Abstract
This review critiques the literature supporting clinical assessment and management of cardiovascular disease (CVD) and CVD risk stratification with brachial-ankle pulse wave velocity (baPWV). First, we outline what baPWV actually measures - arterial stiffness (AS) of both large central elastic arteries and medium-sized muscular peripheral arteries of the lower-limb. Second, we argue that baPWV is not a surrogate for carotid-femoral pulse wave velocity. While both measures are dependent on the properties of the aorta, baPWV is also strongly dependent on the muscular arteries of the lower extremities. Increased lower extremity AS amplifies and hastens wave reflections at the level of the aorta, widens pulse pressure, increases afterload, and reduces coronary perfusion. Third, we used an established evaluation framework to identify the value of baPWV as an independent vascular biomarker. There is sufficient evidence to support: i. proof of concept; ii. prospective validation; iii. incremental value; and iv. clinical utility. However, there is limited or no evidence to support v. clinical outcomes; vi. cost-effectiveness; viii. methodological consensus; or ix. reference values. Fourth, we address future research requirements. The majority of the evaluation criteria (i. proof of concept, ii. prospective validation, iii. incremental value, iv. clinical utility, and ix. reference values) can be supported using existing cohort datasets, whereas the v. clinical outcomes and vi. cost-effectiveness criteria require prospective investigation. The viii. methodological consensus criteria will require an expert consensus statement. Finally, we finish this review by providing an example of a future clinical practice model.
Keywords: Pulse wave velocity, arterial stiffness, biomarker, vascular ageing, cardiovascular disease risk
INTRODUCTION
Cardiovascular disease (CVD) risk management may be improved by incorporating arterial stiffness (AS), a measure of biological vascular aging, within standard clinical practice. Brachial-ankle pulse wave velocity (baPWV, see Figure 1), a composite measure of central and peripheral AS,1 has been widely adopted in parts of East Asia, with the Japanese Society of Hypertension Guidelines recommending use of baPWV for hypertension management.2 Despite incorporation within the 2023 European Society of Hypertension (ESH) guidelines3, in which it is recommended as a screening tool for hypertension mediated organ damage, baPWV has received sparse attention in Western countries. This review focuses on the use of baPWV as a vascular biomarker to aid in the clinical management of CVD risk. Definitions for key terms and concepts used throughout the review are provided in Table 1. After reading this review, readers should be able to better understand:
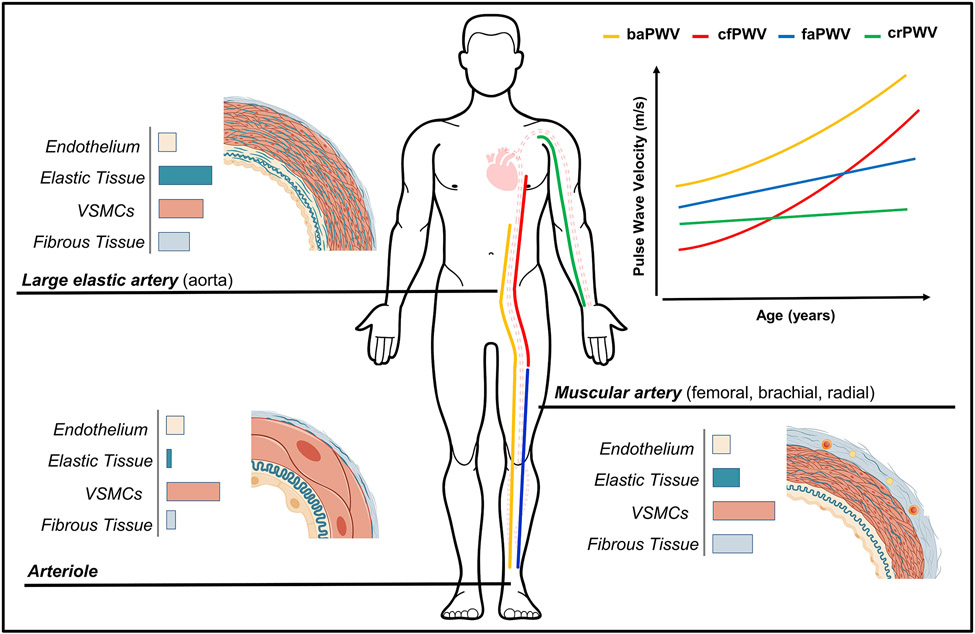
FIGURE 1.
Comparison of wall composition and changes in pulse wave velocity with age between central and peripheral arterial regions. cfPWV, carotid-femoral pulse wave velocity (central); faPWV, femoral-ankle pulse wave velocity (lower-limb); crPWV, carotid-radial pulse wave velocity; baPWV, brachial ankle pulse wave velocity (composite measure). baPWV summates the arterial properties between the descending aorta at the level of the brachial artery and the ankle, comprising of arteries or arterial regions (e.g. the aorta) with different structural and mechanical characteristics.
TABLE 1.
Key terminology definitions.
| Term | Abbreviation> | Definition |
|---|---|---|
| Ankle-brachial index | ABI | Ratio of the systolic blood pressure measured at the ankle (tibial artery) to systolic blood pressure measured at the brachial artery. |
| Area under the curve | AUC | The area of the region enclosed by the curve line and the coordinate axis. |
| Arterial stiffness | AS | Fundamental mechanical behavior or rigidity of the material properties of the artery wall, determined by both structural and functional components. |
| Arterial Stiffening | -- | The loss of arterial compliance or increased rigidity to deformation and/or changes in vessel wall properties. |
| Arteriosclerosis | -- | The remodeling of the vasculature associated with stiffening, thickening, and dilatation of the arteries. |
| Atherosclerosis | -- | An occlusive disease of the vasculature that occurs as a result of the deposition of lipid-laden plaques. |
| Biomarker | -- | A characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. |
| C statistic | -- | The probability of concordance between predicted and observed survival. A measure of goodness of fit for binary outcomes in a logistic regression model. |
| Cardiovascular disease | CVD | A general term that describes heart, circulatory, and cerebrovascular disease. |
| Causal pathway | -- | Steps in the linkage between a cause and outcome. |
| Clinical endpoint | -- | An event or outcome that can be measured objectively to determine whether the intervention being studied is beneficial. |
| Clinical management | -- | A course of action for managing an individual's treatment. |
| Evidence-based science | -- | Science informed by systematic research and underpinned by established theory. |
| Global chi-squared | X2 | A statistical test used to compare observed results with expected results. |
| Hazard ratio | HR | An estimate of the ratio of the hazard rate in the treated versus the control group. |
| Implementation science | -- | A discipline which seeks to understand the barriers and facilitators to clinical practice adoption. |
| Integrated discrimination index | IDI | A tool for evaluating the capacity of a marker to predict a binary outcome of interest. |
| Systolic interarm blood pressure difference | IAD | The absolute value of the difference in systolic blood pressure between left and right arms. |
| Intermediate outcome | -- | A measurable indicator on the causal pathway to the final outcome. |
| Net reclassification index | NRI | A measure to assess the added value of predictor variables or used to assess the relative ability of 2 risk models to distinguish between low- and high-risk individuals. |
| Odds ratio | OR | A measure of association between a treatment/exposure and an outcome. |
| Path length | -- | Length of arterial segment(s) the arterial pulse wave has propagated along. Estimated using body surface measurements or height-based formulas. |
| Pulse wave analysis | PWA | Morphological analysis of blood pressure waveforms. Typically comprises of the recording of peripheral pressure waveforms with subsequent generation of a corresponding central waveform, permitting estimation of central hemodynamic variables including pressure and wave reflection. |
| Pulse wave velocity | PWV | The speed at which the arterial pulse wave propagates along an arterial segment. |
| Quality of life | QoL | The standard of health, comfort, and happiness experienced by an individual or group. |
| Relative risk | RR | The ratio of the risks for an event for the exposure group to the risks for the non-exposure group. |
| Standard care | -- | Treatment approach that is accepted as appropriate and is widely or typically used by healthcare professionals. |
| Vascular ageing | -- | Vascular ageing describes the deterioration in vascular structure and function over time that ultimately leads to damage in the heart, brain kidney, and other organs. Comprises of atherosclerotic and arteriosclerotic processes. |
the physiological principles of baPWV;
baPWV is a unique vascular biomarker and not a surrogate for carotid-femoral pulse wave velocity (cfPWV) or other measures of AS;
the evidence supporting the use of baPWV as a vascular biomarker;
future research requirements.
