Abstract
Purpose
To characterize effects of muscarinic antagonist, atropine (A), and α-adrenergic agonist, phenylephrine (P), on mydriasis and light-evoked signaling in mice anesthetized by ketamine and xylazine (K+X).
Methods
Pupillary areas of anesthetized C57BL/6 mice were measured with or without topical application of either A or A+P. Dark-adapted ERGs were recorded from 2–4 month old C57BL/6 and 7.5 month old albino hrhoG/hrhoG mice following application of A or P singly or in combination before or after induction of K+X anesthesia. Effects of GABA were tested in the hrhoG/hrhoG mice.
Results
K+X anesthesia resulted in maximal mydriasis that was not enhanced by A or A + P. Dark-adapted b-wave amplitudes (−1.3 log sc td s) after K+X anesthesia were similar with or without A or P. A+P in the presence of K+X produced a slow growth in b-wave amplitude, reaching a plateau of two-fold enhancement in 1 h. Recordings with varying flash-energies revealed that the effects of A + P were on the maximum amplitude of the a- and b-waves and not on their sensitivity. Scotopic threshold responses were augmented as well. In photoreceptor-degenerated mice (hrhoG/hrhoG), an electronegative ERG wave recorded with K + X or K + X + A, was converted to a GABA-sensitive response with two electropositive components with A+P after K + X.
Conclusion
Topical administration of A, and P together, but not separately in presence of K+X, leads to a slow, dramatic enhancement of a- and b-waves by an unknown mechanism independent of pupil dilatation.
Indexing terms: Retina, atropine, phenylephrine, Muscarinic receptors, Adrenergic receptors, Mouse
Introduction
Myrdiatics, pharmacological agents used to dilate the pupils of the eye, are a requirement for many diagnostic and therapeutic procedures in ophthalmology (for review, see 1). Selective α-adrenergic agonists such as phenylephrine (a synthetic analog of epinephrine) produce contraction of the radial muscles of the iris causing pupillary dilatation. Muscarinic receptor antagonists such as atropine or tropicamide on the other hand relax the circular muscles to dilate the pupils (for review see 2). These agents can diffuse throughout anterior structures of the eye, and into the systemic circulation, so that even at low-doses used for topical instillation, mydriatics can enter the systemic circulation to affect remote organs such as the heart and lungs 3, 4.
Anesthetics are required for various diagnostic, therapeutic and surgical interventions in humans and animals. Of the different anesthetics used, a combination of, ketamine, an NMDA receptor antagonist 5 and xylazine 6, an α2-receptor agonist are used for a variety of species. Electroretinograms (ERGs) from mice are routinely recorded using combinations of atropine and phyenylephrine for mydriasis, and, ketamine and xylazine for anesthesia (for review see, 7, 8. The mouse has become one of the most important models for studying mammalian vision largely because of the ease with which the mouse genome can be manipulated. In the retina use of genetically altered mice has led to an increased understanding of molecular mechanisms of visual function. Their use has provided insights into mechanisms of retinal dysfunction degeneration, and allowed development of new therapeutic interventions. Interpreting in vivo mouse retinal physiology in normal or pathological states requires the recording and interpretation of their electroretinograms, which require the development of a consistent protocol for their recording to make interpretation dependable and comparable across laboratories.
A previous study reported that there was no significant difference in pupillary mydriasis in anesthetized mice tested with several commonly used topical mydriatics 9. In contrast, another study mentioned the need for a combination of topical α-adrenergic agonist (2.5% phenylephrine HCl) and a muscarinic receptor antagonists (tropicamide (5 mg/mL) for maximal pupillary dilation and optimal electroretinogram amplitudes in anaesthetized mice 8. In optimizing procedures for our studies of ERG in knockout mice 10 we noted that a combination of the muscarinic antagonist, atropine, and phenylephrine was needed to obtain maximal b-wave amplitudes, but did not seem to affect the light sensitivity of the responses as would be expected for an effect primarily due to mydriasis. Therefore we undertook an investigation of the effects of these drugs on pupil dilation and ERG responses in mice.
Methods
Animals
Pupillary measurements and electroretinograms were recorded from 25 adult C57/BL6 mice between 2 and 4 months age. Additionally, electroretinograms were recorded from 7.5 month old albino mice (C57BL/6-Tyrc-Brd background) whose native rhodopsin gene has been replaced with the corresponding human DNA modified to encode an enhanced GFP fusion at the C terminus of rhodopsin (hrhoG/hrhoG mice;11). All mice were reared and housed in a room with a 12 h light (< 40 lux)-12 h dark cycle. All animal procedures conformed to US Public Health Service and Institute for Laboratory Animal Research guidelines and were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. The experimental procedures were in accord with principles of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Recording pupillary diameter
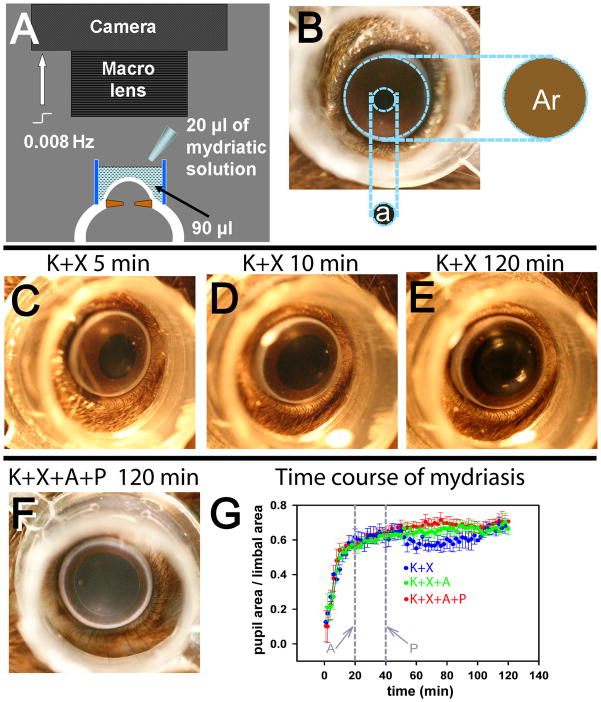
Mice, previously dark-adapted overnight, were anesthetized with a loading dose of ketamine (77 mg/kg), xylazine (16 mg/kg) (both drugs from Vedco, Inc., St Joseph, MO, USA) administered intraperitoneally and maintained with ketamine (56 mg/kg), and xylazine (12 mg/kg) administered subcutaneously. A first maintenance dose was administered after 40–45 min and additional doses were administered if required. The animal’s head was positioned on a well-illuminated elevated platform and optimally positioned to be photographed by a mounted Cannon SLR camera (Canon EOS Digital Rebel Digital camera, Cannon U.S.A, Inc., Lake Success, NY, USA) fitted with an EF-S 60mm f/2.8 macro lens focused at the plane of the pupillary aperture. A transparent cylinder made of polypropylene with ultra-thin side-walls (outer diameter: 8mm; height: 5 mm) was placed on the skin surrounding the eye, and centered on the cornea. The cylinder was filled with 90 μl 0.25% sodium carboxymethyl-cellulose solution (Advanced Vision Research, Woburn, MA, USA), to ensure that the cornea was kept well hydrated, the anterior-chamber maintained throughout the photography session and that the limbus was clearly visible (Figure 1A). Transparency of the cylinder ensured proper illumination of the pupillary plane. Topical mydriatics (20 μl each of 1% atropine Sulfate (Bausch and Lomb Inc., Tampa, FL) and 2.5% phenylephrine HCl (Akorn, Inc., Buffalo Grove, IL)) were instilled into this liquid bath. The filament bulbs that were used to provide illumination for photography provided sufficient heat to the animal and no extra heating pads were used. The average rectal temperature at the end of the recording session was ~ 37.5–38 °C. Image capture by the camera was computer controlled at a 0.008 Hz frequency using Canon Utilities RemoteCapture software (Cannon U.S.A, Inc., Version 2.7.5, Lake Success, New York, USA).
Figure 1. Measurement of pupillary mydriasis.
