Abstract
Background:
Urine cultures collected from catheterized patients have a high likelihood of false positive results due to colonization. We examined the impact of a clinical decision support (CDS) tool that includes catheter information on test utilization and patient-level outcomes.
Methods:
This pre-post intervention study was conducted at three hospitals in North Carolina, USA. In March 2021, CDS was incorporated into urine culture order entry in the electronic health record, providing education about indications for culture and suggesting catheter removal or exchange prior to specimen collection for catheters present greater than seven days. We used an interrupted time series analysis with Poisson regression to evaluate the impact of CDS implementation on utilization of urinalyses and urine cultures, antibiotic use, and other outcomes during the pre- and post-intervention periods.
Results:
CDS was prompted in 38,361 instances of urine cultures ordered in all patients, including 2133 catheterized patients during the post-intervention study period. There was significant decrease in urine culture orders (1.4% decrease/month, p<0.001) and antibiotic use for UTI indications (2.3% decrease/month, p = 0.006), but no significant decline in CAUTI rates in the post-intervention period. Clinicians opted for urinary catheter removal in 183 (8.5%) instances. Evaluation of the safety reporting system revealed no apparent increase in safety events related to catheter removal or reinsertion.
Discussion:
CDS can aid in optimizing urine culture collection practices and serve as a reminder for removal or exchange of long-term indwelling urinary catheters at the time of urine culture collection.
Keywords: urine culture, decision support, CDS, diagnostic stewardship, CAUTI, urinalysis
Introduction:
Urine cultures obtained in the absence of clinical signs or symptoms do not provide actionable information in most patient populations and have a high prevalence of asymptomatic bacteriuria.[1] Positive urine cultures in catheterized patients are difficult to interpret due to high rates of colonization and contamination during specimen collection, and have a low predictive value for true infection.[2] Indwelling urinary catheters are colonized at the rate 3–7% per day.[3, 4] Non-specific clinical signs and symptoms such as fever often trigger urine culture orders in hospitalized patients with indwelling urinary catheters. In addition, subjective findings like color or odor of urine or sediment in tube influence nurses and providers to over order urine cultures in catheterized patients.[5] In many instances, clinicians may not even be aware that the patient has a urinary catheter in place when ordering a urine culture.[6]
Overuse and misuse of urine cultures in catheterized patients leads to inappropriate antibiotic use and artificially inflates the diagnosis of catheter-associated urinary tract infections (CAUTIs).[1, 5, 7] Hence, Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) guidelines recommend replacing long-term urinary catheters before urine specimen collection.[8, 9] However, guidance on optimal timing of catheter exchange at the time of urine collection is unclear. Prior studies have examined time periods ranging from 24 hours to 14 days for catheter replacement at time of urine collection.[3, 10, 11] These interventions, however, primarily focused on the outcome of surveillance CAUTI, without measuring impact on antibiotic use or unintended consequences like catheter trauma.[3, 10–13]
Clinical decision support (CDS) can assist with appropriate urine testing and collection techniques, reduce diagnostic error, and improve antibiotic use. Our goal was to evaluate the effect of a CDS tool on health system-level urine culture volume, antibiotic utilization for urinary tract infection (UTIs), and catheter use, as well as examine safety signals related to catheter trauma among patients who required a catheter exchange.
Methods:
Study Design:
This pre- and post-intervention study examining the impact of CDS tool launched in March 2021 as part of a quality improvement (QI) initiative. The study was divided into two time periods: 12-month pre-intervention period (March 2020 to February 2021) and a 12-month post-intervention period (April 2021 to March 2022). The study was considered exempt by Duke University Institutional Review Board (Pro no. 00108749).
Setting and Population:
This study was conducted in three hospitals in North Carolina including: one academic medical center, Duke University Hospital (1048 inpatient beds), and 2 community hospitals, Duke Raleigh Hospital (175 beds) and Duke Regional hospital (388 beds). All emergency room visits and inpatient admissions of any age were eligible to prompt the CDS. Different base populations were utilized within this cohort to analyze the outcomes outlined below.
Intervention:
The CDS was designed to provide education on appropriate indications for urine culture in all patients regardless of catheter presence, use patient-specific data to prompt catheter exchange when needed, incorporate information from prior urine tests, and ultimately reduce the volume of inappropriate urine cultures. The panel was developed with input from the institutional infection prevention and antimicrobial stewardship teams, pediatrics, urology, and infectious disease clinicians. The four primary features implemented within the clinical decision support panel (Figure 1) were as follows: 1) passive educational information, 2) branching-logic identification of specific patient populations based on coded criteria (i.e., patients with indwelling urinary catheters stratified by duration of existing catheter placement, pediatric intensive care patients (PICU)), 3) an adjustable list of urine culture/nursing orders with the ‘recommended’ action based on the identified patient population listed first, and 4) identification of an existing or pending urinalysis order or result within 24 hours, with a prompt that defaults adding a urinalysis order if none found. When a clinician ordered a urine culture in a patient with an indwelling urinary catheter, the order panel provided education regarding appropriate clinical indications for a urine culture, and recommended catheter removal prior to urine culture for indwelling urinary catheters in place for greater than seven days, after excluding PICU patients and catheters with difficult placement (Supplemental Figure 1). Seven days was chosen as a time frame that would limit harm from rapid removal/replacement of urinary catheters but not allow for significant colonization of long-term indwelling catheters prior to urine culture compared to alternative time frames (i.e., < 24–48 hours after placement or up 14 days after placement). Finally, the intervention was also designed to limit additional ‘clicks’ in the electronic health record (EHR), and in fact for the most commonly encountered clinical scenarios the intervention actually reduced the number of clicks required to order a urine culture.
