Abstract
The composition and location of professional antigen presenting cells (APC) varies in different mucosal surfaces. The cornea, long considered an immune-privileged tissue devoid of APCs, is now known to host a heterogeneous network of bone marrow-derived cells. Here, we utilized transgenic mice that express enhanced green fluorescent protein (EGFP) from the CD11c promoter (pCD11c) in conjunction with immunohistochemical staining to demonstrate an interesting stratification of APCs within non-inflamed murine corneas. pCD11c+ dendritic cells (DCs) reside in the basal epithelium, seemingly embedded in the basement membrane. Most DCs express MHC class II on at least some dendrites, which extend up to 50 µm in length and traverse up 20 µm tangentially towards the apical surface of the epithelium. The DC density diminishes from peripheral to central cornea. Beneath the DCs and adjacent to the stromal side of the basement membrane reside pCD11c− CD11b+ putative macrophages that express low levels of MHC class II. Finally, MHC class II−pCD11c− CD11b+ cells form a network throughout the remainder of the stroma. This highly reproducible stratification of bone marrow-derived cells is suggestive of a progression from an APC function at the exposed corneal surface to an innate immune barrier function deeper in the stroma.
Keywords: cornea, antigen presenting cells
Introduction
Antigen presenting cells (APCs) represent a critical arm of the immune response acting as sentinels in detecting antigens and presenting them to the T and B cells of the adaptive immune system in either a stimulatory or tolerogenic context.1–4 Professional bone marrow-derived APCs, including dendritic cells (DCs), macrophages, and B cells, can exist in differing states of activation. Immature APCs actively endocytose material from their extracellular environment, but are inefficient at presenting processed antigens to naïve T cells in an immunogenic manner. This is because they express low levels of the major histocompatibility complex (MHC) molecules on which foreign peptides are presented and also lack the costimulatory molecules, including CD80 (B7.1) and CD86 (B7.2), that are required to activate T cells and avoid induction of tolerance.1,2 Such immature APCs are thought to maintain tolerance to self antigens and commensal bacterial products that are continuously acquired in the absence of inflammatory mediators associated with APC maturation. However, when immature APCs acquire antigens within an inflammatory environment containing ligands for Toll-like receptors, a maturation process involving up-regulation of MHC and costimulatory molecules renders them highly efficient at immunogenic presentation of antigens to naïve T cells. Moreover, partially matured DCs can differentiate naïve CD4+ T cells into CD4+CD25+ natural T regulatory cells (Tregs) or IL-10-secreting CD4+ type 1 regulatory (Tr1) cells that play an important role in peripheral tolerance.5,6
Until recently, naïve corneas were considered devoid of APCs, a fact thought to contribute to the immune-privileged nature of the cornea. However, this perception was dispelled by two independent and nearly simultaneous reports describing a network of CD11b+ macrophage-like cells in the stroma7 and CD11c+ DCs in the epithelium8 of normal mouse corneas. The presence of DCs in the stroma remains controversial with some groups reporting their presence8,9 and others demonstrating their absence.7,10,11
The cornea resident DCs are heterogeneous with respect to MHC class II expression.7,8,11,12 The DCs in the central cornea reportedly lack expression of both MHC class II and the CD80 and CD86 costimulatory molecules, a unique phenotype as DCs typically constitutively express MHC class II.8 The immature phenotype of corneal DCs might reflect the action of factors such as TGF-β that are present in the micro-environment of the normal cornea and retard DC maturation.13 However, when the cornea becomes inflamed, corneal DCs reportedly mature, migrate to draining lymph nodes, and directly present alloantigens to alloreactive CD4+ T cells.12,14 This finding too is controversial as a recent study by another group was unable to reproduce the migration of DC from corneal transplants or the direct presentation of alloantigens to T cells.15 The reason for these disparate findings remains unclear.
The function of the cornea-resident macrophage-like CD11b+ cells remains largely unexplored. Following whole body irradiation, bone marrow-derived cells begin migrating into the anterior corneal stroma within two weeks and repopulate the remaining stroma by 8 weeks post-depletion.9,16 Recently, the MHC class II+CD11c− presumed macrophages within the naïve corneal stroma were shown to form nanotubular-type connections,11 which may function to transfer calcium fluxes,17 cytoplasmic vesicles, and cell surface proteins,18 with other cells in the stroma.
To more comprehensively define the type and location of APCs within the normal murine cornea, we imaged corneas from transgenic Balb/c mice in which EGFP expression is driven by the DC-specific CD11c promoter.19 EGFP expression was readily detectable in cells possessing dendritic morphology within naïve corneas of live mice using a hand-held in vivo confocal probe as well as an inverted two-photon confocal microscope. In addition, imaging of fixed corneal whole mounts from the same mice allowed a detailed spatial analysis of EGFP+ DCs within the cornea as well as analysis of other APC subsets. Our results revealed an interesting stratification of APCs within normal mouse corneas that would be consistent with progression from antigen presentation capability at the apical surface to a barrier function deeper within the corneal stroma. Our findings provide further support for the view that DCs reside exclusively in the basal layer of the corneal epithelium and that MHC class II positive DC extend into the central cornea.
Materials and Methods
Mice
Six- to ten-week-old transgenic Balb/c mice whose cells express EGFP driven by the CD11c promoter19 were purchased from Jackson Laboratories and bred in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited University of Pittsburgh animal facility. All animal studies were approved by and conducted in accordance with the University of Pittsburgh Institutional Animal Care and Use Committee.
