Abstract
Background:
In patients with heart failure (HF), randomized controlled trials (RCTs) of sodium-glucose transporter-2 inhibitors (SGLT-2is) have proven to be effective in decreasing the primary composite outcome of cardiovascular death and hospitalizations for HF. A recently published meta-analysis showed that the use of SGLT-2is among women with diabetes resulted in less reduction in primary composite outcomes compared with men. This study aims to explore potential sex differences in primary composite outcomes among patients with HF treated with SGLT-2is.
Methods:
We systematically searched the medical database from 2017 to 2022 and retrieved all the RCTs using SGLT-2is with specified cardiovascular outcomes. We used the PRISMA (Preferred Reporting Items for a Review and Meta-analysis) method to screen for eligibility. We evaluated the quality of studies using the Cochrane Risk of Bias tool. We pooled the hazard ratio (HR) of the primary composite outcomes in both sexes, performed a meta-analysis, and calculated the odds ratio (OR) of the primary composite outcomes based on sex.
Results:
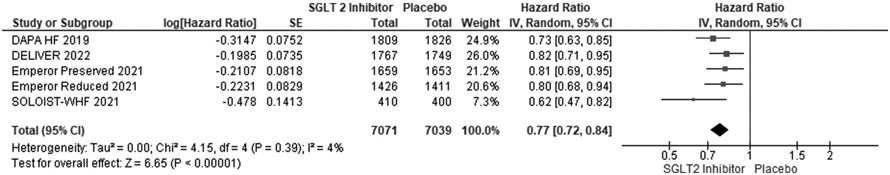
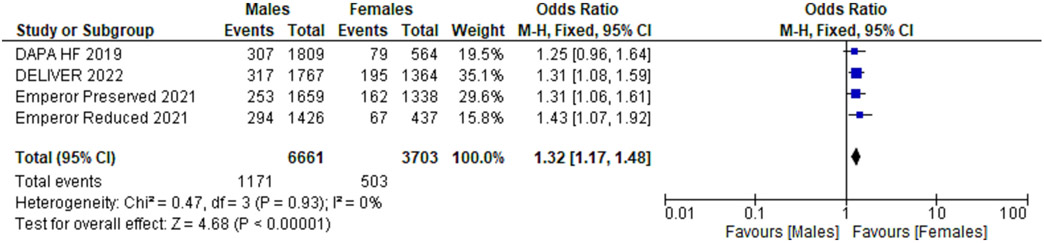
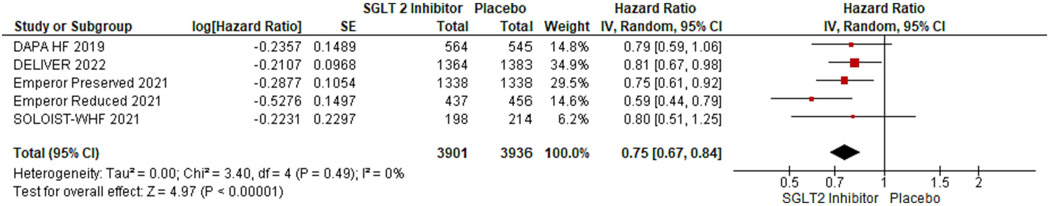
We included 5 RCTs with a total number of 21,947 patients. Of these, 7837 (35.7 %) were females. Primary composite outcomes were significantly lower in males and females taking SGLT-2is compared to placebo (males - HR 0.77; 95 % CI 0.72 to 0.84; p = 0.00001; females - HR 0.75; 95 % CI 0.67 to 0.84; p = 0.00001). Pooled data from four of the RCTs (n = 20,725) revealed a greater occurrence of the primary composite outcomes in females compared with males (OR 1.32; 95 % CI 1.17 to 1.48; p = 0.0002).
Conclusion:
SGLT-2is reduce the risk of primary composite outcomes in patients with HF, regardless of sex; however, the benefits were less pronounced in women. Further research needs to be done to better explain these observed differences in outcomes.
Keywords: SGLT-2 inhibitors, Sex differences, Cardiovascular outcomes, Cardiorenal, Heart failure
1. Introduction
Heart failure (HF) incidence is increasing globally, with almost half of all HF patients being women [1]. The 2022 Heart Disease and Stroke Statistics report by the American Heart Association reported a projected rise in HF prevalence by 46 % from 2012 to 2030, expecting to increase the total percentage of the population from 2.4 % in 2012 to 3 % in 2030 [2]. HF causes significant morbidity and mortality among women [3], and the incidence of HF tripled between ages 65–74 and 75–84 [3]. In women, HF tends to occur at an older age and usually manifests as HF with preserved ejection fraction (HFpEF). Furthermore, women are more likely to be more symptomatic than men [1,3]. Although women are less prone than men to develop coronary artery disease (CAD) at premenopausal age, they reported worse quality of life and increased risk for depression [3]. Guideline-directed medical therapy for HF does not differ in women; however, women are vastly underrepresented in HF trials [3].
