Abstract
Neurotransmitter specification has been shown to depend on genetic programs and electrical activity, however target-dependent regulation also plays important roles in neuronal development. We have investigated the impact of muscle targets on transmitter specification in Xenopus spinal neurons using a neuron-muscle co-culture system. We find that neuron-muscle contact reduces the number of neurons expressing the non-cholinergic transmitters, GABA, glycine and glutamate, while having no effect on the incidence of choline acetyltransferase (ChAT) expression. We show that muscle activity is necessary for target-dependent reduction of non-cholinergic transmitter expression. In addition, we demonstrate that co-culture with muscle cells suppresses early spontaneous calcium spike activity in neurons and the presence of muscle cells abolishes activity-dependent transmitter specification. The results indicate that target-dependent regulation can be crucial in establishing neurotransmitter phenotypes and altering early neuronal excitability.
Keywords: transmitter specification, target-derived factors, muscle electrical activity, cholinergic phenotype, calcium activity, neuron-muscle co-culture
INTRODUCTION
Neurotransmitters provide the fundamental basis for neurons to communicate with their targets and the expression of proper transmitters is critical for normal functioning of the nervous system. Developmental specification of transmitter phenotype is a complex process that involves the interplay of several different mechanisms and pathways. Selective gene expression specifies neuronal identities (Lee & Pfaff, 2001). Misexpression of the homeobox gene MNR2 in chick embryos induces ectopic expression of choline acetyltransferase (ChAT) (Tanabe et al., 1998). LMO4 induces GABAergic differentiation in V2b-interneurons and blocks Lhx3-induced glutamatergic differentiation in V2a-interneurons in mouse spinal cord (Joshi et al., 2009). Early neuronal activity also plays important roles in transmitter specification (Spitzer, 2006). Spontaneous brief elevations of intracellular calcium (calcium spikes) are observed in embryonic Xenopus spinal neurons both in culture and in vivo (Gu et al., 1994). Different classes of neurons express different patterns of calcium spikes, and the frequency of calcium spikes specifies transmitter expression in a homeostatic manner (Borodinsky et al., 2004). Activity is required for expression of tyrosine hydroxylase during development of dopaminergic rat sensory ganglion neurons (Brosenitsch & Katz, 2002) and activation of NMDA receptors specifies the glutamatergic phenotype in cultured rat hypothalamic neurons (Liu et al., 2008).
At embryonic stages, developmentally regulated extrinsic factors determine neural fate in combination with factors intrinsic to the neurons. Target-derived factors regulate axonal and dendritic growth, synapse formation and neuronal survival (Zweifel et al., 2005). Neurotrophic factors can drive acetylcholine expression instead of noradrenaline in mammalian sympathetic ganglion neurons(Furshpan et al., 1976; Landis & Keefe, 1983; Nawa & Patterson, 1990; Francis & Landis, 1999). Neurotrophin transcript expression peaks early in embryonic development and declines at later times (Timmusk et al., 1993), consistent with developmental roles of these factors. Muscle tissue is an important source for many trophic factors including NT-3/4/5, BDNF, NGF, GDNF, CNTF, and LIF (Timmusk et al., 1993; Funakoshi et al., 1995; Fernandes et al., 1998; Fu et al., 1998; Francis & Landis, 1999). Muscle-derived factors are critical for neuronal expression of ChAT (Lams et al., 1988) and acetylcholinesterase (AChE) (Jiang et al., 2003). Eliminating these factors by axotomy or muscle removal often results in cell death or loss of ChAT and AChE expression. The loss can be rescued by application of trophic factors including NT-4/5, BDNF and GDNF (Kou et al., 1995; Fernandes et al., 1998). Muscle-derived factors are also critical for establishing mature properties of synapses (Liou & Fu, 1997; Nick & Ribera, 2000). These studies demonstrate the importance of target-derived factors in neuronal development. However, the interplay between these factors and early neuronal activity, as well as the mechanisms associated with this muscle-dependent regulation of neuronal development, are yet to be understood.
Here we report that cultured muscle cells suppress non-cholinergic transmitter expression in Xenopus spinal cord neurons that grow and make contact with them. We show that retrogradely transported signals and muscle activity are key players in target-dependent transmitter specification. Muscle cells also suppress early calcium spike activity in contacted neurons and abolish activity-dependent transmitter specification without neuronal contact.
METHODS
Cell Culture
Mixed cell cultures containing neurons and muscle cells were prepared from Xenopus laevis neural tube (stage 20) embryos (Ribera & Spitzer, 1989; Holliday & Spitzer, 1990). Myotomes and spinal neural tube were dissected from embryos using jewelers’ forceps and tungsten needles in the presence of 1 mg/ml collagenase (Boehringer Mannheim). Cells were dissociated for 45 min in calcium-magnesium free medium containing (in mM) 58.8 NaCl, 0.67 KCl, 0.4 EDTA, 4.6 Tris, pH adjusted to 7.8 with HCl. Dissociated muscle cells from a single embryo were plated on one side of a 35 mm tissue plastic culture dish, either as a dense lawn or in a striped fashion. Neurons from a single embryo were plated in stripes across the whole dish, 0 or 24 hr after plating muscle cells unless otherwise specified. Neuron-alone cultures were prepared as previously described (Gu & Spitzer, 1995; Borodinsky et al., 2004). Cultures were allowed to grow 24 hr after plating neurons before they were fixed and stained (Fig. 1A, B). Culture medium contained (in mM): 116.6 NaCl, 0.67 KCl, 1.31 MgSO4, 2 CaCl2 and 4.6 Tris, pH adjusted to 7.8 with HCl. There are ~1500 neurons in the spinal neural tube at these stages (Hartenstein, 1989) and only those cultures with more than 100 neurons were scored (Holliday and Spitzer, 1993).
Figure 1.
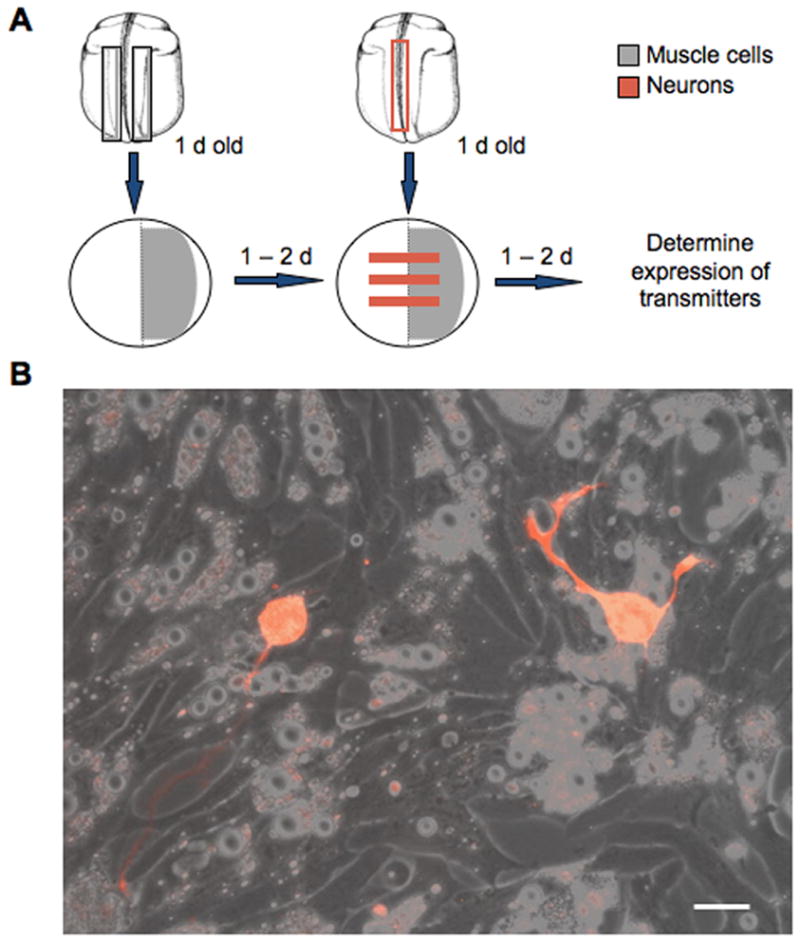
Neuron-muscle co-culture system. A, Diagram illustrating the procedure for establishing neuron-muscle co-cultures. Areas where muscle cells are plated are shown in gray and areas where neurons are located are shown in red. B, Neurons growing on muscle cells. Neurons were dissociated from rhodamine dextran-injected embryos. Scale bar, 10 μm.
Conditioned Medium
Myotomes from 6 neural tube stage embryos were dissected, dissociated, plated and allowed to grow for 48 hr in 3 ml of culture medium. In some experiments, neurons from 6 neural tube stage embryos were dissociated and plated 24 hr following muscle plating. 2.5 ml of conditioned medium were harvested from each dish and transferred to a new culture dish in which neurons from a single neural tube stage embryo were then plated. For the control condition, 3 ml of culture medium were incubated in a culture dish without muscle cells and harvested after 48 hr. Neurons were allowed to grow in each medium for 24 hr before they were fixed and stained.
