Abstract
The influential “premotor theory of attention” proposes that developing oculomotor commands mediate covert visual spatial attention. A likely source of this attentional bias is the frontal eye field (FEF), an area of the frontal cortex involved in converting visual information into saccade commands. We investigated the link between FEF activity and covert spatial attention by recording from FEF visual and saccade-related neurons in monkeys performing covert visual search tasks without eye movements. Here we show that the source of attention signals in the FEF is enhanced activity of visually responsive neurons. At the time attention is allocated to the visual search target, nonvisually responsive saccade-related movement neurons are inhibited. Therefore, in the FEF, spatial attention signals are independent of explicit saccade command signals. We propose that spatially selective activity in FEF visually responsive neurons corresponds to the mental spotlight of attention via modulation of ongoing visual processing.
Keywords: vision, saccade, attention, monkey, physiology, premotor
Introduction
Humans and monkeys are able to select and acquire visual information preferentially within a locus of peripheral vision without shifting gaze (Posner, 1980; Kinchla, 1992; Egeth and Yantis, 1997). This ability, known as covert spatial attention, often is compared metaphorically with a mental spotlight that illuminates a selected area or object for enhanced processing. Behavioral studies have shown that covert spatial attention and overt eye movements are closely linked (Hoffman and Subramaniam, 1995; Kowler et al., 1995; Sheliga et al., 1995a,b; Deubel and Schneider, 1996) and support the premotor theory of attention, which proposes that covert attention arises from latent eye movement commands even when eye movements are not made (Rizzolatti et al., 1987; Sheliga et al., 1995a; Moore et al., 2003). Additional support for this theory comes from studies of the frontal eye field (FEF), an area in the frontal cortex that, in addition to generating saccade commands (Bruce and Goldberg, 1985; Bruce et al., 1985; Hanes and Schall, 1996; Tehovnik et al., 2000), plays a central role in the allocation of spatial attention in both humans (Corbetta et al., 1998; Beauchamp et al., 2001; Corbetta and Shulman, 2002; Grosbras and Paus, 2002; Muggleton et al., 2003; Kincade et al., 2005) and monkeys (Moore et al., 2003; Moore and Fallah, 2004). This evidence has led to the hypothesis that FEF saccade-related movement neurons mediate covert attention by modulating the gain of neurons in extrastriate visual cortex (Hamker, 2005). Although many studies are consistent with the premotor theory of attention, the fact that the FEF plays a role in both covert attention and eye movements does not mean necessarily that covert attention and eye movements originate from the same source; they could be mediated by different processes (Klein and Pontefract, 1994).
In monkeys performing visual search tasks traditionally used to study visual attention, the activity of FEF neurons evolves to identify the target of a search array before a saccade is made (Schall and Hanes, 1993; Schall et al., 1995b; Thompson et al., 1996, 2005; Bichot et al., 2001a,b; Sato et al., 2001; Sato and Schall, 2003). This selection process does not depend on saccade production (Thompson et al., 1997; Murthy et al., 2001; Sato et al., 2003). However, in all of these studies, the saccades were a prominent component of the either the task or the monkeys’ training. Therefore, it could be argued that the selection process could reflect some component of saccade planning.
In this study, we recorded from single neurons in the FEF while monkeys performed a pop-out visual search task requiring a manual response in which there was clear evidence of absence of saccade planning. First, we tested the hypothesis that there is activity in the FEF that corresponds to the locus of attention during visual search that cannot be attributed to previous training or to saccade production. Second, we tested the specific prediction that, when attention shifts covertly to a target in the visual field, motor activity for a saccade toward the locus of attention also should be present. We found that a spatially selective signal that could correspond to the spotlight of attention was present in the activity of most visually responsive FEF neurons, but the activity of movement neurons was suppressed.
Materials and Methods
Data collection
Two experimentally naive male monkeys (Macaca mulatta), weighing 8 kg (monkey S) and 6.5 kg (monkey C), were prepared for electrophysiological recordings. All surgical and experimental protocols were approved by the National Eye Institute Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Sterile surgery was performed under ketamine and isoflurane anesthesia to place a head-holding device, a plastic recording chamber over the left FEF, and a scleral search coil. The FEF was localized within the recording chamber by using low current microstimulation (<50 μA) to evoke saccades and by the presence of saccade-related movement neurons (Bruce and Goldberg, 1985). Recording sites were confirmed to be in the rostral bank of the arcuate sulcus histologically in monkey S and by magnetic resonance imaging in monkey C.
Visual stimulation and behavioral control were done by a computer running the real-time experimentation data acquisition system (REX) (Hays et al., 1982). Visual stimuli were presented on a computer monitor (26 × 21 cm; 1024 × 768 pixel resolution; 85 Hz frame rate) viewed at a distance of 57 cm. Action potential waveforms were recorded with tungsten microelectrodes, digitized, and saved by using a computer-based data acquisition system (Plexon, Dallas, TX). Often two or three units were recorded simultaneously. Off-line spike sorting separated single units on the basis of size and shape of the spike waveforms. Analog eye position and lever position signals were digitized and sampled at 1 kHz.
Behavioral training and tasks
The monkeys used in this experiment had no previous experience in performing behavioral tasks. Monkeys were seated in a primate chair with the head fixed. Using operant conditioning with positive reinforcement, we trained the monkeys to perform a memory-guided saccade task and a covert visual search task. The two tasks were run in separate blocks of trials.
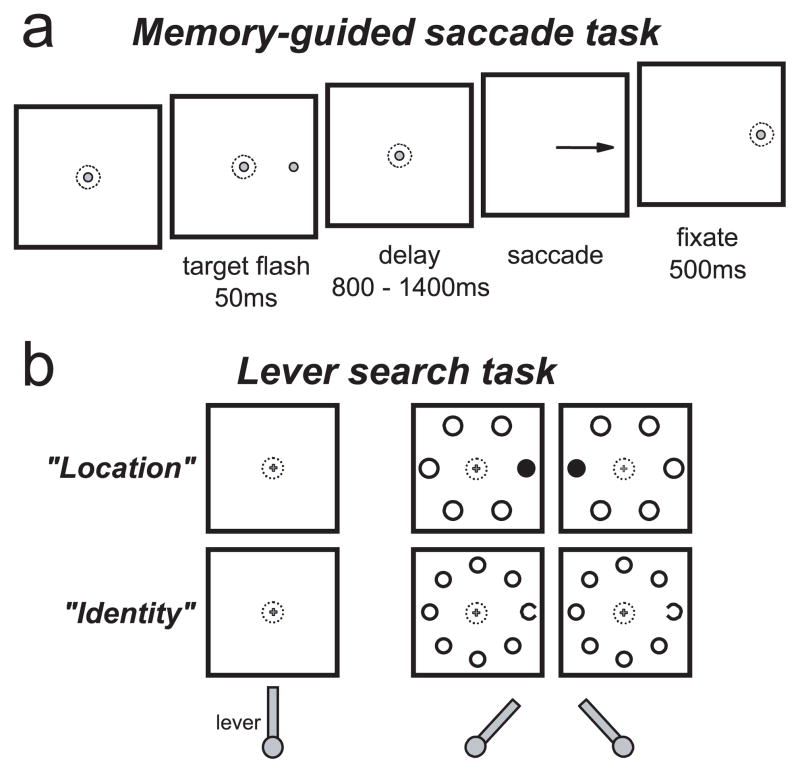
The memory-guided saccade task was used to distinguish visual from movement activity for cell classification and to map the spatial extent of the response field of each neuron (Bruce and Goldberg, 1985) (see Fig. 1a). After the monkey fixated on a 0.3° diameter gray spot on a black background for 400–800 ms, an identical spot was flashed for 50 ms at a peripheral location. The monkeys were required to maintain fixation on the central spot for a random interval ranging from 800 to 1400 ms. After the central spot disappeared, the monkeys were rewarded for making a saccade to the remembered location of the target. Once gaze shifted, the target reappeared to provide feedback and a fixation target for the monkeys.
