Abstract
The objective of this study was to assess if lipoteichoic acid (LTA), produced by Staphylococcus aureus, exacerbates respiratory disease in porcine respiratory coronavirus (PRCV)-infected pigs, as has previously been shown with lipopolysaccharide. Piglets were inoculated with PRCV and 24 h later with S. aureus LTA. Clinical signs, lung virus titres, inflammatory cells and cytokines in bronchoalveolar lavage fluid (BALF) were compared with those of animals in PRCV- and LTA- inoculated control groups.
All PRCV-LTA inoculated pigs except one developed severe respiratory disease, whereas clinical signs in the control groups were minimal or absent. Virus titres and grossly visible pulmonary lesions were similar in the PRCV-LTA- and PRCV-inoculated groups and were not detected in the LTA-group. Neutrophil percentages in BALF were higher in the PRCV-LTA than in the PRCV group. There was no significant difference in interferon (IFN)-γ, interleukin (IL)-1, IL-6, IL-12/IL-23 and tumour necrosis factor (TNF)-α concentrations in BALF between the PRCV-LTA and PRCV groups, but levels of IL-6, IL-12/IL-23 and IFN-γ were higher in the PRCV-LTA-inoculated than in the LTA-inoculated controls.
The findings suggest that the experimentally-induced respiratory disease was not mediated by cytokine over-production, but rather reflected the concerted action of particular cytokine interactions and/or as yet unidentified mediators. This is the first in vivo study to report the synergistic interaction between a virus and LTA in enhancing the severity of respiratory disease in the pig. Given that Gram-positive bacteria, capable of producing LTA, are commonly found in pig accommodation, the role of this compound in the development of the porcine respiratory disease complex requires further investigation.
Keywords: Porcine respiratory coronavirus, Lipoteichoic acid, Respiratory disease, Pigs, Cytokines
Introduction
Porcine respiratory coronavirus (PRCV) is highly prevalent in pigs in Europe (Laude et al., 1993) and in a serological survey in 2008 in Belgium, 698/728 samples tested positive for PRCV (unpublished data). The virus mainly infects the lower respiratory tract, targeting type-2 pneumocytes and, to a lesser extent, bronchiolar epithelium (Atanasova et al., 2008). Although PRCV infection is typically sub-clinical or mild under experimental conditions, it can contribute to naturally occurring respiratory disease, when combined with other viruses or bacteria (Brockmeier et al., 2002).
Bacteria commonly colonise the lower respiratory tract during primary viral infection and their cell-wall components are also present in the dust generated within animal housing (Crook et al., 1991; Donham, 1991; Zhiping et al., 1996; Zucker et al., 2000). Some of the most important inflammatory components of the outer membrane of Gram-negative bacteria are lipopolysaccharides (LPS). PRCV acting in synergy with LPS from Escherichia coli induces severe respiratory disease with excessive production of pro-inflammatory cytokines, such as tumour necrosis factor (TNF)-α and interleukin (IL)-1 in the lungs (Van Reeth et al., 2000).
Gram-positive bacteria also release toxins such as lipoteichoic acids (LTA) that stimulate cytokine production (Ginsburg, 2002). Such bacteria and their cell-wall components can make up > 80% of the total bacterial content of the dust found in pig housing whereas Gram-negative organisms constitute only 2% (Crook et al., 1991; Donham, 1991). Staphylococcus spp. are among the most widely distributed Gram-positive micro-organisms, and the LTA they produce share pathophysiological properties and recognition mechanisms such as CD14 and Toll-like receptors (TLRs), with LPS (Ginsburg, 2002). LTA from S. aureus has powerful cytokine-inducing activity both in vitro (Morath et al., 2002; Kinsner et al., 2006; Deininger et al., 2007) and in vivo in cattle (Rainard et al., 2008).
The objective of the present study was to assess whether LTA from S. aureus exacerbates disease and enhances pulmonary pro-inflammatory cytokine production in PRCV-infected pigs. It was anticipated that the cytokines examined might participate in the pathogenesis of disease (IL-1, IL-6, TNF-α) and/or in the immunological response to infection (interferon [IFN]-γ, IL-12/IL-23). We also evaluated if there was any correlation between the concentrations of the induced cytokines and the severity of clinical signs or pulmonary lesions.
