Abstract
Background:
Cases of adolescents and young adults developing myocarditis following vaccination with SARS-CoV-2-targeted mRNA vaccines have been reported globally, but the underlying immunoprofiles of these individuals have not been described in detail.
Methods:
From January 2021 through February 2022, we prospectively collected blood from 16 patients who were hospitalized at MassGeneral for Children or Boston Children’s Hospital for myocarditis, presenting with chest pain with elevated cardiac troponin T, after SARS-CoV-2 vaccination. We performed extensive antibody profiling, including testing for SARS-CoV-2 specific humoral responses and assessment for autoantibodies or antibodies against the human relevant virome, SARS-CoV-2-specific T cell analysis, and cytokine and SARS-CoV-2 antigen profiling. Results were compared with those from 45 healthy, asymptomatic, age-matched vaccinated controls.
Results:
Extensive antibody profiling and T cell responses in the individuals who developed post-vaccine myocarditis were essentially indistinguishable from vaccinated controls, despite a modest increase in cytokine production. Notably, markedly elevated levels of full-length Spike protein (33.9 ± 22.4 pg/mL), unbound by antibodies, were detected in the plasma of individuals with post-vaccine myocarditis, whereas no free Spike was detected in asymptomatic vaccinated controls (unpaired t-test, p < 0.0001).
Conclusion:
Immunoprofiling of vaccinated adolescents and young adults revealed that the mRNA vaccine-induced immune responses did not differ between individuals that developed myocarditis versus individuals that did not. However, free Spike antigen was detected in the blood of adolescents and young adults who developed post-mRNA vaccine myocarditis, advancing insight into its potential underlying etiology.
Keywords: mRNA vaccine, myocarditis, COVID-19, post-vaccine myocarditis, Spike
Introduction:
Within nine months of COVID-19 reaching pandemic proportions, novel, life-saving SARS-CoV-2-targeted mRNA vaccine technologies received emergency regulatory approvals and were distributed to many developed countries. Following a tiered approach prioritizing high risk individuals, infants ages 6 months and older are now eligible for vaccination with SARS-CoV-2 mRNA vaccines in select countries. SARS-CoV-2 mRNA vaccines have been shown to dramatically reduce disease severity and mortality of COVID-19 in adults1, 2 and children3, 4, and vaccinated children may be less likely to develop the severe, life-threatening post-COVID-19 complication, multisystem inflammatory syndrome in children (MIS-C)5, 6. Rarely, some individuals develop myocarditis following mRNA vaccination. The immune response driving post-vaccine myocarditis has not yet been elucidated. Understanding the immunophenotype associated with mRNA vaccine-induced myocarditis is an essential first step in preventing negative complications resulting from this novel vaccine technology.
Roughly 1–2 cases per 100,000 individuals have developed myocarditis or pericarditis following mRNA vaccination7–9. Prior authors have hypothesized that vaccination may trigger a robust overactive or aberrant innate and acquired immune response in genetically predisposed individuals10, 11, or given the predilection for males, hormones, specifically testosterone, may alter immune responses, eliciting a more aggressive T helper 1 cell-type immune response12. Alternatively, it has been proposed that in certain individuals, vaccination might generate autoantibodies from polyclonal B-cell expansion, immune complex formation and inflammation10, 11, or potentially the molecular mimicry between the Spike protein and self-antigens may result in cardiac-targeted antibodies10. One group performed extensive immunoanalysis on a case of post-mRNA-1273 (Moderna) vaccine myocarditis and compared the findings with vaccinated controls. While the individual with myocarditis displayed increased NK cell subsets, elevated cytokines, and several autoantibodies, a specific signature could not be identified.13 Thus, the cause of post-SARS-CoV-2 mRNA vaccine myocarditis remains unclear. Understanding the immunopathological mechanisms driving post-vaccine myocarditis could help improve safety and guide development of future COVID-19 vaccines.
In this study, blood samples were collected for immunophenotyping from 61 adolescents and young adults who either developed myocarditis or had no vaccine-related complications following vaccination with either the Pfizer BNT162b2 or Moderna mRNA-1273 COVID-19 mRNA vaccine. We performed extensive humoral profiling, quantified and profiled SARS-CoV-2-specific T cell responses, and measured cytokines and SARS-CoV-2 antigens in the collected plasma samples.
Methods:
Patient enrollment and sample collection:
Adolescents or young adults presenting with myocarditis following SARS-CoV-2 mRNA vaccination, along with healthy, vaccinated controls, and children with Multisystem Inflammatory Syndrome in Children (MIS-C) were enrolled in the Institutional Review Board (IRB)-approved Pediatric COVID-19 Biorepository at Massachusetts General Hospital (Mass General Brigham IRB #2020P0000955) 14 or the IRB-approved “Taking on COVID-19 Together” Biorepository at Boston Children’s Hospital (BCH IRB #P00035409). All participant provided written or verbal consent prior to participation, in accordance with the IRB-approved protocols.
The Center for Disease Control’s (CDC) definition for post-vaccine myocarditis was used; both “confirmed” and “probable” cases were included15. Healthy vaccinated, control adults (>18 years of age) were enrolled in a specimen collection study conducted at Brigham and Women’s Hospital16. All studies were Institutional Review Board (IRB)-approved. Samples were collected and processed as outlined in Figure S1.
Immunophenotyping:
In brief, analysis of plasma included anti-SARS-CoV-2 antibody profiling, comprehensive serologic profiling for autoantibodies and antibodies against prior infections, SARS-CoV-2 Spike protein-specific T cell responses, cytokine analysis and hematologic profiling, and testing for circulating SARS-CoV-2 antigens. The data that support the findings of this study are available from the corresponding author upon reasonable request. In-depth descriptions of assays used for single and longitudinal time points are detailed in Supplemental Methods.
Statistical Analysis:
For serologic, cytokine, and antigen comparisons between the myocarditis and control groups, we used two-sided non-parametric Mann Whitney U test. The Benjamini-Hochberg method was used to correct for multiple P-values in the serologic assays. For T cell and hematologic comparison, unpaired t tests were used to analyze comparisons between the myocarditis and control groups.