Figure 1.
The tasks. a, The memory-guided saccade task. After the monkey fixated on a central spot, a peripheral stimulus identical to the fixation spot was flashed for 50 ms at one of six or eight locations. After a delay, the fixation spot was removed, and the monkey was instructed to make a saccade to the remembered target location. b, The manual lever search task. After the monkey grasped a lever in the vertical position, a small fixation cross appeared. After the monkey fixated on the central cross, a search array appeared in which one of the stimuli was different. In the location search (“Location”), the monkey was rewarded for turning the lever in the same direction as a different-colored stimulus in relation to the fixation cross. In the identity search (“Identity”), the monkey was rewarded for turning the lever in the same direction as the gap in the C stimulus.
Covert visual search tasks were used to examine neural activity during visual search without saccades (see Fig. 1b). A lever that could be turned left or right of vertical was attached to the front of the chair within easy reach of the monkey. When no force was applied to the lever, a spring automatically returned it to the vertical position. Although the monkeys were free to use either hand to turn the lever, monkey S was exclusively left-handed and monkey C was exclusively right-handed.
The location and identity variations (see Fig. 1b) of this task had the same temporal structure. After the monkey grasped the lever and positioned it within 10° of vertical, a small (0.3°) central yellow fixation cross appeared on a black background. The different fixation stimulus was used to help distinguish this task from the memory-guided saccade task. In this task, the monkeys were required to maintain fixation on the central stimulus until the reward. After the monkeys fixated on the central cross for a random interval (400–800 ms), a target was presented randomly at one of six or eight isoeccentric locations spaced equally around the fixation cross. The remaining locations were occupied by distractors. Each of the stimuli subtended 1.5° of visual angle, and the eccentricity of the stimuli was adjusted so that at least one of the stimulus locations was inside the receptive field of the neuron. The monkeys were rewarded for making the correct lever turn (>15° from vertical) within 2 s after search array presentation; in practice, the monkeys nearly always turned the lever to the limit of 35° from vertical. If the monkey broke fixation at any time during the trial, released the lever, or made an incorrect lever turn, the trial was aborted immediately. The reward was given immediately after a correct lever turn; however, the fixation spot and search array remained on for an additional 250–500 ms, and during this time the monkeys were free to make saccades without penalty. This was done to probe whether there were latent saccade plans that were being suppressed until after the reward. The intertrial interval from the removal of the visual stimuli and the reappearance of the fixation spot at the beginning of the next trial was at least 500 ms. Longer intertrial intervals occurred when the monkeys did not maintain gaze at the central location between trials or when the lever was not held in the vertical position.
Monkey S was trained to report the location of the color singleton target of the search array. The stimuli were isoluminant green and red disks. The target could be either green or red, but within a block of trials, the color of the target and distractors did not change. Six stimulus locations were used; three were to the left and three were to the right of the fixation cross. A correct response was a lever turn corresponding to the location of the target stimulus relative to the fixation spot.
Monkey C was trained to report the identity of a Landolt C among O distractors. The stimuli were gray rings, with one of them having a 0.5° gap randomly on the left or right. Eight stimulus locations were used. A correct response was a lever turn corresponding to the location of the gap in the Landolt C.
Data analysis
Lever position and eye position were sampled at 1000 Hz. Saccades were detected by using a computer algorithm that searched for elevated eye velocity (>20°/s). Saccade initiations and terminations then were defined as the beginnings and ends of the monotonic changes in eye position that lasted at least 10 ms. A lever turn was defined as a turn >15° from vertical. The beginning and end of each lever turn were defined as the beginning and end of the monotonic change in lever position before and after the 15° threshold was reached. The time of the beginning of the lever turn on each trial was used as the reaction time for that trial.
Activity recorded in the memory-guided saccade task was used for neuron classification. Activity was measured as a spike count per trial occurring in 150 ms time intervals. The visual response was measured between 50 and 200 ms after the target flash. Baseline activity was measured during the last 150 ms before target presentation. The movement response was measured between 100 ms before and 50 ms after saccade initiation. Delay period activity was measured in a 150 ms interval of the delay period beginning 300 ms before the fixation spot disappeared, which cued the monkey to make a saccade to the remembered target location. The nonparametric Wilcoxon rank sum test was used to test for significant differences in spike counts across conditions. A neuron was defined as being visually responsive if the visual response was significantly greater than baseline activity (p <0.05). A neuron was defined as being movement-related if the movement response was significantly greater than the late delay period activity. Neurons were classified as visual, visuomovement, or movement (see Fig. 5) based on these two statistical tests. A neuron was defined as being selective in the covert visual search task if the number of spikes per trial occurring during the interval from 100 to 250 ms after the presentation of the search array was significantly greater (p <0.05) on trials in which the target of the search array fell in the receptive field of the neuron than on trials in which only distractors fell in the receptive field. The average spike density functions shown in Figures 3, 4, and 6 were obtained with a kernel that projects activity forward in time and approximates an EPSP (Thompson et al., 1996) and are used for viewing average spike activity only.
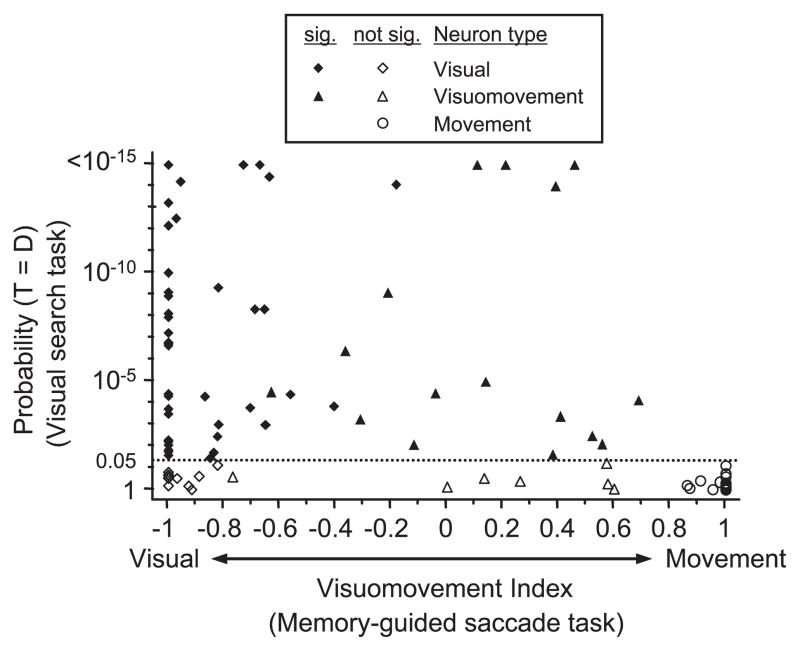
Figure 5.
