Abstract
Background:
Expanding access to naloxone is one of the most impactful interventions in decreasing opioid-related mortality. However, state distribution rates of naloxone are insufficient to meet community need. The current study sought to better understand this gap by focusing on state policies that may facilitate or impede naloxone distribution in four states highly impacted by fatal opioid overdoses – Kentucky, Massachusetts, New York, and Ohio.
Methods:
We provide a descriptive analysis of the policy landscape impacting naloxone distribution through pharmacy and community channels in the four states participating in the HEALing Communities Study (HCS). Publicly available data and the expertise of the research team were used to describe each state’s naloxone access laws (NALs), Medicaid coverage of naloxone, and community overdose education and naloxone distribution infrastructure. Data presented in this study represent the most current policy landscape through September 2022.
Results:
Variation exists between specific components of the NALs of each state, the structure of Medicaid coverage of naloxone, and the community distribution infrastructure networks. Massachusetts and New York have a statewide standing order, but other states use different strategies short of a statewide standing order to expand access to naloxone. Quantity limits specific to naloxone may limit access to Medicaid beneficiaries in some states.
Conclusion:
States participating in the HCS have developed innovative but different mechanisms to ensure naloxone access. Policies were dynamic and moved towards greater access. Research should consider the policy landscape in the implementation and sustainability of interventions as well as the analysis of outcomes.
Keywords: Naloxone, Naloxone access laws, Opioid policy, Overdose prevention, Healing communities study
1. Introduction
The opioid crisis in the United States continues to worsen with more than 107,000 drug overdose deaths occurring in the United States in 2021 (Ahmad et al., 2021). Naloxone, an opioid antagonist, is a prescription medicine that can rapidly reverse an opioid overdose when administered in a timely manner at the appropriate dose. There are several products approved by the U.S. Food and Drug Administration for emergency reversal of known or suspected opioid overdose including user-friendly nasal sprays and injectable formulations. Overdose education and naloxone distribution (OEND) is an evidence-based strategy to reduce opioid-related mortality, with studies showing that expanding access and availability of naloxone in communities is among the most impactful interventions in decreasing opioid overdose deaths (Irvine et al., 2019; Pitt et al., 2018; Rao et al., 2021). Despite this evidence, a 2021 modeling study that set a saturation benchmark of naloxone available at 80% of witnessed overdoses found that nearly every state in the United States was under-saturated with naloxone (Irvine et al., 2022).
Naloxone is made available to communities in the United States through two main mechanisms: dispensing through pharmacies and distribution by community-based organizations (Weiner et al., 2019). Policies play an important role in accessing naloxone, primarily through state and organizational policies including Naloxone Access Laws (NALs), policies that impact community distribution infrastructure for OEND, and payor reimbursement.
As a prescription medication in the United States, naloxone is regulated under the federal Food, Drug and Cosmetic Act and accompanying state laws that govern the distribution and dispensing of prescription medications, both of which currently require a valid prescription from a licensed health care provider before naloxone can be dispensed. To increase access to naloxone, states have implemented NALs that circumvent these regulatory requirements, simplifying the process of obtaining naloxone and expanding who can receive and distribute it. NALs, therefore, play an important role in both pharmacy dispensing and community distribution. While these laws vary by state, they typically include one or more of the following provisions: (1) allowing for non-patient-specific prescriptions through a standing or protocol order; (2) granting prescriptive authority to pharmacists; (3) permitting third-party prescribing that authorizes the prescribing and dispensing of naloxone to people who are not at-risk of overdose themselves, but are likely to witness an overdose and administer naloxone to others; (4) mandatory co-prescribing to persons at high risk for overdose, such as those dispensed high doses of prescription opioids or with a history of substance use disorder; or 5) removal of professional, civil, and/or criminal liability for persons administering, prescribing, or dispensing naloxone (Davis and Carr, 2015, 2017). All 50 states had implemented NALs as of 2017, with rapid adoption beginning in 2013 (Bohler et al., 2021). There is rigorous evidence suggesting NALs increase naloxone availability in communities with mixed evidence of their impact on opioid-related mortality (Mauri et al., 2020; Smart et al., 2020). Some provisions appear to be especially important; those requiring a standing order or allowing third-party prescribing were associated with higher rates of naloxone dispensing from pharmacies (Gertner et al., 2018; Xu et al., 2018). Co-prescription provisions that mandate prescribing naloxone with another prescription for individuals at high risk for an opioid overdose have also been associated with higher naloxone dispensing (Green et al., 2020; Sohn et al., 2019).
Community distribution of naloxone is typically provided by OEND programs. These programs equip people at risk for overdose, family members, friends, and other bystanders with naloxone rescue, usually at no cost, and oftentimes serve high-risk populations, such as people who use drugs and their social networks. Community OEND programs educate and train potential rescuers to respond to an opioid overdose and have been shown to improve potential rescuers’ attitudes toward naloxone; their implementation has been associated with community-level decreases in overdose death rates (Razaghizad et al., 2021). Despite this evidence, implementation, expansion, and sustainability of these programs rely on acquiring adequate and permanent funding (Green et al., 2012; McDonald et al., 2017; Wheeler et al., 2015). These programs are primarily financed through state and federal grant funding mechanisms with some state discretion on how funding is allocated; however, in some communities OEND programs offered by local non-profit harm reduction and recovery organizations may rely solely on volunteer efforts and private donations. Several state and organizational initiatives have emerged to keep the costs of naloxone down for community OEND programs with some states having policies allowing for bulk purchasing of naloxone (Colorado Department of Public Health and Environment, 2022) and special contracts with pharmaceutical companies (NYS Department of Health, 2021). Harm reduction organizations have joined together to increase leverage for price negotiation in the face of increasing naloxone prices (Doe-Simkins et al., 2022). In addition to funding, legal barriers may exist for community OEND programs. These may be addressed in NALs such as standing orders that include community OEND programs, Good Samaritan Laws limiting civil and criminal immunity for layperson administering or others distributing naloxone, and third-party prescribing (Davis and Carr, 2015; Lambdin et al., 2018). In September 2022, the federal U.S. Food and Drug Administration issued new guidance aimed to improve community access to naloxone by exempting harm reduction programs from certain drug supply chain tracking requirements (U.S. Food and Drug Administration, 2022).
Access to health insurance and payor reimbursement also play a role in naloxone distribution. Reimbursement and insurance coverage for naloxone have been increasing among public and private payors (Sohn et al., 2020). However, the price of naloxone without insurance remains high and insurance may only partially cover the expense (Peet et al., 2022). Even though Medicaid beneficiaries face lower costs, these costs may still be a barrier for low-income individuals. The Medicaid population is especially vulnerable to opioid-related overdose, as it is estimated that 4 in 10 nonelderly adults with opioid use disorder are Medicaid beneficiaries (Orgera and Tolbert, 2019). Additional payor restrictions may further reduce naloxone access, such as monthly quantity limits and coverage exclusion of certain naloxone formulations. State policies increasing coverage and reducing costs of naloxone are also likely to increase access. For example, there is evidence that naloxone access increased in states participating in Medicaid expansion, a policy change in the United States where states could opt into expanding public health insurance among low-income individuals (Frank and Fry, 2019; Sohn et al., 2020), and this was the most important state-level naloxone policy (Frank and Fry, 2019), highlighting the importance of having health insurance with low out-of-pocket costs.
Awareness of naloxone policies and how they are interpreted can also impact access. Previous literature across a wide range of states identified policy misunderstandings around NALs’ provisions including standing orders, requirements for identification to receive naloxone, age requirements, and mandates for pharmacies to maintain a continuous supply of naloxone (Evoy et al., 2018; Graves et al., 2019; Jimenez et al., 2019; Thompson et al., 2019; Wu et al., 2020). Lack of clarity around insurance billing when dispensing under a standing order can be problematic (Evoy et al., 2018; Thompson et al., 2019). In addition, given the complexities of NALs, implementation of these laws can be slow, with expert consensus that statewide standing/protocol orders are the most implementable and effective type of NAL provision (Smart and Grant, 2021). There is a delicate balance between a potential portfolio of naloxone polices that are effective at reducing overdose mortality and what is implemented at the state level, with consideration to sustainability, equity, financial viability, political feasibility, and immediate impact.