Results:
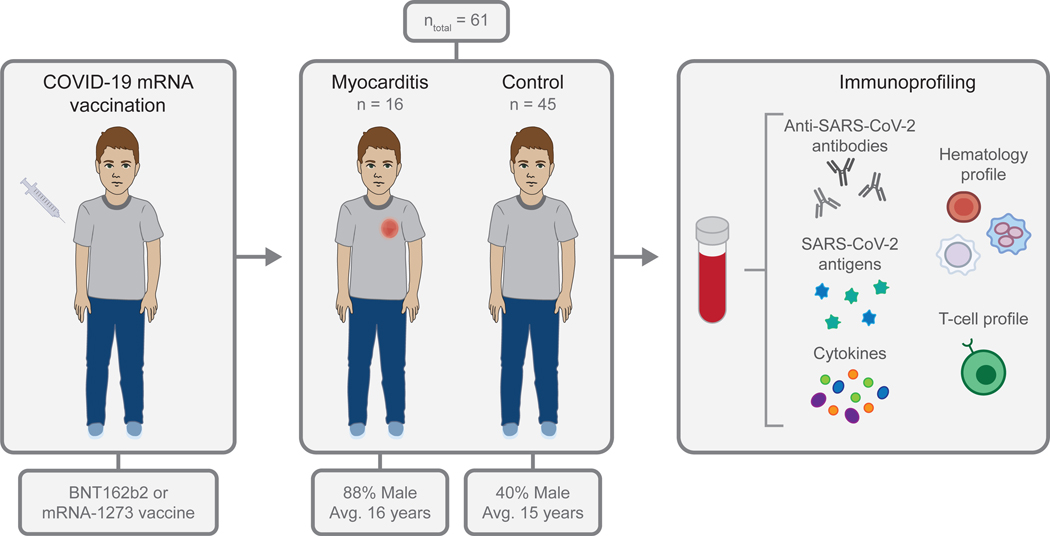
Sixty-one adolescents and young adults between the ages of 12 and 21 years, including 16 individuals with vaccine-associated myocarditis, provided a blood sample for analysis (Figure 1). Demographics are described in Table 1; the majority of individuals with post-vaccine myocarditis were male (n = 13/16, 81%) and symptom onset typically occurred within the first week post-vaccination (median 4 days, range 1–19 days). In the post-vaccine myocarditis cohort, most (n = 12/16, 75%) of the individuals developed myocarditis after the second dose, although two experienced symptoms of myocarditis after the first dose and two after the third “booster” dose. All patients presented with chest pain, and all were found to have elevated cardiac troponin T (median 260, interquartile range (IQR) 215–1114 ng/L; upper limit of normal (ULN) 14 ng/L) and C-reactive protein (CRP) levels (median 29.75, IQR 15.53–50.58 mg/L; ULN 8 mg/L) (Table S1). Samples were also collected from 45 age-matched asymptomatic vaccinated controls, up to 3 weeks following the second mRNA vaccination.
Figure 1. Study overview.
After receiving a COVID-19 mRNA vaccination, blood samples were collected from adolescents and young adults who developed myocarditis or who had no vaccine-related complications. The concentrations of anti-SARS-CoV-2 antibodies, SARS-CoV-2 antigens, and cytokines were measured, and hematology and T cell profiling was performed using the collected blood samples
Table 1:
Demographics of adolescents and young adults who developed myocarditis following COVID-19 vaccination and pediatric vaccinated controls.
| Patient Characteristics | Post-Vaccine Myocarditis (n = 16) | Vaccinated Controls (n = 45) |
|---|---|---|
| Age at Enrollment, average (min, max) | 16 (12, 21) | 15 (12, 20) |
| Male Sex, number (%) | 13 (81) | 18 (40) |
| Race, number (%) | ||
| Asian | 2 (13) | 4 (9) |
| Black | 0 (0) | 1 (2) |
| White | 11 (69) | 23 (51) |
| Other | 2 (13) | 10 (22) |
| Unknown | 1 (6) | 7 (16) |
| Hispanic, number (%) | 5 (31) | 9 (20) |
| Days Between Vaccination and Sample Collection, median (min, max) | 4 (1, 19) | 14 (4, 21) |
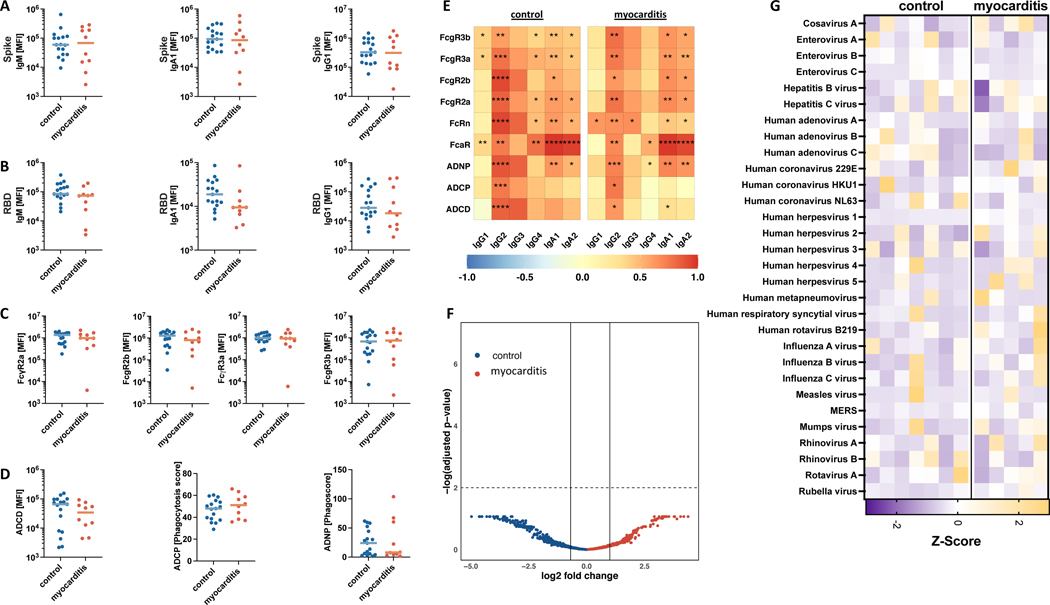
Given the desired goal of humoral protection against SARS-CoV-2 infection, serologic responses were compared between the individuals who developed post-vaccine myocarditis and age-matched, asymptomatic vaccinated controls. Because antibody responses are dynamic over time, only samples collected within 11 days following the second vaccine dose were analyzed to allow a more evenly matched comparison of antibody responses (myocarditis cohort: n = 10; healthy vaccinated controls: n = 17). In this subgroup, no differences were seen in anti-Spike or anti-RBD (anti-receptor binding protein) IgM, IgG or IgA levels (Figure 2A, 2B). To more deeply assess potential qualitative differences in the serologic responses associated with post-vaccine myocarditis, we assessed the antibody’s ability to engage Fc receptors and additional Fc effector functions. No overall difference in binding to FcγR2a, FcγR2b, FcγR3a, or FcγR3b was observed (Figure 2C). Consequently, opsonophagocytic and complement activating potentials were comparable between the groups (Figure 2D). Collectively, these data indicate that individuals who developed myocarditis have a comparable humoral immune response with asymptomatic adolescents and young adults (Figure 2E). We found no indication that a specific antibody response is associated with myocarditis, but instead all adolescents and young adults mounted a substantial immune response conferring protection against SARS-CoV-2 after vaccination.
Figure 2: Humoral reponses.
Humoral responses of adolescents and young adults with post-vaccine myocarditis (n = 10) as compared to age-matched post-vaccine controls (n = 17). (A), (B) IgM/G/A to Spike and RBD, (C) FcRs to Spike, (D) ADCD, ADNP, ADCP; (E) the correlation heatmap; (F) Phip-Seq, (G) heatmap of virscan. A-E only include samples that were collected within eleven days after the second vaccination dose to allow time-equivalent comparison of SARS-CoV-2 specific responses. Analysis by Mann-Whitney U Map, * = p < 0.5; ** = p < 0.01; *** = p < 0.001; **** = p < 0.0001. F-G included five individuals with post-vaccine myocarditis and seven healthy vaccinated controls. ADCD: antibody-dependent complement deposition; ADNP: neutrophil phagocytosis; ADCP cellular phagocytosis
In addition to vaccine-related responses, we looked more broadly at the antibody response in a subset of patients and vaccinated controls by performing PhiP-Seq for self-antibodies (Figure 2F) and a Phip-Seq based VirScan, a phage display approach for in-depth characterization of antibodies to the human relevant virome (Figure 2G). No self-antibodies were significantly noted in the myocarditis group as compared to the healthy vaccinated controls. Strong antibody responses to common pathogens, including other respiratory viruses (respiratory syncytial virus [RSV], influenza, beta-coronaviruses), herpesviruses (herpes simplex virus type 1 [HSV-1], cytomegalovirus [CMV], Epstein-Barr virus [EBV]), and vaccine strains (measles, rubella, mumps) were detected. However, no specific difference tracking with development of myocarditis stood out in this limited cohort.
