatin accessibility and regulate gene expression by 1) promoting site-specific DNA demethylation (mainly enhancers and CGI-rich promoter elements), and enzymatic-dependent activity;16,58,157,173,176,280 and 2) regulating histone modifications via an enzymatic-independent activity by forming chromatin regulatory complexes with OGT, HDACs and/or histone acetyltransferases (HATs)188,208,291,292 Figure 5.
Figure 5. TETs regulate gene expression by both enzyme-dependent and -independent mechanisms.
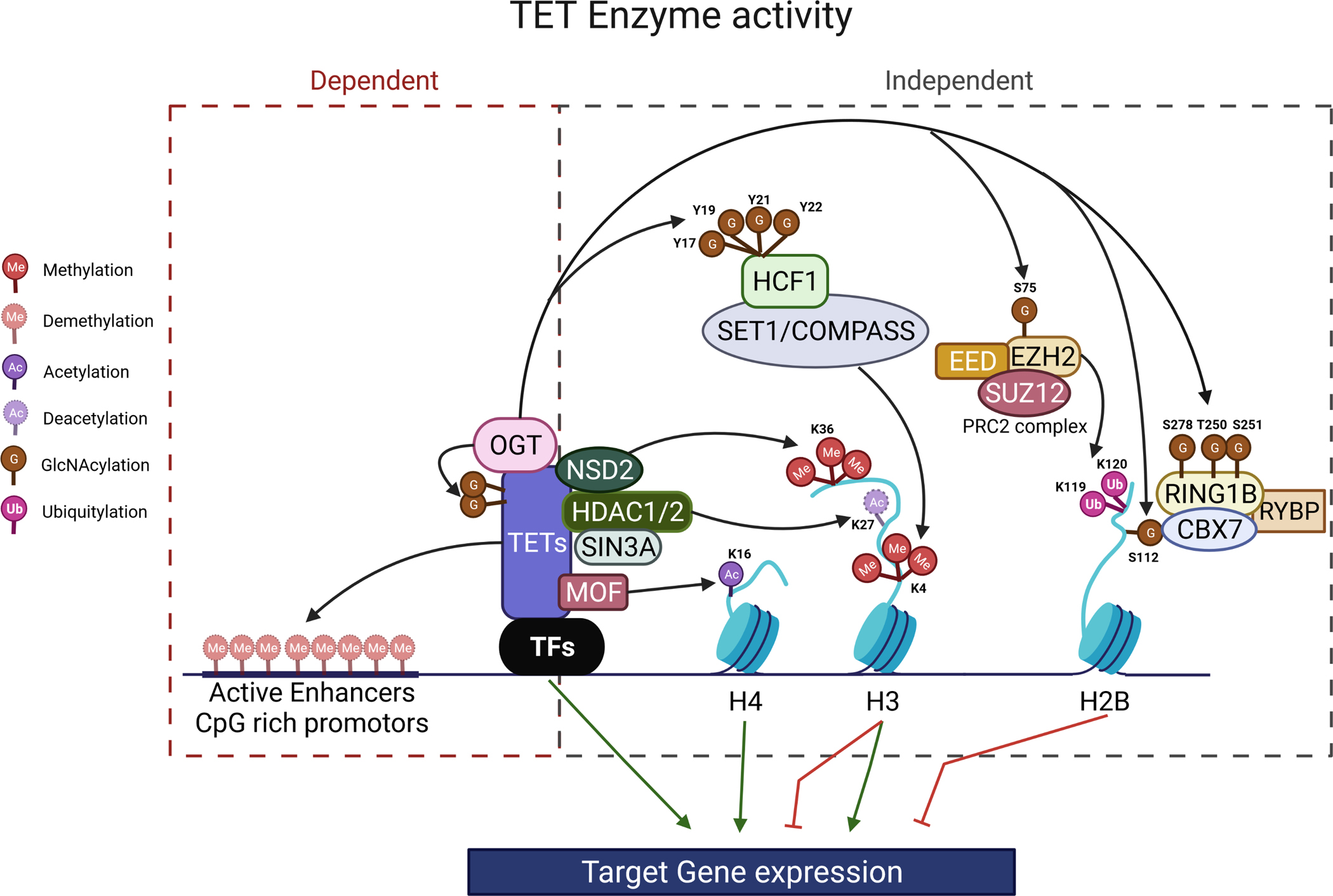
After binding to DNA regulator regions, TETs regulate target gene expression by 1) enzymatic demethylation of 5mC; and 2) recruiting histone modifiers, including OGT, Sin3A/HDACs, or HATs.
TETs regulate gene expression by enzymatic activity-dependent site-specific DNA demethylation.
TET proteins collaborate with lineage-specifying TFs in cells, promoting the expression of cell type-specific genes by demethylation of enhancers of target genes. ChIP-seq assays demonstrated that the top enriched binding motifs of TET proteins in DNA are enriched with binding sites for lineage-specifying TFs of the respective cell types, maintaining 5hmC and demethylation state in the active enhancers of target genes in an enzyme-dependent fashion.12,30,176,223,293,294 For example, in ES cells, TET1/2 together with master self-renewal TFs including SOX2, KLF4, ESRRG, POU5F1, and NANOG bind enhancers of the target genes that are essential for the maintenance of self-renewal.14 In myeloid cells, TET2, together with key myeloid differentiation TFs such as ERG, RUNX1, CEBPA, and GATA1, bind enhancers of genes that are necessary for myeloid lineage commitment and differentiation.14 Loss of TET2 causes down-regulation of cell-type-specific genes due to the widespread reduction of 5hmC and increased methylation of their enhancers, altering cell fate.14,295
In many types of cancers, TET proteins function as tumor suppressors by activating the expression of tumor repressive genes. For example, in gastric cancer, TET1 inhibits the AKT and FAK signaling pathways by demethylation of the PTEN gene promoter.68 In colon cancer, TETs suppress the proliferation of cancer cells by demethylating DKK gene promoters, inhibiting the Wnt signaling cascade.296 In pancreatic cancer, TET1 restricts the cell cycle of cancer cells by upregulating negative cell cycle regulators such as p16.69 In these types of cancers, restoration of the enzymatic activity of TETs is a potent treatment strategy. However, in some other types of cancers, TET proteins function as putative oncoproteins and promote stemness in the cancer stem-like subpopulation that drives aggressiveness and chemoresistance. For example, in ovarian cancers, TET1 induces the expression of cancer stem cell genes, which reprograms epithelial cancer cells into a cancer stem-like state.82 In gliomas, TET1 and TET3 promote stemness and self-renewal of tumor cells by regulating the expression of core stem cell genes.297,298 In breast cancers, TET1 and TET3 cooperatively induce cancer stem-like cells by activating the TNFα-p38-MAPK signaling axis.299 In such types of cancers, high levels of TET proteins promote a subpopulation of the slow-growing chemoresistant stem-like cells that are associated with disease relapse and poor prognosis.300 In addition, TET proteins also regulate EMT and cancer metastasis in a context-dependent manner.98,119,299,301 Thus, targeting TET enzymes for cancer therapy should be also strategized for context-dependence.
TETs regulate gene expression via enzymatic activity-independent histone modifications.
Through their enzymatic-independent activities, TET proteins primarily repress gene expression. For example, TET proteins recruit OGT to histones at the promoters of target genes and regulate target gene expression by mediating OGT-dependent GlcNAc of TFs, epigenetic regulators, and histones.89,90,302 GlcNAc of H2B on Ser112 is required for subsequent Lys120 monoubiquitination and PRC1-mediated gene silencing.303 GlcNAc of RING1B on Ser278 and Thr250/Ser251 residues promotes the binding of RING1B to CBX7 and RYBP to form PRC1, resulting in H2BK119 ubiquitination and silencing of a specific subset of genes.304 In the PRC2 complex, EZH2 is modified by GlcNAc on Ser75, which results in its being stabilized, thus negatively regulating tumor suppressor genes.305 In addition, TET proteins recruit Sin3A/HDAC1/2 to target genes, repressing target gene expression by deacetylation of histone H3K27.206,208 However, TET proteins also promote some target gene expression via enzymatic-independent activity. For example, via OGT-mediated GlcNAc of HCF1 (Tyr17, 19, 21, and 22), the key component of H3K4 methyltransferase SET1/COMPASS complexes, TET proteins promote chromatin binding and H3K4me3, inducing target gene expression.186–188,267,269,306,307 TET1 also upregulates the expression of proliferation and DNA damage repair genes by recruiting the HAT protein MOF to promoters, acetylating H4K16.248 TET3 promotes transcriptional activation in neurons by recruiting NSD2, NSD3, and SETD2, thus mediating H4K36 tri-methylation.
Prospective
The activity of TET proteins shapes the local chromatin environment at enhancers and promoters to facilitate the binding of TFs and affect gene expression patterns.14 Mutations or dysregulation of TET proteins lead to abnormal DNA methylation patterns and epigenetic chromatin modifications, driving disease development. Although TET1 and TET3 have their DNA binding CXXC-domains which mediate a region-specific DNA binding and demethylation, TET2 and the short forms of TET1 and TET3, rely on interactions with TFs for DNA binding. Thus, the regions of DNA binding of these TETs must conform to cell-type specificity, which is controlled by cell-type-specific TFs.
Based on their binding affinity to methylated DNA, TFs form a hierarchy in DNA binding, chromatin modification, and gene regulation. First, the methylation-insensitive pioneer TFs bind to methylated DNA to initiate DNA demethylation by recruiting TET proteins to convert 5mC to 5hmC.308 Next, the secondary level of TFs (5hmC readers) bind to 5hmC-DNA to activate gene expression by recruiting co-activators or to further complete DNA demethylation by recruiting a TET-TDG-BER complex. Finally, the third level TFs (methylation-sensitive) occupy the unmethylated DNA to control gene expression by recruiting co-activators or co-repressors. Such sequential and cooperative binding of TFs and TETs leads to a relatively large open region of chromatin, which forms a super-enhancer in the target genes to determine the fate of cells.232 Many TFs can bind TET proteins. However, the hierarchy of these TFs has not yet been well characterized. Thus the manner in which these TFs cooperate with TET proteins in the regulation of target gene expression needs to be better elucidated in the future.
