Abstract
Background:
The endogenous purine 8-aminoguanine induces diuresis/natriuresis/glucosuria by inhibiting purine nucleoside phosphorylase (PNPase); however, mechanistic details are unknown.
Methods:
Here we further explored in rats 8-aminoguanine’s effects on renal excretory function by combining studies using intravenous 8-aminoguanine, intrarenal artery infusions of PNPase substrates (inosine and guanosine), renal microdialysis, mass spectrometry, selective adenosine-receptor ligands, adenosine receptor knockout rats, laser doppler blood flow analysis, cultured renal microvascular smooth muscle cells, HEK293 cells expressing A2B receptors and homogeneous time resolved fluorescence assay for adenylyl cyclase activity.
Results:
Intravenous 8-aminoguanine caused diuresis/natriuresis/glucosuria and increased renal microdialysate levels of inosine and guanosine. Intrarenal inosine, but not guanosine, exerted diuretic/natriuretic/glucosuric effects. In 8-aminoguanine-pretreated rats, intrarenal inosine did not induce additional diuresis/natriuresis/glucosuria. 8-Aminoguanine did not induce diuresis/natriuresis/glucosuria in A2B-receptor knockout rats, yet did so in A1- and A2A-receptor knockout rats. Inosine’s effects on renal excretory function were abolished in A2B knockout rats. Intrarenal BAY 60-6583 (A2B agonist) induced diuresis/natriuresis/glucosuria and increased medullary blood flow (MBF). 8-Aminoguanine increased MBF, a response blocked by pharmacological inhibition of A2B, but not A2A, receptors. In HEK293 cells expressing A2B receptors, inosine activated adenylyl cyclase, and this was abolished by MRS 1754 (A2B antagonist). In renal microvascular smooth muscle cells, 8-aminoguanine and forodesine (PNPase inhibitor) increased inosine and 3’,5’-cAMP; however, in cells from A2B knockout rats, 8-aminoguanine and forodesine did not augment 3’,5’-cAMP yet increased inosine.
Conclusions:
8-Aminoguanine induces diuresis/natriuresis/glucosuria by increasing renal interstitial levels of inosine which, via A2B receptor activation, increases renal excretory function, perhaps in part by increasing MBF.
Keywords: 8-aminoguanine, purine nucleoside phosphorylase, A2B receptors, inosine, guanosine, medullary blood flow
Graphical Abstract
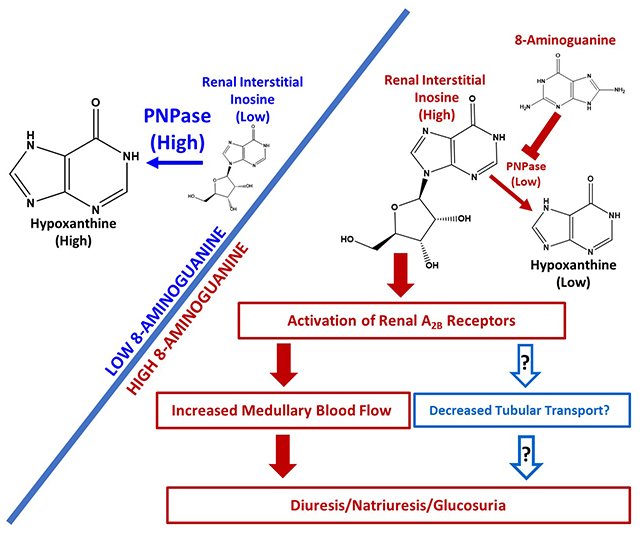
INTRODUCTION
Studies show that 8-aminoguanosine and 8-aminoguanine rapidly increase urine volume, sodium excretion and glucose excretion yet reduce potassium excretion.1 In this regard, systemically administered 8-aminoguanosine is rapidly converted to 8-aminoguanine which mediates most of 8-aminoguanosine’s renal effects (that is 8-aminoguanosine is a prodrug).2 8-Aminoguanine is a naturally occurring purine that likely derives from biomolecules containing 8-nitroguanine.3 Recent studies show that two additional 8-aminopurines, namely 8-aminoinosine and 8-aminohypoxanthine, also induced diuresis, natriuresis and mild glucosuria but not antikaliuresis.4 Similar to 8-aminoguanine, 8-aminoinosine may also occur in vivo.4
The diuretic, natriuretic and glucosuric effects of these 8-aminopurines likely are mediated by inhibition of purine nucleoside phosphorylase (PNPase).5 The evidence for this conclusion is that 8-aminoguanine, 8-aminoinosine, 8-aminohypoxanthine and 9-deazaguanine inhibit PNPase and induce diuresis, natriuresis and glucosuria.4,5 Importantly, unlike 8-aminoguanine, neither 9-deazaguanine, 8-aminoinosine nor 8-aminohypoxanthine alter potassium excretion.4,5 These findings suggest that 8-aminoguanine reduces potassium excretion by a mechanism that does not involve PNPase inhibition.
The diuretic, natriuretic and glucosuric effects of 8-aminopurines appear to be due to PNPase inhibition; however, the mechanistic details linking PNPase inhibition to effects on urine volume and sodium and glucose excretion are unknown. Accordingly, here we sought to further investigate how 8-aminoguanine induces diuresis, natriuresis and glucosuria. This objective was achieved by combining studies using ultra-performance liquid chromatography-tandem mass spectrometry, renal microdialysis, intrarenal artery infusions of PNPase substrates, selective pharmacological inhibitors, rats with specific adenosine receptors knocked out, laser doppler blood flow analysis, cultured renal microvascular smooth muscle cells, HEK293 cells expressing adenosine A2B receptors and a homogeneous time resolved fluorescence assay for 3’,5’-cAMP.
METHODS
For data and for additional information on analytical methods or study materials, contact E.K. Jackson at edj@pitt.edu.
Materials.
See Data Supplement
Animals.
Male and female wild-type Dahl salt-sensitive rats (WT Dahl SS rats) and Dahl salt-sensitive rats with adenosine subtype A1, A2A and A2B receptors knocked out (A1-KO Dahl SS, A2A-KO Dahl SS and A2B-KO Dahl SS rats, respectively) were obtained from colonies maintained at the University of Pittsburgh. Our colonies were established from breeding pairs generated by the MCW Gene Editing Rat Resource Program (Dr. Aron M. Geurts, Department of Physiology and Human Molecular Genetics Center, Medical College of Wisconsin, Milwaukee, WI). We have previously described in detail how these knockout rats were generated and how they were characterized and validated.6 Sprague-Dawley rats were purchased from Charles River (Wilmington, MA). Because experiments with male and female WT Dahl SS, A1-KO Dahl SS, A2A-KO Dahl SS and A2B-KO Dahl SS rats did not indicate sex differences with regard to the effects of 8-aminoguanine on renal excretory function, experiments in Sprague-Dawley rats were conducted in male rats. The Institutional Animal Care and Use Committee approved all procedures. The investigation conforms to National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Animal Preparation.
Rats were anesthetized (Inactin, 90 mg/kg, i.p.) and placed on an isothermal pad. Body temperature was continuously measured with a rectal probe thermometer and was maintained at 37ºC by adjusting the distance of a heat lamp from the surgical preparation. The trachea was cannulated with polyethylene (PE)-240 tubing, and the carotid artery was cannulated with PE-50 tubing, which was connected to a digital blood pressure analyzer (Micro-Med, Inc., Louisville, KY) for continuous monitoring of mean arterial blood pressure (MABP). Either a PE-50 cannula was positioned in the jugular vein for intravenous (IV) administration of test agents or a 30-guage needle connected to a cannula was placed in the left renal artery for intrarenal artery infusions of test agents. Hemodynamic stability was maintained by an infusion of 0.9 % saline (25 μl/min). The left ureter was cannulated with PE-10 tubing for timed collections of urine. Total renal blood flow (RBF) was measured by placing on the left renal artery a 1-mm transit-time flow probe connected to a transit-time flowmeter (model T-206; Transonic Systems, Ithaca, NY). In some experiments, a microdialysis probe (MD 2310, IV-10, 30 kDa cutoff with 10 mm membrane; BASi, West Fafayette, IN) was inserted into the renal cortex of the left kidney and into the renal medulla of the right kidney. In these experiments, microdialysate (0.9% saline) was infused into the probe inlet at 2 μl/min and collected at the probe outlet. Also, in some experiments, a laser doppler needle probe (diameter, 0.48 mm) was inserted into the medulla of the left kidney and connected to a doppler flowmeter (model ALF21; Transonic Systems) for measurement of medullary blood flow (MBF). After instrumentation, animals were allowed a rest period of approximately one hour.
Analysis of Purines in Microdialysate.
Purines in microdialysate samples were analyzed by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) using multiple reaction monitoring. We recently published an updated description of our UPLC-MS/MS purine assays.7
Analysis of Sodium, Potassium and Glucose in Urine.
Concentrations of sodium and potassium in urine were measured by flame photometry (Model IL-943; Instrumentations Laboratory, Lexington, MA). Glucose concentrations in urine were measured using the Cayman Chemical (Ann Arbor, MA) Glucose Colorimetric Assay Kit (catalog number 10009582).
Culture of Renal Microvascular Smooth Muscle Cells (RMSMCs).
RMSMCs were cultured from WT and A2B-KO Dahl SS rats as previously described.8
Inosine Signaling Via A2B Receptors.
The effects of inosine on signaling by A2B receptors was examined in cultured HEK293 cells expressing human A2B receptors (NCBI Reference Sequence: NP_000667.1). For details see Data Supplement.
Statistics.
Statistical analysis was conducted using NCSS Statistical Software version 19.0.2 (Kaysville, Utah). P<0.05 was considered statistically significant. For details see Data Supplement.
RESULTS
Protocol 1. Effects of 8-aminoguanine on renal excretory function and renal microdialysis levels of purines.
