Abstract
Objective
A large number of studies with considerably variable methods have been performed to investigate brain regions involved in the pathophysiology of major depressive disorder. The aim of this study was to use a quantitative meta-analytic technique to synthesise the results of much of this research.
Methods
Three separate quantitative meta-analytical studies were conducted using the Activation Likelihood Estimation technique. Analysis was performed on three types of studies: (1) those conducted at rest comparing brain activation in patients with depression and controls; (2) those involving brain changes following antidepressant treatment; and (3) those comparing brain activation patterns induced by the induction of positive or negative emotion in patients with depression compared with controls.
Results
There appears to be a complex series of areas of the brain implicated in the pathophysiology of depression although limited overlap was found across imaging paradigms. This included a network of regions including frontal and temporal cortex as well as the insula and cerebellum that are hypoactive in depressed subjects and in which there is increase in activity with treatment. There was a corresponding set of subcortical and limbic regions in which opposite changes were found.
Conclusions
There is limited overlap between the brain regions identified using differing imaging methods. The most consistently identified regions include areas of the anterior cingulate, dorsolateral, medial and inferior prefrontal cortex, insula, superior temporal gyrus, basal ganglia and cerebellum. Further research is required to identify if different imaging methods are identifying complementary networks that are equally involved in the disorder.
Keywords: fMRI, depression, meta-analysis
INTRODUCTION
Major depressive disorder (MDD) is a disorder characterised by the recurrence of discrete depressive episodes usually featuring symptoms such as low mood, anhedonia, poor motivation, impaired psychomotor activity, reduced sleep, appetite, energy and libido. Despite the progressive development of new treatments for MDD, symptoms continue to have a considerable impact at the individual and societal level. In addition, there remains the relative lack of a complete and integrated understanding of the pathophysiology and aetiology of this important problem. Over the last 10 years a large number of functional imaging studies have been conducted in an attempt to help elucidate brain processes involved in MDD [for example, Bench et al., 1992, 1993; Drevets, 2000; Mayberg et al., 2000]. These studies have applied a considerable variety of different experimental techniques to help explore differing aspects of the disorder. There are a number of broad categories into which neuroimaging studies in depression may be considered. These include studies conducted at rest (usually of resting blood flow or metabolism), studies examining changes in brain activity over time (usually in response to a treatment intervention), studies examining brain activity in response to a cognitive or emotional activation paradigm and studies using some form of physical or physiological probe such as sleep deprivation or a pharmacological challenge.
Due to the diversity of these techniques and the large number of studies that have been undertaken, it is difficult to gain an integrated understanding of the information the studies provide. In addition, even within each study category there is considerable variation in both the clinical characterisation of patients and the imaging processes that are used. Clinical factors of relevance include issues such as age of illness onset, family history and the behavioural definition of MDD [Drevets, 2001]. However, a number of authors have drawn conclusions from reviewing individual studies and developed sophisticated models describing the potential pathophysiology of depression [for example, Drevets, 2001; Mayberg, 2003]. These models generally involve networks of cortical and subcortical limbic regions and studies have commonly described a divergence of the direction of activity changes [Seminowicz et al., 2004]. More specifically, dorsal prefrontal regions are commonly described as under-active with increases in activity of subgenual cingulate and subcortical regions.
In recent years, a technique has been developed to aid in the understanding and integration of neuroimaging results gathered across studies. This technique, known as function–location meta-analysis, aims to determine the nature of consistent activity across experiments within a certain class of imaging studies [Fox et al., 1998]. The techniques involve searching for locations of functional agreement among statistically significant effects. The most commonly applied of these methods is the activation likelihood estimation (ALE) technique [Turkeltaub et al., 2002]. This technique involves the analysis of concordance between studies by the modelling of each reported significant focus of activation as the centre of a Gaussian probability distribution [Turkeltaub et al., 2002]. Whole brain statistical maps can be generated that estimate the likelihood of activation for each voxel across the entire set of studies. This generates data with an estimate of the probability of the significance of these results, a considerable advantage over traditional methods of tabulating and qualitatively comparing significant results. In recent years the ALE technique has been applied in the study of a variety of imaging areas such as of specific cognitive functions [for example, Laird et al., 2005b; Owen et al., 2005; Price et al., 2005] and illness states [for example, Brown et al., 2005; Glahn et al., 2005]. However, to date this technique has not been applied in the analysis of functional neuroimaging studies of MDD. The aim of this study, therefore, was to quantitatively analyse neuroimaging studies in MDD using the ALE technique. We hypothesised that the quantification of the results of these studies would confirm a pattern of dorsal prefrontal under-activity at rest and reciprocal over-activity in subcortical regions. We hypothesised that changes in the direction of “normalisation” would be found in these regions in treatment studies and that abnormal response would be found in similar overlapping regions during induction paradigms.
METHODS
Literature Search
We conducted multiple Medline searches to initially identify all imaging studies (Positron Emission Tomography (PET), functional magnetic resonance imaging (fMRI), single photon emission computerised tomography (SPECT)) including patients with depressive disorders published until early 2006. The search included the Medical Subheadings (MeSH) term of MDD as well as the keywords of “depressive disorder,” “depression,” “imaging,” “fMRI,” “PET” and “SPECT.” In addition, we searched the reference list of identified articles and several reviews. We excluded studies exclusively of patients with bipolar disorder. A total of 130 studies were initially identified by this process. We attempted to identify multiple reports of single data sets to ensure the inclusion of only one report from each individual study. We then individually screened all the articles for the presence of Talairach or MNI coordinates and tabulated the studies into five groups: (1) “resting studies” (i.e. studies conducted at rest); (2) “emotion activation” studies (studies of the brain response to stimuli designed to activate emotion related brain circuitry); (3) “cognitive induction” studies (studies using a cognitive task); (4) “treatment studies” (i.e. change in brain activity with treatment (pharmacological, ECT, psychotherapy or rTMS)) or (5) other studies, including tryptophan depletion, sleep deprivation and vagal nerve stimulation paradigms. The studies in each category were then analysed to establish whether there was a group or groups of studies with similar methods that would be suitable for meta-analysis. A large number of studies were excluded due to the absence of coordinates or methods which did not include specific comparisons relevant to these five categories. Where multiple studies were present from the same authors, these were analysed to ascertain whether they were of different samples and only included if this appeared to be the case. Sufficient studies were identified in categories 1, 2 and 4 or inclusion in the formal analysis. Studies in category 3 were widely divergent in the cognitive tasks used for induction of brain activation and were not considered sufficiently homogeneous for formal analysis. Although there is no absolute minimum number of studies for ALE analysis, there was no more than two or three studies with similar techniques and even the use of a broader category such as “executive function” did not result in a sufficient group for analysis. There was no homogenous group of studies with coordinates from category 5 that could be included.
Meta-Analysis Procedures
The techniques applied in this analysis have recently been described in detail [Laird et al., 2005a; Turkeltaub et al., 2002]. The procedure involves the modelling of all reported loci of maximum activation as the peaks of a 3D Gaussian probability. Only foci in the source papers reported as significant at P < 0.05 were included. Any coordinates analysed based on the MNI system were converted to Talairach space [Brett, 1999] and coordinates reported in all the tables are in this space. The 3D Gaussian distributions are summed to produce a statistical map that estimated the likelihood of activation for each voxel as determined by all the studies in the analysis. The process then uses standard thresholding techniques applied to this map. We used a Gaussian filter of 12 mm full-width half-maximum (FWHM) and a threshold for false discovery of P < 0.05 (tested with a permutation test of 5,000 permutations). The test was corrected to multiple comparisons using a false discovery rate (FDR) method [Laird et al., 2005a]. All ALE data processing was performed using the BrainMap Search and View software (http://brainmap.org). ALE results were overlaid onto an anatomical template generated by spatially normalizing the International Consortium for Brain Mapping (ICBM) template to Talairach space [Kochunov et al., 2002]. A minimum cluster size of 250 mm3 was applied. Locations of the voxels with peak probabilities within clusters and the cluster sizes were identified. To identify the anatomical location of the voxels identified, these were manually determined by reference to the Talairach and Tourneux stereotaxic neuroanatomical atlas [Talairach and Tournoux, 1988] by two investigators who separately identified all locations. Any discrepancy was resolved by consensus discussion.
