Abstract
Background:
Sexual health service disruptions due to COVID-19 mitigation measures may have decreased gonorrhea screening and biased case-ascertainment towards symptomatic individuals. We assessed changes in reported symptoms and other characteristics among reported gonorrhea cases during- vs. pre-pandemic periods in one city with persistent gonorrhea transmission.
Methods:
Enhanced surveillance data collected on a random sample of gonorrhea cases reported to the Baltimore City Health Department between March 2018–September 2021 was used. Logistic regression assessed differences in case characteristics by diagnosis period (during-pandemic: March 2020–September 2021; pre-pandemic: March 2018–September 2019).
Results:
Analyses included 2,750 (1,090 during-pandemic, 1,660 pre-pandemic) gonorrhea cases, representing 11,904 reported cases. During- vs. pre-pandemic, proportionally fewer cases were reported by sexual health clinics (8.8% vs. 23.2%), and more frequently reported by emergency departments/urgent care centers (23.3% vs. 11.9%). Adjusting for diagnosing provider, fewer cases who were males with urethral infections [aOR: 0.65, 95% CI: (0.55–0.77)], aged <18 [aOR: 0.64, (0.47–0.89)], and females [aOR: 0.84, (0.71–0.99)] were reported, and cases with insurance [aOR: 1.85, (1.40–2.45)], living with HIV [aOR: 1.43, (1.12–1.83)], or recent (≤12 months) gonorrhea history [aOR: 1.25, (1.02–1.53)] were more frequently reported during- vs. pre-pandemic. Reported symptoms and same-day/empiric treatment did not differ across periods.
Conclusions:
We observed no changes in reported symptoms among cases diagnosed during- vs. pre-pandemic. Increased frequency of reported diagnoses who were insured, living with HIV, or with recent gonorrhea history are suggestive of differences in care access and/or care seeking behaviors among populations with high gonorrhea transmission during the pandemic.
Keywords: Gonorrhea, Public Health Surveillance, COVID-19 Pandemic
Summary:
An analysis of characteristics of individuals with reported gonorrhea diagnoses in Baltimore City, Maryland, showed no indication of increased case ascertainment among symptomatic individuals during- compared to pre-COVID-19 pandemic.
Introduction
The impact of COVID-19 mitigation measures on gonorrhea and other bacterial sexually transmitted infections (STI) transmission in the United States (U.S.) remains unknown. STI clinical, laboratory, and prevention service disruptions, particularly among public health-managed sexual health clinics, during the pandemic are well documented.(1–9) These disruptions may have decreased rates of diagnosis and subsequent treatment, increasing population prevalence of infectious individuals, thus providing opportunity for increased transmission.
Routinely collected public health surveillance data traditionally are used to monitor temporal trends in gonorrhea diagnoses. Across the U.S., substantial declines in reported gonorrhea diagnoses were observed early in the pandemic, (April–June 2020) followed by a rebound in diagnoses that surpassed pre-pandemic rates. (1, 10) Compared to 2019, annual gonorrhea diagnoses increased by 7.1% in 2020, but reported chlamydia diagnoses decreased by 14%.(10) Specimens are collected concurrently for chlamydia and gonorrhea diagnostic tests, and gonorrhea infections are more likely to be symptomatic.(11) Sustained decreases in reported chlamydia diagnoses with concurrent increases in reported gonorrhea diagnoses may indicate increased gonorrhea incidence, and also suggests symptomatic gonorrhea cases may be overrepresented in the data. Examining trends in reported symptoms among those diagnosed with gonorrhea during- compared to pre-pandemic may improve understanding of changes in characteristics of diagnoses represented in STI surveillance data, which can inform interpretation of pandemic-era gonorrhea trends.
The objectives were to determine changes in the frequency of 1) monthly case reports; 2) reported symptoms; 3) characteristics consistent with symptomatic infection (males with urethral infections, documentation of same-day/empiric treatment); and 4) other key characteristics (e.g., demographics, diagnosing provider type, health insurance status, HIV co-infection, and gonorrhea history) among individuals aged ≥13 years diagnosed with gonorrhea during- compared to pre-pandemic in one Mid-Atlantic city with a severe and persistent gonorrhea epidemic.
Materials and Methods
Setting
Baltimore City, Maryland has one of the most severe gonorrhea epidemics in the U.S. In 2019, the reported gonorrhea diagnosis rate in Baltimore City was 3.5-fold higher than the national rate (660.9 vs. 188.4 per 100,000) and the third highest among counties and independent cities.(12)
Study Population
We used routine surveillance and STD Surveillance Network (SSuN) data collected on laboratory confirmed gonorrhea diagnoses reported to the Baltimore City Health Department (BCHD) between March 1, 2018–September 30, 2021. SSuN is a sentinel surveillance network of 10 state and local health departments and the Centers for Disease Control and Prevention (CDC) who follow common protocols for enhanced investigations on a random sample of reported gonorrhea diagnoses (heretofore referred to as cases). We based sampling fractions on the number of reported cases and patient survey completion rates, and adjusted as needed to reach annual sample size targets of 350 in 2018–2019 and 400 in 2020–2021. Inclusion criteria included Baltimore City residence and age ≥13 years. We excluded duplicate morbidity reports, (reported diagnoses with positive laboratory tests performed ≤30 days after a previous reported positive test) and cases reported to the BCHD >60 days after the diagnosis date. Individuals could be selected multiple times for SSuN. Reported gonorrhea diagnosis occurring ≥30 days apart were treated as independent events, and each investigation obtained information specific to that diagnosis.
Data Collection
We used information obtained through routine legally mandated laboratory/provider reports and SSuN enhanced surveillance activities. SSuN activities included: a questionnaire administered to diagnosing healthcare providers (provider survey), and separately, individuals (patient survey). Trained interviewers conduced provider surveys via phone or fax-back forms, which ascertained information on clinical findings, anatomic site of infection, treatment date and type, and health insurance. We pursued provider surveys throughout the observation period. Interviewers conducted patient surveys via telephone, which ascertained information on patient demographics, sexual identity, sex partner gender, HIV or pre-exposure prophylaxis (PrEP) care status, reported symptoms, and health insurance. We did not pursue patient surveys for cases reported between May 7–June 7, 2019 (cybersecurity incident involving city government systems), and March 13–August 31, 2020 (staff redirection towards the COVID-19 response). We analyzed information from cases with completed SSuN provider or patient surveys, and among those with completed patient surveys as a subanalysis. For demographics, anatomic site of infection and treatment, we used responses ascertained through SSuN activities, and when missing, supplemented with routine surveillance data. Cases missing information on sex at birth or diagnosing provider type were excluded.
Measures
Pre- and during- pandemic periods
Using diagnosis date, we defined during-pandemic cases as those diagnosed between March 1, 2020–September 30, 2021. To avoid introducing potential bias from seasonal trends, we defined the pre-pandemic comparison group as cases diagnosed between March 1, 2018–September 30, 2019.