A, schematic diagram showing the set-up used for recording pupillary mydriasis. A digital-SLR camera fitted with a macro-lens was focused at the pupillary plane of the anesthetized mouse eye. A transparent polypropylene cylinder (outer diameter: 8mm; height: 5 mm) was centered on the cornea and filled with 90 μl 0.25% sodium carboxymethyl-cellulose solution to which 20 μl of mydriatic solution was added. The camera was computer controlled to take images at 0.008 Hz. B, photograph of a mouse eye showing that pupillary area (a) was expressed as a ratio of the area encircled by the limbus (Ar). C,D,E, representative photographs of a single mouse eye, showing levels of mydriasis 5, 10 and 120 min after ketamine+xylazine anesthesia. F, representative photograph of a mouse eye, taken 120 min after ketamine+xylazine (K+X) anesthesia and topical atropine + phenylephrine administration (topical atropine (A): 20 min and topical phenylephrine (P): 40 min after induction of anesthesia). G, time course of average mydriasis expressed as a ratio of the limbal area, produced by K+X anesthesia (blue circles, n=4), K+X anesthesia followed by A administered at 20 min from induction of anesthesia (green circles, n=3) and K+X anesthesia followed consecutive administration of A at 20 min and P at 40 min from induction of anesthesia (red circles, n=4). Vertical grey lines: time at which A and P were administered.
ERG recording
A custom-built instrument and a procedure described previously10 were used. Animals were dark-adapted over-night in a ventilated light-tight box. The next day they were prepared for recording under red illumination (LED, λ > 620 nm). The mice were initially anaesthetized with an intraperitoneal injection of ketamine (77 mg/kg) and xylazine (14 mg/kg; both drugs from Vedco, Inc., St Joseph, MO, USA). The first dose of maintenance anesthesia, ketamine (56 mg/kg) and xylazine (11.2 mg/kg) was administered after ~45 min, via a subcutaneous needle fixed to the flank, and subsequent doses were given as and when required. Slow administration of maintenance anesthesia (0.1 ml in 1 minute) did not produce any significant alteration in the ERG waveform during continuous recording.
Rectal temperature was maintained between 36 and 37 °C with an electrically heated blanket (CWE, Inc., Ardmore, PA, USA). The animal was housed in an aluminum Faraday cage to insulate the recorded signals from external static electrical fields. The animal’s head was held steady, to reduce noise originating from respiratory and other movements, using an aluminum head holder with a hole for the upper incisors to fix the upper jaw. This fixation ensured that the jaw remained open throughout the recording. Moist room air was pumped through a clear PVC pipe kept close to the open mouth. The head holder also served as the ground. All animals were allowed to recover after the ERG recording session.
Recording of ERGs was started after ~ 20 min following administration of the loading dose and sessions lasted up to 4 hours. ERGs were recorded differentially between DTL fiber electrodes 12 moistened with normal saline and placed on the two eyes. Eyes were covered with contact lenses that were pressure molded from 0.19 mm clear ACLAR film (Ted Pella, Inc. USA) for the stimulated eye and 0.7 mm opaque PVC for the non-stimulated eye (for details see, 13). Both lenses were placed over a cover of 1.2% methylcellulose in 1.2% saline. The signals were amplified (DC to 500 Hz), digitized at 2 KHz, and sent to the computer for averaging, display and storage, and subsequent analysis.
A custom-made LED (λmax, 462 nm; −5.8 to 1.9 log sc td s) based stimulator provided the light stimuli10. The maximum stimulus pulse width was < 5 ms for all normal pigmented C57/BL6 mice but was incremented to 10 ms for the hrhoG/hrhoG mice in order to get recordable signals. The light was collected in a cone internally coated with white paint and sent through a fiber optic cable (0.5 inch diameter, 36 inches long; Edmund Optics, Barrington, NJ) into the Faraday cage. A diffuser and a stainless-steel cone at the distal end of the fiber-optic cable kept very close to the stimulated eye provided a Ganzfeld stimulus. ERGs were recorded using brief full field flashes in darkness. The half time of the pulse width was taken as time zero for the ERG recording. Flash energy was varied by doubling the pulse width of the stimuli and by choosing banks of pre-attenuated LED’s.
Topical mydriatics were administered to both eyes under dim red illumination (LED, λ > 620 nm) with minimal displacement of the contact-lens and the DTL fibre electrode by apposing a drop of the mydriatic solution to the side of the contact lens. The solution was drawn into the tear film between the contact lens and cornea by capillary action and the excess fluid was removed by absorbing it with a cotton-tipped applicator. ERGs were recorded after waiting ~5 min after instillation.
Intravitreal injection
Injections were performed using a trinocular stereo articulating-flexible-boom-arm dissecting microscope (6.6 × magnification) under dim red illumination (> 620 nm) to avoid light-adapting the rods. Pharmacological agents were delivered via a 26 gauge stainless steel needle with a conical style non-coring point fixed on a 10 μl Hamilton microsyringe (Hamilton Company, Reno, NV, USA) and inserted at a 45° angle, into a small pilot hole 0.5 mm behind the limbus (created by a 30 gauge needle). To suppress inner retinal signaling, GABA (1–1.5 μl; final concentration of 35 mM based on an estimated vitreal volume of 20 μl) dissolved in balanced salt solution buffered close to pH 7.4 was delivered slowly over 1 min to avoid excessive local concentrations. This concentration of GABA was found previously to suppress inner-retinal ERG responses (Saszik, S. IOVS, 2002, 43, ARVO E-Abstract, 1817). After injection, the ERG was monitored until the waveform was stable (approximately 40–45 min) before accepting the recording.
Tissue preparation
Retinal tissues were prepared in a manner similar to that detailed in previous publications 14, 15. Briefly, light-adapted adult hrhoG/hrhoG mice were used for immunohistochemical analysis. After ERG recording the animals were euthanized by carbon-dioxide inhalation and the eyes were rapidly excised from the head. The corneas were slit open, the lens was expressed, and the eyes were immersed in 4% formaldehyde in 0.1 M cacodylate buffer (pH 7.4) for 5 minutes at 4 °C during which time the retina was gently peeled out from the sclera using gentle traction. The vitreous humor was removed, and relaxing cuts were made in the retinal margin to allow the retina to flatten. The retina was rinsed in 1x-PBS and immunolabeled free-floating. To observe the photoreceptor morphology in the hrhoG/hrhoG mice, the eyes were fixed in 4% formaldehyde in 1x-PBS for 30 minutes, transferred to a slide and coverslipped with an antifade reagent, Prolong Gold (Molecular Probes, Eugene, OR, USA) and examined under the confocal microscope.
Antibodies/Antisera
The details of all primary antibodies are presented in Table 1. Secondary antisera were raised in donkey and were specific for either rabbit or goat immunoglubulins and conjugated to fluorescent dyes: Alexa Fluor-488 and Alexa Fluor-555 (dilution: 1:200–1:500; Molecular Probes, Eugene, OR, USA).
Table 1.
Antibodies and antisera:
Immunohistochemistry
Immunohistochemistry on retinal wholemounts were performed as described previously 15, 16. Wholemounts were treated with 1–2% NaBH4 for 1–2 min, rinsed in deionized water followed by 1x-PBS and incubated in blocker solution for 1 h at room temperature to block non-specific labeling. Retinas were incubated in primary antibody for 3 days at 4 °C. They were then were rinsed in 1x-PBS for 2 h at room temperature and incubated free-floating in secondary antibody at room temperature for 1 h. Retinas were rinsed in 1x-PBS for 2 h at room temperature, flattened onto microscope slides with the ganglion cell side up, coverslipped with Prolong Gold and examined under the confocal microscope.
Imaging
Confocal images were acquired using a confocal laser scanning microscope (LSM) with a krypton–argon laser (LSM 510; Zeiss, Thornwood, NY). Images were captured using either 40× (numerical aperture = 1.30) or 63× (numerical aperture = 1.40) oil immersion objective lenses. Stacks of serial optical sections were collected at a step size of 0.3 μm. Each image shown is either a maximum projection of an image stack, or a single, representative optical section processed with Zeiss LSM personal computer software and prepared using Adobe Photoshop 6.0 software (Adobe Systems, San Jose, CA)
Image analysis
Pupillary area was measured using ImageJ (v1.37v, W.S. Rasband, NIH, Bethesda, MD) by calculating the pupillary area (in square pixels) from each photograph using the elliptical selection tool (Fig 1B). This measure was normalized to the similarly measured area enclosed by the limbus. The diameter of the limbus was measured after the termination of each experiment.