VASCULAR AGEING AND ARTERIAL STIFFENING
The deterioration in vascular structure and function over time are the hallmarks of vascular ageing. Vascular ageing is attributable to atherosclerosis, the narrowing of the artery by the deposition of atheromatous plaque, and arteriosclerosis, the stiffening and thickening of the arterial wall.4, 5 Although vascular ageing is a natural phenomenon associated with chronological age, there is wide inter-individual variability as a function of lifestyle, environmental and/or genetic factors.6
Arterial stiffness describes the resistance of the arterial wall to deformation. The arterial wall comprises endothelial cells, vascular smooth muscle cells (VSMC), and connective scaffolding proteins - principally collagen and elastin (see Figure 1). Elastin predominates in central arteries, ensuring a high elastic potential and the capacity to cushion the flow and pressure oscillations caused by intermittent cardiac left-ventricular ejection.7 Collagen fibres and VSMCs predominate in muscular peripheral arteries, which means a lower elastic potential but a greater propensity for alterations in tone. The transition from a compliant aorta to relatively stiffer muscular arteries generates an impedance mismatch or stiffness gradient.7, 8 The stiffness gradient attenuates the cyclical forward pressure wave into a smooth consistent blood flow, prevents transmission of pulsatile energy to the micro-circulation, and moderates wave reflection and, therefore, cardiac workload.7, 8
Arterial ‘stiffening’ is an arteriosclerotic process resulting from the gradual fragmentation, calcification, and loss of elastin fibres, the accumulation of stiffer collagen fibres, calcification and senescence of VSMCs, inflammation, and endothelial dysfunction contributing to a loss of elasticity or increase in the myogenic tone of the vessel wall.7, 9 Central arteries are susceptible to stiffening, whereas the stiffening of peripheral arteries is less pronounced (see Figure 1).10 Arterial stiffening, particularly of the aorta, increases systolic and pulse blood pressure and the pulsatile load on the left ventricle, as well as the transmission of pulsatile energy to the micro-circulation, leading to damage of target organs.7 The differing impact of age, lifestyle factors and risk factors on central and peripheral arteries leads to progressive attenuation of the physiological impedance mismatching, with attenuation or reversal of the stiffness gradient.10, 11 This amplifies and hastens wave reflection, increasing cardiac workload and the impairment of coronary perfusion.8
OVERVIEW OF BAPWV
MEASUREMENT OF BAPWV
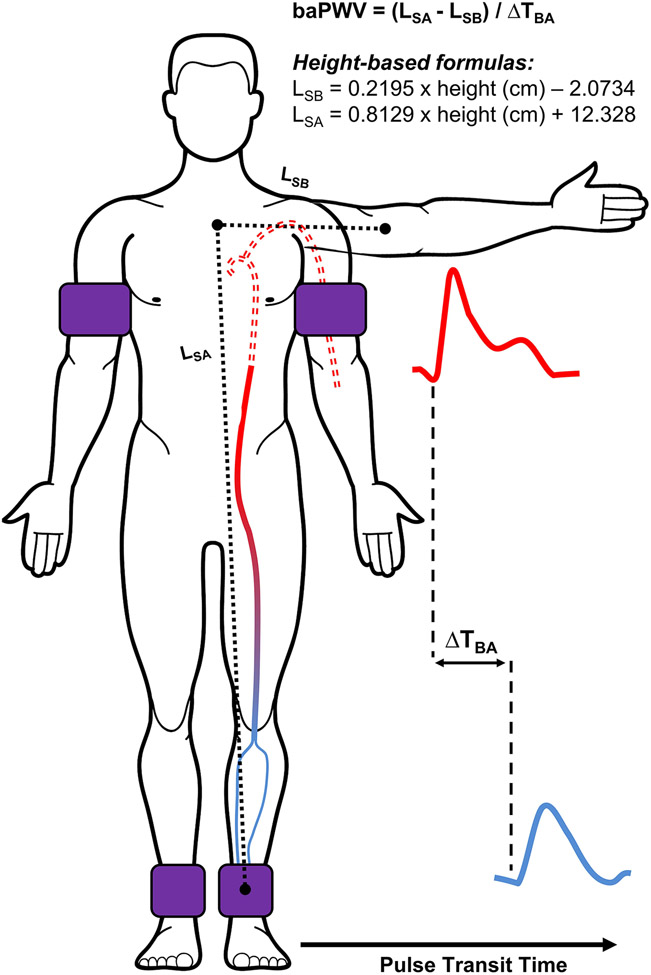
Pulse wave velocity is the speed at which the forward pressure wave propagates along the arterial tree, and is calculated as arterial path length divided by pulse transit time (PTT). A higher PWV indicates a greater (detrimental) arterial stiffness. The PTT for baPWV is commonly measured using blood pressure cuffs equipped with oscillometric and volume-plethysmographic sensors. The cuffs are secured around the upper-arm(s) (brachial artery) and ankle(s) (tibial and peroneal arteries) and used to simultaneously record the underlying pulse pressure waveforms (Figure 2). The PTT is then calculated as the time interval between the brachial and ankle waveforms using the foot-to-foot method, where the ‘foot’ is detected using the ‘intersecting tangent’ method. Arterial path length is typically estimated automatically via height-based equations, but can be estimated using body surface measurements and calculated as the path length from suprasternal notch to the ankle minus the suprasternal notch to the brachium.12
FIGURE 2.
Schematic for the measurement of brachial-ankle pulse wave velocity (baPWV). baPWV is estimated as the length between the sternal notch and the ankle (LSA) minus the sternal notch to brachium (LSB), as determined by body surface measurements or height-based formulas, divided by the time delay between brachial and ankle waveforms (ΔTBA). Note: Solid line denotes effective path length.
WHAT PHYSIOLOGICAL CONSTRUCT DOES BAPWV MEASURE?
There is a prevalent, but mistaken, view that baPWV solely reflects the stiffness of peripheral arteries13. baPWV does incorporate the medium-sized muscular peripheral arteries of the lower-limbs, but also the elastic descending aorta (Figure 1). The proximal portion of the aorta is excluded from baPWV, at least theoretically, which can be attributed to the use of the brachial artery as the proximal measurement site. Following ventricular ejection, pulse pressure waves simultaneously propagate along the heart-brachial and heart-descending aorta arterial segments. It is assumed that the distance propagated and PTT for these pulse pressure waves are equitable. This theoretically results in the descending aorta serving as the start point for baPWV.1, 14 This assumption is made for cfPWV, as the heart-carotid arterial segment is inconsistent with the path of blood flow for the region of interest. While the start point of baPWV is the descending aorta, it is still influenced by the preceding segments and the heart-brachial arterial segment. The importance of upper-limb PWV is often overlooked as it is expected to change little with age,15 but an increase or decrease in heart-brachial PWV may bias the baPWV outcome by increasing or decreasing the brachial-ankle PTT.