Materials and methods
Virus and lipoteichoic acid preparation
A Belgian isolate of PRCV (91V44) was used on second passage in swine testis (ST) cells (Van Reeth and Pensaert, 1994); the inoculation dose was 107 50% tissue culture infective doses (TCID50)/pig. Based on previous experiments, the dose of LTA from S. aureus (Sigma-Aldrich) was 200 μg/kg bodyweight, which, in contrast to a 20 μg/kg dose, results in moderate to severe inflammatory cell infiltration (see Appendix A, Supplementary material, Table 1). In both doses there was no extensive cytokine production. The cytokine concentrations were higher than controls, but comparable with previous data using LPS alone and lower than in PRCV-LPS-inoculated animals (Van Reeth et al., 2000; Van Gucht et al., 2006). Virus and LTA were diluted in pyrogen-free phosphate-buffered saline (PBS) (Gibco) to obtain 3 mL of inoculum.
Experimental design and sampling procedure
Twenty-eight, 3-week-old, caesarean-derived, colostrum-deprived pigs from four sows were individually housed in sterile, Horsefall-type isolation units with positive-pressure ventilation. The animals were fed commercial ultra-high-temperature-treated cow’s milk. Twenty-four pigs were randomly assigned to three groups each containing eight animals.
All inoculations were performed via the intra-tracheal route as described previously (Van Gucht et al., 2006). One group was inoculated with PRCV and 24 h later with LTA (PRCV-LTA group). The remaining two groups were inoculated with either PRCV (PRCV group) or LTA (LTA group), respectively, and served as controls.
One pig from each group was euthanased by intra-peritoneal injection of pentobarbital (Kela Laboratories) at the time-points indicated (Table 1). The four remaining pigs were sham-inoculated with PBS and euthanased 4 h later. The experimental procedure had the authorisation of the Ethical and Animal Welfare Committee of the Faculty of Veterinary Medicine of Ghent University (EC 2004/40; EC 2005/75).
Table 1.
Development of macroscopically visible pulmonary lesions and cytological changes within bronchoalveolar lavage fluid (BALF) of pigs following intra-tracheal inoculation of either porcine respiratory coronavirus (PRCV) only, lipoteichoic acid (LTA) only or a combination of PRCV followed 24 h later by LTA (PRCV-LTA).
| Parameter | Inoculation group | Hours after PRCV/LTA inoculation |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 27/3 | 28/4 | 29/5 | 32/8 | 36/12 | 42/18 | 48/24 | 72/48 | ||
| Macroscopically visible lung lesions a (%) |
PRCV only | 0 | 0.1 | 1.3 | 0.6 | 0.3 | 9.5 | 6.0 | 5.0 |
| LTA only | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| PRCV-LTA | 2.0 | 2.3 | 0 b | 1.0 | 0 b | 4.0 | 2.6 | 8.5 | |
|
| |||||||||
| Total BALF cells (x 106) |
PRCV only | 144 | 173 | 122 | 142 | 181 | 305 | 152 | 138 |
| LTA only | 430 | 550 | 760 | 371 | 260 | 83 | 136 | 235 | |
| PRCV-LTA | 172 | 294 | 105 | 331 | 243 | 223 | 134 | 163 | |
|
| |||||||||
| Neutrophils in BALF (%) |
PRCV only | 2.3 | 1.9 | 3.9 | 6.3 | 5.1 | 4.7 | 3.3 | 3.4 |
| LTA only | 70.8 | 59.0 | 80.6 | 56.7 | 39.3 | 3.6 | 1.6 | 21.0 | |
| PRCV-LTA | 10.9 | 31.2 | 4.7 | 45.4 | 16.6 | 11.4 | 6.2 | 4.2 | |
Macroscopically visible lesions were calculated as the mean of the percentage of affected dorsal and ventral lung surfaces.
Minimal lesions representing <0.05 % of the total lung surface.
Post mortem, bronchoalveolar lavage fluid (BALF) was collected from the right lung and cytological examination was carried out as previously described (Van Gucht et al., 2006). Tissue samples of approximately 1 cm3 were taken from the apical, cardiac and diaphragmatic lobes of the left lung for virological and bacteriological examination. Virus titration was performed on 20% suspensions of pooled samples from the three lung lobes of each animal.
Clinical monitoring and scoring system
The pigs were clinically examined three times daily prior to and every hour after inoculation. Clinical ‘scores’ were allocated for: respiratory rate (0, <60/min; 1, 60-90/min - mild tachypnoea; 2, >90/min - severe tachypnoea); laboured abdominal breathing (0, absent; 1, present); dyspnoea (0, absent; 1, present); depression (0, absent; 1, present), and anorexia (0, absent; 1, present).