Figure 1: Branching Logic to Identify Appropriate Patient Populations for Panel Display.
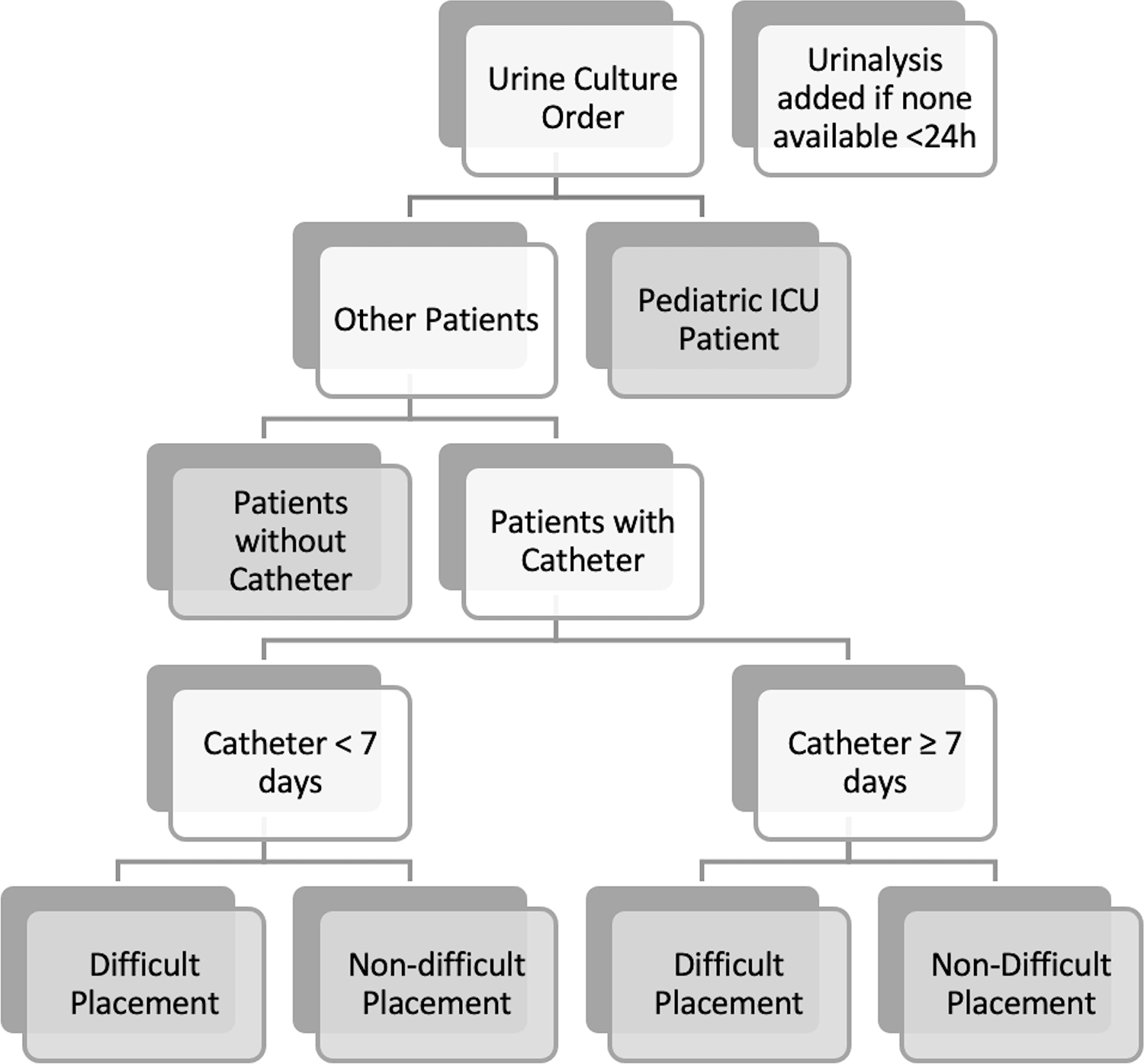
Gray boxes represent a branch-point ‘terminus’ that has unique decision support dependent on the population identified.