Following conduct of the meta-analyses, in an attempt to identify overlapping regions across the analyses, we tabulated the findings (anatomical labels for the coordinates) in the major brain regions. Where we identified a pattern of common regions across study comparisons (for example, regions increased at rest and decreased with treatment), these were overlaid on a standard brain to visually identify actual overlap.
RESULTS
The review process revealed three potential groupings: (1) studies conducted at rest, (2) studies of treatment with a selective serotonin reuptake inhibitor (SSRI) (paroxetine, citalopram or fluoxetine) antidepressant medication, (3) studies utilising an emotional activation paradigm. For group 1, studies were required to identify coordinates for a comparison between blood flow/activation at rest between a group of currently depressed subjects and a health control group. We found nine studies reporting a decrease in activity in patients and seven studies reporting an increase. For group 2, we identified nine papers reporting an increase in activation and nine papers reporting areas of decreased activation with SSRI treatment between two scanning time points (Table I). There were a smaller number of studies in group 3 and with more divergent methods reported. All the studies were required to use a method to induce “positive” or “happy” emotion or “sad” or “negative” emotion. Methods used to do this included the presentation of pictures, scripts of autobiographical memory, words and faces.
TABLE I.
Included studies
| Author | Number in analysis | Imaging method | Contrast |
|---|---|---|---|
| Resting studies | |||
| Bench et al., 1992 | 33/23 | PET | Patients versus controls |
| Bonte et al., 2001 | 21/18 | PET | Patients (children, ages 11–18) versus controls |
| Drevets et al., 1992 | 23/33 | PET | Patients (currently and remitted) versus controls |
| Ito et al., 1996 | 11/9 | PET | Patients versus controls |
| Kimbrell et al., 2002 | 38/37 | PET | Patients versus controls |
| Oda et al., 2003 | 23/25 | SPECT | Patients (with or without hyper-intensities) versus controls |
| Brody et al., 2001 | 24p/16c | PET | Patients versus controls |
| Skaf et al., 2002 | 9/12 | SPECT | Psychotic patients versus controls |
| Kennedy et al., 2001 | 13/24 | PET | Patients versus controls |
| MacHale et al., 2000 | 12/15 | SPECT | Patients versus controls |
| Videbech et al., 2001 | 42/47 | PET | Patients versus controls |
| Perico et al., 2005 | 15/15 | SPECT | Patients versus controls |
| Treatment studies | |||
| Mayberg et al., 1999 | 8 | PET | Fluoxetine treatment |
| Mayberg et al., 2000 | 10 | PET | Fluoxetine treatment |
| Mayberg et al., 2002 | 4 | PET | Fluoxetine responders |
| Kennedy et al., 2001 | 13 | PET | Paroxetine treatment |
| Goldapple et al., 2004 | 13 | PET | Paroxetine treatment |
| Smith et al., 1999 | 6 | PET | Paroxetine treatment |
| Smith et al., 2002 | 6 | PET | Citalopram treatment |
| Brody et al., 1999 | 9 | PET | Paroxetine treatment |
| Brody et al., 2001 | 10 | PET | Paroxetine treatment |
| Vlassenko et al., 2004 | 19 | SPECT | Fluoxetine, paroxetine or amesergide treatment |
| Emotional activation studies | |||
| Canli et al., 2004 | 15/15 | fMRI | Response to happy and sad words (patients versus controls) |
| Gotlib et al., 2005 | 18/18 | fMRI | Response to sad or happy compared with neutral faces (patients versus controls) |
| Keedwell et al., 2005 | 12/12 | fMRI | Response to concurrent happy or sad memory prompts and facial expression (patients versus controls) |
| Kumari et al., 2003 | 6/6 | fMRI | Response to positive negative picture caption pairs compared with neutral (patients versus controls) |
| Lawrence et al., 2004 | 9/11 | fMRI | Response to happy and sad facial expressions of differing intensity (patients versus controls) |
| Surguladze et al., 2005 | 16/14 | fMRI | Response to happy and sad, compared with neutral, facial expressions (patients versus controls) |
Resting Studies
In the nine papers reporting coordinates showing a decrease in activation in depressed subjects compared with controls, there was a total of 14 comparisons considered eligible for inclusion. These contained a total of 55 foci. There were seven papers with eight comparisons and 25 foci demonstrating areas of increased activation in patients compared with controls. The results for these studies are presented in Table II. A total of eight areas were identified where there was decreased activation in patients compared with controls. These included the pregenual anterior and posterior cingulate, bilateral middle frontal gyri, insula and left superior temporal gyrus. Areas identified as “overactive” in patients included a series of deeper brain structures (e.g., thalamus, caudate, medial and inferior frontal gyri) as well as cortical structures including the left superior frontal and right middle frontal gyri.
TABLE II.
Results of resting study analysis
| Region | Hemisphere | BA | Volume (mm3) | Weighted center (x,y,z) | Max ALE value | ||
|---|---|---|---|---|---|---|---|
| Areas of decreased activation in patients | |||||||
| Frontal | |||||||
| Superior temporal gyrus | L | 38 | 504 | −38.45 | 3.49 | −16.53 | 0.0043 |
| Middle frontal gyrus | L | 9 | 648 | −35.96 | 19.91 | 32.36 | 0.0049 |
| Middle frontal gyrus | R | 9 | 1,104 | 30.36 | 47.92 | 34.15 | 0.0049 |
| Pregenual anterior cingulate | L | 24 | 2,544 | −5.29 | 28.67 | 12.72 | 0.0082 |
| Pregenual anterior cingulate | R | 32 | 872 | 0.59 | 48.03 | 3.5 | 0.0060 |
| Dorsal cingulate gyrus | L | 32 | 896 | −14.97 | 20.98 | 38.02 | 0.0057 |
| Claustrum/insula | L | 2,352 | −37.1 | −11.75 | 7.3 | 0.0066 | |
| Posterior cingulate | L | 30 | 768 | −28.58 | −67.02 | 16.55 | 0.0048 |
| Areas of increased activation in patients | |||||||
| Frontal | |||||||
| Inferior frontal gyrus | L | 47 | 288 | −17.68 | 7.91 | −14.14 | 0.0038 |
| Medial frontal gyrus | L | 10 | 272 | −6.95 | 54.83 | 6.1 | 0.0037 |
| Superior frontal gyrus | L | 10 | 272 | −31.1 | 60.16 | 7.16 | 0.0037 |
| Superior frontal gyrus | R | 10 | 256 | 25 | 59 | 3 | 0.0036 |
| Middle frontal gyrus | R | 10 | 312 | 39.08 | 36.85 | 14.04 | 0.0037 |
| Other cortical | |||||||
| Occipital lobe – Lingual gyrus | R | 17 | 336 | 8.32 | −93.52 | −3.75 | 0.0038 |
| Anterior hippocampus/amygdala | R | 312 | 29.86 | −10.83 | −12.86 | 0.0038 | |
| Cerebellum | |||||||
| Anterior lobe | L | 336 | −11.12 | −54.47 | −11 | 0.0036 | |
| Anterior lobe | R | 264 | 4 | −36 | −16 | 0.0038 | |
| Subcortical | |||||||
| Thalamus – Ventral posterior medial nucleus | R | 3,064 | 18.4 | −13.35 | 1.59 | 0.0059 | |
| Thalamus – Pulvinar | L | 336 | −13.04 | −21.95 | 8.12 | 0.0038 | |
| Thalamus | L | 304 | −5.05 | −7.05 | 1.91 | 0.0037 | |
| Thalamus | R | 264 | 13.21 | −3.09 | 12.78 | 0.0037 | |
| Caudate body | L | 336 | −14.91 | 3.91 | 13.79 | 0.0038 | |
Treatment Studies
In regards to treatment studies, we identified nine papers (with 11 experiments and 78 foci) reporting areas of decreased activation following treatment and nine papers with 11 experiments and 68 foci for increased activation with treatment (Table III). Increased activation was found in bilateral middle frontal gyri, cingulate cortex (dorsal and posterior), putamen as well as several other cortical regions. Decreased activation was found in a series of deeper structures (insula, putamen, parahippocampal gyrus (PHG), hippocampus) as well as in pre and subgenual anterior cingulate, inferior medial prefrontal cortex and left superior frontal gyrus.