Results
Effects of anesthesia and mydriatics on the time-course of pupillary dilatation
The mydriasis following a loading dose of K+X anesthesia was rapid for the first 10 min (Fig 1C,D,G-blue plot). The growth of pupillary diameter was insignificant beyond 20 minutes of induction of K+X anesthesia (Fig 1E). The average pupillary diameter (n=4) showed a small dip which started at around 48–50 minutes and troughed at ~70 minutes, correlated with the first maintenance dose of anesthesia administered at 40–45 min following induction. Atropine was instilled into the cylindrical bath after 20 min of K+X injection at which time the mydriasis caused by K+X alone had nearly reached its maximal extent. There was no significant difference in the mydriasis produced by atropine compared to K+X (Fig 1G, green plot). The small decrease in pupillary dilatation seen with K+X anesthesia at 48–50 min was not observed in atropine instilled eyes. Instillation of atropine at 20 min following K+X anesthesia and then a combination of atropine and phenylephrine at 40 min following anesthesia produced similar amplitudes of mydriasis as did atropine instillation alone in the K+X anesthetized mice (Fig 1G, red plot). The pupillary dilation produced at the end of all three experimental paradigms was similar (Fig 1E, F, Fig 1G). We conclude that ketamine and xylaxine anesthesia and mydriatics (used either singly or in combination) produce similar extents of mydriasis.
A combination of atropine and phenylephrine produces a slow-growth in the b-wave amplitude in K+X anesthetized mice
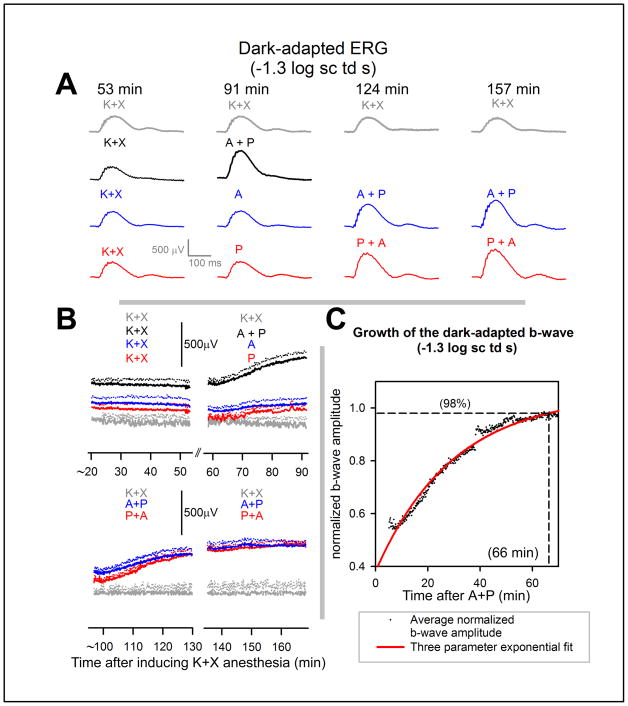
Figure 2A (grey traces) shows representative waveforms from an animal recorded following K+X anesthesia at time points of 53, 91, 124 and 157 min following induction. The waveforms recorded at 53, 91 and 124 min after anesthesia were very similar. The b-wave recorded at 132 minutes showed a decrease in amplitude. The average b-wave amplitude decreased by 11% beyond 130 min after K+X anesthesia compared to the first 33 min of recording (Fig 2B).
Figure 2. Time course of effects of mydriatics on the dark-adapted electroretinogram of anesthetized mice.
A, grey traces, dark-adapted electroretinogram of a single mouse recorded at 53, 91, 124 and 157 min after induction of anesthesia in response to flashes of −1.3 log sc td s. Black traces, dark-adapted electroretinogram in response to flashes of −1.3 log sc td s for a single mice at distinct time intervals. K+X: 53 min after induction of anesthesia; A+P: 91 min after induction of anesthesia and 38 min after instillation of topical A+P. Blue traces, dark-adapted electroretinogram in response to flashes of −1.3 log sc td s for a single mice at distinct time intervals. K+X: 53 min after induction of anesthesia; A: 91 min after induction of anesthesia and 38 min after instillation of topical A; A+P (left): 124 min after induction of anesthesia, 38 min after instillation of topical A and 76 min after instillation of topical A+P. A+P (right): 157 min after induction of anesthesia, 109 min after instillation of topical A and 71 min after instillation of topical A+P. Red traces, dark-adapted electroretinogram in response to flashes of −1.3 log sc td s for a single mice at distinct time intervals. K+X: 53 min after induction of anesthesia; P: 91 min after induction of anesthesia and 38 min after instillation of topical P; A+P (left): 124 min after induction of anesthesia, 38 min after instillation of topical P and 76 min after instillation of topical A+P. A+P (right): 157 min after induction of anesthesia, 109 min after instillation of topical P and 71 min after instillation of topical A+P.
B, time-course of change in dark-adapted b-wave amplitude (−1.3 log sc td s) after induction of K+X anesthesia. Each colored trace represents the same set of animals followed through after administering K+X anesthesia with or without mydriatics. The different data-sets are offset vertically from one another, as the initial baselines for all of them are indistinguishable.. Grey traces, top and bottom panels, average b-wave amplitude of the dark-adapted ERG for 3 animals after K+X anesthesia. Black traces (n=3); top panel, K+X: average b-wave amplitude since K+X anesthesia; top panel, A+P: average b-wave amplitude after administration of topical A+P after 54 min of K+X anesthesia. Blue traces (n=3); top panel, K+X: average b-wave amplitude since K+X anesthesia; top panel, A: average b-wave amplitude after administration of topical A after 54 min of K+X anesthesia; bottom panel, A+P (left): average b-wave amplitude after topical A+P administered after 92 min of K+X anesthesia (after 39 min after A); bottom panel, A+P (right): traces continued from A+P (left). Red traces (n=4); top panel, K+X: average b-wave amplitude since K+X anesthesia; top panel, P: average b-wave amplitude after administration of topical P after 54 min of K+X anesthesia; bottom panel, A+P (left): average b-wave amplitude after topical A+P administered after 92 min of K+X anesthesia (after 39 min after A); bottom panel, A+P (right): traces continued from A+P (left). Dotted lines represent + one S.E.M. A: atropine, P: phenylephrine.
C, Time course of growth of the b-wave amplitude after co-application of atropine and phenylephrine. The dots represent averaged b-wave amplitude (n = 7) normalized to the b-wave Vmax (data from Figure 2B, red and blue traces, bottom panel). These dots were fitted with an exponential with a time constant of 29.7 min (grey curve). Horizontal dashed line represents 98% saturation of the fitted function, which corresponded to a time of 66 min after application of A+P (vertical dashed line).
The black traces in Fig 2A show that representative ERG traces recorded after 38 min following application of topical atropine + phenylephrine (91 min after induction) on a K+X anesthetized mice were of significantly higher amplitudes compared with those recorded after 53 min following induction. At similar times after single application of either atropine (Fig 2A blue traces, top panel) or phenylephrine (Fig 2A red traces) (i.e., 38 min after mydriatic and 91 min following induction) the ERG amplitudes were similar to those produced by K+X anesthesia alone. Application of either topical phenylephrine after application of atropine (Fig 2A blue trace) or vice versa (Fig 2A red trace) produced significantly augmented ERGs as shown in the representative ERG waveforms recorded after 38 and 71 min after application of the mydriatic combination (i.e., 124 and 157 min after induction of K+X anesthesia).