Immunocytochemistry
Cultures were fixed in 4% paraformaldehyde, 0.1% or 0.025% glutaraldehyde, phosphate-buffered saline (PBS; pH 7.4) for 10–15 min at 20°C. Preparations were washed in PBS for 30 min, incubated in a blocking solution of 1% fish gelatin (Sigma) for 0.5 hr at 20°C, and stained overnight at 4°C using the following antibodies: mouse IgG anti-β-tubulin (Sigma), 1:1000; guinea pig anti-GABA (Chemicon), 1:1000; rabbit anti-glycine (Chemicon), 1:50; rabbit anti-glutamate (Sigma), 1:10000; mouse IgM anti-HNK-1 (Sigma), 1:100. Fluorescent secondary antibodies (Invitrogen) were used at 1:300 for 1–2 hr at 20°C. Immunoreactivity was examined on a Zeiss Axioskop with a 40x water objective using a Xenon arc lamp attenuated by neutral density filters, and the appropriate excitation and emission filters for Alexa 488 and Alexa 594 fluorophores. Images were acquired and analyzed with Axiovision (Zeiss). Neurons were considered immunopositive when the average pixel intensity of the neuronal cell body was 10 times higher than that of the background tissue culture plastic dish. The average pixel intensity of immunonegative neurons was less than 5 times that of background.
In situ hybridization
A 1.5 kb probe cDNA template for ChAT was a generous gift from Dr. Margaret Saha (College of William and Mary). It was cloned in a PCR4 vector and anti-sense RNA probe was transcribed using the Megascript Kit (Ambion) in the presence of digoxigenin-labled UTP (Roche). Cell cultures were fixed in 4% PFA for 1 hr, washed for 30 min in PBS and treated with 0.2 M HCl for 10 min. Hybridization was carried out at 60°C overnight with a probe concentration of 40 ng/ml. Preparations were washed in 0.2X saline sodium citrate buffer for 1 hr at 60°C and 30 min at 20°C before staining overnight at 4°C using anti-digoxygenin antibody (coupled to alkaline phosphatase) diluted to a final concentration of 1:1000 in 10% goat serum. Cultures were then rinsed 5 times in 0.5% Triton X-100 in PBS and 2 times in alkaline phosphatase buffer at 20°C and incubated in BM Purple (Roche) for 12–36 hr for signal development. Cultures were washed in PBS for 1 hr before anti-β-tubulin antibody was introduced following the protocol described above, to help visualize neurons.
Imaging
Fluorescence of the calcium indicator Fluo-4AM (Invitrogen) was used to study elevations of [Ca]i in neurons. Cultured cells were incubated for 45 min to 1 hr in culture medium containing 5 μM indicator and 0.01% Pluronic F-127 detergent (Invitrogen) and washed in culture medium prior to imaging. Images were acquired at 0.2 Hz for 30 min periods with a BioRad laser confocal system with a 20× water immersion objective. Image stacks were imported into NIH ImageJ for analysis. Calcium transients were initially identified by eye. For each neuron of interest, the cell body was traced using the line-drawing tools in the program and the average pixel intensity was determined. This measurement was repeated for every image in the stack and the values were exported to Microsoft Excel for analysis. These data were plotted against the time of image acquisition to yield fluorescence kinetics and intensity. Calcium transients were scored as spikes when the amplitude exceeded twice the baseline variation during the previous ten minutes and the rise time was complete within 5 sec. 4–5 cultures were imaged for each experiment and the incidence of spiking neurons was calculated by dividing the total number of neurons that spiked at least once during the 30 min imaging period by the total number of neurons imaged in that culture dish.
Pharmacology
Stock concentrations of drugs were 1 mM veratridine and 100 mM α-bungarotoxin (BgTx). Veratridine and BgTx were added immediately before plating neurons, with final concentrations of 1 μM and 250 nM, respectively.
Channel overexpression
The hKir2.1 construct was a generous gift of Dr. Eduardo Marban (Johns Hopkins University). The gene was subcloned into a Bluescript vector and mRNA was transcribed using the mMessage mMachine kit (Ambion). Capped mRNA (5–10 nl of a 0.01–0.1 mg/ml mRNA solution in 10% MMR, 6% Ficoll) was co-injected with a Cascade Blue or Rhodamine Red 30 kDa dextran (30 mg/ml) into both blastomeres at the 2-cell stage using a picospritzer (Picospritzer III, Parker Instr). Control injections consisted of fluorescent dextran alone (30 mg/ml in 10% MMR).
Statistical analysis
Means and standard errors were calculated using Microsoft Excel. Statistical analyses were performed using online software provided by BrightStat (www.brightstat.com). For comparison among more than two groups, the non-parametric Kruskal-Wallis ANOVA test was used and followed by the non-parametric Conover post-hoc test. For comparison between two groups, the Mann-Whitney U test was used. Data are presented as mean ± s.e.m. Results are considered significant when p<0.05.
RESULTS
Neuron–muscle co-culture reduces the number of neurons expressing non-cholinergic neurotransmitters
To investigate the influence of muscle cells on neurotransmitter expression, we designed a co-culture system in which muscle cells dissociated from a neural tube stage Xenopus embryo were plated in a lawn on one half of a culture dish and spinal neurons dissociated from another neural tube stage embryo were plated in stripes across the whole dish one day later (Fig. 1A, B). This stage is prior to the formation of nerve-muscle contacts in vivo (Kullberg et al, 1977). The procedure allowed neurons on the muscle side to form nerve-muscle contacts while neurons on the blank side grew free of contacts. Muscle contractions were observed on the neuron-muscle side one day after plating neurons, consistent with the formation of neuromuscular junctions (NMJs) during this period (Spitzer & Lamborghini, 1976). The incidence of functional NMJs in this co-culture system is high (Henderson et al., 1984) and contractions were not observed when muscle cells were plated alone.
The numbers of neurons expressing non-cholinergic neurotransmitters, GABA, glycine, and glutamate, were compared between the muscle side and the blank side within the same culture dish, by assaying GABA, glycine and glutamate -immunoreactivity (−IR) at one day in vitro. Neuronal identity was recognized by both morphology and immunostaining for neuron-specific β-tubulin (Fig. 2A). Muscle cells pick up light β-tubulin staining both in vitro and in vivo (S.B. Sann, unpublished data). However this does not affect recognition and counting of neurons because their morphology is distinct from that of muscle cells. Neurons expressing the three non-cholinergic transmitters on the muscle side constitute a significantly smaller percentage of the total neurons scored when compared to the blank side (Fig. 2B). Similar results were obtained when neurons were plated immediately after plating muscle cells. About 10% of neurons grew axons long enough to make contacts with each other, both on the blank and the muscle side. However, scoring transmitter expression in neurons with and without contact with other neurons on either side did not reveal differences between these two groups. Because the culture medium does not contain any amino acids, if neurons release these compounds they may be unable to reload their transmitter pool. Accordingly we examined the effect of muscle cells on the expression of the transmitter-related proteins, glutamic acid decarboxylase (GAD) and vesicular glutamate transporter 1 (VGLuT1). The results demonstrate decreased incidence of staining for GAD and VGluT1 on the muscle side of culture dishes, consistent with staining for GABA and glutamate (Fig. 2C, D).
Figure 2.