Timed collections of urine (from left ureter) and renal cortical and medullary microdiaysate were obtained from anesthetized Sprague Dawley rats from 0-30 min (Period 1), 40-70 min (Period 2) and 85-115 min (Period 3) into the protocol. Rats received an IV bolus injection (1 ml/kg) of either vehicle (0.9% saline containing 0.03 N HCl; control group) or 8-aminoguanine (33.5 μmoles/kg) immediately after Period 1. Vehicle had little or no effect on all measured variables. By contrast, by Period 3 8-aminoguanine had increased urine volume ~4-fold (P=0.0026; Fig. 1A), sodium excretion ~26-fold (P<0.0001; Fig. 1B) and glucose excretion ~12-fold (P<0.0001; Fig. 1C); yet had decreased potassium excretion by ~60% (P=0.0094; Fig. 1D). Basal levels of 8-aminoguanine in renal cortical (Fig. S1A) and medullary (Fig. S1B) microdialysates were at or near the detection limit of our assay system; yet by Period 2 IV 8-aminoguanine had increased both cortical (Fig. S1A) and medullary (Fig. S1B) microdialysate levels of 8-aminoguanine to ~30 μmol/L. 8-Aminoguanine did not significantly affect renal cortical levels of adenosine (Fig. S1C), and only mildly (50%) and transiently (Period 2 only) increased renal medullary adenosine (Fig. S1D). In contrast to adenosine and consistent with the fact that inosine is a PNPase substrate, by Period 2 8-aminoguanine had augmented cortical (P<0.0001; Fig. 1E) and medullary (P<0.0001; Fig. 1F) microdialysate levels of inosine by 5 to 10-fold. High levels of inosine were maintained throughout Period 3. In contrast to inosine and consistent with the fact that hypoxanthine is a product of PNPase, by Period 3 8-aminoguanine had reduced cortical (P=0.0010; Fig. S2A) and medullary (P=0.0400; Fig. S2B) microdialysate levels of hypoxanthine by ~60% and ~30%, respectively. Guanosine, like inosine, is a substrate for PNPase that is converted to guanine. Therefore, one would predict that the qualitative effects of 8-aminoguanine on cortical and medullary levels of guanosine would be similar to the effects of 8-aminoguanine on inosine and that the qualitative effects of 8-aminoguanine on guanine would be similar to the effects of 8-aminoguanine on hypoxanthine. Indeed, 8-aminoguanine did increase cortical (P<0.0001; Fig. S2C) and medullary (P<0.0001; Fig. S2D) levels of guanosine and decreased cortical (P=0.0007; Fig. S2E) and medullary (P=0.0414; Fig. S2F) levels of guanine. Hemodynamically, rats were stable throughout the protocol. At the end of the experiments, MABPs were similar in the two groups (111 ± 3 vs. 112 ± 2 mm Hg, control group vs. 8-aminoguanine group, respectively; mean ± SEM), as were RBFs (9.2 ± 0.9 vs. 8.4 ± 0.8 ml/min, control group vs. 8-aminoguanine group, respectively).
Figure 1. Effects of 8-aminoguanine on renal excretory function and renal cortical and medullary microdialysate levels of inosine.
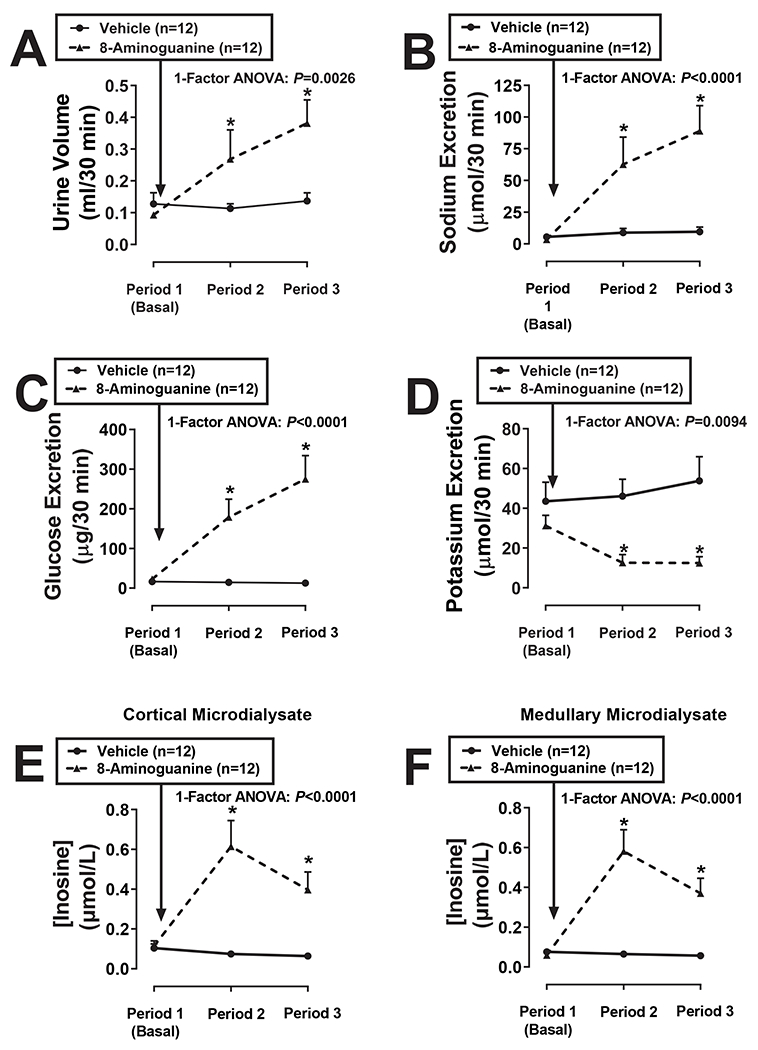
Timed collections of urine from the ureter and renal cortical and medullary microdialysates were obtained from anesthetized rats from 0-30 min (Period 1), 40-70 min (Period 2) and 85-115 min (Period 3) into the protocol. Rats received an IV injection of 8-aminoguanine (33.5 μmoles/kg) immediately after Period 1. Urine volumes (A), excretion rates of sodium (B), glucose (C) and potassium (D) and cortical (E) and medullary (F) concentrations of inosine were determined. Values are means and SEMs for the indicated sample size (n). ANOVA, analysis of variance; *P<0.05 vs Period 1.
Protocol 2. Effects of intrarenal artery infusions of inosine and guanosine on renal excretory function.
Because 8-aminoguanine profoundly increased renal interstitial levels of inosine and guanosine, next we examined the effects of these two purines on renal excretory function. To avoid systemic effects, inosine or guanosine, dissolved in 0.9% saline, was infused directly into the left renal artery of anesthetized Sprague Dawley rats at increasing doses [0 (basal; 0.9% saline only), 0.01, 0.1 and 1 μmol/kg/min]. In preliminary experiments, we showed that the medium dose (0.1 μmol/kg/min) of inosine or guanosine achieved levels of inosine (0.5 ± 0.3 μmol/L; n=6) or guanosine (0.6 ± 0.3 μmol/L; n=6), respectively, in the renal cortical microdialysate of the infused kidney that were comparable to the levels achieved by IV administration of 33.5 μmoles/kg of 8-aminoguanine. Each dose of inosine or guanosine was administered for 30 min and timed collections of urine were obtained from the left ureter between 10 and 30 min after initiating a given dose of inosine or guanosine. Inosine significantly increased urine volume (P=0.0039; Fig. 2A), sodium excretion (P=0.0137; Fig. 2C) and glucose excretion (P=0.0059, Fig. 2E); whereas guanosine had little or no effect on urine volume (Fig. 2B), sodium excretion (Fig. 2D) or glucose excretion (Fig. 2F). Neither inosine (Fig. S3A) nor guanosine (Fig. S2B) affected potassium excretion. We also examined the effects of intrarenal infusions of inosine on renal excretory function in rats pretreated with IV 8-aminoguanine (33.5 μmoles/kg) to increase basal levels of endogenous inosine. As expected, 8-aminoguanine increased urine volume (P=0.0473, Fig. S4A), sodium excretion (P=0.0074, Fig. S4B) and glucose excretion (P=0.0252, Fig. S4C), and tended to decrease potassium excretion (Fig. S4D). In rats pretreated with 8-aminoguanine, intrarenal artery infusions of inosine (0.01, 0.1 and 1 μmol/kg/min) did not affect, relative to the 8-aminoguanine baseline, urine volume (Fig. S4A), sodium excretion (Fig. S4B), glucose excretion (Fig. S4C) or potassium excretion (Fig. S4D). If hypoxanthine decreases renal excretory function, decreases in hypoxanthine levels could theoretically participate in the ability of 8-aminoguanine to increase renal excretory function. Therefore, we also examined the effects of intrarenal artery infusions of hypoxanthine on renal excretory function both in naïve and 8-aminoguanine-pretreated rats. In naïve and 8-aminoguanine-pretreated rats, at the highest dose, hypoxanthine actually increased urine volume (P=0.0076, Fig. S5A; P=0.0008, Fig. S6A, respectively) and sodium excretion (P=0.0002, Fig. S5B; P=0.0005, S6B, respectively), but did not affect glucose excretion (Fig. S5C and S6C, respectively). Hypoxanthine did not affect potassium excretion in naïve rats (Fig. S5D) but did increase potassium excretion in 8-aminoguanine-pretreated rats (P=0.0003, Fig. S6D). As expected, 8-aminoguanine pretreatment increased urine volume (P=0.0011, Fig. S6A), sodium excretion (P=0.0005, Fig. S6B) and glucose excretion (P=0.0161, Fig. S6C), and tended to decrease potassium excretion (Fig. S6D). Hemodynamically, rats were stable throughout the protocol. At the end of the experiments, MABPs (mm Hg; mean ± SEM) were similar in the five groups (inosine group, 121 ± 8.5; guanosine group, 111 ± 7.5; hypoxanthine group, 122 ± 5.2; inosine group pretreated with 8-aminoguanine, 108 ± 5.3; and hypoxanthine group pretreated with 8-aminoguanine, 114 ± 6.4). Also, RBFs (ml/min; mean ± SEM) were similar in the five groups (inosine group, 10.1 ± 0.7; guanosine group, 10.3 ± 1.2; hypoxanthine group, 9.1 ± 0.7; inosine group pretreated with 8-aminoguanine, 9.1 ± 1.4; and hypoxanthine group pretreated with 8-aminoguanine, 9.9 ± 0.9).