In vivo confocal imaging
Corneas of anesthetized naïve mice were imaged with either an Optiscan FIVE 1 handheld fluorescence microscope or a home-built non-descanned two-channel multiphoton system equipped with an Olympus FV300 scan head, IX70 inverted microscope, and Coherent Mira/Verdi Ti-Sapphire laser.
Preparation of cornea whole mounts
Corneas were excised from anesthetized naïve mice and extraneous tissue beyond the limbus was removed with a scalpel. In certain experiments, the corneal epithelium was removed from the stroma following a 20-minute incubation at 37 °C in PBS containing 20 mM EDTA as previously described.7 The corneas were then either cut in half or four small radial incisions were made to flatten the tissue. The tissue was then washed in PBS with 4% FBS for 10 min at 4 °C prior to fixation in CytoFix/CytoPerm (BD) for 1 hr at 4 °C. The tissue was washed three times in PBS/4% FBS prior to incubation with primary non-conjugated or fluorochrome-conjugated antibodies overnight at 4 °C. Following three washes, tissue was incubated with fluorochrome-conjugated secondary antibodies if needed and mounted in Immu-Mount (Thermo Scientific) or Vectashield Mounting Medium with DAPI (Vector Laboratories) prior to confocal imaging. Antibodies used were Pacific Blue- or allophycocyanin-conjugated α-CD11b (eBioscience; clone: M1/70), APC-conjugated α-MHC class II (I-A/I-E; eBioscience, clone: M5/114.15.2), purified rabbit α-laminin (a kind gift from W. Halfter), and Cy3-conjugated α-rabbit IgG (Jackson ImmunoResearch Laboratories).
Confocal imaging of corneal whole mounts
Images were acquired by sequential scanning to avoid fluorescence crossover on an Olympus Fluoview 1000× confocal microscope or a Nikon TiE spinning disk system with a Prairie Sweptfield confocal scan head. Z stacks through the tissue were acquired at Nyquist sampling frequency. All image reconstructions were made using MetaMorph (Molecular Devices; version: 7.5.4.0). The number of DCs were manually counted in random 40× or 60× fields of the central, paracentral, and peripheral regions of the cornea as previously defined.20
Statistics
The density of DCs in different regions of the cornea were compared by ANOVA with Bonferroni post-test using GraphPad Prism 5.0 software.
Results
In vivo detection of DCs in the naïve cornea
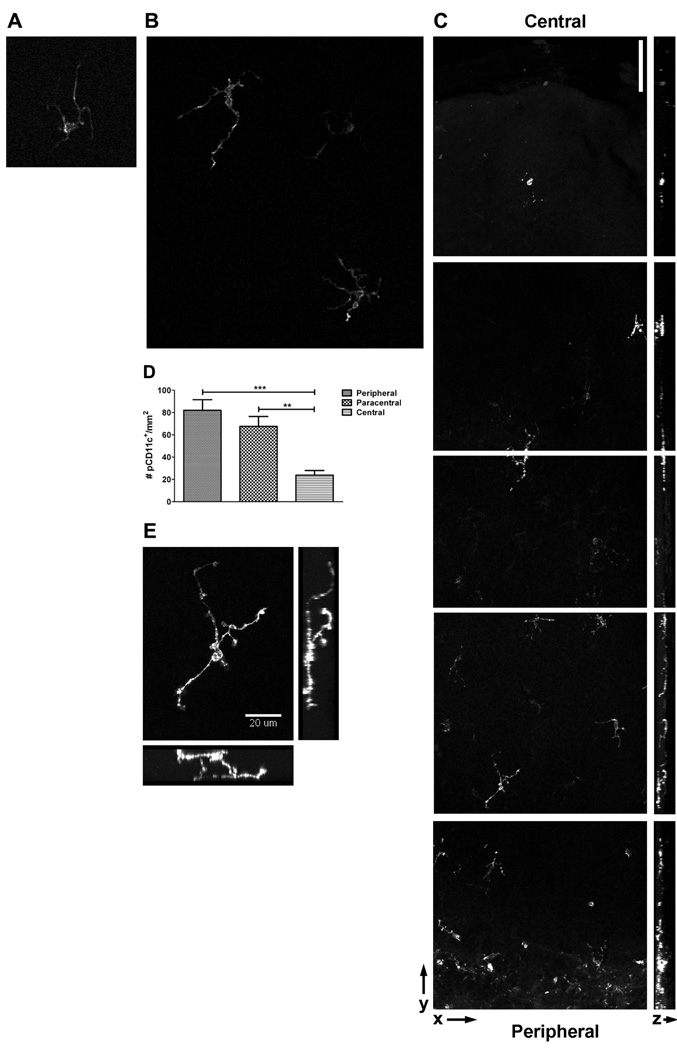
Previous studies have disagreed on the presence of CD11c-expressing cells in the naïve murine corneal stroma.7,8 To definitively demonstrate the presence or absence of CD11c-expressing dendritic cells, we imaged corneas from mice that express EGFP from the CD11c promoter.19 Initial in vivo analyses revealed readily detectable EGFP+ DCs in the normal cornea of live mice (Figs. 1A and B). We first employed a hand-held confocal probe to detect EGFP+ DCs in the corneas of live anesthetized mice (Fig. 1A). Dendritiform EGFP+ cells were observable in every cornea examined with this probe. Due to limitations in magnification and resolution with the hand-held probe, we imaged corneas of the same mice with an inverted two-photon confocal microscope. This method allowed better resolution of EGFP+ DCs in naïve murine corneas (Fig. 1B). However, neither of these in vivo methods allowed reliable positioning of the objective on the murine eye for precise analysis of different regions of the cornea. Thus, we used standard confocal microscopy to image fixed corneal whole mounts, which confirmed a substantial population of EGFP+ DCs within the naïve cornea (Fig. 1C). Essentially all EGFP+ cells possessed a dendritic morphology. The density of CD11c+ DCs was significantly higher in the peripheral and paracentral compared to central regions of the cornea (Fig. 1D). Interestingly, dendritic processes of these cells cover a significant area of the non-inflamed cornea, extending up to approximately 50 µm in length and 20 µm in directions tangential to the cell body and structural layers of the cornea (Fig. 1E).