We then sought to characterize T cell responses by multiparameter flow cytometry. We first compared the distribution of the different memory subsets including all CD4+ and CD8+ T cells between a subset of individuals who developed post-vaccine myocarditis and asymptomatic vaccinated controls using age and sex- matched samples with comparable time from vaccination. Overall, there were no differences in the frequencies of naïve (CD45RA+CCR7+), central memory (CD45RA−CCR7+), effector-memory (CD45RA−CCR7−), and terminally differentiated effector memory (CD45RA+CCR7−) T cells between the two groups except individuals with myocarditis had slightly higher frequencies of effector-memory cells (t-test, p=0.047) (Figure 2S A–B). We also did not see a difference in the proportions of memory subsets in SARS-CoV-2 Spike specific CD4+ T cells (Figure 2S C). Furthermore, the frequencies of IFNγ-secreting and degranulating, as determined by CD107a-expression, CD8+ T cells, and CD4+ T cells upon stimulation with SARS-CoV-2 Spike peptides were indistinguishable between both groups (Figure 2S D–G). The only noticeable difference in the T cell signature was a higher frequency of PD-1 expressing bulk CD4+ T cells in the individuals with post-vaccine myocarditis (unpaired t-test, p=0.02) (Figure 2S H and I) likely reflecting variability in expansion after immunization but potentially also suggesting a higher level of exhaustion in this cell subset.
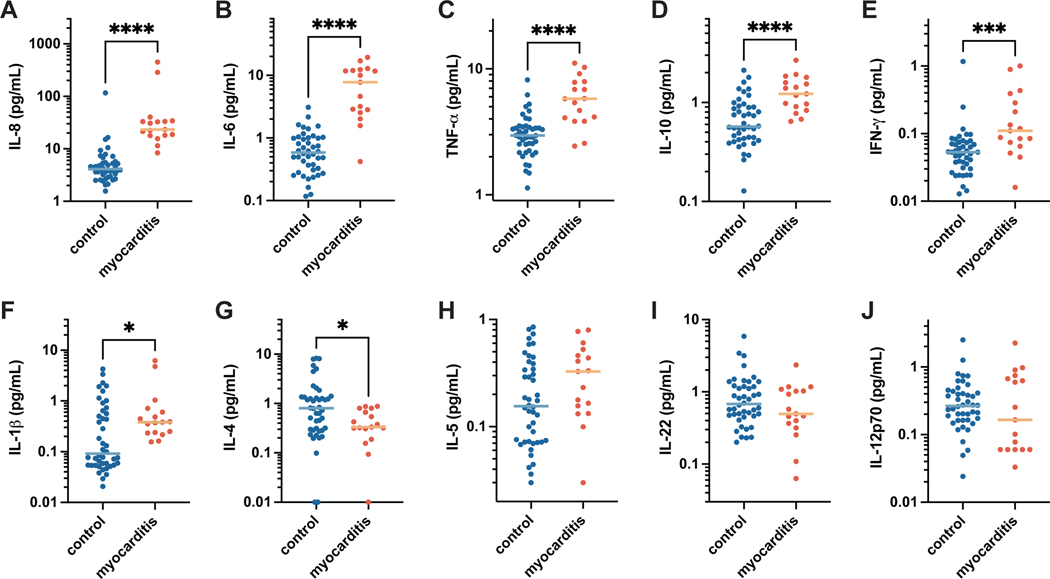
While minimal differences were seen in the T cell populations, individuals with myocarditis displayed distinct cytokine profiles reminiscent of the profile seen in MIS-C17–19, suggesting likely innate inflammatory activation, with significantly elevated levels of IL-8, IL-6, TNF-α, IL-10, IFN-γ, and IL1-β, and lower IL-4 levels as compared to healthy vaccinated controls (Figure 3A–G) with no notable differences in IL-5, IL-12p70, or IL-22 (Figure 3H–K). To further characterize the inflammatory profile, complete blood counts were analyzed. While mostly within normal ranges in both cohorts, total leukocytes, and specifically neutrophils were significantly increased in individuals with post-vaccine myocarditis compared to vaccinated controls (unpaired t-test, p = 0.007, p = 0.01, respectively), while platelet counts were decreased compared to vaccinated controls (unpaired t-test, p = 0.03) (Figure 3S). These results suggest that post-vaccine myocarditis is associated with normal adaptive and T cell immunity but modest innate activation.
Figure 3. Cytokine profiles.
The concentration of cytokines detected in the plasma of adolescents with post-vaccine myocarditis (n = 16) compared to age-matched post-vaccine controls (n = 44). Plots are shown in order of decreasing significance between the mean values of each group for the following cytokines: (A) interleukin (IL)-6, (B) IL-8, (C) tumor necrosis factor (TNF)-α, (D) IL-10, (E) interferon (IFN)-γ, (F) IL-1β, (G) IL-4, (H) IL-5, (I) IL-22, and (J) IL-12p70. All data represents mean values for duplicate measurements. Significance was calculated using the Mann-Whitney U test, where * = p < 0.05, ** = p < 0.01, *** = p < 0.001, and **** = p < 0.0001.
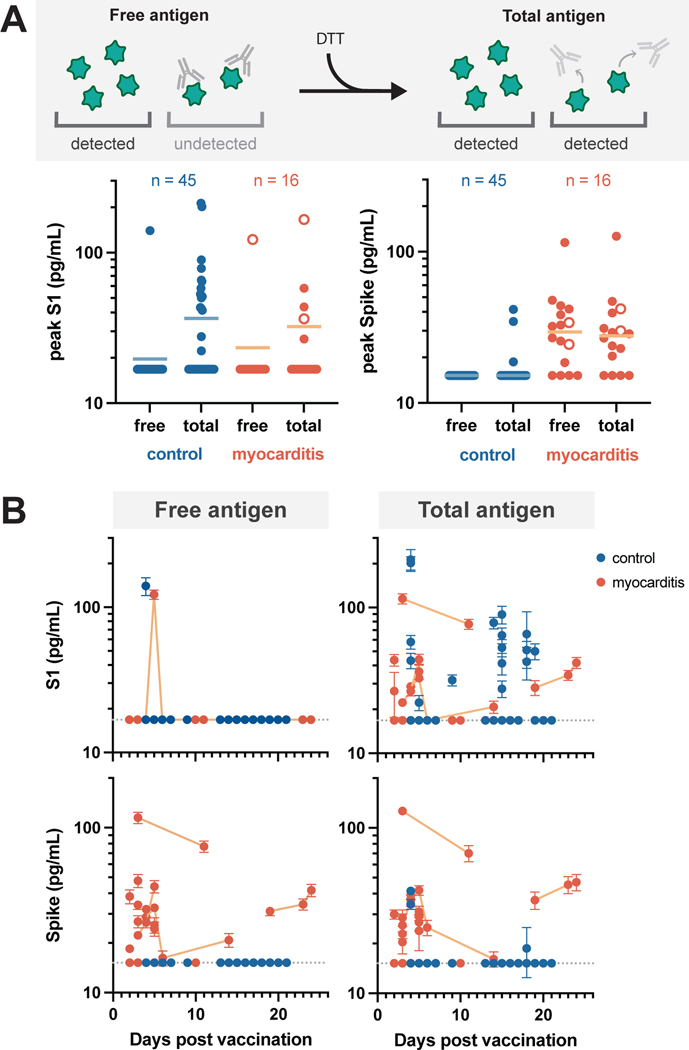
To further characterize immune responses and assess for potential stimuli of innate inflammation, ultrasensitive single molecule array (Simoa) antigen assays were used to measure the vaccine-stimulated production of both the full-length SARS-CoV-2 Spike protein and its cleaved subunit, S1, in the blood following intramuscular vaccination. Given that S1 antigen levels were seen to drop quickly in a healthy adult cohort, we sought to assess the clearance of S1 in our adolescent cohorts. As seen in adults, when analyzing adolescent plasma samples, which were primarily collected following their second vaccination, freely circulating S1 was not detected in most of the samples (Figure 4A).