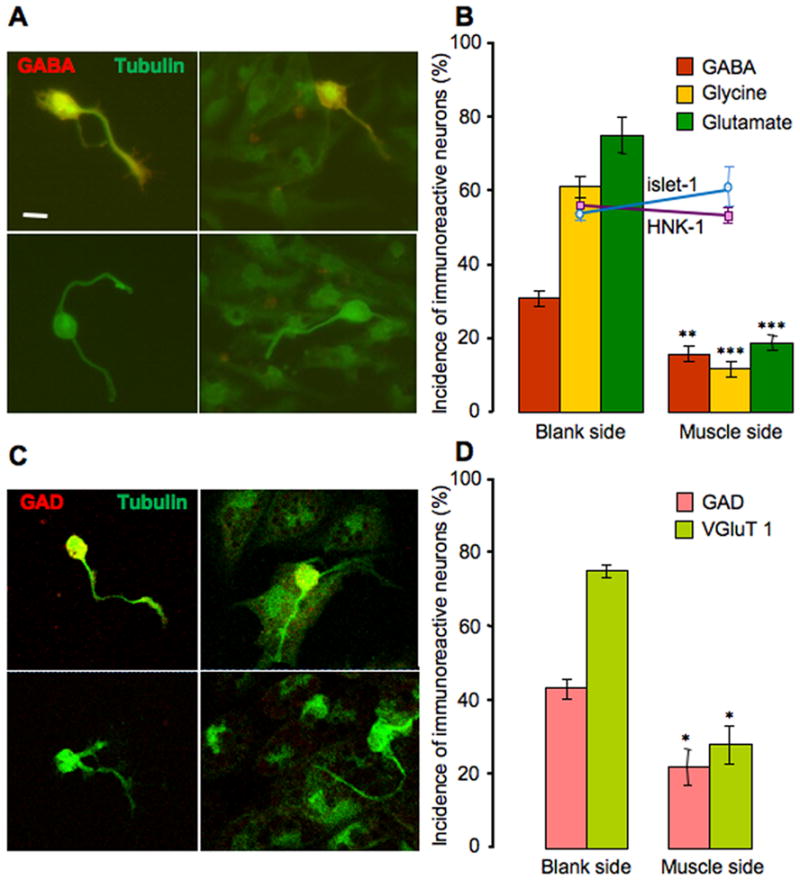
Muscle cells suppress non-cholinergic neurotransmitter expression. A, Immunostaining of neurons for GABA (red) and tubulin (green) on the blank side (left panels) and the muscle side (right panels). Top two panels show positive staining for GABA and the bottom two show negative staining. B, Non-cholinergic transmitter expression is reduced on the muscle side (** p<0.01, ***p<0.001, in comparison to the blank side; n>8 cultures per transmitter, >100 neurons per culture). HNK-1 and islet-1 expression does not differ between the two sides of the culture; the Y-axis applies to the incidence of HNK-1, islet-1 and transmitter expression. Error bars indicate SEM. Scale bar, 10 μm. The Mann-Whitney U test was used to determine statistical significance. C, Immunostaining of neurons for GAD (red) and tubulin (green) on the blank side (left panels) and the muscle side (right panels). Top two panels show positive staining for GAD and the bottom two show negative staining. D, GAD and VGluT1 expression is reduced on the muscle side (* p<0.05, in comparison to the blank side; n>5 cultures per transmitter marker, >100 neurons per culture). Error bars indicate SEM. Scale as for B. The Mann-Whitney U test was used to determine statistical significance.
Thus the presence of muscle cells reduces the fraction of neurons expressing non-cholinergic transmitters only on the muscle side of the culture dish. The incidence of the three non-cholinergic transmitters exceeds 100%, suggesting coexpression. This result is in agreement with previous observations of transmitter coexpression in Xenopus spinal neurons at early stages of development, both in vitro and in vivo (Borodinsky et al., 2004; Root et al., 2008).
The reduction in number of neurons expressing non-cholinergic transmitters could result from a decrease in differentiation or a lower survival rate of neurons that are genetically programmed to express non-cholinergic phenotypes. However the total number of neurons on each side was notsignificantly different (109±10 neurons on the blank side and 103±15 neurons on the muscle side, n=3 cultures). We scored the number of neurons expressing HNK-1, an intrinsic molecular marker for a subtype of non-cholinergic embryonic neurons (Somasekhar & Nordlander, 1997). There was no difference in the percentage of HNK-1-IR neurons between the blank side (55±1%) and the muscle side (53±1%). Staining of islet-1, a molecular marker for non-cholinergic sensory neurons and motor neurons (Pfaff et al., 1996), also revealed no statistically significant difference in its expression in neurons on either side (52±1% on the blank side and 59±3% on the muscle side) (Fig. 2B). These results indicate that cell death is unlikely to play a role in reducing the percentage of non-cholinergic neurons. Data obtained at 6 hr after plating demonstrated that culturing of neurons in the absence of muscle cells does not increase the proportion of the GABA, glycine, and glutamate immunoreactive cells over the 24 h period. Thus muscle cells do not exert their effects by preventing increases in proportions of aminoacidergic neurons.
To examine the impact of neuron–muscle co-culture on cholinergic neurons, we used in situ hybridization to identify neurons expressing choline acetyltransferase (ChAT) (Fig. 3). Neuronal identity was recognized by morphology and fluorescent immunostaining for neuron-specific β-tubulin (not shown). On both sides of the cultures, roughly 30% of the neurons express ChAT (28+2% on the blank side and 31+1% on the muscle side; not statistically different), suggesting that the number of cholinergic neurons is not affected by the presence of muscle cells. In situ signal for ChAT was observed in muscle cells as well, consistent with their release of ACh (Fu et al., 1998).
Figure 3.
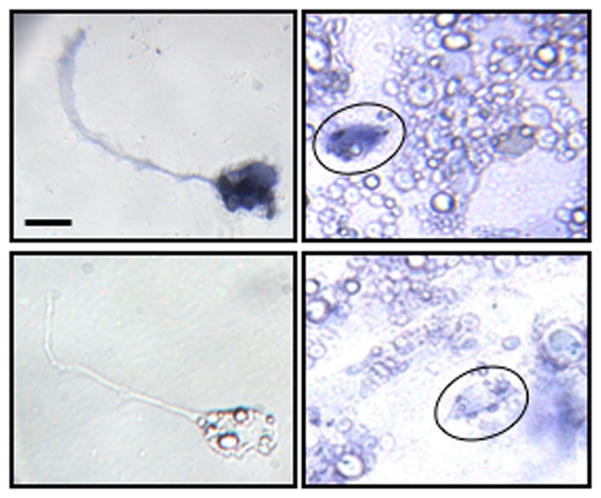
Neuronal ChAT expression is independent of muscle regulation. In situ hybridization for ChAT on the blank side (left panels) and the muscle side (right panels; neurons circled). Top two panels show positive staining and the bottom two show negative staining. Scale bar, 10μm.
Mechanism by which muscle cells induce suppression of non-cholinergic neurotransmitter expression
Muscle cells could regulate neurotransmitter expression by several different means. On the muscle side, direct contact between neuronal somata and muscle cells allows physical interaction between membrane surface molecules, and axon–muscle contact permits retrograde transport of trophic factors that are normally taken up at the distal ends of axons (Zweifel et. al., 2005). Diffusible factors released by muscle are present in the culture medium throughout the entire dish, with higher concentrations on the muscle side compared to the blank side. Extracellular matrix molecules are also released by the muscle and act locally on neurons growing in contact with muscle cells. To determine which of these interactions drives the reduction in expression of non-cholinergic transmitters, we modified the co-culture system to produce a lattice culture in which stripes of neurons are plated on stripes of muscle cells, instead of a muscle lawn (Fig. 4A). These cultures generate three classes of neurons: 1) those with no contact with muscle; 2) those with both axon and soma contacts with muscle (Soma-M group); and 3) those with only axon contact with muscle (Axon-M group).
Figure 4.
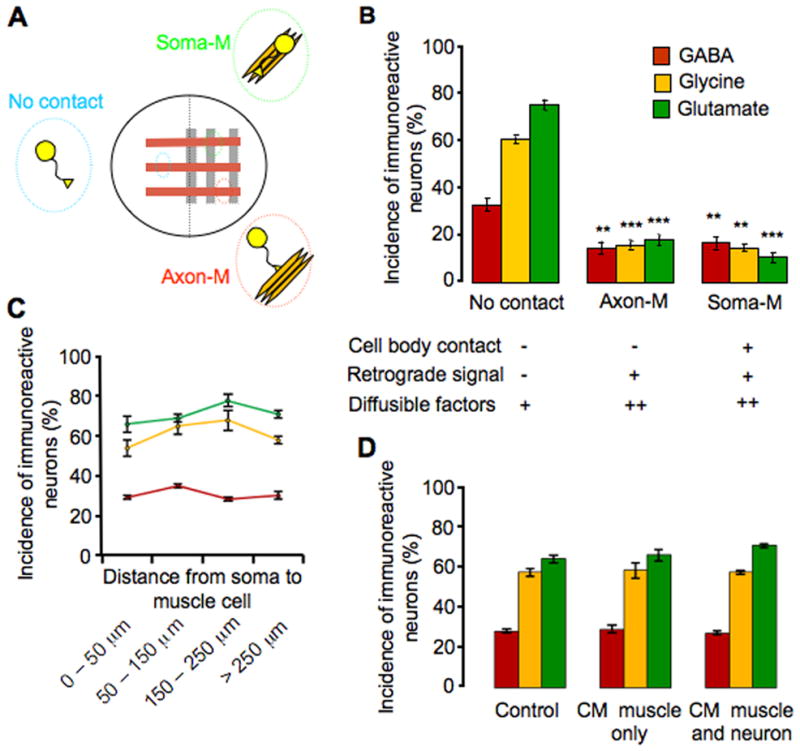
Cell contacts and not diffusible factors regulate muscle-dependent suppression of noncholinergic neurotransmitter expression. A, Schematic view ofthe lattice neuron-muscle co-culture and groups of neurons with different interactions with muscles. B, Neurons with or without cell body interaction with muscle show similar levels of reduction in incidence of non-cholinergic transmitter expression, when compared with the no-contact group. There is no significant difference in non-cholinergic transmitter expression between axon-M and soma-M groups (** p<0.01, *** p<0.001; n>5 cultures per condition per transmitter, >100 neurons per culture). C, The incidence of expression of the three non-cholinergic transmitters in the no-contact group from (B) (GABA in red, glycine in yellow and glutamate in green) does not change as a function of distance from neuronal soma to muscle cells (>100 neurons scored at each distance for each transmitter). D, The incidence of expression of the three non-cholinergic transmitters does not differ between the control condition and conditioned medium condition (muscle alone or muscle co-cultured with neurons) (n>5 cultures per condition per transmitter, >100 neurons per culture). Error bars indicate SEM. The Kruskal-Wallis test and Conover post-hoc test were used to determine statistical significance.