TABLE III.
Results of treatment studies (pre and post SSRI treatment comparisons)
| Region | Hemisphere | BA | Volume (mm3) | Weighted center (x,y,z) | Max ALE value | ||
|---|---|---|---|---|---|---|---|
| Areas of increased activation following treatment | |||||||
| Frontal | |||||||
| Middle frontal gyrus | L | 8/9 | 4,400 | −35.42 | 13.26 | 33.83 | 0.0099 |
| Middle frontal gyrus | R | 46 | 3,032 | 41.32 | 23.73 | 21.09 | 0.0086 |
| Inferior frontal gyrus | L | 47 | 960 | −34.79 | 30.24 | 2.79 | 0.0054 |
| Dorsal anterior cingulate gyrus | R | 24 | 3,960 | 22.73 | 8.47 | 32.85 | 0.0079 |
| Precentral gyrus | L | 4 | 752 | −49.09 | −11.44 | 25.09 | 0.0048 |
| Temporal – Parietal | |||||||
| Supramarginal gyrus | L | 40 | 3,912 | −42.19 | −43.56 | 33.07 | 0.0123 |
| Posterior cingulated | R | 23 | 3,664 | 2.72 | −28.77 | 23.68 | 0.0107 |
| Inferior parietal lobe | R | 40 | 1,808 | 40.82 | −33.09 | 33 | 0.0092 |
| Subcortical | |||||||
| Midbrain | R | 792 | 2.92 | −28.03 | −15.58 | 0.0060 | |
| Putamen | R | 472 | 20 | 1.21 | 13.3 | 0.0048 | |
| Areas of decreased activation following treatment | |||||||
| Frontal | |||||||
| Middle frontal gyrus | L | 10 | 816 | −12.49 | 45.56 | 2.06 | 0.0069 |
| Superior frontal gyrus | L | 9 | 496 | −22.09 | 33.89 | 34.05 | 0.0052 |
| Medial frontal gyrus/gyrus rectus | R | 11/32 | 280 | 5.9 | 24.32 | −10.87 | 0.0044 |
| Subgenual anterior cingulate/fornix | R | 25 | 3,560 | 2.56 | −1.72 | 0.08 | 0.0090 |
| Pregenual anterior cingulate | R | 24 | 1,128 | 6.79 | 38.41 | 4.77 | 0.0061 |
| Medial temporal | |||||||
| Parahippocampal gyrus | R | 28 | 1,224 | 23.48 | −18.23 | −18.37 | 0.0076 |
| Parahippocampal gyrus | L | 35 | 800 | −19.14 | −18 | −14.06 | 0.0068 |
| Hippocampus | R | 368 | 28.29 | −27.88 | −6.9 | 0.0051 | |
| Insula | R | 13 | 11,960 | 32.69 | 1.19 | 8.04 | 0.0130 |
| Subcortical | |||||||
| Putamen | L | 2,472 | −27.42 | −2.73 | 7.53 | 0.0115 | |
| Putamen | L | 2,120 | −20.01 | 18.04 | 4.53 | 0.0076 | |
Emotional Activation Studies
These analyses were considerably smaller. In regards to studies using happy or positive stimuli, there were six papers reporting decreased activation in patients (seven experiments, 25 foci) and three papers reporting three experiments showing increased activation (14 foci) (Table IV). The studies using happy stimuli showed decreased activation in the depressed patients in a large cluster in the posterior cerebellum, in a left orbital-frontal region, in pregenual and posterior cingulate and in both medial and lateral temporal regions including the PHG. Increased activation in patients was seen in subgenual (and posterior) cingulate and a number of medial, inferior and lateral frontal regions.
TABLE IV.
Results of the analysis of emotional activation studies
| Region | Hemisphere | BA | Volume (mm3) | Weighted center (x,y,z) | Max ALE value | ||
|---|---|---|---|---|---|---|---|
| Areas of decreased activation in patients when exposed to “positive” stimuli | |||||||
| Frontal | |||||||
| Posterior orbital gyrus | L | 11/44 | 872 | −21.76 | 11.42 | −14.27 | 0.0040 |
| Pregenual anterior cingulated | L | 24/32 | 304 | −8.14 | 37.95 | 0.95 | 0.0038 |
| Other cortical | |||||||
| Medial-posterior parahippocampal gyrus | R | 36 | 280 | 1.82 | −36.99 | 3.1 | 0.0037 |
| Inferior temporal gyrus | R | 20 | 256 | 57.28 | −5.4 | −30.24 | 0.0037 |
| Fusiform gyrus | R | 19 | 256 | 34.32 | −70.32 | −12.76 | 0.0038 |
| Posterior cingulate gyrus (lateral) | L | 31 | 256 | −25.84 | −37.11 | 35.84 | 0.0038 |
| Cerebellum | |||||||
| Posterior lobe | L | 1568 | −24.11 | −79.44 | −19.68 | 0.0044 | |
| Subcortical | |||||||
| Putamen | R | 280 | 22.11 | 10.8 | −2.2 | 0.0038 | |
| Areas of increased activation in patients when exposed to “positive” stimuli | |||||||
| Frontal | |||||||
| Superior frontal gyrus | R | 8 | 360 | 0.94 | 32.97 | 51.65 | 0.0036 |
| Middle frontal gyrus | R | 8 | 408 | 37.71 | 19.46 | 53.17 | 0.0038 |
| Medial frontal gyrus | R | 10 | 520 | 9.85 | 51.36 | 4.05 | 0.0038 |
| Inferior frontal gyrus | R | 47 | 512 | 22.87 | 9.71 | −20.37 | 0.0038 |
| Anterior cingulate (subgenual) | R | 25 | 1632 | 2.62 | 15.17 | −3.42 | 0.0050 |
| Gyrus rectus | R | 11 | 424 | 20.26 | 27.9 | −11.94 | 0.0038 |
| Inferior frontal gyrus | L | 11/47 | 1152 | −31.37 | 41.46 | −9.06 | 0.0038 |
| Inferior frontal gyrus | L | 44 | 440 | −46 | 6.92 | 20.18 | 0.0038 |
| Anterior cingulate (subgenual) | L | 32 | 392 | −8.06 | 30.91 | −6.84 | 0.0037 |
| Precentral gyrus | L | 6 | 496 | −41.1 | −1.68 | 44.13 | 0.0038 |
| Other | |||||||
| Lingual gyrus | R | 18 | 464 | 13.83 | −81.85 | −14.03 | 0.0038 |
| Posterior cingulate gyrus | R | 23 | 464 | 1.38 | −27 | 30.05 | 0.0037 |
| Areas of decreased activation in patients when exposed to “negative” stimuli | |||||||
| Frontal | |||||||
| Superior frontal gyrus | R | 11 | 448 | 11.12 | 48.22 | −11.91 | 0.0038 |
| Inferior frontal gyrus | R | 45 | 384 | 47.07 | 25.9 | 13.23 | 0.0037 |
| Inferior frontal gyrus | R | 45 | 568 | 54.36 | 17.95 | 1.1 | 0.0037 |