The HEALing (Helping to End Addiction Long-term) Communities Study (HCS) is an ongoing multisite, parallel-group, cluster-randomized control trial in 67 communities that are highly affected by the opioid crisis in four states, Kentucky, Massachusetts, New York, and Ohio (HEALing Communities Study Consortium, 2020). The HCS is examining the impact of the Communities That HEAL (CTH) intervention. CTH is a community-engaged, data-driven planning process promoting the implementation of an integrated set of evidence-based practices across health care, behavioral health, justice, and other community-based settings (Sprague Martinez et al., 2020). The goal of the study is to reduce opioid-related overdose deaths, with naloxone distribution representing a critical component of the CTH (Winhusen et al., 2020). See Winhusen et al. (2020) for more information on naloxone-specific interventions that are part of the CTH.
In the current study, we provide a descriptive analysis of the policy landscape impacting naloxone distribution through pharmacy and community OEND channels in the four states participating in the HCS. We define policy as statutes and regulations at the state level that influence naloxone distribution, specifically NALs, Medicaid coverage and reimbursement, and community OEND infrastructure and funding. We also provide baseline rates for naloxone distribution in HCS communities aggregated at the state level. Given naloxone distribution is substantially impacted by state policies, accounting for this policy landscape may be critical in the HCS efforts to increase access and availability to naloxone.
2. Material and methods
This study conducted a descriptive analysis of policies with the ability to facilitate or impede availability and accessibility of naloxone in the four states participating in the HEALing Communities Study (HCS). Policies included NALs, Medicaid coverage of naloxone, and the community OEND infrastructure. Data presented in this study represent the most current naloxone policy landscape upon completion of this manuscript (September 2022).
2.1. Naloxone access laws
Several sources were used to examine NALs and how nuances within these policies might affect naloxone access and availability. For current provisions we used: (1) Prescription Drug Abuse Policy System, a database providing data up to the end of 2021, (2) a Legislative Analysis and Public Policy Association report summarizing state NALs up to September 2020, and, (3) Nexis Uni to identify relevant state statutes and regulations that were not captured or differed between the first two sources (Legislative Analysis and Public Policy Association, 2020; Prescription Drug Abuse Policy System, 2022). For describing the nuances of NALs, we utilized the expertise of researchers across the four states to highlight the unique features of these laws that might affect naloxone access and availability.
2.2. Medicaid coverage
We conducted web searches for information on each state’s Medicaid program to identify current policies related to the coverage of naloxone, including covered products, preferred vs. non-preferred products, prior authorization (PA) requirements, clinical criteria for use, quantity limits, and beneficiary cost-sharing (i.e., co-pay). We describe coverage for different formulations of naloxone, including injectable or nasal spray, branded or generic, and standard or high dose. Information not clearly identified from web searches was collected via email or telephone communication with state Medicaid Pharmacy Directors.
2.3. Community OEND distribution infrastructure
State Opioid Response (SOR) grant funding, online searches, and the expertise of research teams constituted data sources for OEND infrastructure in each state, including an overview of partnerships and initiatives to support community OEND sites and how they are financed. Public documents were examined including annual congressional reports to better understand the role of SOR grant funding in building and maintaining OEND infrastructure (Substance Abuse and Mental Health Services Administration, 2021). Google searches were conducted with the terms “naloxone”, “community distribution”, “initiative”, and the name of each state to scan publicly available documents describing state initiatives and frameworks that facilitate community distribution of naloxone.
2.4. Naloxone distribution rates
To support the policy landscape analysis between states, we obtained baseline naloxone distribution rates for HCS communities aggregated at the state level before the HCS study began. This data from 2019 was further broken down into pharmacy dispensing rates and community distribution rates. The number of naloxone units (each unit contains two doses of naloxone with dose being defined as the quantity of medication packaged into one naloxone administration) dispensed by community pharmacies was collected from the proprietary IQVIA prescription database Xponent® (Danbury, CT). The number of naloxone units distributed by the community was collected from state administrative sources such as departments of health, hospitals, and emergency departments. For these measures, one unit (two doses) represents one naloxone kit, which often include other items such as face shields and pocket masks. Rates were calculated based on HCS community population derived from either United States Census Data (county level) or the American Community Survey (ZIP code level) and are presented as naloxone distribution rates per 1000 residents. Although there is heterogeneity of HCS communities in each of the four states, all communities were selected based on being highly impacted by opioid-related mortality and efforts were made in the study design of the HCS to increase generalizability of the study findings, such as including both rural and urban communities in each state and selecting states from different regions of the country (El-Bassel et al., 2020; HEALing Communities Study Consortium, 2020).
3. Results
The policy landscape regulating pharmacy dispensing and community distribution of naloxone varies across the four states participating in the HEALing Communities Study (HCS). In this section, we give a summary of the current NALs and regulations specific to each state. This examination includes a nuanced perspective of each state’s NALs and other laws and regulations pertaining to naloxone dispensing and distribution, with a focus on how these policies may impede or facilitate naloxone access (Table 1). Next, we provide a summary of Medicaid policies in relation to reimbursement for naloxone including specific formulations (Table 2). Lastly, we give an overview of the community OEND infrastructure for each state.
Table 1.
Selected naloxone access law provisions for each state participating in the HEALing communities study.
| Kentucky | Massachusetts | New York | Ohio | |
|---|---|---|---|---|
|
| ||||
| Pharmacists can dispense without prescription | √ | √ | √ | √ |
| Statewide standing order | √ | √ | ||
| Third-party Prescribing | √ | √ | √ | √ |
| Co-Prescription Mandate | √ | √ | ||
| Pharmacy must maintain sufficient supply | √ | |||
| Pharmacist educational requirement | √ | |||
| Recipient educational requirement | √ | √ | √ | √ |
| Identification not required for pharmacy dispensing | √ | √ | ||
| Civil/criminal immunity for person administering | √ | √ | √ | √ |
| Immunities for prescriber and dispenser | √ | √ | √ | |
Ohio passed recent legislation to allow pharmacies to obtain and maintain sufficient supply of naloxone for use in emergencies but this is not mandated; See https://codes.ohio.gov/ohio-revised-code/section-4729.515.
Although New York’s NAL does not explicitly address prescriber and dispenser immunity, broad immunity is likely interpretable under the NAL.
New York’s NAL provision for co-prescription became effective in June 2022 and statewide standing order became effective in August 2022.
Table 2.
Naloxone formulations provided by medicaid for each state participating in the healing communities study.
| Product/Strength/Form | Kentucky | Massachusetts | New York ∗ | Ohio ∗∗ |
|---|---|---|---|---|
|
| ||||
| Naloxone HCL 0.4 mg/1mLInjection (1 mL cartridge, 1 mL and 10 mL vials), Generic | PA/QL | √ | √ | √ |
| Narcan®Naloxone HCL 4 mg/0.1mLNasal Spray, Brand | QL | √ | √ | $2 Co-pay |
| Naloxone HCL 4 mg/0.1mLNasal Spray, Generic | QL | Trial of Narcan ® first | √ | √ |
| Kloxxado®Naloxone HCL 8 mg/0.1mLNasal Spray, Brand | QL | PA | √ | $2 Co-pay |
| Zimhi®Naloxone 5 mg/0.5 mL prefilled syringe, Brand | PA | NC | √ | $2 Co-pay |
-NC denotes not covered; PA denotes prior authorization; QL denotes quantity limit.