Symptomatic infection
We defined symptomatic cases (Yes/No) as those for which the provider reported clinical findings of urethritis, proctitis, epididymitis, cervicitis, vaginitis, or pelvic inflammatory disease (PID) or the patient reported discharge/oozing from the penis/vagina, painful/burning urination, or any symptoms/pains believed to be STI-related.
Anatomic site of infection
Urogenital infections are more likely to be symptomatic than extragenital infections and are more likely to be symptomatic among males than females.(11) Cases with urogenital infections had positive laboratory tests reported for urine, urethral, vaginal or cervical specimens. For regression models, we combined sex at birth and testing specimen source to define anatomic site of infection as: males, any urethral; males, extragenital, males unknown site or females, all sites.
Same-day/empiric treatment
We defined same-day/empirically treated (Yes/No) cases as those with documentation of CDC-recommended treatment regimens (ceftriaxone (250 or 500mg) or 400mg cefixime as monotherapy or as dual therapy with either 1g azithromycin or 100mg doxycycline) or treatment with an antimicrobial agent used for gonorrhea-consistent symptoms (azithromycin or doxycycline monotherapy, clindamycin, gentamicin, cefotaxime, cefoxitin, cefpodoxime, cefuroxime, ciprofloxacin, gemifloxacin, levofloxacin, metronidazole) on or before the specimen collection date for laboratory testing.(13, 14)
Demographics
Demographics included age, sex at birth, and race/ethnicity. We categorized age as <18, 18–24, 25–34, 35–44, and ≥45 years, sex at birth as male/female, and race/ethnicity was categorized as non-Hispanic Black; Hispanic or Other Race and Unknown.
Diagnosing provider type
We categorized diagnosing provider types as: sexual health clinics, emergency departments/urgent care centers, hospitals, federally qualified health centers (FQHCs), private healthcare settings, and other (i.e., outreach, corrections facilities, etc.).
HIV and gonorrhea history
We defined individuals living with HIV as those with an HIV diagnosis documented in the Maryland HIV registry on or before their gonorrhea diagnosis date. We defined recent gonorrhea history as a documented gonorrhea diagnosis in the STI registry >30 days and ≤12 months from the SSuN-selected gonorrhea diagnosis.
Health insurance status
We defined insured cases as those for which responses to either the provider or patient survey indicated the patient had public (i.e., Medicaid, Medicare) or private insurance. Uninsured patients were those for which neither survey had indicated the case was insured, and one of the surveys indicated the case was uninsured. All others were categorized as unknown.
Sexual Minorities
We defined cases who self-reported male sex at birth, male gender identity, and either gay/homosexual or bisexual sexual identity or male sex partners as men who have sex with men (MSM).
HIV/PrEP Care Engagement
We defined cases as engaged in HIV or PrEP care if they self-reported current antiretroviral therapy (if living with HIV) or PrEP (if not living with HIV) use.
Statistical Analyses
We generated a monthly time series of all reported cases. Among cases with completed SSuN provider or patient surveys, we generated three monthly time series examining the proportion of cases who were symptomatic, urogenital infected, and same-day/empirically treated; these were stratified by sex at birth.
We generated frequencies of characteristics across pandemic periods. Logistic regression models assessed differences in the odds of characteristics during- compared to pre-pandemic; models were adjusted for: 1) diagnosing provider type; and 2) diagnosing provider and age. As a subanalysis, we repeated regression models restricted to cases with completed patient surveys. We conducted sensitivity analyses to examine the influence of missing data on anatomic site by reassigning all male cases diagnosed by emergency departments/urgent care centers with unknown anatomic site of infection as urethral infections and repeated analyses. Analyses were conducted in R Studio 2021.9.0.35 (R Foundation for Statistical Computing, Vienna, Austria).
Ethical Approval
The SSuN program is conducted for the purposes of public health surveillance and in accordance with the Code of Federal Regulations, Title 45, received an exempt determination from the Johns Hopkins University School of Medicine, and was approved by the BCHD.
Results
Among 13,799 gonorrhea diagnoses reported to the BCHD between March 1, 2018–September 30, 2021, 11,983 were diagnosed during our defined observation periods (pre-pandemic: 5,847 cases; during-pandemic: 6,136 cases); 11,904 (99.3%) had complete information on sex at birth and diagnosing provider type. Among the 11,904, 30.5% (3,626) were selected for SSuN, 3,604 (99.4%) were SSuN eligible, and 2,750 (76.3%) completed SSuN provider or patient surveys (1,790 provider only; 271 patient surveys only; 689 both). Characteristics of the 2,750 SSuN cases were generally similar to those of all reported cases (Table 1) with some differences. Compared to all cases, cases completing SSuN surveys were less frequently diagnosed during-pandemic and more frequently reported non-Hispanic Black race/ethnicity, diagnosed by sexual health clinics, and had recent gonorrhea history.
Table 1.
Characteristics of Individuals with Reported Gonorrhea Diagnoses During Compared to Pre COVID-19 Pandemica, Baltimore City, Maryland.
| Reported Cases (N = 11904) |
Sampled Cases (N = 3626) |
Cases With Completed SSuN Surveysb (N = 2750) |
||||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Time period | ||||||
| Pre-pandemic | 5798 | 48.7% | 2079 | 57.3% | 1660 | 60.4% |
| During pandemic | 6106 | 51.3% | 1547 | 42.7% | 1090 | 39.6% |
| Age | ||||||
| <18 years old | 956 | 8.0% | 277 | 7.6% | 218 | 7.9% |
| 18 – 24 years old | 4139 | 34.8% | 1266 | 34.9% | 984 | 35.8% |
| 25 – 34 years old | 4188 | 35.2% | 1284 | 35.4% | 957 | 34.8% |
| 35 – 44 years old | 1503 | 12.6% | 460 | 12.7% | 330 | 12.0% |
| 45+ years old | 1118 | 9.4% | 339 | 9.4% | 261 | 9.5% |
| Sex at Birth | ||||||
| Female | 4818 | 40.5% | 1476 | 40.7% | 1115 | 40.5% |
| Male | 7086 | 59.5% | 2150 | 59.3% | 1635 | 59.5% |
| Race/Ethnicity | ||||||
| Non-Hispanic Black | 8858 | 74.4% | 2819 | 77.7% | 2233 | 81.2% |
| Hispanic or Other Racec | 1390 | 11.7% | 437 | 12.1% | 323 | 11.7% |
| Unknown | 1656 | 13.9% | 370 | 10.2% | 194 | 7.1% |
| Diagnosing Provider | ||||||
| Sexual Health Clinic | 1488 | 12.5% | 499 | 13.8% | 481 | 17.5% |
| Emergency Department/ Urgent Care Center | 2444 | 20.5% | 737 | 20.3% | 452 | 16.4% |
| Hospital | 2341 | 19.7% | 771 | 21.3% | 669 | 24.3% |
| FQHCd | 1696 | 14.2% | 503 | 13.9% | 386 | 14.0% |
| Private Healthcare Provider | 2108 | 17.7% | 633 | 17.5% | 459 | 16.7% |
| Othere | 1827 | 15.3% | 483 | 13.3% | 303 | 11.0% |
| Living with HIV f | 1370 | 11.5% | 435 | 12.0% | 324 | 11.8% |
| Previous Gonorrhea Diagnosis, past 12 months | 1926 | 16.2% | 609 | 16.8% | 483 | 17.6% |
During pandemic: March 1, 2020 – September 30, 2021; Pre-pandemic: March 1, 2018 – September 30, 2019.