The time course of the averaged b-wave amplitudes measured from the baseline to the b-wave peak for the various experimental protocols used in Fig 2A is illustrated in Fig 2B. The experiments commenced 20 min after induction of K+X anesthesia. The initial 33.3 min of recording shows the variation in b-waves observed after K+X anesthesia. Single application of either atropine or phenylephrine resulted in similar b-wave amplitudes with no observable growth in b-wave amplitudes compared to their pre-mydriatic-instilled control for a 33.3 min recording session. However, application of both atropine and phenylephrine produced a gradual but significant increase in the b-wave amplitudes compared to the pre-mydriatic-instilled control ERGs for the same 33.3 min recording session or ERGs recorded after application of a single mydriatic. To fully characterize this b-wave growth and to check whether it depended on the order of application of mydriatics a second mydriatic was topically instilled in those eyes that had received a single mydriatic and ERGs were then recorded for the next 66.6 min (Fig 2B, bottom panel). The ERG growth occurred only after a second mydriatic was instilled and was independent of the order of application. We did not observe any significant differences in time to peak or changes in the time course of the leading edge of the rod-driven b-wave response (see also, Supplementary Figure 1).
Fig 2C plots the time-course of change of the averaged normalized b-wave amplitude for the experimental paradigms which involved topical instillation of two mydriatics recorded over a 66 min period (i.e., the blue and red traces in Fig 2B, bottom panel). The data-points were fitted by a three-parameter exponential approach to saturation (red curve):
Where, b0 = normalized b-wave amplitude before drug effects, Vmax = normalized maximum amplitude of the b-wave following A+P application, and τ = time constant for the growth in b-wave amplitude. The parameters for the fit (R2=0.94) were: b0 = 0.49; Vmax = 1.06; τ = 29. 7 min corresponding to the growth in b-wave amplitude reaching 98% of its maximal value in 66 min after the application of a combination of atropine and phenylephrine (this includes the 5 min of wait-time after application of the second mydriatic). Thus we conclude that significant ERG amplitude growth occurs only after a combination of atropine and phenylephrine is used and is independent of their order of application. Full growth is seen after 1 hour from application.
Duration of anesthesia but not light exposure is an important factor in producing growth of ERG
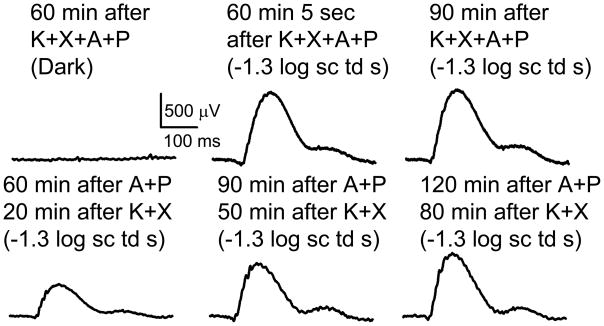
In another set of experiments A+P was instilled on both eyes prior to anesthesia, and the animal was immediately anesthetized and kept in the dark for an hour (Fig. 3, top panel). The first flash itself produced a fully grown b-wave which remained of the same value for another half an hour of recording. This result indicates that subjecting the eye to successive flashes was not a factor in the growth of the ERG. Rather, only the simultaneous presence of A+P in a K+X anesthetized mice for a period of time was required. In another experiment A+P was topically applied to both eyes in a mice 1 hour before anesthesia. In this case, ERGs recorded 20 min after induction of anesthesia produced a b-wave of ~500 μV amplitude, that grew over a period of 1 hour (Fig. 3, bottom panel). Thus K+X anesthesia is required together with A+P over a period of time to produce an augmentation of the ERG. This experiment also excludes slow diffusion of topically applied mydriatics through the ocular media to the retina as the primary reason for the long time required for maximal growth of the b-wave.
Figure 3. Duration of anesthesia is an important factor in producing growth of ERG.
Top panel: Left, ERG trace in the dark; Middle and Right, ERG trace in response to −1.3 log sc td s flash. Bottom panel: Left, Middle and Right ERG trace in ERG trace in response to −1.3 log sc td s flash.
Effects of anesthesia and mydriatics on the voltage-energy relationship of the electroretinogram
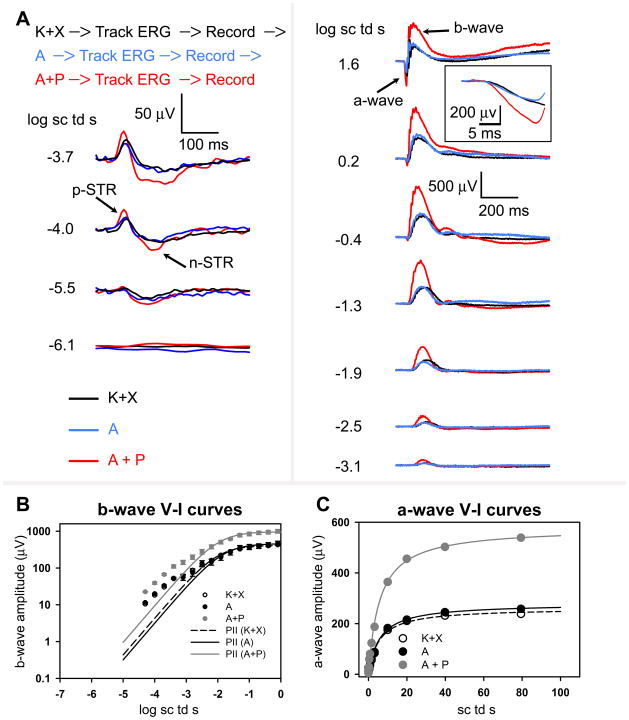
To characterize the effects of anesthesia and mydriatics on the voltage-energy relationship of the ERG it was important to remove the confound of a changing waveform and so the ERG was closely monitored for stability with repetitive test flashes of −1.3 log sc td s every 10 seconds following every mydriatic instillation. Once the ERG waveforms were stable ERGs responses to flashes of incrementing energies (−6.1 log sc td s to 1.9 log sc td s) were recorded. Using this paradigm, we recorded consecutively the ERG responses after anesthesia, topical instillation of atropine and topical instillation of a combination of atropine and phenylephrine. ERGs from a single representative animal are shown in Fig 4A. The ERG responses for all flash energies following K+X anesthesia (black traces) were very similar to those after topical atropine instillation in the anesthetized animal (blue traces). Combined instillation of atropine and phenylephrine produced an increase in ERG amplitudes (Fig 4, red trace). Dark-adapted ERG waveforms originating from the proximal inner-retina in response to very low flash energies, the negative- and positive-scotopic threshold response (nSTR and pSTR) were augmented in amplitude. These ERG waveforms are known to originate from inner-retinal mechanisms in the amacrine and retinal ganglion cells 17–20. The b-waves, known to originate primarily from the depolarization of rod bipolar cell for low flash energies (for example, −2.5 log sc td s) and both rod- and ON cone-bipolar cells for higher flash energies (for example, 1.6 log sc td s) 21 were also significantly increased in amplitude. The amplitude of the a-wave response (originating primarily from light evoked activity of rod photoreceptors in the murine retina) was also considerably increased (1.6 log sc td s; see Fig 4A inset). The averaged normalized ERG waveforms for this set of experiments are shown in Supplementary Figure 1. Although there was an augmentation of the total ERG waveform following a combination of A+P, the time-course of the ERG waveforms for the low and higher scotopic intensities were almost invariant (−4.0, −3.1 and −1.3 log sc td s). However, for higher intensities that involved active rod and cone circuits (1.6 log sc td s), resulted in a change in shape of the later part of the b-wave past its leading edge.
Figure 4. Dark-adapted ERG responses to brief full-field flashes of increasing energy after anesthesia and after consecutive topical application of atropine and atropine + phenylephrine in anesthetized mice.
A, top, order of events in this experimental paradigm. Track ERG: Dark-adapted ERGs were tracked with −1.3 log sc td s flashes (0.2 Hz) until the responses were of uniform amplitude. Record: ERG responses to brief full-field flashes of increasing energy were recorded. Dark-adapted ERGs recorded in a single experimental session from a mouse after anesthesia (K+X, black-trace) and after subsequent instillation of topical atropine (A, blue trace) followed by a combination of topical atropine + phenylephrine (A+P, red trace). Left column: ERG responses to low energy flashes (from bottom to top: −6.1 to −3.7 log sc td s) shows augmentation of the negative scotopic threshold response (nSTR) and the positive STR (pSTR) (arrows) only subsequent to topical application of atropine + phenlyephrine but not for topical atropine in K+X anesthetized mice. Right column: ERG response to flashes of intermediate to high-energy (from bottom to top: −3.1 to 1.6 log sc td s) shows augmentation of the photoreceptor-derived a-wave and the ON bipolar cell derived b-wave only subsequent to topical application of atropine + phenylephrine but not for topical atropine in the K+X anesthetized mice. Inset: The a-wave plotted on an expanded time-scale (1.6 log sc td s).