DISADVATNAGES OF BAPWV
Measurement of baPWV may be inappropriate in some patient populations.
The presence of cardiovascular pathology can impact the accuracy of baPWV measures. Stenosis in the lower-limbs, aortic stenosis, aneurysm, or regurgitation may lead to a pseudo-underestimation of baPWV.16, 17 Stenosis in the subclavian arteries may lead to a pseudo-overestimation of baPWV. Measurement of baPWV may be inappropriate if the patient presents with one of the aforementioned conditions.
Height-based formulas to estimate arterial path length are inaccurate.
Arterial path length is typically estimated using height-based formulas, which may be advantageous for clinical practice but lack accuracy. Compared to magnetic resonance imaging (MRI), height-based formulas overestimated path length by ~11%.18 This contributes to absolute values of baPWV being substantially higher than all other regional PWV measures. MRI and height-based path lengths are highly correlated, indicating this problem may be rectified with an appropriate adjustment factor, but population-specific validation is required.18
ADVANTAGES OF BAPWV
Measurement of baPWV is simple and automated.
A prominent advantage of baPWV is its ease of assessment, with PPT being determined non-invasively and automatically using oscillometry. Limited training is needed for the baPWV operator, since the technique does not differ from any cuff-based blood pressure measurement. This simplicity, combined with an inherent low-patient burden, means baPWV may be easily applied in clinical practice.
Measures of baPWV can be determined with accuracy and precision.
baPWV has shown good agreement (r=0.87) with central PWV obtained by invasive recording.19 baPWV can be determined with good reliability (intra-observer, between-visit day to day) and good reproducibility with low operator bias (inter-observer, same day).20 Comparative studies have reported higher between-day intraclass-correlation coefficients for baPWV (0.84) compared to cfPWV (0.70).20
The ankle brachial index (ABI), systolic interarm blood pressure difference (IAD) and pulse wave analysis (PWA) can be determined concurrently with baPWV.
The ABI is the ratio of the systolic blood pressure measured at the ankle to that measured at the brachial artery, with a threshold of ≤0.9 typically indicating significant stenosis and lower-extremity peripheral artery disease (PAD) or >1.4 indicating vascular calcification.21 An IAD of ≥10 mmHg indicates significant upper extremity stenosis and PAD.22 ABI and IAD can identify the presence of stenosis and inform the suitability of baPWV measurement. PWA entails using a generalized transfer function to estimate the aortic pressure waveform, from which central hemodynamic variables such as central blood pressure and arterial wave reflection indices are derived.23, 24 These complimentary outcomes will be further discussed in the Potential Clinical Practice Model section.
BAPWV IS A STANDALONE MARKER OF CVD RISK
baPWV is commonly perceived as a surrogate of the referent PWV measure, cfPWV. baPWV and cfPWV do reflect overlapping regional arterial properties and functions and share similar associations with CVD risk factors (Figure 1).25, 26 However, baPWV also incorporates the medium-sized muscular arteries of the lower extremities,26 the stiffness of which can increase with age16, 27 and predicts stroke and CVD, including heart failure and myocardial infarction.28 Increased stiffness of the muscular lower-limb arteries may principally contribute to CVD by amplifying and hastening wave reflections at the level of the aorta. This increases systolic blood pressure and widens pulse pressure, augmenting cardiac workload and increasing afterload whilst reducing coronary perfusion. The inclusion of this larger vascular territory means baPWV can provide additional, or at least different, cardiovascular information. Compared to cfPWV, baPWV is more strongly associated with cardiovascular structure and function, including left ventricular mass and diastolic function.29, 30 Although whether this translates to a differentiation of hard outcome prognosis over the longer-term between PWV measures is unclear.
EVIDENCE SUPPORTING USE OF BAPWV AS A VASCULAR BIOMARKER
Within this section we critique whether there is evidence to support the use of baPWV as an intermediate outcome. An intermediate outcome is a surrogate measure for a clinical endpoint, e.g., CVD morbidity or mortality. A vascular biomarker can be considered an intermediate outcome if it is associated with the intended clinical endpoint and is on the causal pathway between exposure (e.g., behaviour or treatment) and effect (i.e., predicts clinical benefit/harm).31 A vascular biomarker can be considered suitable for clinical use if it satisfies the aforementioned, confers meaningful prediction of clinical benefit/harm superior beyond existing biomarkers (e.g., lipids), and is practical for use in the intended setting (i.e., can be implemented). Below we consider the nine criteria for the evaluation of novel biomarkers of CVD risk presented by the American Heart Association (AHA)32 and endorsed by the European Society of Cardiology (ESC) and the Association for Research into Arterial Structure and Physiology (ARTERY) Society.33 For each of the nine criteria we provide a definition, an overview of the key evidence, and indicate important gaps in the literature.
i. PROOF OF CONCEPT
Definition.
Capacity to distinguish between subjects with and without CVD morbidity. Commonly tested with a cross-sectional study and quantified by determining whether the difference between groups is significant. Ideally, these findings are replicated in independent study populations.
Evidence.
Compared to non-diseased controls, baPWV is greater in patients with CAD19, heart failure with preserved ejection fraction34, target organ damage (cardiac, arterial, chronic kidney disease)35, CVD risk factors36, 37, and abnormal cardiovascular structure and function29, 30, 34.
Gaps.
The existing research has predominantly been conducted in East Asia, particularly Japan. Confirmatory research is required but there is no evidence to suggest the utility of baPWV may differ between countries and ethnicities.
ii. PROSPECTIVE VALIDATION
Definition.
Ability to predict the development of future CVD morbidity or mortality. Typically tested with a prospective cohort, nested case-cohort, or case-control study and quantified as an odds ratio (OR), risk ratio (RR) or hazard ratio (HR) adjusted for risk factors including age and blood pressure.
Evidence.
Several meta-analyses have been published summarizing the capacity of baPWV to predict CV events and mortality38-40. In 2017, Ohkuma et al.38 estimated the pooled HR using individual data from 14,673 Japanese adults (across eight studies) without a history of CVD. Following a mean follow-up of 6.4 years, participants in the fifth quintile (≥18.8 m/s) versus first quintile (<12.9 m/s) had a 3.5-fold (95% confidence interval (CI): 2.1 to 5.7) greater risk of developing CVD. These findings by Ohkuma et al. 38 mostly corroborate the 2012 report by Vlachopoulos et al.39 who calculated the pooled RR from 18 cohort studies (total n = 8,169). Following a mean follow-up of 3.6 years, participants with high versus low baPWV (using high/low cut-points specified by each study) were at greater risk for total CV events (RR: 3.0, 95%CI: 1.6 to 5.3) and CV mortality (RR: 5.4, 95%CI: 2.2 to 13.3). And, contrary to the findings by Ohkuma et al.38, were at greater risk for all-cause mortality (RR: 2.5, 95%CI: 1.6 to 3.9). In 2021, Sang et al40 focused on patients with atherosclerotic CVD: compared to low baPWV, participants with high baPWV (using high/low cut-points specified by each study) were at greater risk for CV events (HR: 2.6, 95%CI: 1.6 to 4.0) and cardiovascular mortality (HR: 2.7, 95%CI: 1.9 to 3.8).
Gaps.
baPWV is consistently associated with CV events in participants with and without a history of CVD38-40. Prospective validation studies are required to confirm the applicability of these findings beyond East Asia.
iii. INCREMENTAL VALUE
Definition.
