Abstract
Importance:
Little is known about the potential impact of the rapid transition to telehealth during the pandemic on treatment for opioid use disorder (OUD).
Objective:
To determine the association between telemedicine adoption during the COVID-19 pandemic and OUD treatment use and quality.
Design:
Observational retrospective national cohort study. OUD clinicians were categorized as low, medium, or high telemedicine use groups based on their outpatient visits during the pandemic. Enrollees were attributed to the clinician (and corresponding telemedicine group) with whom they had a plurality of OUD visits. We compared the care provided by low, medium, and high telemedicine clinicians in “pre-pandemic” (3/14/19–3/13/20) and “pandemic” periods (3/14/20–3/13/21).
Participants:
Enrollees with 180 days of continuous enrollment in either commercial insurance or Medicare Advantage plans and are part of the OptumLabs Data Warehouse, a longitudinal dataset with de-identified administrative claims.
Main Outcomes:
OUD visit rates (in-person vs. telemedicine), use of medications for OUD (MOUD), and OUD-related clinical events, including drug overdose, OUD detoxification/rehabilitation treatment, or injection drug use-related infections.
Results:
There were 11,081 enrollees (average age 53.9 [SD 15.7], 50.0% female) treated by 1,768 clinicians. Low vs. high telemedicine clinicians conducted an average of 2.1% vs. 69.9% of their office visits via telemedicine in the pandemic period. While telemedicine use for OUD increased substantially from the pre-pandemic to pandemic periods, total OUD visit volume (in-person plus telemedicine) per patient-episode remained stable among both high and low telemedicine clinicians (3.8 to 3.7 visits per enrollee for low, 2.7 to 2.7 for high, p=0.86). In adjusted analyses comparing the pre-pandemic vs. pandemic periods, there was no differential change in MOUD initiation among patients treated in low vs. high telemedicine clinician groups (OR [95% CI]: 0.93[0.74, 1.15]), in MOUD days supply (−.27[−1.84, 1.3]), nor in OUD-related clinical events (0.95[0.73, 1.24]).
Conclusions and Relevance:
In this observational study of commercially insured and Medicare Advantage enrollees, clinical outcomes were similar among patients treated by high and low telemedicine use clinicians during the pandemic, suggesting that telemedicine is a comparable alternative to in-person OUD care. There was no evidence that telemedicine increased engagement in OUD treatment.
INTRODUCTION
Overdose deaths related to opioid use disorder (OUD) have increased rapidly in the past decade, from 21,000 in 2010 to over 100,000 in 2021.1,2 However, access to OUD treatment has remained limited. Medications for opioid use disorders (MOUD), which include methadone, buprenorphine, and long-acting injectable naltrexone, are considered the most effective treatments available for OUD,3–10 but in 2019, the National Survey on Drug Use and Health found that just 27.8% of individuals needing OUD treatment received MOUD.11 For decades there have been long-standing barriers to OUD care including clinician shortages, stigma, cost, and transportation challenges.12 The situation may have worsened with social isolation and infection control interventions during the COVID-19 pandemic, which appear to have increased demand for OUD treatment and reduced access to in-person care.13,14
Telemedicine has been proposed as a potential solution to improve OUD treatment access, but there was little use prior to 2020.15 During the pandemic, federal and state regulatory changes and expanded reimbursement for telemedicine services facilitated dramatic increases in telemedicine use.16 For patients with OUD, these regulatory changes removed the requirement to meet with a clinician in-person before initiating MOUD,17,18 as required by the Ryan Haight Act.19 In preliminary qualitative and survey evidence, OUD clinicians have reported that telemedicine has improved access to buprenorphine and led to higher rates of MOUD initiation,20–23 in part by removing transportation barriers and relieving burdens faced by those with competing demands, such as child care and work.24,25 While telemedicine for OUD may provide these benefits, there is limited national, empirical literature on the benefits or drawbacks of this shift in care delivery.26,27 More evidence is needed to inform the ongoing debate about regulations and payment for OUD treatment after the COVID-19 public health emergency ends.28
The dramatic shift in care delivery during the pandemic towards more widespread use of telemedicine provides an opportunity to address this knowledge gap. In this study, we used a national database of commercially insured individuals to examine the association between telemedicine use and indicators of OUD treatment quality. We used a difference-in-differences methodologic approach, which reduces bias due to non-random patient selection into “intervention or control” groups. Specifically, we compared patients receiving OUD treatment from clinicians with high vs. low telemedicine use in the pandemic period, adjusting for the outcomes experienced by patients treated by these clinicians during the pre-pandemic period.
METHODS
Data Sources
For this longitudinal cohort study, we used de-identified claims from the OptumLabs Data Warehouse, which contains a national dataset of medical claims and enrollment records for commercial and Medicare Advantage enrollees, linked to county-level characteristics from the US Census. We included claims from March 14th, 2019 to March 13th, 2021, allowing for one year before and one year after the start of the US declaration of the COVID-19 Public Health Emergency (PHE). Loosening of telemedicine restrictions by the Centers for Medicare and Medicaid Services (CMS) happened soon after the PHE declaration.29 Visits from March 14th, 2019, to March 13th, 2020, were labelled as “pre-pandemic” period and visits from March 14th, 2020 to March 13th, 2021 were labelled as “pandemic” period visits. This study was approved by the institutional review board at Harvard Medical School and follows STROBE reporting guidelines for cohort studies.