The first class, growing on the blank side, may receive a low concentration of diffusible factors but their axons and somata remain free of muscle contact; thus other forms of interaction have little if any impact on them. The second class of neurons can receive retrograde signals and a higher concentration of diffusible factors. The neurons in this group can also interact with extracellular matrix and surface-bound molecules. The third class, whose somata normally lie within 20–100 μm of the muscle, can receive retrograde signals and a high concentration of diffusible factors, but their somata have little or no interaction with extracellular matrix or membrane surface molecules.
We scored the incidence of GABA-IR, glycine-IR and glutamate-IR cells for all three classes of neurons and found that both axon-muscle and soma-axon-muscle groups contain significantly fewer non-cholinergic neurons than the no-contact group but are not significantly different from each other (Fig. 4B). This result implies that physical interaction between the neuronal soma and muscle cell body causes reduction of expression of non-cholinergic transmitters. The data also suggest that retrograde signals propagated through axons play the key role in modulating transmitter expression. However our results do not rule out the possibility that extracellular matrix molecules act directly on axons and activate pathways that lead to changes in transmitter expression in neuronal cell bodies (Hall & Sanes, 1993; Mantych & Ferriera, 2001).
To investigate whether a high concentration of diffusible factors contributes to the suppression of non-cholinergic transmitter expression, we divided the no-contact group according to the distance from the neuronal soma to the nearest muscle cell. The concentration of diffusible factors that reaches neurons is expected to decline with distance from the muscle. However, no difference in the number of GABA–IR, glycine–IR and glutamate–IR neurons was observed in neurons located 0–50 μm, 50–150 μm, 150–250 μm and over 250 μm from muscle cells (Fig. 4C). To further address the issue of diffusible factors, we cultured neurons in medium conditioned by muscle tissue for 48 hr, to parallel the time of neuronal exposure in neuron-muscle co-culture. Muscle cells dissected from 6 embryos cover 70–80% of the dish. Trophic factors can be stable and remain active in culture medium for periods up to one week (Barnett et al., 1990). Thus it was expected that the conditioned medium would provide relatively abundant diffusible factors released by the muscle. However, no changes were observed in the number of GABA-IR, glycine-IR and glutamate-IR neurons, when the conditioned medium condition was compared with the control condition in which neurons were cultured in medium without exposure to muscle cells (Fig. 4D). Since the release of muscle-derived diffusible factors can also be dependent on muscle activity, in another set of experiments both neurons and muscle cells from 6 embryos were co-cultured for 48 hr before harvesting conditioned medium. Still no change in non-cholinergic transmitter expression was observed (Fig. 4D). These findings suggest that diffusible factors play little role in suppressing non-cholinergic transmitter expression, although the action of such factors over distances of a few microns or at very high concentrations is not excluded. Taken together, axon-muscle contact alone appears to be sufficient to cause significant suppression of non-cholinergic transmitters on the muscle side of these cultures, and muscle-derived, retrogradely-transported signals are likely to be the prinicpal players in this suppression.
Blocking muscle electrical activity rescues non-cholinergic neurotransmitter expression
To determine whether electrical activity in muscle cells is necessary to suppress non-cholinergic neurotransmitter expression, we used two separate approaches to block muscle activity chronically. We first incubated cultures in 250 nM α-bungarotoxin (BgTx) to block postsynaptic activity mediated by acetylcholine receptors (Berg & Hall, 1975). In parallel experiments we heterologously expressed human Kir channels in embryos from which the muscle cells were dissociated, to hyperpolarize their membrane potential (Borodinsky et al., 2004) (Fig. 5A, B). Neither of these two methods led to a change in non-cholinergic transmitter expression in the neurons on the blank side (Fig. 5C), making it unlikely that the changes in transmitter expression in neurons on the muscle side are due to blockade of neuronal nACh receptors alone. On the muscle side, the percentage of GABA–IR positive neurons was fully restored to the level on the blank side when the muscle was silenced by either BgTx or Kir expression. Significant although partial rescue of glycine-IR and glutamate-IR neurons was also achieved by both of these two methods. The number of cholinergic neurons is not sufficient to account for the increase in non-cholinergic transmitter expression on the basis of direct innervation. Accordingly the paracrine release of ACh or another factor from cholinergic neurons and/or muscle cells (Fu et al., 1998) may exert this effect. Moreover the effective molecules are not exclusively taken up at synapses, although they appear to be taken up by nerve terminals and signals are retrogradely transported. These results demonstrate that muscle activity plays an essential role in suppressing expression of non-cholinergic transmitters. However, the different extents of rescue for GABA, glycine and glutamate indicate that the expression of different transmitters is regulated differently by the muscle target.
Figure 5.
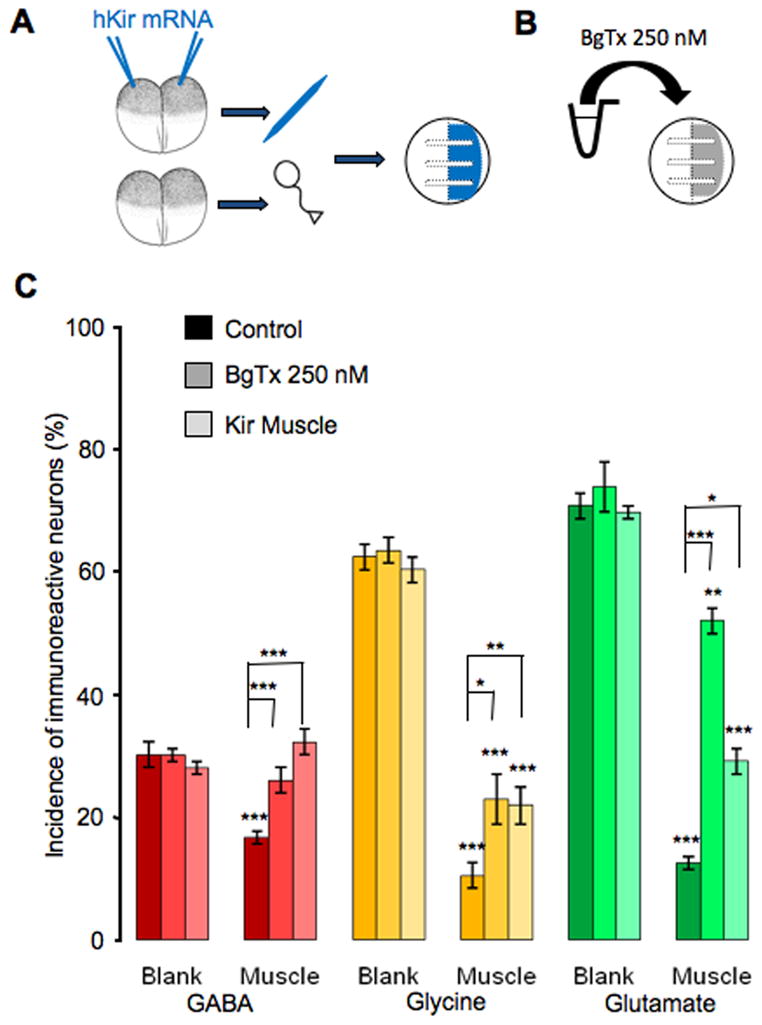
Rescue of non-cholinergic neurotransmitter expression by blockade of muscle activity. A, Diagram illustrating hKir mRNA injection and neuron-muscle co-culture with silenced muscle and wild type neurons. B, Diagram showing method of pharmacological blockade of muscle activity by α-bungarotoxin. C, Complete rescue of GABA expression and partial rescue of glycine and glutamate expression are achieved by either BgTx incubation or Kir expression. Lower asterisks indicate a significant difference between the bar beneath and its counterpart on the blank side. Upper asterisks indicate a significant difference between the bars (n>5 cultures per condition per transmitter, >100 neurons per culture; * p<0.05, ** p<0.01, *** p<0.001). Error bars indicate SEM. The Kruskal-Wallis test and Conover post-hoc test were used to determine statistical significance.