Anorexia was assessed by fasting the pigs for 6-8 h prior to inoculation and then providing each animal with 150-200 mL of milk following the inoculation procedure. Pigs that did not consume the milk were assigned an anorexia score of ‘1’ (sham-inoculated animals had completely consumed their milk ration within 1 h). A fresh quantity of milk was given to each pig every 4 h to prevent bacterial spoilage influencing consumption. The total clinical score for each pig at each time-point was calculated by adding the scores for each clinical parameter.
Post-mortem assessment of lung lesions
Images of the macroscopically visible lesions were transcribed onto a lung diagram (Christensen et al., 1999) and the approximate percentage of the affected dorsal and ventral surfaces estimated. The percentage of lung affected was calculated as the average of the percentages of the affected dorsal and ventral surfaces.
Quantification of pro-inflammatory cytokines in BALF
Concentrations of IL-1, IL-6 and TNF-α were quantified in three separate bioassays and geometric means calculated (Helle et al., 1988; Van Reeth et al., 1999). Interferon-γ was measured by ELISA (Biosource) and IL-12 using a DuoSet ELISA that detects the p40 sub-unit common to both IL-12 and IL-23 (R and D Systems). The assay did not discriminate between IL-12 and IL-23, (Novelli and Casanova, 2004; Bastos et al., 2004; Watford et al., 2004).
Virological and bacteriological examination
Titres of PRCV were determined on ST cells (Van Reeth and Pensaert, 1994) with a detection limit of 101.7 TCID50/g of tissue. Routine bacteriological examination of lung tissue was performed as follows. Samples were plated on bovine blood agar and cultured aerobically. A ‘nurse’-colony of coagulase-positive Staphylococcus spp. was streaked diagonally on each plate. The plates were inspected for bacterial growth after 48 and 72 h. Colonies were identified by standard techniques as mentioned by Van Gucht and colleagues (2003).
Statistical analysis
Results of the three inoculation procedures were compared using the Friedman test with ‘time of euthanasia’ as a stratification factor. Pair-wise comparisons (PRCV-LTA versus PRCV and PRCV-LTA vs. LTA) were carried out using the Wilcoxon signed-rank sum test also stratified using time of euthanasia. Pair-wise comparisons were tested at a comparison-wise significance level of 0.025 (Bonferroni corrected). Statistical analysis was performed using StatXact software.
Results
Clinical signs and pulmonary viral load
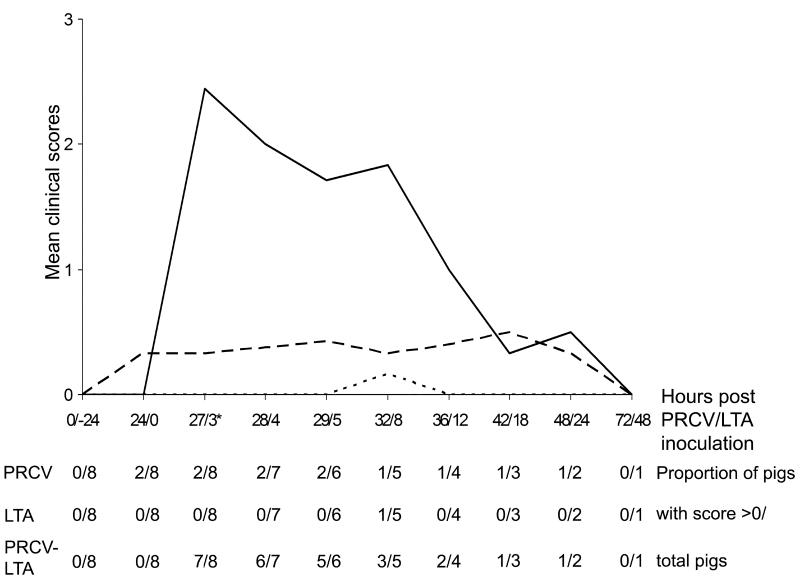
No clinical signs were observed or virus found in the four sham-inoculated controls. Fig. 1 illustrates the development of clinical signs in the experimental groups. Only 2/8 PRCV-only inoculated pigs developed mild depression and occasional anorexia 12 - 24 h post inoculation (PI), which persisted until 48 h PI. Of the eight LTA-only inoculated pigs, four developed mild (lasting <1 h) depression and anorexia at 2 h (n =3) or at 8 h (n =1) PI, respectively. In contrast, the PRCV-LTA inoculated animals exhibited severe tachypnoea, dyspnoea, laboured abdominal breathing, depression, anorexia and occasional vomiting. These signs were evident in 7/8 pigs by 1 h PI and had begun to resolve 4 – 8 h PI (clinical scores relating to individual animals are detailed in Appendix A, Supplementary material, Table 3).