Outcomes:
Primary outcome was urine culture utilization, measured as urine cultures per 1000 patient days among all hospitalized patients. Secondary outcomes included urinalyses per 1000 patient days, days of antibiotic therapy with urinary tract infection (UTI) indication per 1000 patient days and urinary catheter Standardized Utilization Ratio (SUR) among all hospitalized patients. We also measured safety events related to catheter insertion as catalogued by event reports in the Duke Safety Reporting System (SRS). Outcomes measured among catheterized patients included number of catheter removal orders and catheter-associated urinary tract infections (CAUTIs) per 1000 catheter days.
Definitions:
CAUTI was defined according to Centers for Disease Control and Prevention (CDC)/ National Healthcare Safety Network (NHSN) surveillance criteria.[14] SUR was defined as the number of observed device days reported compared to predicted based on the NHSN model.[15] Antibiotic therapy for urinary tract infection (UTI) indication was defined as any hospital-administered antibiotic that had an electronically entered indication category of “genitourinary” or sub-categories of “uncomplicated urinary tract infection,” or “complicated urinary tract infection” at time of order.
Data Collection:
Electronic order entry data and lab result data were extracted from Duke’s Epic Clarity data warehouse. Antibiotic use data was extracted, processed, and cleaned via the Duke Antimicrobial Stewardship Outreach Network central database.[16] Antibiotic use was measured in days of therapy (DOT) per 1000 patient days for inpatient units reported to the National Healthcare Safety Network (NHSN). Urinary catheter SUR was calculated on a monthly basis as reported to the NSHN. Potential adverse events including catheter trauma were extracted via the Duke Safety Reporting System (SRS) database.