| Anterior cingulate gyrus (pregenual/dorsal) | R | 24/32 | 512 | 13.82 | 31.11 | 14.93 | 0.0037 |
| Anterior cingulate gyrus (dorsal) | R | 24/32 | 464 | 15.65 | 17.58 | 28.76 | 0.0038 |
| Middle frontal gyrus | L | 8 | 456 | −24.89 | 31.04 | 42.36 | 0.0037 |
| Middle frontal gyrus | L | 8 | 400 | −43.97 | 11.87 | 28 | 0.0038 |
| Middle frontal gyrus | L | 46 | 336 | −40.25 | 42.96 | 0.96 | 0.0037 |
| Temporal | |||||||
| Middle temporal gyrus | R | 21 | 512 | 45.85 | −2.77 | −18.05 | 0.0038 |
| Superior temporal gyrus | L | 22 | 400 | −54.5 | 5.33 | 0.88 | 0.0037 |
| Posterior Cingulate | L | 31 | 408 | −4.1 | −64.14 | 15.83 | 0.0038 |
| Cerebellum | |||||||
| Posterior lobe | R | 416 | 4.13 | −74.97 | −20.06 | 0.0038 | |
| Anterior lobe | R | 416 | 1.87 | −41.09 | −17.44 | 0.0038 | |
| Insula | L | 13 | 408 | −36.07 | 7.05 | 3.5 | 0.0038 |
| Areas of increased activation in patients when exposed to “negative” stimuli | |||||||
| Frontal | |||||||
| Inferior frontal gyrus | R | 45 | 376 | 45.06 | 25.09 | 18.12 | 0.0037 |
| Middle frontal gyrus | R | 6 | 352 | 42.04 | 3.88 | 42.45 | 0.0038 |
| Inferior frontal gyrus | L | 11 | 408 | −13.91 | 14.97 | −19.53 | 0.0037 |
| Temporal | |||||||
| Superior temporal gyrus | R | 41/42 | 1344 | 53.33 | −20.97 | 14.97 | 0.0044 |
| Superior temporal gyrus | R | 22 | 448 | 47.02 | 3.14 | 1.16 | 0.0036 |
| Fusiform gyrus | R | 37 | 320 | 34.12 | −40.52 | −15.87 | 0.0038 |
| Inferior temporal gyrus | L | 20 | 384 | −45.82 | −30.96 | −10.1 | 0.0038 |
| Parietal | |||||||
| Superior parietal gyrus | L | 7 | 448 | −19 | −57 | 47 | 0.0036 |
| Inferior parietal lobule | R | 40 | 304 | 44.66 | −30.08 | 36.01 | 0.0038 |
| Inferior parietal lobule | L | 40 | 424 | −51.62 | −54.76 | 47.2 | 0.0038 |
| Angular gyrus | L | 39 | 392 | −43.05 | −64 | 22.84 | 0.0037 |
| Posterior cingulate gyrus | L | 31 | 392 | −1.9 | −31.96 | 39.09 | 0.0038 |
| Subcortical | |||||||
| Putamen | L | 392 | −24.63 | 3.88 | −2.21 | 0.0038 | |
| Amygdala/uncus | L | 24/38 | 424 | −24.67 | 0.35 | −23.4 | 0.0038 |
| Cerebellum | |||||||
| Posterior lobe | R | 336 | 28.88 | −58.83 | −17.79 | 0.0037 | |
For studies using sad or negative stimuli, there were five papers reporting decreased activation in patients (six experiments, 15 foci) and five papers reporting increased activation (five experiments, 16 foci). Decreased activation in patients was found in pregenual/dorsal and posterior cingulate, a number of lateral frontal regions as well as cerebellum, insula and superior temporal gyrus. Increased activation was found in a number of temporal and parietal cortical regions, lateral frontal regions, posterior cingulate, amygdala, putamen and cerebellum.
Overlapping Regions
The major areas identified in each analysis are presented in Table V and a comparison of the areas of activation is presented in Figure 1. In Figure 2, we have presented the overlap between two sets of comparisons where there appeared to be a pattern of overlap in the results from the data in Table V. The first includes the following: Decreased activation in patients at rest, increased activation in patients with treatment and decreased activation in patients compared with controls with sad stimuli. This revealed areas of limited overlap between decreased activation at rest and increased activation with treatment and between increased activation with treatment and decreased activation with sad stimuli but no areas of overlap between all three comparisons. The second comparison was between the following: Increased activation in patients at rest, decreased activation in patients with treatment and increased activation in patients compared with controls with sad stimuli. There was overlap between the first two study groups but not the other possible combinations.
TABLE V.
Summary of the major areas of activation
| Comparison | Direction of effect | Cingulate cortical regions | Other frontal regions | Other regions identified |
|---|---|---|---|---|
| Resting studies | Decreased in patients | Pregenual Dorsal |
Bilateral MFG | Insula STG |
| Increased in patients | Bilateral SFG L medial and inferior FG R MFG |
Thalamus Caudate Anterior cerebellum |
||
| Treatment studies | Increased with treatment | Dorsal Posterior |
Bilateral MFG L IFG |
Putamen Midbrain Parietal and precentral |
| Decreased with treatment | Pregenual Subgenual |
L MFG and SFG R medial and inferior |
Insula Putamen Hippocampus/parahippocampal gyrus |
|
| Induction of positive affect | Decreased in patients | Pregenual Posterior |
L orbitofrontal | Medial and lateral temporal Posterior cerebellum |
| Increased in patients | Subgenual Posterior |
R medial Bilateral inferior Lateral (R > L) |
Lingual gyrus Precentral gyrus |
|
| Induction of negative affect | Decreased in patients | Pregenual/dorsal Posterior |
Bilateral MFG R IFG R SFG |
Insula Superior temporal gyrus Anterior and posterior cerebellum |
| Increased in patients | Posterior | Bilateral IFG R MFG |
Lateral temporal cortex Parietal cortex Amygdala, putamen Posterior cerebellum |
Figure 1.