Statistical analysis of spatially selective activity in the covert visual search task as a function of neuron classification. The probability that the activity from 100 to 250 ms after search array presentation is the same on trials in which the target (T) is in the response field and on trials in which distractors (D) are in the response field is plotted as a function of the visuomovement index for each neuron. The visuomovement index is calculated as a contrast ratio of the visual and saccade-related responses recorded during the memory-guided saccade task. Neurons with values near −1 are dominated by a visual response, and neurons near +1 are dominated by saccade-related activity. Values near 0 indicate nearly equivalent visual and saccade-related activation. Visual neurons (diamonds) exhibit significant (sig.) visual responses and a no-movement response. Visuomovement neurons (triangles) have significant visual and movement responses. Movement neurons (circles) have no visual response and significant movement responses. Filled symbols indicate neurons with significantly different activity for the target and distractors in the covert visual search task. The horizontal dotted line indicates the probability threshold for a significant difference (p < 0.05). Six neurons (3 visual and 3 visuomovement) showed p <10−15.
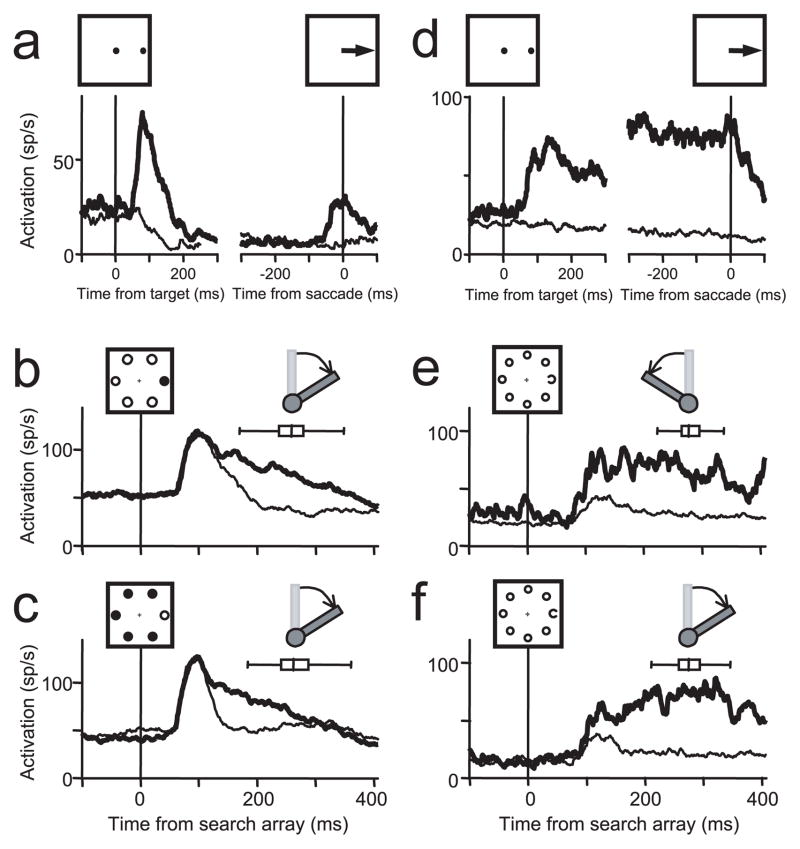
Figure 3.
Representative examples of two visually responsive FEF neurons. a–c, The activity of a visuomovement neuron recorded during the memory-guided saccade task aligned on both target onset and saccade initiation (a) and the two complements of the location search task in which the target was red and the distractors were green (b) or in which the target was green and distractors were red (c). The monkey was rewarded for maintaining fixation on the central cross and indicating the location of the singleton target with a lever turn. d–f, The activity of a visual neuron recorded during the memory-guided saccade task aligned on both target onset and saccade initiation (d) and the identity search task in which the monkey was rewarded for indicating the direction of the C target among O distractors as pointing left (e) or right (f). For all plots, the activity on trials in which the target landed in the receptive field of the neurons (thick line) is plotted with the activity on trials in which no stimulus (a, d, thin line) or in which distractors (b, c, e, f, thin line) landed in the receptive field of the neurons. The box-whisker plot in each search panel indicates the median, quartiles, and range of lever turn reaction times. Diagrams showing the correct direction of the lever turn when the target was in the receptive field of the neurons are above each box-whisker plot.
Figure 4.
A representative example of a movement neuron recorded during the memory-guided saccade task (a) and the location search task (b). Conventions are the same as in Figure 2, with the exception that the activity recorded during the lever search task is plotted until the time of the removal of the search array stimuli, which occurred ~700 ms after search array presentation (b). The box-whisker plot in b indicates the median, quartiles, and range of lever turn reaction times. A diagram showing the correct direction of the lever turn when the target was in the receptive field of the neurons is above the box-whisker plot.
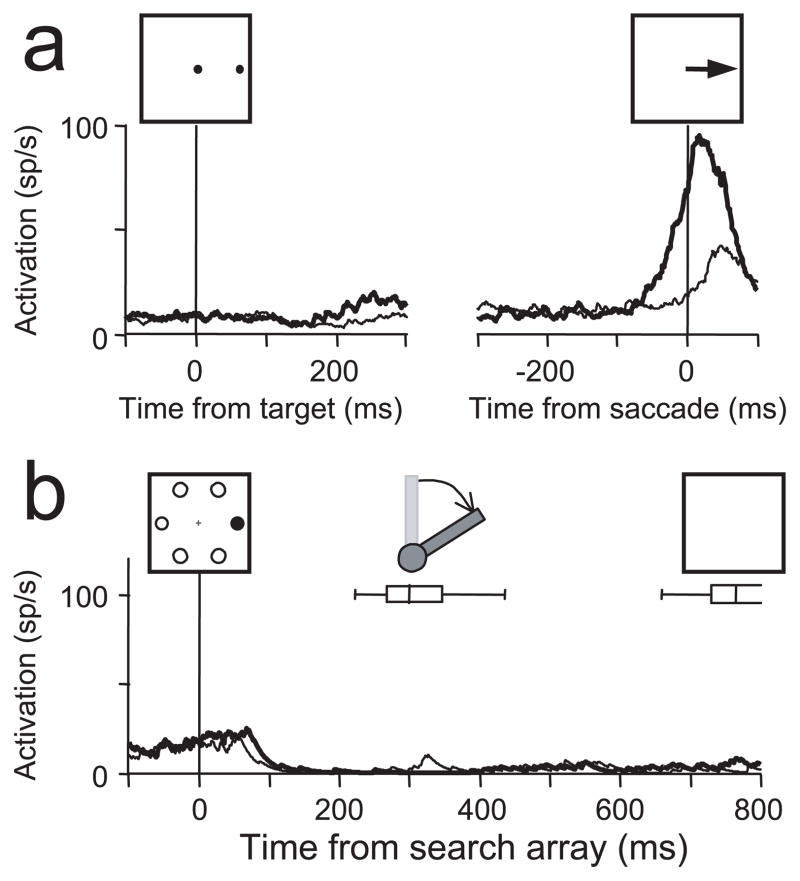
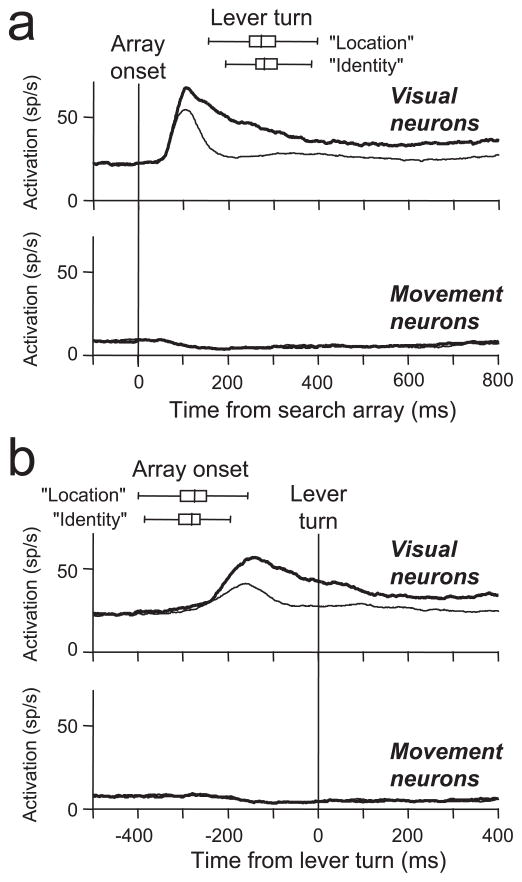
Figure 6.