Clinician Sample
Clinicians functioned as the unit of treatment assignment. The study was limited to clinician specialties most likely to be office-based (i.e., non-Opioid Treatment Program) MOUD prescribers: primary care physicians, psychiatrists, nurse practitioners, anesthesiologists (representing pain medicine specialists), rehabilitation medicine clinicians, neurologists, pediatricians, and obstetricians and gynecologists. We included prescription fills for all possible MOUD in addition to claims for facility administered medications (eTable 1). We defined a “MOUD prescriber” as a clinician with ≥1 MOUD claim in both the pre-pandemic and pandemic periods. We focused on buprenorphine (long acting inectable or oral preparations with naloxone) and naltrexone because they are both available via typical office-based practice while methadone for OUD care can only be dispensed through OTPs.
Defining OUD Visits
Our study sample was composed of outpatient episodes of care for enrollees with OUD. We defined outpatient visits using Healthcare Common Procedure Code Set (HCPCS) codes specific to clinician offices, excluding, for example, emergency department, hospital inpatient, nursing home, or dialysis facility codes (eTable 2). We identified enrollees as having OUD if they had: a) at least two outpatient claims with an International Classification of Diseases-10 (ICD-10) code for OUD (F11.1, F11.2, F11.9) in any diagnosis field; b) at least one inpatient and at least one outpatient claim with an ICD-10 code for OUD, or c) at least one inpatient or outpatient claim with an ICD-10 code for OUD and at least one claim with a confirmatory event (opioid overdose; hepatitis C, an infection potentially secondary to injection drug use; or an inpatient detoxification or rehabilitation treatment; definitions in eTable 3) within 90 days before or after the claim with an OUD diagnosis.
Among enrollees meeting any of the above criteria, the earliest observed visit for OUD following a 90-day clean period (no claims for OUD utilization, MOUD pharmacy, or HCPCS claims) was treated as the index OUD visit. OUD treatment episodes were defined by all claims occurring within 90 days after the index visit. The same enrollee could have episodes of care in both the pre-pandemic and pandemic periods but we included just one episode of care per period. For each episode, enrollees were attributed to the clinician from whom they received a plurality of their OUD visits during that episode. Enrollees were also required to have continuous enrollment in medical and pharmacy benefit for at least 90 days before and after the episode index visit. This was to ensure that we could observe their OUD care utilization, which was necessary to define an index episode following a clean period, and in defining utilization that occurred in the 90-day OUD episodes.
Defining Telemedicine Exposure
We categorized clinicians based on their proportion of telemedicine use during the pandemic period. Telemedicine visits were identified through modifiers GT, GQ, or 95 on eligible outpatient services or CPT codes 99441–99443. Clinicians were then separated into tertiles (low, medium, and high telemedicine use) based on the proportion of all outpatient visits (OUD and non-OUD) conducted by each clinician via telemedicine. We defined clinician telemedicine use by measuring telemedicine use for all outpatient visits (i.e. not limited to OUD, using the same HCPCS codes in eTable 2) to avoid potential misclassification due to small sample sizes of OUD visits within clinicians. Telemedicine use across all outpatient visits was highly correlated with telemedicine use limited to OUD visits within clinician (Pearson ρ=0.71). OUD enrollees were assigned to the telemedicine group (high, medium, low) of their assigned clinician. We used clinician telemedicine group as the key exposure variable, as opposed to comparing in-person vs. telemedicine at the visit level, to avoid selection bias by indication due to a clinician using telemedicine for specific reasons within their own patient population.
Study Outcomes
We examined four outcomes: all outpatient visits, OUD visits, MOUD prescribing, and OUD-related clinical events. For outpatient visits, we captured enrollees’ total, in-person, and telemedicine outpatient visit volume. We then captured total, in-person, and telemedicine OUD visits within 90 days of an enrollee’s index visit. For MOUD prescribing, consistent with prior literature,30 we defined two measures of MOUD initiation: a) the proportion of OUD patients with initiation within 90 days of the index visit, and b) the proportion of OUD patients with initiation within 14 days of the index visit. MOUD retention was defined as having at least one additional MOUD fill within 30–90 days of initiation among those with initiation within 14 days of the index visit. We averaged days-supply of MOUD for fills during their 90-day episode of care (shifting overlapping days forward) across all enrollees, as well as just for enrollees who had at least 1 fill within 14 days. Finally, for OUD-related clinical events, we captured the percentage of enrollees who had a drug overdose, inpatient detoxification or rehabilitation treatment center stay, or an infection potentially secondary to injection drug use within 90 days of index visit (eTable 3).
Study Covariates
We captured age (18–35, 36–50, 51–65, and 66+), documented sex, and insurance type (commercial or Medicare Advantage) from enrollment data. We defined rural-urban classifications using the rural-urban commuting area (RUCA) (metropolitan, micropolitan, small town) system,31 and patient county-level quartiles of race and poverty indicators from US Census data (percentage of population with white race and median household income respectively).32 We also captured clinician specialty.
Statistical Analysis
We used chi-squared tests to test for bivariate differences between patients assigned to low, mid, and high-use telemedicine clinicians during the pre-pandemic and pandemic periods.
To estimate the association between clinician telemedicine use and patient outcomes, we used a difference-in-differences approach. For each outcome, we compared the changes in the pre-pandemic and pandemic periods between the patients being seen by clinicians defined as “low” telemedicine users (i.e., “control” group) and those being seen by clinicians in the “medium” or “high” telemedicine groups (i.e., “intervention” groups). We compared the pre-pandemic and pandemic periods as two time points rather than longitudinal rates per month or quarter to maximize statistical power for the smallest possible minimum detectable effect size for telemedicine. We estimated separate, enrollee-level linear (for visit rates and days’ supply) or logistic (for binary outcomes such as MOUD overdose) models for each outcome including indicators for telemedicine group (high or medium vs. low as reference), pandemic period, and an interaction term of the two variables, adjusting for clinician specialty and all enrollee characteristics. The key variable of interest in each difference-in-differences regression was the coefficient on the interaction term, which represented the differential change in each outcome attributable to clinician telemedicine use during the pandemic period. We clustered standard errors at the clinician level. Analyses were performed in SAS (v. 9.4). The 95% confidence interval around reported estimates reflects 0.025 in each tail or p≤0.05. As a sensitivity analysis we repeated the above analyses, additionally requiring continuous enrollment in the behavioral health plan, but there was no difference in adjusted outcomes.