Figure 4: Circulating SARS-CoV-2 antigen.
Free and total S1 and Spike levels (A) measured in the plasma of the vaccinated control (n = 44) and myocarditis cohorts (n = 16). Antigen data corresponding to all individuals who received the BNT162b2 vaccine (n = 59) are represented as solid circles and those who received the Moderna vaccine (n = 2) are represented as open circles. Free antigen is measured by directly diluting patient plasma, whereas total antigen is measured after treating the plasma with DTT to denature any antigen-bound antibodies. Free and total antigen levels versus days post vaccination (B). Longitudinal measurements associated with the same myocarditis patient are connected with lines. All data points represent mean values for duplicate measurements. Dashed grey lines indicate the limit of detection (LOD) for each assay.
We then sought to determine if S1 was undetectable because the antigen had been effectively cleared or because the antigen was bound and masked by circulating antibodies. To release antibody-bound antigen, we first treated plasma samples with dithiothreitol (DTT) to denature the antibodies. After incubation with DTT, S1 was detected in plasma for 34% of the vaccinated control cohort and 29% of the myocarditis cohort. We repeated this analysis for the adult cohort but S1 antigen remained undetectable in adults following the second vaccine dose (Figure 4S). Although the adult cohort is small (n = 13), these findings may suggest an age-related difference in processing of the mRNA vaccine. Regardless, the frequency with which we could detect S1 as well as the mean concentrations were similar between the two adolescent cohorts and therefore unlikely associated with the development of post-vaccine myocarditis.
In notable contrast, adolescents who developed myocarditis had markedly higher levels of free full-length Spike protein in their plasma (33.9 ± 22.4 pg/mL), unbound by antibodies, (Figure 4A), whereas asymptomatic vaccinated controls had no detectable free Spike protein (unpaired t test, p < 0.0001). While post-vaccine myocarditis clinically occurs more commonly in males, elevated Spike was seen equally in both affected females and males (Figure 5S). We found minimal increase in Spike levels after DTT treatment, suggesting that most of the antigen is freely circulating and unbound by antibodies in the individuals with post-vaccine myocarditis. Antibody-bound Spike was detected in only approximately 6% of the vaccinated controls. Similarly, we were not able to detect any free or antibody-bound Spike in the healthy adult samples (Figure 4S).
When analyzed according to time post vaccination, free S1, which was only detected in one post-myocarditis patient and one vaccinated control, was detected only within the first week, however, antibody-bound S1, which was detected in roughly one-third of both cohorts, could be detected up to three weeks after vaccination (Figure 4B). In contrast, both free and antibody-bound Spike, which was detectable only in patients who developed vaccine-induced myocarditis, remained detectable up to three weeks post-vaccination (Figure 4B). Longitudinal sampling displays a slow decline in both free and antibody-bound Spike, suggesting that collecting blood at only a single time point was unlikely to miss circulating Spike in healthy vaccinated controls. To assess whether this persistence of Spike in myocarditis patients was due to inadequate antibody neutralization, we carried out in vitro Simoa neutralization assays20. We analyzed plasma samples from a subset of myocarditis patients (n = 9) and healthy vaccinated controls (n = 14) for whom plasma samples were collected within one week after the second vaccine dose; however no significant differences in the antibody neutralization capacities were observed (Figure 6S).
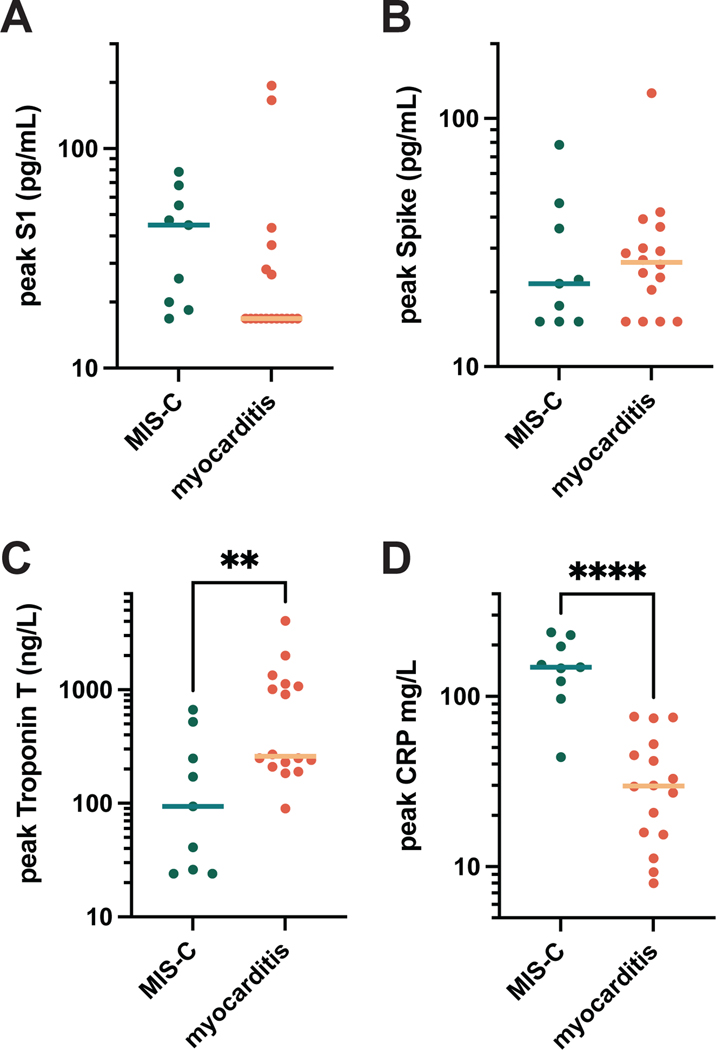
Interestingly, the persistence of circulating Spike in post-vaccine myocarditis patients is similar to the SARS-CoV-2 antigenemia previously reported to be a pathogenic feature of MIS-C18, 21. For that reason, we compared S1, Spike, cardiac troponin T, and CRP levels between individuals who developed myocarditis versus those who developed MIS-C with cardiac complications (Figure 5). There were no significant differences between the mean S1 and Spike concentrations between the two groups. While both MIS-C and post-vaccine myocarditis resulted in elevated cardiac troponin T and CRP compared to healthy vaccinated controls, when comparing MIS-C with post-vaccine myocarditis, cardiac troponin T levels were significantly elevated for the myocarditis cohort and CRP levels were significantly elevated for the MIS-C cohort.