$1 co-pay for New York Medicaid beneficiaries for all brand and generics paid by N–CAP program.
OH Medicaid managed care organizations might impose quantity limits.
3.1. Kentucky
3.1.1. Naloxone access laws
The first NAL in Kentucky became effective in June 2013 authorizing third-party prescribing and granting immunity provisions that provide protection against legal action. It has been amended three times to increase flexibility and legal protections. Currently, Kentucky’s NAL permits licensed health care providers to prescribe and dispense naloxone directly, or via standing order, to individuals and agencies such as jails, emergency medical services, fire departments, harm reduction agencies, and schools. It explicitly authorizes individuals and agencies who receive naloxone prescriptions to possess, administer, or further distribute naloxone as part of a harm reduction program to persons who have been trained on its use. Kentucky’s NAL also provides professional immunity for prescribers and dispensers, and criminal and civil immunity for a person providing or administering naloxone. There is no naloxone co-prescription mandate in Kentucky.
A pharmacist may initiate the dispensing of naloxone to a person or an agency under a physician-approved protocol by applying to the Kentucky Board of Pharmacy to become naloxone-certified. Although there is no statewide standing order, the Medical Director for the Department of Medicaid Services can authorize a protocol for pharmacies that lack a physician to do so. Pharmacists initiating naloxone dispensing under protocol must provide verbal counseling and written educational materials appropriate to the form of naloxone dispensed to each person receiving naloxone under the protocol. Pharmacies with a protocol in place can choose to be listed publicly in the statewide registry. However, due to the voluntary nature of the protocol and the naloxone registry, it is unclear how many pharmacies offer naloxone without a prescription. Previous research suggests that a large portion of pharmacists are reluctant to enter into a protocol agreement to dispense naloxone (Freeman et al., 2017).
3.1.2. Medicaid coverage policies
Kentucky Medicaid implemented a single preferred drug list for use in both the fee-for-service and managed care organization populations in January 2021 and mandated use of a single pharmacy benefits manager to manage prescription drug benefits for all Medicaid beneficiaries in July 2021. All nasal spray formulations are covered without a PA, including generic naloxone 4 mg, brand name Narcan® 4 mg and Kloxxado® 8 mg nasal spray. Zimhi® naloxone 5 mg prefilled syringe and naloxone 0.4 mg/1 mL vials and cartridges for injection require PA. There are quantity limits for all naloxone formulations except for Zimhi®. However, there are no prescription cost-sharing requirements in the Kentucky Medicaid program; therefore, dispensed naloxone is available at no cost to the beneficiary.1
3.1.3. Community OEND distribution infrastructure
The Cabinet for Health and Family Services has partnered with the Kentucky Pharmacists Association for the state’s OEND program. The program supports community OENDs with various agencies, including local health departments, syringe service programs, and law enforcement and other first responder agencies. Additionally, Kentucky supports OEND efforts conducted in a variety of settings, including addiction treatment centers, criminal justice settings, and other community venues and supports OEND efforts of numerous other harm reduction and recovery organizations across the state. These community OEND efforts are funded through a variety of sources, including the SOR-funded Kentucky Opioid Response Effort program and Kentucky’s First Responders-Comprehensive Addiction and Recovery Act grant funded by SAMHSA (High et al., 2020).
3.2. Massachusetts
3.2.1. Naloxone access laws
The first NAL in Massachusetts came into effect in August 2012 and included provisions that allowed third-party prescribing and removed criminal liability for the possession of naloxone without a prescription. The Massachusetts NAL has been amended multiple times primarily to increase pharmacy-based naloxone access. Currently, there is a statewide standing order allowing pharmacies to distribute naloxone without a patient-specific prescription. This type of standing order streamlines naloxone dispensing rather than requiring each pharmacy to secure and file a standing order individually. Current provisions codified through a Board of Registration in Pharmacy regulation require that all pharmacies maintain a continuous, sufficient supply of naloxone and require pharmacists to provide patient education and a pamphlet upon dispensing naloxone under the standing order. Individuals obtaining naloxone at a pharmacy under the statewide standing order are not required to provide identification. Studies have shown that a large majority of Massachusetts’ pharmacies are stocked with naloxone and correctly do not require a prescription, although some pharmacists incorrectly require personal identification and do not provide counseling on naloxone administration (Pollini et al., 2020; Wu et al., 2020). Massachusetts has a remote dispensing policy allows pharmacists to engage in remote dispensing of naloxone if certain requirements are met. For example, after an overdose hotspot is identified, a pharmacy can set up a mobile distribution site outside of a brick-and-mortar pharmacy to distribute naloxone close to where overdoses are occurring.
The NAL is broad in its immunity provisions, providing civil, criminal, and professional immunity to prescribers and dispensers as well as civil and criminal immunity to persons administering naloxone. The current Massachusetts NAL does not have a co-prescribing mandate, and there is a training requirement for pharmacists (Roberts et al., 2019). There are no coverage or cost-sharing requirements placed on insurers, yet the Division of Insurance did circulate a bulletin encouraging commercial insurers to cover naloxone in August 2019. A recent amendment to the NAL in April 2021 adds a provision authorizing a wide range of personnel working in specified community-based organizations to distribute naloxone under the statewide standing order rather than requiring each organization to secure and file a standing order individually. The state has also created the Municipal Bulk Trust Fund allowing municipalities to purchase naloxone at a discounted rate for use by first responders.
3.2.2. Medicaid coverage policies
Massachusetts Medicaid (i.e., MassHealth) has a unified preferred drug list for its fee-for-service, managed care organization, and accountable care organization members. Current information from the MassHealth drug list indicates naloxone HCL 4 mg nasal spray products are covered and that brand name Narcan® is preferred over generic products (i.e., a trial of the preferred drug or clinical rationale for prescribing the non-preferred drug generic equivalent is needed before coverage is provided for the generic). All generic injectable vials and syringes are covered without a PA. The 8 mg nasal spray product (Kloxxado®) requires PA. Zimhi® 5 mg prefilled syringe is not covered. Under MassHealth, covered naloxone products are not subject to cost-sharing requirements and there are no quantity limits for this medication.
3.2.3. Community oend distribution infrastructure
Since 2007, the Massachusetts Department of Public Health has funded OEND agencies across the state to train and distribute naloxone to any person likely to witness an opioid overdose (over 20 agencies in 2022). These programs include every state-supported syringe service program (30+ sites) and a statewide network of support groups for families of people who use drugs (Bagley et al., 2018; Zang et al., 2021). While naloxone is provided at no cost, participants are asked to provide an additional rationale if obtaining more than one kit. Communities where OEND programs were implemented and distributed high volumes of naloxone had a reduction in opioid-related overdose mortality (Walley et al., 2013).
3.3. New york
3.3.1. Naloxone access laws
The first NAL in New York became effective in April 2006 and included provisions giving immunity to parties involved in the process of obtaining and using naloxone and regulated opioid overdose prevention programs. New York’s NAL has been amended several times to increase its flexibility, legal protections, and pharmacy-based access. Up until August 2022, the NAL in New York authorized pharmacies to dispense naloxone under non-patient-specific prescriptions via standing orders issued by individual healthcare prescribers, and chain pharmacies with 20 or more locations were required to secure a non-patient-specific prescription with an authorized health care professional to dispense an opioid antagonist or register with the New York State Department of Health as an opioid overdose prevention program. Recently, the New York Commissioner of Health has issued a statewide standing order allowing pharmacists to dispense naloxone without a patient-specific prescription. The NAL in New York authorizes a wide range of entities to possess, distribute, and administer naloxone, including schools, public libraries, bars and restaurants, theaters, hotels, and organizations registered as opioid overdose prevention programs, such as harm reduction organizations. Organizations registered as opioid overdose prevention programs must have a clinical director to establish appropriate protocols and provide training to individuals receiving naloxone. Entities defined in the NAL that are not registered can obtain naloxone through a third-party prescription.