Figure 2. Effects of intrarenal artery infusions of inosine and guanosine on renal excretory function.
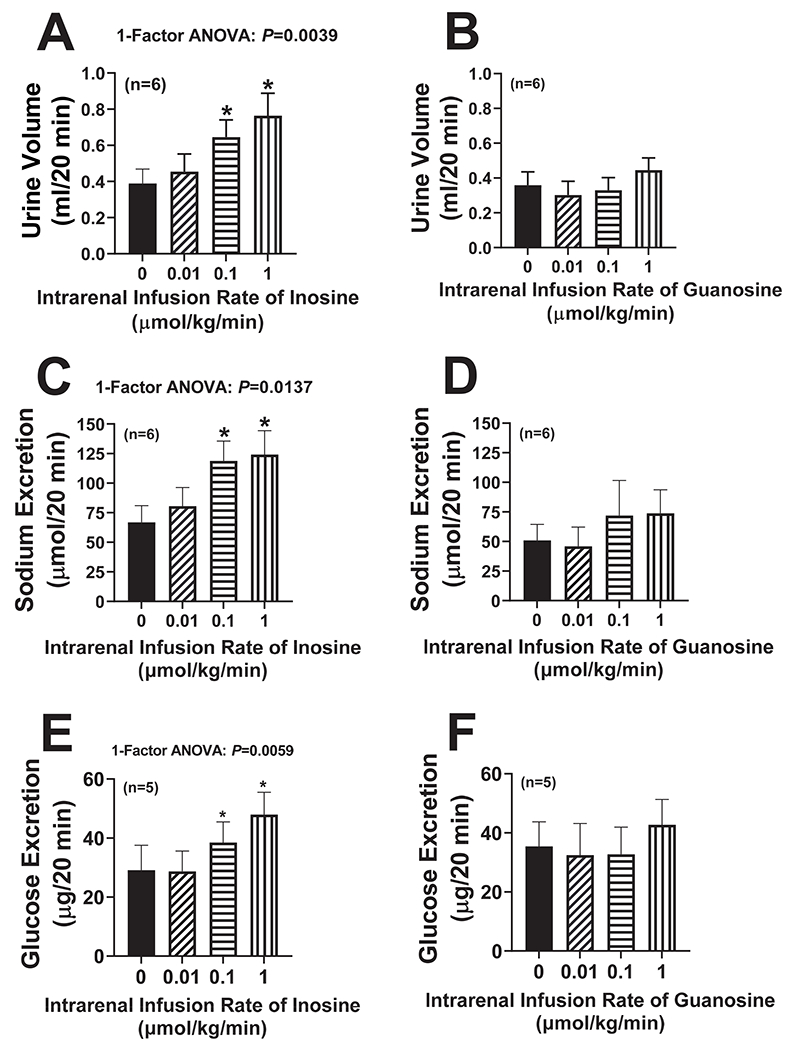
Inosine (A,C,E) or guanosine (B,D,F) was infused directly into the renal artery of anesthetized rats at increasing doses [0 (vehicle only; basal), 0.01, 0.1 and 1 μmol/kg/min]. Each dose of inosine or guanosine was administered for 30 min, timed collections of urine were obtained from the ureter between 10 and 30 min after initiating a given dose of inosine or guanosine, and urine volumes (A,B) and excretion rates of sodium (C,D) and glucose (E,F) were determined. Values are means and SEMs for the indicated sample size (n). ANOVA, analysis of variance; *P<0.05 vs 0 (vehicle; basal).
Protocol 3. Effects of 8-aminoguanine on renal excretory function in wild-type Dahl salt-sensitive rats (WT Dahl SS rats) versus Dahl salt sensitive rats with knockout of either A1, A2A or A2B receptors (A1-KO Dahl SS, A2A-KO Dahl SS and A2B-KO Dahl SS rats, respectively).
Our objectives here were to determine whether 8-aminoguanine has natriuretic effects in a model of sodium retention and whether the renal excretory effects are mediated by adenosine receptors. Here, male and female WT, A1-KO, A2A-KO and A2B-KO Dahl SS rats were placed on a high salt diet (4% NaCl) for 1 week. Next, rats were anesthetized, and timed urine collections were obtained from the left ureter from 0-30 min (Period 1), 40-70 min (Period 2) and 85-115 min (Period 3) into the protocol. Rats received an IV injection of 8-aminoguanine (33.5 μmoles/kg) immediately after Period 1. In WT, A1-KO and A2A-KO Dahl SS rats, 8-aminoguanine increased urine volume (Fig. 3A, S7A and S7B, respectively; P=0.0168, P=0.0757 (near significant) and P=0.0082, respectively), sodium excretion (Fig. 3C, S7C and S7D, respectively; P=0.0350, P=0.0330 and P=0.0132, respectively) and glucose excretion (Fig. 3E, S7E and S7F, respectively; P=0.0003, P=0.0198 and P=0.0006, respectively). By contrast, in A2B-KO Dahl SS rats, 8-aminoguanine did not affect urine volume (Fig. 3B), sodium excretion (Fig. 3D) or glucose excretion (Fig. 3F). The 8-aminoguanine-induced changes (Period 3 minus Period 1) in urine volume, sodium excretion and glucose excretion for the four groups were calculated and compared. Notably, 8-aminoguanine induced similar changes in urine volume (Fig. S8A), sodium excretion (Fig. S8B) and glucose excretion (Fig. S8C) in WT, A1-KO and A2A-KO Dahl SS rats. By contrast, in A2B-KO Dahl SS rats, 8-aminoguanine-induced changes in urine volume (Fig. S8A), sodium excretion (Fig. S8B) and glucose excretion (Fig. S8C) were significantly suppressed relative to WT-KO Dahl SS rats. Indeed, in A2B-KO Dahl SS rats, the 8-aminoguanine-induced changes in urine volume (Fig. S8A) and sodium excretion (Fig. S8B) were abolished. 8-Aminoguanine decreased potassium excretion similarly in all four genotypes (Fig. S8D). Hemodynamically, rats were stable throughout the protocol. However, as previously reported,6 MABPs differed among the four genotypes. At the end of the experiments, MABPs (mm Hg; mean ± SEM) were: WT, 150 ± 5; A1-KO, 112 ± 5.4; A2A-KO, 140 ± 12; and A2B-KO, 115 ± 8; and RBFs (ml/min; mean ± SEM) were WT, 4.3 ± 0.9; A1-KO, 5.1 ± 0.8; A2A-KO, 4.9 ± 0.7; and A2B-KO 7.7 ± 0.9.
Figure 3. Effects of 8-aminoguanine in wild-type Dahl salt-sensitive rats (WT Dahl SS rats) versus Dahl salt sensitive rats with knockout of A2B receptors (A2B-KO Dahl SS rats).
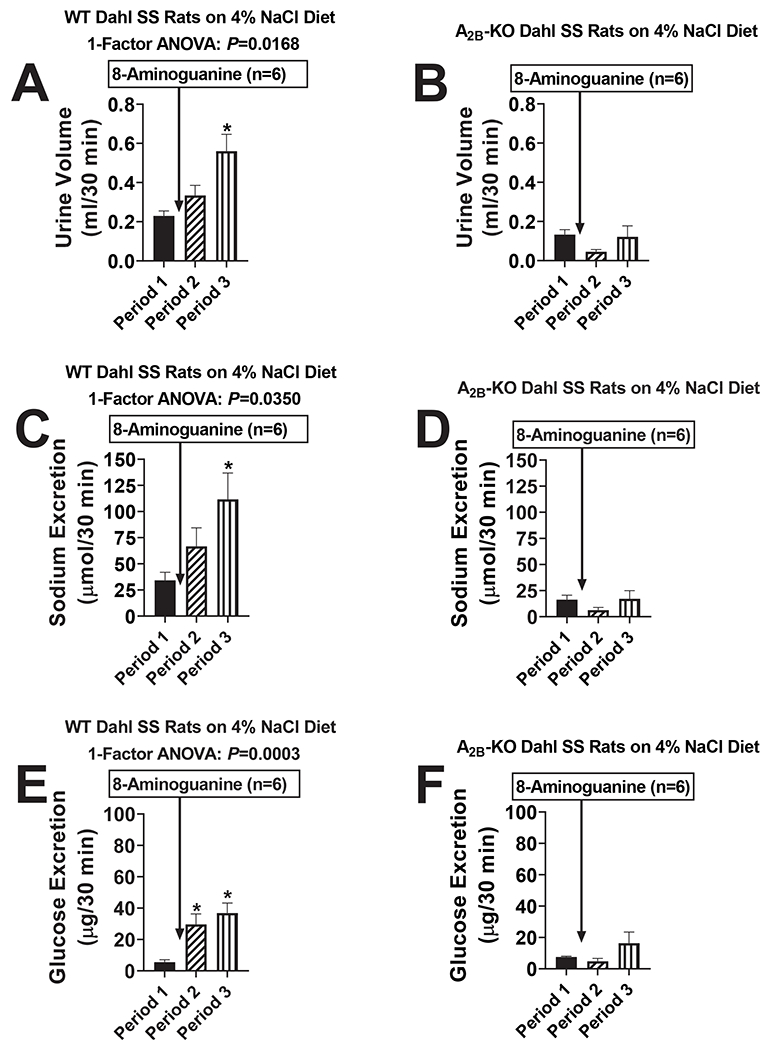
WT Dahl SS rats (A,C,E) and A2B-KO Dahl SS rats (B,D,F) were placed on a high salt diet (4% NaCl) for 1 week. Next, rats were anesthetized, and timed urine collections were obtained from the ureter from 0-30 min (Period 1), 40-70 min (Period 2) and 85-115 min (Period 3) into the protocol. Rats received an IV injection of 8-aminoguanine (33.5 μmoles/kg) immediately after Period 1. Urine volumes (A,B) and sodium (C,D) and glucose (E,F) excretion rates were determined. Values are means and SEMs for the indicated sample size (n). ANOVA, analysis of variance; *indicates P<0.05 vs Period 1.