Analysis:
Outcomes were analyzed using an interrupted time series analysis. Pre-intervention rate trend (March 1, 2020 through February 28 2021) was compared to the post-intervention rate trend (April 1, 2021 through March 31, 2022) using Poisson logistic regression. A difference in rate change in March 2021 was also measured, though the data points for March 2021 were excluded from regression analysis as the CDS was implemented partway through this month. All analysis was performed in Python 3.7.
Results:
A total of 77,608 urine culture orders and 148,694 urinalysis orders were included within the study period analysis (excluding 3511 and 5754 orders, respectively, for March 2021). Poisson regression analysis revealed a significant decrease in urine culture orders per 1000 patient days in the post-intervention period (1.4% decrease per month, p<0.001, Figure 2). During the post intervention period, there was a total estimated reduction of 6,743 urine culture orders when compared to the estimated ‘without intervention’ model. Analysis also indicated an immediate increase of urinalyses orders by 6.2% (p<0.001, Figure 3) with a subsequent monthly decline of 1.4% per month (p<0.001), tracking with the decrease in urine cultures. By the end of the post-intervention period, comparison of the ‘with’ and ‘without intervention’ models indicated a reduction of total urinalyses by 2300 orders.
Figure 2. Trend of Urine Culture Orders in Pre and Post Intervention Period.
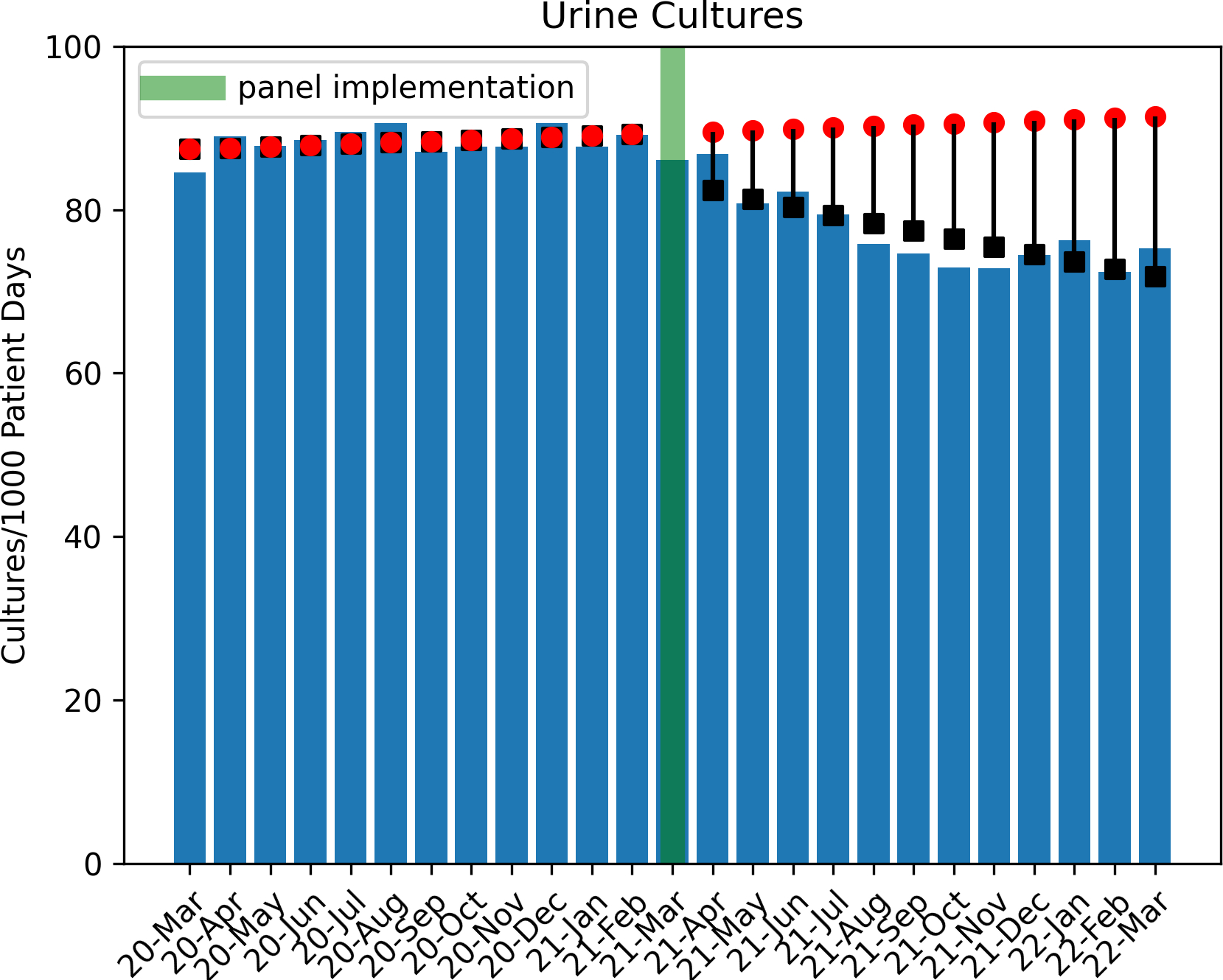
Circles indicate predicted outcome ‘without intervention’
Boxes indicate Poisson regression model estimates.
Figure 3. Trend of Urinalysis Orders in Pre and Post Intervention Period.
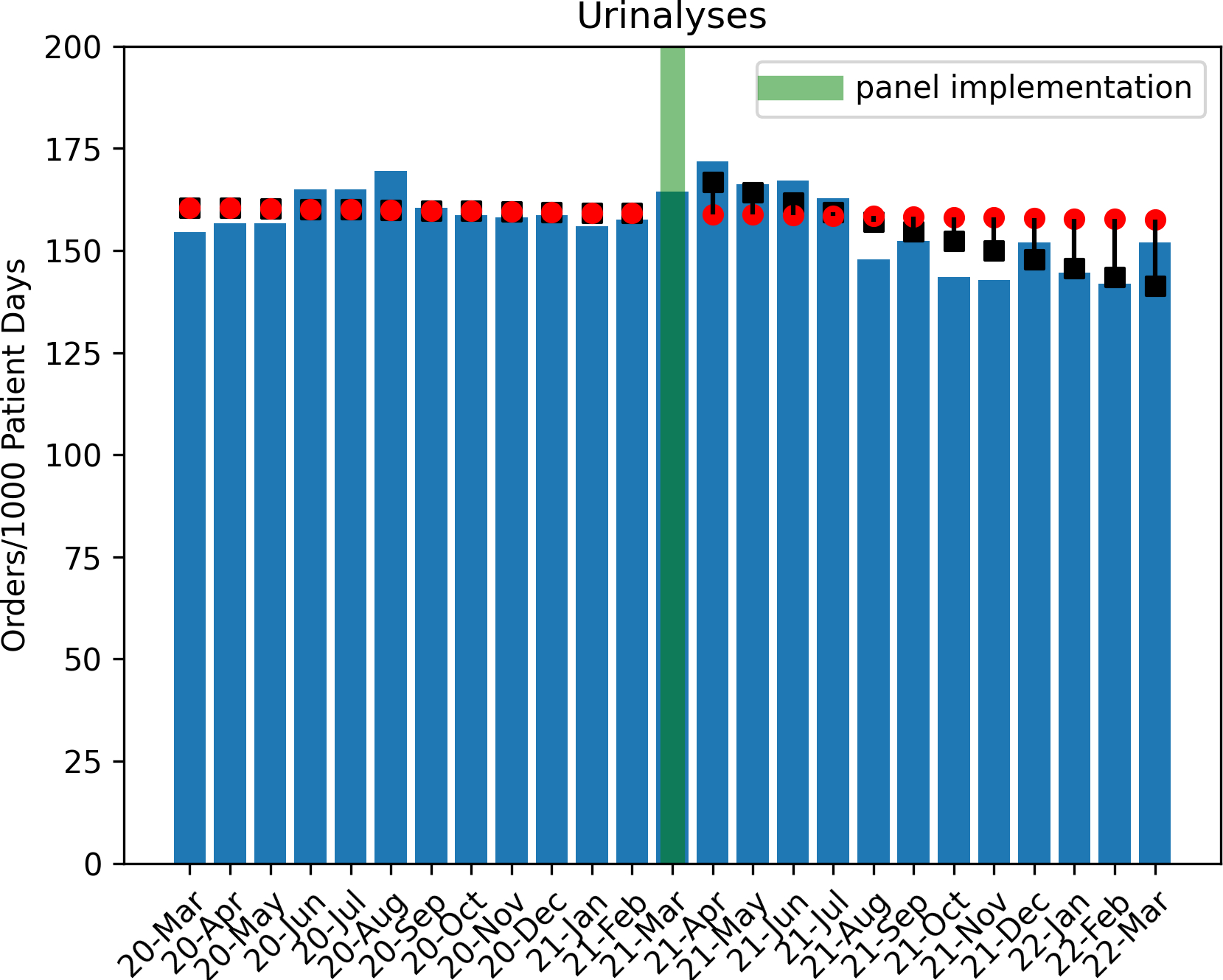
Circles indicate predicted outcome ‘without intervention’
Boxes indicate Poisson regression model estimates.