Immunity from criminal, civil, and administrative liability is provided to persons experiencing an opioid overdose, laypersons administering naloxone, and opioid overdose prevention programs dispensing naloxone, whereas prescriber and dispenser immunity is not explicitly addressed by the NAL. Broad immunity for these stakeholders is likely interpretable under the NAL. The current NAL in New York allows third-party prescribing and has a provision mandating educational requirements to those receiving naloxone through distribution of an informational card or sheet. Legislation signed at the end of 2021 and effective June 2022 amended the NAL mandating co-prescribing of naloxone for opioid prescriptions with a cumulative dose of 90 morphine milligram equivalents (MME) or more per day, with opioid prescriptions that are co-prescribed with benzodiazepines, and for patients with a history of an overdose. New York has an initiative in place, the Naloxone Co-Payment Assistance Program, that covers co-pays up to $40 for insured individuals, though pharmacies must enroll in this program. Pharmacies that participate in the Naloxone Co-Payment Assistance Program are more likely to carry naloxone (Abbas et al., 2021).
3.3.2. Medicaid coverage policies
In October 2021, New York’s Medicaid program adopted a single statewide formulary for opioid dependence agents and opioid antagonists, and the state passed recent legislation removing the requirement for prior authorizations of these medications. Coverage is provided for all naloxone formulations without clinical criteria, PA, or quantity limit, including naloxone syringes and vials, 4 mg generic and brand Narcan® naloxone nasal spray products, 8 mg Kloxxado® naloxone nasal spray, and Zimhi® 5 mg prefilled syringe. In New York, Medicaid beneficiaries are subject to cost-sharing in the form of a $1 co-pay per prescription on preferred brands and generics, with some exceptions. However, beneficiaries can receive naloxone at participating pharmacies at no charge and the pharmacy bills the Naloxone Co-Payment Assistance Program for the co-pay.
3.3.3. Community OEND distribution infrastructure
The state currently has a network of over 800 opioid overdose prevention programs as part of an initiative that began in 2006. These programs include health care facilities, departments of health, clinicians, pharmacies, drug treatment programs, first responders, fixed harm reduction organizations, and mobile units. Opioid overdose prevention programs train individuals in opioid overdose recognition and response and either furnish naloxone to individuals they train in person, by mail, or referral to pharmacies. The trained responders include individuals who are themselves at risk for an overdose. The New York State Department of Health provides naloxone at no cost to these programs.
3.4. Ohio
3.4.1. Naloxone access laws
The first NAL in Ohio became effective in March 2014 and included immunity provisions along with permitting third-party prescribing. Similar to the other states, Ohio’s law has since been amended to allow greater flexibility in who can furnish naloxone and to increase pharmacy-based access. The current NAL in Ohio authorizes a pharmacist or pharmacy intern to dispense naloxone without a prescription in accordance with a physician-approved protocol, essentially functioning as a standing order. This provision can be delivered via a countywide mechanism through the local board of health, and has been associated with increased naloxone dispensing rates, especially in low socioeconomic areas, and the majority of community pharmacies are registered to dispense naloxone without a prescription (Gangal et al., 2020). However, barriers were identified in implementing this provision, such as cost, stigma, and lack of public knowledge (Hincapie et al., 2021). The NAL in Ohio allows healthcare professionals to establish a protocol to authorize an employee, volunteer, or contractor of a service entity to furnish and administer naloxone to individuals so long as certain criteria are met by the protocol. Specifically, the authorized individual must instruct the individual to whom naloxone is furnished to summon emergency services as soon as possible either before or after administering naloxone. Previously, Ohio law required that service entities wanting to distribute naloxone had to obtain a license as a terminal distributor of dangerous drugs, a cumbersome burden that limited the locations where naloxone could be distributed, although a statute effective as of December 2020 exempts service entities that have established a protocol with a physician or board of health from this requirement.
The immunity provisions in Ohio’s NAL are broad, providing civil, criminal, and professional immunity to prescribers and dispensers as well as criminal immunity to administrators of naloxone. Recently adopted provisions give civil immunity to administrators of naloxone with stipulations, namely that these provisions only apply when the individual administering naloxone summons emergency services. There are also limited criminal liability provisions in Ohio’s statutes and regulations for those who summon emergency services, further complicating the decision to respond to an opioid overdose (The Network for Public Health Law, 2018). The pharmacist, or a pharmacy intern under the direct supervision of a pharmacist, must offer overdose education and provide written educational materials to the individual being dispensed naloxone. A co-prescription mandate targeting populations at high risk for an opioid overdose was added to the NAL and became effective in December 2018 mandating that providers offer a prescription of naloxone to patients prescribed an opioid daily dosage that equals or exceeds 80 MME, patients who are prescribed both prescription opioids and benzodiazepines or other sedatives, or when the patient has a history of a nonfatal opioid overdose or a current diagnosis for a substance use disorder.
3.4.2. Medicaid coverage policies
Ohio Medicaid adopted a single pharmacy benefit manager and preferred drug list in January 2022 for all fee-for-service and managed care organization populations. All naloxone products are available without PA. Coverage is provided for both brand and generic 4 mg nasal spray products, brand name Kloxxado® 8 mg nasal spray, and Zimhi® 5 mg pre-filled syringe. No cost-sharing is required for the generic injectable vials and syringes, and the generic nasal spray products. However, the 4 mg Narcan® and 8 mg Kloxxado® brand name nasal spray products and the 5 mg Zimhi® injection are subject to a $2 co-pay per prescription. Quantity limits may be set by some managed care organizations.
3.4.3. Community OEND distribution infrastructure
The Ohio Department of Mental Health and Addiction Services (OhioMHAS) launched Project DAWN (Deaths Avoided with Naloxone) in 2012 to support a network of community OEND programs across the state. Funds allocated to this initiative are used to purchase naloxone for local health departments to provide to local law enforcement, emergency personnel, and first responders. Project DAWN also funds naloxone distribution through partnerships with community-based organizations such as syringe service programs and correctional facilities. In May 2021, OhioMHAS announced it will also allocate nearly $2.5 million in general revenue funds to 23 local Addiction and Mental Health boards to distribute approximately 60,000 additional doses of naloxone in the highest need ZIP codes in their region. Forty-nine of the 90 designated ZIP codes fall in HCS communities. This initiative includes an allocation of $365,000 and 9000 doses to Harm Reduction Ohio to distribute via online mail order or local community networks. As of April 2022, there were 117 Project Dawn programs registered in Ohio, and the number of naloxone kits distributed and people trained through Project DAWN have steadily increased since 2016 (Ohio Department of Health, 2022).
4. Discussion
4.1. Comparative analysis of states participating in the HEALing communities study
Naloxone distribution is an essential component of public health response strategies intended to reduce opioid-related overdose fatalities. This paper highlighted policies impacting naloxone distribution within four states participating in the HEALing Communities Study (HCS). Variation exists between specific components of the NALs of each state, the structure of Medicaid coverage of naloxone, and the community distribution infrastructure networks. In addition, naloxone policies were dynamic in states participating in the HCS over the study period beginning in 2020.