Figure 5. MIS-C versus post-vaccine myocarditis.
S1 (A), Spike (B), cardiac troponin T (C), and C-reactive protein (CRP) (D) levels detected in the blood samples of individuals with MIS-C (n = 9) versus post-vaccine myocarditis (n = 17). All data points represent mean values for duplicate measurements. Significance was calculated using the Mann-Whitney U test; with ** = p < 0.01 and **** = p < 0.0001.
For a subset of post-vaccine myocarditis patients (n=4) from whom we obtained repeat blood sampling, we generated longitudinal immunoprofiles, including cardiac troponin T, S1, Spike, anti-SARS-CoV-2 antibodies, and cytokine levels (Figure 7S). While Spike levels can remain elevated for several days to weeks for all patients, any detected S1 was generally cleared. In all four patients, anti-N IgG was undetectable, suggesting that natural infection with SARS-CoV-2 was unlikely a contributing factor. In contrast, anti-Spike, anti-S1, and anti-RBD IgG, IgA and IgM levels increased as expected following vaccination; patients 1–3 developed myocarditis following the second vaccine dose, patient 4 developed myocarditis after the first dose. Of the cytokines, IL-8 was most prominent, mirroring cardiac troponin T and antigen levels most closely.
Discussion:
While epidemiologic reports describe key clinical features associated with myocarditis following vaccination with BNT162b2 or mRNA-12739, 22, herein, we provide in-depth immunoprofiling of post-vaccine myocarditis patients. We discovered that individuals who developed post-vaccine myocarditis uniquely exhibit elevated levels of free Spike protein in circulation, unbound by anti-Spike antibodies, which appear to correlate with cardiac troponin T levels and innate immune activation with cytokine release. Adaptive immunity and T cell responses, however, were essentially indistinguishable from asymptomatic age-matched vaccinated controls. The post-vaccine myocarditis immunoprofile is distinct, though, from acute SARS-CoV-2 infection and the delayed post inflammatory illness, MIS-C. While these findings might provide insight into the immunophenotype of vaccine-related myocarditis, they do not alter the risk-benefit ratio strongly favoring vaccination10 to protect against severe COVID-19 related complications.
Notably, Spike, which remained intact by evading cleavage and clearance, was associated with myocarditis in this cohort. Whether the circulating Spike protein in the setting of mRNA vaccination was pathogenic is unclear. In post-vaccine myocarditis, the Spike protein appears to evade antibody recognition because the anti-Spike antibodies that are generated are produced in adequate quantities with normal functional and neutralization capacity. There is growing in vitro evidence that Spike itself can stimulate cardiac pericytes dysfunction23 or inflame the endothelium, potentially by down regulating ACE2 expression or impairing endothelial nitric oxide bioavailability24, or by activating integrin-mediated inflammation with hyperpermeability of the endothelial cell layer25. Thus, the Spike antigen itself, which evades antibody recognition rather than invoking immune hyperactivation, may contribute myocarditis in these individuals.
T cells subsets have been implicated in other forms of myocarditis, such as auto-immune myocarditis and dilated cardiomyopathy26. While we did not observe any difference in SARS-CoV-2 specific CD4+ and CD8+ T cells, nor did in vitro stimulation reveal any differences in IFN-γ response between the two cohorts, we cannot rule out T cell involvement based on this analysis alone. However, a slight increase in PD-1 expressing bulk CD4+ T cells in post-vaccine myocarditis may indicate some degree of T cell activation or exhaustion, although it appears distinct from SARS-CoV-2 specific responses. Additionally, although we did not detect any autoantibodies as has been seen in other forms of myocarditis, this does not necessarily rule out the potential for T cell involvement. Our data suggest, however, that there is a potentially unique mechanism of myocardial injury following SARS-CoV-2 mRNA vaccination associated with a robust innate immune response. Future studies are needed to fully investigate the contribution of T cells in post-vaccine myocarditis.
Vaccine-related myocarditis is not unique to the mRNA vaccines; myocarditis can also occur following other vaccines27, 28, including the non-mRNA COVID-19 vaccines, such as influenza and smallpox vaccines. Therefore, it is possible that circulating Spike is a biomarker of immune dysregulation leading to myocarditis rather than a causal agent. However, we discovered distinct differences in how adolescents respond to mRNA vaccination as compared to adults, which warrant further investigation. It has previously been shown that following the first inoculation of the mRNA-1273 vaccine, the cleaved S1 subunit of Spike can be detected in the plasma of healthy adults16. However, after the second dose no antigen was detected16, presumably because there are higher levels of circulating anti-SARS-CoV-2 antibodies, which quickly bind any circulating antigen, facilitating its clearance. In contrast, one-third of the adolescents displayed antibody-bound S1 antigenemia following the second vaccination, regardless of the development of myocarditis, a finding not seen in our smaller sample of adults. This suggests that either the immune system of adults responds more quickly to the vaccine-induced production of Spike or due to differences in body mass, the levels of S1 fall below the limit of detection for adults. Alternatively, increased levels of free Spike compared to free S1 may be due to differences in renal clearance rates, where S1 would be expected to clear faster with a molecular weight of 76 kD, approximately half that of full spike (180 kD). Since both adults and the adolescents included in our cohort all received adult dosing of the mRNA vaccine, this finding suggests an age-related capacity for handling vaccine-introduced antigen. Importantly, the vast majority of circulating S1 was bound by specific anti-S1 antibodies, indicating an appropriate immune response for targeting and clearing S1.
Identification of SARS-CoV-2 viral particles in the blood is not unique to post-vaccine myocarditis. However, when antigenemia is detected in severe COVID-1929 or MIS-C18, 21 a more pronounced hyperinflammatory, superantigen-like response is classically seen, with spike immune complexes triggering hyperactivation of monocyte phagocytosis30 and neutrophil extracellular trap formation31. In vaccine-induced myocarditis, though, Spike does not appear to be bound to antibodies, and there is no evidence of excessive immune complex activation of neutrophils, complement or monocytes. Additionally, in MIS-C, CRP levels are markedly elevated as compared to post-vaccine myocarditis32. This distinction in hyperinflammation may result from the source of antigenemia. In vaccine-associated myocarditis, antigenemia results after sterile intramuscular inoculation with the liposomally protected mRNA transcript. In contrast, in MIS-C, antigens may leak into the circulation due to dysbiosis in the gut, which signals the zonulin-mediated loss of tight junctions, as evidenced by elevated zonulin levels, LPS binding protein, and soluble CD1418. It is also possible that associated endotoxemia exacerbates the inflammatory response against SARS-CoV-2 antigens, accounting for severe hyperinflammatory response characterizing MIS-C. Furthermore, in MIS-C, the superantigen-like motif33 of Spike/S1 engages with the immune system to produce T cell receptor skewing34 and a profound hyperinflammatory response35; in post-vaccine myocarditis, the circulating free Spike antigen appears to evade antibody recognition. Correspondingly, post-mRNA vaccine myocarditis follows a more subdued acute course8, 36, but long-term outcomes remain to be seen.