Capacity of a novel biomarker to add predictive information beyond established biomarkers or models, such as the American College of Cardiology (ACC)/American Heart Association (AHA) atherosclerotic cardiovascular disease risk score. Typically tested with a prospective cohort study, in which the participants are followed over time and the number of outcome events (i.e., CVD morbidity or mortality) is recorded. The incremental value of the new biomarker can be quantified by comparing the change in OR, RR, HR, or the global chi-squared (X2). Though it should be noted that these statistics are not standardized and can be misleading for prognosis analysis. An alternative statistic is the c-index, which is used to determine how well a risk marker discriminates between individuals at different risk levels. For binary outcome variables the c-index is equivalent to the area under the receiver operating characteristic curve (AUC).
Evidence.
In the largest study to date,41 6,359 Korean adults (40–79 yrs) without a documented history of CVD were followed for a median period of 4 years. The AUC for CV events was superior for baPWV (AUC: 0.70, 95%CI: 0.68 to 71) compared to the 2013 ACC/ AHA a risk score (AUC: 0.62, 0.61 to 0.63). baPWV also conferred incremental value to the 2013 ACC/AHA risk score, improving the X2 from 21 to 50.41 For patients with a history of CVD or suspected CAD, baPWV also conferred additional prognostic value to computed tomography angiography42, single-photon emission computed tomography,43 flow-mediated dilation (endothelial function),44, 45 hs-CRP,46 and ABI.47
Gaps.
Only two prospective studies have investigated the general population. Additional prospective studies are warranted, particularly in general populations beyond East Asia.
iv. CLINICAL UTILITY
Definition.
Usefulness to clinical practice, in particular the ability of the new biomarkers to move patients from one risk category to another and thereby modify treatment. As a more powerful alternative to the c-statistic, the integrated discrimination improvement (IDI) and net reclassification improvement (NRI) tests are often used to test whether the new biomarker improves the ability to discriminate between groups.
Evidence.
Among a general population of 4,251 Chinese adults, with and without a history of CVD, baPWV was added to a model which included mean arterial pressure as the initial exposure variable and fatal/nonfatal CV and cerebrovascular endpoints as the outcomes.48 The addition of baPWV improved discrimination (IDI: 1.6%, 95%CI: 0.2 to 2.9) and net reclassification (NRI: 52%, 95%CI: 29 to 75). This supports the findings from previous individual-participant data meta-analysis by Ohkuma et al.38 While no known studies have investigated whether baPWV measurements can improve disease management, a limited number of studies have investigated the baPWV response to treatment. A meta-analysis of 17 randomized controlled clinical trials reported non-significant changes in baPWV or cfPWV following treatment with angiotensin-converting enzyme inhibitors.49 However, baPWV improved (i.e., decreased) by 1.3 m/s (95%: −2.1 to −0.5) when Japanese adults (n = 80) with type 2 diabetes were treated for 2 years with a sodium-glucose cotransporter 2 inhibitor (tofogliflozin), when compared to a usual care group (n = 74).50 A meta-analysis (n = 6 baPWV studies)51 reported that diet plus exercise therapy had a moderate effect on baPWV (standardized mean difference = −0.5, 95%CI: −0.8 to −0.2), a systematic review (n=7 studies)52 reported that combined resistance and aerobic training in post-menopausal women (n=16 to 101, mean age = 54 to 79 years) reduced baPWV by 0.6–2.1 m/s, and a 20-week quasi-experimental study53 reported that combined exercise plus statin treatment decreased baPWV from 17.5 m/s (SD: 3.6) to 16.3 m/s (SD: 2.7) despite a non-significant change in blood pressure.
Gaps.
Additional prospective studies, in independent populations and beyond East Asia, are required to quantify whether baPWV improves risk stratification.
v. CLINICAL OUTCOMES
Definition.
Whether use of the biomarker improves clinical outcomes. Optimally tested with a randomized controlled clinical trial and quantified by comparing outcomes for participants who are clinically managed using a new biomarker versus participants who are managed using standard care.
Evidence.
No known direct evidence exists to support the clinical outcomes criteria. However, the 2019 Japanese Society of Hypertension Guidelines for the Management of Hypertension recommend measurement of baPWV for managing hypertension2. The recommendation was based on the available research supporting the predictive ability (i.e., prospective validation) and incremental value of baPWV.38 baPWV was also included in the 2023 ESH guidelines.3
Gaps.
Clinical trials are required to determine whether treatment strategies based on baPWV measurement improve clinical outcomes.
vi. COST-EFFECTIVENESS
Definition.
Whether use of the biomarker improves clinical outcomes sufficiently to justify the additional costs. Typically determined by comparing the incremental cost to the incremental benefit. The incremental benefit is typically quantified as the change in life expectancy, the quality adjusted life-years (QALY), and the incremental cost-effectiveness ratio (ICER). The incremental costs include the downstream costs that can be attributed to the new biomarker, including follow-up testing and therapies prescribed.
Evidence.
No known studies have investigated the cost-effectiveness of baPWV. Cost-effectiveness is likely high, considering clinical utility and low operation costs, namely: (i) a single oscillometric device could be used to simultaneously measure blood pressure, ABI, IAD and baPWV; (ii) consumable requirements are minimal, including reusable cuffs; and (iii) minimal operator training requirements – akin to ABI. While baPWV cost-effectiveness data is unavailable, data is available for ABI. A recent Markov model54 focused on 10-year CV event risk among women in the general Italian population. The additional cost associated with ABI screening, when added to the Framingham Risk Score, was €110/patient, the incremental QALY was 0.0039/patient, and the ICER was €27.99/QALY.
Gaps.
baPWV-specific cost-effectiveness are required.
vii. EASE OF USE
Definition.
Whether measurement procedures are simple enough to permit widespread adoption.
Evidence.
The most prominent advantage of baPWV is its simplicity of use, with automated measurement following cuff placement.
Gaps.
Implementation studies are warranted to determine best practice guidelines for implementing baPWV within clinical practice.
viii. METHODOLOGICAL CONSENSUS
Definition.
Uniformity of biomarker measurements in different clinics. Required to compare obtained measurement values against reference values.
Evidence.
Though some scientific societies have provided guidelines on the clinical use of different techniques assessing AS,55 no specific guidelines exist to standardize baPWV methodology.
Gaps.
A consensus statement is required to help ensure measurement procedures are uniform across clinics, including consideration to: the required operator training; quality control; subject preparation; testing environment; data collection; cuff placement; outcome reporting; and path length determination.
ix. REFERENCE VALUES
Definition.
The availability of published reference or cut-off values.
Evidence.
Reference values, relative to age and blood pressure, have been published for the Japanese population.36 Several East Asian studies have suggested cut-points for CV risk assessment, albeit the cut-point range is wide, 14 to 18 m/s, and a limited number of adults older than 70 years of age have been included.36, 56, 57
Gaps.
Additional studies, including in non-Asian populations, are required to determine the expected change in baPWV with age, with respect to biological sex and blood pressure.
FUTURE DIRECTION
Below, we summarize the important gaps in the literature, provide suggestions for addressing the gaps, then finish with an example of a potential clinical practice model.
GAPS IN THE LITERATURE
As summarized above and in Table 2, there is sufficient evidence to support criteria i to iv. The priority scores were driven up (worse) by the need for data beyond East Asia and moderated by the fact that between-country differences in the association between baPWV and CV outcomes are likely attributable to modifiable risk factors rather than race/ethnicity.
TABLE 2.