Pooled average activity from FEF neurons recorded during the lever search tasks aligned on the time of the search array presentation (a) and the time of the initiation of the lever turn (b). The activity of target-selective visually responsive neurons and movement neurons is shown separately. Thick lines plot the average activity on trials in which the target landed in the response field. Thin lines plot the average activity on trials in which only distractors landed in the response field. The spatial extent of the response field was based on activity recorded during the memory-guided saccade task (see Figs. 3a,d, 4a). For the movement neurons, the target-related and distractor-related activity was nearly identical and cannot be differentiated in the plots. The box-whisker plots show the median, quartile, and ranges of the lever turn reaction times (a) and search array presentation times (b) separately for the location and identity search tasks.
Results
In two monkeys, we controlled the locus of exogenously driven covert attention with a pop-out search task without eye movements (Fig. 1b). A salient oddball stimulus was presented among homogeneous distractors. The location of the target was randomized from trial to trial. In this situation, the target stimulus popped out, automatically attracting attention (Theeuwes, 1994; Joseph and Optican, 1996; Egeth and Yantis, 1997; Nothdurft, 1999; Turatto and Galfano, 2000; Turatto et al., 2004). In addition, the salient target stimulus was behaviorally relevant, which also encouraged subjects to focus attention to the location of the target for visual analysis (Nothdurft, 2002). The monkeys were trained to report with a manual lever turn either the location (monkey S) or the orientation (monkey C) of a singleton target among distractors without shifting gaze from a central fixation spot (Fig. 1b). Because the monkeys were required to recognize the target stimulus to guide their behavior, we are confident that their attention was directed to the location of the target stimulus before the manual report.
Although the two monkeys were trained on different versions of the covert visual search task, their behavioral performance was similar. Overall, the monkeys performed correctly on 80% of the trials (monkey S, 82.3% correct; monkey C, 77.3% correct), which was well above the chance level of 50% (p < 0.001). The reaction times measured from the presentation of the search array to the beginning of the lever turn averaged 283.9 ms (SD = 72.8 ms) for monkey S in reporting the location of the singleton color target and 297.4 ms (SD = 91.3 ms) for monkey C in reporting the identity of the Landolt C among O distractors.
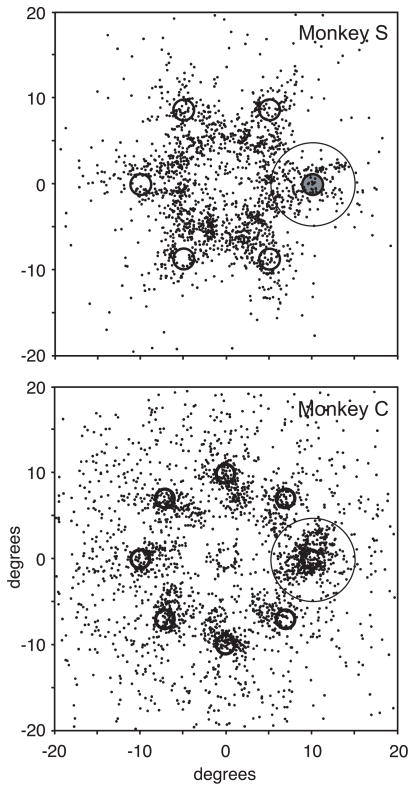
An analysis of gaze behavior does not show evidence for latent saccade planning to the singleton target while the monkeys performed the manual lever search tasks (Fig. 2). For both monkeys, the reward was given within 100 ms of a correct lever turn. If the monkey broke fixation at any time before the liquid reward, the trial was aborted. However, after reward delivery, the search array and the fixation cross remained on the video screen for an additional 250 ms for monkey S and an additional 500 ms for monkey C. During this time, the monkeys were free to shift gaze without penalty. Nevertheless, they nearly always maintained fixation on the central fixation spot until the search array was removed (monkey S, 96% of trials; monkey C, 82% of trials). Even when the fixation spot and the search stimuli were removed from the video screen, the monkeys still tended to maintain fixation at the center of the screen, awaiting the beginning of the next trial that started 500 ms later. By 500 ms after the removal of the fixation spot and search stimuli, monkey S continued to maintain fixation at the central position after 69% of the trials and monkey C after 52% of the trials.
Figure 2.
Saccade behavior of monkey S during the location search task (top) and monkey C during the identity search task (bottom). Each point plots the endpoint of the first postreward saccade on each trial in which a saccade was made within a time window ending 500 ms after the search array was removed. The oddball stimulus is shown at the right horizontal position at 10° eccentricity, and the saccade endpoints were rotated and scaled accordingly for display. The circle around the target represents the 5° window in which saccades were counted as being made to the target location after the reward. These trials were removed from the neural activity analysis. For monkey S, a saccade was made after 1460 of 4710 trials (31%) and landed within 5° of the target on 188 trials (4%). For monkey C, a saccade was made after 2384 of 4966 trials (48%) and landed within 5° of the target on 394 trials (8%).