Neuron–muscle co-culture suppresses embryonic neuronal calcium spike activity
We next investigated the interplay between muscle-derived factors and spontaneous activity in transmitter specification. Spontaneous neuronal calcium spike activity is observed at early stages of development and plays an important role in transmitter specification both in vitro and in vivo (Gu & Spitzer, 1995; Borodinsky et al., 2004, Dulcis & Spitzer, 2008). Calcium spikes are produced by calcium-dependent action potentials generated by voltage-dependent calcium channels and involve calcium-induced calcium release from calcium stores. They are usually first detected at the neural tube stage (stage 20) (Spitzer & Lamborghini, 1976; Holiday & Spitzer, 1990; Gu & Spitzer, 1993; Borodinsky et al., 2004), prior tosynapse formation (Hayes & Roberts, 1973), and are generated for 10–12 hr until the early larval stage (~stage 32; Root et al., 2008). Calcium spike activity disappears as the action potential matures to a brief sodium-dependent impulse. Spontaneous activity is also observed during a limited time window in culture. It peaks from 5 to 10 hr after plating and gradually decreases afterwards (Gu et al., 1994; Gu & Spitzer, 1995; Chang & Spitzer, 2009).
To determine whether the presence of muscle cells affects this neuronal activity, we imaged intracellular calcium in neurons dissociated from neural tube stage Xenopus embryos and grown on the muscle side and the blank side of co-cultures. Neurons growing in neuronal cultures were also imaged, as a further control (Fig. 6A). Images were acquired for a 30 min period, starting 4 to 8 hr after plating. The incidence of spiking neurons was calculated by dividing the number of neurons exhibiting spike activity during an imaging period by the total number of neurons imaged. We observed a significantly lower percentage of spiking neurons on the muscle side of the culture dishes, when compared with those on the blank side or in neuronal cultures (5% vs 32% and 28%; Fig. 6B).
Figure 6.
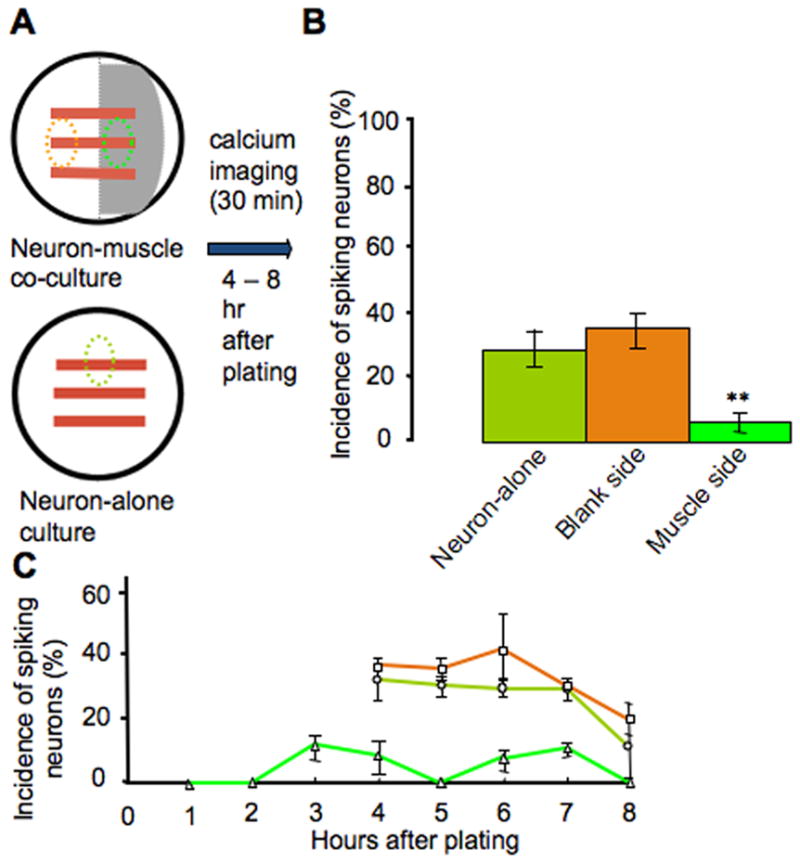
Muscle cells suppress early neuronal spontaneous calcium spike activity. A, Diagram showing areas of interest where neurons were imaged. B, Neurons grown on muscle cells exhibit a significantly lower incidence of spiking at 4–8 hr in vitro (n=10 cultures, >50 neurons per condition; ** p<0.01). C, Neurons grown on muscle cells exhibit reduced incidence of spiking during each hr after plating (n>10 cultures, >50 neurons per hour). A higher percentage of neurons exhibit calcium spikes from 4 to 8 hr on the blank side of co-cultures and in neuron-alone cultures. Imaging at earlier times was not carried out with the two control groups due to lack of morphological distinction between neurons prior to axon outgrowth and the presence of other cell types. Error bars indicate SEM. The Kruskal-Wallis test and Conover post-hoc test were used to determine statistical significance.
On the muscle side, neurons extend axons more rapidly and grow longer than on the blank side during the first 8 hr after plating. To determine whether those neurons mature more rapidly and shift the period during which they generate spikes to earlier times in culture, we imaged them at 1–4 hr after plating. However, only 6% of neurons grown on the muscle side generated spikes during this period (Fig. 6C). This result suggests that shifting of the spiking window toward earlier times is unlikely.
Muscle cells abolish veratridine-induced neuronal activity-dependent neurotransmitter specification
Manipulations of neuronal calcium activity alter neurotransmitter expression both in cultures prepared from neural plate stage embryos and in vivo (Gu and Spitzer, 1995; Borodinsky et al., 2004). We repeated the in vitro experiment with neuronal cultures dissociated from neural tube stage embryos. Enhancing spiking activity by chronic incubation with 1 μM veratridine, a drug blocking sodium channel inactivation, led to an increase in the number of neurons expressing the inhibitory transmitters, GABA and glycine, and a decrease in the number of neurons expressing the excitatory transmitter, glutamate (Fig. 7A, B).
Figure 7.
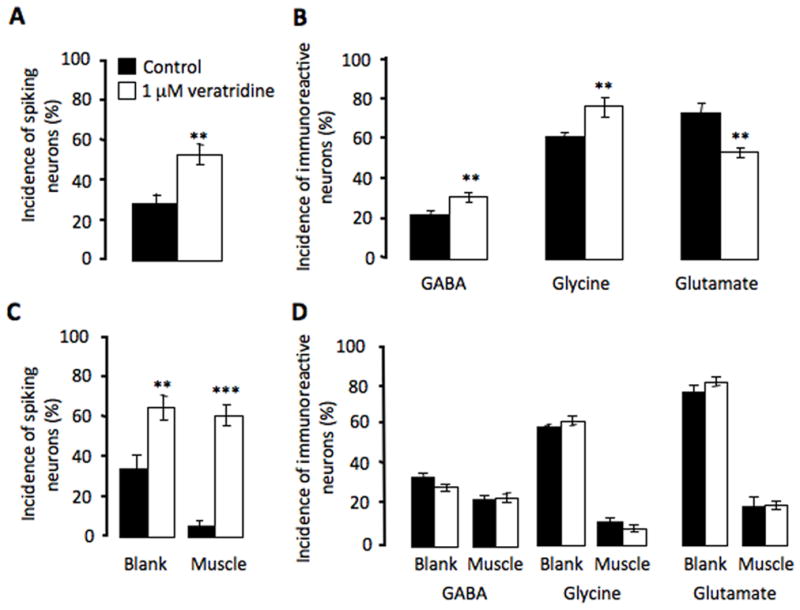
Muscle cells abolish activity-dependent neurotransmitter specification induced by veratridine. A, Veratridine increases the incidence of spiking neurons in neuron-alone cultures. B, In neuron-alone cultures, veratridine increases the incidence of neurons expressing the inhibitory transmitters, GABA and glycine, and reduces incidence of neurons expressing the excitatory transmitter, glutamate. C, Veratridine increases the incidence of spiking neurons in neuron-muscle co-cultures, both on the blank side and the muscle side. D, In neuron-muscle co-cultures, veratridine fails to induce changes in either inhibitory or excitatory transmitter expression (n>5 cultures per condition per transmitter, >100 neurons per culture; ** p<0.01, *** p<0.001). Error bars indicate SEM. The Mann-Whitney U test (A–C) and the Kruskal-Wallis test (D) were used to determine statistical significance.