Summary of evidence, gaps in knowledge, and research priorities.
| Evidence | Gaps in Knowledge | |||||
|---|---|---|---|---|---|---|
| Criteria | Definition | Evidence Summary | Score | Gaps | Priority | |
| i | Proof of concept | Ability to distinguish between subjects | Distinguishes CAD, HF, TOD, CV pathology | 4 | Data: beyond East Asia | 1 |
| ii | Prospective validation | Future CVD morbidity/mortality predictive ability | High vs. low baPWV: CVD morbidity (HR: 2.6-3.4, RR: 3.0), CVD mortality (HR: 1.2-2.6, RR: 5.4), all-cause mortality (HR: 1.8-2.5) | 4 | Data: beyond East Asia | 2 |
| iii | Incremental value | Predictive information beyond standard risk markers | CV event prediction ↑ when added to ACC/AHA risk score (X2: 21 to 50), ABI (AUC: 0.72 to 0.83), CTTA (X2: 132 to 154), SPECT (X2: 24 to 27), FMD (AUC: 0.71 to 0.75), & hs-CRP (X2: 126 to 167) | 4 | Data: beyond East Asia & in general populations | 3 |
| iv | Clinical utility | Ability to move patients from one risk category to another | CV events: IDI = 1.6%, NRI = 52%. | 3 | Limited data from prospective studies in independent populations | 3 |
| v | Clinical outcomes | Impact of clinical management with biomarker on clinical outcomes | No data available | 1 | Clinical management: support for aiding treatment | 4 |
| vi | Cost-effectiveness | Incremental costs vs incremental benefits (life expectancy and QoL) | No data available, though likely high considering clinical utility & low examination costs | 2 | Clinical management: impact on life expectancy & QoL | 2 |
| vii | Ease of use | Simplicity of measurement procedures | Measurements automated following cuff placement | 3 | Implementation studies: best practices for implementing within clinical workflow | 5 |
| viii | Methodological consensus | Uniformity of measurements in different clinical settings. | No known guidelines exist | 1 | Consensus statement: measurement conformity across clinics, facilitating comparison to reference values | 5 |
| ix | Reference values | Availability of published reference or cut-off values | Reference values for Chinese populations | 2 | Data: beyond East Asia | 4 |
| Median evidence score | 4 | |||||
Evidence Score / Gaps Priority Likert scale: 1 = very low; 2 = low; 3 = medium, 4 = high, 5 = very high. Abbreviations: ACC/AHA, American College of Cardiology/American Heart Association; CAD, coronary artery disease; CV, cardiovascular; CVD, cardiovascular disease; FMD, flow-mediated dilation; HF, heart failure; hs-CRP, high-sensitivity C-reactive protein; HR, hazard ratio; IDI, integrated discrimination improvement; NRI, net reclassification index; OR, odds ratio; RR, relative risk; QoL, quality of life; SPECT, single-photon emission computed tomography; TOD, target organ damage; X2, global chi squared.
There is limited or no evidence to support the v, vi, viii and ix. Studies are required to discern the usefulness of baPWV in guiding treatment decisions, and to determine whether the baPWV-guided treatment improves life expectancy and quality of life. Additionally, to guide clinical management with baPWV, data are required to generate reference values that are applicable to diverse populations. However, the reference values will only be of true value if there is measurement conformity across clinics. As such, a consensus statement is required to standardize the way baPWV is measured. The priority for addressing the cost-effectiveness criteria has been scored 2 (low), considering the expected clinical utility and low operating costs. The priority for addressing clinical outcomes, methodological consensus, and reference values have been scored 4 (high) to 5 (very high), considering that these are major impediments for persuading clinicians to change their clinical practice.
Criteria vii has been scored a 3 (medium). The score was driven up due to the low operator training requirements and moderated by the lack of best practice guidelines for clinical practice adoption. Adopting a new clinical management tool is disruptive to existing clinical practices and many barriers may exist, including but not limited to where/how the new tool fits within existing workflow models, potential changes to infrastructure, and alterations to clinical decision making. To address this gap, the current focus on evidence-based research needs to extend to implementation science. Most evidence-based research is not incorporated into routine clinical practice. Implementation science is a discipline which seeks to understand the barriers and facilitators to clinical practice adoption, and design and test different strategies to scale evidence-based practices.
ADDRESSING THE GAPS IN THE LITERATURE
This section has been broken down into two parts. Part one (short-term) considers research that can be conducted to address the evaluation criteria. Part two (long term) goes beyond the evaluation criteria, considering future refinement of the methodology itself.
SHORT-TERM
A number of the evaluation criteria, particularly i to iv and ix, can be further supported using existing cohort datasets. Example datasets include the Atherosclerosis Risk in Communities (ARIC), Multi-Ethnic Study of Atherosclerosis (MESA), Jackson Heart Study (JHS), Hispanic Community Health Study / Study of Latinos (HCHS/SOL), Framingham Heart Study (FHS), German National Cohort (NAKO), The Study of Health in Pomerania (SHIP), the COmPLETE Health Study, and The Swiss Study on Air Pollution and Lung Disease in Adults (SAPALDIA) 3 study.
The viii. methodological consensus criteria can largely be addressed by bringing together an expert team and adapting guidelines, which have been developed for cfPWV. However, there are some unique considerations for baPWV which may require additional research, including a path length formula suited to Western populations. The NAKO study,58 which calculated baPWV using direct body surface measurements in more than 200,000 adults, may assist with validating a path length formula.
The v. clinical outcomes and vi. cost-effectiveness criteria will require prospective investigation, whereby primary care clinicians are randomized to adopt baPWV or to persist with usual patient care for a defined period. This will facilitate determination of the impact of baPWV on clinical management, including therapeutic prescription, and CV outcomes.
LONG-TERM
The above (short-term) discussion considers baPWV as standalone biomarker, which may not be the best approach for clinical management. Consider that the same device can be used to measure blood pressure, ABI, IAD and baPWV, each of which provides different CV information. Further research is required to determine whether the combined use of these biomarkers improves risk stratification and clinical management. There is also scope for utilizing artificial intelligence/machine learning to distinguish CV phenotypes and more objective treatment recommendations.