If saccades were being planned covertly, one would expect that the saccades that were made after either the reward or the removal of the fixation cross would tend to land near the target location. However, this was not the case. Postreward saccades tended to land near one of the search stimulus locations. However, they did not tend to be directed toward the target. Also, many saccades were made to the edge of the video screen or other objects within the monkeys’ field of vision (Fig. 2). The saccades that were made between the time of reward delivery and 500 ms after the removal of the fixation cross and search stimuli landed within 5° of the target location on only 4% of the trials for monkey S and 8% of the trials for monkey C. Because we were interested in FEF activity without saccade planning, these trials were removed from the neural activity analysis because of the possibility that saccades to the target may have been programmed during those trials. Trials in which the target was presented near the edge of the receptive field were not used in the neural activity analysis. As a result, on average, only 1.2 trials for monkey S and 2.2 trials for monkey C were removed from the analysis of activity for each neuron because of the saccade endpoint being near the target. The removal of these rare trials did not change the results in any way. Also, the results did not change when all trials in which saccades were made within 500 ms after the removal of the search array were removed from the analysis.
We recorded the activity of 152 neurons in the FEFs of the two monkeys performing a memory-guided saccade task (Fig. 1a) and one of the manual search tasks (Fig. 1b). Activity recorded during the memory-guided saccade task was used for neuron classification (Bruce and Goldberg, 1985) and for mapping the extent of the receptive field. A total of 101 neurons (50 from monkey S and 51 from monkey C) exhibited visual and/or saccade-related activity during the memory-guided saccade task. These neurons provided the data for the analysis of activity collected during the manual search tasks.
Visually responsive neurons
In total, 80 neurons were visually responsive. Figure 3 plots the activity of two typical visually responsive neurons, one from each monkey. The neuron shown in Figure 3a–c is from monkey S. In the memory-guided saccade task (Fig. 3a), this neuron had a phasic visual response after the target flash, no activity during the delay period before the instruction to make a saccade, and a growth of activity before the saccade to the remembered target location. Many visually responsive neurons, like this one, were classified as visuomovement neurons (n = 23) because they exhibited increased activation before saccades during the memory-guided saccade task (Fig. 3a). The neuron shown in Figure 3d–f is from monkey C and is an example of a visual neuron. In the memory-guided saccade task (Fig. 2d), this neuron had a sustained visual response after the target flash that lasted through the delay period and no activity increase before the saccade. In the manual lever search task (Fig. 3b,c,e,f), the activity of both neurons evolved to select the location of the singleton target before the lever turn.
The majority (77.5%) of visually responsive neurons exhibited significantly greater responses (Wilcoxon rank sum test; p < 0.05) before the lever turn when the singleton target was in the receptive field than when distractors were in the receptive field. The results from monkey S performing the location search task (27 of 38 visually responsive neurons were selective, 71%) and from monkey C performing the identity search task (35 of 42 visually responsive neurons were selective, 83%) were similar. Because all previous studies showing spatial selectivity in the FEF incorporated saccades as part of the task, it was unknown whether FEF activity would select the singleton target of a search array without saccades. A real possibility was that spatial selectivity occurs in the FEF only when saccades are produced. Therefore, the first main result of this study is that the activity of the majority of visually responsive neurons evolved to select the location of the singleton target before the monkey turned the lever without any behavioral or physiological evidence of saccade planning. The physiological evidence for the absence of saccade planning will be presented in the next section.
FEF visual responses typically do not exhibit selectivity to a specific feature of a visual stimulus (Mohler et al., 1973; Schall et al., 1995b); however, under some training conditions, FEF neurons can exhibit color selectivity (Bichot et al., 1996). This possibility was addressed in monkey S. This monkey was trained to report the location of the singleton color with a corresponding lever turn. When the target was to left of the fixation spot, the correct response was to turn the lever leftward and to turn the lever rightward when it was on the right. For 10 of the visually responsive neurons that exhibited significant selection, we were able to collect data while the monkey performed a block of red-target-among-green distractor trials and a block of green-target-among-red distractor trials. The neuron shown in Figure 3a–c is one example. Figure 3b shows the activity when the singleton target was red, and Figure 3c shows the activity of the same neuron when the singleton target was green. In this neuron, as in all 10 neurons tested with both complements of the search array, the activity was greater when the singleton target was in the receptive field than when only distractors were in the receptive field regardless of color.
Although it has been shown that FEF neurons are not active for arm movements (Mushiake et al., 1996), the possibility that the neurons were responding to the direction of the lever turn was addressed in monkey C. This monkey was trained to report the orientation of the Landolt C, which varied randomly from trial to trial. Thus a target at the same location could result in opposite manual responses. The neuron shown in Figure 3d–f is one example. Figure 3e shows the activity from trials in which the C was oriented leftward and the lever turns were leftward, and Figure 3f shows the activity from trials in which the C was oriented rightward and the lever turns were rightward. In this neuron, as in all 35 of the selective neurons tested with this version of the task, the activity was greater when the C was in the receptive field than when only O distractors were in the receptive field, regardless of direction of the manual lever turn.
Movement neurons
Twenty-one movement neurons were recorded in the manual lever search task, 12 from monkey S and 9 from monkey C. Movement neurons are those neurons that did not exhibit a visual response after the target flash and had a growth of activity before the saccade in the memory-guided saccade task (Bruce and Goldberg, 1985). The neuron shown in Figure 4 is one example. The second main result of this study is that none of the 21 purely movement neurons in the sample showed any selectivity during the manual lever search task. The neuron shown in Figure 4b showed a significant decrease in activity after the presentation of the search array that lasted throughout the trial, even after the lever turn and the reward, until the search array was removed. The suppression was not spatially specific; it occurred equally on trials in which the singleton target of the search array was in the response field (Fig. 4b, thick line) and on trials in which distractors were in the response field (Fig. 4b, thin line). Across the entire sample, 57% (12 of 21) of the movement neurons exhibited a similar significant reduction in activity (p < 0.05). When we considered only those neurons with baseline activity (before the presentation of the search array) of >4 spikes/s, the percentage of movement neurons that had a significant reduction in activity during the search trials increased to 86% (12 of 14).
Population activity
In memory-guided saccade tasks, FEF neurons respond along a visuomotor continuum, exhibiting differing degrees of visual and saccade-related activation (Bruce and Goldberg, 1985). At one end of the continuum, visual neurons respond to the presentation of a visual stimulus in their receptive field, but they do not exhibit an increase in activity before saccades (Fig. 3d). At the other end of the continuum, movement neurons exhibit increased activity before and during saccades, but no activity after the presentation of a visual stimulus (Fig. 4a). Visuomovement neurons are intermediate, exhibiting both visual and saccade-related activity (Fig. 3a). To quantify this continuum, we calculated a visuomovement index for each neuron as the contrast ratio between the visual response and the movement response in the memory-guided saccade task [(movement − visual)/(movement + visual)] (for details, see Materials and Methods). For this calculation, the baseline activity was subtracted from the visual response, and late delay period activity was subtracted from the movement response. Negative visual or movement responses were rounded to zero.
To determine whether neuron type was related to spatial selectivity during the covert visual search task, we plotted the probability that the target response was equal to the distractor response in the covert visual search task as a function of visuomovement index (Fig. 5). Each neuron also was classified as visual, visuomovement, or movement, based on whether it exhibited statistically significant visual-related and/or saccade-related activity in the memory-guided saccade task. There was no significant difference in the results from visual neurons (46 of 57 visual neurons were selective) and visuomovement neurons (18 of 23 visuomovement neurons were selective) (χ2; p = 0.25). In addition, all neurons that exhibited spatial selectivity in the manual lever search task had significant visual responses in the memory-guided saccade task. The nonvisually responsive movement neurons were the only neuron population that did not exhibit any selectivity in the manual lever search task. These results show that an FEF neuron must be visually responsive to signal spatial selectivity without saccade production. Nonvisually responsive movement neurons do not exhibit activity associated with covert attention.