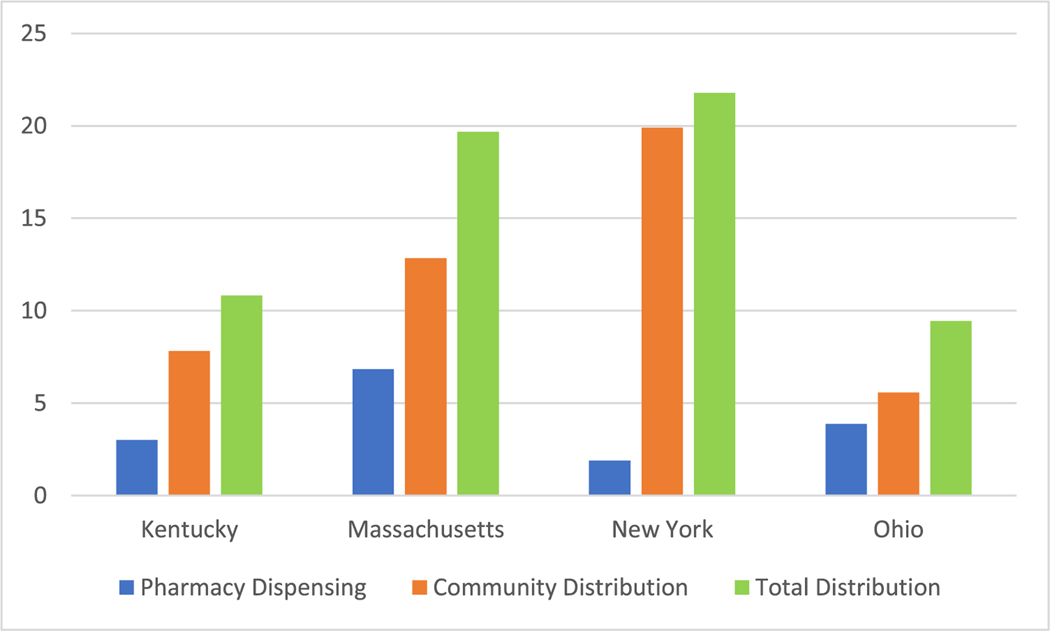
Massachusetts had the most comprehensive NAL at the beginning of the Communities that HEAL (CTH) intervention, supported by having the highest naloxone dispensing rate of the four states before the study period began (see Fig. 1). Essential provisions include a statewide standing order covering pharmacists and mandating pharmacies to maintain a sufficient supply of naloxone (see Table 1). New York, having the oldest NAL established in 2006, added several critical provisions during the study period (2020–2022), including a statewide standing order and a co-prescribing mandate. Currently, only Massachusetts and New York have a statewide standing order, although NALs in Kentucky and Ohio used strategies short of a statewide standing order to expand access to naloxone. For example, the Deputy Commissioner for the Kentucky Department for Public Health or the Medical Director for the Department of Medicaid Services can sign a protocol for pharmacists in Kentucky, and Ohio has a county-wide mechanism through the local board of health. Each state’s NAL has mechanisms in place for community-based organizations to possess, distribute, and administer naloxone, but only Massachusetts has a statewide standing order for these entities, which was implemented during the study period. Other notable NAL differences were that New York and Ohio had a co-prescribing mandate, although a nuanced but important distinction was that Ohio’s NAL requires prescribers to offer a prescription for naloxone whereas New York’s NAL requires prescribers to provide a prescription for naloxone, and Massachusetts and New York do not require identification for an individual to receive naloxone from pharmacies. All states provide civil and criminal immunity to persons administering naloxone and all except New York provide professional immunity to prescribers and dispensers of naloxone. It is unclear, however, if these provisions have an impact on naloxone distribution (Smart et al., 2020). In addition to the provisions themselves, mechanisms of NAL may codify requirements of pharmacies, counties, or prescribers that increase administrative burden, decreasing access and uptake at the pharmacy level that might translate to reduced naloxone access.
Fig. 1.
Pharmacy dispensing, community distribution, and total distribution rates per 1000 residents of naloxone in HCS communities in Kentucky, Massachusetts, New York, and Ohio in 2019.
Notes:
*County-defined community population estimates based on Unites States Census for Kentucky and Ohio.
*ZIP code-defined community population estimates based on American Community Survey for Massachusetts.
*New York HCS community population estimates based on American Community Survey (n = 3) and the United States Census (n = 13).
All four of the states participating in the HCS are Medicaid expansion states; however, Medicaid coverage of naloxone varies, illustrating the heterogenous nature of state Medicaid programs (see Table 2). New York’s Medicaid program appears to have the best coverage for all formulations of naloxone. Notable facilitators of naloxone access include no cost-sharing in Kentucky, Massachusetts, and New York (through pharmacies participating in the Naloxone Co-Payment Assistance Program) and coverage of at least one commonly used nasal spray and injectable formulation in each state. Notable barriers of naloxone access include prior authorizations, co-pays, and quantity limits. Ohio has a co-pay for some naloxone formulations, Kentucky has a prior authorization for injectable formulations, and several states require prior authorizations for newer formulations of naloxone. Kentucky does have quantity limits on some naloxone formulations, such as commonly used injectable formulations, and managed care organizations in Ohio can impose quantity limits. Although general prescription limits on Medicaid beneficiaries may have minimal impact on naloxone access (Roberts et al., 2021; Talbert et al., 2021), specific quantity limits on naloxone are more likely to restrict access to this life-saving medication.
All four states support community-based infrastructure to distribute naloxone through some facet of the state department of health. Kentucky has a unique partnership between the Cabinet for Health and Family Services and the Kentucky Pharmacists Association to support community OEND programs, Massachusetts funds more than 20 agencies through the Massachusetts Department of Public Health and these agencies have stablished a network of OEND sites across the state, New York has a robust network of over 800 opioid overdose prevention programs, and is financially supported by the New York State Department of Health, and Ohio has a state initiative to coordinate 117 OEND programs across the state. Common distribution venues across the states include health departments, addiction treatment programs, criminal justice settings, and harm reduction programs. States provide both direct funds as well as distributing federal SAMHSA funding to purchase naloxone. New York had the highest community distribution rate of any state before the study period began, and these rates were higher than pharmacy dispensing rates for all states, highlighting the importance of community access to naloxone as well as the potential of inadequate and under-implemented pharmacy access laws circa 2019. In addition, a robust OEND network providing naloxone at no cost to consumers could partially explain low utilization of pharmacy-based naloxone access, as in the case of New York.
4.2. Naloxone policies and the HEALing communities study
There were important differences in naloxone policies between the four states participating in the HCS, and these policies were dynamic in each state during the study period beginning in 2020. For example, Massachusetts may have had the most favorable policy landscape for naloxone distribution at the beginning of the CTH intervention, while New York implemented a co-prescribing mandate and a statewide standing order during the study period. Naloxone policy changes in all states moved towards facilitating naloxone access. Researchers should con- sider the evolving and varied policy landscape when implementing and evaluating the CTH intervention as well as similar interventions in the future. Researchers would also benefit from accounting for implementation factors when measuring the impact of opioid policies, such as NALs. For example, a state may use strategies short of a statewide standing order to increase naloxone distribution, as seen in this study, and these nuances are typically not captured in policy impact analyses.
Expanding access to naloxone is an important goal of the HCS. As such, policies supporting the broadest access via pharmacies and other community organizations, funding for purchasing a range of formulations, and infrastructure for naloxone distribution provide the strongest platform for the study. For example, having a statewide standing order for naloxone and requiring pharmacies to maintain a sufficient supply could go a long way toward ensuring those seeking naloxone after seeing an HCS communication campaign on OEND can gain access. Likewise, requiring some form of identification to obtain naloxone in pharmacies or other community-based venues could serve as a barrier to those at highest risk of overdose as well as individuals who might perceive a negative impact of a naloxone prescription being reported to a life insurance policy (Green et al., 2020). In addition, the health insurance mix of a state may play an important role in accessing naloxone. For example, Massachusetts has the lowest uninsured rate of the four states but also has the highest rate of private insurance beneficiaries for whom out-of-pocket costs can be a significant barrier to obtaining naloxone through a pharmacy (Kaiser Family Foundation, 2022).