POTENTIAL CLINICAL PRACTICE MODEL
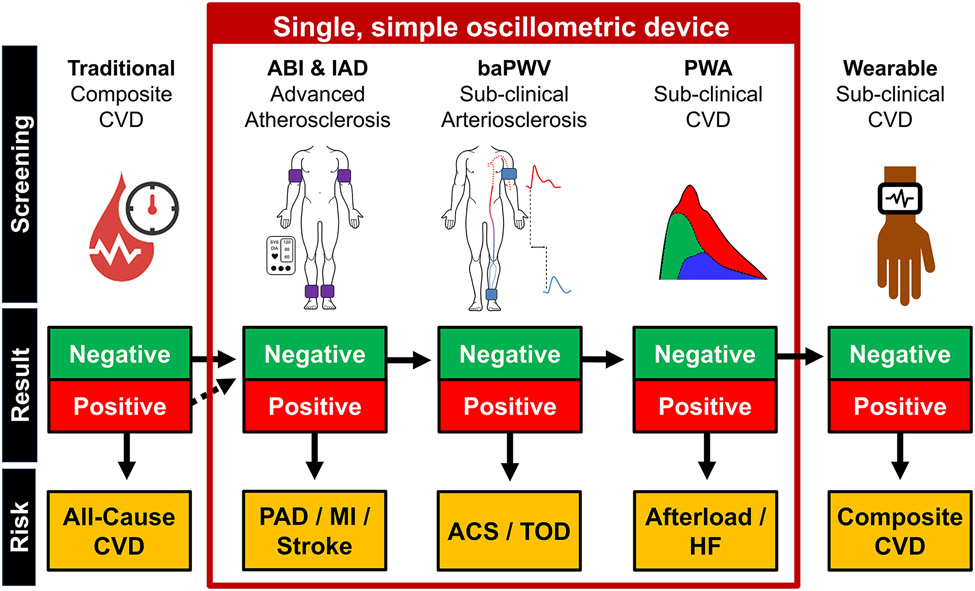
The intent of this section is not to make specific recommendations pertaining to the implementation of baPWV within clinical practice, but rather to stimulate much needed discourse. The simplicity and automation of measurement, low cost of measurement device, and inherent low-patient burden, means baPWV may be readily integrated into clinical practice and succeed where other PWV biomarkers have yet to gain traction. Nonetheless, in addition to satisfying the nine evaluation criteria discussed above, thought needs to be afforded to how baPWV will be implemented. In this section we present a theoretical model to indicate how baPWV may be used in clinical practice as part of a screening routine by a primary care physician to gain an holistic snapshot of a patient’s cardiovascular health. This model encompasses ABI, IAD and PWA, and although the evidence supporting their use is not unequivocal59-62, these measures can be assessed concurrently with baPWV and may enhance reclassification beyond risk scores.22, 63, 64 PWA derived outcomes including central blood pressure and arterial wave reflection may be particularly useful for enhancing diagnostic accuracy of heart failure65 and improving clinical care.66
Figure 3 outlines a potential clinical practice model in the form of a decision tree. Traditional risk factors are initially used to stratify all-cause CVD risk, followed by the measurement of ABI and IAD. A positive (i.e. unhealthy) ABI (< 0.9 or > 1.4) or IAD (≥ 10 mmHg systolic BP) test would indicate advanced peripheral atherosclerosis and very high risk for myocardial infarction and stroke. A patient may be prescribed advanced imaging and then intensive treatment in the form of lifestyle behaviors, medication, and/or surgery. If the ABI and IAD are ‘negative’ (healthy), additional information provided by baPWV and PWA will be considered. If the baPWV score is positive (see Reference Values section) the patient may be allocated to intensive preventive treatments for all-cause CV event risk, acute coronary syndrome, and/or target organ damage risk. A positive PWA score may indicate the need to prescribe medications to decrease cardiac afterload, such as anti-hypertensive medications66. If both the baPWV and PWA scores are negative, the patient may be sent home with a wearable patch which continuously sends sensitive PWA and baPWV data to the cloud.67 The in-clinic and ambulatory data will be harmonized in clinical management software and used to generate a CV phenotype and treatment plan. The first generation of the software may use a decision tree-based machine learning algorithm,68 advancing to more complex algorithms or artificial intelligence with subsequent generations.
FIGURE 3.
Example of a potential future clinical practice model in the form of a decision tree. In this example, traditional risk factors will be measured and used to stratify all-cause cardiovascular disease (CVD) risk. Subsequently, ankle brachial index (ABI) and systolic interarm blood pressure difference (IAD) are measured. Positive ABI and/or IAD (i.e., unhealthy, ABI score < 0.9 or > 1.4 and/or IAD ≥ 10 mmHg) scores indicates peripheral arterial disease (PAD) and increased myocardial infarction and stroke risk. If the ABI and IAD scores are negative (i.e. healthy, ABI: between 0.9 and 1.4; IAD < 10 mmHg), the additional information provided by brachial-ankle pulse wave velocity (baPWV) and pulse wave analysis (PWA) will be considered. A positive baPWV indicates increased risk for all-cause cardiovascular events, acute coronary syndrome (ACS) and/or target organ damage (TOD) risk. A positive PWA score may indicate increased afterload and increased heart failure (HF) risk. If both the baPWV and PWA scores are negative, the participant is sent home with a wearable device to collect ambulatory PWA and baPWV data, from which a composite CVD risk score is derived.
CONCLUSIONS
The overarching goal of this article was to harmonize and critique the literature supporting clinical management of CVD risk with baPWV. In the introduction we listed four aims, each of which are restated and addressed below, related to a better understanding of:
1. Physiological principles of baPWV.
baPWV characterizes the AS of large central elastic arteries and medium-sized muscular peripheral arteries of the lower-limb (see Figure 1).
2. baPWV is a unique vascular biomarker.
While baPWV and cfPWV strongly agree, due to both incorporating the aorta,25, 26 baPWV is dependent on the medium-sized peripheral muscular arteries of the lower extremities (see Figure 2). Increased lower extremity AS may principally contribute to CV by amplifying and hastening wave reflections at the level of the aorta, widening pulse pressure, increasing afterload, and reducing coronary perfusion. baPWV and cfPWV are distinct biomarkers and attempts to quantify baPWV as a surrogate for cfPWV undermine the true values of both measures.
3. Evidence supporting the use of baPWV as a vascular biomarker.
To address this aim we used the nine criteria presented by the AHA32 and endorsed by the ESC and ARTERY.33 The evidence is summarized in Table 2. There is sufficient evidence to support the criteria: i. proof of concept; ii. prospective validation; iii. incremental value; and iv. clinical utility. There is limited or no evidence to support the criteria: v. clinical outcomes; vi. cost-effectiveness; viii. methodological consensus; or ix. reference values.
4. Future research requirements.
The majority of framework criteria (i. proof of concept, ii. prospective validation, iii. incremental value, iv. clinical utility, and ix. reference values) can be supported using existing cohort datasets. Prospective investigation is required for criteria v. clinical outcomes and vi. cost-effectiveness. The viii. methodological consensus will require an expert consultation.
We finish this review by providing an example future clinical practice model (see Figure 3). This model assumes that the optimal value of baPWV will be extracted when it is not considered as a standalone biomarker, but rather is incorporated with traditional risk factors and complimentary outcomes.
SOURCES OF FUNDING
This article is based upon work from COST Action VascAgeNet CA18216 supported by COST (European Cooperation in Science and Technology, www.cost.eu). Dr Keeron Stone is supported by the Health Care Research Wales funded National Cardiovascular Research Network, Wales. Dr Michelle Meyer is supported by the National Institute for Health Grant R01AG061088. Dr Rachel E Climie is supported by the National Health and Medical Research Council of Australia (reference: 2009005) and by a National Heart Foundation Future Leader Fellowship (reference: 105636). L Stoner is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Numbers R01HL157187 and R01HL162805.
Footnotes
DISCLOSURES
There are no conflicts of interest to declare.