Limitations of this study include the relatively small sample size, because post-vaccine myocarditis is a rare complication with approximately 18 cases occurring for every one million vaccine doses administered. Our cohorts were not evenly balanced between the BNT162b2 and mRNA-1273 vaccines: all of our adolescent control cohort and the majority of our myocarditis cohort received the BNT162b2 vaccine (n = 15). Furthermore, a more extensive analysis of T cell subsets, including Th17 cells which have previously been implicated in non-vaccine-induced myocarditis/dilated cardiomyopathy26, or cardiac biopsy tissue analysis would be informative in the future. Nonetheless, our multicenter collaboration allowed for the meaningful analysis of a considerably sized post-vaccine myocarditis cohort. Additionally, ultrasensitive antigen detection via Simoa29 does not distinguish whether Spike antigenemia is the cause or consequence, and not all patients with myocarditis had detectable antigenemia; rather, immunophenotyping lays important groundwork for further understanding of mRNA vaccine-associated complications and provides rationale for future research to aid in vaccine design and dosage. These findings also suggest that administration of anti-Spike antibodies, if Spike antigenemia is detected, could potentially prevent or reverse post-vaccine myocarditis.
Reassuringly, neither the BNT162b2 nor mRNA-1273 vaccine induce abnormal adaptive immunity or T cell responses associated with immune activation targeting the myocardium. However, we detected free Spike antigen in the blood of adolescents and young adults who developed post-mRNA vaccine myocarditis. While the implications of this finding must be better understood, these results do not alter the risk benefit ratio favoring vaccination against COVID-19 to prevent severe clinical outcomes.
Supplementary Material
Clinical Perspective.
What is new?
Adolescents and young adults who developed myocarditis after SARS-CoV-2 mRNA vaccination display persistently elevated circulating levels of full-length Spike protein, unbound by antibodies.
No evidence of autoantibody production, concomitant viral infections, or excessive antibody responses to the anti-SARS-CoV-2 mRNA vaccines were identified in post-vaccine myocarditis cases.
What are the clinical implications?
Understanding the immunopathological mechanisms associated with post-vaccine myocarditis will help improve safety and guide development of future COVID-19 vaccines.
These results do not alter the risk benefit ratio favoring vaccination against COVID-19 to prevent severe clinical outcomes.
Acknowledgments:
We thank the children and families who participated in this research. We thank Jeni Melo, BS of Boston Children’s Hospital for her assistance in collecting clinical data.
Funding Sources:
This research was supported by the National Institute Health: National Heart, Lung, and Blood Institute (5K08HL143183 to LMY), the National Institute of Diabetes and Digestive and Kidney Diseases (DK104344 to AF), National Institute of Child Health and Human Development (R01HD100022-02S2 to AE), National Institute of Allergy and Infectious Diseases (3R01AI072726-10S1 to MA) and (3R37AI080289-11S1, R01AI146785 and U19AI42790-01, U19AI135995-02, 1U01CA260476-01, CIVIC75N93019C00052 to GA). We also report funding from the Regione Campania Italy (CUP G58D20000240002 - SURF 20004BP000000011 to AF), Boston Children’s Hospital’s Taking on COVID-19 Together Study (to AR), and MassGeneral for Children (to LMY). Funding for the SARS-CoV-2 antigen measurements came from a generous donation from Barbara and Amos Hostetter and the Chleck Foundation. We thank Nancy Zimmerman, Mark and Lisa Schwartz, an anonymous donor (financial support), Terry and Susan Ragon, and the Samana Cay Massachusetts General Hospital Research Scholars award for their support. We acknowledge support from the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk foundation, and the Gates Foundation Global Health Vaccine Accelerator Platform funding (OPP1146996 and INV-001650).
Nonstandard abbreviations and nonstandard acronyms:
- CMV
cytomegalovirus
- COVID-19
coronavirus disease
- CRP
C-reactive protein
- DTT
dithiothreitol
- EBV
Epstein-Barr virus
- HSV
herpes simplex virus type 1
- IQR
interquartile range
- MIS-C
multisystem inflammatory syndrome in children
- RBD
receptor binding protein
- RSV
respiratory syncytial virus
- Simoa
single molecule array
- ULN
upper limit of normal
Footnotes
Disclosures:
David Walt has a financial interest in Quanterix Corporation, a company that develops an ultra-sensitive digital immunoassay platform. He is an inventor of the Simoa technology, a founder of the company and also serves on its Board of Directors. Dr. Walt’s interests were reviewed and are managed by BWH and MassGeneral Brigham in accordance with conflict of interest policies. Galit Alter has been employed by Moderna since Oct 2022; her contributions to this manuscript preceded her employment by Moderna. Dr. Alter is also a founder and equity holder of Seromyx Systems, a company developing a platform technology to profile antibody immunity. Drs. Juelg and Alter are employees and equity holders of Leyden Labs, a company developing pandemic prevention therapeutics. Drs. Juelg and Alter’s interests were reviewed and are managed by Massachusetts General Hospital and MassGeneral Brigham in accordance with their conflict of interest policies. Dr. Randolph received funding (to Boston Children’s Hospital) from the US Center for Disease Control and Prevention to study COVID-19 complications in children outside of this work.
All other authors have declared that no conflicts of interest exist.
References
- 1.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Perez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr., Hammitt LL, Tureci O, Nell H, Schaefer A, Unal S, Tresnan DB, Mather S, Dormitzer PR, Sahin U, Jansen KU, Gruber WC and Group CCT. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T and Group CS. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson SM, Newhams MM, Halasa NB, Price AM, Boom JA, Sahni LC, Pannaraj PS, Irby K, Walker TC, Schwartz SP, Maddux AB, Mack EH, Bradford TT, Schuster JE, Nofziger RA, Cameron MA, Chiotos K, Cullimore ML, Gertz SJ, Levy ER, Kong M, Cvijanovich NZ, Staat MA, Kamidani S, Chatani BM, Bhumbra SS, Bline KE, Gaspers MG, Hobbs CV, Heidemann SM, Maamari M, Flori HR, Hume JR, Zinter MS, Michelson KN, Zambrano LD, Campbell AP, Patel MM, Randolph AG and Overcoming Covid I. Effectiveness of BNT162b2 Vaccine against Critical Covid-19 in Adolescents. N Engl J Med. 2022;386:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walter EB, Talaat KR, Sabharwal C, Gurtman A, Lockhart S, Paulsen GC, Barnett ED, Munoz FM, Maldonado Y, Pahud BA, Domachowske JB, Simoes EAF, Sarwar UN, Kitchin N, Cunliffe L, Rojo P, Kuchar E, Ramet M, Munjal I, Perez JL, Frenck RW Jr., Lagkadinou E, Swanson KA, Ma H, Xu X, Koury K, Mather S, Belanger TJ, Cooper D, Tureci O, Dormitzer PR, Sahin U, Jansen KU, Gruber WC and Group CCT. Evaluation of the BNT162b2 Covid-19 Vaccine in Children 5 to 11 Years of Age. N Engl J Med. 2022;386:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy M, Recher M, Hubert H, Javouhey E, Flechelles O, Leteurtre S and Angoulvant F. Multisystem Inflammatory Syndrome in Children by COVID-19 Vaccination Status of Adolescents in France. JAMA. 2022;327:281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zambrano LD, Newhams MM, Olson SM, Halasa NB, Price AM, Boom JA, Sahni LC, Kamidani S, Tarquinio KM, Maddux AB, Heidemann SM, Bhumbra SS, Bline KE, Nofziger RA, Hobbs CV, Bradford TT, Cvijanovich NZ, Irby K, Mack EH, Cullimore ML, Pannaraj PS, Kong M, Walker TC, Gertz SJ, Michelson KN, Cameron MA, Chiotos K, Maamari M, Schuster JE, Orzel AO, Patel MM, Campbell AP, Randolph AG and Overcoming C-I. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA Vaccination Against Multisystem Inflammatory Syndrome in Children Among Persons Aged 12–18 Years - United States, July-December 2021. MMWR Morb Mortal Wkly Rep. 2022;71:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlstad O, Hovi P, Husby A, Harkanen T, Selmer RM, Pihlstrom N, Hansen JV, Nohynek H, Gunnes N, Sundstrom A, Wohlfahrt J, Nieminen TA, Grunewald M, Gulseth HL, Hviid A and Ljung R. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022;7:600–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD and Kornowski R. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med. 2021;385:2132–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/myocarditis.html.