1.1. Sodium-glucose transporter-2 inhibitors and heart failure
Sodium-glucose transporter-2 inhibitors (SGLT-2is) are hypoglycemic agents which block the sodium-dependent glucose transporter-2 in the early proximal renal tubule of the kidneys. Their primary effect is increasing urinary glucose excretion and decreasing blood glucose levels. Additional effects of SGLT-2is, especially on the cardiovascular system, have been increasingly reported in the literature. In early 2022, the US Food and Drug Administration approved empagliflozin to treat HF. SGLT-2is use is currently one of the four pillars in managing patients with HF with reduced ejection fraction (HFrEF) [4]. In patients with HF and preserved ejection fraction (HFpEF), empagliflozin showed a 21 % lower relative risk in the composite of cardiovascular death or hospitalization for HF [5]. In the recently published 2022 American Heart Association/American College of Cardiology/Heart Failure Society of America (AHA/ACC/HSFA) heart failure guideline, SGLT-2is received a class I recommendation for patients with HFrEF, including those at risk for HF (Stage A) [6]. Similarly, the 2021 European Society of Cardiology guidelines for acute and chronic HF highlighted the importance of SGLT-2is (class IA recommendation) in reducing HF hospitalization and mortality risk in patients with HFrEF [7]. Possible mechanisms of cardiovascular benefits of SGLT-2 inhibitors are changes in energy metabolism, reduction of blood pressure (BP) and vessel stiffness, increase in hemoglobin and hematocrit, improvement of myocardial remodeling, and weight loss [8].
Men and women may differ in myocardial adaptation after a cardiac event [9]. HFpEF is more common in women than in men. Furthermore, women with HFpEF have more comorbidities than those with HFrEF, with >60 % having at least four comorbidities [9]. White women with HFpEF had the highest proportion of hospitalization for HF [3]. A meta-analysis by Singh et al. showed that the reduction in primary composite outcomes with SGLT-2is appears to be significantly less in women with diabetes than in men [10]. However, to our knowledge, no study has investigated whether there are sex differences in cardiovascular (CV) outcomes among HF patients on SGLT-2is. We conducted a thorough systematic review and meta-analysis of all the CV outcomes studied with SGLT-2is in patients with HF with or without type 2 diabetes mellitus (DM) to study the primary composite outcomes based on the sex of the patients.
1.2. Objective
This study aimed to determine the effect of SGLT-2is on primary composite outcomes among patients with HF, with or without type 2 DM, stratified by sex, through a systematic review and meta-analysis of relevant randomized controlled trials.
1.3. Data sources
A comprehensive search and review of the PubMed, SCOPUS, and Cochrane databases was conducted from 2017 to 2022. Results were limited to randomized clinical trials and clinical trials only. Relevant keywords such as “SGLT-2 inhibitors,” “heart failure,” “chronic heart failure,” and “acute heart failure” and their combinations were used in the search. All subsequent articles cited in these studies were also considered and reviewed.
1.4. Study selection
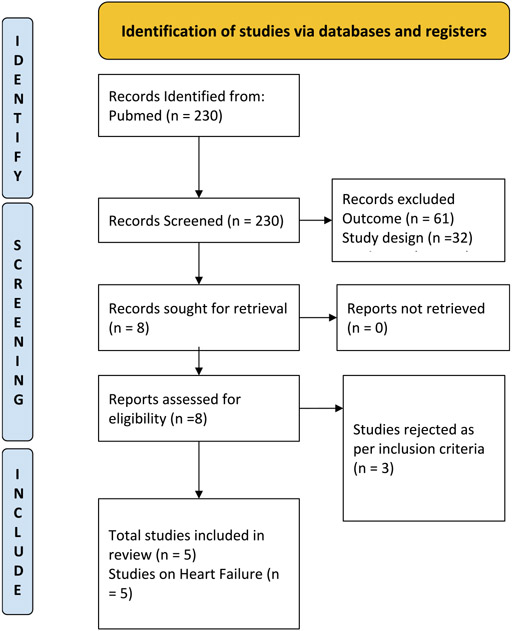
Only dedicated cardiovascular outcome trials conducted with SGLT-2is investigating the primary outcome of the composite of cardiovascular death, hospitalization for heart failure, and urgent visit for heart failure, and published the results of subgroup analysis based on sex were included. Studies with either HFrEF or HFpEF participants were included. Other studies which did not have cardiovascular death and HF hospitalizations as the primary endpoint and did not have a randomized controlled trial design were excluded from the analysis. Studies that have met the specified inclusion and exclusion criteria were compiled. The study was done in line with the statements mentioned in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (11). A detailed PRISMA diagram for the process of inclusion and exclusion for selecting key studies that have been used in this meta-analysis has been depicted in Fig. 1.