REFERENCES
- 1.Tomiyama H, Shiina K. State of the art review: Brachial-ankle pwv. Journal of atherosclerosis and thrombosis. 2020;27:621–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The japanese society of hypertension guidelines for the management of hypertension (jsh 2019). Hypertension research : official journal of the Japanese Society of Hypertension. 2019;42:1235–1481 [DOI] [PubMed] [Google Scholar]
- 3.Mancia G, Kreutz R, Brunström M, Burnier M, Grassi G, Januszewicz A, et al. 2023 esh guidelines for the management of arterial hypertension the task force for the management of arterial hypertension of the european society of hypertension endorsed by the european renal association (era) and the international society of hypertension (ish). Journal of Hypertension. 2023: 10.1097/HJH.0000000000003480 [DOI] [PubMed] [Google Scholar]
- 4.Climie RE, Alastruey J, Mayer CC, Schwarz A, Laucyte-Cibulskiene A, Voicehovska J, et al. Vascular ageing - moving from bench towards bedside. Eur J Prev Cardiol. 2023:DOI. 10.1093/eurjpc/zwad1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone K, Fryer S, Faulkner J, Meyer ML, Heffernan K, Kucharska-Newton A, et al. Associations of lower-limb atherosclerosis and arteriosclerosis with cardiovascular risk factors and disease in older adults: The atherosclerosis risk in communities (aric) study. Atherosclerosis. 2022;340:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruno RM, Nilsson PM, Engstrom G, Wadstrom BN, Empana JP, Boutouyrie P, et al. Early and supernormal vascular aging: Clinical characteristics and association with incident cardiovascular events. Hypertension. 2020;76:1616–1624 [DOI] [PubMed] [Google Scholar]
- 7.Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in health and disease: Jacc state-of-the-art review. Journal of the American College of Cardiology. 2019;74:1237–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, et al. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: The framingham heart study. Hypertension (Dallas, Tex. : 1979). 2004;43:1239–1245 [DOI] [PubMed] [Google Scholar]
- 9.Lacolley P, Regnault V, Segers P, Laurent S. Vascular smooth muscle cells and arterial stiffening: Relevance in development, aging, and disease. Physiological reviews. 2017;97:1555–1617 [DOI] [PubMed] [Google Scholar]
- 10.Yu S, McEniery CM. Central versus peripheral artery stiffening and cardiovascular risk. Arteriosclerosis, thrombosis, and vascular biology. 2020;40:1028–1033 [DOI] [PubMed] [Google Scholar]
- 11.Stone K, Fryer S, Meyer ML, Kucharska-Newton A, Faulkner J, Zieff G, et al. The aortic-femoral arterial stiffness gradient: An atherosclerosis risk in communities (aric) study. J Hypertens. 2021;39:1370–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomiyama H, Hashimoto H, Hirayama Y, Yambe M, Yamada J, Koji Y, et al. Synergistic acceleration of arterial stiffening in the presence of raised blood pressure and raised plasma glucose. Hypertension (Dallas, Tex. : 1979). 2006;47:180–188 [DOI] [PubMed] [Google Scholar]
- 13.Sugawara J, Tanaka H. Brachial-ankle pulse wave velocity: Myths, misconceptions, and realities. Pulse (Basel, Switzerland). 2015;3:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munakata M. Brachial-ankle pulse wave velocity: Background, method, and clinical evidence. Pulse (Basel, Switzerland). 2016;3:195–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEniery CM, Yasmin, Hall IR, Qasem A, Wilkinson IB, Cockcroft JR. Normal vascular aging: Differential effects on wave reflection and aortic pulse wave velocity: The anglo-cardiff collaborative trial (acct). Journal of the American College of Cardiology. 2005;46:1753–1760 [DOI] [PubMed] [Google Scholar]
- 16.Wohlfahrt P, Krajčoviechová A, Seidlerová J, Galovcová M, Bruthans J, Filipovský J, et al. Lower-extremity arterial stiffness vs. Aortic stiffness in the general population. Hypertension research : official journal of the Japanese Society of Hypertension. 2013;36:718–724 [DOI] [PubMed] [Google Scholar]
- 17.Ato D. Pitfalls in the ankle-brachial index and brachial-ankle pulse wave velocity. Vascular health and risk management. 2018;14:41–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugawara J, Hayashi K, Tanaka H. Arterial path length estimation on brachial-ankle pulse wave velocity: Validity of height-based formulas. Journal of hypertension. 2014;32:881–889 [DOI] [PubMed] [Google Scholar]
- 19.Yamashina A, Tomiyama H, Takeda K, Tsuda H, Arai T, Hirose K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertension research : official journal of the Japanese Society of Hypertension. 2002;25:359–364 [DOI] [PubMed] [Google Scholar]
- 20.Meyer ML, Tanaka H, Palta P, Patel MD, Camplain R, Couper D, et al. Repeatability of central and peripheral pulse wave velocity measures: The atherosclerosis risk in communities (aric) study. American journal of hypertension. 2016;29:470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: A scientific statement from the american heart association. Circulation. 2012;126:2890–2909 [DOI] [PubMed] [Google Scholar]
- 22.Clark CE, Warren FC, Boddy K, McDonagh STJ, Moore SF, Goddard J, et al. Associations between systolic interarm differences in blood pressure and cardiovascular disease outcomes and mortality. Hypertension. 2021;77:650–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townsend RR, Black HR, Chirinos JA, Feig PU, Ferdinand KC, Germain M, et al. Clinical use of pulse wave analysis: Proceedings from a symposium sponsored by north american artery. J Clin Hypertens (Greenwich). 2015;17:503–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kesten S, Qasem A, Avolio A. Viewpoint: The case for non-invasive central aortic pressure monitoring in the management of hypertension. Artery Research. 2022:128–139 [Google Scholar]
- 25.Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. Journal of hypertension. 2009;27:2022–2027 [DOI] [PubMed] [Google Scholar]
- 26.Sugawara J, Hayashi K, Yokoi T, Cortez-Cooper MY, DeVan AE, Anton MA, et al. Brachial-ankle pulse wave velocity: An index of central arterial stiffness? Journal of human hypertension. 2005;19:401–406 [DOI] [PubMed] [Google Scholar]
- 27.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern chinese urban community. Circulation. 1983;68:50–58 [DOI] [PubMed] [Google Scholar]
- 28.Kawai T, Ohishi M, Onishi M, Ito N, Takeya Y, Oguro R, et al. Prognostic impact of regional arterial stiffness in hypertensive patients. Heart and vessels. 2015;30:338–346 [DOI] [PubMed] [Google Scholar]
- 29.Chow B, Rabkin SW. The relationship between arterial stiffness and heart failure with preserved ejection fraction: A systemic meta-analysis. Heart failure reviews. 2015;20:291–303 [DOI] [PubMed] [Google Scholar]
- 30.Yu WC, Chuang SY, Lin YP, Chen CH. Brachial-ankle vs carotid-femoral pulse wave velocity as a determinant of cardiovascular structure and function. Journal of human hypertension. 2008;22:24–31 [DOI] [PubMed] [Google Scholar]
- 31.Wolff TA, Krist AH, LeFevre M, Jonas DE, Harris RP, Siu A, et al. Update on the methods of the u.S. Preventive services task force: Linking intermediate outcomes and health outcomes in prevention. American journal of preventive medicine. 2018;54:S4–S10 [DOI] [PubMed] [Google Scholar]
- 32.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the american heart association. Circulation. 2009;119:2408–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the european society of cardiology working group on peripheral circulation: Endorsed by the association for research into arterial structure and physiology (artery) society. Atherosclerosis. 2015;241:507–532 [DOI] [PubMed] [Google Scholar]
- 34.Aizawa Y, Okumura Y, Saito Y, Ikeya Y, Nakai T, Arima K. Association of renal resistance index and arterial stiffness on clinical outcomes in patients with mild-to-moderate renal dysfunction and presence or absence of heart failure with preserved ejection fraction. Heart and vessels. 2020;35:1699–1708 [DOI] [PubMed] [Google Scholar]
- 35.Lu Y, Zhu M, Bai B, Chi C, Yu S, Teliewubai J, et al. Comparison of carotid-femoral and brachial-ankle pulse-wave velocity in association with target organ damage in the community-dwelling elderly chinese: The northern shanghai study. Journal of the American Heart Association. 2017;6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamashina A, Tomiyama H, Arai T, Koji Y, Yambe M, Motobe H, et al. Nomogram of the relation of brachial-ankle pulse wave velocity with blood pressure. Hypertension research : official journal of the Japanese Society of Hypertension. 2003;26:801–806 [DOI] [PubMed] [Google Scholar]