Both positive and negative regulatory effects of TET proteins on target gene expression have been reported. TET proteins activate the expression of target genes primarily by enzymatic activity mediated DNA demethylation and/or by OGT-regulated SET1/COMPAS-mediated H3K4 trimethylation, while they repress the expression of target genes by recruiting SIN3A/HDAC1/2 or OGT-regulated PRC1. It is still unknown how such positive and negative regulatory mechanisms are coordinated in regulating target gene expression. Furthermore, the activity of TET proteins is regulated by many types of posttranslational modifications. Detailed study of the molecular mechanisms by which the activities of TET proteins are regulated and how TET proteins selectively regulate target gene expression will provide useful information for designing medications for a new generation of TET-related disease treatment.
Key points:
TET proteins play a key role in regulating both normal and disease tissue regeneration;
TET proteins regulate the dynamic differentiation and fate determination of tissue stem and progenitor cells;
TET proteins regulate target gene expression by demethylation of enhancers/promoters and modification of histones of the target genes;
The function of TET proteins is regulated by interacting partners and posttranslational modifications.
Funding
This work was supported by NIH grants R01 HL133560 and R01 CA223194 through Loyola University Chicago, as well as Loyola program development funds to Jiwang Zhang.
Abbreviations used
- TET
Ten-eleven translocation
- α-KG
α-ketoglutarate
- 5mC
5-methylcytosine
- 5hmC
5-hydroxymethylcytosine
- 5fC
5-formylcytosine
- 5caC
5-carboxylcytosine
- TFs
Transcription factors
- ARCH
Age-related clonal hematopoiesis
- MDS
Myelodysplastic syndromes
- AML
Acute myeloid leukemia
- ALL
Acute lymphoblastic leukemia
- DLBCLs
Diffuse large B cell lymphomas
- PTCL
Peripheral T-cell lymphoma
- IFN-γ
Interferon-γ
- TNF-α
Tumor necrosis factor-α
- TIS
Transcriptional initiation sites
- DSBH
Double-stranded beta-helix domain
- TDG
Thymine DNA glycosylase
- BER
Base excision repair
- ES
Embryonic stem cells
- IDHs
Isocitrate dehydrogenases
- AP site
Apyrimidinic site
- AID
Activation-induced cytidine deaminase
- 5hmU
5-hydroxymethyluracil
- GC
Germinal center
- CSR
Class-switch recombination
- DSBs
Double-strand breaks
- PGCs
Primordial germ cells
- CGIs
CpG islands
- OGT
O-linked GlcNAc transferase
- SID
Sin3A interacts with the Sin3-interaction domain
- CoA
Coactivators
- PTM
Post-translational modifications
- AMPK
AMP-activated protein kinase
- C/EBPα
CCAAT/enhancer-binding protein alpha
- KLF4
Kruppel-like factor-4
- TFCP2l1
Transcription factor CP2 like-1
- YBX1
Y box-binding protein-1
- FOXK2
Forkhead box protein K-2
- KZF1
DNA-binding protein Ikaros 1
- NFIL3
Nuclear factor interleukin-3-regulated protein
- ATRX
Alpha-thalassemia/mental retardation syndrome X-linked transcriptional regulator
- CUX1
Homeobox protein cut-like-1
- YY2
Yin and yang-2 transcription factor
- IκBζ
Inhibitory-kappa-B-zeta
Footnotes
Conflicts of interest
The authors declare that they have no competing financial or professional interests.
Ethics approval
This is not applicable for this review.
Consent to participate
This is not applicable for this review.
Consent for publication
This is not applicable for this review.
Availability of data and material
This is not applicable for this review.
Code availability
This is not applicable for this review.
References
- 1.McKinney-Freeman S et al. The transcriptional landscape of hematopoietic stem cell ontogeny. Cell Stem Cell 11, 701–714, doi: 10.1016/j.stem.2012.07.018 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Long HK, Prescott SL & Wysocka J Ever-Changing Landscapes: Transcriptional Enhancers in Development and Evolution. Cell 167, 1170–1187, doi: 10.1016/j.cell.2016.09.018 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kulis M et al. Whole-genome fingerprint of the DNA methylome during human B cell differentiation. Nat Genet 47, 746–756, doi: 10.1038/ng.3291 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barwick BG, Scharer CD, Bally APR & Boss JM Plasma cell differentiation is coupled to division-dependent DNA hypomethylation and gene regulation. Nat Immunol 17, 1216–1225, doi: 10.1038/ni.3519 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith ZD & Meissner A DNA methylation: roles in mammalian development. Nat Rev Genet 14, 204–220, doi: 10.1038/nrg3354 (2013). [DOI] [PubMed] [Google Scholar]
- 6.Apostolou E & Hochedlinger K Chromatin dynamics during cellular reprogramming. Nature 502, 462–471, doi: 10.1038/nature12749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin Y et al. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science 356, doi: 10.1126/science.aaj2239 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schubeler D Function and information content of DNA methylation. Nature 517, 321–326, doi: 10.1038/nature14192 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Hu S et al. DNA methylation presents distinct binding sites for human transcription factors. eLife 2, e00726, doi: 10.7554/eLife.00726 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klose RJ & Bird AP Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci 31, 89–97, doi: 10.1016/j.tibs.2005.12.008 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Greenberg MVC & Bourc’his D The diverse roles of DNA methylation in mammalian development and disease. Nat Rev Mol Cell Biol 20, 590–607, doi: 10.1038/s41580-019-0159-6 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki J et al. TET2 Mutations Affect Non-CpG Island DNA Methylation at Enhancers and Transcription Factor-Binding Sites in Chronic Myelomonocytic Leukemia. Cancer Res 75, 2833–2843, doi: 10.1158/0008-5472.CAN-14-0739 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lea AJ et al. Genome-wide quantification of the effects of DNA methylation on human gene regulation. eLife 7, doi: 10.7554/eLife.37513 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rasmussen KD et al. TET2 binding to enhancers facilitates transcription factor recruitment in hematopoietic cells. Genome Res 29, 564–575, doi: 10.1101/gr.239277.118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Z & Zhang Y Role of Mammalian DNA Methyltransferases in Development. Annu Rev Biochem 89, 135–158, doi: 10.1146/annurev-biochem-103019-102815 (2020). [DOI] [PubMed] [Google Scholar]
- 16.Wu X & Zhang Y TET-mediated active DNA demethylation: mechanism, function and beyond. Nat Rev Genet 18, 517–534, doi: 10.1038/nrg.2017.33 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Broome R et al. TET2 is a component of the estrogen receptor complex and controls 5mC to 5hmC conversion at estrogen receptor cis-regulatory regions. Cell reports 34, 108776, doi: 10.1016/j.celrep.2021.108776 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruz-Rodriguez N, Combita AL & Zabaleta J Epigenetics in Hematological Malignancies. Methods Mol Biol 1856, 87–101, doi: 10.1007/978-1-4939-8751-1_5 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Hu D & Shilatifard A Epigenetics of hematopoiesis and hematological malignancies. Genes Dev 30, 2021–2041, doi: 10.1101/gad.284109.116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Y, Dominguez PM & Melnick AM The many layers of epigenetic dysfunction in B-cell lymphomas. Curr Opin Hematol 23, 377–384, doi: 10.1097/MOH.0000000000000249 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Wahab O et al. Genetic characterization of TET1, TET2, and TET3 alterations in myeloid malignancies. Blood 114, 144–147, doi: 10.1182/blood-2009-03-210039 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delhommeau F et al. Mutation in TET2 in myeloid cancers. N Engl J Med 360, 2289–2301, doi: 10.1056/NEJMoa0810069 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Smith AE et al. Next-generation sequencing of the TET2 gene in 355 MDS and CMML patients reveals low-abundance mutant clones with early origins, but indicates no definite prognostic value. Blood 116, 3923–3932, doi: 10.1182/blood-2010-03-274704 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Kosmider O et al. TET2 gene mutation is a frequent and adverse event in chronic myelomonocytic leukemia. Haematologica 94, 1676–1681, doi: 10.3324/haematol.2009.011205 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swierczek SI et al. Extent of hematopoietic involvement by TET2 mutations in JAK2V(6)(1)(7)F polycythemia vera. Haematologica 96, 775–778, doi: 10.3324/haematol.2010.029678 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tefferi A et al. TET2 mutations and their clinical correlates in polycythemia vera, essential thrombocythemia and myelofibrosis. Leukemia 23, 905–911, doi: 10.1038/leu.2009.47 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tefferi A et al. Detection of mutant TET2 in myeloid malignancies other than myeloproliferative neoplasms: CMML, MDS, MDS/MPN and AML. Leukemia 23, 1343–1345, doi: 10.1038/leu.2009.59 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jankowska AM et al. Loss of heterozygosity 4q24 and TET2 mutations associated with myelodysplastic/myeloproliferative neoplasms. Blood 113, 6403–6410, doi: 10.1182/blood-2009-02-205690 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langemeijer SM et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat Genet 41, 838–842, doi: 10.1038/ng.391 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Asmar F et al. Genome-wide profiling identifies a DNA methylation signature that associates with TET2 mutations in diffuse large B-cell lymphoma. Haematologica 98, 1912–1920, doi: 10.3324/haematol.2013.088740 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy A et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 171, 481–494 e415, doi: 10.1016/j.cell.2017.09.027 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dominguez PM et al. TET2 Deficiency Causes Germinal Center Hyperplasia, Impairs Plasma Cell Differentiation, and Promotes B-cell Lymphomagenesis. Cancer discovery 8, 