- 10.Bozkurt B, Kamat I and Hotez PJ. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;144:471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heymans S and Cooper LT. Myocarditis after COVID-19 mRNA vaccination: clinical observations and potential mechanisms. Nat Rev Cardiol. 2022;19:75–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heymans S, Eriksson U, Lehtonen J and Cooper LT Jr. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J Am Coll Cardiol. 2016;68:2348–2364. [DOI] [PubMed] [Google Scholar]
- 13.Muthukumar A, Narasimhan M, Li QZ, Mahimainathan L, Hitto I, Fuda F, Batra K, Jiang X, Zhu C, Schoggins J, Cutrell JB, Croft CL, Khera A, Drazner MH, Grodin JL, Greenberg BM, Mammen PPA, Morrison SJ and de Lemos JA. In-Depth Evaluation of a Case of Presumed Myocarditis After the Second Dose of COVID-19 mRNA Vaccine. Circulation. 2021;144:487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lima R, Gootkind EF, De la Flor D, Yockey LJ, Bordt EA, D’Avino P, Ning S, Heath K, Harding K, Zois J, Park G, Hardcastle M, Grinke KA, Grimmel S, Davidson SP, Forde PJ, Hall KE, Neilan AM, Matute JD, Lerou PH, Fasano A, Shui JE, Edlow AG and Yonker LM. Establishment of a pediatric COVID-19 biorepository: unique considerations and opportunities for studying the impact of the COVID-19 pandemic on children. BMC Med Res Methodol. 2020;20:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gargano JW, Wallace M, Hadler SC, Langley G, Su JR, Oster ME, Broder KR, Gee J, Weintraub E, Shimabukuro T, Scobie HM, Moulia D, Markowitz LE, Wharton M, McNally VV, Romero JR, Talbot HK, Lee GM, Daley MF and Oliver SE. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices - United States, June 2021. MMWR Morb Mortal Wkly Rep. 2021;70:977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogata AF, Cheng CA, Desjardins M, Senussi Y, Sherman AC, Powell M, Novack L, Von S, Li X, Baden LR and Walt DR. Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients. Clin Infect Dis. 2022;74:715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diorio C, Henrickson SE, Vella LA, McNerney KO, Chase J, Burudpakdee C, Lee JH, Jasen C, Balamuth F, Barrett DM, Banwell BL, Bernt KM, Blatz AM, Chiotos K, Fisher BT, Fitzgerald JC, Gerber JS, Gollomp K, Gray C, Grupp SA, Harris RM, Kilbaugh TJ, John ARO, Lambert M, Liebling EJ, Paessler ME, Petrosa W, Phillips C, Reilly AF, Romberg ND, Seif A, Sesok-Pizzini DA, Sullivan KE, Vardaro J, Behrens EM, Teachey DT and Bassiri H. Multisystem inflammatory syndrome in children and COVID-19 are distinct presentations of SARS-CoV-2. J Clin Invest. 2020;130:5967–5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yonker LM, Gilboa T, Ogata AF, Senussi Y, Lazarovits R, Boribong BP, Bartsch YC, Loiselle M, Rivas MN, Porritt RA, Lima R, Davis JP, Farkas EJ, Burns MD, Young N, Mahajan VS, Hajizadeh S, Lopez XIH, Kreuzer J, Morris R, Martinez EE, Han I, Griswold K Jr., Barry NC, Thompson DB, Church G, Edlow AG, Haas W, Pillai S, Arditi M, Alter G, Walt DR and Fasano A. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter MJ, Fish M, Jennings A, Doores KJ, Wellman P, Seow J, Acors S, Graham C, Timms E, Kenny J, Neil S, Malim MH, Tibby SM and Shankar-Hari M. Peripheral immunophenotypes in children with multisystem inflammatory syndrome associated with SARS-CoV-2 infection. Nat Med. 2020;26:1701–1707. [DOI] [PubMed] [Google Scholar]
- 20.Gilboa T, Cohen L, Cheng CA, Lazarovits R, Uwamanzu-Nna A, Han I, Griswold K Jr., Barry N, Thompson DB, Kohman RE, Woolley AE, Karlson EW and Walt DR. A SARS-CoV-2 Neutralization Assay Using Single Molecule Arrays. Angew Chem Int Ed Engl. 2021;60:25966–25972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacco K, Castagnoli R, Vakkilainen S, Liu C, Delmonte OM, Oguz C, Kaplan IM, Alehashemi S, Burbelo PD, Bhuyan F, de Jesus AA, Dobbs K, Rosen LB, Cheng A, Shaw E, Vakkilainen MS, Pala F, Lack J, Zhang Y, Fink DL, Oikonomou V, Snow AL, Dalgard CL, Chen J, Sellers BA, Montealegre Sanchez GA, Barron K, Rey-Jurado E, Vial C, Poli MC, Licari A, Montagna D, Marseglia GL, Licciardi F, Ramenghi U, Discepolo V, Lo Vecchio A, Guarino A, Eisenstein EM, Imberti L, Sottini A, Biondi A, Mato S, Gerstbacher D, Truong M, Stack MA, Magliocco M, Bosticardo M, Kawai T, Danielson JJ, Hulett T, Askenazi M, Hu S, Group NIRtC, Chile MISCG, Pavia Pediatric C-G, Cohen JI, Su HC, Kuhns DB, Lionakis MS, Snyder TM, Holland SM, Goldbach-Mansky R, Tsang JS and Notarangelo LD. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat Med. 2022;28:1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Truong DT, Dionne A, Muniz JC, McHugh KE, Portman MA, Lambert LM, Thacker D, Elias MD, Li JS, Toro-Salazar OH, Anderson BR, Atz AM, Bohun CM, Campbell MJ, Chrisant M, D’Addese L, Dummer KB, Forsha D, Frank LH, Frosch OH, Gelehrter SK, Giglia TM, Hebson C, Jain SS, Johnston P, Krishnan A, Lombardi KC, McCrindle BW, Mitchell EC, Miyata K, Mizzi T, Parker RM, Patel JK, Ronai C, Sabati AA, Schauer J, Sexson Tejtel SK, Shea JR, Shekerdemian LS, Srivastava S, Votava-Smith JK, White S and Newburger JW. Clinically Suspected Myocarditis Temporally Related to COVID-19 Vaccination in Adolescents and Young Adults: Suspected Myocarditis After COVID-19 Vaccination. Circulation. 2022;145:345–356. [DOI] [PubMed] [Google Scholar]