To investigate whether reduced spontaneous neuronal activity is responsible for muscle-dependent transmitter specification, we performed a rescue experiment by enhancing neuronal activity. Chronic treatment of co-cultures with 1 μM veratridine increased the incidence of spiking neurons on the blank side by 100% and restored the incidence of spiking neurons on the muscle side (Fig. 7C). Veratridine does not elicit calcium transients in muscle cells in nerve-muscle co-cultures (Borodinsky and Spitzer, 2007). However, even with complete rescue of neuronal activity, there was no difference in the percentage of neurons expressing GABA, glycine or glutamate, on either side of the dish (Fig. 7D). This result suggests that suppression of neuronal activity by muscle contact does not account for muscle-dependent suppression of non-cholinergic transmitter expression. In addition, the surprisingly unaltered transmitter expression on the blank side of the dish shows that diffusible muscle-derived factors are likely to be sufficient to eliminate veratridine-induced, activity-dependent transmitter specification. Since presynaptic activity may be involved in stimulating the release of muscle-derived factors, the high level of neuronal activity induced by veratridine may cause muscle to dominate the regulation of transmitter expression.
Blocking trk receptors does not rescue non-cholinergic neurotransmitter expression
Muscle-derived members of the neurotrophin family are important for motor neuron development, including the induction and maintenance of ChAT expression (Chiu et al., 1994; Zurn et al., 1996) and establishment of mature electrophysiological properties of the NMJ (Funakoshi et al., 1995; Liou & Fu, 1997; Xie et al., 1997; Nick & Ribera, 2000). To determine whether neurotrophins play a role in suppressing non-cholinergic transmitters, cultures were incubated with 10 nM K252a, a trk receptor blocker. K252a was applied 5 hr after plating, to minimize prevention of neuronal differentiation. At this concentration no rescue of GABA, glycine or glutamate expression was achieved (Fig. 8). At higher concentrations or when applied immediately before plating neurons, K252a reduced the number of morphologically distinguishable neurons while leaving the percentage of tubulin-positive cells unaltered; neurons displayed short neurites and abnormal hairy morphology both on cell bodies and along axons. Although morphological differentiation was clearly abnormal, concentrations of K252a up to 10 μM were equally ineffective in rescuing expression of the three non-cholinergic transmitters and no concentration-dependent effect of K252a on transmitter expression was observed (data not shown).
Figure 8.
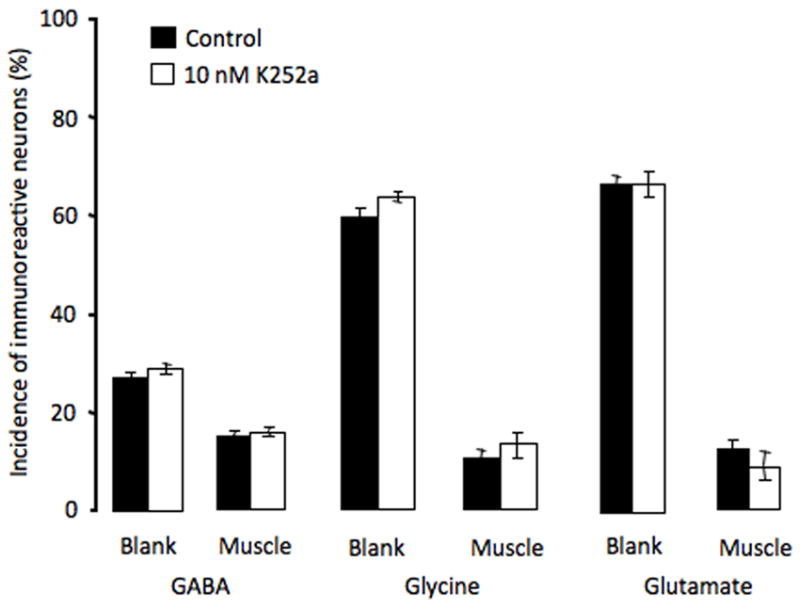
Blockade of trk receptors does not rescue expression of non-cholinergic transmitters. 10 nM K252a were applied from 5–24 hr. No significant difference in expression of GABA, glycine or glutamate was observed between the blank and muscle sides of the dish (n>5 cultures per condition per transmitter, >100 neurons per culture). Higher concentrations (up to 10 μM) were also ineffective. Error bars indicate SEM. The Kruskal-Wallis test was used to determine statistical significance.
DISCUSSION
The presence of muscle cells has a striking impact on differentiation of neurons in co-cultures. Contact with muscle cells significantly suppresses non-cholinergic transmitter expression. Investigation of the mechanisms underlying target-dependent regulation suggests an important role for muscle activity-dependent retrograde signals. Contact with muscle cells also suppresses early neuronal calcium spike activity. Even when neurons are free of contacts, muscle cells abolish activity-dependent transmitter specification in co-cultured neurons, most likely via diffusible factors. These results demonstrate multi-level, muscle-dependent regulation of transmitter specification.
Muscle cell-dependent neurotransmitter specification
Previous studies demonstrated that muscle cells are critical to support neuronal survival and maintain ChAT expression in adult motor neurons (Lams et al., 1988; Kou et al., 1995). However, the initial expression of ChAT in motor neurons is thought to be target-independent, since rat thoracic spinal motor neurons express ChAT before they can be retrogradely labeled through their targets (Phelps et al., 1991). In Xenopus laevis, however, the onset of ChAT expression in spinal motor neurons is first detected at the tailbud stage (stage 30) by immunocytochemistry (Root et al., 2008), almost 12 hr after axons start to contact developing muscle cells at the neural tube stage (stage 21) (Kullberg et al., 1977). Despite apparent differences between rat and Xenopus in the order of onset of ChAT expression and establishment of muscle contact, we found that the number of neurons expressing ChAT transcripts is not altered by contact with muscle cells. These results suggest that ChAT expression in embryonic Xenopus spinal neurons is indeed independent of muscle-derived factors.
Muscle-derived signals appear to have a profound effect on non-cholinergic transmitter expression. In vivo, Xenopus spinal neurons start to express GABA and glutamate at neural plate stages, well before synapse formation (Root et al., 2008). Accordingly, when Xenopus neurons are dissociated and subsequently cultured from stage 20 embryos, it is expected that GABA and glutamate are present while glycine is absent. After 1 d in culture, expression of all three non-cholinergic transmitters is much higher in neurons grown free of muscle contact, when compared with those grown on muscle cells. Thus nerve-muscle contact seems to be effective both in reducing preexisting non-cholinergic transmitter (GABA and glutamate) expression and in preventing neurons from acquiring a new non-cholinergic transmitter (glycine). The expression of the intrinsic molecular markers HNK-1 and Islet-1 does not differ between the two groups of neurons, suggesting that muscle-dependent transmitter specification is independent of cell death.
In vivo, GABA and glutamate expression peak at the neural tube stage, declining thereafter, and are expressed in neurons that are positive for lim-3, a motor neuron transcription factor (Root et al., 2008). Since the reduction of GABA and glutamate expression occurs at the time when motor neurons start to contact muscle cells, muscle-derived retrograde signals may contribute to suppression of ectopic expression of GABA and glutamate in motor neurons in vivo. In addition, early tailbud stage (stage 25) neurons that are positive for lim-3 never exhibit glycine-IR. Consequently, neuron-muscle contact may also help to prevent motor neurons from acquiring the wrong transmitter in vivo.
Role of muscle cell activity and candidate molecules
Many target-derived factors are released in an activity-dependent fashion (Xie et al., 1997). Postsynaptic excitability is critical in forming functional synapses and in synapse maturation; synaptic activity often modulates synaptic efficiency, both at early developmental stages and in adults. Electrical stimulation of the rat sciatic nerve leads to increased levels of NT4 mRNA in both the soleus and gastrocnemius muscles and blocking postsynaptic activity blocks factor release and thus abolishes factor-dependent regulation of development (Funakoshi et al., 1995; Nick & Ribera, 2000). We find that blocking muscle activity suppresses reductions in expression of non-cholinergic transmitters. The rescue is complete for GABA and partial for glutamate and glycine. This suggests that multiple factors or mechanisms are responsible for target-dependent regulation and that different transmitters are regulated in different ways. Because BgTx blocks nicotinic acetylcholine receptors that mediate transmission at the NMJ and rescues expression of non-cholinergic transmitters on the muscle side of co-cultures, presynaptic release of acetylcholine may lead to suppression of non-cholinergic transmitter expression.
Since the lattice culture and conditioned medium experiments argue for the role of retrogradely-transported trophic factors, it will be useful to identify the target-derived factors that are involved in regulating the changes in transmitter specification we have observed. Several neurotrophins have been shown to play important roles in muscle-dependent motor neuron differentiation. BDNF can rescue ChAT expression in adult mammalian hypoglossal neurons following transection and induce ChAT expression in cultured embryonic mouse neurons (Zurn et al., 1996; Liou & Fu, 1997). Both muscle-derived NT-3 and NT-4 regulate presynaptic electrophysiological properties in an activity-dependent manner (Funakoshi et al., 1995; Nick & Ribera, 2000). Although chronic blockade of trk receptors by K252a failed to rescue expression of the non-cholinergic transmitters, we cannot exclude the possibility that different members of the neurotrophin family act differently or have opposing effects, in which case blocking them all could mask changes elicited by each one. Knocking down individual receptors would provide a more definitive test.