1632–1653, doi: 10.1158/2159-8290.CD-18-0657 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z et al. Deletion of Tet2 in mice leads to dysregulated hematopoietic stem cells and subsequent development of myeloid malignancies. Blood 118, 4509–4518, doi: 10.1182/blood-2010-12-325241 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lemonnier F et al. Recurrent TET2 mutations in peripheral T-cell lymphomas correlate with TFH-like features and adverse clinical parameters. Blood 120, 1466–1469, doi: 10.1182/blood-2012-02-408542 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Sakata-Yanagimoto M et al. Somatic RHOA mutation in angioimmunoblastic T cell lymphoma. Nat Genet 46, 171–175, doi: 10.1038/ng.2872 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Odejide O et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood 123, 1293–1296, doi: 10.1182/blood-2013-10-531509 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palomero T et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nat Genet 46, 166–170, doi: 10.1038/ng.2873 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitz R et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N Engl J Med 378, 1396–1407, doi: 10.1056/NEJMoa1801445 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soucie E et al. In aggressive forms of mastocytosis, TET2 loss cooperates with c-KITD816V to transform mast cells. Blood 120, 4846–4849, doi: 10.1182/blood-2011-12-397588 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Tefferi A et al. Frequent TET2 mutations in systemic mastocytosis: clinical, KITD816V and FIP1L1-PDGFRA correlates. Leukemia 23, 900–904, doi: 10.1038/leu.2009.37 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coltro G et al. Clinical, molecular, and prognostic correlates of number, type, and functional localization of TET2 mutations in chronic myelomonocytic leukemia (CMML)-a study of 1084 patients. Leukemia 34, 1407–1421, doi: 10.1038/s41375-019-0690-7 (2020). [DOI] [PubMed] [Google Scholar]
- 42.Yao WQ et al. Angioimmunoblastic T-cell lymphoma contains multiple clonal T-cell populations derived from a common TET2 mutant progenitor cell. The Journal of Pathology 250, 346–357, doi: 10.1002/path.5376 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shih AH, Abdel-Wahab O, Patel JP & Levine RL The role of mutations in epigenetic regulators in myeloid malignancies. Nat Rev Cancer 12, 599–612, doi: 10.1038/nrc3343 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Xie M et al. Age-related mutations associated with clonal hematopoietic expansion and malignancies. Nat Med 20, 1472–1478, doi: 10.1038/nm.3733 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaiswal S et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371, 2488–2498, doi: 10.1056/NEJMoa1408617 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Genovese G et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 371, 2477–2487, doi: 10.1056/NEJMoa1409405 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang CRC et al. Inflammatory cytokines promote clonal hematopoiesis with specific mutations in ulcerative colitis patients. Exp Hematol 80, 36–41 e33, doi: 10.1016/j.exphem.2019.11.008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hormaechea-Agulla D et al. Chronic infection drives Dnmt3a-loss-of-function clonal hematopoiesis via IFNgamma signaling. Cell Stem Cell, doi: 10.1016/j.stem.2021.03.002 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buscarlet M et al. DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions. Blood 130, 753–762, doi: 10.1182/blood-2017-04-777029 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Jaiswal S & Ebert BL Clonal hematopoiesis in human aging and disease. Science 366, doi: 10.1126/science.aan4673 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steensma DP et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 126, 9–16, doi: 10.1182/blood-2015-03-631747 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun D et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell 14, 673–688, doi: 10.1016/j.stem.2014.03.002 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang W et al. Isoform Switch of TET1 Regulates DNA Demethylation and Mouse Development. Mol Cell 64, 1062–1073, doi: 10.1016/j.molcel.2016.10.030 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Good CR et al. A novel isoform of TET1 that lacks a CXXC domain is overexpressed in cancer. Nucleic Acids Res 45, 8269–8281, doi: 10.1093/nar/gkx435 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iyer LM, Abhiman S & Aravind L Natural history of eukaryotic DNA methylation systems. Prog Mol Biol Transl Sci 101, 25–104, doi: 10.1016/B978-0-12-387685-0.00002-0 (2011). [DOI] [PubMed] [Google Scholar]
- 56.Sohni A et al. Dynamic switching of active promoter and enhancer domains regulates Tet1 and Tet2 expression during cell state transitions between pluripotency and differentiation. Mol Cell Biol 35, 1026–1042, doi: 10.1128/MCB.01172-14 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu N et al. Intrinsic and extrinsic connections of Tet3 dioxygenase with CXXC zinc finger modules. PLoS One 8, e62755, doi: 10.1371/journal.pone.0062755 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ito S et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science 333, 1300–1303, doi: 10.1126/science.1210597 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He YF et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 333, 1303–1307, doi: 10.1126/science.1210944 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito S et al. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature 466, 1129–1133, doi: 10.1038/nature09303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu Y et al. Genome-wide regulation of 5hmC, 5mC, and gene expression by Tet1 hydroxylase in mouse embryonic stem cells. Mol Cell 42, 451–464, doi: 10.1016/j.molcel.2011.04.005 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hahn MA et al. Dynamics of 5-hydroxymethylcytosine and chromatin marks in Mammalian neurogenesis. Cell reports 3, 291–300, doi: 10.1016/j.celrep.2013.01.011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin SG et al. Tet3 Reads 5-Carboxylcytosine through Its CXXC Domain and Is a Potential Guardian against Neurodegeneration. Cell reports 14, 493–505, doi: 10.1016/j.celrep.2015.12.044 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shekhawat J et al. Ten-eleven translocase: key regulator of the methylation landscape in cancer. Journal of cancer research and clinical oncology 147, 1869–1879, doi: 10.1007/s00432-021-03641-3 (2021). [DOI] [PubMed] [Google Scholar]
- 65.Kunimoto H & Nakajima H TET2: A cornerstone in normal and malignant hematopoiesis. Cancer Sci 112, 31–40, doi: 10.1111/cas.14688 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bray JK, Dawlaty MM, Verma A & Maitra A Roles and Regulations of TET Enzymes in Solid Tumors. Trends in cancer 7, 635–646, doi: 10.1016/j.trecan.2020.12.011 (2021). [DOI] [PubMed] [Google Scholar]
- 67.Dziaman T et al. Characteristic profiles of DNA epigenetic modifications in colon cancer and its predisposing conditions-benign adenomas and inflammatory bowel disease. Clinical epigenetics 10, 72, doi: 10.1186/s13148-018-0505-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pei YF et al. TET1 inhibits gastric cancer growth and metastasis by PTEN demethylation and re-expression. Oncotarget 7, 31322–31335, doi: 10.18632/oncotarget.8900 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu J et al. TET1-mediated DNA hydroxymethylation activates inhibitors of the Wnt/beta-catenin signaling pathway to suppress EMT in pancreatic tumor cells. J Exp Clin Cancer Res 38, 348, doi: 10.1186/s13046-019-1334-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eyres M et al. TET2 Drives 5hmc Marking of GATA6 and Epigenetically Defines Pancreatic Ductal Adenocarcinoma Transcriptional Subtypes. Gastroenterology 161, 653–668 e616, doi: 10.1053/j.gastro.2021.04.044 (2021). [DOI] [PubMed] [Google Scholar]
- 71.Spans L et al. Genomic and epigenomic analysis of high-risk prostate cancer reveals changes in hydroxymethylation and TET1. Oncotarget 7, 24326–24338, doi: 10.18632/oncotarget.8220 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Q et al. MicroRNA-29a induces loss of 5-hydroxymethylcytosine and promotes metastasis of hepatocellular carcinoma through a TET-SOCS1-MMP9 signaling axis. Cell Death Dis 8, e2906, doi: 10.1038/cddis.2017.142 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lian CG et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 150, 1135–1146, doi: 10.1016/j.cell.2012.07.033 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu J et al. Global DNA 5-Hydroxymethylcytosine and 5-Formylcytosine Contents Are Decreased in the Early Stage of Hepatocellular Carcinoma. Hepatology 69, 196–208, doi: 10.1002/hep.30146 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Zahid OK et al. Solid-state nanopore analysis of human genomic DNA shows unaltered global 5-hydroxymethylcytosine content associated with early-stage breast cancer. Nanomedicine : nanotechnology, biology, and medicine 35, 102407, doi: 10.1016/j.nano.2021.102407 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haffner MC et al. Global 5-hydroxymethylcytosine content is significantly reduced in tissue stem/progenitor cell compartments and in human cancers. Oncotarget 2, 627–637, doi: 10.18632/oncotarget.316 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kraus TF et al. Loss of 5-hydroxymethylcytosine and intratumoral heterogeneity as an epigenomic hallmark of glioblastoma. Tumour Biol 36, 8439–8446, doi: 10.1007/s13277-015-3606-9 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Thomson JP et al. Loss of Tet1-Associated 5-Hydroxymethylcytosine Is Concomitant with Aberrant Promoter Hypermethylation in Liver Cancer. Cancer Res 76, 3097–3108, doi: 10.1158/0008-5472.CAN-15-1910 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang H et al. Tumor development is associated with decrease of TET gene expression and 5-methylcytosine hydroxylation. Oncogene 32, 663–669, doi: 10.1038/onc.2012.67 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsai KW et al. Reduction of global 5-hydroxymethylcytosine is a poor prognostic factor in breast cancer patients, especially for an ER/PR-negative subtype. Breast Cancer Res Treat 153, 219–234, doi: 10.1007/s10549-015-3525-x (2015). [DOI] [PubMed] [Google Scholar]