B, left: ERG b-wave amplitudes (group mean ± one S.E.M.) plotted as a function of stimulus energy after ketamine + xylazine anesthesia (K+X) (open circles) and after subsequent instillation of topical atropine (A, black circles) followed by a combination of topical atropine + phenylephrine (A+P, grey circles). The data-points, fitted with the Fulton-Rushton function represent the voltage-energy relationship of the PII after ketamine + xylazine anesthesia (K+X) (dashed curve) and PII after subsequent instillation of topical atropine (A, black curve) followed by a combination of topical atropine + phenylephrine (A+P, grey curve). Right: ERG a-wave amplitudes (group mean ± one S.E.M.) plotted as a function of stimulus energy after ketamine + xylazine anesthesia (K+X) (open circles) and after subsequent instillation of topical atropine (A, black circles) followed by a combination of topical atropine + phenylephrine (A+P, grey circles). The data-points were fitted with the Fulton-Rushton function to characterize the voltage-energy relationship for ketamine + xylazine anesthesia (K+X) (dashed curve) and after subsequent instillation of topical atropine (A, black curve) followed by a combination of topical atropine + phenylephrine (A+P, grey curve).
Figure 4B plots the b-wave amplitude measured at 110 ms as a function of scotopic-intensities for the dark adapted ERG. The Fulton-Rushton hyperbolic equation 22 was used to fit the data in order to represent the scotopic PII, similar to another study 20:
where V = ERG response amplitude, Vmax = the maximum amplitude of the response, I0 = flash energy that elicits a half-maximal response, and I= flash energy that elicits the response, V.
Responses to flash energies between −4.5 to 0 log sc td s were used to produce the fit, to reduce the effects of the STRs in the fit for low intensities and to minimize the influence of the cone-driven responses at higher intensities 21. Even though the Fulton-Rushton equation does not provide a good fit for the curves at lower intensities, pharmacological experiments involving blockade of inner-retinal circuitry with GABA has revealed that it can suitably represent the rod-bipolar cell-derived PII component of the b-wave originating from the light-evoked activity of rod-bipolar cells 20. The sensitivity and Vmax of the curve for the anesthetized animal (Fig 4B, black circles) and the anesthetized animal administered atropine (Fig 4B, open circles) were almost indistinguishable. There was a greater than two-fold increase in the Vmax (see Table 2) after topical application of a combination of atropine and phenylephrine compared to atropine alone. However, the sensitivities for all three conditions were very similar. The unchanged sensitivity of the b-wave voltage-energy plots further verifies that mydriasis was not a factor in producing an increase in Vmax following atropine + phenylephrine application, consistent with our direct observations of the lack of A + P effect on mydriasis. Our observations on the amplitudes and sensitivity of the b-wave are restricted only to the initial rising limb (rod-driven) of the b-wave until its first saturation.
Table 2.
Parameters of the Fulton-Rushton function for dark-adapted b-wave and a-waves:
| b-wave (n=4) | |||
| K+X | A | A+P | |
| Vmax (μV) | 413.58 | 460.66 | 969.79 |
| I0 (log sc td s) | −2.02 | −1.87 | −1.99 |
| R2 | 0.92 | 0.91 | 0.92 |
| a-wave (n=4) | |||
| K+X | A | A+P | |
| Vmax (μV) | 261.20 | 278.99 | 578.59 |
| I0 (log sc td s) | 0.73 | 0.76 | 0.76 |
| R2 | 0.99 | 0.99 | 0.99 |
The a-wave amplitudes plotted as a function of stimulus energy are shown in figure 4C. There was no significant difference in the Vmax and sensitivity of the Fulton-Rushton fits for the a-wave energy-response function after K+X anesthesia and topical application of atropine following anesthesia. Combined application of atropine and phenylephrine produced a greater than two-fold increase in the amplitude of the a-wave compared to topical application of atropine alone. Similar to the flash-energy response function for the b-wave, the sensitivity of the a-waves remained unaltered (see Table 2).
Combination of mydriatics can alter light-evoked inner-retinal signaling in severe photoreceptor degeneration
To characterize the effects of the combination of atropine and phenylephrine on light-evoked signaling in states of retinal degeneration we compared the ERG signals after topical application of atropine with those following topical application of phenylephrine in K+X anesthetized albino hrhoG/hrhoG mice. Previous studies have shown that mice whose native rhodopsin gene was replaced with the corresponding human DNA modified to encode an enhanced GFP fusion at the C terminus of rhodopsin (hrhoG/hrhoG mice;11) undergo severe progressive photoreceptor degeneration such that there are virtually no photoreceptor outer-segments by 3–5 months. We chose a time point of 7.5 months to record ERGs from 4 hrhoG/hrhoG mice, to ensure that the ERG records were taken when photoreceptor degeneration was very severe. Because these animals were albino, pupillary pigmentation was not expected to cause a significant alteration to the electroretinogram.
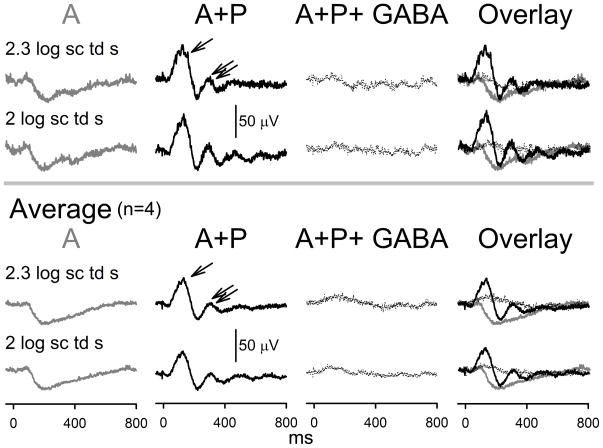
ERGs recorded from these animals (Fig 5) produced low-amplitude (40μV), slow (time to peak: 200ms) electronegative signals for very high stimulus energies, with maximum amplitudes reached at 2 to 2.3 log sc td s. Similar dark-adapted electronegative ERG signals have been observed in other photoreceptor degeneration models such as RCS rat and are known to originate from light-evoked activity in the inner-retina 23. At 1 h after instillation of a combination of atropine and phenylephrine on the cornea, these electronegative waveforms were replaced by a waveform with two prominent positive peaks. The first positive peak was of larger amplitude (~50 μV, measured from base-line to peak) than the second one (~30 μV, measured from trough to peak). The first peak also had a faster time course (time to peak: 110–120 ms) peak compared to the second peak (time to peak: 0.3 s). There were minor peaks visible at later times in the record. Intravitreal GABA injection extinguished these waveforms to be replaced by a very low-amplitude (~10 μV) positive potential (time to peak 110–120 ms), indicating that the oscillating waveform produced by A + P treatment arose from electrical activity in the inner retina. This low-amplitude positive potential most likely represents the residual PII. We conclude that a combination of atropine and phenylephrine results in alteration of inner-retinal potentials (likely originating proximal to retinal bipolar cells) in a photoreceptor-degenerated retina. Confocal microscopy showed the presence of a sparse population of photoreceptors that lacked visible outer segments (Supplementary Fig 2, A–D). Calretinin and VChat immunofluoresence was present in the neurons in the proximal inner-nuclear layer and ganglion cell layer indicating the presence of viable inner-retinal neurons and cholinergic cells (Supplementary Fig 2, E–G).
Figure 5. Dark-adapted ERG responses to full-field flashes after consecutive topical application of atropine and atropine + phenylephrine and intravitreal GABA injection in 7.5-month-old hRhoG/hRhoG anesthetized mice.