Many other factors also play important roles in motor neuron development (Zurn et al., 1996; Dryer et al., 2003). A number of target-derived factors, including CNTF, LIF, and GDNF have been proposed as potential candidates regulating the switch of rat sympathetic neurons from a noradrenergic to a cholinergic phenotype during development (Francis & Landis, 1999; Zweifel et al., 2005). BMP-9 is effective in inducing and maintaining the neuronal cholinergic phenotype in the central nervous system (Lopez-Coviella et al., 2000). Pharmacological blockade and targeted gene knock-down or knock-out of specific receptors may be expected to identify muscle-derived factors responsible for transmitter specification in neuronal development.
Muscle cell inhibition of early neuronal calciumspike activity
Spontaneous calcium-dependent spikes are characteristic of the early development of Xenopus spinal neurons, both in vivo and in vitro. During the first 8 hr after plating, calcium spikes are rare in neurons grown on muscle although they are relatively common in neurons free of muscle contact. This result indicates that muscle cells prevent neurons from generating spontaneous spikes. It remains to be determined whether this result is due to the action of retrogradely transported signals or a high concentration of diffusible factors. Because veratridine not only rescues but also enhances spike incidence on the muscle side to the same extent as the blank side, calcium channels are not functionally impaired; however, the spike trigger mechanism may be suppressed. Spontaneous calcium spikes disappear as synapses mature in vivo, but this also happens in dissociated cell cultures in the absence of cell-cell interactions (Gu & Spitzer, 1995). It is not yet clear whether termination of this spontaneous activity is due to genetic programs or target-derived factors or to both.
Muscle cells override activity-dependent transmitter specification
Modulation of spontaneous calcium spike activity respecifies transmitter expression in a homeostatic manner. Altering this activity pharmacologically or by ion channel overexpression results in changes in transmitter expression that tend to counterbalance the effects of activity manipulation. Like spontaneous calcium spike activity, activity-dependent transmitter specification occurs only during a brief time window during development (Borodinsky et al., 2004; Root et al., 2008) prior to synapse formation.
In neuron-muscle co-cultures, however, even when neuronal activity is altered, no change in transmitter expression takes place. More remarkably, abolition of activity-dependent transmitter specification occurs with or without physical contact between neurons and muscle cells, as long as they are placed in the same dish, arguing for regulation by diffusible factors. Because diffusible factors seem to play little or no role in reducing non-cholinergic transmitter expression, it appears that different factors and target-dependent mechanisms are responsible for these two aspects of neuronal differentiation. In addition, because target-derived factors can be released in an activity-dependent fashion, it is likely that veratridine enhances neuronal activity and in turn enhances postsynaptic muscle activity, which leads to increased release of trophic factors that abolish activity-dependent transmitter specification. These results suggest that muscle provides factors that prevent neurons from resuming activity-dependent transmitter specification even if calcium-dependent neuronal activity were to return at a later time.
Model for target-dependent regulation of neurotransmitter expression
We propose a model in which different combinations of intrinsic genetic coding, neuronal activity and target-derived factors at different stages define the pattern of neurotransmitter expression. The pattern is initially specified genetically through the activity of transcription factors and modulated by environmental influences through changes in spontaneous calcium-dependent activity (Fig. 9A). Once neurons have made contact with their targets, the influence of target-derived factors, including both retrogradely transported signals and diffusible factors, overrides that of early activity. Targets block spontaneous calcium spike activity and further modulate the action of transcription factors to refine the neuronal transmitter expression phenotype (Fig. 9B). Thus, several mechanisms are utilized to ensure that the correct transmitter phenotype is established and maintained throughout development.
Figure 9.
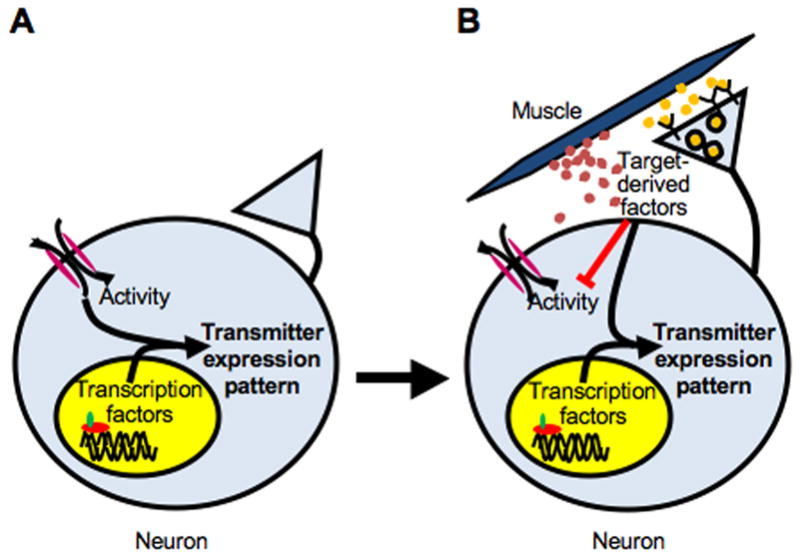
Model of neurotransmitter specification by transcription factors, activity and target-derived factors. A, Early in development, when neurons have not yet established contact with their targets, spontaneous calcium spike activity and transcription factors work together to specify their transmitter expression profile. B, After neurons contact their targets, target-derived factors, including both retrograde factors (yellow dots) and diffusible factors (red dots), suppress expression of non-cholinergic transmitters, block spontaneous calcium activity and disrupt evoked activity-dependent transmitter specification. These factors work in combination with transcription factors to further refine and lock inthe transmitter expression phenotype.
Acknowledgments
This research was supported by National Institutes of Health Grant R01NS15918 to N.C.S. We thank members of our laboratory for helpful discussions and technical assistance; Darwin Berg, Davide Dulcis, Kurt Marek and Xavier Nicol for comments on the manuscript; and A. de la Torre and I. Hsieh for technical support.
References
- Barnett J, Baecker P, Routledge-Ward C, Bursztyn-Pettegrew H, Chow J, Nguyen B, Bach C, Chan H, Tuszynski MH, Yoshida K, Rubalcava R, Gage FH. Human beta nerve growth factor obtained from a baculovirus expression system has potent in vitro and in vivo neurotrophic activity. Exp Neurol. 1990;110:11–24. doi: 10.1016/0014-4886(90)90047-v. [DOI] [PubMed] [Google Scholar]
- Berg DK, Hall ZW. Increased extrajunctional acetylcholine sensitivity produced by chronic acetylcholine sensitivity produced by chronic post-synaptic neuromuscular blockade. J Physiol. 1975;244:659–676. doi: 10.1113/jphysiol.1975.sp010818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodinsky LA, Root CM, Cronin JA, Sann SB, Gu X, Spitzer NC. Activity-dependent homeostatic specification of transmitter expression in embryonic neurons. Nature. 2004;420:523–530. doi: 10.1038/nature02518. [DOI] [PubMed] [Google Scholar]
- Borodinsky LA, Spitzer NC. Activity-dependent neurotransmitter-receptor matching at the neuromuscular junction. Proc Natl Acad Sci USA. 2007;104:335–340. doi: 10.1073/pnas.0607450104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosenitsch TA, Katz DM. Expression of Phox2 transcription factors and induction of the dopaminergic phenotype in primary sensory neurons. Mo l Cell Neurosci. 2002;20:447–457. doi: 10.1006/mcne.2002.1135. [DOI] [PubMed] [Google Scholar]
- Chang LW, Spitzer NC. Spontaneous calcium spike activity in embryonic spinal neurons is regulated by developmental expression of the Na+, K+-ATPase β3 subunit. J Neurosci. 2009;29:7877–7885. doi: 10.1523/JNEUROSCI.4264-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu AY, Chen EW, Loera S. Distinct neurotrophic responses of axotomized motor neurons to BDNF and CNTF in adult rats. Neuroreport. 1994;5:693–696. doi: 10.1097/00001756-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Dulcis D, Spitzer NC. Illumination controls differentiation of dopamine neurons regulating behavior. Nature. 2008;456:195–201. doi: 10.1038/nature07569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryer SE, Lhuillier L, Cameron JS, Martin-Caraballo M. Expression of KCa channels in identified populations of developing vertebrate neurons: role of neurotrophic factors and activity. J Physiol Paris. 2003;97:49–58. doi: 10.1016/j.jphysparis.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Fernandes KJL, Kobayashi NR, Jasmin BJ, Tetzlaff W. Acetylcholinesterase gene expression in axotomized rat facial motoneurons is differentially regulated by neurotrophins: correlation with trkB and trkC mRNA levels and isoforms. J Neurosci. 1998;18:9936–9947. doi: 10.1523/JNEUROSCI.18-23-09936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis NJ, Landis SC. Cellular and molecular determinants of sympathetic neuron development. Annu Rev Neurosci. 1999;22:541–566. doi: 10.1146/annurev.neuro.22.1.541. [DOI] [PubMed] [Google Scholar]