Sampled cases with a completed SSuN provider or patient survey were included.
Other Race includes White, Asian, American Indian/Alaska Native, Hawaiian/Pacific Islander, Multi-race or Other
Federally Qualified Health Center
Includes outreach, schools, correctional facilities, laboratories, and reproductive health facilities.
Documented HIV diagnosis reported to Maryland electronic HIV/AIDS reporting system on or before the gonorrhea diagnosis date.
Among the 2,750 SSuN cases, the majority was diagnosed during-pandemic (60.4%), male (59.5%), and non-Hispanic Black (81.0%). About one-third were aged 18–24 years (35.8%, 984); another third were aged 25–34 years (34.8%, 957). Twelve percent (11.8%, 324) were living with HIV and 17.6% (483) had recent gonorrhea history. One quarter (24.3%, 669) were diagnosed in hospital settings, and about 17% were diagnosed by: sexual health clinics (17.5%, 481); emergency departments/urgent care centers (16.4%, 452); and private healthcare providers (16.7%, 459).
Figure 1 shows the monthly time series of all reported cases. Between March 2018–May 2019, monthly case reports ranged between 251–311, then increased by 38.3% to 430 cases in October 2019. Reported cases reached a nadir of 223 cases in April 2020, representing a 48.1% decrease from October 2019 and a 34.4% decrease from February 2020. After April 2020, monthly reported cases steadily increased, but generally were similar to those observed pre-pandemic; ranging from 283–401 between June 2020–September 2021.
Figure 1. Reported Gonorrhea Diagnoses During Compared to Pre COVID-19 Pandemic, Baltimore City, Maryland.
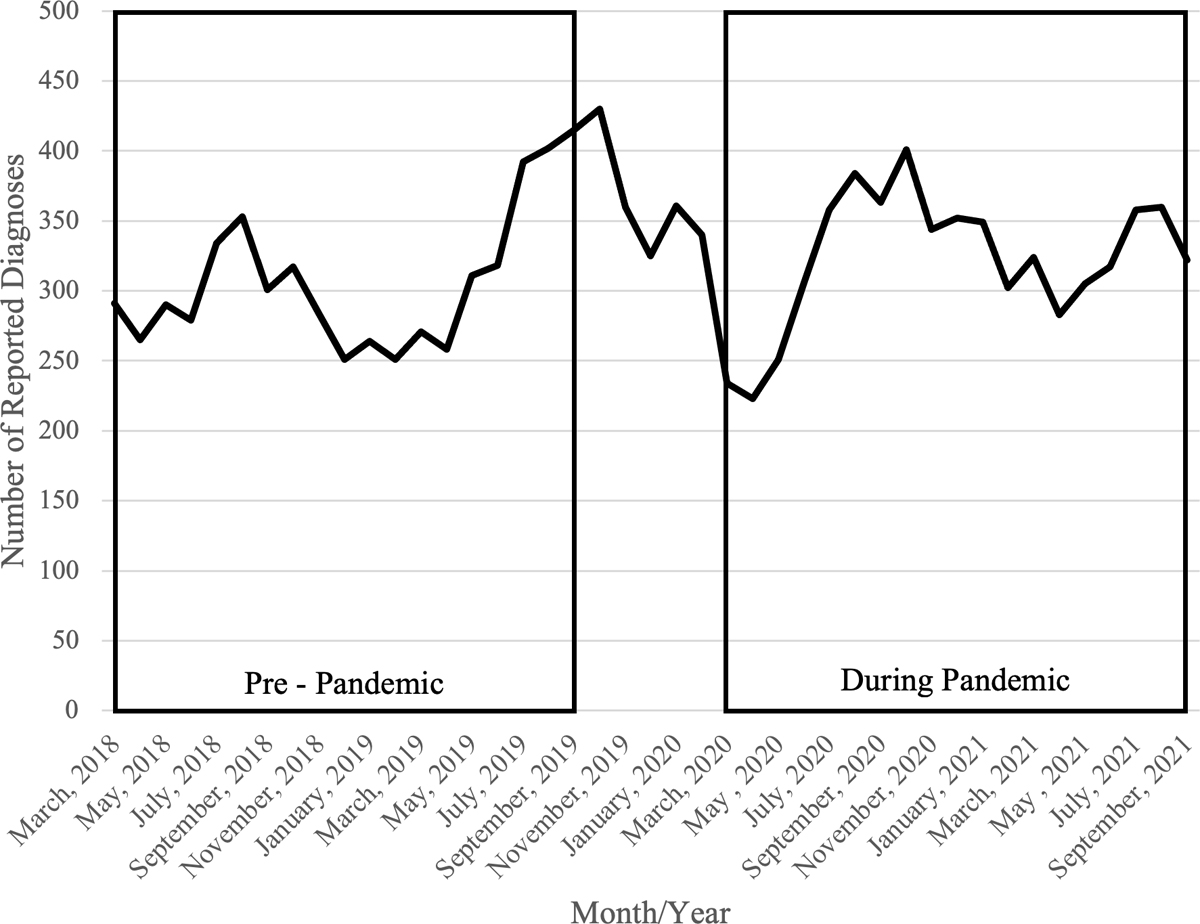
During pandemic: March 1, 2020 – September 30, 2021; Pre-pandemic: March 1, 2018 – September 30, 2019.
Among both sexes, temporal trends in the proportion of cases reporting symptoms (Figure 2A) during compared to pre-pandemic were similar, though there was a marked decline in June of 2020. (Males during-pandemic range: 39.1%-85.2%, pre-pandemic range: 50.9%-79.5%; Females during-pandemic range: 33.3%-69.2%, pre-pandemic range: 23.1%-70.4%). The monthly proportion of urogenital diagnoses among males and females (Figure 2B) were stable pre-pandemic. Among males, during-pandemic, reported urogenital diagnoses peaked in April 2020 at 78.3%, then decreased to 34.4% in December 2020. Among females, urogenital diagnoses ranged from 59.1%-91.7%. Among both sexes, trends in the proportions of cases who received same-day/empiric treatment (Figure 2C) during- vs. pre-pandemic mirrored trends in symptomatic cases.
Figure 2. Proportion of Reported Gonorrhea Diagnoses with Symptoms and Characteristics Indicative of Symptomatic Infection by Sex at Birth During Compared to Pre COVID-19 Pandemic, Baltimore City, Maryland.