Antibiotic use for UTIs was increasing by 1.1 % per month in the pre-intervention period. At the time of panel intervention, there was an immediate decrease of 7.1% in antibiotic use for UTIs after March 2021 followed by a decreasing monthly trend of approximately 2.3% DOT per 1000 patient days in the post-intervention period (p=0.006, Figure 3). During the urine culture order entry process, when a catheter was detected (of any duration) by the panel logic, clinicians selected catheter removal in a total of 183 of 2133 instances (8.5%). Standardized Utilization Ratio (SUR) for urinary catheters and number of CAUTIs did not change significantly for the study period (Table 1, supplementary figures 1 and 2)
Table 1:
Impact of clinical decision support on urine test utilization and other outcomes in pre and post intervention period
| Interrupted Time Series Models with Poisson Regression | ||||
|---|---|---|---|---|
| Intercept | Baseline Trend RR (95% CI) |
Trend Change RR (95% CI) |
Level Change RR (95% CI) |
|
|
Urine Cultures (Urine cultures/1000 patient days per month) |
86.287 (83.044–89.657) |
1.002 (0.9999–1.005) |
0.986
*
(0.982–0.990) |
0.933
*
(0.906–0.962) |
|
Urinalyses (Urinalyses/1000 patient days per month) |
161.397 (156.876–166.048) |
0.999 (0.997–1.001) |
0.986
*
(0.983–0.989) |
1.064
*
(1.042–1.087) |
|
Antibiotic Use with GU or UTI Indication
(DOT/1000pd per month) |
64.592 (61.684– 67.636) |
1.011
*
(1.007–1.014) |
0.977
*
(0.973–0.982) |
0.929
*
(0.897–0.962) |
|
CAUTIs (CAUTIs/1000 foley days per month) |
0.057 (0.210–1.555) |
1.049 (0.976–1.128) |
0.956 (0.869–1.051) |
0.900 (0.468–1.734) |
|
SUR (SUR Rate per month) |
0.706 (0.224–2.221) |
1.000 (0.900–1.111) |
0.995 (0.798–1.241) |
1.038 (0.168–6.414) |
indicates p-value < 0.05
RR: Rate Ratio, DOT: Days of therapy, CAUTI: Catheter Associated Urinary Tract Infection, GU: Geintoruinary, UTI: Urinary Tract Infection, SUR: Standardized Utilization Ratio.
Evaluation of Duke Safety Reporting System (SRS) identified three events during the study period related to orders for removal or replacement or a urinary catheter. Two of the events occurred in the pre-intervention period, and a single event occurred in the post-intervention period. The event in the post-intervention period was related to catheter insertion, and was not associated with an order for catheter exchange from the urine culture order panel.
Discussion:
Clinical decision support for ordering urine cultures led to a decrease in overall utilization of urine cultures across our health system. The logic-based decision support also provided a simple, direct method for clinicians to identify and remove or exchange long-term indwelling catheters prior to obtaining a urine culture. Following implementation of this CDS, there was also a decrease in antibiotic use with UTI indications. Additionally, there were no instances of urethral trauma from replacement of long-standing catheters at or after day 7 using our logic.