Top panel: Dark-adapted ERGs in response to response saturating high-energy flashes (2–2.3 log sc td s) recorded in a single experimental session from a single anesthetized hRhoG/hRhoG mice after the following (temporal sequence from left to right): 30 min of instillation of topical atropine (A, grey trace), 1 hour of instillation of topical atropine + phenylephrine (A+P, black trace) and 1 hour of intravitreal injection of GABA in the A+P instilled eye (traced with black dots). Bottom panel: Averaged dark-adapted ERGs in response to high-energy flashes (2–2.3 log sc td s) recorded from single experimental sessions from anesthetized hRhoG/hRhoG mice (n=4) after the following (temporal sequence from left to right): instillation of topical atropine (A, grey trace), instillation of topical atropine + phenylephrine (A+P, black trace) and intravitreal injection of GABA (traced with black dots).
Discussion
We found that a combination of ketamine and xylazine can produce mydriasis in mice in the anesthetic doses used in this study, very likely by stimulation of the α2-adrenergic receptors in the iris and CNS in a dose dependent manner as seen in rats 24. Although K+X anesthesia can produce mydriasis, variations in the extent of mydriasis can occur possibly due to the changing systemic anesthetic concentration. At least one mydriatic is necessary to maintain the constancy of pupillary dilation while recording ERGs over a prolonged duration (i.e., >40–45 min from the initial loading dose in our case).
Anticholinergic drugs and adrenergic agonists have been reported to influence the electroretinogram individually in rabbits25, 26 but, we found no evidence of significant alteration of the ERG b-wave with the singular use of atropine in K+X anesthetized mice compared with K+X anesthesia alone. The major waves of the ERG, the photoreceptor derived a-wave and the ON bipolar cell derived b-wave, had increased amplitudes but no change in sensitivity following stable ERG recording after a combination of A+P in K+X anesthetized mice. Although the exact mechanism for producing these effects remains to be determined, the most economical explanation for these changes in the ERG is that the combined effects of muscarinic antagonism and alpha adrenergic agonism in mice that were anesthetized with K+X, resulted in a decrease in resistance across the retina at least for the rod-driven ERGs where the waveforms were invariant in their time course. This decrease in resistance could potentially occur by altering the conductances of ion-channels involved in light-evoked signaling in retinal neurons. Changes in the resistance of other ocular structures can affect the ERG amplitudes (e.g., retinal pigment epithelium: 27–30; vitreous: 31, 32). However, the effects of the drugs on the light-evoked waveforms, for dark adapted ERGs involving both rod and cone circuits, and in the mice suffering advanced retinal degeneration cannot be explained simply by a decrease in resistance, suggesting that changes in responses in individual retinal cell types are involved.
The ERG augmentation requires the presence of a combination of A+P in K+X anesthetized mice for an extended period of time. The slow growth of the waveforms could not be a result of slow diffusion of A+P because A+P application 1 hour before K+X anesthesia did not produce a fully grown b-wave when ERGs were recorded 20 min after K+X anesthesia. Moreover, extended duration of K+X anesthesia is not by itself responsible for the slow augmentation of the ERG because even if the animal was anesthetized with K+X for an hour slow growth of the ERGs occurred only after topical A+P application. These results suggest that the interaction of mydriatic combination with anesthesia probably triggered a secondary slowly evolving mechanism that was responsible for producing the augmentation of the ERG waveforms. Because topical mydriatics are known to enter the systemic circulation and cause side-effects 3, 4, the route of by which a combination of mydriatics could produce its effect on the retina could either be via the systemic circulation or via diffusion across the cornea, anterior chamber and vitreous (for example, 33, 34). The slowly evolving nature of the drug-induced changes is suggestive of effects on paracrine or endocrine mechanisms within the retina or elsewhere in the body. Paracrine mechanisms in the retina that produce an augmentation of the ERG waveforms have been reported for amacrine cells that release dopamine, a global modulator of retinal activity that is known to produce a change in all the major ERG waves (for review, 35). Parkinsons disease patients, reported to have low retinal dopamine levels 36, manifest abnormally high amplitude ERG waveforms when un-treated, but the amplitudes rapidly revert to normal levels with L-DOPA treatment 37. Although it is not obvious how muscarinic antagonists and alpha-adrenergic agonists in the presence of K+X anesthesia could affect the dopaminergic amacrine cells in the murine retina, the receptor types on which they act are known to be closely related to dopaminergic amacrine cell activity in different species (for example, guinea-pig: 38; tiger salamander and chicks 39, 40; rats: 41). Ketamine is an antagonist for NMDA receptors that are known to be distributed mainly in the inner-retinal neurons (for review see 42). Although, blockade of NMDA receptors itself is not known to cause significant change in the rodent ERG 17 it may do so indirectly in combination with other mechanisms. Xylazine, an α2-adrenergic receptor agonist, may add somewhat to the effects produced by phenylephrine, a non-specific α-adrenergic receptor agonist. Cholinergic amacrine cells (43–47) and epinephrine containing amacrine cells (48–50) are endogenous sources for acetylcholine and epinephrine in the retina. Expression of both α-adrenergic and cholinergic receptors has been reported in retina of various species (for example, α-adrenergic receptors: 51–59; and muscarinic receptors: 60, 61). It seems likely that both acetylcholine and adrenergic systems may have modulatory functions in the retina and could conceivably affect neuronal systems that could influence the generators of the a- and b-waves either directly or indirectly which may have been unmasked with the use of K+X anesthesia. The possibility of systemic effects on the ERG following these pharmacological combinations cannot be ruled out, given the fact that substances such as corticosteroids or high levels of carbon dioxide can lead to an augmented ERG response 62, 63.
We found that the ERG response characteristics for topically administered atropine and phenylephrine combination in K+X anesthetized mice with severe photoreceptor degeneration, was remarkably different from those observed after topical administration of atropine alone. Recording ERGs in albino mice ensured that the changes observed were not a consequence of variable pupillary dilatation in this animal. The conversion of an electronegative potential in response to saturating light to a waveform with positive potentials upon administration of the atropine and phenylephrine combination cannot be explained on the basis of a decreased light-evoked resistance. The altered waveform seen after a combination of mydriatics originated in the inner-retina indicating that in states of photoreceptor degeneration mydriatic combination could potentially alter inner-retinal signaling. The presence of a very small PII remnant after GABA injection conforms to a previous finding that there was a relative lack of functioning bipolar cells in this degenerated retina 64. The combination of atropine and phenylephrine most likely caused an alteration of light-evoked signaling in the extremely active amacrine and ganglion cell circuits 64. Another significant implication of our finding is that a standardized mydriatic protocol is required for ERG recording in pathological conditions, perhaps with the inclusion of only one mydriatic agent.
Considerable additional work will be needed to determine the mechanism(s) and site(s) of action of the effects we have observed, and to determine the extent to which they occur in other species, including humans. The extensive use of related drugs, including anti-cholinergic drugs, adgrenergic agonists and NMDA antagonists for a range of therapeutic and diagnostic applications, and their dramatic effects on the ERG argue that these efforts will be well warranted.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant R01-EY11900 (TGW) and the Welch Foundation Q0035.
Footnotes
Financial disclosures: There are no financial disclosures
Conflict of Interest: There are no conflicts to report.