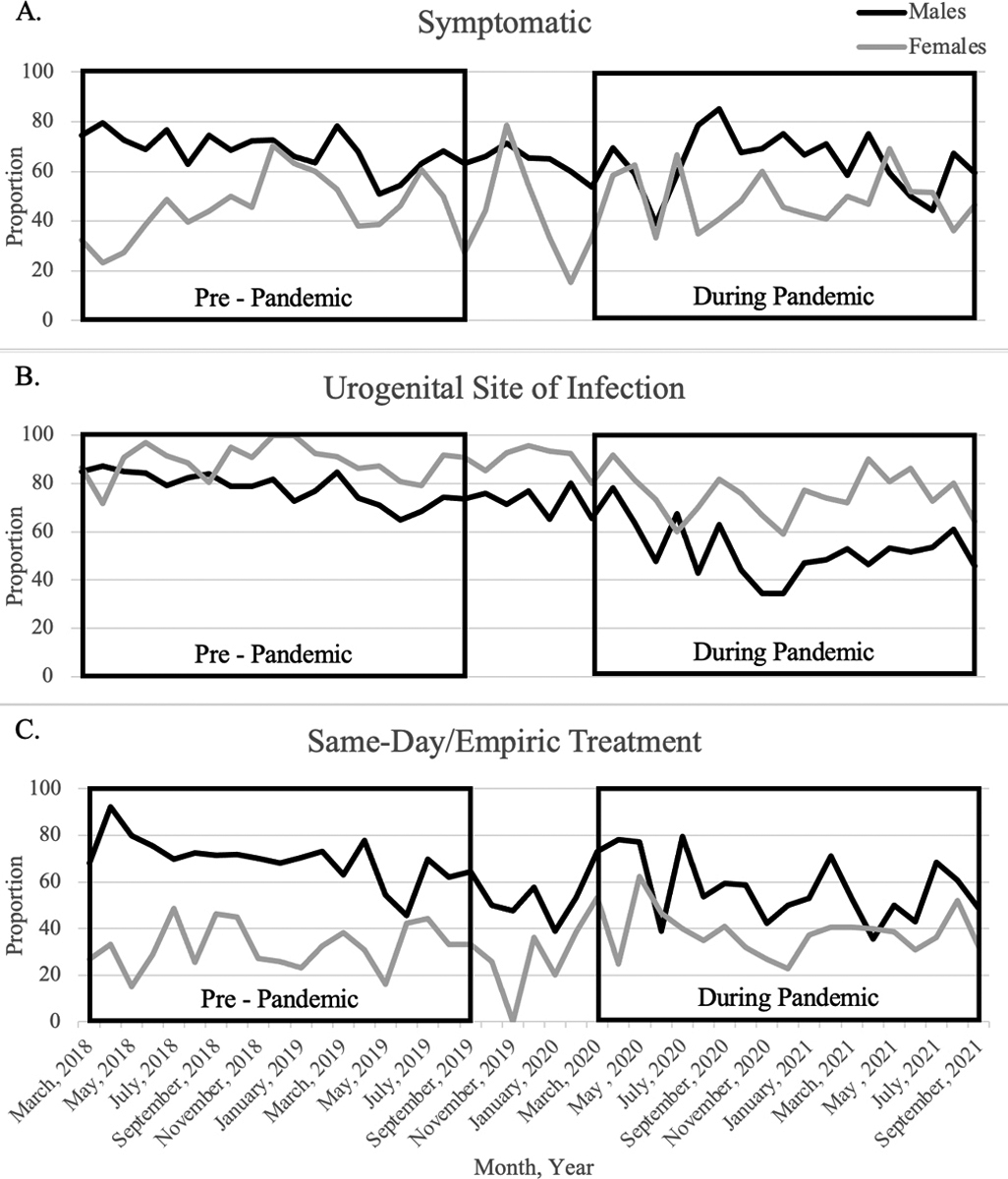
Panel A shows the proportion of cases with symptoms (urethritis, proctitis, epididymitis, PID, discharge, or other STI-related clinical findings reported by the diagnosing provider or patient self-reported discharge, dysuria, or other symptoms/pains believed to be caused by an STI). Panel B shows the proportion of cases whose had positive laboratory tests from urogenital (urine, urethral, vaginal or cervical) specimens. Panel C shows the proportion of cases with documentation of receipt of CDC-recommended antimicrobial regimens for gonorrhea treatment or other microbial regimens commonly used to treat symptoms consistent with gonorrhea on or before the date a specimen was collected for laboratory testing. All proportions are calculated among cases with completed SSuN provider or patient surveys and stratified by sex with male cases represented in black and female cases represented in grey.
During pandemic: March 1, 2020 – September 30, 2021; Pre-pandemic: March 1, 2018 – September 30, 2019.
In unadjusted analyses, we observed no differences in the proportion of cases reporting symptoms across periods (Odds Ratio (OR): 0.93, 95% CI [0.79–1.08]). During- (vs. pre-) pandemic, fewer cases were males with urethral diagnoses (OR: 0.53, [0.45–0.62]) and received same-day/empiric treatment (OR: 0.82, [0.71–0.96]) (Table 2). Generally, demographic characteristics were similar across periods, though there were significantly fewer older cases (aged ≥45 vs. 25–34 years) (OR: 0.72, [0.54–0.97]). The proportion of cases diagnosed by sexual health clinics during- (vs. pre-) pandemic declined by 62% (8.8% vs. 28.5%), and the proportion diagnosed by emergency departments/urgent care centers doubled (23.3% vs. 11.9%). Relative to sexual health clinics, cases were significantly more frequently diagnosed in nearly all other diagnosing providers. Notably, cases were more frequently diagnosed by emergency departments/urgent care centers during- vs. pre-pandemic, (OR: 5.14, [3.85–6.88]). During- (vs. pre-) pandemic cases were more frequently insured (OR: 2.27, [1.74–2.96]), living with HIV (OR: 1.34, [1.06–1.70]), and had recent gonorrhea history (OR: 1.24, [1.01–1.51]).
Table 2.