Protocol 4. Effects of intrarenal artery infusions of inosine on renal excretory function in wild-type Dahl salt-sensitive rats (WT Dahl SS rats) versus Dahl SS rats with knockout of A2B receptors (A2B-KO Dahl SS rats).
Since A2B receptors appear to mediate the renal excretory effects (with the exception of potassium excretion) of 8-aminoguanine and since inosine appears to be involved in the renal excretory effects of 8-aminoguanine, here we sought to determine whether the renal excretory effects of inosine are mediated by A2B receptors. Inosine was infused at increasing doses [0 (basal), 0.01, 0.1 and 1 μmol/kg/min] directly into the renal artery of either anesthetized WT Dahl SS or A2B-KO Dahl SS rats. Each dose of inosine was administered for 30 min and timed collections of urine were obtained from the left ureter between 10 and 30 min after initiating a given dose of inosine. In WT Dahl SS rats, inosine significantly increased urine volume (P=0.0002; Fig. S9A) and sodium excretion (P=0.0038; Fig. S9C). By contrast, in A2B-KO Dahl SS rats, direct intrarenal artery infusions of inosine did not alter either urine volume (Fig. S9B) or sodium excretion (Fig. S9D). Inosine did not affect potassium excretion in either WT or A2B-KO Dahl SS rats, and the stimulatory effects of inosine on glucose excretion were abolished in A2B-KO Dahl SS rats (data not shown). Hemodynamically, rats were stable throughout the protocol. At the end of the experiments, MABPs (mm Hg; mean ± SEM) were similar in the two groups (WT Dahl SS rats, 124 ± 4; A2B-KO Dahl SS rats, 126 ± 5).
Protocol 5. Effects of 8-aminoguanine on renal excretory function and renal medullary blood flow (MBF) in naïve rats vs. rats pretreated with an A2A receptor antagonist, an A2B receptor antagonist or both.
To further test the role of adenosine receptors in the renal effects of 8-aminoguanine, we examined the effects of adenosine receptor antagonists on the renal actions of 8-aminoguanine. Anesthetized Sprague Dawley rats were pretreated intravenously with either vehicle, 10 mg/kg of ZM 241385 (selective A2A receptor antagonist9), 30 mg/kg of PSB 1115 (selective A2B receptor antagonist10) or ZM 241385 plus PSB 1115, and 15 min later timed (30 min) urine collections were obtained from the left ureter. Next, rats received an IV injection of 8-aminoguanine (33.5 μmoles/kg), and after approximately 1 hr 30-min urine collections were again obtained. As expected, in naïve rats 8-aminoguanine increased urine volume (P=0.0195; Fig. S10A) and sodium excretion (P=0.0055; Fig. S10B). Notably, in naïve rats 8-aminoguanine also increased MBF (P=0.0216; Fig. 4A). In rats pretreated with an A2A antagonist, 8-aminoguanine still increased urine volume (P=0.0485; Fig. S10C), tended to increase sodium excretion (P=0.0779; Fig. S10D) and still increased MBF (P=0.0320; Fig. 4B). Treatment of rats with an A2B antagonist abolished the effects of 8-aminoguanine on urine volume (Fig. S10E), sodium excretion (Fig. S10F) and MBF (Fig. 4C). Co-pretreatment of rats with both an A2B antagonist plus an A2A antagonist also abolished the effects of 8-aminoguanine on urine volume (Fig. S10G), sodium excretion (Fig. S10H) and MBF (Fig. 4D). The 8-aminoguanine-induced changes in urine volume, sodium excretion and MBF were calculated and compared. The changes in urine volume (Fig. S11A) and sodium excretion (Fig. S11B) were not different in naïve vs. A2A antagonist-treated rats; however, pretreatment with an A2B antagonist abolished the 8-aminoguanine-induced changes in urine volume (P=0.0006; Fig. S11A) and sodium excretion (P=0.0462; Fig. S11B). Likewise, pretreatment with the combination of an A2B plus A2A antagonist abolished the 8-aminoguanine-induced changes in urine volume (P=0.0124; Fig. S11A) and sodium excretion (P=0.0048; Fig. S11B). The 8-aminoguanine-induced increases in MBF were similar in naïve rats vs. rats pretreated with an A2A antagonist but were reduced in rats pretreated with an A2B antagonist or the combination of an A2B plus A2A antagonist (P=0.0298; Fig. S11C). 8-Aminoguanine reduced potassium excretion in all groups (Fig. S12A–S12D) and the 8-aminoguanine-induced reductions in potassium excretion were similar among the four groups (Fig. S12E). Hemodynamically, rats were stable throughout the protocol. At the end of the experiments, MABPs (mm Hg; mean ± SEM) were similar in the four groups (naïve group, 113 ± 4; A2A antagonist pretreated group, 117 ± 4; A2B antagonist pretreated group, 126 ± 6; combination A2A plus A2B antagonist pretreated group, 115 ± 4).
Figure 4. Effects of 8-aminoguanine on medullary blood flow (MBF) in rats pretreated with an A2A receptor antagonist, an A2B receptor antagonist or both an A2A and A2B receptor antagonist.
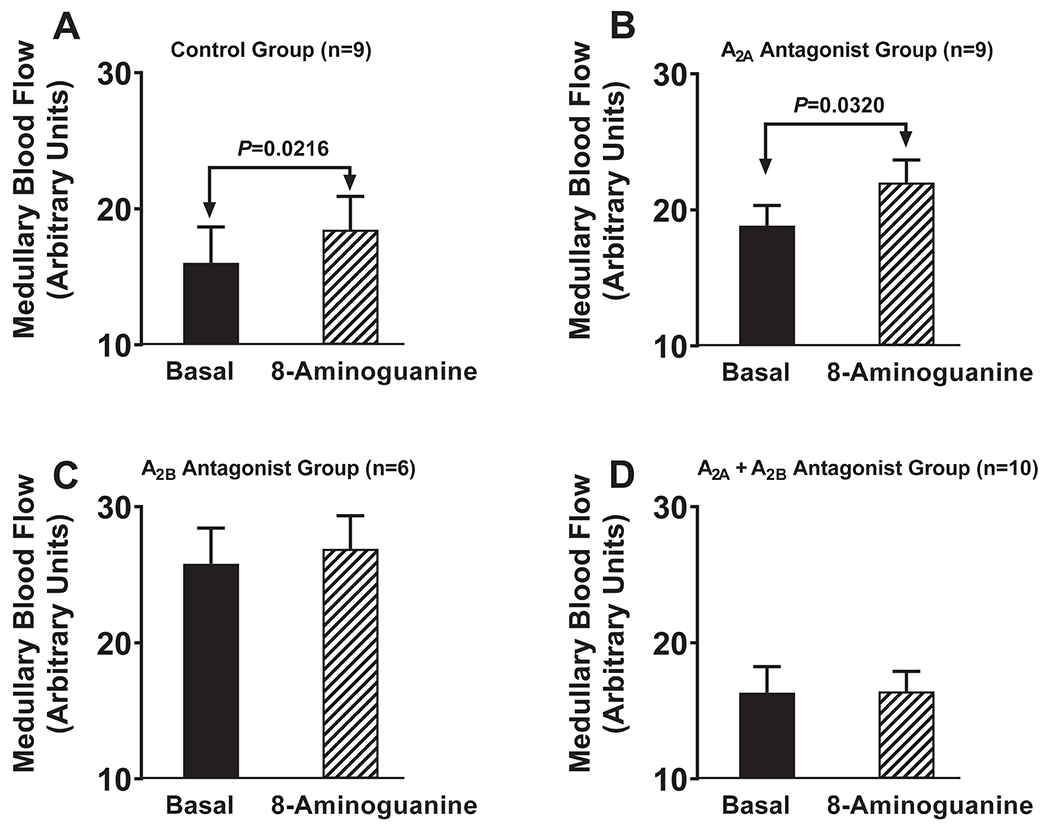
Anesthetized rats were pretreated intravenously with either vehicle [A; Control Group], 10 mg/kg of ZM 241385 (B; A2A Antagonist Group), 30 mg/kg of PSB 1115 (C; A2B Antagonist Group) or ZM 241385 plus PSB 1115 (D; A2A + A2B Antagonist Group), and 15 min later MBF was averaged over 30 min (Basal). Next, rats received an IV injection of 8-aminoguanine (33.5 μmoles/kg), and after approximately 1 hr MBF was averaged over 30 min (8-Aminoguanine). P-values are from paired t-tests. Values are means and SEMs for the indicated sample size (n).
Protocol 6. Effects of BAY 60-6583 on renal excretory function and MBF.
Because the effects of 8-aminoguanine appeared to be mediated by A2B receptors, we next tested the effects of the selective A2B receptor agonist, BAY 60-6583,11 on renal excretory function and MBF. To avoid systemic effects, BAY 60-6583, dissolved in 0.9% saline, was infused directly into the renal artery of anesthetized rats at increasing doses [0 (0.9% saline only; basal), 1 and 3 nmol/kg/min]. Each dose of Bay 60-6538 was administered for 40 min and timed collections of urine were obtained from the left ureter between 10 and 40 min after initiating a given dose of BAY 60-6538. Similar to 8-aminoguanine, BAY 60-6538 increased urine volume (P<0.0001; Fig. 5A), sodium excretion (P<0.0001; Fig. 5B) and MBF (P=0.0174; Fig. 5D). In contrast to 8-aminoguanine, BAY 60-6538 increased, rather than decreased, potassium excretion (P=0.0257; Fig. 5C).
Figure 5. Effects of BAY 60-6583 on renal excretory function.