Figure 6 shows the pooled activity from all 80 visually responsive neurons and all 21 nonvisually responsive movement neurons recorded during the manual lever search task. After the presentation of the search array (Fig. 6a), on average, visual neurons initially responded equally well to the target and distractors, but after ~100 ms, the activity evolved to indicate whether or not the singleton target was in the receptive field. This is the typical response of FEF visual neurons previously shown to occur during visual search tasks in which saccades were made to the target of the search array (Schall and Hanes, 1993; Schall et al., 1995b; Thompson et al., 1996; Bichot et al., 2001a,b; Sato et al., 2001). As in the studies in which saccades were made, in this experiment the greatest selectivity occurred before the monkeys’ behavioral report of the target (Fig. 6b). Overall, after the lever turn a smaller selective response continued as long as the search array was present. However, this maintained selectivity was not observed in all of the neurons (Fig. 3b,c). Additional study is needed to determine the reason for this difference across neurons. Nevertheless, the main result is clear: a majority of FEF visually responsive neurons exhibit selectivity for the location of a salient target before a manual report even when there is no evidence of saccade planning. In contrast, movement neurons are not active in this task, and the overall activity is suppressed after the presentation of the search array (Fig. 6a). This suppression is not spatially specific, and it continues throughout the time in which the search array remains on the screen after the manual lever turn and reward. The presence of postreward saccades on some trials did not affect the results. Identical results were obtained when all trials with postreward saccades were removed from the analysis.
Discussion
Although the locus of the monkeys’ attention was not probed directly, it is reasonable to assert that exogenously driven attention was directed covertly to the location of the singleton pop-out target in the manual lever search tasks. Numerous behavioral studies have shown that attention is directed automatically to the pop-out singleton of a search array (Theeuwes, 1994; Joseph and Optican, 1996; Egeth and Yantis, 1997; Nothdurft, 1999; Turatto and Galfano, 2000; Turatto et al., 2004; Thompson et al., 2005), especially when the stimulus is used to guide behavior (Nothdurft, 2002). Also, the perception of a pop-out search singleton can be impaired when subjects are required to focus attentional resources to visual stimuli presented at the central fixation point (Joseph et al., 1997). Therefore, attention is critical for the recognition of the singleton target even in pop-out search. By making the monkeys recognize the singleton target of a pop-out search array, we maximized the probability that attention was directed to the target stimulus and away from the distractor stimuli on every trial.
Evidence is growing that the FEF plays a key role in covert orienting. In humans, functional imaging studies show that the FEF is active during the allocation of attention with and without eye movements (Beauchamp et al., 2001; Corbetta and Shulman, 2002), and transcranial magnetic stimulation over the FEF facilitates visual perception (Grosbras and Paus, 2002) and modulates performance in visual search tasks without saccades (Muggleton et al., 2003). Recently, Moore and colleagues demonstrated that weak electrical stimulation of the FEF below the threshold for producing saccades improves the perceptual abilities of monkeys (Moore and Fallah, 2001) and produces enhanced responses in extrastriate visual cortex that resembles the effects of directed spatial attention (Moore and Armstrong, 2003). However, with electrical stimulation, we cannot distinguish those neurons that mediate the observed effect from those that do not. We now have shown that stimulus-driven (exogenous) covert orienting and saccade production are separate processes that are mediated by separate populations of neurons. We propose that the selective activity we observed in FEF visually responsive neurons corresponds to the spotlight of attention and mediates the covert spatial attention-related modulations observed in visual cortex (Connor et al., 1997; McAdams and Maunsell, 2000; Ogawa and Komatsu, 2004), presumably via the strong feedback connections from the FEF to extrastriate visual cortex (Schall et al., 1995a).
The dissociation of visual selection from saccade production
Previous studies have shown a dissociation of visual selection in the FEF from saccade production. First, the time of selection in easy search tasks, such as the one used in this study, does not predict the time of saccades to the target (Thompson et al., 1996; Sato et al., 2001; Sato and Schall, 2003). Also, when the same monkeys that were trained to make saccades to the singleton target performed a NoGo search task in which they viewed the search array passively (Thompson et al., 1997) or an anti-search task in which they made a saccade opposite to the location of the target, the neurons still exhibited selective activity for the oddball stimulus (Sato and Schall, 2003). This was similar to a study that showed enhanced responses in the FEF to a peripheral visual stimulus when saccades were made to it and when it was attended during fixation (Kodaka et al., 1997). In another visual search experiment in which the target unpredictably switched places with one of the distractors, the activity of FEF visual neurons represented accurately the new location of the salient target, but not the goal of the next saccade (Murthy et al., 2001). In that experiment, however, after an incorrect saccade to the first target location, the monkeys usually made a second saccade to the new target location. This behavior introduces the possibility that the selective activation was related to the production of the second corrective saccade. In fact, in all of the previous studies showing a dissociation of selection from saccade production, the monkeys had been trained extensively to make saccades to the singleton target of the search array. Therefore, it cannot be ruled out that, even in the experiments in which saccades were not made (Kodaka et al., 1997; Thompson et al., 1997; Sato and Schall, 2003; Sato et al., 2003), saccades actively were being planned but suppressed, especially given the well known role that the FEF plays in the saccade production (Bruce and Goldberg, 1985; Bruce et al., 1985; Hanes and Schall, 1996; Hanes et al., 1998; Tehovnik et al., 2000). This assumption frames the hypothesis of motor origins of selective attention (Moore and Fallah, 2004; Hamker, 2005).
In this study, considerable effort was made to address whether or not the selective activation observed in the FEF during visual search is attributable to latent saccade planning. First, we minimized the chance for behavioral training to affect the results. The monkeys were experimentally naive; they had never been rewarded for making a saccade in a visual search task. Second, we used a fixation stimulus unique to the covert visual search task to instruct the monkeys to maintain fixation until the reward was given. Third, if the monkeys were planning saccades before the lever turn, then there should have been a bias in their gaze behavior after the reward while the search array remained on and there was no penalty for making a saccade. Although saccades were made after some trials, there was no behavioral evidence that the monkeys were inclined to make saccades to the target of the search array. Finally, perhaps the strongest evidence that saccades were not being planned is the nonspatially selective inhibition of activity in saccade-related movement neurons. In previous studies that used visual search tasks involving saccades, FEF movement neurons exhibited selective activity similar to that of visual neurons (Schall et al., 1995b; Thompson et al., 2005) even when saccades were not made to the response field of the neurons (Bichot et al., 2001). Also, when an instruction is given to countermand a partially prepared saccade, there is a growth of saccade-related activity in the FEF before it is suppressed (Hanes et al., 1998). Therefore, in tasks that use saccades as the behavioral report, FEF saccade-related movement neurons become active even when saccades are suppressed or canceled. In this study, however, there was no behavioral or physiological evidence of saccade planning, and spatially selective activity corresponding to spatial attention was observed only in the activity of visually responsive neurons.