- 37.Endes S, Caviezel S, Schaffner E, Dratva J, Schindler C, Kunzli N, et al. Associations of novel and traditional vascular biomarkers of arterial stiffness: Results of the sapaldia 3 cohort study. PLoS One. 2016;11:e0163844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: An individual participant data meta-analysis. Hypertension (Dallas, Tex. : 1979). 2017;69:1045–1052 [DOI] [PubMed] [Google Scholar]
- 39.Vlachopoulos C, Aznaouridis K, Terentes-Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension (Dallas, Tex. : 1979). 2012;60:556–562 [DOI] [PubMed] [Google Scholar]
- 40.Sang T, Lv N, Dang A, Cheng N, Zhang W. Brachial-ankle pulse wave velocity and prognosis in patients with atherosclerotic cardiovascular disease: A systematic review and meta-analysis. Hypertension research : official journal of the Japanese Society of Hypertension. 2021;44:1175–1185 [DOI] [PubMed] [Google Scholar]
- 41.Kim HM, Rhee TM, Kim HL. Integrated approach of brachial-ankle pulse wave velocity and cardiovascular risk scores for predicting the risk of cardiovascular events. PloS one. 2022;17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang IC, Jin KN, Kim HL, Kim YN, Im MS, Lim WH, et al. Additional prognostic value of brachial-ankle pulse wave velocity to coronary computed tomography angiography in patients with suspected coronary artery disease. Atherosclerosis. 2018;268:127–137 [DOI] [PubMed] [Google Scholar]
- 43.Lee HS, Kim HL, Kim H, Hwang D, Choi HM, Oh SW, et al. Incremental prognostic value of brachial-ankle pulse wave velocity to single-photon emission computed tomography in patients with suspected coronary artery disease. Journal of atherosclerosis and thrombosis. 2015;22:1040–1050 [DOI] [PubMed] [Google Scholar]
- 44.Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, et al. Endothelial dysfunction, increased arterial stiffness, and cardiovascular risk prediction in patients with coronary artery disease: Fmd-j (flow-mediated dilation japan) study a. Journal of the American Heart Association. 2018;7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugamata W, Nakamura T, Uematsu M, Kitta Y, Fujioka D, Saito Y, et al. Combined assessment of flow-mediated dilation of the brachial artery and brachial-ankle pulse wave velocity improves the prediction of future coronary events in patients with chronic coronary artery disease. Journal of cardiology. 2014;64:179–184 [DOI] [PubMed] [Google Scholar]
- 46.Kim HL, Lim WH, Seo JB, Kim SH, Zo JH, Kim MA. Improved prognostic value in predicting long-term cardiovascular events by a combination of high-sensitivity c-reactive protein and brachial-ankle pulse wave velocity. Journal of clinical medicine. 2021;10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park HW, Kim HR, Kang MG, Kim K, Koh JS, Park JR, et al. Predictive value of the combination of brachial-ankle pulse wave velocity and ankle-brachial index for cardiovascular outcomes in patients with acute myocardial infarction. Coronary artery disease. 2020;31:157–165 [DOI] [PubMed] [Google Scholar]
- 48.Lu YC, Lyu P, Zhu HY, Xu DX, Tahir S, Zhang HF, et al. Brachial-ankle pulse wave velocity compared with mean arterial pressure and pulse pressure in risk stratification in a chinese population. Journal of hypertension. 2018;36:528–536 [DOI] [PubMed] [Google Scholar]
- 49.Li X, Chang P, Wang Q, Hu H, Bai F, Li N, et al. Effects of angiotensin-converting enzyme inhibitors on arterial stiffness: A systematic review and meta-analysis of randomized controlled trials. Cardiovascular therapeutics. 2020;2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katakami N, Mita T, Yoshii H, Shiraiwa T, Yasuda T, Okada Y, et al. Effect of tofogliflozin on arterial stiffness in patients with type 2 diabetes: Prespecified sub-analysis of the prospective, randomized, open-label, parallel-group comparative utopia trial. Cardiovascular diabetology. 2021;20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petersen KS, Blanch N, Keogh JB, Clifton PM. Effect of weight loss on pulse wave velocity: Systematic review and meta-analysis. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:243–252 [DOI] [PubMed] [Google Scholar]
- 52.Manojlović M, Protić-Gava B, Maksimović N, Šćepanović T, Poček S, Roklicer R, et al. Effects of combined resistance and aerobic training on arterial stiffness in postmenopausal women: A systematic review. International journal of environmental research and public health. 2021;18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toyama K, Sugiyama S, Oka H, Iwasaki Y, Sumida H, Tanaka T, et al. Combination treatment of rosuvastatin or atorvastatin, with regular exercise improves arterial wall stiffness in patients with coronary artery disease. PLoS One. 2012;7:e41369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cortesi PA, Maloberti A, Micale M, Pagliarin F, Antonazzo IC, Mazzaglia G, et al. Costs and effects of cardiovascular risk reclassification using the ankle-brachial index (abi) in addition to the framingham risk scoring in women. Atherosclerosis. 2021;317:59–66 [DOI] [PubMed] [Google Scholar]
- 55.Park JB, Sharman JE, Li Y, Munakata M, Shirai K, Chen CH, et al. Expert consensus on the clinical use of pulse wave velocity in asia. Pulse (Basel). 2022;10:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ninomiya T, Kojima I, Doi Y, Fukuhara M, Hirakawa Y, Hata J, et al. Brachial-ankle pulse wave velocity predicts the development of cardiovascular disease in a general japanese population: The hisayama study. Journal of hypertension. 2013;31:477–483 [DOI] [PubMed] [Google Scholar]
- 57.Tomiyama H, Koji Y, Yambe M, Shiina K, Motobe K, Yamada J, et al. Brachial -- ankle pulse wave velocity is a simple and independent predictor of prognosis in patients with acute coronary syndrome. Circulation journal : official journal of the Japanese Circulation Society. 2005;69:815–822 [DOI] [PubMed] [Google Scholar]
- 58.German National Cohort C. The german national cohort: Aims, study design and organization. Eur J Epidemiol. 2014;29:371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guirguis-Blake JM, Evans CV, Redmond N, Lin JS. Screening for peripheral artery disease using the ankle-brachial index: Updated evidence report and systematic review for the us preventive services task force. JAMA. 2018;320:184–196 [DOI] [PubMed] [Google Scholar]
- 60.Qadura M, Syed MH, Anand S, Bosch J, Connolly S, Aboyans V, et al. The predictive value of interarm systolic blood pressure differences in patients with vascular disease: Sub-analysis of the compass trial. Atherosclerosis. 2023;372:41–47 [DOI] [PubMed] [Google Scholar]
- 61.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: The framingham heart study. Circulation. 2010;121:505–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dart AM, Gatzka CD, Kingwell BA, Willson K, Cameron JD, Liang Y-L, et al. Brachial blood pressure but not carotid arterial waveforms predict cardiovascular events in elderly female hypertensives. Hypertension. 2006;47:785–790 [DOI] [PubMed] [Google Scholar]
- 63.Vlachopoulos C, Aznaouridis K, O'Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis. Eur Heart J. 2010;31:1865–1871 [DOI] [PubMed] [Google Scholar]
- 64.Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, et al. Ankle brachial index combined with framingham risk score to predict cardiovascular events and mortality: A meta-analysis. JAMA. 2008;300:197–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weber T, Wassertheurer S, O'Rourke MF, Haiden A, Zweiker R, Rammer M, et al. Pulsatile hemodynamics in patients with exertional dyspnea: Potentially of value in the diagnostic evaluation of suspected heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;61:1874–1883 [DOI] [PubMed] [Google Scholar]
- 66.Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: Principal results of the conduit artery function evaluation (cafe) study. Circulation. 2006;113:1213–1225 [DOI] [PubMed] [Google Scholar]
- 67.Charlton PH, Kyriaco PA, Mant J, Marozas V, Chowienczyk P, Alastruey J. Wearable photoplethysmography for cardiovascular monitoring. Proc IEEE Inst Electr Electron Eng. 2022;110:355–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bikia V, Fong T, Climie RE, Bruno RM, Hametner B, Mayer C, et al. Leveraging the potential of machine learning for assessing vascular ageing: State-of-the-art and future research. Eur Heart J Digit Health. 2021;2:676–690 [DOI] [PMC free article] [PubMed] [Google Scholar]