- 81.Deng M et al. TET-Mediated Sequestration of miR-26 Drives EZH2 Expression and Gastric Carcinogenesis. Cancer Res 77, 6069–6082, doi: 10.1158/0008-5472.CAN-16-2964 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Chen LY et al. TET1 reprograms the epithelial ovarian cancer epigenome and reveals casein kinase 2alpha as a therapeutic target. J Pathol 248, 363–376, doi: 10.1002/path.5266 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Filipczak PT et al. p53-Suppressed Oncogene TET1 Prevents Cellular Aging in Lung Cancer. Cancer Res 79, 1758–1768, doi: 10.1158/0008-5472.CAN-18-1234 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Muller T et al. Nuclear exclusion of TET1 is associated with loss of 5-hydroxymethylcytosine in IDH1 wild-type gliomas. Am J Pathol 181, 675–683, doi: 10.1016/j.ajpath.2012.04.017 (2012). [DOI] [PubMed] [Google Scholar]
- 85.Good CR et al. TET1-Mediated Hypomethylation Activates Oncogenic Signaling in Triple-Negative Breast Cancer. Cancer Res 78, 4126–4137, doi: 10.1158/0008-5472.CAN-17-2082 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lio CJ, Yuita H & Rao A Dysregulation of the TET family of epigenetic regulators in lymphoid and myeloid malignancies. Blood 134, 1487–1497, doi: 10.1182/blood.2019791475 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cimmino L et al. TET1 is a tumor suppressor of hematopoietic malignancy. Nat Immunol 16, 653–662, doi: 10.1038/ni.3148 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morin RD et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature 476, 298–303, doi: 10.1038/nature10351 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pasqualucci L et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat Genet 43, 830–837, doi: 10.1038/ng.892 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okosun J et al. Integrated genomic analysis identifies recurrent mutations and evolution patterns driving the initiation and progression of follicular lymphoma. Nat Genet 46, 176–181, doi: 10.1038/ng.2856 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H et al. Mutations in linker histone genes HIST1H1 B, C, D, and E; OCT2 (POU2F2); IRF8; and ARID1A underlying the pathogenesis of follicular lymphoma. Blood 123, 1487–1498, doi: 10.1182/blood-2013-05-500264 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Keersmaecker K et al. Exome sequencing identifies mutation in CNOT3 and ribosomal genes RPL5 and RPL10 in T-cell acute lymphoblastic leukemia. Nat Genet 45, 186–190, doi: 10.1038/ng.2508 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cancer Genome Atlas Research, N. et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. The New England journal of medicine 368, 2059–2074, doi: 10.1056/NEJMoa1301689 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Palomero T et al. Recurrent mutations in epigenetic regulators, RHOA and FYN kinase in peripheral T cell lymphomas. Nature genetics 46, 166–170, doi: 10.1038/ng.2873 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Quesada V et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet 44, 47–52, doi: 10.1038/ng.1032 (2012). [DOI] [PubMed] [Google Scholar]
- 96.Nickerson ML et al. TET2 binds the androgen receptor and loss is associated with prostate cancer. Oncogene 36, 2172–2183, doi: 10.1038/onc.2016.376 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Neri F et al. TET1 is controlled by pluripotency-associated factors in ESCs and downmodulated by PRC2 in differentiated cells and tissues. Nucleic Acids Res 43, 6814–6826, doi: 10.1093/nar/gkv392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsai YP et al. TET1 regulates hypoxia-induced epithelial-mesenchymal transition by acting as a co-activator. Genome Biol 15, 513, doi: 10.1186/s13059-014-0513-0 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang YA et al. FOXA1 potentiates lineage-specific enhancer activation through modulating TET1 expression and function. Nucleic Acids Res 44, 8153–8164, doi: 10.1093/nar/gkw498 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yosefzon Y et al. An epigenetic switch repressing Tet1 in gonadotropes activates the reproductive axis. Proc Natl Acad Sci U S A 114, 10131–10136, doi: 10.1073/pnas.1704393114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li L et al. Epigenetic inactivation of the CpG demethylase TET1 as a DNA methylation feedback loop in human cancers. Scientific reports 6, 26591, doi: 10.1038/srep26591 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Agirre X et al. Whole-epigenome analysis in multiple myeloma reveals DNA hypermethylation of B cell-specific enhancers. Genome Res 25, 478–487, doi: 10.1101/gr.180240.114 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun M et al. HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer growth and metastasis. Proc Natl Acad Sci U S A 110, 9920–9925, doi: 10.1073/pnas.1305172110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Forloni M et al. Oncogenic EGFR Represses the TET1 DNA Demethylase to Induce Silencing of Tumor Suppressors in Cancer Cells. Cell reports 16, 457–471, doi: 10.1016/j.celrep.2016.05.087 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Collignon E et al. Immunity drives TET1 regulation in cancer through NF-kappaB. Science advances 4, eaap7309, doi: 10.1126/sciadv.aap7309 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Noreen F et al. DNA methylation instability by BRAF-mediated TET silencing and lifestyle-exposure divides colon cancer pathways. Clinical epigenetics 11, 196, doi: 10.1186/s13148-019-0791-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hsu CH et al. TET1 suppresses cancer invasion by activating the tissue inhibitors of metalloproteinases. Cell reports 2, 568–579, doi: 10.1016/j.celrep.2012.08.030 (2012). [DOI] [PubMed] [Google Scholar]
- 108.Teslow EA et al. Obesity-induced MBD2_v2 expression promotes tumor-initiating triple-negative breast cancer stem cells. Mol Oncol 13, 894–908, doi: 10.1002/1878-0261.12444 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang H et al. MiR-29b/TET1/ZEB2 signaling axis regulates metastatic properties and epithelial-mesenchymal transition in breast cancer cells. Oncotarget 8, 102119–102133, doi: 10.18632/oncotarget.22183 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen N et al. A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol 19, 218, doi: 10.1186/s13059-018-1594-y (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu Y et al. Oct4 and the small molecule inhibitor, SC1, regulates Tet2 expression in mouse embryonic stem cells. Molecular biology reports 40, 2897–2906, doi: 10.1007/s11033-012-2305-5 (2013). [DOI] [PubMed] [Google Scholar]
- 112.Koh KP et al. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell 8, 200–213, doi: 10.1016/j.stem.2011.01.008 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fischer AP & Miles SL Silencing HIF-1alpha induces TET2 expression and augments ascorbic acid induced 5-hydroxymethylation of DNA in human metastatic melanoma cells. Biochem Biophys Res Commun 490, 176–181, doi: 10.1016/j.bbrc.2017.06.017 (2017). [DOI] [PubMed] [Google Scholar]
- 114.Zhang LY, Li PL, Wang TZ & Zhang XC Prognostic values of 5-hmC, 5-mC and TET2 in epithelial ovarian cancer. Archives of gynecology and obstetrics 292, 891–897, doi: 10.1007/s00404-015-3704-3 (2015). [DOI] [PubMed] [Google Scholar]
- 115.Tucker DW et al. Epigenetic Reprogramming Strategies to Reverse Global Loss of 5-Hydroxymethylcytosine, a Prognostic Factor for Poor Survival in High-grade Serous Ovarian Cancer. Clin Cancer Res 24, 1389–1401, doi: 10.1158/1078-0432.CCR-17-1958 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rawluszko-Wieczorek AA et al. Clinical significance of DNA methylation mRNA levels of TET family members in colorectal cancer. Journal of cancer research and clinical oncology 141, 1379–1392, doi: 10.1007/s00432-014-1901-2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Carella A et al. Epigenetic downregulation of TET3 reduces genome-wide 5hmC levels and promotes glioblastoma tumorigenesis. Int J Cancer 146, 373–387, doi: 10.1002/ijc.32520 (2020). [DOI] [PubMed] [Google Scholar]
- 118.Mo HY, An CH, Choi EJ, Yoo NJ & Lee SH Somatic mutation and loss of expression of a candidate tumor suppressor gene TET3 in gastric and colorectal cancers. Pathology, research and practice 216, 152759, doi: 10.1016/j.prp.2019.152759 (2020). [DOI] [PubMed] [Google Scholar]
- 119.Ye Z et al. TET3 inhibits TGF-beta1-induced epithelial-mesenchymal transition by demethylating miR-30d precursor gene in ovarian cancer cells. J Exp Clin Cancer Res 35, 72, doi: 10.1186/s13046-016-0350-y (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cao T, Pan W, Sun X & Shen H Increased expression of TET3 predicts unfavorable prognosis in patients with ovarian cancer-a bioinformatics integrative analysis. Journal of ovarian research 12, 101, doi: 10.1186/s13048-019-0575-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Song SJ et al. MicroRNA-antagonism regulates breast cancer stemness and metastasis via TET-family-dependent chromatin remodeling. Cell 154, 311–324, doi: 10.1016/j.cell.2013.06.026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cheng J et al. An extensive network of TET2-targeting MicroRNAs regulates malignant hematopoiesis. Cell reports 5, 471–481, doi: 10.1016/j.celrep.2013.08.050 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fu X et al. MicroRNA-26a targets ten eleven translocation enzymes and is regulated during pancreatic cell differentiation. Proc Natl Acad Sci U S A 110, 17892–17897, doi: 10.1073/pnas.1317397110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Loriot A et al. A novel cancer-germline transcript carrying pro-metastatic miR-105 and TET-targeting miR-767 induced by DNA hypomethylation in tumors. Epigenetics 9, 1163–1171, doi: 10.4161/epi.29628 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chuang KH et al. MicroRNA-494 is a master epigenetic regulator of multiple invasion-suppressor microRNAs by targeting ten eleven translocation 1 in invasive human hepatocellular carcinoma tumors. Hepatology 62, 466–480, doi: 10.1002/hep.27816 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang W et al. MiR-520b suppresses proliferation of hepatoma cells through targeting ten-eleven translocation 1 (TET1) mRNA. Biochem Biophys Res Commun 460, 793–798, doi: 10.1016/j.bbrc.2015.03.108 (2015). [DOI] [PubMed] [Google Scholar]