Figure 1. Detection of CD11c-expressing dendritic cells in live and fixed corneas.
A) Corneas of live anesthetized alb/c mice expressing EGFP from the CD11c promoter were imaged with a hand-held confocal microscopic probe. Original magnification, ~20×. B) Corneas of live anesthetized Balb/c mice expressing EGFP from the CD11c promoter were imaged on an inverted two-photon confocal microscope. Original magnification, 40×. C–E) Corneas from Balb/c mice expressing EGFP from the CD11c promoter were excised and corneal whole mounts were imaged through the entire thickness of the tissue. C) Montage of images spanning from center (top) to peripheral (bottom) cornea. Left: Single plane 3D reconstructions of stacks through the entire thickness of the cells. Right: The full-thickness stacks were reconstructed in the yz plane, with the epithelium to the left and the endothelium to the right. Bar, 75 µm. D) Plot of the density of EGFP+ DCs in the peripheral, paracentral, and central regions of the non-inflamed cornea. Numbers calculated by counting the number of cells per field and adjusting it to the number per mm. ANOVA analysis revealed significant differences between the density of DCs in peripheral versus central (***, 95% CI = 28.71 to 87.56) as well as paracentral versus central (**, 95% CI = 14.29 to 73.15) regions of the cornea. E) High resolution image of a single EGFP+ DC shown as a single-plane 3D reconstruction of images through the entire thickness of the cell as well as en face views.
CD11c-expressing DCs reside in the basal layer of the epithelium
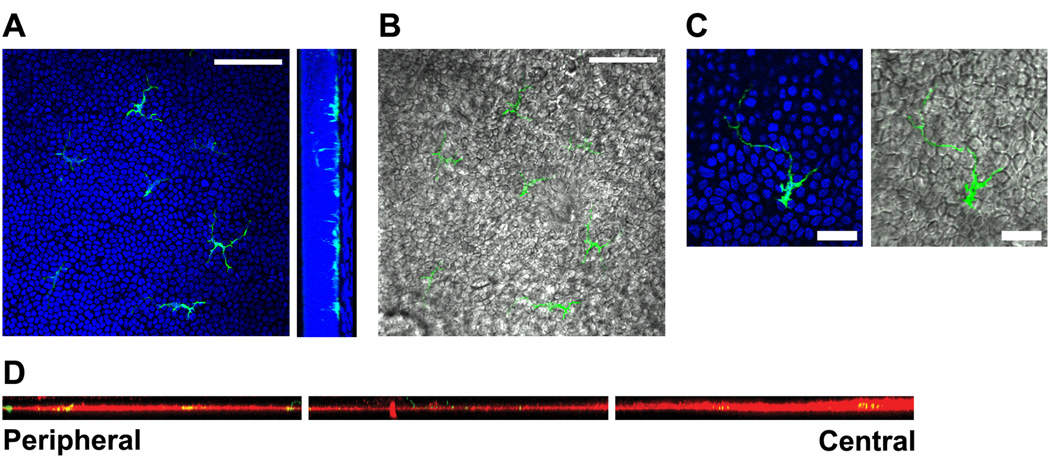
To determine whether these CD11c-expressing DCs resided in the epithelium, stroma, or both, we stained corneal whole mounts with DAPI to demark the epithelial from stromal layers. Whether in the peripheral, paracentral, or central regions of the cornea, all of the EGFP+ DC bodies were found in the basal layer of the corneal epithelium, with most dendrites extending toward the ocular surface (Fig. 2A). Dendrites were found to interdigitate between epithelial cells from the basal to the more superficial layers (Figs. 2B and C and Movie 1).
Figure 2. CD11c-expressing dendritic cells reside in the basal epithelium.
A–C) Whole corneas from Balb/c mice expressing EGFP from the CD11c promoter (green) were stained with DAPI (blue) and imaged by confocal microscopy. A) Left: Representative slice through basal epithelial layer of the paracentral region of the cornea. Right: Images were reconstructed in the yz plane to show the position of EGFP+ dendritic cells in the basal layer of the corneal epithelium demarked by the heavy density of cell nuclei. Bar, 75 µm. B) Same optical section as in A (left) with green DCs overlaid on corresponding differential interference contrast (DIC) image. Bar, 75 µm. C) High magnification image of a DC in the basal epithelial layer. Bar, 25 µm. D) Corneal epithelial sheets were dissociated from whole corneas by treatment with EDTA, stained for laminin, a marker of the epithelial basement membrane, and imaged by confocal microscopy. The images were reconstructed in the xz plane and form a montage from the peripheral cornea on the left to the center of the cornea on the right, with the ocular surface above the images.