Fig. 1.
PRISMA diagram on selection of studies.
1.5. Data extraction and synthesis
The full text and all supplementary appendices were obtained, screened, and reviewed using the Cochrane Risk of Bias tool. The tool evaluates studies on seven domains, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Three authors (FBR, DDL, and VST) independently extracted data using a standardized data collection form. Differences in rating given by the reviewers were discussed by all authors and resolved with a consensus. Statistical analysis was completed using Cochrane Review Manager (RevMan) version 5.4. Inverse variance weighted averages of logarithmic hazard ratio using random effects model were used to calculate for study weight. The pooled hazard ratio [HR], stratified by sex, and the 95 % confidence interval (CI) were based on intention-to-treat analysis. Odds Ratio [OR] was then computed between pooled HR to compare primary composite outcomes between sexes. Heterogeneity was evaluated using the I2 method and Cochrane Q statistics. Results were classified as low (<25 %), moderate (25 to 50 %), and high (>50 %) heterogeneity based on the 12. All reported two-sided p values are considered significant if <0.05.
1.6. Main outcomes and measures
The primary outcome of interest was a composite of the following outcomes: cardiovascular death, hospitalization for heart failure, and urgent visit for heart failure.
2. Results
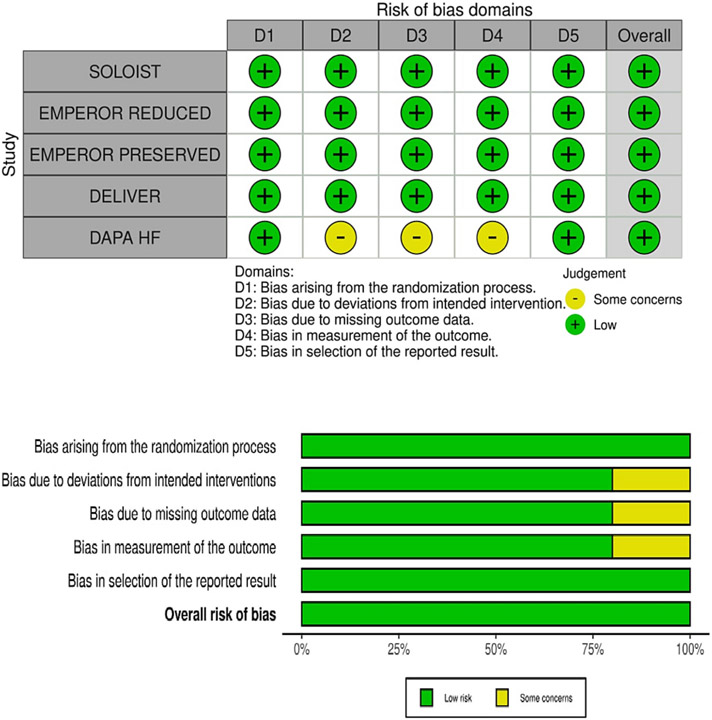
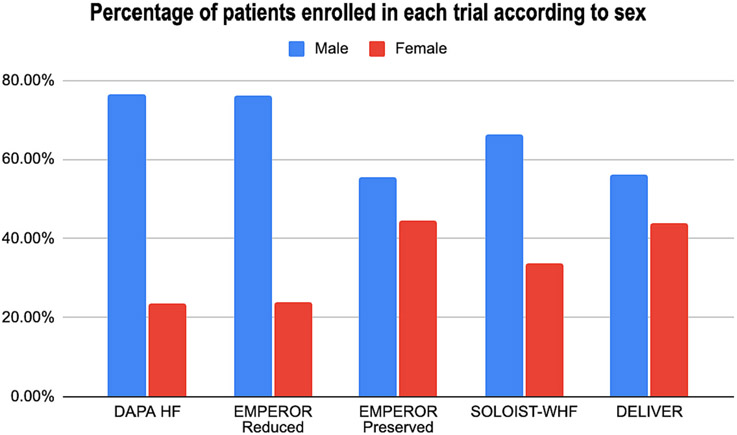
Five RCTs were included in the meta-analysis after rigorous screening described in the PRISMA diagram (Fig 1). These studies are generally found to be of good quality, using the Cochrane Risk of Bias Tool, summarized in Fig. 2. One study, in particular, the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure (DAPA-HF) RCT, presented minor issues on dose adjustments for trial drug empagliflozin. Although >10 % of patients from the control and intervention group discontinued from this study, this was addressed by utilizing intention to treat analysis. A funnel plot was not done considering the quality and size of the trials included in the study and the results of the Cochrane tool. The final meta-analysis included 21,947 participants from five published RCTs comparing SGLT2-is with placebo and performed across multiple centers worldwide (see Table 1 and Figs. 4-6) [5,11-14]. The study-level baseline characteristics are presented in Table 2. The participants included in the studies ranged from 65 to 81 years old. Empagliflozin in Heart Failure with a Preserved Ejection Fraction (EMPEROR-PRESERVED) and Dapagliflozin in Heart Failure with mildly reduced or preserved ejection fraction (DELIVER) trials enrolled relatively older participants. All 5 RCTs had predominantly male participants. In general, women were underrepresented (35.71 %). However, more women were recruited for EMPEROR-PRESERVED (44.69 %) and DELIVER trials (43.86 %) compared to the others. Most patients were Whites; Asians and Black populations account for approximately 20 %. Most were NYHA Class II. Hypertension and diabetes were the predominant comorbidities. Patients with HFpEF (EMPEROR-Preserved and DELIVER) and HFrEF (DAPA-HF, Empagliflozin in Heart Failure with a Reduced Ejection Fraction (EMPEROR-Reduced), Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure (SOLOIST-WHF) were adequately represented. Among those with HFrEF, most were on beta-blockers and mineralocorticoid receptor antagonists, but only a minority were on neprilysin inhibitors.