- 23.Avolio E, Carrabba M, Milligan R, Kavanagh Williamson M, Beltrami AP, Gupta K, Elvers KT, Gamez M, Foster RR, Gillespie K, Hamilton F, Arnold D, Berger I, Davidson AD, Hill D, Caputo M and Madeddu P. The SARS-CoV-2 Spike protein disrupts human cardiac pericytes function through CD147 receptor-mediated signalling: a potential non-infective mechanism of COVID-19 microvascular disease. Clin Sci (Lond). 2021;135:2667–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, Zhang Y, Yin Q, Cho Y, Andrade L, Shadel GS, Hepokoski M, Lei T, Wang H, Zhang J, Yuan JX, Malhotra A, Manor U, Wang S, Yuan ZY and Shyy JY. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ Res. 2021;128:1323–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robles JP, Zamora M, Adan-Castro E, Siqueiros-Marquez L, Martinez de la Escalera G and Clapp C. The spike protein of SARS-CoV-2 induces endothelial inflammation through integrin alpha5beta1 and NF-kappaB signaling. J Biol Chem. 2022;298:101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myers JM, Cooper LT, Kem DC, Stavrakis S, Kosanke SD, Shevach EM, Fairweather D, Stoner JA, Cox CJ and Cunningham MW. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight. 2016;1:e85851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling RR, Ramanathan K, Tan FL, Tai BC, Somani J, Fisher D and MacLaren G. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir Med. 2022;10:679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neff J, Modlin J, Birkhead GS, Poland G, Robertson RM, Sepkowitz K, Yancy C, Gardner P, Gray GC, Maurer T, Siegel J, Guerra FA, Berger T, Flanders WD, Shope R, Advisory Committee on Immunization P and Armed Forces Epidemiological B. Monitoring the safety of a smallpox vaccination program in the United States: report of the joint Smallpox Vaccine Safety Working Group of the advisory committee on immunization practices and the Armed Forces Epidemiological Board. Clin Infect Dis. 2008;46 Suppl 3:S258–70. [DOI] [PubMed] [Google Scholar]
- 29.Ogata AF, Maley AM, Wu C, Gilboa T, Norman M, Lazarovits R, Mao CP, Newton G, Chang M, Nguyen K, Kamkaew M, Zhu Q, Gibson TE, Ryan ET, Charles RC, Marasco WA and Walt DR. Ultra-Sensitive Serial Profiling of SARS-CoV-2 Antigens and Antibodies in Plasma to Understand Disease Progression in COVID-19 Patients with Severe Disease. Clin Chem. 2020;66:1562–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartsch YC, Wang C, Zohar T, Fischinger S, Atyeo C, Burke JS, Kang J, Edlow AG, Fasano A, Baden LR, Nilles EJ, Woolley AE, Karlson EW, Hopke AR, Irimia D, Fischer ES, Ryan ET, Charles RC, Julg BD, Lauffenburger DA, Yonker LM and Alter G. Humoral signatures of protective and pathological SARS-CoV-2 infection in children. Nat Med. 2021;27:454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boribong BP, LaSalle TJ, Bartsch YC, Ellett F, Loiselle ME, Davis JP, Gonye ALK, Sykes DB, Hajizadeh S, Kreuzer J, Pillai S, Haas W, Edlow AG, Fasano A, Alter G, Irimia D, Sade-Feldman M and Yonker LM. Neutrophil Profiles of Pediatric COVID-19 and Multisystem Inflammatory Syndrome in Children. Cell Reports Medicine. [DOI] [PMC free article] [PubMed]
- 32.Henderson LA, Canna SW, Friedman KG, Gorelik M, Lapidus SK, Bassiri H, Behrens EM, Ferris A, Kernan KF, Schulert GS, Seo P, Son MBF, Tremoulet AH, Yeung RSM, Mudano AS, Turner AS, Karp DR and Mehta JJ. American College of Rheumatology Clinical Guidance for Multisystem Inflammatory Syndrome in Children Associated With SARS-CoV-2 and Hyperinflammation in Pediatric COVID-19: Version 2. Arthritis Rheumatol. 2021;73:e13–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng MH, Zhang S, Porritt RA, Noval Rivas M, Paschold L, Willscher E, Binder M, Arditi M and Bahar I. Superantigenic character of an insert unique to SARS-CoV-2 spike supported by skewed TCR repertoire in patients with hyperinflammation. Proc Natl Acad Sci U S A. 2020;117:25254–25262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porritt RA, Paschold L, Rivas MN, Cheng MH, Yonker LM, Chandnani H, Lopez M, Simnica D, Schultheiss C, Santiskulvong C, Van Eyk J, McCormick JK, Fasano A, Bahar I, Binder M and Arditi M. HLA class I-associated expansion of TRBV11–2 T cells in multisystem inflammatory syndrome in children. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Porritt RA, Binek A, Paschold L, Rivas MN, McArdle A, Yonker LM, Alter G, Chandnani HK, Lopez M, Fasano A, Van Eyk JE, Binder M and Arditi M. The autoimmune signature of hyperinflammatory multisystem inflammatory syndrome in children. J Clin Invest. 2021;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dionne A, Sperotto F, Chamberlain S, Baker AL, Powell AJ, Prakash A, Castellanos DA, Saleeb SF, de Ferranti SD, Newburger JW and Friedman KG. Association of Myocarditis With BNT162b2 Messenger RNA COVID-19 Vaccine in a Case Series of Children. JAMA Cardiol. 2021;6:1446–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. https://covid.cdc.gov/covid-data-tracker/#mis-national-surveillance.
- 38.Norman M, Gilboa T, Ogata AF, Maley AM, Cohen L, Busch EL, Lazarovits R, Mao CP, Cai Y, Zhang J, Feldman JE, Hauser BM, Caradonna TM, Chen B, Schmidt AG, Alter G, Charles RC, Ryan ET and Walt DR. Ultrasensitive high-resolution profiling of early seroconversion in patients with COVID-19. Nat Biomed Eng. 2020;4:1180–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mina MJ, Kula T, Leng Y, Li M, de Vries RD, Knip M, Siljander H, Rewers M, Choy DF, Wilson MS, Larman HB, Nelson AN, Griffin DE, de Swart RL and Elledge SJ. Measles virus infection diminishes preexisting antibodies that offer protection from other pathogens. Science. 2019;366:599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borducchi EN, Cabral C, Stephenson KE, Liu J, Abbink P, Ng’ang’a D, Nkolola JP, Brinkman AL, Peter L, Lee BC, Jimenez J, Jetton D, Mondesir J, Mojta S, Chandrashekar A, Molloy K, Alter G, Gerold JM, Hill AL, Lewis MG, Pau MG, Schuitemaker H, Hesselgesser J, Geleziunas R, Kim JH, Robb ML, Michael NL and Barouch DH. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature. 2016;540:284–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keng CT, Zhang A, Shen S, Lip KM, Fielding BC, Tan TH, Chou CF, Loh CB, Wang S, Fu J, Yang X, Lim SG, Hong W and Tan YJ. Amino acids 1055 to 1192 in the S2 region of severe acute respiratory syndrome coronavirus S protein induce neutralizing antibodies: implications for the development of vaccines and antiviral agents. J Virol. 2005;79:3289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rivnak AJ, Rissin DM, Kan CW, Song L, Fishburn MW, Piech T, Campbell TG, DuPont DR, Gardel M, Sullivan S, Pink BA, Cabrera CG, Fournier DR and Duffy DC. A fully-automated, six-plex single molecule immunoassay for measuring cytokines in blood. J Immunol Methods. 2015;424:20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.