- 127.Song SJ et al. The oncogenic microRNA miR-22 targets the TET2 tumor suppressor to promote hematopoietic stem cell self-renewal and transformation. Cell Stem Cell 13, 87–101, doi: 10.1016/j.stem.2013.06.003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Takeshima H et al. TET repression and increased DNMT activity synergistically induce aberrant DNA methylation. J Clin Invest 130, 5370–5379, doi: 10.1172/JCI124070 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Scherm MG et al. miRNA142–3p targets Tet2 and impairs Treg differentiation and stability in models of type 1 diabetes. Nat Commun 10, 5697, doi: 10.1038/s41467-019-13587-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jiang S, Yan W, Wang SE & Baltimore D Dual mechanisms of posttranscriptional regulation of Tet2 by Let-7 microRNA in macrophages. Proc Natl Acad Sci U S A 116, 12416–12421, doi: 10.1073/pnas.1811040116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maiti A & Drohat AC Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: potential implications for active demethylation of CpG sites. J Biol Chem 286, 35334–35338, doi: 10.1074/jbc.C111.284620 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cortellino S et al. Thymine DNA glycosylase is essential for active DNA demethylation by linked deamination-base excision repair. Cell 146, 67–79, doi: 10.1016/j.cell.2011.06.020 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bhutani N et al. Reprogramming towards pluripotency requires AID-dependent DNA demethylation. Nature 463, 1042–1047, doi: 10.1038/nature08752 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Popp C et al. Genome-wide erasure of DNA methylation in mouse primordial germ cells is affected by AID deficiency. Nature 463, 1101–1105, doi: 10.1038/nature08829 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nawy T Dynamics of DNA demethylation. Nat Methods 10, 466, doi: 10.1038/nmeth.2506 (2013). [DOI] [PubMed] [Google Scholar]
- 136.Muramatsu M et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563, doi: 10.1016/s0092-8674(00)00078-7 (2000). [DOI] [PubMed] [Google Scholar]
- 137.Xu Z, Zan H, Pone EJ, Mai T & Casali P Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat Rev Immunol 12, 517–531, doi: 10.1038/nri3216 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Barreto G et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 445, 671–675, doi: 10.1038/nature05515 (2007). [DOI] [PubMed] [Google Scholar]
- 139.Schmitz KM et al. TAF12 recruits Gadd45a and the nucleotide excision repair complex to the promoter of rRNA genes leading to active DNA demethylation. Mol Cell 33, 344–353, doi: 10.1016/j.molcel.2009.01.015 (2009). [DOI] [PubMed] [Google Scholar]
- 140.Ma DK et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science 323, 1074–1077, doi: 10.1126/science.1166859 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hajkova P et al. Genome-wide reprogramming in the mouse germ line entails the base excision repair pathway. Science 329, 78–82, doi: 10.1126/science.1187945 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dominguez PM et al. DNA Methylation Dynamics of Germinal Center B Cells Are Mediated by AID. Cell reports 12, 2086–2098, doi: 10.1016/j.celrep.2015.08.036 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lio CJ et al. TET enzymes augment activation-induced deaminase (AID) expression via 5-hydroxymethylcytosine modifications at the Aicda superenhancer. Science immunology 4, doi: 10.1126/sciimmunol.aau7523 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Delker RK, Fugmann SD & Papavasiliou FN A coming-of-age story: activation-induced cytidine deaminase turns 10. Nat Immunol 10, 1147–1153, doi: 10.1038/ni.1799 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kunimoto H et al. Aid is a key regulator of myeloid/erythroid differentiation and DNA methylation in hematopoietic stem/progenitor cells. Blood 129, 1779–1790, doi: 10.1182/blood-2016-06-721977 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bachman M et al. 5-Hydroxymethylcytosine is a predominantly stable DNA modification. Nature chemistry 6, 1049–1055, doi: 10.1038/nchem.2064 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bachman M et al. 5-Formylcytosine can be a stable DNA modification in mammals. Nat Chem Biol 11, 555–557, doi: 10.1038/nchembio.1848 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu H, Wu X, Shen L & Zhang Y Single-base resolution analysis of active DNA demethylation using methylase-assisted bisulfite sequencing. Nat Biotechnol 32, 1231–1240, doi: 10.1038/nbt.3073 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Spruijt CG et al. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell 152, 1146–1159, doi: 10.1016/j.cell.2013.02.004 (2013). [DOI] [PubMed] [Google Scholar]
- 150.Mellen M, Ayata P, Dewell S, Kriaucionis S & Heintz N MeCP2 binds to 5hmC enriched within active genes and accessible chromatin in the nervous system. Cell 151, 1417–1430, doi: 10.1016/j.cell.2012.11.022 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Takai H et al. 5-Hydroxymethylcytosine plays a critical role in glioblastomagenesis by recruiting the CHTOP-methylosome complex. Cell reports 9, 48–60, doi: 10.1016/j.celrep.2014.08.071 (2014). [DOI] [PubMed] [Google Scholar]
- 152.Yildirim O et al. Mbd3/NURD complex regulates expression of 5-hydroxymethylcytosine marked genes in embryonic stem cells. Cell 147, 1498–1510, doi: 10.1016/j.cell.2011.11.054 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen R et al. The 5-Hydroxymethylcytosine (5hmC) Reader UHRF2 Is Required for Normal Levels of 5hmC in Mouse Adult Brain and Spatial Learning and Memory. J Biol Chem 292, 4533–4543, doi: 10.1074/jbc.M116.754580 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tsagaratou A et al. Dissecting the dynamic changes of 5-hydroxymethylcytosine in T-cell development and differentiation. Proc Natl Acad Sci U S A 111, E3306–3315, doi: 10.1073/pnas.1412327111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pastor WA et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Nature 473, 394–397, doi: 10.1038/nature10102 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stroud H, Feng S, Morey Kinney S, Pradhan S & Jacobsen SE 5-Hydroxymethylcytosine is associated with enhancers and gene bodies in human embryonic stem cells. Genome Biol 12, R54, doi: 10.1186/gb-2011-12-6-r54 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Williams K et al. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature 473, 343–348, doi: 10.1038/nature10066 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Madzo J et al. Hydroxymethylation at gene regulatory regions directs stem/early progenitor cell commitment during erythropoiesis. Cell reports 6, 231–244, doi: 10.1016/j.celrep.2013.11.044 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guilhamon P et al. Meta-analysis of IDH-mutant cancers identifies EBF1 as an interaction partner for TET2. Nat Commun 4, 2166, doi: 10.1038/ncomms3166 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mellen M, Ayata P & Heintz N 5-hydroxymethylcytosine accumulation in postmitotic neurons results in functional demethylation of expressed genes. Proc Natl Acad Sci U S A 114, E7812–E7821, doi: 10.1073/pnas.1708044114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.He B et al. Tissue-specific 5-hydroxymethylcytosine landscape of the human genome. Nat Commun 12, 4249, doi: 10.1038/s41467-021-24425-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dawlaty MM et al. Loss of Tet enzymes compromises proper differentiation of embryonic stem cells. Dev Cell 29, 102–111, doi: 10.1016/j.devcel.2014.03.003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li C et al. Overlapping Requirements for Tet2 and Tet3 in Normal Development and Hematopoietic Stem Cell Emergence. Cell reports 12, 1133–1143, doi: 10.1016/j.celrep.2015.07.025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dawlaty MM et al. Combined deficiency of Tet1 and Tet2 causes epigenetic abnormalities but is compatible with postnatal development. Dev Cell 24, 310–323, doi: 10.1016/j.devcel.2012.12.015 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hu L et al. Structural insight into substrate preference for TET-mediated oxidation. Nature 527, 118–122, doi: 10.1038/nature15713 (2015). [DOI] [PubMed] [Google Scholar]
- 166.Huang Y et al. Distinct roles of the methylcytosine oxidases Tet1 and Tet2 in mouse embryonic stem cells. Proc Natl Acad Sci U S A 111, 1361–1366, doi: 10.1073/pnas.1322921111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tan L & Shi YG Tet family proteins and 5-hydroxymethylcytosine in development and disease. Development 139, 1895–1902, doi: 10.1242/dev.070771 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Song CX et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol 29, 68–72, doi: 10.1038/nbt.1732 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Putiri EL et al. Distinct and overlapping control of 5-methylcytosine and 5-hydroxymethylcytosine by the TET proteins in human cancer cells. Genome Biol 15, R81, doi: 10.1186/gb-2014-15-6-r81 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yamaguchi S, Shen L, Liu Y, Sendler D & Zhang Y Role of Tet1 in erasure of genomic imprinting. Nature 504, 460–464, doi: 10.1038/nature12805 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu H & Zhang Y Reversing DNA methylation: mechanisms, genomics, and biological functions. Cell 156, 45–68, doi: 10.1016/j.cell.2013.12.019 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gu TP et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature 477, 606–610, doi: 10.1038/nature10443 (2011). [DOI] [PubMed] [Google Scholar]