References
- 1.Moroi SE, Lichter PR. Ocular Pharmacology. In: Goodman LS, Hardman JG, Limbird LE, Gilman AG, editors. Goodman & Gilman’s the Pharmacological Basis of Therapeutics. New York: McGraw-Hill; 2001. p. xxvii.p. 2148. [Google Scholar]
- 2.Bartlett JD, Jaanus SD. Clinical Ocular Pharmacology. 5. Oxford: Butterworth-Heinemann; 2008. p. xvi.p. 793. [Google Scholar]
- 3.Isenberg S, Everett S. Cardiovascular effects of mydriatics in low-birth-weight infants. J Pediatr. 1984;105:111–112. doi: 10.1016/s0022-3476(84)80373-x. [DOI] [PubMed] [Google Scholar]
- 4.Mirmanesh SJ, Abbasi S, Bhutani VK. Alpha-adrenergic bronchoprovocation in neonates with bronchopulmonary dysplasia. J Pediatr. 1992;121:622–625. doi: 10.1016/s0022-3476(05)81159-x. [DOI] [PubMed] [Google Scholar]
- 5.Harrison NL, Simmonds MA. Quantitative studies on some antagonists of N-methyl D-aspartate in slices of rat cerebral cortex. Br J Pharmacol. 1985;84:381–391. doi: 10.1111/j.1476-5381.1985.tb12922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwartz DD, Clark TP. Affinity of detomidine, medetomidine and xylazine for alpha-2 adrenergic receptor subtypes. J Vet Pharmacol Ther. 1998;21:107–111. doi: 10.1046/j.1365-2885.1998.00113.x. [DOI] [PubMed] [Google Scholar]
- 7.Peachey NS, Ball SL. Electrophysiological analysis of visual function in mutant mice. Doc Ophthalmol. 2003;107:13–36. doi: 10.1023/a:1024448314608. [DOI] [PubMed] [Google Scholar]
- 8.Weymouth AE, Vingrys AJ. Rodent electroretinography: methods for extraction and interpretation of rod and cone responses. Prog Retin Eye Res. 2008;27:1–44. doi: 10.1016/j.preteyeres.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Lyubarsky AL, Daniele LL, Pugh EN., Jr From candelas to photoisomerizations in the mouse eye by rhodopsin bleaching in situ and the light-rearing dependence of the major components of the mouse ERG. Vision Res. 2004;44:3235–3251. doi: 10.1016/j.visres.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Mojumder DK, Qian Y, Wensel TG. Two R7 regulator of G-protein signaling proteins shape retinal bipolar cell signaling. J Neurosci. 2009;29:7753–7765. doi: 10.1523/JNEUROSCI.1794-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan F, Bradley A, Wensel TG, Wilson JH. Knock-in human rhodopsin-GFP fusions as mouse models for human disease and targets for gene therapy. Proc Natl Acad Sci U S A. 2004;101:9109–9114. doi: 10.1073/pnas.0403149101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson WW, Trick GL, Litzkow CA. Improved electrode for electroretinography. Invest Ophthalmol Vis Sci. 1979;18:988–991. [PubMed] [Google Scholar]
- 13.Sagdullaev BT, DeMarco PJ, McCall MA. Improved contact lens electrode for corneal ERG recordings in mice. Doc Ophthalmol. 2004;108:181–184. doi: 10.1007/s10633-004-5734-1. [DOI] [PubMed] [Google Scholar]
- 14.Mojumder DK. Capillary-contacting horizontal cells in the rodent retina. J Anat Soc India. 2008;57:34–36. [PMC free article] [PubMed] [Google Scholar]
- 15.Mojumder DK, Wensel TG, Frishman LJ. Subcellular compartmentalization of two calcium binding proteins, calretinin and calbindin-28 kDa, in ganglion and amacrine cells of the rat retina. Mol Vis. 2008;14:1600–1613. [PMC free article] [PubMed] [Google Scholar]
- 16.Mojumder DK, Frishman LJ, Otteson DC, Sherry DM. Voltage-gated sodium channel alpha-subunits Na(v)1.1, Na(v)1.2, and Na(v)1.6 in the distal mammalian retina. Mol Vis. 2007;13:2163–2182. [PubMed] [Google Scholar]
- 17.Bui BV, Fortune B. Ganglion cell contributions to the rat full-field electroretinogram. J Physiol. 2004;555:153–173. doi: 10.1113/jphysiol.2003.052738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mojumder DK, Sherry DM, Frishman LJ. Contribution of voltage-gated sodium channels to the b-wave of the mammalian flash electroretinogram. J Physiol. 2008;586:2551–2580. doi: 10.1113/jphysiol.2008.150755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naarendorp F, Sieving PA. The scotopic threshold response of the cat ERG is suppressed selectively by GABA and glycine. Vision Res. 1991;31:1–15. doi: 10.1016/0042-6989(91)90068-g. [DOI] [PubMed] [Google Scholar]
- 20.Saszik SM, Robson JG, Frishman LJ. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. J Physiol. 2002;543:899–916. doi: 10.1113/jphysiol.2002.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toda K, Bush RA, Humphries P, Sieving PA. The electroretinogram of the rhodopsin knockout mouse. Vis Neurosci. 1999;16:391–398. doi: 10.1017/s0952523899162187. [DOI] [PubMed] [Google Scholar]
- 22.Fulton AB, Rushton WA. Rod ERG of the mudpuppy: effect of dim red backgrounds. Vision Res. 1978;18:785–792. doi: 10.1016/0042-6989(78)90118-9. [DOI] [PubMed] [Google Scholar]
- 23.Machida S, Raz-Prag D, Fariss RN, Sieving PA, Bush RA. Photopic ERG negative response from amacrine cell signaling in RCS rat retinal degeneration. Invest Ophthalmol Vis Sci. 2008;49:442–452. doi: 10.1167/iovs.07-0291. [DOI] [PubMed] [Google Scholar]
- 24.Hsu WH, Lee P, Betts DM. Xylazine-induced mydriasis in rats and its antagonism by alpha-adrenergic blocking agents. J Vet Pharmacol Ther. 1981;4:97–101. doi: 10.1111/j.1365-2885.1981.tb00717.x. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa T, Kurasaki S, Masuda T, Ukai K, Kubo S, Kadono H. Effects of some psychotropic drugs on the b-wave of the electroretinogram in isolated rabbit retina. Jpn J Pharmacol. 1988;46:97–100. doi: 10.1254/jjp.46.97. [DOI] [PubMed] [Google Scholar]
- 26.Czepita D. Influence of alpha and beta-adrenergic stimulators and blockers on the electroretinogram and visually evoked potentials of the rabbit. Biomed Biochim Acta. 1990;49:509–513. [PubMed] [Google Scholar]
- 27.Brindley GS. The passive electrical properties of the frog’s retina, choroid and sclera for radial fields and currents. J Physiol. 1956;134:339–352. doi: 10.1113/jphysiol.1956.sp005647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brindley GS, Hamasaki DI. The properties and nature of the R membrane of the frog’s eye. J Physiol. 1963;167:599–606. doi: 10.1113/jphysiol.1963.sp007170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byzov AL. Localization of the R-membrane in the frog eye by means of an electrode marking method. Vision Res. 1968;8:697–700. doi: 10.1016/0042-6989(68)90043-6. [DOI] [PubMed] [Google Scholar]
- 30.Ogden TE, Ito H. Avian retina. II. An evaluation of retinal electrical anisotropy. J Neurophysiol. 1971;34:367–373. doi: 10.1152/jn.1971.34.3.367. [DOI] [PubMed] [Google Scholar]
- 31.Arden GB, Brown KT. Some properties of components of the cat electroretinogram revealed by local recording under oil. J Physiol. 1965;176:429–461. doi: 10.1113/jphysiol.1965.sp007560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doslak MJ, Plonsey R, Thomas CW. The effects of variations of the conducting media inhomogeneities on the electroretinogram. IEEE Trans Biomed Eng. 1980;27:88–94. doi: 10.1109/TBME.1980.326712. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser T, Werner A, Baumer W, Kietzmann M. Tissue distribution of dexamethasone in canine ocular compartments following topical application of dexamethasone-21-isonicotinate and oxytetracycline HCl. Vet Ophthalmol. 2008;11:335–339. doi: 10.1111/j.1463-5224.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 34.Mizuno K, Koide T, Shimada S, Mori J, Sawanobori K, Araie M. Route of Penetration of Topically Instilled Nipradilol into the Ipsilateral Posterior Retina. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.08-2922. [DOI] [PubMed] [Google Scholar]