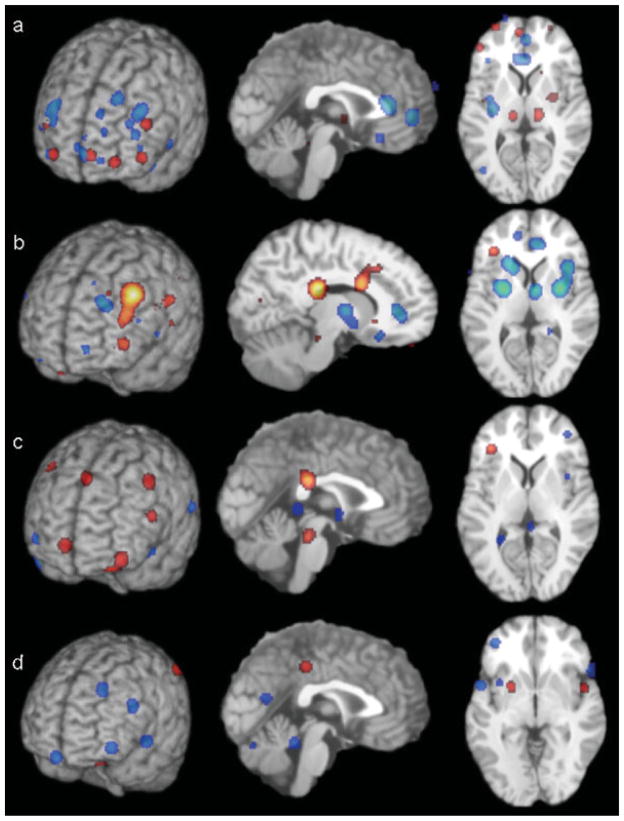
Demonstration of the areas of significant differences across the four analyses. (a) Decreased (blue) and increased (red) activation in depressed patients at rest compared with controls (x = 0, z = +9). (b) Increased activation (red) and decreased activation (blue) with SSRI treatment in depressed patients (x = −10, z = +9). (c) Increased (red) and decreased (blue) activation in depressed patients compared with controls in response to happy stimuli (x = 0, z = +9). (d) Decreased (blue) and increased (red) activation in depressed patients compared with controls in response to sad stimuli (x = 0, z = +1). Images are neurological.
Figure 2.
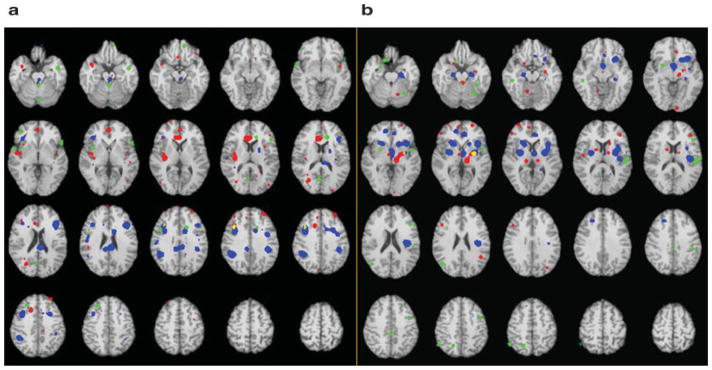
Demonstration of the areas of overlap between analyses. (a) Decreased activation in patients at rest (red); increased activation in patients with treatment (blue); decreased activation in patients compared with controls with sad stimuli (green); overlap between decreased activation at rest and increased activation with treatment (yellow); overlap between increased activation with treatment and decreased activation with sad stimuli (pink). There were no areas of overlap between decreased at rest and decreased with sad stimuli or for the three comparisons. (b) Increased activation in patients at rest (red); decreased activation in patients with treatment (blue); increased activation in patients compared with controls with sad stimuli (green); overlap between increased activation at rest and decreased activation with treatment (yellow). There were no areas of overlap between increased activation with treatment and decreased activation with sad stimuli, decreased at rest and decreased with sad stimuli or for the three comparisons.
DISCUSSION
The aim of this study was to quantitatively analyse the results of a large number of neuroimaging studies performed in the investigation of the pathophysiology of MDD. Our findings suggest that despite the complexity and diversity of the imaging methods studied, there appears to be a pattern of distributed brain regions involved in the pathophysiology of this illness that may be identified and characterised with these techniques. However, as there was quite limited overlap between the areas found across imaging methods, it is critical to acknowledge that the pathophysiology as potentially identified with these techniques appears complex and not reducible to simple models or understandable with single imaging methods. Depression appears to involve a considerable number of diverse cortical and subcortical brain regions and there are significant differences in the way in which differing regions are abnormally active in the disorder and differences both between and within regions identifiable with differing methods of study.
Although there is considerable complexity in the regions identified in this analysis, a number of interesting patterns may be tentatively identified that support the notion that MDD involves a series of specific related networks [Mayberg, 1997]. The first of these involves a series of regions including the dorsal/pregenual anterior cingulate, bilateral middle frontal gyrus (dorsolateral prefrontal cortex (DLPFC)), insula and the superior temporal gyrus (Table V). These regions appear to be characterised by decreased activity in resting studies, a relative lack of activation during induction of negative affect and an increase in activation with SSRI treatment. Although many of these findings have also been reported in individual studies, some inconsistently reported effects, for example the increase in DLPFC activity with treatment [for example, reported in Mayberg et al., 2000, but not in Davies et al., 2003], appear to be significant based on our analysis of pooled results. Although some studies in the literature have suggested lateralised differences, for example, left DLPFC hypoactivity [Bench et al., 1992], and most of the findings of reduced activity in depressed subjects at rest are left sided, no clear laterality is apparent across the other comparisons and study types. Interestingly, the exception to the left predominance of findings in the resting studies is the finding of bilateral hypoactivity in DLPFC. By contrast, changes seen with treatment are much less consistently lateralised and there is no clearly lateralised pattern of difference in response to negative emotion induction.
It has been proposed that the involvement of dorsal frontal regions in depression is closely related to the cognitive symptoms of the disorder [Dolan et al., 1993]. Indeed, in controls, these regions are activated by tasks such as working memory, word generation and planning that have been shown to be performed more poorly in depressed subjects [for example, Fincham et al., 2002; Rajah and D’Esposito, 2005] and studies using some of these tasks report under-activation of DLPFC in depressed subjects [for example, Elliott et al., 1997; Okada et al., 2003]. Due to the lack of studies using consistent methods, it was not possible to conduct a qualitative analysis of cognitive activation studies to confirm this observation. Importantly, dorsal frontal regions were also under-active in patients during the induction of negative affect. Dorsal prefrontal cortex, especially the medial component of BA9, has been found to be activated in response to emotional stimuli in normal controls by both positive and negative emotional stimuli [Lane et al., 1997a,b]. This region may be involved in the modulation of emotional responses [Drevets, 1999], suggesting that dysfunction of this region will produce heightened or abnormal physiological and or psychological responses to stressful stimuli. Our finding of a failure of patients to normally activate this area in response to negative emotional stimuli would be consistent with the interpretation that dysfunction of this area contributes to an abnormal appraisal and response to negative stimuli possibly leading to secondary effects in other brain regions including increased activation in cortical-limbic circuitry.
It is in cortical-limbic regions that the second most obvious network identified across these analyses was found involving regions including medial and inferior frontal cortex and the basal ganglia (caudate or putamen). These areas appear to be relatively consistently overactive at rest, overactive during induction of negative affect and display a reduction in activity with SSRI treatment. Additional regions identified in some but not all of these analyses included the amygdala and thalamus. This pattern of baseline and change related activation appears consistent with the previously proposed involvement of limbic-thalamo-cortical circuits in depression [Drevets, 1998]. This series of connected limbic components appears to be clearly hyperactive in the depressed state and hyperresponsive to the induction of both negative and positive emotion. There is also a complementary finding that some orbitofrontal and temporal cortical regions fail to activate normally in depressed subjects in response to positive emotional stimuli. Collectively, this implies that a substantive dysfunction of the circuitry involved in emotional control and regulation is at least in part modifiable with successful antidepressant therapy.