Resources from the HCS have been used to identify gaps in laws and regulations, funding, and distribution networks and, when possible, provide additional support, in the context of urban-rural differences where appropriate. For example, HCS communities in all four states are working with jails to aid them in developing OEND programs for people returning to the community. Additionally, two states are offering stipends to people using drugs to distribute naloxone kits to their at-risk peers, a model shown in other states to prioritize naloxone distribution to high-risk individuals (Meyerson et al., 2021). Academic detailing is being provided to pharmacists in HCS communities to address barriers to naloxone among other things. These creative strategies are intended to supplement state and local efforts and move communities toward ensuring the supply of naloxone meets the need.
Increasing naloxone distribution is not just a goal of the HCS. The World Health Organization has released guidelines calling for increased naloxone distribution globally (World Health Organization, 2014). Naloxone policy landscapes vary considerably internationally. For example, Australia offers naloxone as an over-the-counter medication that can be dispensed by a pharmacist at no cost (New South Wales Ministry of Health, 2022) and in Kazakhstan naloxone has been added to the list of essential medicines in the country but funding inhibits its needed supply (Gilbert et al., 2018), whereas restrictive legal environments in many countries present a significant barrier to increasing naloxone distribution (Harm Reduction International, 2020). Although each country is likely to have different regulatory frameworks and legislative systems, how the four states participating in the HCS have navigated a complicated federalist system may offer lessons for how other countries can move towards policy landscapes that increase access to naloxone.
4.3. Limitations
The current examination of the policy climate in the context of the HCS has limitations. First, the association between state naloxone policies and naloxone distribution rates should be interpreted with caution. Some policies were implemented after the 2019 baseline data collection, data were not at the state level but aggregated for HCS communities in each state, and this study did not examine the myriad barriers important in naloxone access, including stigma and structural racism. However, the focus was narrow and intended to inform how policy might interact with HCS efforts to increase naloxone distribution. It also should be noted there may be sources of community naloxone distribution not captured in state administrative data, resulting in an underestimation of community-distributed naloxone. Second, all four of the states participating in the HCS are Medicaid expansion states thus no comparison is made with non-expansion states, although previous research has shown that Medicaid expansion facilitates naloxone access (Frank and Fry, 2019; Sohn et al., 2020). Third, only payer policies for Medicaid were examined in this study. Private health plans typically have large variation in naloxone coverage and uninsured individuals are increasingly burdened with out-of-pocket costs (Peet et al., 2022). However, given that all four states are Medicaid-expansion states, a large portion of individuals at risk for opioid overdose are likely covered by Medicaid. Fourth, naloxone distribution rates lacked individual-level data such as race and ethnicity. Although equitable access to naloxone is critical given the sharp increase in overdose death rates among non-whites (Friedman and Hansen, 2022), this was unable to be examined in this study. Finally, this study lacks a national perspective, and the state-level perspective could be strengthened by further qualitative research from relevant stakeholders, although several individuals on the research team are involved in naloxone distribution.
5. Conclusion
Naloxone policies play a critical role in naloxone access. These policies vary among states participating in the HEALing Communities Study (HCS) and may affect implementation and sustainability of overdose prevention interventions and the primary outcome of the study, opioid-related overdose deaths. In this selection of four states, there is evidence of variability in responsiveness to naloxone access barriers and innovation in mechanisms to ensure access to naloxone at pharmacies and OEND programs across many sectors (e.g., health care, criminal justice). State health authorities and state policymakers should continue to lower naloxone access barriers and may consider some of the strategies of states participating in the HCS to do so, while adapting to new changes in federal policy permitting novel distribution pathways directly benefiting harm reduction organizations (U.S. Food and Drug Administration, 2022) and preparing for a potential change that may allow non-prescription distribution of naloxone (Federal Register, 2022). Research should consider the policy landscape in both the implementation of interventions and the analysis of outcomes, including reach and whether there is equitable access across race/ethnicity, geography, and community settings alongside critical public health outcomes such as overdose mortality. Future work could also consider if there are specific policies that are more impactful in particular sectors and venues, such as criminal justice and detention centers/jails where there is a particularly high rate of fatal opioid-involved overdose deaths upon release.
Acknowledgments
This research was supported by the National Institutes of Health and the Substance Abuse and Mental Health Services Administration through the NIH HEAL (Helping to End Addiction Long-term SM) Initiative under award numbers UM1DA049394, UM1DA049406, UM1DA049412, UM1DA049415, UM1DA049417 (ClinicalTrials.gov Identifier: NCT04111939). This study protocol (Pro00038088) was approved by Advarra Inc., the HEALing Communities Study single Institutional Review Board. We wish to acknowledge the participation of the HEALing Communities Study communities, community coalitions, and Community Advisory Boards and state government officials who partnered with us on this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Substance Abuse and Mental Health Services Administration or the NIH HEAL InitiativeSM.
Footnotes
Author disclosures
Disclaimer
The views and opinions expressed in this manuscript are those of the authors only and do not necessarily represent the views, official policy or position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies.
Declaration of Competing Interest
No conflicts declared.
Kentucky recently implemented a naloxone co-pay program for those who are uninsured or have commercial insurance; see https://www.kphanet.org/copay.
References
- Abbas B, Marotta PL, Goddard-Eckrich D, Huang D, Schnaidt J, El-Bassel N, Gilbert L, 2021. Socio-ecological and pharmacy-level factors associated with naloxone stocking at standing-order naloxone pharmacies in New York City. Drug Alcohol Depend. 218, 108388. doi: 10.1016/j.drugalcdep.2020.108388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad FB, Rossen LM, Sutton P, 2021. Provisional Drug Overdose Death Counts. National Center for Health Statistics. [Google Scholar]
- Bagley SM, Forman LS, Ruiz S, Cranston K, Walley AY, 2018. Expanding access to naloxone for family members: the Massachusetts experience. Drug Alcohol Rev. 37 (4), 480–486. doi: 10.1111/dar.12551. [DOI] [PubMed] [Google Scholar]
- Bohler RM, Hodgkin D, Kreiner PW, Green TC, 2021. Predictors of US states’ adoption of naloxone access laws, 2001–2017. Drug Alcohol Depend. 225, 108772. doi: 10.1016/j.drugalcdep.2021.108772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colorado Department of Public Health & Environment. (2022). Naloxone bulk purchase fund opportunity. https://cdphe.colorado.gov/naloxone-bulk-purchase-fund.