- 173.Pastor WA, Aravind L & Rao A TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nat Rev Mol Cell Biol 14, 341–356, doi: 10.1038/nrm3589 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dawlaty MM et al. Tet1 is dispensable for maintaining pluripotency and its loss is compatible with embryonic and postnatal development. Cell Stem Cell 9, 166–175, doi: 10.1016/j.stem.2011.07.010 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wu H et al. Dual functions of Tet1 in transcriptional regulation in mouse embryonic stem cells. Nature 473, 389–393, doi: 10.1038/nature09934 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hon GC et al. 5mC oxidation by Tet2 modulates enhancer activity and timing of transcriptome reprogramming during differentiation. Mol Cell 56, 286–297, doi: 10.1016/j.molcel.2014.08.026 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fidalgo M et al. Zfp281 Coordinates Opposing Functions of Tet1 and Tet2 in Pluripotent States. Cell Stem Cell 19, 355–369, doi: 10.1016/j.stem.2016.05.025 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Piccolo FM et al. Different roles for Tet1 and Tet2 proteins in reprogramming-mediated erasure of imprints induced by EGC fusion. Mol Cell 49, 1023–1033, doi: 10.1016/j.molcel.2013.01.032 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Jin C et al. TET1 is a maintenance DNA demethylase that prevents methylation spreading in differentiated cells. Nucleic Acids Res 42, 6956–6971, doi: 10.1093/nar/gku372 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Iyer LM, Tahiliani M, Rao A & Aravind L Prediction of novel families of enzymes involved in oxidative and other complex modifications of bases in nucleic acids. Cell Cycle 8, 1698–1710, doi: 10.4161/cc.8.11.8580 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Frauer C et al. Different binding properties and function of CXXC zinc finger domains in Dnmt1 and Tet1. PLoS One 6, e16627, doi: 10.1371/journal.pone.0016627 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang H et al. TET1 is a DNA-binding protein that modulates DNA methylation and gene transcription via hydroxylation of 5-methylcytosine. Cell Res 20, 1390–1393, doi: 10.1038/cr.2010.156 (2010). [DOI] [PubMed] [Google Scholar]
- 183.Greer CB et al. Tet1 Isoforms Differentially Regulate Gene Expression, Synaptic Transmission, and Memory in the Mammalian Brain. J Neurosci 41, 578–593, doi: 10.1523/JNEUROSCI.1821-20.2020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chandru A, Bate N, Vuister GW & Cowley SM Sin3A recruits Tet1 to the PAH1 domain via a highly conserved Sin3-Interaction Domain. Scientific reports 8, 14689, doi: 10.1038/s41598-018-32942-w (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhu F et al. Sin3a-Tet1 interaction activates gene transcription and is required for embryonic stem cell pluripotency. Nucleic Acids Res 46, 6026–6040, doi: 10.1093/nar/gky347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Vella P et al. Tet proteins connect the O-linked N-acetylglucosamine transferase Ogt to chromatin in embryonic stem cells. Mol Cell 49, 645–656, doi: 10.1016/j.molcel.2012.12.019 (2013). [DOI] [PubMed] [Google Scholar]
- 187.Deplus R et al. TET2 and TET3 regulate GlcNAcylation and H3K4 methylation through OGT and SET1/COMPASS. EMBO J 32, 645–655, doi: 10.1038/emboj.2012.357 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chen Q, Chen Y, Bian C, Fujiki R & Yu X TET2 promotes histone O-GlcNAcylation during gene transcription. Nature 493, 561–564, doi: 10.1038/nature11742 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hrit J et al. OGT binds a conserved C-terminal domain of TET1 to regulate TET1 activity and function in development. eLife 7, doi: 10.7554/eLife.34870 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Teif VB et al. Nucleosome repositioning links DNA (de)methylation and differential CTCF binding during stem cell development. Genome Res 24, 1285–1295, doi: 10.1101/gr.164418.113 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dubois-Chevalier J et al. A dynamic CTCF chromatin binding landscape promotes DNA hydroxymethylation and transcriptional induction of adipocyte differentiation. Nucleic Acids Res 42, 10943–10959, doi: 10.1093/nar/gku780 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wiehle L et al. DNA (de)methylation in embryonic stem cells controls CTCF-dependent chromatin boundaries. Genome Res 29, 750–761, doi: 10.1101/gr.239707.118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Perera A et al. TET3 is recruited by REST for context-specific hydroxymethylation and induction of gene expression. Cell reports 11, 283–294, doi: 10.1016/j.celrep.2015.03.020 (2015). [DOI] [PubMed] [Google Scholar]
- 194.Ko M et al. Modulation of TET2 expression and 5-methylcytosine oxidation by the CXXC domain protein IDAX. Nature 497, 122–126, doi: 10.1038/nature12052 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ayaz G et al. CXXC5 as an unmethylated CpG dinucleotide binding protein contributes to estrogen-mediated cellular proliferation. Scientific reports 10, 5971, doi: 10.1038/s41598-020-62912-0 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ravichandran M et al. Rinf Regulates Pluripotency Network Genes and Tet Enzymes in Embryonic Stem Cells. Cell reports 28, 1993–2003 e1995, doi: 10.1016/j.celrep.2019.07.080 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Abbas S, Erpelinck-Verschueren CA, Goudswaard CS, Lowenberg B & Valk PJ Mutant Wilms’ tumor 1 (WT1) mRNA with premature termination codons in acute myeloid leukemia (AML) is sensitive to nonsense-mediated RNA decay (NMD). Leukemia 24, 660–663, doi: 10.1038/leu.2009.265 (2010). [DOI] [PubMed] [Google Scholar]
- 198.Wang Y et al. WT1 recruits TET2 to regulate its target gene expression and suppress leukemia cell proliferation. Mol Cell 57, 662–673, doi: 10.1016/j.molcel.2014.12.023 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rampal R et al. DNA hydroxymethylation profiling reveals that WT1 mutations result in loss of TET2 function in acute myeloid leukemia. Cell reports 9, 1841–1855, doi: 10.1016/j.celrep.2014.11.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Pan F, Weeks O, Yang FC & Xu M The TET2 interactors and their links to hematological malignancies. IUBMB Life 67, 438–445, doi: 10.1002/iub.1389 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lazarenkov A & Sardina JL Dissecting TET2 Regulatory Networks in Blood Differentiation and Cancer. Cancers (Basel) 14, doi: 10.3390/cancers14030830 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Muller U, Bauer C, Siegl M, Rottach A & Leonhardt H TET-mediated oxidation of methylcytosine causes TDG or NEIL glycosylase dependent gene reactivation. Nucleic Acids Res 42, 8592–8604, doi: 10.1093/nar/gku552 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cartron PF et al. Identification of TET1 Partners That Control Its DNA-Demethylating Function. Genes Cancer 4, 235–241, doi: 10.1177/1947601913489020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Costa Y et al. NANOG-dependent function of TET1 and TET2 in establishment of pluripotency. Nature 495, 370–374, doi: 10.1038/nature11925 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zheng L et al. Modification of Tet1 and histone methylation dynamics in dairy goat male germline stem cells. Cell Prolif 49, 163–172, doi: 10.1111/cpr.12245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zheng L et al. The Modification of Tet1 in Male Germline Stem Cells and Interact with PCNA, HDAC1 to promote their Self-renewal and Proliferation. Scientific reports 6, 37414, doi: 10.1038/srep37414 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zhang X, Yang C, Peng X, Chen X & Feng Y Acute WT1-positive promyelocytic leukemia with hypogranular variant morphology, bcr-3 isoform of PML-RARalpha and Flt3-ITD mutation: a rare case report. Sao Paulo medical journal = Revista paulista de medicina 135, 179–184, doi: 10.1590/1516-3180.2016.020104102016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhang Q et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 525, 389–393, doi: 10.1038/nature15252 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen LL et al. SNIP1 Recruits TET2 to Regulate c-MYC Target Genes and Cellular DNA Damage Response. Cell reports 25, 1485–1500 e1484, doi: 10.1016/j.celrep.2018.10.028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sardina JL et al. Transcription Factors Drive Tet2-Mediated Enhancer Demethylation to Reprogram Cell Fate. Cell Stem Cell 23, 905–906, doi: 10.1016/j.stem.2018.11.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhang YW et al. Acetylation Enhances TET2 Function in Protecting against Abnormal DNA Methylation during Oxidative Stress. Mol Cell 65, 323–335, doi: 10.1016/j.molcel.2016.12.013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Song C et al. PML Recruits TET2 to Regulate DNA Modification and Cell Proliferation in Response to Chemotherapeutic Agent. Cancer Res 78, 2475–2489, doi: 10.1158/0008-5472.CAN-17-3091 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nakagawa T et al. CRL4(VprBP) E3 ligase promotes monoubiquitylation and chromatin binding of TET dioxygenases. Mol Cell 57, 247–260, doi: 10.1016/j.molcel.2014.12.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Qiao Y et al. AF9 promotes hESC neural differentiation through recruiting TET2 to neurodevelopmental gene loci for methylcytosine hydroxylation. Cell discovery 1, 15017, doi: 10.1038/celldisc.2015.17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lio CW et al. Tet2 and Tet3 cooperate with B-lineage transcription factors to regulate DNA modification and chromatin accessibility. eLife 5, doi: 10.7554/eLife.18290 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.de la Rica L et al. PU.1 target genes undergo Tet2-coupled demethylation and DNMT3b-mediated methylation in monocyte-to-osteoclast differentiation. Genome Biol 14, R99, doi: 10.1186/gb-2013-14-9-r99 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Suzuki T et al. RUNX1 regulates site specificity of DNA demethylation by recruitment of DNA demethylation machineries in hematopoietic cells. Blood advances 1, 1699–1711, doi: 10.1182/bloodadvances.2017005710 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zeng Y et al. Lin28A Binds Active Promoters and Recruits Tet1 to Regulate Gene Expression. Mol Cell 61, 153–160, doi: 10.1016/j.molcel.2015.11.020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Sun Z et al. EGR1 recruits TET1 to shape the brain methylome during development and upon neuronal activity. Nat Commun 10, 3892, doi: 10.1038/s41467-019-11905-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Guan W et al. Methylcytosine dioxygenase TET3 interacts with thyroid hormone nuclear receptors and stabilizes their association to chromatin. Proc Natl Acad Sci U S A 114, 8229–8234, doi: 10.1073/pnas.1702192114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Fujiki K et al. PPARgamma-induced PARylation promotes local DNA demethylation by production of 5-hydroxymethylcytosine. Nat Commun 4, 2262, doi: 10.1038/ncomms3262 (2013). [DOI] [PubMed] [Google Scholar]