Another important region identified in this analysis is the subgenual anterior cingulate cortex. We identified this region as showing a decrease in activity with SSRI treatment and there were differences in response activation in this region with positive stimuli in patients. We did not, however, identify an increase in activity in this area at rest. Individual studies have reported a decrease in activity in this site [Drevets et al., 1997] that may be partially explained by reduced volume of this site: this effect of volume could easily confound analyses evaluating the degree of resting change across studies. Activity relative to volume may actually be increased [Drevets, 1999; Drevets et al., 1998], which would be consistent with our finding of an overall reduction in activity with SSRI treatment.
Whilst there has been a considerable focus on frontal and subcortical regions in depression, we identified the involvement of a number of parietal and temporal regions as well as the posterior cingulate and cerebellum. In regards to the posterior cingulate, we found differences in response to both positive and negative emotional stimuli (both increased and decreased activation was identified) as well as an increase in activity with SSRI treatment. Although not commonly highlighted in the imaging literature on depression, involvement of this region is not surprising given that there is a direct connection between ventral posterior cingulate and subgenual anterior cingulate as well as a link to medial orbitofrontal cortex [Vogt et al., 2006] and that it is activated by emotional stimuli [George et al., 1995; Vogt et al., 2003]. It has been proposed that this region of the posterior cingulate is involved in self-assessment, in particular the assessment of emotional content (in collaboration with subgenual anterior cingulate) arising from inputs from the ventral visual stream [Vogt et al., 2006]. Abnormal functioning of a region involved in self monitoring could be consistent with the apparently contradictory findings of both increased and decreased activation with positive and negative affect. This could also be consistent with a “normalising” increase in abnormally reduced activation with SSRI treatment.
Cerebellar regions were also identified in a number of analyses. There was an increase in resting anterior cerebellar activity as well as abnormal responses in cerebellar activation to both positive and negative affect. The cerebellum is now recognised as being connected to both cortical and limbic brain regions important for mood regulation [Schmahmann and Pandya, 1997] and probably plays an important role in the perception of emotional stimuli [Bermpohl et al., 2006]. Studies suggest that the cerebellum is differentially activated by positive emotion, at least in males, [Canli et al., 2004; Habel et al., 2005] and we found a reduction in normal posterior cerebellar activation in the depressed group. However, other studies have reported changes in cerebellar responsively to negative stimuli, suggesting that the dysfunction in this region in not valence specific, as is supported by our analysis.
The study also produced an interesting, if tentative, finding in regards to medial temporal lobe (MTL) structures and their interconnections arising from analysis of the emotional activation studies. In particular, the induction of negative affect resulted in increased MTL activation whilst the opposite (positive affect induction) resulted in decreased MTL activation when compared with controls. The MTL (especially the PHG) has substantial connections to several regions also identified in the analyses. The PHG is the major inflow tract to the hippocampus which is widely identified in structural imaging studies as being reduced in volume in depression [Campbell and Macqueen, 2004; Sheline et al., 2002]. In addition, one of the major input pathways to the PHG arises in the inferior parietal lobe [Clower et al., 2001; Ding et al., 2000; Gaffan and Hornak, 1997], especially in the angular gyrus, an area where we found increased activation in response to negative stimuli. The MTL is also reciprocally connected to the claustrum [Crick and Koch, 2005] which lies anatomically adjacent to the insula which was also identified as abnormally active in a number of these analyses. It is possible that disruption of this circuitry may well underlie, or be a consequence, of altered appraisal of the emotional content of visual stimuli. This inference may be drawn from studies of visual neglect, which has been identified as arising from damage to angular gyrus and its connections to the PHG [Mort et al., 2003]. Similar pathways may be involved in this condition to those that are affected in the disruption of emotional experience in depression. Indirect support for this is also provided by the observation that unilateral caloric vestibular stimulation, which is known to transiently reverse neglect [Bottini et al., 2005; Cappa et al., 1987] by the activation of structures in the contralateral hemisphere including those in the circuits described [Fasold et al., 2002; Suzuki et al., 2001], may also have mood altering properties in patients with clinical mood disorder [Dodson, 2004].
Although there was some consistency in the identification of the regions discussed on purely an anatomical descriptive level, as is apparent from Figure 2, there were few regions identified “in common” between the study types. This is even the case within specific regions: for example, the dorsal anterior cingulate region identified as “under-active” at rest appears not to be exactly the same as the area in which treatment results in increased activity (Fig. 1). It is possible that this results from methodological issues: however, it is not clear why there would be a systematic difference between study types where consistent overlapping activation is found within study groups as reflected as the significant areas of the activation in each separate analysis. Alternatively, these intra-regional variations truly reflect differences in the involvement of subregions in the illness and the process through which these regions are activated. For example, this seems quite reasonable when we consider the involvement of regions imaged at rest and with emotional activation. Pathophysiological disruption of a broad cortical region, for example, dorsal cingulate, may be differently apparent depending on the scanning paradigm and the local circuitry evoked by this. However, currently our understanding of the complexity of interregional physiology may be insufficient to allow us to fully explain why some regions may show both increases and decreases in activation, for example in the right middle frontal gyrus in the studies conducted at rest. Finally, although it is not apparent from us on inspection of the inclusion criteria of the studies, it is possible that there are substantial differences in the types of patients selected into different types of studies which may underlie some of these differences.
There are a number of limitations to be considered in interpretation of these results. The most significant of these is in the nature of the studies that were available to be included in the analysis. Achieving a sufficiently homogenous sample of studies for each analysis required the exclusion of many studies in each category. For example, in the area of treatment studies, we thought it was necessary to limit the studies to a single therapeutic paradigm to constrain potential heterogeneity resulting from the impacts of different treatments as diverse as psychotherapy and ECT, on brain activation patterns. For the analysis of emotional induction, it was necessary to include studies using a variety of methods to induce positive and negative affect due to the limited research that has been carried out in this area. As such, the possibility that heterogenous methods of mood induction alter activity in divergent brain regions cannot be ruled out. Consequently, these results should be treated as relatively preliminary. In contrast, compromises were also required in studies included in the analyses to ensure a sufficient sample of studies. The most obvious of these was the inclusion of a study in an adolescent population in the analysis of studies conducted at rest [Bonte et al., 2001]. This raises concerns about the appropriateness of the comparability of data across age groups and methodological issues related to scan normalisation although the authors seem to have normalised to the standard adult template. In addition, there is considerable variability in the quality of the primary data. For example, in the methods within studies for dealing with multiple comparisons and variability in the clinical definitions; these issues are likely to be reflected in the final quality of the analyses. However, despite the small number of studies and these other issues, the analyses clearly identified significant ALE clusters; these were plausible and broadly consistent with proposed regions of involvement in depressive disorders and there was overlap between identified regions across paradigms. Finally, we have no capacity to judge whether the results are affected by unpublished papers (the “file drawer problem”). However, there is less apparent motivation for the nonpublication of studies that fail to show a specific activation pattern than may be the motivation for the nonpublication of negative treatment studies, and differences in the methods for function/location meta-analyses protect these studies from some of the methodological issues found with standard clinical trial/effect size meta-analysis studies [Fox et al., 1998].
In conclusion, the study has identified a complex network of brain regions involved in the pathophysiology of MDD. First, there appears to be a network involving the dorsal/pregenual anterior cingulate, bilateral middle frontal gyrus, insula and the superior temporal gyrus where there is decreased activity at rest which is increased with SSRI treatment and a relative lack of activation during induction of negative affect. In contrast, the medial and inferior frontal cortex and the basal ganglia (caudate or putamen) as well as potentially the amygdala and thalamus are overactive at rest, overactive during induction of negative affect and display a reduction in activity with SSRI treatment. In addition, depression seems to involve posterior cingulate, medial temporal lobe and the cerebellum. Further research is required to try and untangle the role of these regions and networks, in particular to try and delineate the brain areas involved in the primary pathophysiological change and areas in which changes may be secondary.