- 35.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 36.Harnois C, Di Paolo T. Decreased dopamine in the retinas of patients with Parkinson’s disease. Invest Ophthalmol Vis Sci. 1990;31:2473–2475. [PubMed] [Google Scholar]
- 37.Terziivanov D, Filipova M, Janku I, Balik J, Filip V, Stika L. Changes in electroretinogram and serum potassium during L-DOPA treatment in parkinsonism. Arch Psychiatr Nervenkr. 1983;232:507–513. doi: 10.1007/BF00344065. [DOI] [PubMed] [Google Scholar]
- 38.Weber B, Schlicker E. Modulation of dopamine release in the guinea-pig retina by G(i)- but not by G(s)- or G(q)-protein-coupled receptors. Fundam Clin Pharmacol. 2001;15:393–400. doi: 10.1046/j.1472-8206.2001.00056.x. [DOI] [PubMed] [Google Scholar]
- 39.Hare WA, Owen WG. Similar effects of carbachol and dopamine on neurons in the distal retina of the tiger salamander. Vis Neurosci. 1995;12:443–455. doi: 10.1017/s0952523800008348. [DOI] [PubMed] [Google Scholar]
- 40.Schwahn HN, Kaymak H, Schaeffel F. Effects of atropine on refractive development, dopamine release, and slow retinal potentials in the chick. Vis Neurosci. 2000;17:165–176. doi: 10.1017/s0952523800171184. [DOI] [PubMed] [Google Scholar]
- 41.Iuvone PM, Rauch AL. Alpha 2-adrenergic receptors influence tyrosine hydroxylase activity in retinal dopamine neurons. Life Sci. 1983;33:2455–2463. doi: 10.1016/0024-3205(83)90640-9. [DOI] [PubMed] [Google Scholar]
- 42.Shen Y, Liu XL, Yang XL. N-methyl-D-aspartate receptors in the retina. Mol Neurobiol. 2006;34:163–179. doi: 10.1385/MN:34:3:163. [DOI] [PubMed] [Google Scholar]
- 43.O’Malley DM, Sandell JH, Masland RH. Co-release of acetylcholine and GABA by the starburst amacrine cells. J Neurosci. 1992;12:1394–1408. doi: 10.1523/JNEUROSCI.12-04-01394.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Voigt T. Cholinergic amacrine cells in the rat retina. J Comp Neurol. 1986;248:19–35. doi: 10.1002/cne.902480103. [DOI] [PubMed] [Google Scholar]
- 45.Masland RH, Cassidy C. The resting release of acetylcholine by a retinal neuron. Proc R Soc Lond B Biol Sci. 1987;232:227–238. doi: 10.1098/rspb.1987.0071. [DOI] [PubMed] [Google Scholar]
- 46.Masland RH, Mills JW, Cassidy C. The functions of acetylcholine in the rabbit retina. Proc R Soc Lond B Biol Sci. 1984;223:121–139. doi: 10.1098/rspb.1984.0086. [DOI] [PubMed] [Google Scholar]
- 47.Hayden SA, Mills JW, Masland RM. Acetylcholine synthesis by displaced amacrine cells. Science. 1980;210:435–437. doi: 10.1126/science.7433984. [DOI] [PubMed] [Google Scholar]
- 48.Hadjiconstantinou M, Mariani AP, Panula P, Joh TH, Neff NH. Immunohistochemical evidence for epinephrine-containing retinal amacrine cells. Neuroscience. 1984;13:547–551. doi: 10.1016/0306-4522(84)90247-1. [DOI] [PubMed] [Google Scholar]
- 49.Osborne NN. Noradrenaline, a transmitter candidate in the retina. J Neurochem. 1981;36:17–27. doi: 10.1111/j.1471-4159.1981.tb02372.x. [DOI] [PubMed] [Google Scholar]
- 50.Malmfors T. Evidence of adrenergic neurons with synaptic terminals in the retina of rats demonstrated with fluorescence and electron microscopy. Acta Physiol Scand. 1963;58:99–100. doi: 10.1111/j.1748-1716.1963.tb02632.x. [DOI] [PubMed] [Google Scholar]
- 51.Wikberg-Matsson A, Uhlen S, Wikberg JE. Characterization of alpha(1)-adrenoceptor subtypes in the eye. Exp Eye Res. 2000;70:51–60. doi: 10.1006/exer.1999.0753. [DOI] [PubMed] [Google Scholar]
- 52.Matsuo T, Cynader MS. Localization of alpha-2 adrenergic receptors in the human eye. Ophthalmic Res. 1992;24:213–219. doi: 10.1159/000267170. [DOI] [PubMed] [Google Scholar]
- 53.Kalapesi FB, Coroneo MT, Hill MA. Human ganglion cells express the alpha-2 adrenergic receptor: relevance to neuroprotection. Br J Ophthalmol. 2005;89:758–763. doi: 10.1136/bjo.2004.053025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishimoto I, Kiyama H, Hamano K, et al. Co-localization of adrenergic receptors and vitamin-D-dependent calcium-binding protein (calbindin) in the dopaminergic amacrine cells of the rat retina. Neurosci Res. 1989;7:257–263. doi: 10.1016/0168-0102(89)90020-5. [DOI] [PubMed] [Google Scholar]
- 55.Kiyama H, Tohyama M. Morphological demonstration of retinal neuroreceptors and mRNA: immunohistochemical demonstration of adrenergic receptor and visualization of preprotachykinin A mRNA by in situ hybridization histochemistry. Neurosci Res Suppl. 1988;8:S167–181. doi: 10.1016/0921-8696(88)90015-1. [DOI] [PubMed] [Google Scholar]
- 56.Lograno MD, Tricarico D, Masciopinto V, Scuderl AC. Specific binding of nicergoline on an alpha1-like adrenoreceptor in the rat retina. J Pharm Pharmacol. 2000;52:207–211. doi: 10.1211/0022357001773706. [DOI] [PubMed] [Google Scholar]
- 57.Wikberg-Matsson A, Wikberg JE, Uhlen S. Characterization of alpha 2-adrenoceptor subtypes in the porcine eye: identification of alpha 2A-adrenoceptors in the choroid, ciliary body and iris, and alpha 2A- and alpha 2C-adrenoceptors in the retina. Exp Eye Res. 1996;63:57–66. doi: 10.1006/exer.1996.0091. [DOI] [PubMed] [Google Scholar]
- 58.Berlie JR, Iversen LJ, Blaxall HS, Cooley ME, Chacko DM, Bylund DB. Alpha-2 adrenergic receptors in the bovine retina. Presence of only the alpha-2D subtype. Invest Ophthalmol Vis Sci. 1995;36:1885–1892. [PubMed] [Google Scholar]
- 59.Woldemussie E, Wijono M, Pow D. Localization of alpha 2 receptors in ocular tissues. Vis Neurosci. 2007;24:745–756. doi: 10.1017/S0952523807070605. [DOI] [PubMed] [Google Scholar]
- 60.Wasselius J, Johansson K, Bruun A, Zucker C, Ehinger B. Correlations between cholinergic neurons and muscarinic m2 receptors in the rat retina. Neuroreport. 1998;9:1799–1802. doi: 10.1097/00001756-199806010-00023. [DOI] [PubMed] [Google Scholar]
- 61.Yamada ES, Dmitrieva N, Keyser KT, Lindstrom JM, Hersh LB, Marshak DW. Synaptic connections of starburst amacrine cells and localization of acetylcholine receptors in primate retinas. J Comp Neurol. 2003;461:76–90. doi: 10.1002/cne.10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Heckenlively JR, Nusinowitz S. Hyperabnormal (supranormal) electroretinographic responses. In: Heckenlively JR, GBA, editors. Principles and practice of clinical electrophysiology of vision. The MIT Press; Cambridge, MA: 2006. pp. 533–540. [Google Scholar]
- 63.Tanabe J, Shirao Y, Oda N, Kawasaki K. Evaluation of retinal integrity in eyes with retained intraocular metallic foreign body by ERG and EOG. Doc Ophthalmol. 1992;79:71–78. doi: 10.1007/BF00160133. [DOI] [PubMed] [Google Scholar]
- 64.Marc RE, Jones BW, Anderson JR, et al. Neural reprogramming in retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:3364–3371. doi: 10.1167/iovs.07-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pasteels B, Rogers J, Blachier F, Pochet R. Calbindin and calretinin localization in retina from different species. Vis Neurosci. 1990;5:1–16. doi: 10.1017/s0952523800000031. [DOI] [PubMed] [Google Scholar]
- 66.Winsky L, Nakata H, Martin BM, Jacobowitz DM. Isolation, partial amino acid sequence, and immunohistochemical localization of a brain-specific calcium-binding protein. Proc Natl Acad Sci U S A. 1989;86:10139–10143. doi: 10.1073/pnas.86.24.10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.