- Davis CS, Carr D, 2015. Legal changes to increase access to naloxone for opioid overdose reversal in the United States. Drug Alcohol Depend. 157, 112–120. doi: 10.1016/j.drugalcdep.2015.10.013. [DOI] [PubMed] [Google Scholar]
- Davis CS, Carr D, 2017. State legal innovations to encourage naloxone dispensing. J. Am. Pharm. Assoc. 57 (2), S180–S184. doi: 10.1016/j.japh.2016.11.007, (Wash. DC). [DOI] [PubMed] [Google Scholar]
- Doe-Simkins M, Wheeler EJ, Figgatt MC, Jones ST, Bell A, Davidson PJ, & Dasgupta N.(2022). Naloxone Buyers club: overlooked critical public health infrastructure for preventing overdose deaths. MedRxiv Preprint. https://www.medrxiv.org/content/medrxiv/early/2021/11/16/2021.11.14.21266221.full.pdf. [Google Scholar]
- El-Bassel N, Jackson RD, Samet J, Walsh SL, 2020. Introduction to the special issue on the HEALing communities study. Drug Alcohol Depend. 217, 108327. doi: 10.1016/j.drugalcdep.2020.108327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evoy KE, Hill LG, Groff L, Mazin L, Carlson CC, Reveles KR, 2018. Naloxone accessibility without a prescriber encounter under standing orders at community pharmacy chains in Texas. JAMA 320 (18), 1934–1937. doi: 10.1001/jama.2018.15892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Register Federal. (2022). Safety and effectiveness of certain naloxone hydrochloride drug products for nonprescription use. a notice by the food and drug administration. https://www.federalregister.gov/documents/2022/11/16/2022-24874/safety-and-effectiveness-of-certain-naloxone-hydrochloride-drug-products-for-nonprescription-use. [Google Scholar]
- Frank RG, Fry CE, 2019. The impact of expanded Medicaid eligibility on access to naloxone. Addiction 114 (9), 1567–1574. doi: 10.1111/add.14634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman PR, Goodin A, Troske S, Strahl A, Fallin A, Green TC, 2017. Pharmacists’ role in opioid overdose: kentucky pharmacists’ willingness to participate in naloxone dispensing. J. Am. Pharm. Assoc. JAPhA 57 (2S), S28–S33. doi: 10.1016/j.japh.2016.12.064. [DOI] [PubMed] [Google Scholar]
- Friedman JR, Hansen H, 2022. Evaluation of increases in drug overdose mortality rates in the US by race and ethnicity before and during the COVID-19 pandemic. JAMA Psychiatry 79 (4), 379–381. doi: 10.1001/jamapsychiatry.2022.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangal NS, Hincapie AL, Jandarov R, Frede SM, Boone JM, MacKinnon NJ, Koechlin K, DeFiore-Hyrmer J, Holthusen A, Heaton PC, 2020. Association between a state law allowing pharmacists to dispense naloxone without a prescription and naloxone dispensing rates. JAMA Netw. Open 3 (1), e1920310. doi: 10.1001/jamanetworkopen.2019.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertner AK, Domino ME, Davis CS, 2018. Do naloxone access laws increase outpatient naloxone prescriptions? Evidence from medicaid. Drug Alcohol Depend. 190, 37–41. doi: 10.1016/j.drugalcdep.2018.05.014. [DOI] [PubMed] [Google Scholar]
- Gilbert L, Hunt T, Primbetova S, Terlikbayeva A, Chang M, Wu E, McCrimmon T, El-Bassel N, 2018. Reducing opioid overdose in Kazakhstan: a randomized controlled trial of a couple-based integrated HIV/HCV and overdose prevention intervention “Renaissance. Int. J. Drug Policy 54, 105–113. doi: 10.1016/j.drugpo.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves RL, Andreyeva E, Perrone J, Shofer FS, Merchant RM, Meisel ZF, 2019. Naloxone availability and pharmacy staff knowledge of standing order for naloxone in Pennsylvania pharmacies. J. Addict. Med. 13 (4), 272–278. doi: 10.1097/ADM.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Davis C, Xuan Z, Walley AY, Bratberg J, 2020a. Laws mandating coprescription of naloxone and their impact on naloxone prescription in five US States, 2014–2018. Am. J. Public Health 110 (6), 881–887. doi: 10.2105/AJPH.2020.305620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Donovan E, Klug B, Case P, Baird J, Burstein D, Tapper A, Walley AY, Bratberg J, 2020b. Revisiting pharmacy-based naloxone with pharmacists and naloxone consumers in 2 states: 2017 perspectives and evolving approaches. J. Am. Pharm. Assoc. JAPhA 60 (5), 740–749. doi: 10.1016/j.japh.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Martin EG, Bowman SE, Mann MR, Beletsky L, 2012. Life after the ban: an assessment of US syringe exchange programs’ attitudes about and early experiences with federal funding. Am. J. Public Health 102 (5), e9–16. doi: 10.2105/AJPH.2011.300595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harm Reduction International. (2020). Global state of harm reduction 2020. https://hri.global/wp-content/uploads/2022/10/Global_State_HRI_2020_BOOK_FA_Web−1.pdf. [Google Scholar]
- HEALing Communities Study Consortium, 2020. The HEALing (Helping to End Addiction Long-term SM) Communities Study: protocol for a cluster randomized trial at the community level to reduce opioid overdose deaths through implementation of an integrated set of evidence-based practices. Drug Alcohol Depend. 217, 108335. doi: 10.1016/j.drugalcdep.2020.108335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High PM, Marks K, Robbins V, Winograd R, Manocchio T, Clarke T, Wood C, Stringer M, 2020. State targeted response to the opioid Crisis grants (opioid STR) program: preliminary findings from two case studies and the national cross-site evaluation. J. Subst. Abuse Treat. 108, 48–54. doi: 10.1016/j.jsat.2019.06.008. [DOI] [PubMed] [Google Scholar]
- Hincapie AL, Hegener M, Heaton PC, Fish G, Fetters K, Sneed GT, Koechlin K, DeFiore-Hyrmer J, Holthusen A, MacKinnon NJ, 2021. Challenges and facilitators of implementing a physician-approved naloxone protocol: a mixed-methods study. J. Addict. Med. 15 (1), 40–48. [DOI] [PubMed] [Google Scholar]
- Irvine MA, Kuo M, Buxton JA, Balshaw R, Otterstatter M, Macdougall L, Milloy M−J, Bharmal A, Henry B, Tyndall M, Coombs D, Gilbert M, 2019. Modelling the combined impact of interventions in averting deaths during a synthetic-opioid overdose epidemic. Addiction 114 (9), 1602–1613. doi: 10.1111/add.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine MA, Oller D, Boggis J, Bishop B, Coombs D, Wheeler E, Doe-Simkins M, Walley AY, Marshall BDL, Bratberg J, Green TC, 2022. Estimating naloxone need in the USA across fentanyl, heroin, and prescription opioid epidemics: a modelling study. Lancet Public Health doi: 10.1016/S2468-2667(21)00304-2, S2468–2667(21)00304–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez DE, Singer MR, Adesman A, 2019. Availability of naloxone in pharmacies and knowledge of pharmacy staff regarding dispensing naloxone to younger adolescents. J. Adolesc. Health Off. Public. Soc. Adolesc. Med. 65 (5), 698–701. doi: 10.1016/j.jadohealth.2019.07.009. [DOI] [PubMed] [Google Scholar]
- Kaiser Family Foundation. (2022). Health insurance coverage of the total population. https://www.kff.org/other/state-indicator/health-insurance-coverage-of-the-total-population-cps/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. [Google Scholar]
- Lambdin BH, Davis CS, Wheeler E, Tueller S, Kral AH, 2018. Naloxone laws facilitate the establishment of overdose education and naloxone distribution programs in the United States. Drug Alcohol Depend. 188, 370–376. doi: 10.1016/j.drugalcdep.2018.04.004. [DOI] [PubMed] [Google Scholar]
- Legislative Analysis and Public Policy Association. (2020). Naloxone access: summary of state laws. https://legislativeanalysis.org/wp-content/uploads/2020/10/Naloxone-summary-of-state-laws-FINAL-9.25.2020.pdf.
- Mauri AI, Townsend TN, Haffajee RL, 2020. The association of state opioid misuse prevention policies with patient- and provider-related outcomes: a scoping review. Milbank Q. 98 (1), 57–105. doi: 10.1111/1468-0009.12436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald R, Campbell ND, Strang J, 2017. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-conception and maturation. Drug Alcohol Depend. 178, 176–187. doi: 10.1016/j.drugalcdep.2017.05.001. [DOI] [PubMed] [Google Scholar]
- Meyerson BE, Moehling TJ, Agley JD, Coles HB, Phillips J, 2021. Insufficient access: naloxone availability to laypeople in Arizona and Indiana, 2018. J. Health Care Poor Underserved 32 (2), 819–829. doi: 10.1353/hpu.2021.0107. [DOI] [PubMed] [Google Scholar]
- New South Wales Ministry of Health. (2022). Take-home naloxone program. https://www.health.nsw.gov.au/aod/programs/Pages/naloxone.aspx.
- NYS Department of Health. (2021). Single source procurement: opioid overdose prevention program. https://www.health.ny.gov/funding/single_source/c033007_2.htm.