RESULTS
Study Sample
The study sample contained 1,768 clinicians caring for 5,990 and 5,811 enrollees with an episode in the pre- and pandemic periods, respectively (Table 1). Average enrollee age was 53.9 [SD 15.7]; 50.0% of enrollees were female, 50.0% were male. (Table 1). Regardless of telemedicine group, the highest percentage of enrollees were aged 51–65 (37.0% in low and 35.0% in high). Patients seeing high telemedicine clinicians were less likely to reside in the lowest income counties compared to low telemedicine clinicians (20.5% in high vs. 37.3% in low). Primary care clinicians were more likely to be in the low telemedicine group (47.3% in low vs. 26.2% in high), while psychiatrists were more likely to be in the high group (24.1% low vs. 39.8% high) (Table 1).
Table 1:
Patient and Clinician Characteristics
| Clinician Telemedicine Use Group | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Low | Mid | High | Low vs. Mid | Low vs. High | ||
|
| ||||||
| Count (%) | Count (%) | Count (%) | P-Valuea | P-Value | ||
| OUD Patients (n) | 4197 | 4308 | 3296 | |||
| Clinicians (n) | 589 | 590 | 589 | |||
|
| ||||||
| Age | <.001 | 0.001 | ||||
| 18–35 | 699 (16.7) | 551 (12.8) | 608 (18.5) | |||
| 36–50 | 947 (22.6) | 927 (21.5) | 791 (24.0) | |||
| 51–65 | 1551 (37.0) | 1559 (36.2) | 1153 (35.0) | |||
| 66+ | 1000 (23.8) | 1271 (29.5) | 744 (22.6) | |||
|
| ||||||
| Documented Sex | <.001 | 0.001 | ||||
| Male | 2204 (52.5) | 2069 (48.0) | 1629 (49.4) | |||
| Female | 1993 (47.5) | 2239 (52.0) | 1667 (50.6) | |||
|
| ||||||
| Rurality | <.001 | <.001 | ||||
| Metropolitan | 3376 (80.4) | 3683 (85.5) | 2897 (87.9) | |||
| Micropolitan | 493 (11.8) | 330 (7.7) | 262 (8.0) | |||
| Small Town | 229 (5.5) | 205 (4.8) | 89 (2.7) | |||
| Rural | 99 (2.4) | 90 (2.1) | 48 (1.5) | |||
|
| ||||||
| Insurance Type | 0.88 | <.001 | ||||
| Commercial | 2092 (49.9) | 2060 (47.8) | 1900 (57.7) | |||
| Medicare Advantage | 2105 (50.2) | 2248 (52.2) | 1396 (42.4) | |||
|
| ||||||
| Median Household Income in County | <.001 | <.001 | ||||
| 1 (low) | 1565 (37.3) | 1305 (30.3) | 677 (20.5) | |||
| 2 | 1044 (24.9) | 1080 (25.1) | 879 (26.7) | |||
| 3 | 907 (21.6) | 1078 (25.0) | 872 (26.5) | |||
| 4 (high) | 681 (16.2) | 845 (19.6) | 868 (26.3) | |||
|
| ||||||
| % White Population in County | <.001 | <.001 | ||||
| 1 (low) | 449 (10.7) | 517 (12.0) | 416 (12.6) | |||
| 2 | 1187 (28.3) | 1328 (30.8) | 1053 (32.0) | |||
| 3 | 1104 (26.3) | 1346 (31.2) | 901 (27.3) | |||
| 4 (high) | 1457 (34.7) | 1117 (25.9) | 926 (28.1) | |||
|
| ||||||
| Clinician Specialty | <.001 | <.001 | ||||
| Primary Care | 286 (48.6) | 303 (51.4) | 156 (26.5) | |||
| Psychiatrist | 132 (22.4) | 117 (19.8) | 219 (37.2) | |||
| RN Special Service | 59 (10.0) | 52 (8.8) | 85 (14.4) | |||
| Anesthesiology | 61 (10.4) | 78 (13.2) | 85 (14.4) | |||
| Rehabilitation Medicine | 34 (5.8) | 34 (5.8) | 37 (6.3) | |||
| Otherb | 17 (2.9) | 6 (1.0) | 7 (1.2) | |||
Unadjusted p values were estimated with the use of chi-square tests
Other = Neurology, Pediatrics and Obstetrics and Gynecology
Outpatient and OUD Visit Utilization
In the pandemic period, low telemedicine clinicians conducted an average of 2.1% of all office visits (i.e., not just OUD visits) via telemedicine while high telemedicine clinicians conducted an average of 69.5% of their visits virtually (p<0.001) ( eTable 4). Clinician telemedicine use for OUD visits followed a similar pattern: 2.4% vs. 62.3% telemedicine in the low vs. high groups for this subset of all visits (p<0.001) (Table 2).