Since we could not detect CD11c+ cells in noninflamed corneal stromas in a previous study, we prepared corneas from mice expressing EGFP from the CD11c promoter in the same fashion as those used in our previous study.7 Specifically, we incubated the corneas in EDTA, removed the epithelium, stained for laminin to demark the epithelial basement membrane and imaged both the dissociated epithelial sheet and the remaining stroma separately for EGFP+ DC. All EGFP+ DC bodies were found embedded within the basement membrane of the dissociated epithelial sheet with dendrites extending toward the ocular surface (Fig. 2D). No EGFP+ DCs were found in the remaining stroma except in the very peripheral regions (data not shown).
The majority of DCs express MHC class II in the normal cornea
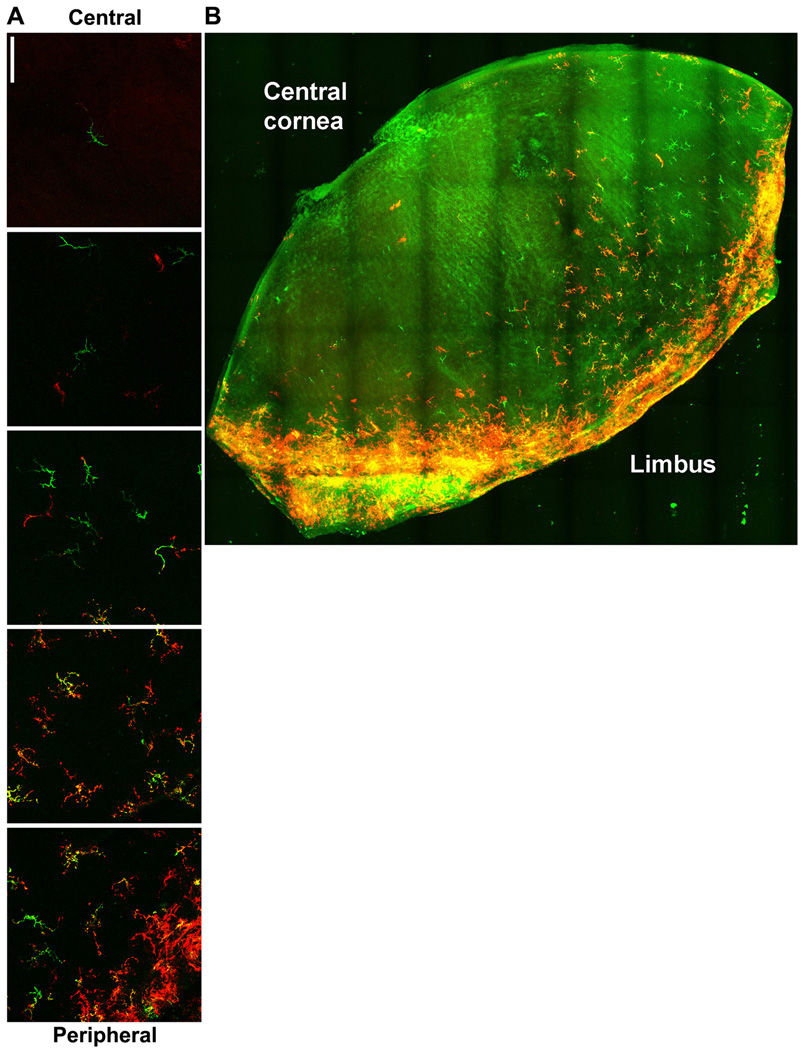
Having defined the presence and location of CD11c-expressing DCs in the normal cornea, we next investigated whether these cells expressed MHC class II. Indeed, the majority of EGFP+ DCs coexpressed MHC class II (Fig. 3A). MHC class II expression was found on EGFP+ DCs in all regions of the cornea, with the highest frequency of MHC class II+ DCs residing in the periphery. MHC class II expression was often restricted to individual DC processes. Employing state-of-the-art confocal technology, we were able to obtain z sections and reconstruct montages illustrating MHC class II+ DCs within a large segment of a normal mouse cornea (Fig. 3B). This figure recapitulates the observation that MHC class II+ DCs are present throughout the cornea, but also demonstrates regions of the cornea that are populated with DCs of varying densities and MHC class II expression.
Figure 3. MHC class II expression on corneal DCs.
Corneal whole mounts from mice expressing EGFP from the CD11c promoter were stained for MHC class II and imaged by confocal microscopy. A) Montage of images showing CD11c promoter activity (green) and surface MHC class II expression (red) from central (top) to peripheral (bottom) cornea. Bar, 75 µm. B) Montage of images from a large portion of cornea with CD11c promoter in green and MCH call II expression in red.