Selected Characteristics of Individuals with Reported Gonorrhea Diagnosesa During Compared to Pre COVID-19 Pandemicb, Baltimore City, Maryland
| Pre – Pandemic (N = 1660) |
During Pandemic (N = 1090) |
Unadjusted Odds Ratio | Odds Ratio Adjusted for Diagnosing Provider Typec | Odds Ratio Adjusted for Diagnosing Provider Type and Aged | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR | 95% CI | aOR | 95% CI | aOR | 95% CI | |
| Symptomatic e | 971 | 58.5% | 617 | 56.6% | 0.93 | (0.79, 1.08) | 1.02 | (0.87, 1.21) | 1.02 | (0.86, 1.20) |
| Anatomic Site of Infection | ||||||||||
| Males, any urethralf | 766 | 46.1% | 339 | 31.1% | 0.48 | (0.37, 0.62)‡ | 0.49 | (0.37, 0.64)‡ | 0.51 | (0.39, 0.67)‡ |
| Males, extragenital only | 172 | 10.4% | 159 | 14.6% | Ref | Ref | Ref | |||
| Males, unknown site | 49 | 3.0% | 150 | 13.8% | 3.31 | (2.25, 4.89)‡ | 2.14 | (1.41, 3.26)‡ | 2.21 | (1.45, 3.37)‡ |
| Female, all site | 673 | 40.5% | 442 | 40.6% | 0.71 | (0.56, 0.91)† | 0.58 | (0.44, 0.76)‡ | 0.63 | (0.47, 0.82)‡ |
| Same-Day/Empiric Treatment g | 901 | 54.3% | 539 | 49.4% | 0.82 | (0.71, 0.96)† | 0.97 | (0.82, 1.15) | 0.96 | (0.81, 1.14) |
| Age | ||||||||||
| <18 years old | 143 | 8.6% | 75 | 6.9% | 0.76 | (0.56, 1.03) | 0.64 | (0.47, 0.89)† | ||
| 18–24 years old | 602 | 36.3% | 382 | 35.0% | 0.92 | (0.77, 1.10) | 0.86 | (0.71, 1.04) | ||
| 25–34 years old | 566 | 34.1% | 391 | 35.9% | Ref | Ref | ||||
| 35–44 years old | 175 | 10.5% | 155 | 14.2% | 1.28 | (1.00, 1.65) | 1.25 | (0.96, 1.62) | ||
| 45+ years old | 174 | 10.5% | 87 | 8.0% | 0.72 | (0.54, 0.97)* | 0.80 | (0.60, 1.09) | ||
| Female Sex at Birth | 673 | 40.5% | 442 | 40.6% | 1.00 | (0.86, 1.17) | 0.84 | (0.71, 0.99)* | 0.88 | (0.74, 1.04) |
| Race/Ethnicity | ||||||||||
| Non-Hispanic Black | 1357 | 81.7% | 876 | 80.4% | 1.01 | (0.79, 1.28) | 1.13 | (0.88, 1.45) | 1.19 | (0.93, 1.54) |
| Hispanic or Other Raceh | 197 | 11.9% | 126 | 11.6% | Ref | Ref | Ref | |||
| Unknown | 106 | 6.4% | 88 | 8.1% | 1.30 | (0.91, 1.86) | 1.03 | (0.71, 1.50) | 1.13 | (0.77, 1.64) |
| Diagnosing Provider Type | ||||||||||
| Sexual Health Clinic | 385 | 23.2% | 96 | 8.8% | Ref | |||||
| Emergency Department / Urgent Care Center | 198 | 11.9% | 254 | 23.3% | 5.14 | (3.85, 6.88)‡ | ||||
| Hospital | 473 | 28.5% | 196 | 18.0% | 1.66 | (1.26, 2.20)‡ | ||||
| FQHCi | 201 | 12.1% | 185 | 17.0% | 3.69 | (2.74, 4.98)‡ | ||||
| Private Healthcare Provider | 260 | 15.7% | 199 | 18.3% | 3.07 | (2.30, 4.10)‡ | ||||
| Otherj | 143 | 8.6% | 160 | 14.7% | 4.49 | (3.27, 6.16)‡ | ||||
| Living with HIV k | 175 | 10.5% | 149 | 13.7% | 1.34 | (1.06, 1.70)* | 1.43 | (1.12, 1.83)† | 1.33 | (1.04, 1.72)* |
| Gonorrhea Diagnosis, past 12m | 271 | 16.3% | 212 | 19.4% | 1.24 | (1.01, 1.51)* | 1.25 | (1.02, 1.53)* | 1.25 | (1.02, 1.54)* |
| Insurance Status | ||||||||||
| Insured | 1008 | 60.7% | 850 | 78.0% | 2.27 | (1.74, 2.96)‡ | 1.85 | (1.40, 2.45)‡ | 1.88 | (1.42, 2.48)‡ |
| Uninsured | 226 | 13.6% | 84 | 7.7% | Ref | Ref | Ref | |||
| Unknown | 426 | 25.7% | 156 | 14.3% | 0.99 | (0.72, 1.34)‡ | 1.01 | (0.73, 1.39) | 1.02 | (0.74, 1.40) |
Among randomly sampled cases with a completed SSuN provider or patient survey.
During pandemic: March 1, 2020 – September 30, 2021; Pre-pandemic: March 1, 2018 – September 30, 2019.
Values shown are odds ratios for each characteristic adjusted for diagnosing provider type.
Values shown are odds ratios for each characteristic adjusted for diagnosing provider type and age.
Cases with provider documented urethritis, proctitis, epididymitis, PID, discharge, or other STI-related clinical findings or who self-reported: discharge, dysuria, or other symptoms/pains believed to be caused by an STI.
Cases with reported positive laboratory tests from urine/urethral specimens.
Cases with documentation of receipt of CDC-recommended antimicrobial regimens for gonorrhea treatment or other microbial commonly used to treat symptoms consistent with gonorrhea on or before the date a specimen collected for laboratory testing.
Other Race includes White, Asian, American Indian/Alaska Native, Hawaiian/Pacific Islander, Multi-race or Other
Federally Qualified Health Center
Includes outreach, schools, correctional facilities, laboratories, and reproductive health facilities.
Documented HIV diagnosis reported to Maryland electronic HIV/AIDS reporting system on or before the gonorrhea diagnosis date.
p < 0.05
p < 0.01
p < 0.001
Adjusting for diagnosing provider, the proportion of cases who were symptomatic, males with urethral infections and received same-day/empiric treatment remained similar across periods (Table 2). Differences in demographics emerged. Fewer cases aged <18 years (vs. 25–34 years) were diagnosed (aOR: 0.64, [0.47–0.89]) during- vs. pre-pandemic, and the proportional change of older adults diagnosed during-pandemic was no longer statistically significant. Fewer female (vs. male) diagnoses were reported during- compared to pre-pandemic (aOR: 0.84, [0.71–0.99]). The proportion of cases with insurance, living with HIV, and with recent gonorrhea history diagnosed during- compared to pre-pandemic adjusted for diagnosing provider were similar to that observed in bivariate analyses. Results were similar when adjusting for diagnosing provider and age, except the relationship between pandemic period and female sex was no longer statistically significant.
Results among 960 cases with completed patient surveys were mostly similar to those observed among the full analytic cohort (Table 3). Notably, fewer cases completing patient surveys during vs. pre-pandemic were males with urethral infections (OR: 0.29 [0.19–0.44]). Diagnoses among MSM (OR: 1.59, [1.18–2.13]), and those engaged in HIV care (OR: 2.10, [1.35–3.28]) and PrEP care (OR: 2.08, [1.42–3.06]) were more frequently reported during the pandemic. These associations remained in adjusted models.
Table 3.