Fig. 1.
Development of mean clinical scores and the proportion of pigs exhibiting clinical signs in the group inoculated with both porcine respiratory coronavirus (PRCV) and lipoteichoic acid (LTA) (PRCV-LTA) (continuous line) compared to groups inoculated with either PRCV (long-dashed line) or LTA (short-dashed line). * one animal from each group was euthanased from this time onwards. The X axis signifies hours after inoculation with PRCV (before the slash) and LTA (after the slash).
The three treatment groups differed significantly from each other (P = 0.0014) when the maximum scores/pig in each group were compared. The PRCV-LTA group had a significantly higher maximum score than either the LTA (P = 0.0156) or PRCV (P = 0.0156) group.
Virus was detected in the lungs of all the PRCV-inoculated pigs. Titres were similar (P = 1.00) in the PRCV (4.1 – 7.3 log10 TCID50/g tissue) and PRCV-LTA (4.8 – 8.0 log10 TCID50/g tissue) groups (individual animal titres detailed in Appendix A, Supplementary material, Table 2). Virus was not detected in animals in the LTA group. No bacteria were cultured from any of the lungs from any group.
Assessment of lung lesions and cytological examination of BALF
Grossly visible red regions of consolidation were scattered predominantly throughout the cranial and cardiac lobes of the PRCV-only and PRCV-LTA inoculated groups. Table 1 details the percentages of affected lung area, BALF cell counts and neutrophil influx as percent of BALF cells. Lesions were not observed in the sham-inoculated animals. The BALF cell count and the neutrophil percentages in these pigs ranged from 34 - 161 × 106 and from 0 - 0.9 %, respectively.
In the PRCV-only group, pulmonary lesions were seen in < 9.5 % of the lung surface area. The total BALF cell count and neutrophil percentage also remained constant despite individual animal variation. Although the LTA group did not exhibit lesions, the total BALF cell count in 6/8 of these pigs and the neutrophil percentage in all eight animals exceeded the maximal values observed in sham-inoculated controls. The PRCV-LTA group developed lung lesions 27 h after PRCV inoculation but the overall pattern of lesion development did not differ from that of the PRCV-only group (P = 1.0).
The total BALF cell counts did not differ significantly between either the PRCV-LTA and PRCV groups (P = 0.25), or between the PRCV-LTA and LTA groups (P = 0.078). The percentage of neutrophils in BALF in the PRCV-LTA group, while significantly higher than in the PRCV group (P = 0.0078), was not significantly different from that in the LTA group (P = 0.039).
Quantification of pro-inflammatory cytokines in BALF
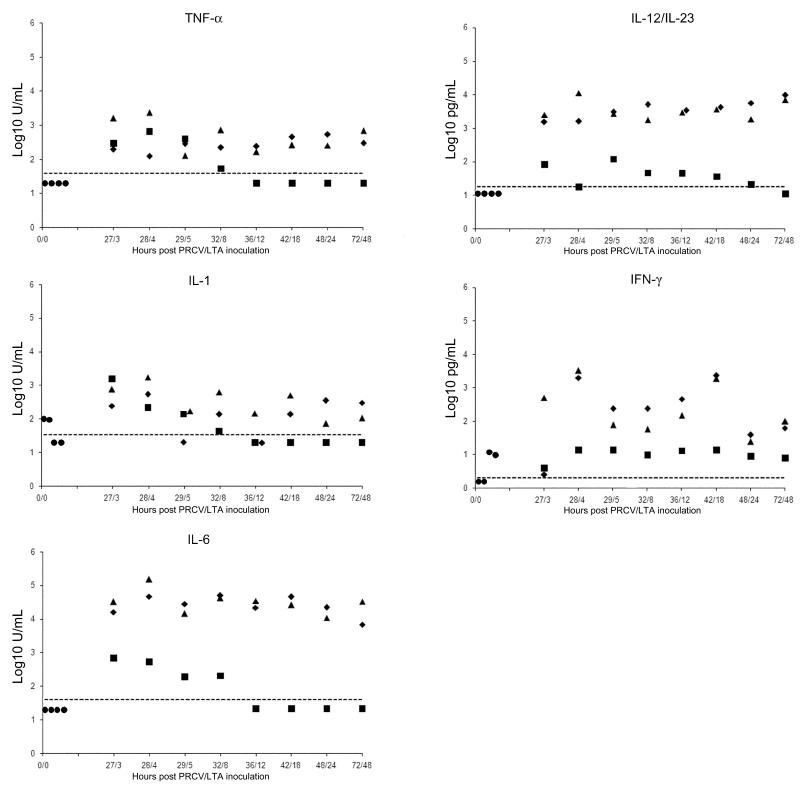
The concentrations of pro-inflammatory cytokines in BALF are illustrated in Fig. 2. No TNF-α, IL-6 or IL-12/IL-23 were detected in any of the sham-inoculated controls. Only low levels of IL-1 (102 and 96 U/mL) and IFN-γ (10 and 12 pg/mL) were detected in 2/4 of the sham-inoculated controls. Inoculation of PRCV alone induced higher levels of IL-6 (4662-46353 U/mL), IL-12/IL-23 (11-121 pg/mL) and TNF-α (124-545 U/mL) than in the sham-inoculated pigs, and the concentrations of IL-1 (< 40-544 U/mL) and IFN-γ (0 - 2329 pg/mL) in 6/8 of the PRCV-inoculated animals were higher than the highest levels recorded in the sham-inoculates.