Stratification of APCs within the normal corneal stroma
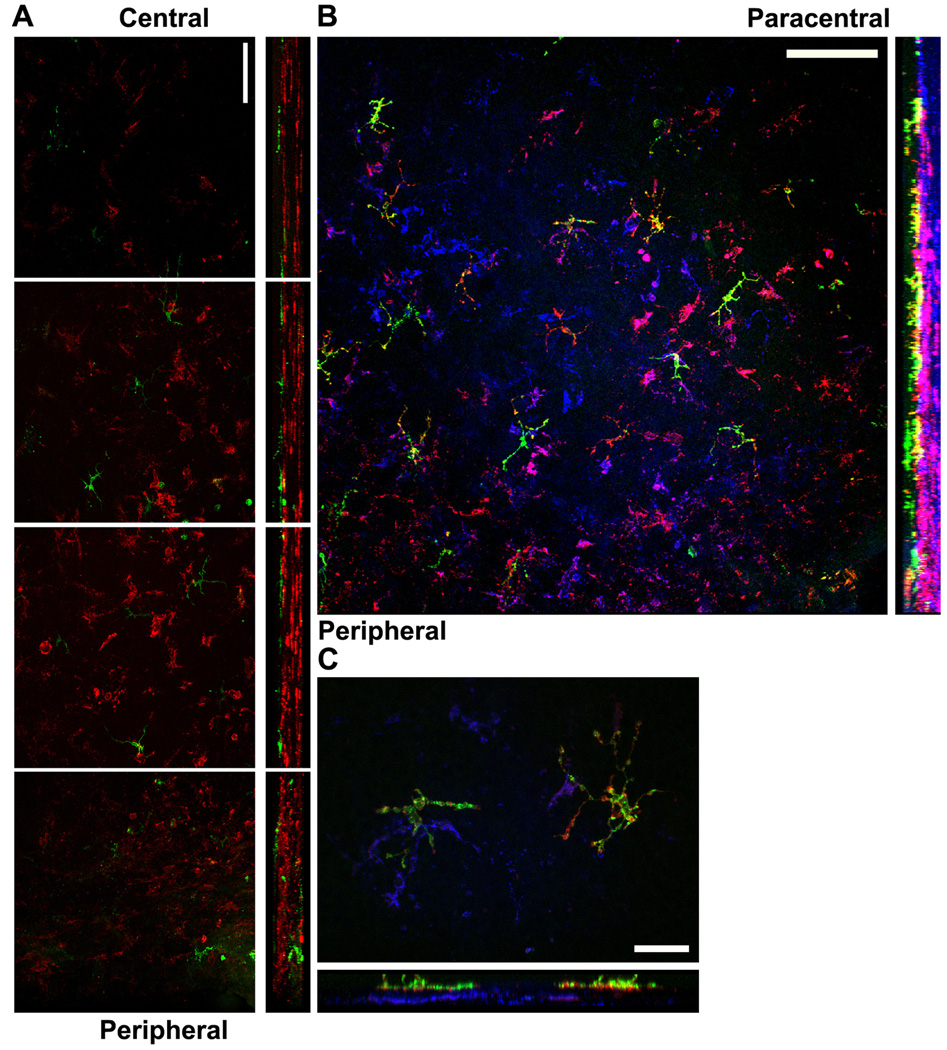
Given the heterogeneity of APCs within normal corneas with regard to expression of CD11c, CD11b (a macrophage marker), and MHC class II, it was of interest to simultaneously localize cells expressing combinations of these markers within the cornea. Accordingly, we determined the presence and location of CD11b+ and MHC class II+ cells in corneas of mice that express EGFP from the CD11c promoter. These studies revealed a very interesting stratification of APCs within the naïve cornea. As noted above, the CD11c (EGFP)-expressing cells were aligned along the basal layer of the corneal epithelium, and as previously reported,7,8,10,16 a substantial population of CD11b+ cells was found throughout the normal corneal stroma (Fig. 4 A). However, the CD11c+ DCs and CD11b+ presumed macrophages were situated in disparate layers of the cornea with essentially no observable overlap. Some CD11b+ cells co-expressed low levels of MHC class II, and most of these CD11b+MHC class II+ presumed macrophages were situated just posterior to the layer of EGFP+MHC class II+ DCs (Figs. 4B and C). Below the layer of MHC class IIdimCD11b+ cells were cells that expressed CD11b but no detectable MHC class II (Fig. 4B).
Figure 4. Stratification of APCs within the normal cornea.
Corneal whole mounts from mice expressing EGFP from the CD11c promoter were stained for CD11b alone (A) or CD11b and MHC class II (B and C) and imaged by confocal microscopy. A) Corneal whole mounts from mice expressing EGFP from the CD11c promoter (green) were stained for surface CD11b expression (red). Montage of images (xy plane on the left; yz plane on the right with epithelium toward the left) from the corneal center (top) to periphery (bottom). Bar, 75 µm. B and C) Corneal whole mounts from mice expressing EGFP from the CD11c promoter (green) were stained for surface MHC class II (red) and CD11b (blue). B) Representative low-magnification image of the peripheral (lower left) and paracentral (upper right) cornea. Bar, 100 µm C) Representative high-magnification image from the peripheral/paracentral region. Bar, 25 µm.