Table 2:
Unadjusted OUD Utilization and Outcomes by Clinician Telemedicine Group
| Clinician Telemedicine Use Group | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Low | Medium | High | ||||
|
|
||||||
| Prea | Postb | Pre | Post | Pre | Post | |
| Clinicians (n) | 589 | 589 | 590 | 590 | 589 | 589 |
| Enrollees with OUD (n) | 2095 | 2102 | 2240 | 2068 | 1655 | 1641 |
| OUD Outpatient Visits | ||||||
| Total within 90 days post earliest visit (n) | 6,445 | 6,848 | 5,910 | 5,442 | 4,387 | 4,424 |
| Percent Telemedicine (%) | 0.2 | 2.4 | 0.1 | 23.4 | 1.2 | 62.3 |
| OUD Visit Volume c | ||||||
| Total | 3.1 | 3.3 | 2.6 | 2.6 | 2.6 | 2.7 |
| In-person | 3.1 | 3.2 | 2.6 | 2.1 | 2.5 | 1.1 |
| Telemedicine | 0.0 | 0.1 | 0.0 | 0.5 | 0.1 | 1.6 |
| MOUD Initiation and Supply (%) | ||||||
| ≥ 1 MOUD fill within 14 days | 15.3 | 15.2 | 13.8 | 14.6 | 14.7 | 13.7 |
| ≥ 1 MOUD fill within 90 days | 19.1 | 18.5 | 18.0 | 18.1 | 19.9 | 17.5 |
| ≥ 1 MOUD fill within 30–90 days among those with a fill within 14 days | 76.9 | 68.8 | 68.2 | 68.1 | 75.0 | 65.8 |
| MOUD Days-Supply | ||||||
| Among those ≥ 1 MOUD fill | 20.2 | 20.5 | 20.8 | 20.9 | 18.8 | 18.2 |
| OUD-Related Event within 90 Days Following Index Visit (%) | ||||||
| Overdose | 2.3 | 2.1 | 1.7 | 1.9 | 1.9 | 1.8 |
| Detoxification/Rehabilitation | 4.1 | 3.4 | 2.3 | 2.0 | 3.0 | 3.0 |
| Injection-Related Infection | 9.0 | 10.2 | 8.3 | 8.3 | 9.8 | 7.9 |
| Any OUD Related Event | 14.7 | 14.6 | 11.4 | 11.7 | 14.0 | 12.1 |
OUD: Opioid Use Disorder, MOUD: Medication for Opioid Use Disorder
Pre: March 14th, 2019 – March 13th, 2020
Post: March 14th, 2020 – March 13th, 2021
This refers to the total number of OUD visits an enrollee had in their 90 day episode.
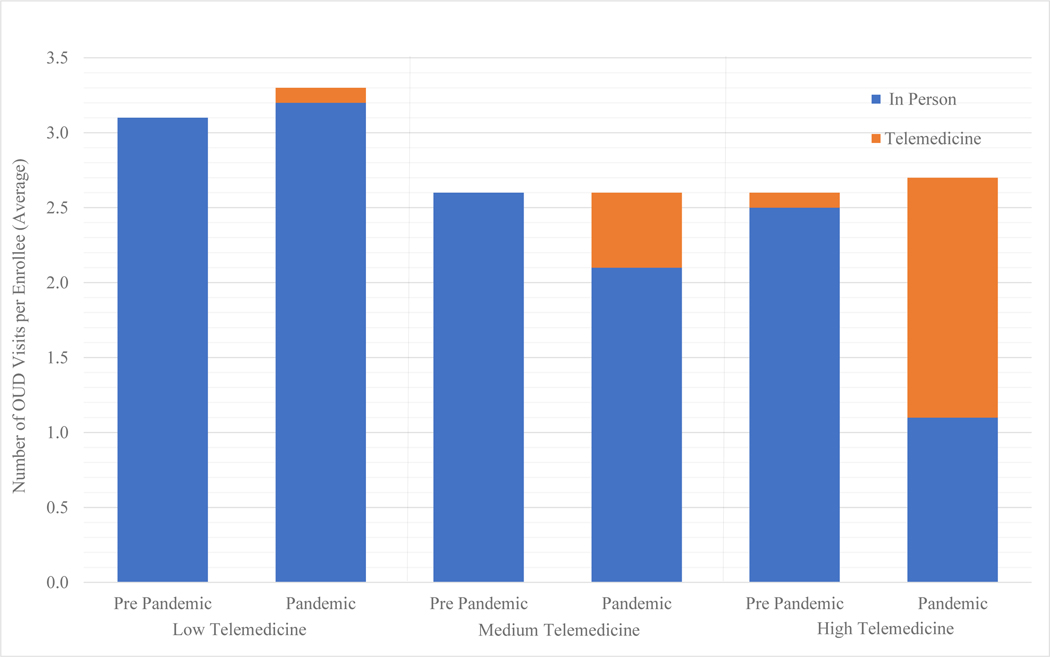
The average number of OUD visits per episode remained stable in both the low and high telemedicine clinician groups over time (Figure 1). Enrollees seen by low telemedicine clinicians had an average of 3.1 OUD visits per patient-episode pre-pandemic and 3.3 OUD visits per patient-episode during the pandemic (Table 2). For high telemedicine volume clinicians, there were 2.6 visits per patient episode in the pre-pandemic and and 2.7 in the pandemic period. In the adjusted model, there was no differential change in visit volume per enrollee from pre-pandemic to pandemic among high vs. low telemedicine clinicians (coefficient[95%CI]: −0.01[−0.28 – 0.26]) (Table 3).
Figure 1:
Average Number of OUD Visits per Enrollee with OUD by 12-Month Time Period, Unadjusted
The low, medium, and high telemedicine groups are based on clinicians’ telemedicine use across all outpatient visits. The pre-pandemic period is March 14th, 2019 to March 13th, 2020 and the pandemic period is March 14th, 2020 to March 13th, 2021.