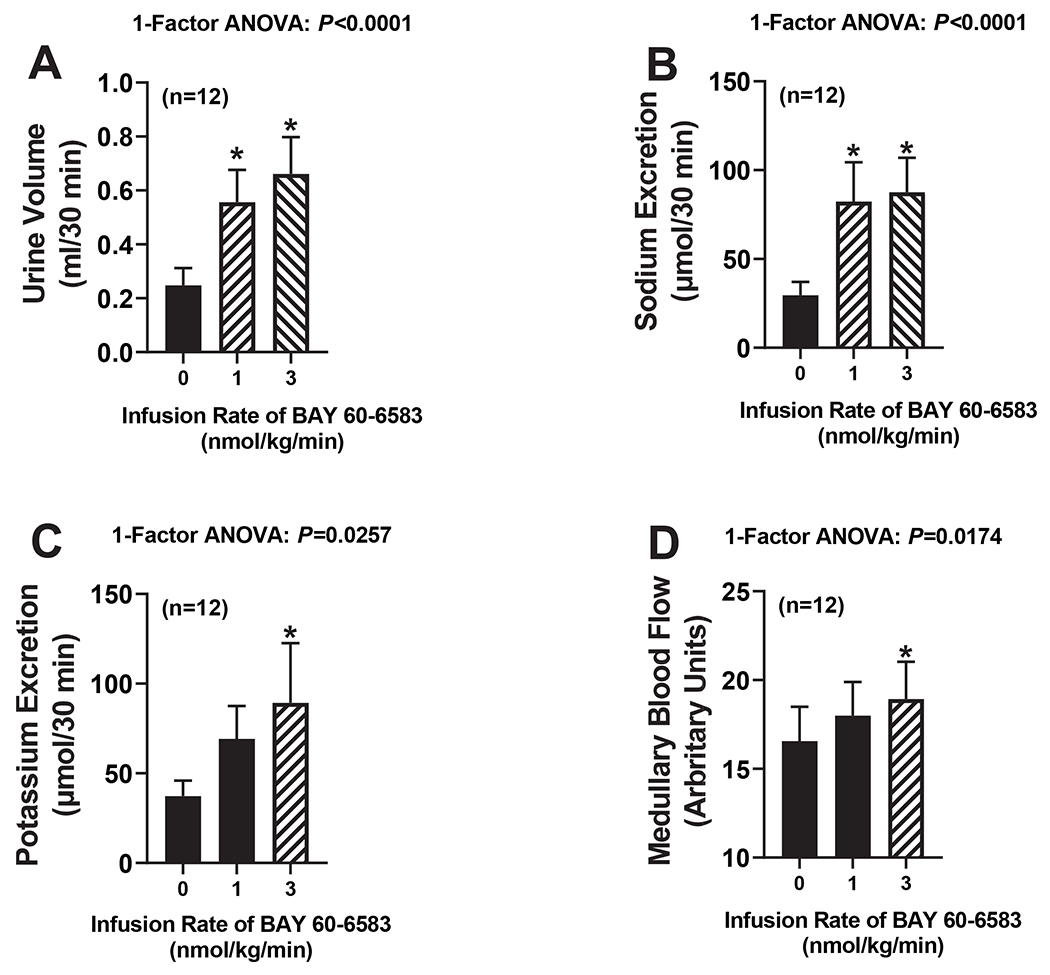
BAY 60-6583 was infused directly into the renal artery of anesthetized rats at increasing doses [0 (vehicle; basal), 1 and 3 nmol/kg/min]. Each dose of Bay 60-6538 was administered for 40 min and timed collections of urine were obtained from the ureter between 10 and 40 min after initiating a given dose of BAY 60-6538, and urine volumes (A) and excretion rates of sodium (B) and potassium (C) were determined. Also shown are medullary blood flows during the infusions (D). Values are means and SEMs for the indicated sample size (n). ANOVA, analysis of variance; *indicates P<0.05 vs 0 (vehicle; basal).
Protocol 7. Effects of 8-aminoguanine and forodesine on 3’,5’-cAMP and inosine in wild-type (WT) vs. A2B-KO renal microvascular smooth muscle cells (RMSMCs).
RMSMCs were obtained from WT and A2B-KO Dahl SS rats and were grown to confluence in 6-well plates. Cells were incubated for 30 min with vehicle or forskolin to sensitize adenylyl cyclase to receptor-mediated activation. Experiments were performed in the absence and presence of either 8-aminoguanine (100 μmol/L) or forodesine (10 μmol/L; alternative PNPase inhibitor). Medium was collected and analyzed for 3’,5’-cAMP and inosine by UPLC-MS/MS. In WT RMSMCs, addition of either 8-aminoguanine (P=0.0003; Fig. S13A) or forodesine (P=0.0004; Fig. S13A) to forskolin-treated cells significantly increased levels of 3’,5’-cAMP. However, in A2B-KO RMSMCs, neither 8-aminoguanine (Fig. S13B) nor forodesine (Fig. S13B) affected 3’,5’-cAMP levels in forskolin-treated cells. In both WT (Fig. S11C) and A2B-KO (Fig. S11D) RMSMCs both 8-aminoguanine and forodesine increased inosine levels.
Protocol 8. Effects of inosine on A2B receptor signaling induced by 5’-N-ethylcarboxamidoadenosine (NECA).
Next, we sought to determine whether inosine is a positive allosteric modulator of A2B receptors. Human A2B receptors were expressed in HEK293 cells and stimulated by the A2B receptor agonist NECA.12 Receptor activation was monitored by measuring intracellular 3’,5’-cAMP (as an index of adenylyl cyclase activity) using a highly sensitive assay (homogeneous time resolved fluorescence). The concentration-response curve to NECA (performed in duplicate) was similar in cells incubated without inosine (Fig. 6A) versus with either 30 μmol/L (Fig. 6B) or 100 μmol/L (Fig. 6C) of inosine; thus, indicating that inosine is not a positive allosteric modulator of A2B receptors. Although inosine did not act as a positive allosteric modulator of A2B receptors, inosine per se activated adenylyl cyclase (see shift in basal adenylyl cyclase activity in Fig. 6B and Fig. 6C versus Fig. 6A). Fig. 6D directly compares the effects of inosine [0 (basal), 30 and 100 μmol/L] on activation of adenylyl cyclase in HEK293 cells expressing A2B receptors. Inosine markedly and significantly (P<0.0001) increased adenylyl cyclase activity.
Figure 6. Effects of inosine on A2B receptor signaling.
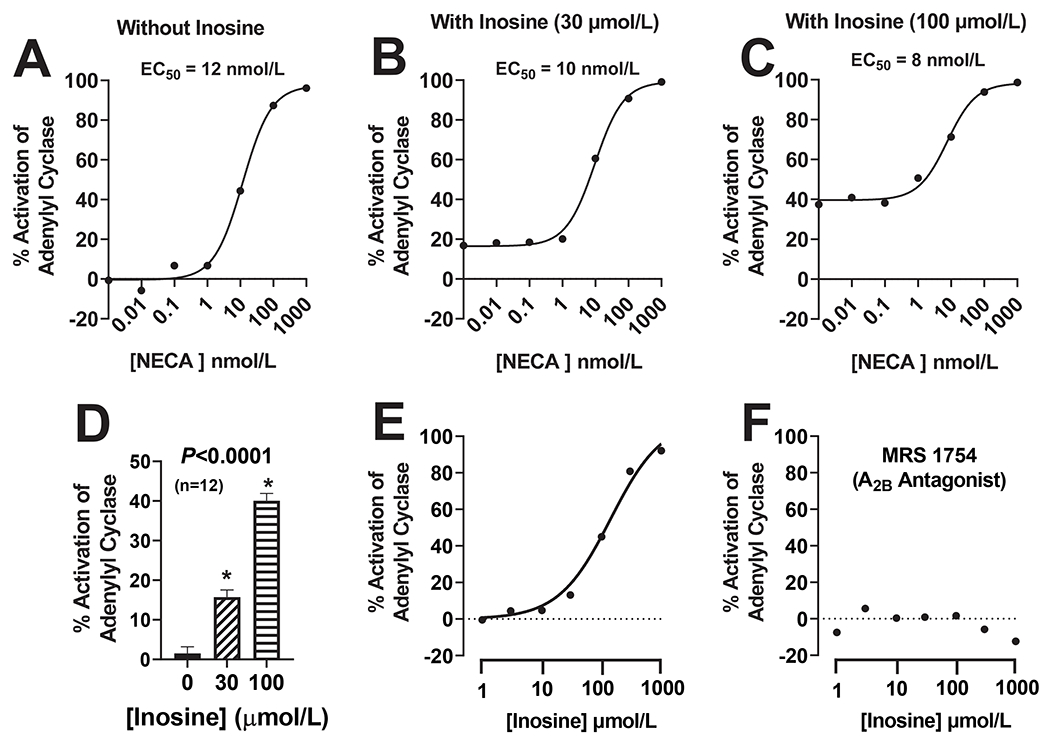
Human A2B receptors were expressed in HEK293 cells and intracellular 3’,5’-cAMP was measured using a highly sensitive assay (homogeneous time resolved fluorescence). In some experiments (in duplicate), cells were treated with a range of concentrations of the A2B receptor agonist 5’-N-ethylcarboxamidoadenosine (NECA) without (A) or with inosine [either 30 (B) or 100 (C) μmol/L]. Also shown are the shifts in basal levels of 3’,5’-cAMP induced by inosine at 30 and 100 μmol/L (D). In additional experiments (in duplicate), cells were treated with a range of concentrations of inosine in the absence (E) and presence (F) of MRS 1754 (1 μmol/L; highly selective A2B receptor antagonist). ANOVA, analysis of variance; *indicates P<0.05 vs 0 (no inosine). In (D) values are means and SEMs for the indicated sample size (n).
Protocol 9. Concentration-response relationship for agonism of A2B receptors by inosine.