- 222.Baubec T, Ivanek R, Lienert F & Schubeler D Methylation-dependent and -independent genomic targeting principles of the MBD protein family. Cell 153, 480–492, doi: 10.1016/j.cell.2013.03.011 (2013). [DOI] [PubMed] [Google Scholar]
- 223.Peng L et al. MBD3L2 promotes Tet2 enzymatic activity for mediating 5-methylcytosine oxidation. J Cell Sci 129, 1059–1071, doi: 10.1242/jcs.179044 (2016). [DOI] [PubMed] [Google Scholar]
- 224.Okashita N et al. PRDM14 promotes active DNA demethylation through the ten-eleven translocation (TET)-mediated base excision repair pathway in embryonic stem cells. Development 141, 269–280, doi: 10.1242/dev.099622 (2014). [DOI] [PubMed] [Google Scholar]
- 225.Soufi A et al. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell 161, 555–568, doi: 10.1016/j.cell.2015.03.017 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Boller S et al. Pioneering Activity of the C-Terminal Domain of EBF1 Shapes the Chromatin Landscape for B Cell Programming. Immunity 44, 527–541, doi: 10.1016/j.immuni.2016.02.021 (2016). [DOI] [PubMed] [Google Scholar]
- 227.Liu Y et al. Structural basis for Klf4 recognition of methylated DNA. Nucleic Acids Res 42, 4859–4867, doi: 10.1093/nar/gku134 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Hashimoto H et al. Wilms tumor protein recognizes 5-carboxylcytosine within a specific DNA sequence. Genes Dev 28, 2304–2313, doi: 10.1101/gad.250746.114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vanzan L et al. High throughput screening identifies SOX2 as a super pioneer factor that inhibits DNA methylation maintenance at its binding sites. Nat Commun 12, 3337, doi: 10.1038/s41467-021-23630-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Quenneville S et al. In embryonic stem cells, ZFP57/KAP1 recognize a methylated hexanucleotide to affect chromatin and DNA methylation of imprinting control regions. Mol Cell 44, 361–372, doi: 10.1016/j.molcel.2011.08.032 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Wang D et al. MAX is an epigenetic sensor of 5-carboxylcytosine and is altered in multiple myeloma. Nucleic Acids Res 45, 2396–2407, doi: 10.1093/nar/gkw1184 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Domcke S et al. Competition between DNA methylation and transcription factors determines binding of NRF1. Nature 528, 575–579, doi: 10.1038/nature16462 (2015). [DOI] [PubMed] [Google Scholar]
- 233.Xiong J et al. Cooperative Action between SALL4A and TET Proteins in Stepwise Oxidation of 5-Methylcytosine. Mol Cell 64, 913–925, doi: 10.1016/j.molcel.2016.10.013 (2016). [DOI] [PubMed] [Google Scholar]
- 234.Looney TJ et al. Systematic mapping of occluded genes by cell fusion reveals prevalence and stability of cis-mediated silencing in somatic cells. Genome Res 24, 267–280, doi: 10.1101/gr.143891.112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Polo JM et al. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell 151, 1617–1632, doi: 10.1016/j.cell.2012.11.039 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Okashita N et al. PRDM14 Drives OCT3/4 Recruitment via Active Demethylation in the Transition from Primed to Naive Pluripotency. Stem cell reports 7, 1072–1086, doi: 10.1016/j.stemcr.2016.10.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Iwafuchi-Doi M & Zaret KS Cell fate control by pioneer transcription factors. Development 143, 1833–1837, doi: 10.1242/dev.133900 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mayran A & Drouin J Pioneer transcription factors shape the epigenetic landscape. J Biol Chem 293, 13795–13804, doi: 10.1074/jbc.R117.001232 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Larson ED, Marsh AJ & Harrison MM Pioneering the developmental frontier. Mol Cell 81, 1640–1650, doi: 10.1016/j.molcel.2021.02.020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Balsalobre A & Drouin J Pioneer factors as master regulators of the epigenome and cell fate. Nat Rev Mol Cell Biol, doi: 10.1038/s41580-022-00464-z (2022). [DOI] [PubMed] [Google Scholar]
- 241.Iwafuchi-Doi M The mechanistic basis for chromatin regulation by pioneer transcription factors. Wiley interdisciplinary reviews. Systems biology and medicine 11, e1427, doi: 10.1002/wsbm.1427 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Chronis C et al. Cooperative Binding of Transcription Factors Orchestrates Reprogramming. Cell 168, 442–459 e420, doi: 10.1016/j.cell.2016.12.016 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Mayran A et al. Pioneer and nonpioneer factor cooperation drives lineage specific chromatin opening. Nat Commun 10, 3807, doi: 10.1038/s41467-019-11791-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sonmezer C et al. Molecular Co-occupancy Identifies Transcription Factor Binding Cooperativity In Vivo. Mol Cell 81, 255–267 e256, doi: 10.1016/j.molcel.2020.11.015 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Rishi V et al. CpG methylation of half-CRE sequences creates C/EBPalpha binding sites that activate some tissue-specific genes. Proc Natl Acad Sci U S A 107, 20311–20316, doi: 10.1073/pnas.1008688107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.van Oevelen C et al. C/EBPalpha Activates Pre-existing and De Novo Macrophage Enhancers during Induced Pre-B Cell Transdifferentiation and Myelopoiesis. Stem cell reports 5, 232–247, doi: 10.1016/j.stemcr.2015.06.007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Xue S et al. TET3 Inhibits Type I IFN Production Independent of DNA Demethylation. Cell reports 16, 1096–1105, doi: 10.1016/j.celrep.2016.06.068 (2016). [DOI] [PubMed] [Google Scholar]
- 248.Zhong J et al. TET1 modulates H4K16 acetylation by controlling auto-acetylation of hMOF to affect gene regulation and DNA repair function. Nucleic Acids Res 45, 672–684, doi: 10.1093/nar/gkw919 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Bauer C et al. Phosphorylation of TET Proteins Is Regulated via O-GlcNAcylation by the O-Linked N-Acetylglucosamine Transferase (OGT). Journal of Biological Chemistry 290, 4801–4812, doi: 10.1074/jbc.m114.605881 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhang YW et al. Acetylation Enhances TET2 Function in Protecting against Abnormal DNA Methylation during Oxidative Stress. Molecular Cell 65, 323–335, doi: 10.1016/j.molcel.2016.12.013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Li X et al. SIRT1 Deacetylates TET2 and Promotes Its Ubiquitination Degradation to Achieve Neuroprotection Against Parkinson’s Disease. Front Neurol 12, 652882, doi: 10.3389/fneur.2021.652882 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Wu D et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature 559, 637–641, doi: 10.1038/s41586-018-0350-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Rao VK et al. Phosphorylation of Tet3 by cdk5 is critical for robust activation of BRN2 during neuronal differentiation. Nucleic Acids Res 48, 1225–1238, doi: 10.1093/nar/gkz1144 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bauer C et al. Phosphorylation of TET proteins is regulated via O-GlcNAcylation by the O-linked N-acetylglucosamine transferase (OGT). J Biol Chem 290, 4801–4812, doi: 10.1074/jbc.M114.605881 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Coulter JB et al. TET1 deficiency attenuates the DNA damage response and promotes resistance to DNA damaging agents. Epigenetics 12, 854–864, doi: 10.1080/15592294.2017.1359452 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Zhang T et al. Phosphorylation of TET2 by AMPK is indispensable in myogenic differentiation. Epigenetics Chromatin 12, 32, doi: 10.1186/s13072-019-0281-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Fiedler EC & Shaw RJ AMPK Regulates the Epigenome through Phosphorylation of TET2. Cell Metab 28, 534–536, doi: 10.1016/j.cmet.2018.09.015 (2018). [DOI] [PubMed] [Google Scholar]
- 258.Chen H et al. TET2 stabilization by 14–3-3 binding to the phosphorylated Serine 99 is deregulated by mutations in cancer. Cell Res 29, 248–250, doi: 10.1038/s41422-018-0132-5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kundu A et al. 14–3-3 proteins protect AMPK-phosphorylated ten-eleven translocation-2 (TET2) from PP2A-mediated dephosphorylation. J Biol Chem 295, 1754–1766, doi: 10.1074/jbc.RA119.011089 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Giovannucci E et al. Diabetes and cancer: a consensus report. CA: a cancer journal for clinicians 60, 207–221, doi: 10.3322/caac.20078 (2010). [DOI] [PubMed] [Google Scholar]
- 261.Brower V Illuminating the diabetes-cancer link. J Natl Cancer Inst 104, 1048–1050, doi: 10.1093/jnci/djs322 (2012). [DOI] [PubMed] [Google Scholar]