- Ohio Department of Health. (2022). Project DAWN data. https://odh.ohio.gov/know-our-programs/project-dawn/project-dawn-data. [Google Scholar]
- Orgera K, Tolbert J, 2019. The Opioid Epidemic and Medicaid’s Role in Facilitating Access to Treatment. Kaiser Family Foundation. [Google Scholar]
- Peet ED, Powell D, Pacula RL, 2022. Trends in out-of-pocket costs for naloxone by drug brand and payer in the US, 2010–2018. JAMA Health Forum 3 (8), e222663. doi: 10.1001/jamahealthforum.2022.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt AL, Humphreys K, Brandeau ML, 2018. Modeling health benefits and harms of public policy responses to the US opioid epidemic. Am. J. Public Health 108 (10), 1394–1400. doi: 10.2105/AJPH.2018.304590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollini RA, Joyce R, Ozga-Hess JE, Xuan Z, Green TC, Walley AY, 2020. Assessing pharmacy-based naloxone access using an innovative purchase trial methodology. J. Am. Pharm. Assoc. JAPhA 60 (6), 853–860. doi: 10.1016/j.japh.2020. 05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescription Drug Abuse Policy System. (2022). Naloxone overdose prevention laws. https://www.pdaps.org/datasets/laws-regulating-administration-of-naloxone-1501695139.
- Rao IJ, Humphreys K, Brandeau ML, 2021. Effectiveness of policies for addressing the US opioid epidemic: a model-based analysis from the stanford-lancet commission on the North American opioid crisis. Lancet Regional Health Am. 3, 100031. doi: 10.1016/j.lana.2021.100031, (Engl ed). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razaghizad A, Windle SB, Filion KB, Gore G, Kudrina I, Paraskevopoulos E, Kimmelman J, Martel MO, Eisenberg MJ, 2021. The effect of overdose education and naloxone distribution: an umbrella review of systematic reviews. Am. J. Public Health 111 (8), 1516–1517. doi: 10.2105/AJPH.2021.306306a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Carpenter DM, Smith A, Look KA, 2019. Reviewing state-mandated training requirements for naloxone-dispensing pharmacists. Res. Soc. Admin. Pharm. 15 (2), 222–225. doi: 10.1016/j.sapharm.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AW, Look KA, Trull G, Carpenter DM, 2021. Medicaid prescription limits and their implications for naloxone accessibility. Drug Alcohol Depend. 218, 108355. doi: 10.1016/j.drugalcdep.2020.108355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart R, Grant S, 2021. Effectiveness and implementability of state-level naloxone access policies: expert consensus from an online modified-Delphi process. Int. J. Drug Policy 98, 103383. doi: 10.1016/j.drugpo.2021.103383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart R, Pardo B, Davis CS, 2020. Systematic review of the emerging literature on the effectiveness of naloxone access laws in the United States. Addiction doi: 10.1111/add.15163.add.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn M, Talbert JC, Delcher C, Hankosky ER, Lofwall MR, Freeman PR, 2020. Association between state Medicaid expansion status and naloxone prescription dispensing. Health Serv. Res. 55 (2), 239–248. doi: 10.1111/1475-6773.13266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn M, Talbert JC, Huang Z, Lofwall MR, Freeman PR, 2019. Association of naloxone coprescription laws with naloxone prescription dispensing in the United States. JAMA Netw. Open 2 (6), e196215. doi: 10.1001/jamanetworkopen.2019.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague Martinez L, Rapkin BD, Young A, Freisthler B, Glasgow L, Hunt T, Salsberry PJ, Oga EA, Bennet-Fallin A, Plouck TJ, Drainoni M−L, Freeman PR, Surratt H, Gulley J, Hamilton GA, Bowman P, Roeber CA, El-Bassel N, Battaglia T, 2020. Community engagement to implement evidence-based practices in the HEALing communities study. Drug Alcohol Depend. 217, 108326. doi: 10.1016/j.drugalcdep.2020.108326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2021). 2021 Report to Congress on the State Opioid Response Grants (SOR). https://www.samhsa.gov/sites/default/files/2021-state-opioid-response-grants-report.pdf.
- Talbert J, Bohler R, Frazier L, El-Bassel N, Freeman PR, 2021. Letter to the Editor regarding: medicaid prescription limits and their implications for naloxone accessibility (by Roberts et al. , 2021). Drug Alcohol Depend. 226, 108888. doi: 10.1016/j.drugalcdep.2021.108888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Network for Public Health Law. (2018). Naloxone Access and Overdose Good Samaritan Law in Ohio. https://www.networkforphl.org/wp-content/uploads/2020/01/Ohio-Naloxone-Good-Sam-Laws-Fact-Sheet.pdf.
- Thompson EL, Rao PSS, Hayes C, Purtill C, 2019. Dispensing Naloxone Without a Prescription: survey Evaluation of Ohio Pharmacists. J Pharm Pract 32 (4), 412–421. doi: 10.1177/0897190018759225. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration. (2022). Exemption and exclusion from certain requirements of the drug supply chain security act for the distribution of FDA-approved naloxone products during the opioid public health emergency guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/exemption-and-exclusion-certain-requirements-drug-supply-chain-security-act-distribution-fda.
- Walley AY, Xuan Z, Hackman HH, Quinn E, Doe-Simkins M, Sorensen-Alawad A, Ruiz S, Ozonoff A, 2013. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ 346, f174. doi: 10.1136/bmj.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner J, Murphy SM, & Behrends C.(2019). Expanding access to naloxone: a review of distribution strategies (p. 9). Penn LDI/CHERISH Issue Brief. https://ldi.upenn.edu/sites/default/files/pdf/LDI%20CHERISH%20Brief_May2019.pdf. [Google Scholar]
- Wheeler E, Jones TS, Gilbert MK, Davidson PJ, 2015. Opioid overdose prevention programs providing naloxone to laypersons-United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 64 (23), 631–635. [PMC free article] [PubMed] [Google Scholar]
- Winhusen T, Walley A, Fanucchi LC, Hunt T, Lyons M, Lofwall M, Brown JL, Freeman PR, Nunes E, Beers D, Saitz R, Stambaugh L, Oga EA, Herron N, Baker T, Cook CD, Roberts MF, Alford DP, Starrels JL, Chandler RK, 2020. [Google Scholar]
- The opioid-overdose reduction continuum of care approach (ORCCA): evidence-based practices in the HEALing communities study. Drug Alcohol Depend. 217, 108325. doi: 10.1016/j.drugalcdep.2020.108325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2014). Community management of opioid overdose. https://apps.who.int/iris/bitstream/handle/10665/137462/9789241548816_eng.pdf. [PubMed]
- Wu C, Brown T, Moreno JL, 2020. Access to naloxone at community pharmacies under the Massachusetts statewide standing order. J. Am. Pharm. Assoc. JAPhA 60 (4), 647–652. doi: 10.1016/j.japh.2019.11.009. [DOI] [PubMed] [Google Scholar]
- Xu J, Davis CS, Cruz M, Lurie P, 2018. State naloxone access laws are associated with an increase in the number of naloxone prescriptions dispensed in retail pharmacies. Drug Alcohol Depend. 189, 37–41. doi: 10.1016/j.drugalcdep.2018.04.020. [DOI] [PubMed] [Google Scholar]
- Zang X, Macmadu A, Krieger MS, Behrends CN, Green TC, Morgan JR, Murphy SM, Nolen S, Walley AY, Schackman BR, Marshall BD, 2021. Targeting community-based naloxone distribution using opioid overdose death rates: a descriptive analysis of naloxone rescue kits and opioid overdose deaths in Massachusetts and Rhode Island. Int. J. Drug Policy 98, 103435. doi: 10.1016/j.drugpo.2021.103435. [DOI] [PMC free article] [PubMed] [Google Scholar]