Fig. 2.
The concentrations of various cytokines in the bronchoalveolar lavage fluid of pigs in the porcine respiratory coronavirus (PRCV) and lipoteichoic acid (LTA)-inoculated group (PRCV-LTA) (triangles), and in groups given either PRCV (diamonds) or LTA (squares). The concentrations in the four sham-inoculated controls are represented as circles at time-point 0/0. The X axis signifies hours after inoculation with PRCV (before the slash) and LTA (after the slash).
Inoculation of LTA alone resulted in the induction of IL-1, IL-6 and TNF-α for a short period. By 12 h PI, these cytokines were no longer detectable in these pigs. The concentrations of IFN-γ in this group never exceeded those in sham-inoculated animals and remained constant throughout the experiment. The levels of IL-12/IL-23 in 3/8 LTA-inoculated animals exceeded the maximal levels observed in the sham-inoculates. Cytokine concentrations were similar in the PRCV-LTA and PRCV groups (P > 0.025), and the levels of IL-6, IL-12/IL-23 and IFN-γ were higher than those of the LTA group (P < 0.025). The concentrations of all cytokines peaked at 28/4 h after PRCV/LTA inoculation and at the end of the study the levels were still higher than in the sham-inoculated controls.
Discussion
The results of this experiment demonstrate that LTA from S. aureus enhances the clinical severity of PRCV infection in pigs in a manner similar to that of LPS from E. coli (Van Reeth et al., 2000). However, there was no evidence that this clinical exacerbation was associated with over-production of pro-inflammatory cytokines such as TNF-α, as previously hypothesised in the context of PRCV infection and LPS (Van Reeth et al., 2000). The concentration of TNF-α was higher in PRCV-LPS inoculated relative to PRCV- and LPS-inoculated controls, and there was a temporal association between peak TNF-α production and the severity of clinical signs. Other cytokines, in addition to TNF-α, were elevated in PRCV-LTA-inoculated pigs and there was no significant difference in the concentration of this cytokine between the PRCV-only and the PRCV-LTA groups.
Differences in the modes of action of LTA and LPS may relate to the different ways these compounds are recognised by the host (Draing et al., 2008). It is likely that LTA is recognised by TLR-2 (Hoogerwerf et al., 2008; Knapp et al., 2008; Meron-Sudai et al., 2008), whereas LPS recognition is mediated through TLR-4 (Triantafilou and Triantafilou, 2005). Pure LTA appears to be a less potent cytokine-inducer than LPS and the ability of LTA to induce higher concentrations of cytokines has been attributed to its synergistic interactions with other bacterial components such as muramyl dipeptide (Yang et al., 2001), peptidoglycan or host macromolecules such as glycosphingolipids (Meron-Sudai et al., 2008).
Recently, the recognition of LTA has been shown to involve not only TLR-2 and CD14 (also a co-receptor for LPS), but also CD36. Blocking of this co-receptor and CD14 inhibited the binding of LTA and LTA-induced TNF-α release by human monocytes (Nilsen et al., 2008). Further research is required to determine if PRCV infection induces increased expression of LTA receptors such as TLR-2 and CD36, as has been shown for CD14 (Van Gucht et al., 2006). In general, the mechanisms through which Gram-positive bacteria effect host immunological activation are less clearly understood than for Gram-negative infections (Sriskandan and Cohen, 1999; Draing et al., 2008).