Acknowledgments
Contract grant sponsor: Human Brain Project; Contract grant number: 1 R01 NIH/MH074457-01A1; Contract grant sponsors: National Health and Medical Research Council (NHMRC) Practitioner Fellowship, CIHR Clinician Scientist Award, National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator awards, Neurosciences Australia Clinical Neurobiology of Psychiatry Platform.
References
- Bench CJ, Friston KJ, Brown RG, Scott LC, Frackowiak RS, Dolan RJ. The anatomy of melancholia: Focal abnormalities of cerebral blood flow in major depression. Psychol Med. 1992;22:607–615. doi: 10.1017/s003329170003806x. [DOI] [PubMed] [Google Scholar]
- Bench CJ, Friston KJ, Brown RG, Frackowiak RS, Dolan RJ. Regional cerebral blood flow in depression measured by positron emission tomography: The relationship with clinical dimensions. Psychol Med. 1993;23:579–590. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- Bermpohl F, Pascual-Leone A, Amedi A, Merabet LB, Fregni F, Gaab N, Alsop D, Schlaug G, Northoff G. Dissociable networks for the expectancy and perception of emotional stimuli in the human brain. Neuroimage. 2006;30:588–600. doi: 10.1016/j.neuroimage.2005.09.040. [DOI] [PubMed] [Google Scholar]
- Bonte FJ, Trivedi MH, Devous MD, Sr, Harris TS, Payne JK, Weinberg WA, Haley RW. Occipital brain perfusion deficits in children with major depressive disorder. J Nucl Med. 2001;42:1059–1061. [PubMed] [Google Scholar]
- Bottini G, Paulesu E, Gandola M, Loffredo S, Scarpa P, Sterzi R, Santilli I, Defanti CA, Scialfa G, Fazio F, Vallar G. Left caloric vestibular stimulation ameliorates right hemianesthesia. Neurology. 2005;65:1278–1283. doi: 10.1212/01.wnl.0000182398.14088.e8. [DOI] [PubMed] [Google Scholar]
- Brett M. The MNI brain and the Talairach atlas, Cambridge Imagers. 1999 Available at http://www.mrc-cbu.cam.ac.uk/Imaging/mnispace.html.
- Brody AL, Saxena S, Silverman DH, Alborzian S, Fairbanks LA, Phelps ME, Huang SC, Wu HM, Maidment K, Baxter LR., Jr Brain metabolic changes in major depressive disorder from pre- to post-treatment with paroxetine. Psychiatry Res. 1999;91:127–139. doi: 10.1016/s0925-4927(99)00034-7. [DOI] [PubMed] [Google Scholar]
- Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, Phelps ME, Huang SC, Wu HM, Ho ML, Ho MK, Au SC, Maidment K, Baxter LR., Jr Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: Preliminary findings. Arch Gen Psychiatry. 2001;58:631–640. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: An ALE meta-analysis of functional neuroimaging studies. Hum Brain Mapp. 2005;25:105–117. doi: 10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci. 2004;29:417–426. [PMC free article] [PubMed] [Google Scholar]
- Canli T, Sivers H, Thomason ME, Whitfield-Gabrieli S, Gabrieli JD, Gotlib IH. Brain activation to emotional words in depressed vs. healthy subjects. Neuroreport. 2004;15:2585–2588. doi: 10.1097/00001756-200412030-00005. [DOI] [PubMed] [Google Scholar]
- Cappa S, Sterzi R, Vallar G, Bisiach E. Remission of hemineglect and anosognosia during vestibular stimulation. Neuropsychologia. 1987;25:775–782. doi: 10.1016/0028-3932(87)90115-1. [DOI] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL. The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci. 2001;21:6283–6291. doi: 10.1523/JNEUROSCI.21-16-06283.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick FC, Koch C. What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci. 2005;360:1271–1279. doi: 10.1098/rstb.2005.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J, Lloyd KR, Jones IK, Barnes A, Pilowsky LS. Changes in regional cerebral blood flow with venlafaxine in the treatment of major depression. Am J Psychiatry. 2003;160:374–376. doi: 10.1176/appi.ajp.160.2.374. [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen G, Rockland KS. Inferior parietal lobule projections to the presubiculum and neighboring ventromedial temporal cortical areas. J Comp Neurol. 2000;425:510–530. doi: 10.1002/1096-9861(20001002)425:4<510::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Dodson MJ. Vestibular stimulation in mania: A case report. J Neurol Neurosurg Psychiatry. 2004;75:168–169. [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ, Bench CJ, Liddle PF, Friston KJ, Frith CD, Grasby PM, Frackowiak RS. Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity? J Neurol Neurosurg Psychiatry. 1993;56:1290–1294. doi: 10.1136/jnnp.56.12.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Functional neuroimaging studies of depression: The anatomy of melancholia. Annu Rev Med. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Prefrontal cortical-amygdalar metabolism in major depression. Ann NY Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res. 2000;126:413–431. doi: 10.1016/S0079-6123(00)26027-5. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: Implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–249. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–3641. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Ongur D, Price JL. Neuroimaging abnormalities in the subgenual prefrontal cortex: Implications for the pathophysiology of familial mood disorders. Mol Psychiatry. 1998;3:220–226. 190–191. doi: 10.1038/sj.mp.4000370. [DOI] [PubMed] [Google Scholar]
- Elliott R, Baker SC, Rogers RD, DAOL, Paykel ES, Frith CD, Dolan RJ, Sahakian BJ. Prefrontal dysfunction in depressed patients performing a complex planning task: A study using positron emission tomography. Psychol Med. 1997;27:931–942. doi: 10.1017/s0033291797005187. [DOI] [PubMed] [Google Scholar]
- Fasold O, von Brevern M, Kuhberg M, Ploner CJ, Villringer A, Lempert T, Wenzel R. Human vestibular cortex as identified with caloric stimulation in functional magnetic resonance imaging. Neuroimage. 2002;17:1384–1393. doi: 10.1006/nimg.2002.1241. [DOI] [PubMed] [Google Scholar]
- Fincham JM, Carter CS, van Veen V, Stenger VA, Anderson JR. Neural mechanisms of planning: A computational analysis using event-related fMRI. Proc Natl Acad Sci USA. 2002;99:3346–3351. doi: 10.1073/pnas.052703399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox PT, Parsons LM, Lancaster JL. Beyond the single study: Function/location metanalysis in cognitive neuroimaging. Curr Opin Neurobiol. 1998;8:178–187. doi: 10.1016/s0959-4388(98)80138-4. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Hornak J. Visual neglect in the monkey. Representation and disconnection. Brain. 1997;120(Pt 9):1647–1657. doi: 10.1093/brain/120.9.1647. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995;152:341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: A quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25:60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, Mayberg H. Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. doi: 10.1001/archpsyc.61.1.34. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JD, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Habel U, Klein M, Kellermann T, Shah NJ, Schneider F. Same or different? Neural correlates of happy and sad mood in healthy males. Neuroimage. 2005;26:206–214. doi: 10.1016/j.neuroimage.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Ito H, Kawashima R, Awata S, Ono S, Sato K, Goto R, Koyama M, Sato M, Fukuda H. Hypoperfusion in the limbic system and prefrontal cortex in depression: SPECT with anatomic standardization technique. J Nucl Med. 1996;37:410–414. [see comments] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. A double dissociation of ventromedial prefrontal cortical responses to sad and happy stimuli in depressed and healthy individuals. Biol Psychiatry. 2005;58:495–503. doi: 10.1016/j.biopsych.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Evans KR, Kruger S, Mayberg HS, Meyer JH, McCann S, Arifuzzman AI, Houle S, Vaccarino FJ. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry. 2001;158:899–905. doi: 10.1176/appi.ajp.158.6.899. [DOI] [PubMed] [Google Scholar]