Frequencies and Odds of Selected Characteristics of Gonorrhea Cases with Completed SSuN Patient Interviewsa During Compared to Pre COVID-19 Pandemicb, Baltimore City, Maryland
| Pre – Pandemic (N = 512) |
During Pandemic (N = 448) |
(A) Unadjusted Odds Ratio |
(B) Models Adjusted for Diagnosing Provider Typec | (C) Models Adjusted for Diagnosing Provider Type and Aged | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | OR | 95% CI | aOR | 95% CI | aOR | 95% CI | |
| Symptomatic e | 336 | 65.6% | 293 | 65.4% | 0.99 | (0.76, 1.29) | 1.11 | (0.84, 1.47) | 1.12 | (0.85, 1.49) |
| Anatomic Site of Infection | ||||||||||
| Males, any urethralf | 225 | 43.9% | 109 | 24.3% | 0.29 | (0.19, 0.44)‡ | 0.36 | (0.23, 0.56)‡ | 0.36 | (0.23, 0.56)‡ |
| Males, extragenital only | 51 | 10.0% | 85 | 19.0% | Ref | Ref | Ref | |||
| Males, unknown site | 33 | 6.5% | 80 | 17.9% | 1.45 | (0.85, 2.48) | 1.25 | (0.69, 2.24) | 1.22 | (0.68, 2.20) |
| Female, all sites | 203 | 39.6% | 174 | 38.8% | 0.51 | (0.34, 0.77)† | 0.53 | (0.35, 0.81)† | 0.53 | (0.34, 0.83)† |
| Same-Day/Empiric Treatment g | 272 | 53.1% | 167 | 37.3% | 0.52 | (0.40, 0.68)‡ | 0.66 | (0.50, 0.87)† | 0.67 | (0.50, 0.89)† |
| Age | ||||||||||
| <18 years old | 27 | 5.3% | 29 | 6.5% | 1.20 | (0.68, 2.11) | 1.17 | (0.65, 2.10) | ||
| 18–24 years old | 181 | 35.4% | 145 | 32.4% | 0.89 | (0.66, 1.21) | 0.89 | (0.65, 1.21) | ||
| 25–34 years old | 183 | 35.7% | 164 | 36.6% | Ref | Ref | ||||
| 35–44 years old | 63 | 12.3% | 71 | 15.8% | 1.26 | (0.84, 1.87) | 1.38 | (0.90, 2.09) | ||
| 45+ years old | 58 | 11.3% | 39 | 8.7% | 0.75 | (0.47, 1.19) | 0.84 | (0.52, 1.35) | ||
| Female Sex at Birth | 203 | 39.6% | 174 | 38.8% | 0.97 | (0.75, 1.25) | 0.90 | (0.68, 1.18) | 0.91 | (0.68, 1.22) |
| Race/Ethnicity | ||||||||||
| Non-Hispanic Black | 437 | 85.4% | 372 | 83.0% | 0.85 | (0.60, 1.20) | 0.97 | (0.68, 1.39) | 1.01 | (0.70, 1.46) |
| Hispanic, or Other Raceh | 75 | 14.6% | 76 | 17.0% | Ref | Ref | Ref | |||
| Diagnosing Provider Type | ||||||||||
| Sexual Health Clinic | 94 | 18.4% | 41 | 9.2% | Ref | |||||
| Emergency Department / Urgent Care Center | 78 | 15.2% | 109 | 24.3% | 3.20 | (2.01, 5.12)‡ | ||||
| Hospital | 141 | 27.5% | 56 | 12.5% | 0.91 | (0.56, 1.47) | ||||
| FQHCi | 61 | 11.9% | 89 | 19.9% | 3.35 | (2.05, 5.46)‡ | ||||
| Private Healthcare Provider | 88 | 17.2% | 90 | 20.1% | 2.34 | (1.47, 3.75)‡ | ||||
| Otherj | 50 | 9.8% | 63 | 14.1% | 2.89 | (1.71, 4.87)‡ | ||||
| Living with HIV k | 39 | 7.6% | 64 | 14.3% | 2.02 | (1.33, 3.08)‡ | 1.95 | (1.26, 3.03)† | 1.91 | (1.22, 3.00)† |
| Previous Gonorrhea Diagnosis, past 12m | 67 | 13.1% | 86 | 19.2% | 1.58 | (1.11, 2.24)† | 1.55 | (1.08, 2.23)* | 1.56 | (1.09, 2.25)* |
| Insurance | ||||||||||
| Insured | 428 | 83.6% | 413 | 92.2% | 2.32 | (1.48, 3.62) | 1.89 | (1.18, 3.03)† | 1.90 | (1.18, 3.05)† |
| Uninsured | 72 | 14.1% | 30 | 6.7% | Ref | Ref | Ref | |||
| Unknown | 12 | 2.3% | 5 | 1.1% | 1.00 | (0.32, 3.09)‡ | 0.91 | (0.28, 2.93) | 0.90 | (0.28, 2.93) |
| MSM l | 104 | 20.3% | 129 | 28.8% | 1.59 | (1.18, 2.13)† | 1.51 | (1.10, 2.07)† | 1.50 | (1.08, 2.08)* |
| In Care | ||||||||||
| HIV Carem | 36 | 7.0% | 56 | 12.5% | 2.10 | (1.35, 3.28)‡ | 2.07 | (1.30, 3.31)† | 2.07 | (1.28, 3.35)† |
| PrEP Caren | 50 | 9.8% | 77 | 17.2% | 2.08 | (1.42, 3.06)‡ | 2.21 | (1.47, 3.32) ‡ | 2.23 | (1.48, 3.35)‡ |
| None Documented | 426 | 83.2% | 315 | 70.3% | Ref | Ref | Ref | |||
SSuN enhanced surveillance activities, which include a survey completed by the diagnosing provider and a patient interview, are conducted on a random sample of the total reported gonorrhea diagnoses.
During pandemic: March 1, 2020 – September 30, 2021; Pre-pandemic: March 1, 2018 – September 30, 2019
Values shown are odds ratios for each characteristic adjusted for diagnosing provider type.
Values shown are odds ratios for each characteristic adjusted for diagnosing provider type and age.
Symptomatic patients were those that providers documented as having urethritis, proctitis, epididymitis, PID, discharge, or other STD-related findings during the exam or patients that self-reported discharge/oozing from the penis/vagina, painful/burning urination, or symptoms/pains believed to be caused by an STD.
Cases with reported positive laboratory tests from urine/urethral specimens.
Cases with documentation of receipt of CDC-recommended antimicrobial regimens for gonorrhea treatment or other microbial commonly used to treat symptoms consistent with gonorrhea on or before the date a specimen collected for laboratory testing.
Other Race includes White, Asian, American Indian/Alaska Native, Hawaiian/Pacific Islander, Multi-race or Other; Four individuals with unknown race were collapsed into this category.
Federally Qualified Health Center
Includes outreach, schools, correctional facilities, laboratories, and reproductive health facilities.
Documented HIV diagnosis reported to Maryland electronic HIV/AIDS reporting system on or before the gonorrhea diagnosis date.
Cases reporting male sex at birth and male gender identity as well as either gay/homosexual or bisexual identity OR male sex partners.
Cases living with HIV who self-report taking anti-retroviral treatment (ART).
Cases reporting not living with HIV and self-report taking HIV pre-exposure prophylactic treatment (PrEP).
p < 0.05
p < 0.01
p < 0.001
Cases with unknown anatomic site of infection were more frequently reported during pandemic (Tables 2 and 3). One-third of cases with unknown anatomic site of infection were diagnosed in emergency departments/urgent care centers, the majority of which (63.5%) were male. After reclassifying anatomic site of infection among these cases to urethral infections, we still observed a statistically significant decrease in the proportion of male urethral infections reported during- compared to pre-pandemic.