Prior literature has shown that computerized CDS can improve inpatient antimicrobial utilization and contribute to diagnostic stewardship.[17] While some prior research highlighting CDS relied on urine culture indications as entered by clinicians, a process which increases the steps (or ‘clicks’) an end-user must take to order a test, we implemented a panel that added no additional workload to the ordering clinician. [18] Our findings of decreased diagnostic test (urine culture and urinalysis) utilization and UTI-specific antibiotic use are consistent with other studies using CDS at time of order entry. [18–20] As the large majority of tests ordering during the study period are on patients without a urinary catheter in place, our significant outcomes of decreased test utilization and antimicrobial stewardship are likely a result of the passive educational decision support component of the implemented order panel. Less literature exists describing CDS for catheter removal in the context of urine culture ordering. Frontera et al, found that a protocol requiring catheter removal at the time of urine sampling led to a significant reduction in CAUTI rates. However, this protocol involved replacing catheters in place for 24 hours at time of urine sampling, without measuring unintended consequences like catheter trauma.[3] In our initiative, we opted to allow for a longer duration of indwelling catheters before prompting removal to limit clinical scenarios requiring catheter replacement and potential trauma. This longer duration likely decreased a potential impact on CAUTI rates as catheter removal was not as strongly prompted within the 7-day period.
To our knowledge, this study is the first description of implementation of branching-logic CDS panel for the urine culture order process. A primary goal of this quality improvement initiative was to provide real-time education and relevant patient-centered clinical information while also streamlining the order process and reducing overall clicks required from clinicians to achieve the original desired outcome (urine culture order). Streamlining workflow in the EHR is often difficult to achieve in stewardship efforts which frequently require hard stops or additional clicks such as indication fields on antimicrobials or diagnostic tests. The results of this study indicate what may be the beginning of a sustained culture shift in ordering less urine cultures for patients that results in lower antimicrobial use with UTI indications, while avoiding the frustration inherent with the addition of new steps in EHR workflows. The branching-logic features also allowed clinicians to identify long-term indwelling catheters and prompt removal in safe and appropriate populations as identified by pediatric and adult infectious diseases and urology specialists.
While catheter removal was only selected in a fraction of patients with indwelling catheters (8.5%), catheter removal was not the original ‘intent’ of the clinician when entering into the urine culture order as prior to the logic-based pane implementation, catheter removal would not have bene part of the urine culture order workflow. Prior data has shown that over 20% of clinicians may not be aware that their patient has an indwelling urinary catheter.[6] Hence, our CDS intervention also serves as a subtle reminder to remove the catheter in these instances when a urine culture is being ordered, but the clinician is either unaware of the presence of the catheter, or has not assessed the ongoing need.
Our study is limited by its implementation at a single health system. Further, the pre- and post- intervention analysis is subject to time-associated confounding, including other stewardship efforts to reduce inappropriate urine culture ordering and antibiotic use for suspected UTIs. In addition, while we were able to evaluate to total number of instances that a clinician ordered catheter replacement when the panel detected one in place, due to limitations in data extraction we were not able to distinguish if these 183 orders occurred when the catheter was in place less than or greater than seven days. Finally, adverse events related to catheter trauma are difficult to capture and may be underrepresented by the SRS system (both before and after the intervention).
Our study is unique in that our primary outcomes include volume of urine culture orders and antibiotic use. Additionally, our data captured unintended consequences like catheter trauma. Our interval of seven days for prompting catheter removal or replacement may provide a more feasible time frame to balance early inappropriate removal vs. unnecessary longstanding catheters, as compared to prior studies that have recommended replacement early (24–48 hours) or late (14 days). However, the optimal duration until prompting clinicians to remove catheters with decision support, is unknown and future studies are needed to address this knowledge gap.
Many EHR-based interventions are implemented as QI initiatives, and thus it is difficult to disentangle the effect of multiple features of a decision support process that are simultaneous. Future studies that evaluate decision support for urine cultures in a randomized, controlled manner could identify the potential efficacy and effect size of the various decision support features. However, based on the results of our pre-/post- analysis of the QI initiative, our CDS tool was associated with reduced urine culture utilization and antibiotic use.
Supplementary Material
Supplemental Figure 1. Example Order Panel Display
Example order panel for a patient with indwelling catheter that had an uncomplicated insertion great than 7 days prior. © 2022 Epic Systems Corporation.
Supplemental Figure 2. Panel Urine Culture Order Entry Outcomes
Supplemental Figure 3. Trend of Catheter Associated Urinary Tract Infections in Pre and Post Intervention Period
Boxes indicate Poisson regression model estimates.
CAUTI - Catheter Associated Urinary Tract Infections
Figure 4. Trend of Antibiotic Utilization in Pre and Post Intervention Period.