Fig. 2.
Cochrane risk of bias assessment.
Table 1.
Summary of trials.
| Trial | Total (N) |
Year | Author | Duration | Drug | Primary endpoint | Secondary endpoint | Results |
|---|---|---|---|---|---|---|---|---|
| DAPA HF | 4744 | 2019 | McMurray et al. | 18.2 months | Dapagliflozin | Composite of worsening heart failure and death from cardiovascular causes | Cardiovascular death, heart failure hospitalization, changes in KCCQ, worsening renal function, death from any case | Primary outcome occurred in 386 of 2373 patients (16.3 %) in the dapagliflozin group and in 502 of 2371 patients (21.2 %) in the placebo group |
| EMPEROR Reduced | 3730 | 2020 | Packer et al. | 16 months | Empagliflozin | Composite of cardiovascular death and hospitalization for heart failure | Occurrence of all adjudicated hospitalization for heart failure and rate of decline in estimated GFR | Primary outcome event occurred in 361 of 1863 patients (19.4 %) in the empagliflozin group and in 462 of 1867 patients (24.7 %) in the placebo group |
| EMPEROR Preserved | 5988 | 2021 | Anker et al. | 26.2 months | Empagliflozin | Composite of cardiovascular death and hospitalization for heart failure | Occurrence of all adjudicated hospitalization for heart failure and rate of decline in estimated GFR | Primary outcome event occurred in 415 of 2997 patients (13.8 %) in the empagliflozin group and in 511 of 2991 patients (17.1 %) in the placebo group |
| SOLOIST WHF | 1222 | 2021 | Bhatt et al. | 9.0 months | Sotagliflozin | Total number of deaths from cardiovascular causes, hospitalizations and urgent visits for heart failure | Total number of hospitalizations and urgent visits for heart failure, incidence of death from CV and any cause, total number of deaths from CV cause, nonfatal MI, nonfatal stroke, chance in KCCQ, events of HF during hospitalization | Primary outcome occurred in 245 of 608 patients in the sotagliflozin group and in 355 of 614 patients in the placebo group. |
| DELIVER | 6263 | 2022 | Solomon et al. | 27 months | Dapagliflozin | Composite of worsening heart failure or Cardiovascular death | Total number of worsening heart failure events and cardiovascular deaths, the change from baseline in the total symptom score on the KCCQ scores, cardiovascular death, death from any cause | Primary outcome occurred in 512 of 3131 patients (16.4 %) in the dapagliflozin group and in 610 of 3132 patients (19.5 %) in the placebo group. |
Abbreviations:
DAPA HF = Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; EMPEROR Reduced = Empagliflozin in Heart Failure with a Reduced Ejection Fraction; EMPEROR Preserved = Empagliflozin in Heart Failure with a Preserved Ejection Fraction; SOLOIST WHF = Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure; DELIVER = Dapagliflozin in Heart Failure with mildly reduced or preserved ejection fraction; KCCQ = Kansas City Cardiomyopathy Questionnaire
CV = Cardiovascular; GFR = Glomerular filtration rate.
Fig. 4.
Primary composite outcomes in males receiving SGLT-2is vs. placebo.
Fig. 6.
Primary composite outcomes in males vs. females receiving SGLT-2is.
Table 2.