Table 3:
Adjusted Differential Change in OUD Outcomes, High and Medium vs. Low Telemedicine Groups
| Medium Telemedicine vs. Low | High Telemedicine vs. Low | |||
|---|---|---|---|---|
| Continuous Outcomes | Change in Outcome in Post-Pandemic Period (95% CI) |
p-value | Change in Outcome in Post-Pandemic Period (95% CI) |
p-value |
| OUD Visit Volume | ||||
| Total | −0.13 (−0.42 – 0.16) | 0.38 | −0.01 (−0.28 – 0.26) | 0.94 |
| In-person | −0.55 (−0.85 – −0.27) | 0.002 | −1.51 (−1.79 – −1.24) | <.001 |
| Telemedicine | 0.43 (0.37 – 0.49) | <.001 | 1.51 (1.37 – 1.65) | <.001 |
| MOUD Days-Supply | ||||
| Among those ≥ 1 MOUD fill | 0.29 (−1.09 – 1.68) | 0.68 | −0.27 (−1.84 – 1.30) | 0.73 |
| Binary Outcomes | Odd Ratio (95% CI) |
P-value | Odds Ratio (95% CI) |
P-value |
| MOUD Initiation and Supply | ||||
| ≥ 1 MOUD within 14 days | 1.01 (0.87 – 1.16) | 0.93 | 1.00 (0.84 – 1.19) | 0.96 |
| ≥ 1 MOUD within 90 days | 1.00 (0.84 – 1.18) | 0.97 | 0.91 (0.74 – 1.12) | 0.38 |
| ≥ 1 MOUD fills within 30–90 days | 0.79 (0.57 – 1.10) | 0.16 | 1.09 (0.74 – 1.60) | 0.67 |
| Healthcare Utilization within 90 Days | ||||
| Overdose | 1.12 (0.66 – 1.91) | 0.68 | 1.14 (0.72 – 1.83) | 0.58 |
| Detoxification/Rehabilitation | 0.92 (0.45 – 1.88) | 0.82 | 0.84 (0.53 – 1.34) | 0.46 |
| Injection-Related Infection | 0.91 (0.71 – 1.16) | 0.44 | 0.81 (0.58 – 1.11) | 0.19 |
| Any OUD related Event | 1.01 (0.72 – 1.36) | 0.95 | 0.95 (0.73 – 1.24) | 0.69 |
OUD: Opioid Use Disorder, MOUD: Medication for Opioid Use Disorder
Continuous outcomes were modeled with linear regressions and binary outcomes were modeled with logistic regressions. Adjusted values in the table are the coefficient on the interaction term, representing the difference in outcomes between low vs. medium and low vs. high telemedicine groups during the pandemic. Each statistical model adjusted for age, documented sex, rurality, insurance type, median household income in county, racial demographics in county, clinician specialty, and state. We clustered standard errors by state.
MOUD Initiation and Retention
Among patients seen by low telemedicine clinicians, 15.3% and 15.2% initiated MOUD within 14 days of the index visit in the pre-pandemic and pandemic periods, respectively. For patients seen by clinicians in the high telemedicine group, these rates were 14.7% pre-pandemic and 13.7% pandemic (adjusted OR[95%CI]: 1.00[0.84 – 1.19]) (Tables 2 and 3). In both groups, enrollees who initiated MOUD within 14 days were equally likely to have at least one subsequent prescription in the 30–90 days after index visit in the pandemic period (68.8% vs. 65.8% respectively, adjusted OR[95%CI]: 0.91[0.74 – 1.12]). The average days-supply for enrollees with at least 1 fill within 90 days of the index visit was consistent for both low and high telemedicine groups across the pre- and pandemic periods — 20.2 and 20.5 among low telemedicine clinicians, and 18.8 and 18.2 among high telemedicine clinicians (coefficient[95% CI]: −0.27[−1.84 – 1.30]).
OUD-Related Events
The percentage of enrollees with at least one OUD-related clinical event was lower in the pandemic period compared to pre-pandemic for enrollees in both the low and high telemedicine clinician groups — from 14.7% to 14.6% in the low group and 14.0% to 12.1% in the high group, and in adjusted analyses there was no differential change between groups (adjusted OR[95% CI] = 1.01[0.72 – 1.36]) (Tables 2 and 3). There was also no difference between low and high telemedicine groups when looking at each OUD-related event individually. The percentage of enrollees with an overdose went from 2.3% to 2.1% in the low group and 1.9% to 1.8% in the high (adjusted OR[95%CI]: 1.14[0.72 – 1.83]). Detoxification/rehabilitation admissions went from 4.1% to 3.4% in the pre- vs. pandemic periods and in the high group remained at 3.0% in both periods (adjusted OR[95%CI]: 0.84[0.52 – 1.34]) (Tables 2 and 3).
DISCUSSION
In a national sample of commercially insured and Medicare Advantage patients with OUD, we found that being treated by a clinician with high telemedicine use was not associated with a difference in the pattern of outpatient care or OUD-related events compared to clinicians with low telemedicine use. The total number of OUD visits per episode was consistent across the pre-pandemic and pandemic periods regardless of telemedicine uptake, showing that telemedicine use was almost entirely substituting, rather than supplementing, care. Overall, based on measures observable in claims data, telemedicine was comparable to in-person care, with no evidence of differential harm or benefit to patients who were seen by clinicians with high versus low telemedicine usage.