The premotor theory of attention
The previously cited evidence linking the FEF to visual attention (Beauchamp et al., 2001; Moore and Fallah, 2001; Corbetta and Shulman, 2002; Grosbras and Paus, 2002; Moore and Armstrong, 2003; Muggleton et al., 2003) has been cited in support of the premotor theory of attention (Rizzolatti et al., 1987), which postulates that saccade programming in the FEF and other oculomotor structures provides the basis for covert orienting (Findlay and Gilchrist, 2003; Moore et al., 2003; Moore and Fallah, 2004; Hamker, 2005). This theory also has been supported by the results of behavioral studies that show that attention is directed obligatorily to a saccade target (Hoffman and Subramaniam, 1995; Kowler et al., 1995; Deubel and Schneider, 1996). However, the inhibition of activity in movement neurons during our visual search task without eye movements is evidence against the view that the activity of FEF movement neurons mediates covert orienting (Hamker, 2005). This is supported by a recent study that used electrical microstimulation in the FEF to reveal the state of saccade preparation in an anti-saccade task, which showed that covert attention is not related to the monkeys’ state of saccade preparation (Juan et al., 2004).
Evidence suggests that the spatially selective activity observed in FEF visual responses functions as a visual salience map that identifies potential targets for eye movements (Thompson and Bichot, 2005) but is not an explicit saccade plan. In addition to the evidence of a dissociation of visual selection in the FEF from saccade production (see above), visual activity in the FEF does not drive saccade-related activity directly (Sato and Schall, 2003) and does not modulate in time to control gaze shifts (Hanes et al., 1998).
Nevertheless, the selection of potential saccade targets is an essential part of saccade planning, and therefore our results do not invalidate the premotor theory of attention. Previous studies have shown that, during tasks in which saccades are made to the attended location, the spatially selective signal that develops in visual neurons is transferred in a continuous manner to motor processes for saccade production (Gold and Shadlen, 2000; Bichot et al., 2001a; Thompson et al., 2005). The suppression of activity in movement neurons in our study during conditions in which covert attention is deployed suggests that the flow of information from visual selection to motor planning within the FEF can be controlled according to task demands. Our results demonstrate that spatial attention corresponds to the visual selection stage of saccade production; it is a precursor to the motor activity that leads directly to saccade generation and therefore can affect eye movements (Sheliga et al., 1995a,b). Nevertheless, the spatially selective visual activity in the FEF is not by itself a saccade plan (Klein and Pontefract, 1994; Deubel and Schneider, 1996). This view is consistent with the idea that there is a common origin for spatial attention and eye movements. The importance of the present results is that we have identified that the functional divergence of exogenously driven spatial attention and eye movements takes place between the visual selection and motor selection processes in the FEF.
In conclusion, although spatial attention and saccade generation probably are linked within the neural circuitry of the FEF, the spatial attention signal in the FEF during a pop-out visual search task is related more closely to visual processing than to motor processing. A correlate of exogenous covert orienting was found in the activity of visually responsive FEF neurons without any behavioral or physiological evidence of a saccade plan. Currently it is unknown whether the same activity patterns occur in the FEF during endogenously controlled attention. Our data suggest that the functional link between attention and eye movements is gated within the FEF. Nevertheless, it is likely that covert attention is distributed across multiple visual and motor structures that include areas of the parietal cortex (Astafiev et al., 2003; Bisley and Goldberg, 2003; Wardak et al., 2004) and the superior colliculus (Cavanaugh and Wurtz, 2004; Ignashchenkova et al., 2004; Muller et al., 2005). Additional work is needed to clarify the relationships between these areas and the neural mechanisms underlying spatial attention and its role in guiding goal-directed actions.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health–National Eye Institute. We thank N. Bichot, J. Schall, and our colleagues in the Laboratory of Sensorimotor Research (J. Cavanaugh, N. Port, B. Sheliga, M. Sommer, R. Wurtz, and H. Zhou) for helpful discussions and valuable comments.
References
- Astafiev SV, Shulman GL, Stanley CM, Snyder AZ, Van Essen DC, Corbetta M. Functional organization of human intraparietal and frontal cortex for attending, looking, and pointing. J Neurosci. 2003;23:4689–4699. doi: 10.1523/JNEUROSCI.23-11-04689.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. NeuroImage. 2001;14:310–321. doi: 10.1006/nimg.2001.0788. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Schall JD, Thompson KG. Visual feature selectivity in frontal eye fields induced by experience in mature macaques. Nature. 1996;381:697–699. doi: 10.1038/381697a0. [DOI] [PubMed] [Google Scholar]
- Bichot NP, Thompson KG, Chenchal Rao S, Schall JD. Reliability of macaque frontal eye field neurons signaling saccade targets during visual search. J Neurosci. 2001a;21:713–725. doi: 10.1523/JNEUROSCI.21-02-00713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot NP, Chenchal Rao S, Schall JD. Continuous processing in macaque frontal cortex during visual search. Neuropsychologia. 2001b;39:972–982. doi: 10.1016/s0028-3932(01)00022-7. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Goldberg ME. Neuronal activity in the lateral intraparietal area and spatial attention. Science. 2003;299:81–86. doi: 10.1126/science.1077395. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Bruce CJ, Goldberg ME, Stanton GB, Bushnell MC. Primate frontal eye fields. II. Physiological and anatomical correlates of electrically evoked eye movements. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci. 2004;24:11236–11243. doi: 10.1523/JNEUROSCI.3724-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor CE, Preddie DC, Gallant JL, Van Essen DC. Spatial attention effects in macaque area V4. J Neurosci. 1997;17:3201–3214. doi: 10.1523/JNEUROSCI.17-09-03201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res. 1996;36:1827–1837. doi: 10.1016/0042-6989(95)00294-4. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annu Rev Psychol. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Findlay JM, Gilchrist ID. Active vision: the psychology of looking and seeing. Oxford: Oxford UP; 2003. [Google Scholar]
- Gold JI, Shadlen MN. Representation of a perceptual decision in developing oculomotor commands. Nature. 2000;404:390–394. doi: 10.1038/35006062. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T. Transcranial magnetic stimulation of the human frontal eye field: effects on visual perception and attention. J Cogn Neurosci. 2002;14:1109–1120. doi: 10.1162/089892902320474553. [DOI] [PubMed] [Google Scholar]