Patient characteristics.
| DAPA HF | EMPEROR reduced | EMPEROR preserved | SOLOIST-WHF | DELIVER | |
|---|---|---|---|---|---|
| N | 4744 | 3730 | 5988 | 1222 | 6263 |
| Mean age | 66.3 | 67.35 | 71.85 | 69.50 | 71.65 |
| Female sex no. (%) | 1109 (23.4) | 893 (23.9) | 2676 (44.7) | 412 (33.7) | 2747 (43.9) |
| Race no. (%) | |||||
| Asian | 1116 (23.5) | 672 (18.0) | 824 (13.8) | 15 (1.2) | 1274 (20.3) |
| Black | 226 (4.8) | 257 (6.9) | 258 (4.3) | 50 (4.1) | 159 (2.5) |
| White | 3333 (70.3) | 2629 (70.5) | 4542 (75.9) | 1139 (93.2) | 4439 (70.9) |
| NYHA class — no. (%) | |||||
| II | 3203 (67.5) | 2800 (75.1) | 4883 (81.6) | – | 4713 (75.3) |
| III | 1498 (31.6) | 910 (24.4) | 1083 (18.1) | – | 1531 (24.5) |
| IV | 43 (0.9) | 20 (0.5) | 18 (0.3) | – | 18 (0.3) |
| Left ventricular ejection fraction | – | ||||
| Mean | 31.05 | 27.45 | 54.3 | 35 | 54.15 |
| ≤49 % | – | – | 1983 (33.1) | – | 2116 (33.8) |
| 50–59 % | – | – | 2058 (34.4) | – | 2256 (36.0) |
| ≥60 % | – | – | 1947 (32.5) | – | 1891 (30.2) |
| Median NT-proBNP (IQR) — pg/ml | 1437 | 1906.52 | 970.02 | 1778.71 | – |
| Atrial fibrillation | 1818 (38.3) | 1369 (36.7) | 3057 (51.1) | – | – |
| Type 2 diabetes mellitus no. (%) | 1983 (41.8) | 1856 (49.8) | 2938 (49.1) | – | 2806 (44.8) |
| Hypertension no. (%) | – | 2698 (72.3) | 5424 (90.6) | – | 5553 (88.7) |
| Mean eGFR | 65.75 | 62.00 | 60.6 | 49.85 | 61 |
| BMI | 28.15 | 27.90 | 29.83 | 30.75 | – |
| Medications | |||||
| Neprilysin inhibitor | 508 (10.7) | 727 (19.5) | – | 205 (16.8) | – |
| Beta-blocker | 4558 (96.1) | 3533 (94.7) | – | 1125 (92.1) | – |
| Mineralocorticoid receptor antagonist | 3370 (71.0) | 2661 (71.3) | – | 788 (64.5) | – |
| Device therapy | |||||
| Implantable cardioverter–defibrillator | 1242 (26.2) | 1171 (31.4) | – | – | – |
| Cardiac resynchronization therapy | 354 (7.5) | 442 (11.9) | – | – | – |
Abbreviations:
DAPA HF = Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure; EMPEROR Reduced = Empagliflozin in Heart Failure with a Reduced Ejection Fraction; EMPEROR Preserved = Empagliflozin in Heart Failure with a Preserved Ejection Fraction; SOLOIST WHF = Effect of Sotagliflozin on Cardiovascular Events in Patients with Type 2 Diabetes Post Worsening Heart Failure; DELIVER = Dapagliflozin in Heart Failure with mildly reduced or preserved ejection fraction.
Using forest plot to analyze composite outcomes for the 5 RCTs, the study revealed significantly lower primary composite outcomes both in males and females taking SGLT-2is (males - HR 0.77; 95 % CI 0.72 to 0.84; p = 0.00001; females - HR 0.75; 95 % CI 0.67 to 0.84; p = 0.00001). Pooled data from four of the RCTs (n = 20,725) were compared between males and females, which revealed a greater occurrence of the primary composite outcomes among females compared to males (OR 1.32; 95 % CI: 1.17–1.48) (see Fig. 6).