Reassuringly, these results suggest that using telemedicine for OUD care was not associated with significantly lower rates of MOUD initiation or refills. These findings are consistent with pre-pandemic studies showing that buprenorphine delivered virtually has had comparable patient retention and medication adherence to buprenorphine delivered in-person.33–35 This study extends prior literature to the pandemic era of telemedicine expansion and its “real world” implementation and suggests on a larger scale that telemedicine can safely be used to expand access to OUD care. While we were unable to observe visit appropriateness, our results also do not suggest that telemedicine led to a spike in unnecessary or inefficient utilization, an important concern raised by critics of telemedicine expansion.36
However, neither did we find evidence of differential benefit. Higher telemedicine use did not improve access, as measured by visit volume, given the consistent number of OUD visits across pre-pandemic and pandemic periods. Higher telemedicine use was also not associated with increased MOUD initiation, refills, or days-supply. The low rates of MOUD use both before and during the pandemic were consistent with prior literature on access to OUD treatment among commercially insured populations.37,38 While telemedicine access may be part of a comprehensive policy package to promote MOUD access, there is still significant progress needed to increase access and telemedicine is unlikely to be sufficient alone.
It is important to note that enrollees receiving OUD care from high telemedicine clinicians were concentrated in higher income, metropolitan counties with greater racial diversity. This could be consistent with concerns about a “digital divide” separating lower income and rural areas in the US from mainstream technological advances that require broadband or other resources.39–41 In addition, given that high telemedicine use was not associated with changes in OUD care, it is possible that populations accessing providers with high telemedicine usage had more resources to begin with, compared to patients accesssing providers with lower telemedicine use. Therefore, the digital divide may lower the potential of telemedicine to advance treatment access if additional measures are not taken to make telemedicine availability more equitable. High telemedicine use also was associated primarily with psychiatrists, aligning with previous reports of greater telemedicine usage among behavioral health clinicians.42
Limitations
First, this was an observational study; we are only able to report associations and cannot provide conclusive evidence of any causal relationships. However, we mitigate the selection bias that can occur in an observational study through our difference-in-differences design. Additionally, our findings may not be generalizable to other commercially insured populations, individuals enrolled in Medicaid and other Medicare programs, and those who are uninsured (a notable population since around a fifth of adults with OUD are uninsured).43 Our outcomes only capture part of the complex process of access to care, and it is possible that telemedicine provided benefits (or drawbacks) that we did not observe. While we were able to measure an individual’s receipt of MOUD, visit volume, and some OUD-treatment related utilization or adverse events, we were unable to measure receipt of long acting buprenorphine implants and other important clinical outcomes, such as OUD relapse or patient functioning. In addition, the rates of overdose were limited to those who initiated OUD treatment and therefore part of our cohort.
Conclusions
We found that after telemedicine expansion during the COVID-19 pandemic, patients with OUD received similar patterns of care and had similar outcomes whether they were treated by clinicians who predominantly used telemedicine or in-person care. There was no evidence suggesting that telemedicine was unsafe or overused comparing high vs. low telemedicine clinicians. Conversely, there was no evidence that telemedicine facilitated greater access or improved quality of care. These results imply that telemedicine is a safe alternative for delivering care for OUD, but not one that will drastically change quality or access in the short term.
Supplementary Material
KEY POINTS.
Question:
Is provider telemedicine use associated with differences in OUD care (visits, MOUD, OUD related events)?
Findings:
In this observational study, we did not find significant differences in outcomes, regardless of their provider’s telemedicine usage, among 11,081 enrollees with OUD. This includes similar rates of change between the pre-pandemic and pandemic periods in each telemedicine group for total OUD visits, MOUD initiation, and adverse outcomes.
Meaning:
Our findings suggest that telemedicine is a comparable alternative to in-person OUD care. There was no evidence that telemedicine was unsafe or overused, but there was also no evidence that it increased engagement in OUD care.
ACKNOWLEDGMENTS
Ruth Hailu and Michael Barnett had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Supported by grants from the National Institute on Aging of the National Institutes of Health (K23 AG058806) and the National Institute on Drug Abuse (R01 DA048533 and P30 DA035772).
Contributor Information
Ruth Hailu, Department of Health Care Policy, Harvard Medical School.
Ateev Mehrotra, Department of Health Care Policy, Harvard Medical School; Division of General Medicine, Beth Israel Deaconess Medical Center.
Haiden A. Huskamp, Department of Health Care Policy, Harvard Medical School.
Alisa B. Busch, Department of Health Care Policy, Harvard Medical School; Boston, Massachusetts; McLean Hospital, Belmont, MA.
Michael L. Barnett, Department of Health Policy and Management, Harvard T. H. Chan School of Public Health; Division of General Internal Medicine and Primary Care, Department of Medicine, Brigham and Women’s Hospital.