- 262.Wang T et al. Diabetes Risk Reduction Diet and Survival after Breast Cancer Diagnosis. Cancer Res 81, 4155–4162, doi: 10.1158/0008-5472.CAN-21-0256 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Jeong JJ et al. Cytokine-Regulated Phosphorylation and Activation of TET2 by JAK2 in Hematopoiesis. Cancer discovery 9, 778–795, doi: 10.1158/2159-8290.CD-18-1138 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Jin Z et al. FGFR3 big up tri, open7–9 promotes tumor progression via the phosphorylation and destabilization of ten-eleven translocation-2 in human hepatocellular carcinoma. Cell Death Dis 11, 903, doi: 10.1038/s41419-020-03089-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mancini M et al. Cytoplasmatic compartmentalization by Bcr-Abl promotes TET2 loss-of-function in chronic myeloid leukemia. J Cell Biochem 113, 2765–2774, doi: 10.1002/jcb.24154 (2012). [DOI] [PubMed] [Google Scholar]
- 266.Jiang D, Wei S, Chen F, Zhang Y & Li J TET3-mediated DNA oxidation promotes ATR-dependent DNA damage response. EMBO Rep 18, 781–796, doi: 10.15252/embr.201643179 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ito R et al. TET3-OGT interaction increases the stability and the presence of OGT in chromatin. Genes Cells 19, 52–65, doi: 10.1111/gtc.12107 (2014). [DOI] [PubMed] [Google Scholar]
- 268.Zhang Q et al. Differential regulation of the ten-eleven translocation (TET) family of dioxygenases by O-linked beta-N-acetylglucosamine transferase (OGT). J Biol Chem 289, 5986–5996, doi: 10.1074/jbc.M113.524140 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Shi FT et al. Ten-eleven translocation 1 (Tet1) is regulated by O-linked N-acetylglucosamine transferase (Ogt) for target gene repression in mouse embryonic stem cells. J Biol Chem 288, 20776–20784, doi: 10.1074/jbc.M113.460386 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lv L et al. Vpr Targets TET2 for Degradation by CRL4(VprBP) E3 Ligase to Sustain IL-6 Expression and Enhance HIV-1 Replication. Mol Cell 70, 961–970 e965, doi: 10.1016/j.molcel.2018.05.007 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sun J et al. SIRT1 Activation Disrupts Maintenance of Myelodysplastic Syndrome Stem and Progenitor Cells by Restoring TET2 Function. Cell Stem Cell 23, 355–369 e359, doi: 10.1016/j.stem.2018.07.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Wang Y & Zhang Y Regulation of TET protein stability by calpains. Cell reports 6, 278–284, doi: 10.1016/j.celrep.2013.12.031 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Bamezai S et al. TET1 promotes growth of T-cell acute lymphoblastic leukemia and can be antagonized via PARP inhibition. Leukemia 35, 389–403, doi: 10.1038/s41375-020-0864-3 (2021). [DOI] [PubMed] [Google Scholar]
- 274.Ciccarone F et al. Poly(ADP-ribosyl)ation is involved in the epigenetic control of TET1 gene transcription. Oncotarget 5, 10356–10367, doi: 10.18632/oncotarget.1905 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Ciccarone F, Valentini E, Zampieri M & Caiafa P 5mC-hydroxylase activity is influenced by the PARylation of TET1 enzyme. Oncotarget 6, 24333–24347, doi: 10.18632/oncotarget.4476 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Thienpont B et al. Tumour hypoxia causes DNA hypermethylation by reducing TET activity. Nature 537, 63–68, doi: 10.1038/nature19081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Cimmino L et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell 170, 1079–1095 e1020, doi: 10.1016/j.cell.2017.07.032 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Chen J et al. Vitamin C modulates TET1 function during somatic cell reprogramming. Nat Genet 45, 1504–1509, doi: 10.1038/ng.2807 (2013). [DOI] [PubMed] [Google Scholar]
- 279.Yin R et al. Ascorbic acid enhances Tet-mediated 5-methylcytosine oxidation and promotes DNA demethylation in mammals. Journal of the American Chemical Society 135, 10396–10403, doi: 10.1021/ja4028346 (2013). [DOI] [PubMed] [Google Scholar]
- 280.Tahiliani M et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935, doi: 10.1126/science.1170116 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Hu L et al. Crystal Structure of TET2-DNA Complex: Insight into TET-Mediated 5mC Oxidation. Cell 155, 1545–1555, doi: 10.1016/j.cell.2013.11.020 (2013). [DOI] [PubMed] [Google Scholar]
- 282.Sciacovelli M et al. Fumarate is an epigenetic modifier that elicits epithelial-to-mesenchymal transition. Nature 537, 544–547, doi: 10.1038/nature19353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Bledea R et al. Functional and topographic effects on DNA methylation in IDH1/2 mutant cancers. Scientific reports 9, 16830, doi: 10.1038/s41598-019-53262-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Morin A et al. TET-Mediated Hypermethylation Primes SDH-Deficient Cells for HIF2alpha-Driven Mesenchymal Transition. Cell reports 30, 4551–4566 e4557, doi: 10.1016/j.celrep.2020.03.022 (2020). [DOI] [PubMed] [Google Scholar]
- 285.Chen LL et al. Itaconate inhibits TET DNA dioxygenases to dampen inflammatory responses. Nat Cell Biol 24, 353–363, doi: 10.1038/s41556-022-00853-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Jakubek M et al. Hydrazones as novel epigenetic modulators: Correlation between TET 1 protein inhibition activity and their iron(II) binding ability. Bioorganic chemistry 88, 102809, doi: 10.1016/j.bioorg.2019.02.034 (2019). [DOI] [PubMed] [Google Scholar]
- 287.Vissers MCM & Das AB Potential Mechanisms of Action for Vitamin C in Cancer: Reviewing the Evidence. Frontiers in physiology 9, 809, doi: 10.3389/fphys.2018.00809 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Brabson JP, Leesang T, Mohammad S & Cimmino L Epigenetic Regulation of Genomic Stability by Vitamin C. Frontiers in genetics 12, 675780, doi: 10.3389/fgene.2021.675780 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Lee Chong T, Ahearn EL & Cimmino L Reprogramming the Epigenome With Vitamin C. Frontiers in cell and developmental biology 7, 128, doi: 10.3389/fcell.2019.00128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Agathocleous M et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 549, 476–481, doi: 10.1038/nature23876 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Chrysanthou S et al. The DNA dioxygenase Tet1 regulates H3K27 modification and embryonic stem cell biology independent of its catalytic activity. Nucleic Acids Res 50, 3169–3189, doi: 10.1093/nar/gkac089 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ito K et al. Non-catalytic Roles of Tet2 Are Essential to Regulate Hematopoietic Stem and Progenitor Cell Homeostasis. Cell reports 28, 2480–2490 e2484, doi: 10.1016/j.celrep.2019.07.094 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ko M et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 468, 839–843, doi: 10.1038/nature09586 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Rasmussen KD et al. Loss of TET2 in hematopoietic cells leads to DNA hypermethylation of active enhancers and induction of leukemogenesis. Genes Dev 29, 910–922, doi: 10.1101/gad.260174.115 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Thurman RE et al. The accessible chromatin landscape of the human genome. Nature 489, 75–82, doi: 10.1038/nature11232 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Neri F et al. TET1 is a tumour suppressor that inhibits colon cancer growth by derepressing inhibitors of the WNT pathway. Oncogene 34, 4168–4176, doi: 10.1038/onc.2014.356 (2015). [DOI] [PubMed] [Google Scholar]
- 297.Prasad P, Mittal SA, Chongtham J, Mohanty S & Srivastava T Hypoxia-Mediated Epigenetic Regulation of Stemness in Brain Tumor Cells. Stem Cells 35, 1468–1478, doi: 10.1002/stem.2621 (2017). [DOI] [PubMed] [Google Scholar]
- 298.Herrmann A et al. Integrin alpha6 signaling induces STAT3-TET3-mediated hydroxymethylation of genes critical for maintenance of glioma stem cells. Oncogene 39, 2156–2169, doi: 10.1038/s41388-019-1134-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wu MZ et al. Hypoxia Drives Breast Tumor Malignancy through a TET-TNFalpha-p38-MAPK Signaling Axis. Cancer Res 75, 3912–3924, doi: 10.1158/0008-5472.CAN-14-3208 (2015). [DOI] [PubMed] [Google Scholar]
- 300.Puig I et al. TET2 controls chemoresistant slow-cycling cancer cell survival and tumor recurrence. J Clin Invest 128, 3887–3905, doi: 10.1172/JCI96393 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Su PH et al. TET1 promotes 5hmC-dependent stemness, and inhibits a 5hmC-independent epithelial-mesenchymal transition, in cervical precancerous lesions. Cancer Lett 450, 53–62, doi: 10.1016/j.canlet.2019.01.033 (2019). [DOI] [PubMed] [Google Scholar]
- 302.Hart GW, Housley MP & Slawson C Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022, doi: 10.1038/nature05815 (2007). [DOI] [PubMed] [Google Scholar]
- 303.Fujiki R et al. GlcNAcylation of histone H2B facilitates its monoubiquitination. Nature 480, 557–560, doi: 10.1038/nature10656 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Maury JJ et al. RING1B O-GlcNAcylation regulates gene targeting of polycomb repressive complex 1 in human embryonic stem cells. Stem cell research 15, 182–189, doi: 10.1016/j.scr.2015.06.007 (2015). [DOI] [PubMed] [Google Scholar]
- 305.Chu CS et al. O-GlcNAcylation regulates EZH2 protein stability and function. Proc Natl Acad Sci U S A 111, 1355–1360, doi: 10.1073/pnas.1323226111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Capotosti F et al. O-GlcNAc transferase catalyzes site-specific proteolysis of HCF-1. Cell 144, 376–388, doi: 10.1016/j.cell.2010.12.030 (2011). [DOI] [PubMed] [Google Scholar]
- 307.Li HJ et al. Roles of ten-eleven translocation family proteins and their O-linked beta-N-acetylglucosaminylated forms in cancer development. Oncology letters 21, 1, doi: 10.3892/ol.2020.12262 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Iwafuchi-Doi M & Zaret KS Pioneer transcription factors in cell reprogramming. Genes Dev 28, 2679–2692, doi: 10.1101/gad.253443.114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]