Because inosine per se appeared to activate A2B receptors, next we examined the full concentration-response relationship of inosine on adenylyl cyclase activity in HEK293 cells expressing human A2B receptors. These experiments were performed both in the presence and absence of MRS 1754 (1 μmol/L; highly selective A2B receptor antagonist13). Inosine caused a concentration-dependent stimulation of adenylyl cyclase with an activation threshold of 3 to 10 μmol/L (Fig. 6E). The ability of inosine to activate adenylyl cyclase was abolished by MRS 1754 (Fig. 6F) suggesting that the activation of adenylyl cyclase by inosine was mediated by activation of A2B receptors.
DISCUSSION
Previously, we observed that 8-aminoguanine, a naturally occurring 8-aminopurine,3 rapidly alters renal excretory function.1 Specifically, 8-aminoguanine increases urine volume and sodium and glucose excretion while decreasing potassium excretion.1 The diuretic, natriuretic and glucosuric effects of 8-aminoguanine appear to be mediated by inhibition of PNPase,5 while the antikaliuretic actions of 8-aminoguanine are independent of PNPase inhibition.4,5 The goal of the present study was to investigate how inhibition of PNPase by 8-aminoguanine induces diuresis, natriuresis and glucosuria.
Our first experimental series, which employed a large sample size (n=12), confirmed our conclusion that 8-aminoguanine is a diuretic, natriuretic, glucosuric and antikaliuretic agent. We view these results as critically important because they demonstrate the reproducibility of our previous findings. In addition to confirming our previous results, we used this opportunity to examine, via renal microdialysis combined with UPLC-MS/MS, how a diuretic/natriuretic/glucosuric dose (i.e., an effective dose) of 8-aminoguanine affects renal interstitial levels of purines. The in vivo recovery rate of an analyte into microdialysate depends on a number of conditions14 and is a fraction of the actual concentration of the analyte in the interstitium14. Thus, microdialysate levels of purines should not be interpreted as absolute levels of purines in the interstitial compartment. Nonetheless, using a standardized protocol, microdialysate levels of purines provide an accurate assessment of the directional and fold changes in interstitial purine levels induced by an intervention.
Importantly, our first experimental series revealed that an effective IV dose of 8-aminoguanine increased 8-aminoguanine levels in the renal microdialysate to approximately 30 μmol/L. Of note, we recently showed that the Ki of 8-aminoguanine for recombinant human PNPase is approximately 2.8 μmol/L.4 Thus, in this study the renal interstitial levels of 8-aminoguanine after administration of an effective dose of 8-aminoguanine were undoubtably sufficient to inhibit PNPase. Consistent with this conclusion, an effective dose of 8-aminoguanine increased by several fold renal interstitial levels of PNPase substrates (i.e., inosine and guanosine), yet decreased renal interstitial levels of PNPase products (i.e., hypoxanthine and guanine). 8-Aminoguanine had no detectable effects on adenosine levels in the renal cortex and caused only a slight and brief increase in adenosine in the renal medulla. Mammalian PNPase exists in several different multimeric complexes,15 most of which do not process adenosine to adenine.15 However, there is evidence in rats that under some conditions a form of PNPase can convert adenosine to adenine.16 There is also evidence that guanosine can increase extracellular levels of adenosine by blocking the disposition of adenosine from the extracellular compartment.17 Nonetheless, given the limited (in location, magnitude and time) effects of 8-aminoguanine on renal interstitial levels of adenosine, it is unlikely that the renal excretory effects of PNPase inhibition are mediated by adenosine.
Because 8-aminoguanine caused large fold increases in inosine and guanosine, we entertained the hypothesis that the diuretic/natriuretic/glucosuric effects of 8-aminoguanine were mediated by one or both of these purines. To test this, we infused directly into the renal artery doses of inosine or guanosine that provided renal microdialysate levels that were comparable to those achieved by an effective dose of 8-aminoguanine. Here we noted that inosine, but not guanosine, increased urine volume, sodium excretion and glucose excretion, without affecting potassium excretion. We view this as strong evidence that 8-aminoguanine induces diuresis, natriuresis and glucosuria by elevating, as a consequence of PNPase inhibition, renal interstitial levels of inosine. That potassium excretion was not affected by inosine is consistent with our previous findings that PNPase inhibition does not mediate the antikaliuretic effects of 8-aminoguanine.5 If inosine mediates the effects of 8-aminoguanine on renal excretory function, exogenous inosine should have little or no effect on renal excretory function in animals pretreated with an effective dose of 8-aminoguanine that elevates endogenous inosine levels. Consistent with this prediction, inosine did not affect renal excretory function in rats pretreated with an effective dose of 8-aminoguanine.
Because hypoxanthine induces oxidative stress18 that could compromise renal excretory function,19 it is conceivable that the reduction in renal hypoxanthine levels by 8-aminoguanine contributes to the renal excretory effects of 8-aminoguanine. If so, exogenous hypoxanthine should decrease renal excretory function. However, intrarenal artery infusions of hypoxanthine did not reduce renal excretory function in naïve rats or rats pretreated with 8-aminoguanine to reduce endogenous hypoxanthine levels. Thus, it is unlikely that 8-aminoguanine’s effects on renal excretory function are mediated by reductions in renal hypoxanthine levels.
Next, we hypothesized that adenosine receptors mediate the renal excretory effects of 8-aminoguanine. This hypothesis was motivated by published evidence that inosine may directly, or indirectly, activate A1 20–22, A2A23,24 or A2B25–27 receptors. To test this hypothesis, we examined the effects of 8-aminoguanine on renal excretory function in wild-type, A1-KO, A2A-KO and A2B-KO rats that were generated by Dr. Aron M. Geurts (Department of Physiology, Medical college of Wisconsin), with colonies maintained at the University of Pittsburgh.6 These knockout rats were generated on a Dahl SS background, which provided the opportunity to not only test the role of adenosine receptors in the renal effects of 8-aminoguanine, but also to test whether 8-aminoguanine is an effective diuretic/natriuretic in an animal model that has a genetic predisposition to retain sodium. Importantly, 8-aminoguanine was an effective diuretic/natriuretic/glucosuric/antikaliuretic agent in wild-type Dahl SS rats on a 4% NaCl diet. This confirms the utility of 8-aminoguanine as a diuretic/natriuretic in a challenging model of salt retention, thus underscoring the potential of 8-aminoguanine in salt-retaining states. Equally important, we observed that renal excretory responses to 8-aminoguanine were similar in wild-type vs. A1-KO vs. A2A-KO rats; however, in A2B-KO rats, 8-aminoguanine did not increase urine volume or sodium or glucose excretion. As expected, however, the antikaliuretic effects of 8-aminoguanine were maintained in A1-KO, A2A-KO and A2B-KO rats. To confirm the mechanistic links between PNPase inhibition, increased inosine levels and A2B receptor activation, we also compared the effects of intrarenal artery infusions of inosine in wild-type vs. A2B-KO rats. Notably, inosine increased renal excretory function in wild-type, but not A2B-KO, rats.
To further test the concept that A2B receptors mediate the effects of 8-aminoguanine on renal excretory function, we also examined the effects of 8-aminoguanine on renal excretory function in Sprague Dawley rats before and after administration of a selective A2A receptor antagonist, a selective A2B receptor antagonist or both. Consistent with the results in knockout rats, the ability of 8-aminoguanine to induce diuresis and natriuresis was abolished by blockade of A2B, but not A2A, receptors; however, once again the antikaliuretic effects of 8-aminoguanine were not affected by suppressing A2B receptors.
Although compelling, our proposed mechanistic pathway of PNPase inhibition leading to increased renal interstitial levels of inosine with subsequent activation of A2B receptors was still incomplete in two aspects. First, there was the question as to whether 8-aminoguanine and inosine activate A2B receptor signaling. Indeed, the literature regarding the effects of inosine on A2 receptors is inconsistent. For example, Fredholm et al. reported that inosine did not activate either A2A or A2B receptors expressed in CHO cells;20 however, Welihinda et al. reported that inosine activated A2A, but not A2B, receptors expressed in CHO-K1 cells and that this effect of inosine was enhanced by positive allosteric modulation.23,24 Valada et al. observed that inosine did not displace radioligand binding to the orthosteric site of A2A (3H-CGS21680) or A2B (3H-NECA) receptors expressed in CHO cells.28 By contrast, inosine was found to attenuate spontaneous activity in the rat neurogenic bladder via an A2B receptor-mediated pathway,25,26 and da Rocha Lapa et al. reported that A2B receptors mediate in part the anti-inflammatory effects of inosine.27 The complexity of A2B receptor pharmacology is underscored by the recent findings of Voss et al. showing that A2B receptors couple to multiple G-proteins in an agonist specific manner,29 which suggests that inosine-induced activation of A2B receptors may depend on the precise G-protein environment of the receptor.
In the present study, we tested, using a model system in which A2B receptors were expressed in HEK293 cells, the ability of inosine to function as a positive allosteric modulator of A2B receptors. In this system, inosine did not alter the concentration-response (i.e., adenylyl cyclase activity) relationship to NECA but did per se activate adenylyl cyclase. To follow up on this observation, we examined the full concentration-response relationship to inosine on A2B receptor-mediated activation of adenylyl cyclase. Here we observed that inosine at a threshold of 3 to 10 μmol/L stimulated adenylyl cyclase, and at higher concentrations fully activated the A2B-adenylyl cyclase axis. This conclusion was confirmed by the observation that a selective A2B receptor antagonist abolished the ability of inosine to activate adenylyl cyclase. Further, we observed that 8-aminoguanine and forodesine (a structurally different PNPase inhibitor) increased extracellular levels of inosine and stimulated adenylyl cyclase activity in RMSMCs isolated from wild-type rats. In RMSMCs lacking A2B receptors, both 8-aminoguanine and forodesine increased inosine levels, yet both compounds failed to activate adenylyl cyclase in A2B-KO RMSMCs. These finding support the conclusion that PNPase inhibition increases inosine levels which then activate the A2B-adenylyl cyclase axis. Although it is possible that inosine somehow indirectly activates the A2B-adenylyl cyclase axis; it is also conceivable that inosine activates A2B receptors by direct binding to A2B receptors. For example, there could be more than one independent agonist orthosteric binding site on A2B receptors such that inosine does not compete with NECA binding but still activates the A2B receptor via its own orthosteric binding domain.