In general, bacterial cell-wall components are effective in inducing the production of chemokines such as IL-8. Our finding that up to 81% of the total BALF cells were neutrophils in LTA-inoculated pigs indicates that this bacterial component may be highly effective in activating pulmonary neutrophil infiltration in this species. A similar rapid influx of neutrophils into the milk of cows occurs following inoculation of 10 to 100 μg LTA into the mammary gland (Rainard et al., 2008). In this study the neutrophil influx was accompanied by increased secretion of TNF-α and IL-1β and of chemokines like CXCL-2 and IL-8. In the current study, the finding that the total BALF cell count and neutrophil percentages in the PRCV-LTA pigs were slightly, though not significantly, lower than in LTA-inoculated animals during the first 8 h PI may possibly have been due to their more rapid and effective destruction in the dually-inoculated pigs (Medan et al., 2002; Fuchs et al., 2007; Zhang et al., 2008).
We did not investigate the possible role of histamine in the pathogenesis of disease in our experimental model. In addition to mast cells, neutrophils can produce histamine during mycoplasma infection in mice (Xu et al., 2006). Histamine can interact with LPS in the expression of cyclooxygenase-2 and of prostaglandin I2 and E2 by human endothelial cells (Tan et al., 2007) and can also increase the sensitivity of these cells to the effects of Gram-positive and Gram-negative bacteria through inducing the expression of TLR-2, TLR-4 (Talreja et al., 2004), IL-8 and IL-6 (Li et al., 2001). Furthermore, histamine-mediated respiratory disease closely resembles the pattern of clinical signs observed in our study (White et al., 1987; White, 1990).
Histamine causes broncho-constriction leading to dyspnoea, tachypnoea and laboured abdominal breathing. Moreover, its ability to induce the production of TNF-α, IL-6 and other cytokines may also contribute to the severity of these clinical features. Leaving aside this speculation as to a possible role for histamine in our infection model, we can conclude that the severity of the induced disease was not linked to the effect of TNF-α, but most likely reflected an integrated, concerted interaction between the examined cytokines and possibly other (so far unidentified) mediators.
Conclusions
To our knowledge this is the first in vivo study to report the synergistic interaction between a virus and LTA in enhancing the severity of respiratory disease in the pig. Given that Gram-positive bacteria capable of producing LTA are commonly found in high quantities in the dust within pig accommodation, its role in the development of porcine respiratory disease complex merits further investigation.
Supplementary Material
Acknowledgements
The authors would like to thank Fernand De Backer, Nele Dennequin, Peter Meerts, Geert Opsomer and Lieve Sys for their excellent technical assistance. This work was supported by grant 1R01 AI060739-01 from the National Institutes of Health, USA.
Appendix A Supplementary material
Supplementary data associated with this article can be found, in the online version, at doi:......................................
Footnotes
Conflict of interest statement None of the authors of this paper has a financial or personal relationship with other people or organisations that could inappropriately influence or bias the content of the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atanasova K, Van Gucht S, Barbé F, Lefebvre D, Chiers K, Van Reeth K. Lung cell tropism and inflammatory cytokine-profile of porcine respiratory coronavirus infection. Open Veterinary Science Journal. 2008;2:117–126. [Google Scholar]
- Bastos K, Marinho C, Barboza R, Russo M, Alvarez J, D’Imperio Lima M. What kind of message does IL-12/IL-23 bring to macrophages and dendritic cells? Microbes and Infection. 2004;6:630–636. doi: 10.1016/j.micinf.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Brockmeier S, Halbur P, Thacker E. Porcine respiratory disease complex. In: Brogden K, Guthmiller J, editors. Polymicrobial Diseases. American Society for Microbiology; Washington, D.C.: 2002. pp. 231–258. [Google Scholar]
- Christensen G, Sorensen V, Mousing J. Diseases of the respiratory system. In: Straw B, D’Allaire S, Mengeling W, Taylor D, editors. Diseases of Swine. Iowa State University Press; Ames, Iowa, U.S.A.: 1999. pp. 913–940. [Google Scholar]
- Crook B, Robertson J, Glass S, Botheroyd E, Lacey J, Topping M. Airborne dust, ammonia, microorganisms, and antigens in pig confinement houses and the respiratory health of exposed farm workers. American Industrial Hygiene Association Journal. 1991;52:271–279. doi: 10.1080/15298669191364721. [DOI] [PubMed] [Google Scholar]