- Fu WM, Liou HC, Chen YH, Wang SM. Release of acetylcholine from embryonic myocytes in Xenopus cell cultures. J Physiol. 1998;509:497–506. doi: 10.1111/j.1469-7793.1998.497bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Furshpan EJ, MacLeish PR, O’Lague PH, Potter DD. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual function neurons. Proc Natl Acad Sci USA. 1976;73:4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Olson EC, Spitzer NC. Spontaneous neuronal calcium spikes and waves during early differentiation. J Neurosci. 1994;14:6325–6335. doi: 10.1523/JNEUROSCI.14-11-06325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Low-threshold Ca2+ current and its role in spontaneous elevations of intracellular Ca2+ in developing Xenopus neurons. J Neurosci. 1993;13:4936–4948. doi: 10.1523/JNEUROSCI.13-11-04936.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375:784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- Hall ZW, Sanes JR. Synaptic structure and development: the neuromuscular junction. Cell [Suppl] 1993;72:99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- Hartenstein V. Early neurogenesis in Xenopus: the spatio-temporal pattern of proliferation and cell lineages in the embryonic spinal cord. Neuron. 1989;3:399–411. doi: 10.1016/0896-6273(89)90200-6. [DOI] [PubMed] [Google Scholar]
- Hayes BP, Roberts A. Synaptic junction development in the spinal cord of an amphibian embryo: an electron microscope study. Z Zellforsch Mikrosk Anat. 1973;137:251–269. doi: 10.1007/BF00307433. [DOI] [PubMed] [Google Scholar]
- Henderson LP, Smith MA, Spitzer NC. The absence of calcium blocks impulse-evoked release of acetylcholine but not de novo formation of functional neuromuscular synaptic contacts in culture. J Neurosci. 1984;4:3140–3150. doi: 10.1523/JNEUROSCI.04-12-03140.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday J, Spitzer NC. Spontaneous calcium influx and its roles in differentiation of spinal neurons in culture. Dev Biol. 1990;141:13–23. doi: 10.1016/0012-1606(90)90098-4. [DOI] [PubMed] [Google Scholar]
- Holliday J, Spitzer NC. Calcium regulates neuronal differentiation both directly and via co- cultured myocytes. J Neurobio. 1993;24:506–514. doi: 10.1002/neu.480240408. [DOI] [PubMed] [Google Scholar]
- Jiang JXS, Choi RCY, Siow NL, Lee HHC, Wan DCC, Tsim KWK. Muscle induces neuronal expression of acetylcholinesterase in neuron-muscle co-culture. J Bio Chem. 2003;278:45435–45444. doi: 10.1074/jbc.M306320200. [DOI] [PubMed] [Google Scholar]
- Joshi K, Lee S, Lee B, Lee JW, Lee SK. LMO4 Controls the balance between excitatory and inhibitory spinal V2 interneurons. Neuron. 2009;61:839–851. doi: 10.1016/j.neuron.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou SY, Chiu AY, Patterson PH. Differential regulation of motor neuron survival and choline acetyltransferase expression following axotomy. J Neurobio. 1995;27:561–572. doi: 10.1002/neu.480270410. [DOI] [PubMed] [Google Scholar]
- Kullberg RW, Lentz TL, Cohen MW. Development of the myotomal neuromuscular junction in Xenopus Laevis: an electrophysiological and fine-structural study. Dev Bio. 1977;60:101–129. doi: 10.1016/0012-1606(77)90113-0. [DOI] [PubMed] [Google Scholar]
- Lams BE, Isacson O, Sofroniew MV. Loss of transmitter-associated enzyme staining following axotomy does not indicate death of brain-stem cholinergic neurons. Brain Res. 1988;475:401–406. doi: 10.1016/0006-8993(88)90635-x. [DOI] [PubMed] [Google Scholar]
- Landis SC, Keefe D. Evidence for neurotransmitter plasticity in vivo: developmental changes in properties of cholinergic sympathetic neurons. Dev Biol. 1983;98:349–372. doi: 10.1016/0012-1606(83)90365-2. [DOI] [PubMed] [Google Scholar]
- Lee SK, Pfaff SL. Transcriptional networks regulating neuronal identity in the developing spinal cord. Nature Neurosci. 2001;4:1183–1191. doi: 10.1038/nn750. [DOI] [PubMed] [Google Scholar]
- Liou JC, Fu WM. Regulation of quantal secretion from developing motoneurons by postsynaptic activity-dependent release of NT-3. J Neurosci. 1997;17:2459–2468. doi: 10.1523/JNEUROSCI.17-07-02459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Popescu IR, Denisova JV, Neve RL, Corriveau RA, Belousov AB. Regulation of cholinergic phenotype in developing neurons. J Neurophysiol. 2008;99:2443–2455. doi: 10.1152/jn.00762.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Coviella I, Berse B, Krauss R, Thies RS, Blusztajn JK. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science. 2000;289:313–316. doi: 10.1126/science.289.5477.313. [DOI] [PubMed] [Google Scholar]
- Mantych KB, Ferreira A. Agrin differentially regulates the rates of axonal and dendritic elongation in cultured hippocampal neurons. J Neurosci. 2001;21:6802–6809. doi: 10.1523/JNEUROSCI.21-17-06802.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawa H, Patterson PH. Separation and partial characterization of neuropeptide-inducing factors in heart cell conditioned medium. Neuron. 1990;4:169–277. doi: 10.1016/0896-6273(90)90101-k. [DOI] [PubMed] [Google Scholar]
- Nick TA, Ribera AB. Synaptic activity modulates presynaptic excitability. Nature Neurosci. 2000;3:142–149. doi: 10.1038/72082. [DOI] [PubMed] [Google Scholar]
- Pfaff SL, Mendelsohn M, Stewart CL, Edlund T, Jessell TM. Requirement for LIM homeobox gene Isl1 in motor neuron generation reveals a motor neuron-dependent step in interneuron differentiation. Cell. 1996;84:309–320. doi: 10.1016/s0092-8674(00)80985-x. [DOI] [PubMed] [Google Scholar]
- Phelps PE, Barber RP, Vaughn JE. Embryonic development of choline acetyltransferase in thoracic spinal motor neurons: Somatic and autonomic neurons may be derived from a common cellular group. J Comp Neurol. 1991;307:77–86. doi: 10.1002/cne.903070108. [DOI] [PubMed] [Google Scholar]
- Ribera AB, Spitzer NC. A critical period of transcription required for differentiation of the action potential of spinal neurons. Neuron. 1989;2:1055–1062. doi: 10.1016/0896-6273(89)90229-8. [DOI] [PubMed] [Google Scholar]
- Root CM, Velazquez-Ulloa NA, Monsalve GC, Minakova E, Spitzer NC. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J Neurosci. 2008;28:4777–4784. doi: 10.1523/JNEUROSCI.4873-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somasekhar T, Nordlander RH. Selective early innervation of a subset of epidermal cells in Xenopus may be mediated by chondroitin sulfate proteoglycans. Brain Res Dev Brain Res. 1997;99:208–215. doi: 10.1016/s0165-3806(97)00011-4. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Lamborghini JE. The development of action potential mechanism of amphibian neurons isolated in cell culture. Proc Natl, Acad Sci USA. 1976;73:1641–1645. doi: 10.1073/pnas.73.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe Y, William C, Jessell TM. Specification of motor neuron identity by the MNR2 homeodomain protein. Cell. 1998;95:67–80. doi: 10.1016/s0092-8674(00)81783-3. [DOI] [PubMed] [Google Scholar]
- Timmusk T, Belluardo N, Metsis M, Persson H. Widespread and developmentally regulated expression of neurotrophin-4 mRNA in rat brain and peripheral tissues. Eur J Neurosci. 1993;5:605–613. doi: 10.1111/j.1460-9568.1993.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Xie K, Wang T, Olafsson P, Mizuno K, Lu B. Activiy-dependent expression of NT-3 in muscle cells in culture: implications in development of neuromuscular junctions. J Neurosci. 1997;17:2947–2958. doi: 10.1523/JNEUROSCI.17-09-02947.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurn AD, Winkel L, Menoud A, Djabali K, Aebischer P. Combined effects of GDNF, BDNF and CNTF on motoneuron differentiation in vitro. J Neurosci Res. 1996;44:133–141. doi: 10.1002/(SICI)1097-4547(19960415)44:2<133::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Kuruvilla R, Ginty DD. Functions and mechanisms of retrograde neurotrophin signaling. Nature Rev Neurosci. 2005;6:615–625. doi: 10.1038/nrn1727. [DOI] [PubMed] [Google Scholar]