2.1. Discussion
Cardiovascular disease (CVD) is the leading cause of death in females in the US. SGLT2-is has become a major pillar in treating heart failure across the spectrum of phenotypes. The findings of this study showed that the treatment effect of these drugs on women is markedly less compared to men. This difference may be explained by various factors – the clinical characteristics of women with heart failure, the representation of women in major heart failure trials, peculiarities of female physiology, and the pharmacodynamics of SGLT2-is. Women with heart failure present with a different clinically picture compared to men with heart failure. At age 40, the lifetime risk of developing HF without antecedent MI is one in six for women and one in nine for men making women more likely to develop non-ischemic HF compared to men [15]. The most common underlying mechanism of HF in women is myocardial hypertrophy from uncontrolled hypertension [15,16]. In addition, hypertension is more challenging to control in women despite no difference in response to antihypertensive medications between men and women [16]. Compared to men, women with HF are often older, are less likely to smoke, and have more HFpEF [16]. Lastly, women have more comorbidities such as diabetes, renal disease, and arthritis [16]. The clinical course and patient-reported outcomes for acute HF are also different. The study of Blumer et al. reported that among patients with HFrEF, women were less likely to receive guideline-directed medical therapy [17]. Signs and symptoms of HF were similar in women and men, but women experienced less in-hospital weight loss and urine output (all p < 0.01) [17]. Women were also reported to have a significantly lower EuroQol-5D (EQ-5D) utility and visual analogue scores at admission, discharge, and 30 days, and continued to have significantly lower EQ-5D scores at all in-hospital and post-discharge time points after adjustment for clinical characteristics [17]. These results imply that women experience a lower quality of life with more significant functional impairment secondary to HF and have higher rates of dyspnea on exertion and difficulty exercising than men [3,17]. When men and women with HFrEF are compared, women are more symptomatic despite both sexes having similarly poor outcomes [3]. Despite this, women are less likely to receive treatment compared to men for the same risk factors [15]. Women are also less likely to be referred for procedures such as implantable cardioverter defibrillators, cardiac resynchronization therapy, or mechanical circulatory support [3,17].
Women have traditionally been underrepresented in clinical trials [15]. RCTs supporting current HF management guidelines have recruited predominantly men subjects with a lack of prospective sex-specific analyses [15]. Across major clinical trials investigating the effect of SGLT-2is on patients with HF, women have made up less than a quarter of the total number of study participants. The EMPEROR-PRESERVED and DELIVER trials, two of the largest HF trials using SGLT2-is, enrolled a relatively higher number of women, highlighting the greater percentage of women afflicted by HFpEF [5,11-14]. In our study, we noted a significant difference in the number of participants based on sex, with the male sex predominating in all trials (see Fig. 3). A recent meta-analysis investigating the effect of SGLT2-is on MACE reduction by sex among diabetic patients also revealed no significant benefit for women that was attributed partly also to a similar underrepresentation of women in the included trials [10].
Fig. 3.
Percentage of patients enrolled in each trial according to sex.
The five RCTs included in this meta-analysis did not report the treatment effect of SGLT2-is, stratified by sex, for each of the individual outcomes (i.e., cardiovascular death, hospitalization for heart failure, urgent care visit for heart failure) comprising the primary composite outcome. However, it is notable that the benefit of SGLT2-is on the primary composite outcome was driven primarily by reductions in hospitalizations for heart failure, which was largely consistent across the spectrum of heart failure phenotypes. Several studies have shown that women are disproportionately more likely to experience recurrent hospitalizations for heart failure compared to men, despite similar rates of in-hospital mortality [18-21]. Future studies investigating the benefits of SGLT2-is among heart failure patients should stratify the individual components of the primary composite outcome by sex to explore any differences in the treatment effect of the drug.
The anatomical and physiological differences between men and women have CV implications. Coronary artery disease is thought to occur more frequently in men than in women, owing to the cardioprotective effects of estrogen [22,23]. However, as women enter the menopausal stage, this protection wanes as estrogen significantly decreases due to reproductive senescence.
Sex differences in pharmacokinetics and pharmacodynamics of SGLT-2is may also play an important role. The interplay of these concepts is affected by many factors; therefore, detecting sex differences in the pharmacokinetics and pharmacodynamics of drugs is reasonable [23-25]. In terms of body composition, women typically have lower plasma volume and higher body fat percentage compared to men. In particular, increased body fat may contribute to a higher volume of distribution of lipophilic drugs such as SGLT2-is [10]. The pharmacodynamic effect of SGLT-2 inhibitors involves inciting glycosuria by lowering the maximum renal glucose resorptive capacity and the glucose-resorptive threshold in the apical membrane of the proximal convoluted tubules [26,27]. Among the SGLT-2is, empagliflozin has the highest selectivity (>2500-fold) for the SGLT-2 receptors [28]. It is rapidly absorbed via the oral route with a peak action of 1.5 h and a half-life of 12.4 h, making it suitable for once-a-day dosing. Its bioavailability is around 78 %, and it binds to proteins by about 86 % [28]. Its excretion is both fecal (40 %) and renal (50 %); nevertheless, it is still well tolerated in those with mild to moderate chronic kidney disease [28]. There are currently no studies on sex differences in the pharmacokinetic and pharmacodynamic properties of SGLT-2is.