Discussion
This analysis sought to improve understanding as to how the COVID-19 pandemic may have impacted trends in gonorrhea diagnoses in one mid-Atlantic U.S. city with a persistent and severe gonorrhea epidemic. Using enhanced surveillance data collected on a random sample of all reported gonorrhea cases, during- compared to pre-pandemic, we observed no differences in reported symptoms and fewer cases with characteristics associated with symptomatic infections (male urethral infections and same-day/empiric treatment). During- vs. pre-pandemic, the proportion of cases diagnosed in public health managed sexual health clinics declined by 62%, while those diagnosed in emergency departments/urgent care centers nearly doubled. After adjusting for diagnosing provider, proportionally fewer reported cases were female or aged <18 years, while cases who were insured, living with HIV, or who had recent gonorrhea history were more frequently reported during- compared to pre-pandemic. These findings have important implications for understanding gonorrhea transmission and interpreting surveillance data throughout the COVID-19 pandemic.
Our observed trends in monthly case reports – precipitous decreases during the early months of the pandemic followed by a rebound to pre-pandemic levels during the summer of 2020 – are consistent with prior reports throughout the U.S.(1, 10, 15) Other studies have reported sharp declines in STI laboratory testing.(2, 16) One study, using data from one U.S. commercial laboratory, estimated that decreased laboratory testing resulted in 5,577 undiagnosed gonorrhea cases, which supports hypotheses that observed pandemic-era declines in gonorrhea diagnoses may be partially attributed to decreased access to testing. We expected to observe both decreases in reported diagnoses and proportional increases in diagnoses reporting symptoms. Conversely, we observed no proportional increases in symptomatic infections, male urethral infections, or same-day/empiric treatment, and instead, symptomatic infections may be underrepresented in pandemic-era surveillance data. There are several possible explanations for these findings. First, care disruption in settings other than sexual health clinics may not have been as severe or prolonged as expected. Second, patients who, prior to the pandemic, would have sought care at sexual health clinics instead may have sought care at other acute care providers such as emergency departments and urgent care centers. Third, shifts to telemedicine necessitated syndromic management protocol implementation (treatment based on symptoms without laboratory confirmation).(4) Since diagnoses without laboratory confirmation would not be reported, the proportion of symptomatic diagnoses during the pandemic may be underestimated. Up to 86.4%–92.6% of urethral gonorrhea infections in males are symptomatic, and symptomatic patients are more likely than asymptomatic patients to receive same-day treatment.(11, 17) Increased syndromic management during the pandemic may explain observed proportional declines in male urethral diagnoses and empiric/same day treatment. Fourth, observed declines in reported cases early in the pandemic may, in part, be due to population-level sexual behavior change.(18) This also may explain findings of no change in the frequency of reported symptomatic diagnoses during the pandemic.
Alternatively, observed crude declines in same-day/empiric treatment may be explained by decreased gonorrhea diagnoses at sexual health clinics, as we observed no change in same-day/empiric treatment during- compared to pre-pandemic when adjusting for diagnosing provider. A majority (75% of males and 50% of females) of patients diagnosed with gonorrhea or chlamydia in BCHD sexual health clinics receive same-day treatment.(19) These clinics also disproportionately serve racial and sexual minorities, youth, and patients who are un- or underinsured.(20, 21) Continued service disruptions at sexual health clinics, including provision of empiric/same-day treatment may contribute to increased gonorrhea transmission in these populations and exacerbate existing disparities.
We observed important changes in demographic and clinical characteristics of reported gonorrhea cases during the pandemic. Proportionally fewer female cases and cases aged <18 years were reported, and cases who were insured, living with HIV, had recent gonorrhea history, MSM, or engaged in HIV/PrEP care were more frequently reported. Prior work has shown that over 60% of persons living with HIV are insured, and among MSM, PrEP users were more frequently insured compared to non-PrEP users.(22, 23) Among MSM, gonorrhea incidence has increased for years, particularly among MSM living with HIV and PrEP users.(24–26) Prior work also suggests that one-third of 2020 gonorrhea cases occurred among MSM.(27) Our findings, therefore, may in part reflect a continuation of trends in increased gonorrhea incidence among MSM.
In contrast, prior work has reported larger increases in reported gonorrhea diagnoses among females relative to males between 2019–2020.(27) Our finding of increased frequency of reported diagnoses among MSM could be due to ascertainment bias. Standards of care for HIV and PrEP care include frequent routine STI screening, and the CDC recommends annual STI screening for MSM.(13, 28, 29) Moreover, the BCHD sexual health clinics, in an effort to mitigate decreased capacity for in-person care during the pandemic, prioritized some in-person visits for individuals who were: enrolled in HIV or PrEP continuity care programs; newly diagnosed with HIV or syphilis, or referred through HIV or syphilis partner notification services (Dr. Elizabeth Gilliams, personal communication). Prioritizing HIV and syphilis care, though warranted, may have contributed to ascertainment bias of gonorrhea cases among MSM and MSM living with HIV among those diagnosed in sexual health clinics. However, patient prioritization policies in other clinics are unknown. Additionally, chlamydia diagnoses, many of which are detected through routine asymptomatic screening of women aged ≤24, declined throughout 2020.(10, 30) Since gonorrhea and chlamydia specimens are collected concurrently, our results support hypotheses that a substantial proportion of gonorrhea infections among young women may have remained undiagnosed, increasing risk of severe sequalae such as PID and infertility. This underscores the need to implement interventions that increase access to STI screening and treatment among women, including interventions that do not require in-person evaluation (i.e., self-collected testing kits, expedited partner therapy).
Several important limitations should be considered when interpreting results. This analysis was performed on a random sample of all reported gonorrhea cases, which would minimize selection bias. There were some differences between cases completing SSuN activities and all reported cases. Notably, proportionally fewer cases diagnosed during- vs. pre-pandemic had completed SSuN activities. This can be attributed to decreased sampling fractions in 2020–2021 compared to 2018–2019 and suspension of SSuN patient surveys between March 13–August 31, 2020 due to staff redirection to the COVID-19 response. This may have led to underestimation of some characteristics of early pandemic cases, namely symptoms. Also, patient reported symptoms may be subject to recall bias. These biases should be minimized; surveys are usually conducted within 30 days of report, and we ascertained clinical findings through provider surveys. Misclassification may also impact our results. Higher proportions of cases diagnosed during- vs. pre-pandemic were missing information on anatomic site of infection, potentially underestimating frequencies of male urethral diagnoses during-pandemic. Sensitivity analyses suggest our findings are robust to this potential misclassification. Inferences can only be drawn regarding differences in reported cases, not transmission, as negative test results are not routinely reported to health authorities. Finally, this analysis was conducted in one urban area with a majority Black/African American population, high poverty rates, and persistent gonorrhea transmission among both heterosexual and MSM populations; results may not be generalizable to other settings.