Another “knowledge gap” in our proposed mechanism relates to the question of how activation of A2B receptors could increase renal excretory function. Here, we proposed that 8-aminoguanine via A2B receptor activation, leads to an increase in MBF, which in turn increases renal excretory function. This hypothesis is based on reports by: 1) Zou et al. who showed that A2 receptors increase MBF and sodium excretion;30 2) Grenz et al. who demonstrated that within the kidney A2B receptors are expressed predominantly in the renal vasculature;31 3) Feng and Navar32 and Cooper et al.33 who showed that A2B receptors are the A2 receptor subtype that mediates renal vasodilation; and 4) reports by many investigators that decreases or increases in MBF decrease or increase, respectively, renal excretory function.34–36 In support of this aspect of our proposed mechanism, we observed that 8-aminoguanine increases MBF and that this response is blocked by antagonism of A2B, but not A2A, receptors. Our hypothesis is further corroborated by the finding that intrarenal artery infusions of BAY 60-6583 (a selective partial agonist for A2B receptors) increase urine volume, sodium excretion and MBF.
It is possible that changes in MBF do not fully account for the renal excretory effects of 8-aminoguanine and that A2B receptor activation engages direct tubular mechanisms that contribute to 8-aminoguanine-induced effects on urine volume and sodium and glucose excretion. Although MBF does affect sodium excretion, and sodium and glucose reabsorption are linked in proximal tubules by SGLT2,37 a relationship between MBF and glucose excretion has not been established. Also, Rajagopal and Pao demonstrated that A2B receptors in renal inner medullary collecting duct epithelium promote chloride excretion via cystic fibrosis transmembrane conductance regulator chloride channels and suggested that this is a mechanism for enhancing urine NaCl excretion.38 In addition, Battistone et al. reported that A2B receptors stimulate vacuolar ATPase-dependent proton secretion in renal medullary type A intercalated cells.39 Together, the evidence suggests that increases in MBF may account for some, but not all, of the renal excretory effects of 8-aminoguanine. The mechanism by which 8-aminoguanine reduces potassium excretion remains unclear. 8-Aminoguanine, 8-aminoinosine, 8-aminohypoxanthine and 9-deazaguanine inhibit PNPase and induce diuresis, natriuresis and glucosuria, yet only 8-aminoguanine reduces potassium excretion.4,5 This indicates that the antikaliuretic effects of 8-aminoguanine are not due to inhibition of PNPase. We have observed that 8-aminoguanine is a weak inhibitor of Rac1 and that Nsc23766 (blocks activation of Rac1) mimics the antikaliuretic effects of 8-aminoguanine.5 Because Rac1 activates mineralocorticoid receptors,40,41 this mechanism could explain, in part, 8-aminoguanine’s antikaliuretic effects. However, since 8-aminoguanine is not a potent Rac1 inhibitor and the effects of Nsc23766 on potassium excretion could be due to off target actions of this Rac1 inhibitor, it is possible that 8-aminoguanine affects potassium excretion mostly by additional mechanisms that are yet to be discovered. These additional mechanism may also contribute to the effects of 8-aminoguanine on other aspects of renal excretory function.
Perspectives.
Taken together, the results of this investigation support the concept that inhibition of PNPase by 8-aminoguanine induces diuresis/natriuresis/glucosuria by increasing renal interstitial levels of inosine which, via A2B receptor activation, increases renal excretory function, perhaps in part by increasing MBF. Previously, we reported that 8-aminoguanine exerts antihypertensive activity in DOCA/salt-induced hypertension1; we have also found that chronic administration of 8-aminoguanine attenuates salt-induced hypertension and strokes in Dahl SS rats (unpublished results). Likely the mechanisms revealed here mediate, at least in part, the antihypertensive actions of 8-aminoguanine. We have also observed that 8-aminoguanine has beneficial effects in other diseases including the metabolic syndrome,42 pulmonary hypertension43, sickle cell disease44, age-associated bladder dysfunction,45 age-associated retinal degeneration46 and RHO-associated retinitis pigmentosa.46 Likely, the mechanisms of action of 8-aminoguanine in these other diseases will share some common elements.
Supplementary Material
Pathophysiological Novelty and Relevance.
What Is New?
8-Aminoguanine, a naturally occurring purine, increases renal interstitial levels of inosine and guanosine while suppressing renal interstitial levels of hypoxanthine and guanine. Thus 8-aminoguanine “rebalances” the renal interstitial purine metabolome. In this regard, the largest effect of 8-aminoguanine is to increase renal interstitial levels of inosine.
8-Aminoguanine reproducibly increases urine volume and sodium and glucose excretion; also 8-aminoguanine increases renal medullary blood flow and 3’,5’-cAMP production by renal microvascular smooth muscle cells. All of these effects of 8-aminoguanine are mediated by adenosine A2B receptors, which are well known to stimulate adenylyl cyclase.
Like 8-aminoguanine, inosine induces diuresis/natriuresis/glucosuria and activates the A2B receptor/adenylyl cyclase/3’,5’-cAMP pathway.
The totality of evidence indicates that 8-aminoguanine induces diuresis/natriuresis/glucosuria at least in part by inhibiting PNPase, which massively increases renal interstitial levels of inosine. In turn, inosine activates the A2B receptor/adenylyl cyclase/3’,5’-cAMP pathway, which augments renal medullary blood flow and renal excretory function.
What Is Relevant?
Administration of 8-aminoguanine, a naturally occurring purine, reproducibly increases urine volume and sodium and glucose excretion while conserving potassium.
8-Aminoguanine affects renal excretory function even in animals that are genetically predisposed to retain sodium and challenged by a high salt diet.
Clinical/Pathophysiological Implications?
8-Aminoguaine, a naturally occurring PNPase inhibitor, causes diuresis/natriuresis/glucosuria by “rebalancing” the renal interstitial purine metabolome in favor of inosine, thus resulting in A2B receptor activation that increases renal medullary blood flow.
8-Aminoguanine causes diuresis/natriuresis/glucosuria by a unique mechanism that may have implications for the treatment of not only hypertension but also a broad array of vascular diseases.
SOURCES OF FUNDING
The work was supported by the National Institutes of Health [HL109002 and DK079307].
Nonstandard Abbreviations and Acronyms.
- A1 receptor
adenosine receptor subtype A1
- A2A receptor
adenosine receptor subtype A2A
- A2B receptor
adenosine receptor subtype A2B
- A1-KO Dahl SS rat
Dahl salt-sensitive rat with adenosine subtype A1 receptors knocked out
- A2A-KO Dahl SS rat
Dahl salt-sensitive rat with adenosine subtype A2A receptors knocked out
- A2B-KO Dahl SS rat
Dahl salt-sensitive rat with adenosine subtype A2B receptors knocked out
- ANOVA
analysis of variance
- IV
intravenous
- MBF
medullary blood flow
- MABP
mean arterial blood pressure
- PE
polyethylene
- PNPase
purine nucleoside phosphorylase
- RBF
renal blood flow
- UPLC-MS/MS
ultra-performance liquid chromatography-tandem mass spectrometry
- WT Dahl SS rat
wild-type Dahl salt-sensitive rat
Footnotes
Disclosures: None
REFERENCES
- 1.Jackson EK, Gillespie DG, Mi Z. 8-Aminoguanosine and 8-aminoguanine exert diuretic, natriuretic, glucosuric, and antihypertensive activity. J Pharmacol Exp Ther. 2016;359:420–435. doi: 10.1124/jpet.116.237552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson EK, Mi Z. 8-Aminoguanosine exerts diuretic, natriuretic, and glucosuric activity via conversion to 8-aminoguanine, yet has direct antikaliuretic effects. J Pharmacol Exp Ther. 2017;363:358–366. doi: 10.1124/jpet.117.243758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson EK, Menshikova EV, Ritov VB, Gillespie DG, Mi Z. Biochemical pathways of 8-aminoguanine production in Sprague-Dawley and Dahl salt-sensitive rats. Biochem Pharmacol. 2022;201:115076. doi: 10.1016/j.bcp.2022.115076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson EK, Menshikova EV, Ritov VB, Mi Z, Birder LA. 8-Aminoinosine and 8-aminohypoxanthine inhibit purine nucleoside phosphorylase and exert diuretic and natriuretic activity. J Pharmacol Exp Ther. 2022. doi: 10.1124/jpet.122.001221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson EK, Mi Z, Kleyman TR, Cheng D. 8-Aminoguanine induces diuresis, natriuresis, and glucosuria by inhibiting purine nucleoside phosphorylase and reduces potassium excretion by inhibiting Rac1. J Am Heart Assoc. 2018;7:e010085. doi: 10.1161/jaha.118.010085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson EK, Gillespie DG, Mi Z, Cheng D. Adenosine receptors influence hypertension in Dahl salt-sensitive rats: Dependence on receptor subtype, salt diet, and sex. Hypertension. 2018;72:511–521. doi: 10.1161/hypertensionaha.117.10765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson EK, Kitsios GD, Lu MY, Schaefer CM, Kessinger CJ, McVerry BJ, Morris A, Macatangay BJC. Suppressed renoprotective purines in COVID-19 patients with acute kidney injury. Sci Rep. 2022;12:17353. doi: 10.1038/s41598-022-22349-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu X, Jackson EK. RACK1 regulates angiotensin II-induced contractions of SHR preglomerular vascular smooth muscle cells. Am J Physiol Renal Physiol. 2017;312:F565–F576. doi: 10.1152/ajprenal.00547.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keddie JR, Poucher SM, Shaw GR, Brooks R, Collis MG. In vivo characterisation of ZM 241385, a selective adenosine A2A receptor antagonist. Eur J Pharmacol. 1996;301:107–113. doi: 10.1016/0014-2999(96)00020-9 [DOI] [PubMed] [Google Scholar]