Discussion
As of a decade ago, the “immune-privileged” status of the cornea was attributed in part to a relative absence of APCs.21 However, several investigative teams have now identified and have begun to characterize populations of APCs within naïve murine corneas.7,10–12 Although researchers agree that normal corneas possess a substantial population of CD11b+ presumed macrophages and CD11c+ DCs, there is disagreement on the exact phenotype and location of these cells. Previous studies have concluded that APCs in the central or paracentral regions of the normal murine cornea lacked MHC class II expression.8,14 Furthermore, some studies reported the presence of CD11c+ bone marrow-derived cells in the anterior portion of non-inflamed corneal stromas,8,9 while other studies failed to detect CD11c+ cells in the corneal stroma.7,10,11 Previous reports have even concluded that staining for surface CD11c poses a technical difficulty that has prevented the definitive characterization of DCs in corneal whole mounts.16 One potential explanation for this difficulty is that, while the available anti-CD11c antibodies have proven useful for staining cells in suspension for flow cytometric analysis, their use for immunohistochemical staining of sections or whole mounts of tissue may be unreliable. Therefore, we used mice whose cells express EGFP from the active CD11c promoter to histologically define a population of dendritic cells in the normal murine cornea. Previous studies using these mice have definitively shown that all EGFP+ cells also co-express surface CD11c protein via flow cytometry.19,22 However, it is currently unknown whether CD11c promoter activity correlates exactly with CD11c surface protein expression in other tissues, such as the cornea.
In the present study, we definitively demonstrate a population of cells actively expressing the CD11c promoter residing in the basal layer of the corneal epithelium of naïve corneas, where most appear to be anchored to the basal lamina of the basal epithelium. The density of these cells was greatest in the periphery and decreased centripetally, but cells were present in all regions. No CD11c promoter-positive cells were detected below the basement membrane of the corneal epithelium in any normal corneas imaged. Additionally, we establish MHC class II expression on DCs within all regions of the cornea. Interestingly, MHC class II expression was heterogeneous even on individual cells, where certain dendrites expressed high levels of MHC class II while others expressed none. Therefore, without the combination of intracellular EGFP and surface MHC class II staining, the structure may not have been identifiable as a cell.
These MHC class II+ DCs reportedly lack expression of the CD80 and CD86 co-stimulatory molecules in a non-inflamed cornea, thus resembling typical immature DCs.13 In this activation state, the corneal DCs could present antigens to effector or memory T cells but would be inefficient at activating naïve T cells due to a lack of co-stimulation. However, it is unclear whether these DCs ever encounter naïve T cells in a non-inflamed cornea. Such encounters would most likely take place in regional lymph nodes, which would require DC migration through a significant expanse of corneal epithelium to reach lymphatic vessels in the corneal limbus. In doing so, they would be essentially “swimming upstream” against the centripetal flow of epithelial cells. Resident DCs in corneal buttons reportedly mature by up-regulating co-stimulatory molecules and migrate to the lymph nodes following corneal transplantation to an inflamed corneal bed.14 However, this finding was not reproduced in a recent similar study.15 Thus, the function of cornea-resident DCs remains uncertain. It is possible that these cells function primarily to present antigens to effector or memory T cells that infiltrate the cornea by sampling ocular surface bacterial antigens as recently demonstrated for DCs in the gut that also send processes to the gut lumen.23 These cells might also function in a non-immunologic capacity by contributing to normal corneal physiology. For instance, DCs in the intestinal epithelium were recently shown to contribute to epithelial wound healing by producing IL-22 that activates a Stat3 signaling pathway in epithelial cells.24
Consistent with previous reports,7,8,10 we observed a substantial population of CD11b+ putative macrophages in the noninflamed corneal stroma. This correlates with the visualization of CD45+CD11b+F4/80+CD68+Cx3cr1+ macrophages in whole mounts of murine corneal stromas in previous studies from one of our laboratories.11,16 In the present study, some of these cells expressed MHC class II; although, the intensity of staining was lower than that seen on EGFP+ DCs. Furthermore, the MHC class II+CD11b+ cells were found exclusively in the anterior stroma directly underlying the DC layer. Recent evidence from experiments using transgenic macrophage Fas-induced apoptosis (Mafia) mice (whose macrophages express EGFP and a suicide gene under control of the c-fms promoter so that treatment with the FK506 dimerizer AP20187 causes Fas-mediated apoptosis of monocytic cells) revealed that AP20187-treated mice had significantly fewer EGFP+ cells in the cornea and a significantly impaired neutrophil response following corneal LPS challenge compared to untreated mice.25 These results demonstrated a central role for corneal macrophages in innate immune responses in the murine cornea.
MHC class II−CD11b+ cells were found throughout most of the thickness of the remaining corneal stroma. It is not clear if MHC class II expression defines functionally distinct subpopulations of macrophages within the cornea or if the corneal epithelium and anterior stroma possess a cytokine milieu that promotes MHC class II expression.