This analysis provides important information on gonorrhea trends during- compared to pre-pandemic in one U.S. urban area. We found no evidence of increases in the proportion of reported diagnoses with symptoms or factors suggestive of symptomatic infection during the pandemic. This may be a consequence of utilizing syndromic management without laboratory confirmation through telemedicine. Observed changes in demographic and clinical characteristics of cases during the pandemic could be used to inform mathematical modeling studies examining the pandemic’s impact on transmission. Results also could inform future work exploring potential impact on transmission of other mass disruptions/changes in healthcare seeking behaviors and/or healthcare delivery practices. Research exploring temporal differences in characteristics among those treated empirically without laboratory confirmation and those screened asymptomatically is needed to improve understanding of the pandemic’s impact on gonorrhea transmission.
Footnotes
Conflicts of Interest and Sources of Support:
This study was supported by the U.S. Centers for Disease Control and Prevention STD Surveillance Network (NH25PS005187, NH25PS004259). The authors report no conflicts of interest.
References
- 1.Berzkalns A, Thibault CS, Barbee LA, Golden MR, Khosropour C, Kerani RP. Decreases in Reported Sexually Transmitted Infections During the Time of COVID-19 in King County, WA: Decreased Transmission or Screening? Sex Transm Dis. 2021;48(8S):S44–S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pinto CN, Niles JK, Kaufman HW, et al. Impact of the COVID-19 Pandemic on Chlamydia and Gonorrhea Screening in the U.S. Am J Prev Med. 2021;61(3):386–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachmann LH, Bolan G. Dear Colleague Letter: Shortage of STI Diagnostic Test Kits and Laboratory Supplies. September 8, 2020. https://www.cdc.gov/general/DCL-Diagnostic-Test-Shortages.pdf.
- 4.Bachmann LH, Thorpe P, Bolan G, Mermin J. Dear Colleague Letter: STD Treatment Options April 6, 2020. https://www.cdc.gov/std/dstdp/DCL-STDTreatment-COVID19-04062020.pdf.
- 5.Tao J, Napoleon SC, Maynard MA, et al. Impact of the COVID-19 Pandemic on Sexually Transmitted Infection Clinic Visits. Sex Transm Dis. 2021;48(1):e5–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Coalition of STD Directors. COVID-19 & the State of the STD Field: Phase III. January 2021. https://www.ncsddc.org/wp-content/uploads/2021/01/COVID19-State-of-STD-Field-Phase-III-Report-1.28.21-FINAL-1.pdf
- 7.National Coalition of STD Directors. Sexual Health Clinics and Our Nation’s COVID-19 Response. October 23, 2020. https://www.ncsddc.org/wp-content/uploads/2020/10/Clinic-Call-Report-10.23.2020-final.pdf.
- 8.Bonett S, Petsis D, Dowshen N, Bauermeister J, Wood SM. The Impact of the COVID-19 Pandemic on Sexually Transmitted Infection/Human Immunodeficiency Virus Testing Among Adolescents in a Large Pediatric Primary Care Network. Sex Transm Dis. 2021;48(7):e91–e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogers B, Tao J, Murphy M, Chan PA. The COVID-19 Pandemic and Sexually Transmitted Infections: Where Do We Go From Here? Sex Transm Dis. 2021;48(7):e94–e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagaoa M, Grey J, Torrone E, Kreisel K, Stenger M, Weinstock H. Trends in Nationally Notifiable Sexually Transmitted Disease Case Reports During the US COVID-19 Pandemic, January to December 2020. Sex Transm Dis. 2021;48(10):798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and Treatment of Sexually Transmitted Infections: A Review. JAMA. 2022;327(2):161–72. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2019. US Department of Health and Human Services. Atlanta; 2021. [Google Scholar]
- 13.Workowski KA, Bolan GA, Centers for Disease C, Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 14.St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s Treatment Guidelines for Gonococcal Infection, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang JJ, Chen Q, Dionne-Odom J, Hechter RC, Bruxvoort KJ. Changes in testing and diagnoses of sexually transmitted infections and HIV during the COVID-19 pandemic. Sex Transm Dis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiridou M, Heijne J, Adam P, et al. How the Disruption in Sexually Transmitted Infection Care Due to the COVID-19 Pandemic Could Lead to Increased Sexually Transmitted Infection Transmission Among Men Who Have Sex With Men in The Netherlands: A Mathematical Modeling Study. Sex Transm Dis. 2022;49(2):145–53. [DOI] [PubMed] [Google Scholar]
- 17.Kirkcaldy RD, Weston E, Segurado AC, Hughes G. Epidemiology of gonorrhoea: a global perspective. Sex Health. 2019;16(5):401–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumacher CM, Thornton N, Wagner J, et al. Sexually Transmitted Infection Transmission Dynamics During the Coronavirus Disease 2019 (COVID-19) Pandemic Among Urban Gay, Bisexual, and Other Men Who Have Sex With Men. Clin Infect Dis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stracker NGE, Williford SL, Greenbaum A,; Schumacher C Risk Factors for Untreated Gonorrhea and Chlamydia among Public Sexual Health Clinic Patients, Baltimore, Maryland 2018–2019. National STD Prevention Conference. Virtual; 2020. [Google Scholar]
- 20.Mehtani NJ, Schumacher CM, Johnsen LE, et al. Continued Importance of Sexually Transmitted Disease Clinics in the Era of the Affordable Care Act. Am J Prev Med. 2016;51(3):364–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golden MR, Kerndt PR. Improving clinical operations: can we and should we save our STD clinics? Sex Transm Dis. 2010;37(4):264–5. [DOI] [PubMed] [Google Scholar]
- 22.Rozin I, Sayles H, Anderson MJ, et al. HIV-Infected Patient Knowledge, Attitudes, and Beliefs Regarding the Affordable Care Act. AIDS Res Hum Retroviruses. 2015;31(6):581–6. [DOI] [PubMed] [Google Scholar]
- 23.Marks SJ, Merchant RC, Clark MA, et al. Potential Healthcare Insurance and Provider Barriers to Pre-Exposure Prophylaxis Utilization Among Young Men Who Have Sex with Men. AIDS Patient Care STDS. 2017;31(11):470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenger MR, Pathela P, Anschuetz G, et al. Increases in the Rate of Neisseria gonorrhoeae Among Gay, Bisexual and Other Men Who Have Sex With Men-Findings From the Sexually Transmitted Disease Surveillance Network 2010–2015. Sex Transm Dis. 2017;44(7):393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stenger MR, Pathela P, Schumacher C, et al. Trends in HIV prevalence by self-report among MSM diagnosed and reported with gonorrhea in six United States jurisdictions from 2010 to 2019. AIDS. 2021;35(15):2523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansen K, Steffen G, Potthoff A, et al. STI in times of PrEP: high prevalence of chlamydia, gonorrhea, and mycoplasma at different anatomic sites in men who have sex with men in Germany. BMC Infect Dis. 2020;20(1):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanne JH. Covid-19: Sexually transmitted diseases surged in US during pandemic. BMJ. 2022;377:o1275. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. US Public Health Service: Preexposure prophylaxis for the prevention of HIV in the United States - 2021 Update: a clinical practice guideline. 2021. Accessed September 16, 2022. https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
- 29.Workowski KA, Bachmann LH, Chan PA, et al. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2020. US Department of Health and Human Services. Atlanta; 2022. [Google Scholar]