Biological differences in men and women have important implications for disease etiology and manifestation, treatment response, and outcomes. As previously mentioned, women are underrepresented in many CV RCTs [29]. The reason for this disparity is multifaceted. Historically, studies were performed on male subjects and extrapolated to females to avoid the burden of consideration of female hormonal fluctuations and unknown harm in pregnancy [30]. Other studies cited that decreased female recruitment is due to decreased referral rates, heightened worry of harm, family and childcare responsibilities, and low likelihood of trial retention [31,32]. Despite efforts to ensure the inclusion of women as subjects in clinical trials, the lack of sex diversity in cardiology and clinical trial leadership perpetuates sex disparities in clinical research [33,34]. This paucity of sex-specific data may translate into suboptimal health outcomes as clinical practice guidelines are driven by clinical trial data from predominantly male subjects. Addressing these gender gaps may lead to more robust data in sex-specific analysis and therapy which may improve outcomes in both sexes.
2.2. Strength and limitations
The primary strength of this study is the standardized approach used to systematically review the data from all the RCTs with the same endpoints of the use of SGLT-2is in HF patients. There have been other studies on the effect of the sex of patients on CV outcomes for those taking SGLT-2is, but these analyses have been limited to a few observational studies. To our knowledge, this study is the first meta-analysis involving five large RCTs examining the sex differences in SGLT-2i cardiovascular outcomes. Limitations to the study include the limited availability of individual data specific to sex-stratified outcomes. Furthermore, the analysis was based on published aggregate data, not individual patient-level data. Patient-level data would have allowed for more in-depth analysis and better adjustment for confounding factors. Additionally, the SOLOIST – WHF trial was excluded from the comparison of effects on males versus females due to the unavailability of data on actual events per subgroup. The main strength of the analysis is in the availability of these RCTs for SGLT-2is and their effect on cardiovascular outcomes.
2.3. Conclusion
SLGT-2is are a very effective treatment for HF regardless of sex. Compared to men, women receive less benefit from these drugs. Further research needs to be done to better explain these observed outcome differences. Appropriate patients should be started on these medications.
Fig. 5.
Primary composite outcomes in females receiving SGLT-2is vs. placebo.
Funding
This research did not receive any specific grant from the public, commercial, or not-for-profit funding agencies.
Footnotes
CRediT authorship contribution statement
Credit roles: Frederick B. Rivera MD: Conceptualization; Data curation; Validation; Writing - original draft; Writing - review & editing; Deogracias Villa De Luna, MD, Vincent Tang, MD: Data curation; Formal analysis; Methodology; Frederick Berro Rivera, MD: Validation, Writing-review, and editing original draft; Anabelle S. Volgman, MD, FACC, FAHA; Amir Kazory, MD; Nilay S. Shah, MD, MPH; Krishnaswami Vijayaraghavan, MD; Validation; Writing - review & editing. Annabelle Santos Volgman, MD, FACC, FAHA.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Statement of ethics
Ethics approval for this study was not required.
Data availability statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
References
- [1].Taylor AL, Heart failure in women, Curr. Heart Fail. Rep 12 (2) (2015) 187–195. [DOI] [PubMed] [Google Scholar]
- [2].Virani SS, et al. , Heart disease and stroke Statistics-2021 update: a report from the American Heart Association, Circulation 143 (8) (2021) e254–e743. [DOI] [PubMed] [Google Scholar]
- [3].Bozkurt B, Khalaf S, Heart failure in women, Methodist Debakey Cardiovasc. J 13 (4) (2017) 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abdin A, et al. , Timely and individualized heart failure management: need for implementation into the new guidelines, Clin. Res. Cardiol 110 (8) (2021) 1150–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Anker SD, et al. , Empagliflozin in heart failure with a preserved ejection fraction, N. Engl. J. Med 385 (16) (2021) 1451–1461. [DOI] [PubMed] [Google Scholar]
- [6].Heidenreich PA, et al. , 2022 AHA/ACC/HFSA guideline for the Management of Heart Failure: a report of the american College of Cardiology/American Heart Association joint committee on clinical practice guidelines, Circulation 145 (18) (2022) e895–e1032. [DOI] [PubMed] [Google Scholar]
- [7].McDonagh TA, et al. , 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure, Eur. Heart J 42 (36) (2021) 3599–3726. [DOI] [PubMed] [Google Scholar]
- [8].Yang F, Meng R, Zhu DL, Cardiovascular effects and mechanisms of sodium-glucose cotransporter-2 inhibitors, Chronic Dis. Transl. Med 6 (4) (2020) 239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kimmelstiel CD, Konstam MA, Heart failure in women, Cardiology 86 (4) (1995) 304–309. [DOI] [PubMed] [Google Scholar]