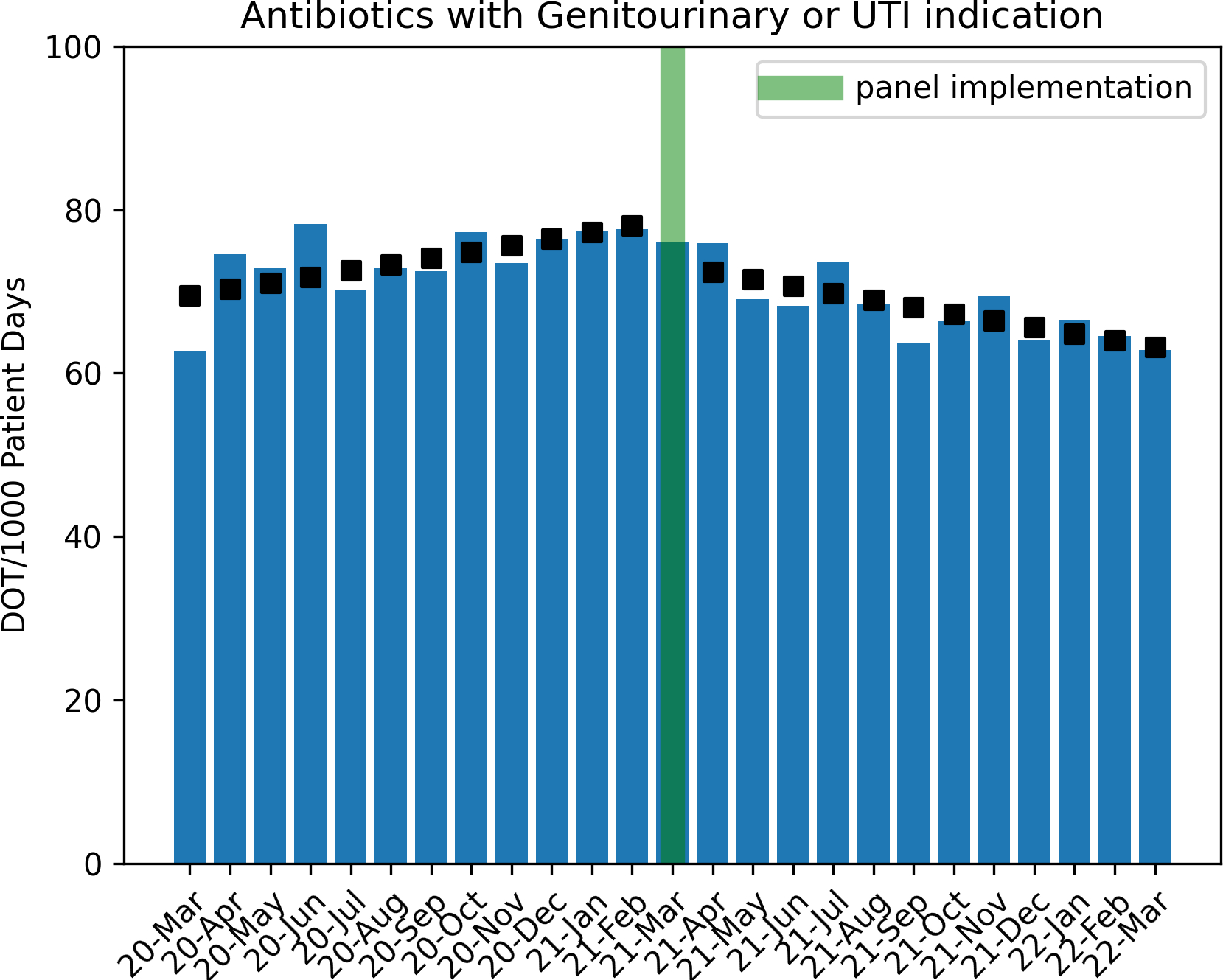
Boxes indicate Poisson regression model estimates.
UTI = Urinary Tract Indication
DOT = Days of Therapy
Acknowledgements:
This work was presented at IDWeek 2022 on Oct 22, 2022 at Washington, DC as a poster presentation #2240 titled “Implementation of a Clinical Decision Support Panel for Urine Culture Ordering”
Funding:
S.A. is supported by NIH-NIDDK K12DK100024 (KURe) for her effort.
Footnotes
Conflicts of interest. S.A. reports grants from the CDC, NIH-NIDDK K12DK100024, NIA/Pepper Center, and consulting fees from IDSA, Sysmex America, Locus Biosciences, GSK, Biomerieux, and co-ownership of IPEC Experts, LLC (unrelated to this work).