- Hamker FH. The reentry hypothesis: the putative interaction of the frontal eye field, ventrolateral prefrontal cortex, and areas V4, IT for attention and eye movement. Cereb Cortex. 2005;15:431–447. doi: 10.1093/cercor/bhh146. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Schall JD. Neural control of voluntary movement initiation. Science. 1996;274:427–430. doi: 10.1126/science.274.5286.427. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Patterson WF, Schall JD. Role of frontal eye fields in countermanding saccades: visual, movement, and fixation activity. J Neurophysiol. 1998;79:817–834. doi: 10.1152/jn.1998.79.2.817. [DOI] [PubMed] [Google Scholar]
- Hays AV, Richmond BJ, Optican LM. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf Proc. 1982;2:1–10. [Google Scholar]
- Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys. 1995;57:787–795. doi: 10.3758/bf03206794. [DOI] [PubMed] [Google Scholar]
- Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci. 2004;7:56–64. doi: 10.1038/nn1169. [DOI] [PubMed] [Google Scholar]
- Joseph JS, Optican LM. Involuntary attentional shifts due to orientation differences. Percept Psychophys. 1996;58:651–665. doi: 10.3758/bf03213098. [DOI] [PubMed] [Google Scholar]
- Joseph JS, Chun MM, Nakayama K. Attentional requirements in a “preattentive” feature search task. Nature. 1997;387:805–807. doi: 10.1038/42940. [DOI] [PubMed] [Google Scholar]
- Juan CH, Shorter-Jacobi SM, Schall JD. Dissociation of spatial attention and saccade preparation. Proc Natl Acad Sci USA. 2004;101:15541–15544. doi: 10.1073/pnas.0403507101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. J Neurosci. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinchla RA. Attention. Annu Rev Psychol. 1992;43:711–742. doi: 10.1146/annurev.ps.43.020192.003431. [DOI] [PubMed] [Google Scholar]
- Klein RM, Pontefract A. Does oculomotor readiness mediate cognitive control of visual attention? Revisited! In: Umilta C, Moskovitch M, editors. Attention and performance. XV. Cambridge, MA: MIT; 1994. pp. 333–350. [Google Scholar]
- Kodaka Y, Mikami A, Kubota K. Neuronal activity in the frontal eye field of the monkey is modulated while attention is focused on to a stimulus in the peripheral visual field, irrespective of eye movement. Neurosci Res. 1997;28:291–298. doi: 10.1016/s0168-0102(97)00055-2. [DOI] [PubMed] [Google Scholar]
- Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Maunsell JH. Attention to both space and feature modulates neuronal responses in macaque area V4. J Neurophysiol. 2000;83:1751–1755. doi: 10.1152/jn.2000.83.3.1751. [DOI] [PubMed] [Google Scholar]
- Mohler CW, Goldberg ME, Wurtz RH. Visual receptive fields of frontal eye field neurons. Brain Res. 1973;61:385–389. doi: 10.1016/0006-8993(73)90543-x. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci USA. 2001;98:1273–1276. doi: 10.1073/pnas.021549498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40:671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Muggleton NG, Juan CH, Cowey A, Walsh V. Human frontal eye fields and visual search. J Neurophysiol. 2003;89:3340–3343. doi: 10.1152/jn.01086.2002. [DOI] [PubMed] [Google Scholar]
- Muller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci USA. 2005;102:524–529. doi: 10.1073/pnas.0408311101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy A, Thompson KG, Schall JD. Dynamic dissociation of visual selection from saccade programming in frontal eye field. J Neurophysiol. 2001;86:2634–2637. doi: 10.1152/jn.2001.86.5.2634. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Fujii N, Tanji J. Visually guided saccade versus eye–hand reach: contrasting neuronal activity in the cortical supplementary and frontal eye fields. J Neurophysiol. 1996;75:2187–2191. doi: 10.1152/jn.1996.75.5.2187. [DOI] [PubMed] [Google Scholar]
- Nothdurft HC. Focal attention in visual search. Vision Res. 1999;39:2305–2310. doi: 10.1016/s0042-6989(99)00006-1. [DOI] [PubMed] [Google Scholar]
- Nothdurft HC. Attention shifts to salient targets. Vision Res. 2002;42:1287–1306. doi: 10.1016/s0042-6989(02)00016-0. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Komatsu H. Target selection in area V4 during a multidimensional visual search task. J Neurosci. 2004;24:6371–6382. doi: 10.1523/JNEUROSCI.0569-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians: evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Sato T, Murthy A, Thompson KG, Schall JD. Search efficiency but not response interference affects visual selection in frontal eye field. Neuron. 2001;30:583–591. doi: 10.1016/s0896-6273(01)00304-x. [DOI] [PubMed] [Google Scholar]
- Sato TR, Schall JD. Effects of stimulus–response compatibility on neural selection in frontal eye field. Neuron. 2003;38:637–648. doi: 10.1016/s0896-6273(03)00237-x. [DOI] [PubMed] [Google Scholar]
- Sato TR, Watanabe K, Thompson KG, Schall JD. Effect of target-distractor similarity on FEF visual selection in the absence of the target. Exp Brain Res. 2003;151:356–363. doi: 10.1007/s00221-003-1461-1. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP. Neural basis of saccade target selection in frontal eye field during visual search. Nature. 1993;366:467–469. doi: 10.1038/366467a0. [DOI] [PubMed] [Google Scholar]
- Schall JD, Morel A, King DJ, Bullier J. Topography of visual cortex connections with frontal eye field in macaque: convergence and segregation of processing streams. J Neurosci. 1995a;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci. 1995b;15:6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Rizzolatti G. Spatial attention and eye movements. Exp Brain Res. 1995a;105:261–275. doi: 10.1007/BF00240962. [DOI] [PubMed] [Google Scholar]
- Sheliga BM, Riggio L, Craighero L, Rizzolatti G. Spatial attention-determined modifications in saccade trajectories. NeuroReport. 1995b;6:585–588. doi: 10.1097/00001756-199502000-00044. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Sommer MA, Chou IH, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Brain Res Rev. 2000;32:413–448. doi: 10.1016/s0165-0173(99)00092-2. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. Stimulus-driven capture and attentional set: selective search for color and visual abrupt onsets. J Exp Psychol Hum Percept Perform. 1994;20:799–806. doi: 10.1037//0096-1523.20.4.799. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP. A visual salience map in the primate frontal eye field. Prog Brain Res. 2005;147:251–262. doi: 10.1016/S0079-6123(04)47019-8. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Hanes DP, Bichot NP, Schall JD. Perceptual and motor processing stages identified in the activity of macaque frontal eye field neurons during visual search. J Neurophysiol. 1996;76:4040–4055. doi: 10.1152/jn.1996.76.6.4040. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Schall JD. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J Neurophysiol. 1997;77:1046–1050. doi: 10.1152/jn.1997.77.2.1046. [DOI] [PubMed] [Google Scholar]
- Thompson KG, Bichot NP, Sato TR. Frontal eye field activity before visual search errors reveals the integration of bottom-up and top-down salience. J Neurophysiol. 2005;93:337–351. doi: 10.1152/jn.00330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turatto M, Galfano G. Color, form, and luminance capture attention in visual search. Vision Res. 2000;40:1639–1643. doi: 10.1016/s0042-6989(00)00061-4. [DOI] [PubMed] [Google Scholar]
- Turatto M, Galfano G, Gardini S, Mascetti GG. Stimulus-driven attentional capture: an empirical comparison of display-size and distance methods. Q J Exp Psychol A. 2004;57:297–324. doi: 10.1080/02724980343000242. [DOI] [PubMed] [Google Scholar]
- Wardak C, Olivier E, Duhamel JR. A deficit in covert attention after parietal cortex inactivation in the monkey. Neuron. 2004;42:501–508. doi: 10.1016/s0896-6273(04)00185-0. [DOI] [PubMed] [Google Scholar]