- Kimbrell TA, Ketter TA, George MS, Little JT, Benson BE, Willis MW, Herscovitch P, Post RM. Regional cerebral glucose utilization in patients with a range of severities of unipolar depression. Biol Psychiatry. 2002;51:237–252. doi: 10.1016/s0006-3223(01)01216-1. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Lancaster J, Thompson P, Toga AW, Brewer P, Hardies J, Fox P. An optimized individual target brain in the Talairach coordinate system. Neuroimage. 2002;17:922–927. [PubMed] [Google Scholar]
- Kumari V, Mitterschiffthaler MT, Teasdale JD, Malhi GS, Brown RG, Giampietro V, Brammer MJ, Poon L, Simmons A, Williams SC, Checkley SA, Sharma T. Neural abnormalities during cognitive generation of affect in treatment-resistant depression. Biol Psychiatry. 2003;54:777–791. doi: 10.1016/s0006-3223(02)01785-7. [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Price CJ, Glahn DC, Uecker AM, Lancaster JL, Turkeltaub PE, Kochunov P, Fox PT. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Hum Brain Mapp. 2005a;25:155–164. doi: 10.1002/hbm.20136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT. A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp. 2005b;25:6–21. doi: 10.1002/hbm.20129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Ahern GL, Schwartz GE, Davidson RJ. Neuroanatomical correlates of happiness, sadness, and disgust. Am J Psychiatry. 1997a;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Bradley MM, Lang PJ, Ahern GL, Davidson RJ, Schwartz GE. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia. 1997b;35:1437–1444. doi: 10.1016/s0028-3932(97)00070-5. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- MacHale SM, Lawrie SM, Cavanagh JT, Glabus MF, Murray CL, Goodwin GM, Ebmeier KP. Cerebral perfusion in chronic fatigue syndrome and depression. Br J Psychiatry. 2000;176:550–556. doi: 10.1192/bjp.176.6.550. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: A proposed model of depression. J Neuropsychiatry Clin Neurosci. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: Serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Silva JA, Brannan SK, Tekell JL, Mahurin RK, McGinnis S, Jerabek PA. The functional neuroanatomy of the placebo effect. Am J Psychiatry. 2002;159:728–737. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- Mort DJ, Malhotra P, Mannan SK, Rorden C, Pambakian A, Kennard C, Husain M. The anatomy of visual neglect. Brain. 2003;126 (Pt 9):1986–1997. doi: 10.1093/brain/awg200. [DOI] [PubMed] [Google Scholar]
- Oda K, Okubo Y, Ishida R, Murata Y, Ohta K, Matsuda T, Matsushima E, Ichimiya T, Suhara T, Shibuya H, Nishikawa T. Regional cerebral blood flow in depressed patients with white matter magnetic resonance hyperintensity. Biol Psychiatry. 2003;53:150–156. doi: 10.1016/s0006-3223(02)01548-2. [DOI] [PubMed] [Google Scholar]
- Okada G, Okamoto Y, Morinobu S, Yamawaki S, Yokota N. Attenuated left prefrontal activation during a verbal fluency task in patients with depression. Neuropsychobiology. 2003;47:21–26. doi: 10.1159/000068871. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perico CA, Skaf CR, Yamada A, Duran F, Buchpiguel CA, Castro CC, Soares JC, Busatto GF. Relationship between regional cerebral blood flow and separate symptom clusters of major depression: A single photon emission computed tomography study using statistical parametric mapping. Neurosci Lett. 2005;384:265–270. doi: 10.1016/j.neulet.2005.04.088. [DOI] [PubMed] [Google Scholar]
- Price CJ, Devlin JT, Moore CJ, Morton C, Laird AR. Meta-analyses of object naming: Effect of baseline. Hum Brain Mapp. 2005;25:70–82. doi: 10.1002/hbm.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, D’Esposito M. Region-specific changes in pre-frontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain. 2005;128 (Pt 9):1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. The cerebrocerebellar system. Int Rev Neurobiol. 1997;41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, Rafi-Tari S. Limbic-frontal circuitry in major depression: A path modeling metanalysis. Neuroimage. 2004;22:409–418. doi: 10.1016/j.neuroimage.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Mittler BL, Mintun MA. The hippocampus and depression. Eur Psychiatry. 2002;17 (Suppl 3):300–305. doi: 10.1016/s0924-9338(02)00655-7. [DOI] [PubMed] [Google Scholar]
- Skaf CR, Yamada A, Garrido GE, Buchpiguel CA, Akamine S, Castro CC, Busatto GF. Psychotic symptoms in major depressive disorder are associated with reduced regional cerebral blood flow in the subgenual anterior cingulate cortex: A voxel-based single photon emission computed tomography (SPECT) study. J Affect Disord. 2002;68:295–305. doi: 10.1016/s0165-0327(00)00365-7. [DOI] [PubMed] [Google Scholar]
- Smith GS, Reynolds CF, III, Pollock B, Derbyshire S, Nofzinger E, Dew MA, Houck PR, Milko D, Meltzer CC, Kupfer DJ. Cerebral glucose metabolic response to combined total sleep deprivation and antidepressant treatment in geriatric depression. Am J Psychiatry. 1999;156:683–689. doi: 10.1176/ajp.156.5.683. [DOI] [PubMed] [Google Scholar]
- Smith GS, Kramer E, Hermann CR, Goldberg S, Ma Y, Dhawan V, Barnes A, Chaly T, Belakhleff A, Laghrissi-Thode F, Greenwald B, Eidelberg D, Pollock BG. Acute and chronic effects of citalopram on cerebral glucose metabolism in geriatric depression. Am J Geriatr Psychiatry. 2002;10:715–723. [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kitano H, Ito R, Kitanishi T, Yazawa Y, Ogawa T, Shiino A, Kitajima K. Cortical and subcortical vestibular response to caloric stimulation detected by functional magnetic resonance imaging. Brain Res Cogn Brain Res. 2001;12:441–449. doi: 10.1016/s0926-6410(01)00080-5. [DOI] [PubMed] [Google Scholar]
- Talairach J, Thournoux P. Co-planar stereotaxic atlas of the human brain. Stuttgart, Germany: Thieme Medical Publishers; 1988. [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. Neuroimage. 2002;16(3, Pt 1):765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B, Pedersen AR, Egander A, Landbo B, Rasmussen NA, Andersen F, Stodkilde-Jorgensen H, Gjedde A, Rosenberg R. The Danish PET/depression project: PET findings in patients with major depression. Psychol Med. 2001;31:1147–1158. doi: 10.1017/s0033291701004469. [DOI] [PubMed] [Google Scholar]
- Vlassenko A, Sheline YI, Fischer K, Mintun MA. Cerebral perfusion response to successful treatment of depression with different serotoninergic agents. J Neuropsychiatry Clin Neurosci. 2004;16:360–363. doi: 10.1176/jnp.16.3.360. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. Eur J Neurosci. 2003;18:3134–3144. doi: 10.1111/j.1460-9568.2003.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA, Vogt L, Laureys S. Cytology and functionally correlated circuits of human posterior cingulate areas. Neuroimage. 2006;29:452–466. doi: 10.1016/j.neuroimage.2005.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]