- [10].Singh AK, Singh R, Gender difference in cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in type 2 diabetes: a systematic review and meta-analysis of cardio-vascular outcome trials, Diabetes Metab. Syndr 14 (3) (2020) 181–187. [DOI] [PubMed] [Google Scholar]
- [11].Solomon SD, et al. , Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction, N. Engl. J. Med 387 (12) (2022) 1089–1098. [DOI] [PubMed] [Google Scholar]
- [12].McMurray JJV, et al. , A trial to evaluate the effect of the sodium-glucose cotransporter 2 inhibitor dapagliflozin on morbidity and mortality in patients with heart failure and reduced left ventricular ejection fraction (DAPA-HF), Eur. J. Heart Fail 21 (5) (2019) 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Packer M, et al. , Effect of empagliflozin on the clinical stability of patients with heart failure and a reduced ejection fraction: the EMPEROR-reduced trial, Circulation 143 (4) (2021) 326–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bhatt DL, et al. , Sotagliflozin in patients with diabetes and recent worsening heart failure, N. Engl. J. Med 384 (2) (2021) 117–128. [DOI] [PubMed] [Google Scholar]
- [15].McSweeney J, et al. , Disparities in heart failure and other cardiovascular diseases among women, Women's Health (Lond. Engl.) 8 (4) (2012) 473–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hopper I, et al. , Comorbidities in heart failure: are there gender differences? Curr. Heart Fail. Rep 13 (1) (2016) 1–12. [DOI] [PubMed] [Google Scholar]
- [17].Blumer V, et al. , Sex differences in clinical course and patient-reported outcomes among patients hospitalized for heart failure, JACC Heart Fail 9 (5) (2021) 336–345. [DOI] [PubMed] [Google Scholar]
- [18].Shah KS, et al. , Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes, J. Am. Coll. Cardiol 70 (20) (2017) 2476–2486. [DOI] [PubMed] [Google Scholar]
- [19].Badran HM, Elgharably MA, Faheem N, Clinical characteristics and in-hospital outcome of heart failure in women: a single center registry from Egyptian cardiac care unit, Egypt Heart J 71 (1) (2019) 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Parissis JT, et al. , Gender-related differences in patients with acute heart failure: management and predictors of in-hospital mortality, Int. J. Cardiol 168 (1) (2013) 185–189. [DOI] [PubMed] [Google Scholar]
- [21].Sotomi Y, et al. , Sex differences in heart failure with preserved ejection fraction, J. Am. Heart Assoc 10 (5) (2021), e018574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Jochmann N, et al. , Female-specific aspects in the pharmacotherapy of chronic cardiovascular diseases, Eur. Heart J 26 (16) (2005) 1585–1595. [DOI] [PubMed] [Google Scholar]
- [23].Kuhl H, Pharmacology of estrogens and progestogens: influence of different routes of administration, Climacteric 8 (Suppl. 1) (2005) 3–63. [DOI] [PubMed] [Google Scholar]
- [24].Tamargo J, et al. , Gender differences in the effects of cardiovascular drugs, Eur. Heart J. Cardiovasc. Pharmacother 3 (3) (2017) 163–182. [DOI] [PubMed] [Google Scholar]
- [25].Rosano GM, et al. , Gender differences in the effect of cardiovascular drugs: a position document of the working group on pharmacology and drug therapy of the ESC, Eur. Heart J 36 (40) (2015) 2677–2680. [DOI] [PubMed] [Google Scholar]
- [26].Xu B, et al. , The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management, Cardiovasc. Diabetol 21 (1) (2022) 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Heerspink HJ, et al. , Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications, Circulation 134 (10) (2016) 752–772. [DOI] [PubMed] [Google Scholar]
- [28].Anker SD, Butler J, Empagliflozin, calcium, and SGLT1/2 receptor affinity: another piece of the puzzle, in: ESC Heart Fail, 2018, pp. 549–551. England. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Reza N, Gruen J, Bozkurt B, Representation of women in heart failure clinical trials: barriers to enrollment and strategies to close the gap, Am Heart J Plus (2022) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Söderström M, Why researchers excluded women from their trial populations, Lakartidningen 98 (13) (2001) 1524–1528. [PubMed] [Google Scholar]
- [31].Cho L, et al. , Increasing participation of women in cardiovascular trials: JACC council perspectives, J. Am. Coll. Cardiol 78 (7) (2021) 737–751. [DOI] [PubMed] [Google Scholar]
- [32].Kim ES, Menon V, Status of women in cardiovascular clinical trials, Arterioscler. Thromb. Vasc. Biol 29 (3) (2009) 279–283. [DOI] [PubMed] [Google Scholar]
- [33].Steinberg JR, et al. , Analysis of female enrollment and participant sex by burden of disease in US clinical trials between 2000 and 2020, JAMA Netw. Open 4 (6) (2021), e2113749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Denby KJ, et al. , Representation of women in cardiovascular clinical trial leadership, JAMA Intern. Med 180 (10) (2020) 1382–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.