- 10.Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A, Müller CE. Antinociceptive effects of novel A2B adenosine receptor antagonists. J Pharmacol Exp Ther. 2004;308:358–366. doi: 10.1124/jpet.103.056036 [DOI] [PubMed] [Google Scholar]
- 11.Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, et al. Cardioprotection by ecto-5’-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/circulationaha.106.669697 [DOI] [PubMed] [Google Scholar]
- 12.Hinz S, Alnouri WM, Pleiss U, Müller CE. Tritium-labeled agonists as tools for studying adenosine A2B receptors. Purinergic Signal. 2018;14:223–233. doi: 10.1007/s11302-018-9608-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji X, Kim YC, Ahern DG, Linden J, Jacobson KA. [3H]MRS 1754, a selective antagonist radioligand for A2B adenosine receptors. Biochem Pharmacol. 2001;61:657–663. doi: 10.1016/s0006-2952(01)00531-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen DK, Davies MI, Lunte SM, Lunte CE. Pharmacokinetic and metabolism studies using microdialysis sampling. J Pharm Sci. 1999;88:14–27. doi: 10.1021/js9801485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bzowska A, Kulikowska E, Shugar D. Purine nucleoside phosphorylases: properties, functions, and clinical aspects. Pharmacol Ther. 2000;88:349–425. [DOI] [PubMed] [Google Scholar]
- 16.Jackson EK, Gillespie DG, Cheng D, Mi Z, Menshikova EV. Characterization of the N6-etheno-bridge method to assess extracellular metabolism of adenine nucleotides: detection of a possible role for purine nucleoside phosphorylase in adenosine metabolism. Purinergic Signal. 2020;16:187–211. doi: 10.1007/s11302-020-09699-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson EK, Cheng D, Jackson TC, Verrier JD, Gillespie DG. Extracellular guanosine regulates extracellular adenosine levels. Am J Physiol Cell Physiol. 2013;304:C406–C421. doi: 10.1152/ajpcell.00212.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Battelli MG, Polito L, Bortolotti M, Bolognesi A. Xanthine oxidoreductase-derived reactive species: Physiological and pathological effects. Oxid Med Cell Longev. 2016;2016:3527579. doi: 10.1155/2016/3527579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowley AW Jr., Abe M, Mori T, O’Connor PM, Ohsaki Y, Zheleznova NN. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol Renal Physiol. 2015;308:F179–197. doi: 10.1152/ajprenal.00455.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fredholm BB, Irenius E, Kull B, Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem Pharmacol. 2001;61:443–448. [DOI] [PubMed] [Google Scholar]
- 21.Hasko G, Kuhel DG, Nemeth ZH, Mabley JG, Stachlewitz RF, Virag L, Lohinai Z, Southan GJ, Salzman AL, Szabo C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol. 2000;164:1013–1019. [DOI] [PubMed] [Google Scholar]
- 22.de Oliveira ED, Schallenberger C, Bohmer AE, Hansel G, Fagundes AC, Milman M, Silva MD, Oses JP, Porciuncula LO, Portela LV, et al. Mechanisms involved in the antinociception induced by spinal administration of inosine or guanine in mice. Eur J Pharmacol. 2016;772:71–82. doi: 10.1016/j.ejphar.2015.12.034 [DOI] [PubMed] [Google Scholar]
- 23.Welihinda AA, Kaur M, Raveendran KS, Amento EP. Enhancement of inosine-mediated A2AR signaling through positive allosteric modulation. Cell Signal. 2018;42:227–235. doi: 10.1016/j.cellsig.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welihinda AA, Kaur M, Greene K, Zhai Y, Amento EP. The adenosine metabolite inosine is a functional agonist of the adenosine A2A receptor with a unique signaling bias. Cell Signal. 2016;28:552–560. doi: 10.1016/j.cellsig.2016.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle C, Cristofaro V, Sullivan MP, Adam RM. Inosine - a multifunctional treatment for complications of neurologic injury. Cell Physiol Biochem. 2018;49:2293–2303. doi: 10.1159/000493831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doyle C, Cristofaro V, Sack BS, Lukianov SN, Schäfer M, Chung YG, Sullivan MP, Adam RM. Inosine attenuates spontaneous activity in the rat neurogenic bladder through an A2B pathway. Sci Rep. 2017;7:44416. doi: 10.1038/srep44416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.da Rocha Lapa F, da Silva MD, de Almeida Cabrini D, Santos AR. Anti-inflammatory effects of purine nucleosides, adenosine and inosine, in a mouse model of pleurisy: evidence for the role of adenosine A2 receptors. Purinergic Signal. 2012;8:693–704. doi: 10.1007/s11302-012-9299-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valada P, Hinz S, Vielmuth C, Lopes CR, Cunha RA, Müller CE, Lopes JP. The impact of inosine on hippocampal synaptic transmission and plasticity involves the release of adenosine through equilibrative nucleoside transporters rather than the direct activation of adenosine receptors. Purinergic Signal. 2022. doi: 10.1007/s11302-022-09899-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voss JH, Mahardhika AB, Inoue A, Müller CE. Agonist-dependent coupling of the promiscuous adenosine A2B receptor to Gα protein subunits. ACS Pharmacol Transl Sci. 2022;5:373–386. doi: 10.1021/acsptsci.2c00020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou AP, Nithipatikom K, Li PL, Cowley AW. Role of renal medullary adenosine in the control of blood flow and sodium excretion. Am J Physiol Regul Integr Comp Physiol. 1999;276:R790. [DOI] [PubMed] [Google Scholar]
- 31.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Medicine / Public Library of Science. 2008;5:e137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng M-G, Navar LG. Afferent arteriolar vasodilator effect of adenosine predominantly involves adenosine A2B receptor activation. Am J Physiol Renal Physiol. 2010;299:F310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper SL, Wragg ES, Pannucci P, Soave M, Hill SJ, Woolard J. Regionally selective cardiovascular responses to adenosine A2A and A2B receptor activation. Faseb j. 2022;36:e22214. doi: 10.1096/fj.202101945R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cowley AW Jr., Mori T, Mattson D, Zou AP. Role of renal NO production in the regulation of medullary blood flow. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1355–1369. doi: 10.1152/ajpregu.00701.2002 [DOI] [PubMed] [Google Scholar]
- 35.Mattson DL. Importance of the renal medullary circulation in the control of sodium excretion and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2003;284:R13–27. doi: 10.1152/ajpregu.00321.2002 [DOI] [PubMed] [Google Scholar]
- 36.Roman RJ, Zou AP. Influence of the renal medullary circulation on the control of sodium excretion. Am J Physiol. 1993;265:R963–973. doi: 10.1152/ajpregu.1993.265.5.R963 [DOI] [PubMed] [Google Scholar]
- 37.Braunwald E Gliflozins in the Management of Cardiovascular Disease. N Engl J Med. 2022;386:2024–2034. doi: 10.1056/NEJMra2115011 [DOI] [PubMed] [Google Scholar]
- 38.Rajagopal M, Pao AC. Adenosine activates a2b receptors and enhances chloride secretion in kidney inner medullary collecting duct cells. Hypertension. 2010;55:1123–1128. doi: 10.1161/hypertensionaha.109.143404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Battistone MA, Nair AV, Barton CR, Liberman RN, Peralta MA, Capen DE, Brown D, Breton S. Extracellular Adenosine Stimulates Vacuolar ATPase-Dependent Proton Secretion in Medullary Intercalated Cells. J Am Soc Nephrol. 2018;29:545–556. doi: 10.1681/asn.2017060643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata S, Mu S, Kawarazaki H, Muraoka K, Ishizawa K-i, Yoshida S, Kawarazaki W, Takeuchi M, Ayuzawa N, Miyoshi J, et al. Rac1 GTPase in rodent kidneys is essential for salt-sensitive hypertension via a mineralocorticoid receptor–dependent pathway. The Journal of Clinical Investigation. 2011;121:3233–3243. doi: 10.1172/JCI43124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibata S, Nagase M, Yoshida S, Kawarazaki W, Kurihara H, Tanaka H, Miyoshi J, Takai Y, Fujita T. Modification of mineralocorticoid receptor function by Rac1 GTPase: implication in proteinuric kidney disease. Nature Medicine. 2008;14:1370. doi: 10.1038/nm.1879 [DOI] [PubMed] [Google Scholar]
- 42.Tofovic SP, Hu J, Jackson E. Purine nucleoside phosphorylase inhibition attenuates the metabolic syndrome and associated pulmonary hypertension and right ventricular dysfunction. In: American Thoracic Society; 2019:A4197. [Google Scholar]
- 43.Tofovic SP, Hu J, Milosevic J, Jackson EK. Inhibition of purine nucleoside phosphorylase retards the progression of angioproliferative pulmonary hypertension in female rats. In: American Thoracic Society; 2018:A2883. [Google Scholar]
- 44.Tofovic SP, Jackson EK, Ihrig LL, Mutchler S, Novelli EM. Purine nucleoside phosphorylase inhibition attenuates the progression of anemia and organ damage in sickle cell mice. Blood. 2022;140:2519–2520. doi: 10.1182/blood-2022-15978436480218 [DOI] [Google Scholar]
- 45.Birder LA, Wolf-Johnston A, Wein AJ, Cheng F, Grove-Sullivan M, Kanai AJ, Watson AM, Stoltz D, Watkins SC, Robertson AM, et al. Purine nucleoside phosphorylase inhibition ameliorates age-associated lower urinary tract dysfunctions. JCI Insight. 2020;5. doi: 10.1172/jci.insight.140109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clinger O, Xi Y, Vats A, Wolf-Johnson A, Birder L, Jackson E, Sahel JA, Chen Y. Potent retinal protection by oral administration of a purine metabolite against age-related retinal degeneration and RHO-associated retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2022;63:1777–F0326. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