- Deininger S, Figueroa-Perez I, Sigel S, Stadelmaier A, Schmidt R, Hartung T, von Aulock S. Use of synthetic derivatives to determine the minimal active structure of cytokine-inducing lipoteichoic acid. Clinical and Vaccine Immunology. 2007;14:1629–1633. doi: 10.1128/CVI.00007-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donham K. Association of environmental air contaminants with disease and productivity in swine. American Journal of Veterinary Research. 1991;52:1723–1730. [PubMed] [Google Scholar]
- Draing C, Sigel S, Deininger S, Traub S, Munke R, Mayer C, Hareng L, Hartung T, von Aulock S, Hermann C. Cytokine induction by Gram-positive bacteria. Immunobiology. 2008;213:285–296. doi: 10.1016/j.imbio.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Fuchs T, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. Journal of Cellular Biology. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg I. Role of lipoteichoic acid in infection and inflammation. The Lancet Infectious Diseases. 2002;2:171–179. doi: 10.1016/s1473-3099(02)00226-8. [DOI] [PubMed] [Google Scholar]
- Helle M, Boeije L, Aarden L. Functional discrimination between interleukin 6 and interleukin 1. European Journal of Immunology. 1988;18:1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Hoogerwerf J, de Vos A, Bresser P, van der Zee J, Pater J, de Boer A, Tanck M, Lundell D, Her-Jenh C, Draing C, von Aulock S, van der Poll T. Lung inflammation induced by lipoteichoic acid or lipopolysaccharide in humans. American Journal of Respiratory and Critical Care Medicine. 2008;178:34–41. doi: 10.1164/rccm.200708-1261OC. [DOI] [PubMed] [Google Scholar]
- Kinsner A, Boveri M, Hareng L, Brown G, Coecke S, Hartung T, Bal-Price A. Highly purified lipoteichoic acid induced pro-inflammatory signalling in primary culture of rat microglia through Toll-like receptor 2: selective potentiation of nitric oxide production by muramyl dipeptide. Journal of Neurochemistry. 2006;99:596–607. doi: 10.1111/j.1471-4159.2006.04085.x. [DOI] [PubMed] [Google Scholar]
- Knapp S, von Aulock S, Leendertse M, Haslinger I, Draing C, Golenbock D, van der Poll T. Lipoteichoic acid-induced lung inflammation depends on TLR2 and the concerted action of TLR4 and the platelet-activating factor receptor. Journal of Immunology. 2008;180:3478–3484. doi: 10.4049/jimmunol.180.5.3478. [DOI] [PubMed] [Google Scholar]
- Laude H, Van Reeth K, Pensaert M. Porcine respiratory coronavirus: molecular features and virus-host interactions. Veterinary Research. 1993;24:125–150. [PubMed] [Google Scholar]
- Li Y, Chi L, Stechschulte D, Dileepan K. Histamine-induced production of interleukin-6 and interleukin-8 by human coronary artery endothelial cells is enhanced by endotoxin and tumor necrosis factor-alpha. Microvascular Research. 2001;61:253–262. doi: 10.1006/mvre.2001.2304. [DOI] [PubMed] [Google Scholar]
- Medan D, Wang L, Yang X, Dokka S, Castranova V, Rojanasakul Y. Induction of neutrophil apoptosis and secondary necrosis during endotoxin-induced pulmonary inflammation in mice. Journal of Cellular Physiology. 2002;191:320–326. doi: 10.1002/jcp.10105. [DOI] [PubMed] [Google Scholar]
- Meron-Sudai S, Matityahou A, Keisari Y, Cox K, Hasty D, Ofek I. Lipoteichoic acid synergizes with glycosphingolipids to potently stimulate IL-6 secretion from human blood cells. Clinical Vaccine Immunology. 2008;15:1309–1315. doi: 10.1128/CVI.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morath S, Stadelmaier A, Geyer A, Schmidt R, Hartung T. Synthetic lipoteichoic acid from Staphylococcus aureus is a potent stimulus of cytokine release. Journal of Experimental Medicine. 2002;195:1635–1640. doi: 10.1084/jem.20020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen N, Deininger S, Nonstad U, Skjeldal F, Husebye H, Rodionov D, von Aulock S, Hartung T, Lien E, Bakke O, Espevik T. Cellular trafficking of lipoteichoic acid and Toll-like receptor 2 in relation to signaling; role of CD14 and CD36. Journal of Leukocyte Biology. 2008;84:280–291. doi: 10.1189/jlb.0907656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novelli F, Casanova J. The role of IL-12, IL-23 and IFN-gamma in immunity to viruses. Cytokine and Growth Factor Reviews. 2004;15:367–377. doi: 10.1016/j.cytogfr.2004.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainard P, Fromageau A, Cunha P, Gilbert F. Staphylococcus aureus lipoteichoic acid triggers inflammation in the lactating bovine mammary gland. Veterinary Research. 2008;39:52. doi: 10.1051/vetres:2008034. [DOI] [PubMed] [Google Scholar]