REFERENCES
- 1.Ahmad FB, Cisewski JA, Rossen LM, Sutton P. Provisional drug overdose death counts. National Center for Health Statistics, Centers for Disease Control and Prevention. Updated February 9, 2022. Accessed November 23, 2022. https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm [Google Scholar]
- 2.O’Donnell J, Gladden RM, Mattson CL, Kariisa M. Notes from the Field: Overdose Deaths with Carfentanil and Other Fentanyl Analogs Detected - 10 States, July 2016-June 2017. MMWR Morb Mortal Wkly Rep. 2018;67(27):767–768. Published 2018 Jul 13. doi: 10.15585/mmwr.mm6727a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larochelle MR, Bernson D, Land T, et al. Medication for Opioid Use Disorder After Nonfatal Opioid Overdose and Association With Mortality: A Cohort Study. Ann Intern Med. 2018;169(3):137–145. doi: 10.7326/M17-3107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deck D, Wiitala W, McFarland B, et al. Medicaid coverage, methadone maintenance, and felony arrests: outcomes of opiate treatment in two states. J Addict Dis. 2009;28(2):89–102. doi: 10.1080/10550880902772373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63–75. doi: 10.1097/HRP.0000000000000075 [DOI] [PubMed] [Google Scholar]
- 6.Alderks CE. Trends in the Use of Methadone, Buprenorphine, and Extended-Release Naltrexone at Substance Abuse Treatment Facilities: 2003–2015 (Update). In: The CBHSQ Report. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); August 22, 2017.1–8. [PubMed] [Google Scholar]
- 7.Larochelle MR, Wakeman SE, Ameli O, et al. Relative Cost Differences of Initial Treatment Strategies for Newly Diagnosed Opioid Use Disorder: A Cohort Study. Med Care. 2020;58(10):919–926. doi: 10.1097/MLR.0000000000001394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orgera K, Tolbert J. Key Facts about Uninsured Adults with Opioid Use Disorder. KFF. Published July 5, 2019. Accessed November 23, 2022. https://www.kff.org/uninsured/issue-brief/key-facts-about-uninsured-adults-with-opioid-use-disorder/
- 9.Wakeman SE, Larochelle MR, Ameli O, et al. Comparative Effectiveness of Different Treatment Pathways for Opioid Use Disorder. JAMA Netw Open. 2020;3(2):e1920622. doi: 10.1001/jamanetworkopen.2019.20622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309–318. doi: 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauro PM, Gutkind S, Annunziato EM, Samples H. Use of Medication for Opioid Use Disorder Among US Adolescents and Adults With Need for Opioid Treatment, 2019 [published correction appears in JAMA Netw Open. 2022 Jul 1;5(7):e2227817]. JAMA Netw Open. 2022;5(3):e223821. doi: 10.1001/jamanetworkopen.2022.3821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackey K, Veazie S, Anderson J, Bourne D, Peterson K. Barriers and Facilitators to the Use of Medications for Opioid Use Disorder: a Rapid Review. J Gen Intern Med. 2020;35(Suppl 3):954–963. doi: 10.1007/s11606-020-06257-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgartner JC, Radley DC. Overdose Deaths Surged in the First Half of 2021, Underscoring Urgent Need for Action. To the Point (blog), Commonwealth Fund. 2022. 10.26099/tmae-je82 [DOI] [Google Scholar]
- 14.Vieson J, Yeh AB, Lan Q, Sprague JE. During the COVID-19 Pandemic, Opioid Overdose Deaths Revert to Previous Record Levels in Ohio. J Addict Med. 2022;16(2):e118–e122. doi: 10.1097/ADM.0000000000000874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huskamp HA, Busch AB, Souza J, et al. How Is Telemedicine Being Used In Opioid And Other Substance Use Disorder Treatment?. Health Aff (Millwood). 2018;37(12):1940–1947. doi: 10.1377/hlthaff.2018.05134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medicare Telemedicine Health Care Provider Fact Sheet. Centers for Medicare & Medicaid Services. Published, March 17, 2020. Accessed November 23, 2022. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet
- 17.Ng J, Niles L, Kinderknecht K, Strohmeyer J, Olin S. Changes in Access to Medication Treatment during COVID-19 Telehealth Expansion and Disparities in Telehealth Use for Medicare Beneficiaries with Opioid Use Disorder. Office of Minority Health (OMH) Data Highlight No. 28. Centers for Medicare & Medicaid Services (CMS). 2022. [Google Scholar]
- 18.Ferrante TB, Levine SJ. COVID-19: DEA Confirms Public Health Emergency Exception for Telemedicine Prescribing of Controlled Substances. Foley & Lardner LLP. Published March 18, 2020. Accessed November 23, 2022. https://www.foley.com/en/insights/publications/2020/03/covid19-public-health-exception-telemedicine
- 19.Supporting Access to Telehealth For Addiction Services. American Society of Addiction Medicine. Updated September 8, 2020. Accessed November 23, 2022. https://www.asam.org/quality-care/clinical-guidelines/covid/supporting-access-to-telehealth-for-addiction-services
- 20.Clark SA, Davis C, Wightman RS, et al. Using telehealth to improve buprenorphine access during and after COVID-19: A rapid response initiative in Rhode Island. J Subst Abuse Treat. 2021;124:108283. doi: 10.1016/j.jsat.2021.108283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Weiss J, Ryan EB, Waldman J, Rubin S, & Griffin JL (2021). Telemedicine increases access to buprenorphine initiation during the COVID-19 pandemic. Journal of Substance Abuse Treatment, 124, 108272. doi: 10.1016/j.jsat.2020.108272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riedel L, Uscher-Pines L, Mehrotra A, et al. Use of telemedicine for opioid use disorder treatment - Perceptions and experiences of opioid use disorder clinicians. Drug Alcohol Depend. 