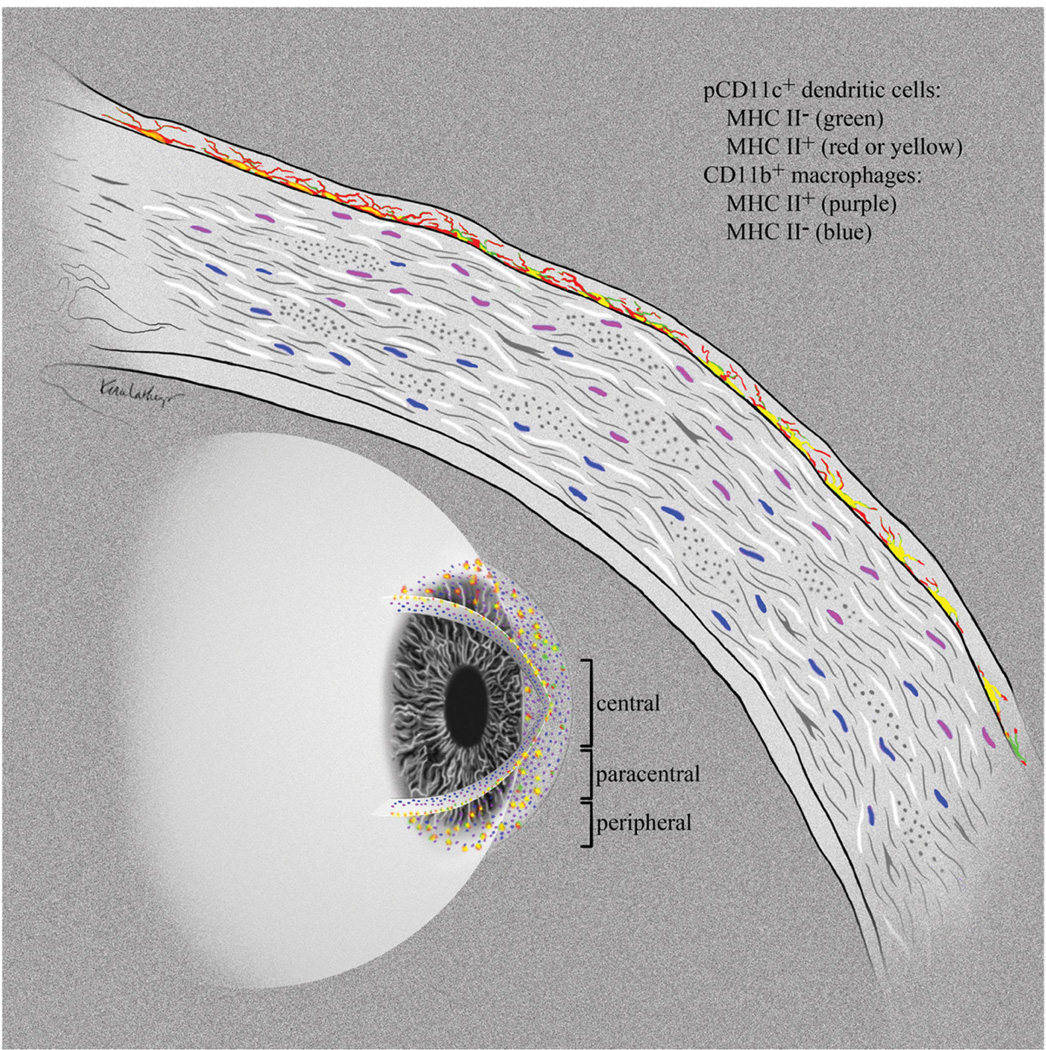
Taken together, these findings reveal a unique stratification of APCs within the noninflamed murine cornea (Fig. 5). The high degree of uniformity of this APC stratification in normal corneas suggests functional relevance. MHC class IIhi DCs reside in the basal layer of the epithelium with tortuous dendrites up to 50 microns in length extending between epithelial cells nearly to the apical surface of the cornea. These cells may be able to survey large areas of the corneal epithelium for foreign material that has penetrated superficial corneal epithelial layers or is present on the apical surface of the epithelium. Directly underlying the epithelial basement membrane in the anterior stroma is a population of CD11b+CD11c− putative macrophages, some expressing low levels of MHC class II. These cells might serve as a backup to the DCs, capturing and presenting antigens that penetrate the corneal epithelial basement membrane. Finally, a rather extensive network of CD11b+MHC class II− macrophages extends from the anterior stroma to the corneal endothelium. These cells might serve primarily in a barrier capacity by responding directly to bacterial threats and preventing penetration of pathogens through the cornea or potentially in a non-immunologic capacity, such as physiologic wound healing.
Figure 5. Stratification of APCs within the normal cornea.
Schematic diagram showing the stratification of APCs within the normal cornea. EGFP+ (green) DCs that co-express MHC class II (red or yellow) reside within the epithelial basement membrane extending dendrites toward the ocular surface. Below the epithelial basement membrane, MHC class IIdim CD11b+ putative macrophages (purple) sit in the anterior stroma, while MHC class II− CD11b+ cells (blue) fill the remainder of the posterior stroma. This unique stratification of APCs suggests progression from an antigen presentation function at the exposed corneal surface to an innate immune barrier function deeper in the stroma.
Supplementary Material
Acknowledgments
We thank K. Lathrop (Department of Ophthalmology), G. Papworth (Center for Biologic Imaging), and G. Gibson (Center for Biologic Imaging) for technical assistance with microscopy. We also thank K. Lathrop for preparation of Figure 5.
Grant Information
Supported by National Institutes of Health grants F30 NS061471 (JEK), R01EY010359 (RLH), P30EY08098 (RLH), U54RR022241 (SCW), a Research to Prevent Blindness Medical Student Fellowship (JEK), unrestricted grants from Research to Prevent Blindness and the Eye and Ear Foundation of Pittsburgh (RLH), and a University of Western Australia and Lions Eye Institute NH&MRC Project Grant 572709 (PGM).