- Sriskandan S, Cohen J. Gram-positive sepsis. Mechanisms and differences from Gram-negative sepsis. Infectious Disease Clinics of North America. 1999;13:397–412. doi: 10.1016/s0891-5520(05)70082-9. [DOI] [PubMed] [Google Scholar]
- Talreja J, Kabir M, Filla M, Stechschulte D, Dileepan K. Histamine induces Toll-like receptor 2 and 4 expression in endothelial cells and enhances sensitivity to Gram-positive and Gram-negative bacterial cell wall components. Immunology. 2004;113:224–233. doi: 10.1111/j.1365-2567.2004.01946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan X, Essengue S, Talreja J, Reese J, Stechschulte D, Dileepan K. Histamine directly and synergistically with lipopolysaccharide stimulates cyclooxygenase-2 expression and prostaglandin I2 and E2 production in human coronary artery endothelial cells. Journal of Immunology. 2007;179:7899–7906. doi: 10.4049/jimmunol.179.11.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triantafilou M, Triantafilou K. The dynamics of LPS recognition: complex orchestration of multiple receptors. Journal of Endotoxin Research. 2005;11:5–11. doi: 10.1179/096805105225006641. [DOI] [PubMed] [Google Scholar]
- Van Gucht S, Van Reeth K, Pensaert M. Interaction between porcine reproductive-respiratory syndrome virus and bacterial endotoxin in the lungs of pigs: potentiation of cytokine production and respiratory disease. Journal of Clinical Microbiology. 2003;41:960–966. doi: 10.1128/JCM.41.3.960-966.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gucht S, Atanasova K, Barbe F, Cox E, Pensaert M, Van Reeth K. Effect of porcine respiratory coronavirus infection on lipopolysaccharide recognition proteins and haptoglobin levels in the lungs. Microbes and Infection. 2006;8:1492–1501. doi: 10.1016/j.micinf.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K, Pensaert MB. Porcine respiratory coronavirus-mediated interference against influenza virus replication in the respiratory tract of feeder pigs. American Journal of Veterinary Research. 1994;55:1275–1281. [PubMed] [Google Scholar]
- Van Reeth K, Labarque G, Nauwynck H, Pensaert M. Differential production of proinflammatory cytokines in the pig lung during different respiratory virus infections: correlations with pathogenicity. Research in Veterinary Science. 1999;67:47–52. doi: 10.1053/rvsc.1998.0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reeth K, Nauwynck H, Pensaert M. A potential role for tumour necrosis factor-alpha in synergy between porcine respiratory coronavirus and bacterial lipopolysaccharide in the induction of respiratory disease in pigs. Journal of Medical Microbiology. 2000;49:613–620. doi: 10.1099/0022-1317-49-7-613. [DOI] [PubMed] [Google Scholar]
- Watford W, Hissong B, Bream J, Kanno Y, Muul L, O’Shea J. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunological Reviews. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- White M, Slater J, Kaliner A. Histamine and asthma. American Journal of Respiratory Disease. 1987;135:1165–1176. doi: 10.1164/arrd.1987.135.5.1165. [DOI] [PubMed] [Google Scholar]
- White M. The role of histamine in allergic diseases. Journal of Allergy and Clinical Immunology. 1990;86:599–605. doi: 10.1016/s0091-6749(05)80223-4. [DOI] [PubMed] [Google Scholar]
- Xu X, Zhang D, Zhang H, Wolters P, Killeen N, Sullivan B, Locksley R, Lowell C, Caughey G. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. Journal of Experimental Medicine. 2006;203:2907–2917. doi: 10.1084/jem.20061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Tamai R, Akashi S, Takeuchi O, Akira S, Sugawara S, Takada H. Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infection and Immunity. 2001;69:2045–2053. doi: 10.1128/IAI.69.4.2045-2053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Cherryholmes G, Shively J. Neutrophil secondary necrosis is induced by LL-37 derived from cathelicidin. Journal of Leukocyte Biology. 2008;84:780–788. doi: 10.1189/jlb.0208086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhiping W, Malmberg P, Larsson B, Larsson K, Larsson L, Saraf A. Exposure to bacteria in swine-house dust and acute inflammatory reactions in humans. American Journal of Respiratory and Critical Care Medicine. 1996;154:1261–1266. doi: 10.1164/ajrccm.154.5.8912733. [DOI] [PubMed] [Google Scholar]
- Zucker B, Trojan S, Muller W. Airborne Gram-negative bacterial flora in animal houses. Journal of Veterinary Medicine. 2000;47:37–46. doi: 10.1046/j.1439-0450.2000.00308.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.