References
- 1.Nicolle LE, Gupta K, Bradley SF, et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin Infect Dis 2019; 68(10): e83–e110. [DOI] [PubMed] [Google Scholar]
- 2.Whelan P, Nelson A, Kim CJ, et al. Investigating Risk Factors for Urine Culture Contamination in Outpatient Clinics: A New Avenue for Diagnostic Stewardship. Antimicrob Steward Healthc Epidemiol 2022; 2(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frontera JA, Wang E, Phillips M, et al. Protocolized Urine Sampling is Associated with Reduced Catheter-associated Urinary Tract Infections: A Pre- and Postintervention Study. Clin Infect Dis 2021; 73(9): e2690–e6. [DOI] [PubMed] [Google Scholar]
- 4.Lo E, Nicolle LE, Coffin SE, et al. Strategies to prevent catheter-associated urinary tract infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014; 35(5): 464–79. [DOI] [PubMed] [Google Scholar]
- 5.Advani SD, Gao CA, Datta R, et al. Knowledge and Practices of Physicians and Nurses Related to Urine Cultures in Catheterized Patients: An Assessment of Adherence to IDSA Guidelines. Open Forum Infect Dis 2019; 6(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saint S, Wiese J, Amory JK, et al. Are physicians aware of which of their patients have indwelling urinary catheters? Am J Med 2000; 109(6): 476–80. [DOI] [PubMed] [Google Scholar]
- 7.Belton PJ, Litofsky NS, Humphries WE. Effect of Empiric Treatment of Asymptomatic Bacteriuria in Neurosurgical Trauma Patients on Surgical Site and Clostridium difficile Infection. Neurosurgery 2019; 85(5): 664–71. [DOI] [PubMed] [Google Scholar]
- 8.Hooton TM, Bradley SF, Cardenas DD, et al. Diagnosis, Prevention, and Treatment of Catheter-Associated Urinary Tract Infection in Adults: 2009 International Clinical Practice Guidelines from the Infectious Diseases Society of America. Clinical Infectious Diseases 2010; 50(5): 625–63. [DOI] [PubMed] [Google Scholar]
- 9.Lo E, Nicolle LE, Coffin SE, et al. Strategies to Prevent Catheter-Associated Urinary Tract Infections in Acute Care Hospitals: 2014 Update. Infection Control & Hospital Epidemiology 2014; 35(S2): S32–S47. [PubMed] [Google Scholar]
- 10.Raz R, Schiller D, Nicolle LE. Chronic indwelling catheter replacement before antimicrobial therapy for symptomatic urinary tract infection. J Urol 2000; 164(4): 1254–8. [PubMed] [Google Scholar]
- 11.Babich T, Zusman O, Elbaz M, et al. Replacement of Urinary Catheter for Urinary Tract Infections: A Prospective Observational Study. J Am Geriatr Soc 2018; 66(9): 1779–84. [DOI] [PubMed] [Google Scholar]
- 12.Lesho EP, Clifford R, Vore K, et al. Sustainably reducing device utilization and device-related infections with DeCATHlons, device alternatives, and decision support. Infection Control & Hospital Epidemiology 2020; 41(11): 1344–7. [DOI] [PubMed] [Google Scholar]
- 13.Cooper FPM, Alexander CE, Sinha S, Omar MI. Policies for replacing long-term indwelling urinary catheters in adults. Cochrane Database of Systematic Reviews 2016; (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CDC. Urinary Tract Infection (Catheter-Associated Urinary Tract Infection [CAUTI] and Non-Catheter-Associated Urinary Tract Infection [UTI]) Events Available at: https://www.cdc.gov/nhsn/pdfs/pscmanual/7psccauticurrent.pdf. Accessed 10/1/22.
- 15.CDC. A Guide to the SUR. Available at: https://www.cdc.gov/nhsn/pdfs/ps-analysis-resources/nhsn-sur-guide-508.pdf.
- 16.Moehring RW, Yarrington ME, Davis AE, et al. Effects of a Collaborative, Community Hospital Network for Antimicrobial Stewardship Program Implementation. Clin Infect Dis 2021; 73(9): 1656–63. [DOI] [PubMed] [Google Scholar]
- 17.McGregor JC, Weekes E, Forrest GN, et al. Impact of a computerized clinical decision support system on reducing inappropriate antimicrobial use: a randomized controlled trial. J Am Med Inform Assoc 2006; 13(4): 378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watson KJ, Trautner B, Russo H, et al. Using clinical decision support to improve urine culture diagnostic stewardship, antimicrobial stewardship, and financial cost: A multicenter experience. Infect Control Hosp Epidemiol 2020; 41(5): 564–70. [DOI] [PubMed] [Google Scholar]
- 19.Keller SC, Feldman L, Smith J, Pahwa A, Cosgrove SE, Chida N. The Use of Clinical Decision Support in Reducing Diagnosis of and Treatment of Asymptomatic Bacteriuria. J Hosp Med 2018; 13(6): 392–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirley D, Scholtz H, Osterby K, Musuuza J, Fox B, Safdar N. Optimizing Inpatient Urine Culture Ordering Practices Using the Electronic Medical Record: A Pilot Study. Infection Control & Hospital Epidemiology 2017; 38(4): 486–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Example Order Panel Display
Example order panel for a patient with indwelling catheter that had an uncomplicated insertion great than 7 days prior. © 2022 Epic Systems Corporation.
Supplemental Figure 2. Panel Urine Culture Order Entry Outcomes
Supplemental Figure 3. Trend of Catheter Associated Urinary Tract Infections in Pre and Post Intervention Period
Boxes indicate Poisson regression model estimates.
CAUTI - Catheter Associated Urinary Tract Infections