Footnotes
This is an open access article distributed under the terms of the Creative Commons Attribution License (http://www.creativecommons.org/licenses/by/2.0) which permits unrestricted use, distribution and reproduction provided the original work is properly cited.
Disclosures
The authors report no conflicts of interest.
References
- 1.Banchereau JF, Briere C, Caux J, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 2.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 3.Thomson AW, Lu L. Dendritic cells as regulators of immune reactivity: implications for transplantation. Transplantation. 1999;68:1–8. doi: 10.1097/00007890-199907150-00001. [DOI] [PubMed] [Google Scholar]
- 4.Thomson AW, Lu L, Wan Y, et al. Identification of donor-derived dendritic cell progenitors in bone marrow of spontaneously tolerant liver allograft recipients. Transplantation. 1995;60:1555–1559. doi: 10.1097/00007890-199560120-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Vigouroux S, Yvon E, Biagi E, Brenner MK. Antigen-induced regulatory T cells. Blood. 2004;104:26–33. doi: 10.1182/blood-2004-01-0182. [DOI] [PubMed] [Google Scholar]
- 7.Brissette-Storkus CS, Reynolds SM, Lepisto AJ, Hendricks RL. Identification of a novel macrophage population in the normal mouse corneal stroma. Invest Ophthalmol Vis Sci. 2002;43:2264–2271. [PMC free article] [PubMed] [Google Scholar]
- 8.Hamrah P, Liu Y, Zhang Q, Dana MR. The corneal stroma is endowed with a significant number of resident dendritic cells. Invest Ophthalmol Vis Sci. 2003;44:581–589. doi: 10.1167/iovs.02-0838. [DOI] [PubMed] [Google Scholar]
- 9.Nakamura T, Ishikawa F, Sonoda KH, et al. Characterization and distribution of bone marrow-derived cells in mouse cornea. Invest Ophthalmol Vis Sci. 2005;46:497–503. doi: 10.1167/iovs.04-1154. [DOI] [PubMed] [Google Scholar]
- 10.Sosnova M, Bradl M, Forrester JV. CD34+ corneal stromal cells are bone marrow-derived and express hemopoietic stem cell markers. Stem Cells. 2005;23:507–515. doi: 10.1634/stemcells.2004-0291. [DOI] [PubMed] [Google Scholar]
- 11.Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: Membrane nanotubes in vivo: a feature of MHC class II+ cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamrah P, Huq SO, Liu Y, Zhang Q, Dana MR. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J Leukoc Biol. 2003;74:172–178. doi: 10.1189/jlb.1102544. [DOI] [PubMed] [Google Scholar]
- 13.Shen L, Barabino S, Taylor AW, Dana MR. Effect of the ocular microenvironment in regulating corneal dendritic cell maturation. Arch Ophthalmol. 2007;125:908–915. doi: 10.1001/archopht.125.7.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Hamrah P, Zhang Q, Taylor AW, Dana MR. Draining lymph nodes of corneal transplant hosts exhibit evidence for donor major histocompatibility complex (MHC) class II-positive dendritic cells derived from MHC class II-negative grafts. J Exp Med. 2002;195:259–268. doi: 10.1084/jem.20010838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuffova L, Netukova M, Duncan L, Porter A, Stockinger B, Forrester JV. Cross presentation of antigen on MHC class II via the draining lymph node after corneal transplantation in mice. J Immunol. 2008;180:1353–1361. doi: 10.4049/jimmunol.180.3.1353. [DOI] [PubMed] [Google Scholar]
- 16.Chinnery HR, Humphries T, Clare A, et al. Turnover of bone marrow-derived cells in the irradiated mouse cornea. Immunology. 2008;125:541–548. doi: 10.1111/j.1365-2567.2008.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watkins SC, Salter RD. Functional connectivity between immune cells mediated by tunneling nanotubules. Immunity. 2005;23:309–318. doi: 10.1016/j.immuni.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Onfelt B, Nedvetzki S, Yanagi K, Davis DM. Cutting edge: Membrane nanotubes connect immune cells. J Immunol. 2004;173:1511–1513. doi: 10.4049/jimmunol.173.3.1511. [DOI] [PubMed] [Google Scholar]
- 19.Jung S, Unutmaz D, Wong P, et al. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinnery HR, Ruitenberg MJ, Plant GW, Pearlman E, Jung S, McMenamin PG. The chemokine receptor CX3CR1 mediates homing of MHC class II-positive cells to the normal mouse corneal epithelium. Invest Ophthalmol Vis Sci. 2007;48:1568–1574. doi: 10.1167/iovs.06-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Streilein JW. Regional immunity and ocular immune privilege. Chem Immunol. 1999;73:11–38. doi: 10.1159/000058741. [DOI] [PubMed] [Google Scholar]
- 22.Cordier-Dirikoc S, Chabry J. Temporary depletion of CD11c+ dendritic cells delays lymphoinvasion after intraperitonal scrapie infection. J Virol. 2008;82:8933–8936. doi: 10.1128/JVI.02440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickert G, Neufert C, Leppkes M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009 doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chinnery HR, Carlson EC, Sun Y, et al. Bone marrow chimeras and c-fms conditional ablation (Mafia) mice reveal an essential role for resident myeloid cells in lipopolysaccharide/TLR4-induced corneal inflammation. J Immunol. 2009;182:2738–2744. doi: 10.4049/jimmunol.0803505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.