2021;228:108999. doi: 10.1016/j.drugalcdep.2021.108999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huskamp HA, Riedel L, Uscher-Pines L, et al. Initiating Opioid Use Disorder Medication via Telemedicine During COVID-19: Implications for Proposed Reforms to the Ryan Haight Act. J Gen Intern Med. 2022;37(1):162–167. doi: 10.1007/s11606-021-07174-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eaves E, Trotter R 2nd, Baldwin J. Another silver lining?: Anthropological perspectives on the promise and practice of relaxed restrictions for telemedicine and medication-assisted treatment in the context of COVID-19. Hum Organ. 2020;79(4):292–303. doi: 10.17730/1938-3525-79.4.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aronowitz SV, Engel-Rebitzer E, Dolan A. et al. Telehealth for opioid use disorder treatment in low-barrier clinic settings: an exploration of clinician and staff perspectives. Harm Reduct J 18, 119 (2021). 10.1186/s12954-021-00572-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samson LW, Tarazi W, Turrini G, Sheingold S. Medicare beneficiaries’ use of telehealth in 2020: trends by beneficiary characteristics and location. Washington, DC: Office of the Assistant Secretary for Planning and Evaluation. Published December 3, 2021. Accessed November 23, 2022. https://aspe.hhs.gov/reports/medicare-beneficiaries-use-telehealth-2020 [Google Scholar]
- 27.Lin LA, Fortney JC, Bohnert ASB, Coughlin LN, Zhang L, Piette JD. Comparing telemedicine to in-person buprenorphine treatment in U.S. veterans with opioid use disorder. J Subst Abuse Treat. 2022;133:108492. doi: 10.1016/j.jsat.2021.108492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Academy of Medicine Action Collaborative on Countering the U.S. Opioid Epidemic: Research Agenda. National Academy of Medicine. Published March 2021. Accessed November 23, 2022. https://nam.edu/programs/action-collaborative-on-countering-the-u-s-opioid-epidemic/opioid-collaborative-agenda/
- 29.Medicare Telemedicine Health Care Provider Fact Sheet. Centers for Medicare & Medicaid Services. Published, March 17, 2020. Accessed November 23, 2022. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet
- 30.Larochelle MR, Wakeman SE, Ameli O, et al. Relative Cost Differences of Initial Treatment Strategies for Newly Diagnosed Opioid Use Disorder: A Cohort Study. Med Care. 2020;58(10):919–926. doi: 10.1097/MLR.0000000000001394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrill R, Cromartie J, Hart G. Rural-Urban Commuting Code Database. Accessed November 23, 2022. https://depts.washington.edu/uwruca/index.php
- 32.WWAMI RUCA Rural Health Research Center. University of Washington, WWAMI RUCA Rural Health Research Center. Accessed November 23, 2022. http://depts.washington.edu/uwruca/ruca-maps.php [Google Scholar]
- 33.Eibl JK, Gauthier G, Pellegrini D, et al. The effectiveness of telemedicine-delivered opioid agonist therapy in a supervised clinical setting. Drug Alcohol Depend. 2017;176:133–138. doi: 10.1016/j.drugalcdep.2017.01.048 [DOI] [PubMed] [Google Scholar]
- 34.Guille C, Simpson AN, Douglas E, et al. Treatment of Opioid Use Disorder in Pregnant Women via Telemedicine: A Nonrandomized Controlled Trial. JAMA Netw Open. 2020;3(1):e1920177. doi: 10.1001/jamanetworkopen.2019.20177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng W, Nickasch M, Lander L, et al. Treatment Outcome Comparison Between Telepsychiatry and Face-to-face Buprenorphine Medication-assisted Treatment for Opioid Use Disorder: A 2-Year Retrospective Data Analysis. J Addict Med. 2017;11(2):138–144. doi: 10.1097/ADM.0000000000000287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mehrotra A, Wang B, and Snyder G. Telemedicine: What Should the Post-Pandemic Regulatory and Payment Landscape Look Like? Commonwealth Fund Aug. 2020. 10.26099/7ccp-en63 [DOI]
- 37.Kilaru AS, Xiong A, Lowenstein M, et al. Incidence of Treatment for Opioid Use Disorder Following Nonfatal Overdose in Commercially Insured Patients. JAMA Netw Open. 2020;3(5):e205852. doi: 10.1001/jamanetworkopen.2020.5852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huskamp HA, Busch AB, Uscher-Pines L, Barnett ML, Riedel L, Mehrotra A. Treatment of Opioid Use Disorder Among Commercially Insured Patients in the Context of the COVID-19 Pandemic. JAMA. 2020;324(23):2440–2442. doi: 10.1001/jama.2020.21512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koma W, Cubanski J, Neuman T. Medicare, and Telehealth: Coverage and Use During the COVID-19 Pandemic and Options for the Future. KFF. Published May 19, 2021. Accessed November 23, 2022. https://www.kff.org/medicare/issue-brief/medicare-and-telehealth-coverage-and-use-during-the-covid-19-pandemic-and-options-for-the-future/
- 40.Patel SY, Mehrotra A, Huskamp HA, Uscher-Pines L, Ganguli I, Barnett ML. Variation In Telemedicine Use And Outpatient Care During The COVID-19 Pandemic In The United States. Health Aff (Millwood). 2021;40(2):349–358. doi: 10.1377/hlthaff.2020.01786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karimi M, Lee EC, Couture SJ, et al. National survey trends in telehealth use in 2021: disparities in utilization and audio vs. video services. Office of the Assistant Secretary for Planning and Evaluation. Published February 1, 2022. Accessed November 23, 2022. https://aspe.hhs.gov/reports/hps-analysis-telehealth-use-2021
- 42.Lo J, Rae M, Amin K, Cox C, Panchal N, Miller BF. Telehealth Has Played an Outsized Role Meeting Mental Health Needs During the COVID-19 Pandemic. KFF. Published Mar 15, 2020. Accessed November 23, 2022. https://www.kff.org/coronavirus-covid-19/issue-brief/telehealth-has-played-an-outsized-role-meeting-mental-health-needs-during-the-covid-19-pandemic/
- 43.Orgera K, Tolbert J. Key Facts about Uninsured Adults with Opioid Use Disorder. KFF. Published July 15, 2019. Accessed November 23, 2022. https://www.kff.org/uninsured/issue-brief/key-facts-about-uninsured-adults-with-opioid-use-disorder/#:~:text=The%20number%20of%20deaths%20is,access%20to%20treatment%20and%20care
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.