Abstract
Background
Tobacco related lung diseases including chronic obstructive pulmonary disease (COPD), are major causes of lung-related disability and death worldwide. Acute exacerbation of COPD (AE-COPD) is commonly associated with upper and lower respiratory viral infections and may result in respiratory failure in those with advanced lung disease.
Objective
We sought to determine the mechanism underlying COPD exacerbation, and host response to pathogen-derived factors.
Methods
Over a 24 months period, we assessed the viral causes for upper and lower respiratory infections in COPD (n=155) and control (n=103) subjects. We collected nasal and bronchoalveolar lavage (BAL) fluid and peripheral blood under baseline and exacerbated condition. We determined the effect of human rhinovirus (HRV) proteinases on T cell activation in humans, and in mice.
Results
HRVs are isolated from nasal and lung fluid from subjects with AE-COPD. BAL fluid, and CD4 T cells from COPD patients exhibited a type 1 T helper (Th1), and Th2 cell cytokine phenotype during acute infection. HRV-encoded proteinase 2A activated monocyte-derived dendritic cells in vitro, and induced strong Th1, and Th2 immune responses from CD4 T cells. Intranasal administration of recombinant rhinovirus proteinase 2A in mice resulted in an increase in airway hyperreactivity, lung inflammation, and IL-4 and IFN-γ production from CD4 T cells.
Conclusion
Our findings suggest that patients with severe COPD show Th1 and Th2 bias responses during AE-COPD. HRV-encoded proteinase 2A, like other microbial proteinases, could provide a Th1 and Th2-biasing adjuvant factor during upper and lower respiratory infection in patients with severe COPD. Alteration of the immune response to secreted viral proteinases may contribute to worsening of dyspnea and respiratory failure in COPD.
Keywords: Th1/Th2 cells, COPD, proteinase, lung inflammation
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by irreversible reduction of maximum expiratory airflow and is currently the fourth leading cause of death in the U.S. Worldwide, COPD is the 12th most prevalent disease and is estimated to rise to the 5th in the next two decades 1, 2. Frequent hospital and clinic-based visits for treatment of episodic worsening of shortness of breath accounts for a large proportion of the medical expenditures and portends a poor outcome in patients with COPD 3.
Respiratory tract infections are a leading cause of morbidity, hospitalization, and antibiotic use in patients with chronic lung disease, in particular COPD; however, in the past the etiological agents responsible for respiratory failure remained obscure in 20 to 50% of the cases 4–6. Most viral infections evoke a strong type 1 T helper (Th1) and/or cytotoxic T lymphocyte (CTL) responses that are required for efficient eradication of the organism, however a number of respiratory viruses, including HRVs, has been associated with comparatively weak Th1 immunity and more robust Th2-biased allergic inflammation 7, 8. Th1-biased immunity has previously been linked to the immunopathology of COPD and emphysema, but whether respiratory viral infections exacerbate Th1 immunity or evoke an aberrant systemic or lung-specific Th2 response remains unclear.
A major difficulty in understanding the etiology of AE-COPD is the mechanism(s) by which respiratory failure occurs. Acute worsening in airway obstruction in patients with advanced COPD is clearly linked to an increase in the work of breathing that is reminiscent of acute exacerbations of asthma. We and others have shown that the presence of active proteinases in environmentally relevant allergens is critical for the development of the allergic response and for inducing symptoms of airway obstruction in humans and in experimental asthma 9–11. Indeed, neutralization of proteinases renders allergens inactive because in the absence of proteolytic activity, allergens fail to induce requisite lung Th2 responses 12. Previously, we demonstrated that dendritic cells are activated by allergenic proteinases to induce Th2 cell differentiation 13.
In this study, we investigated whether the host immune response to rhinoviral proteinases could contribute to the immunopathology and disease exacerbation linked to acute viral infection in COPD. We show that HRVs are linked to AE-COPD in persons with advanced lung disease. Moreover, we demonstrate that rhinoviral proteinase 2A, but not 3C has potent immunomodulatory activity on human dendritic cells that favors production of Th1, and Th2 cytokines from CD4 T cells in the peripheral system. Rhinoviral proteinase 2A further induced allergic disease in mice when administered to the upper respiratory tract. Together, our findings support a mechanism for AE-COPD in which proteinases derived from rhinovirus and potentially other viruses could create an allergic lung immune environment that leads to airway obstruction induced by Th1, and Th2 cell-derived cytokines.
Methods
Clinical and demographic characteristic of the study participants
The clinical and demographic characteristics of subjects are shown in Online Repository Table E1. We recruited 258 subjects as part of the Longitudinal Exacerbation Study of COPD (LES-COPD). At the time of enrolment, subjects were free of respiratory symptoms and had no history of antibiotic use or systemic corticosteroids in the past 6 weeks. Peripheral blood, nasal, and throat swab samples were collected from subjects during the initial enrollment and at the time of acute exacerbation of COPD (AE-COPD). COPD exacerbation was defined as a respiratory illness marked by the presence of symptoms of rhinitis or pharyngitis, and increased cough, shortness of breath, or sputum production and/or change in sputum color in the presence or absence of fever (temperature >37.7 °C) 4. Subjects underwent bronchoscopy when clinically stable; alternatively, patients that were admitted to the Ben Taub General Hospital with a diagnosis of AE-COPD and were placed on ventilator support underwent bronchoscopy with alveolar fluid lavage sampling as we have described previously 5. All studies were approved by the Institutional Review Board at Baylor College of Medicine and written informed consent was obtained from all study participants.
Detection of respiratory viruses from nasal swab and BAL samples
Nasal and throat swab samples were collected from the LES-COPD participants (Online Repository Table E1) at the time of entry into the study (baseline) and during an episode of AE-COPD. We used the commercially available rapid multiplex PCR system (Multicode-PLX system; Eragen, Biosciences, Madison, WI) for the detection of seventeen sets of respiratory viruses, that included: human rhinovirus (HRV), respiratory syncytial virus (RSV), parainfluenza virus (PIV), influenza virus (InfV), metapneumovirus, adenovirus (Ad), coronavirus, and enterovirus according to the manufacturer’s instructions and described previously 14. Bronchoalveolar fluid (BAL) samples were obtained from intubated subjects that were admitted to the MICU at the Ben Taub General Hospital for an AE-COPD, from clinically stable COPD patients, and from control subjects, as described previously 15.
Detection of cytokines in BAL and in PBMC derived CD4 T cells
Concentration of cytokines in the BAL fluid was detected using antibody-based standard ELISA or Luminex assay. Briefly, total protein from BAL samples were measured and all samples were normalized to the lowest protein concentration using standard protein measurement assays (Pierce BCA Protein Assay; Thermo Scientific USA), and standard ELISA (to detect CXCL9, CXCL10), Luminex-100 (BioRad), and Beadlyte multiplex assays from Millipore (Charlottesville, Virginia; to detect cytokines) were used to measure supernatant concentrations of IL-4, IL-13, IL-10, and IFN-γ, according to the manufacturer (R&D systems and BD Bioscience).
For the analysis of in vitro T cell activation, we used CD4 T cell depleted PBMCs (2 × 104/well) that were irradiated (3,000 rads) and were used as antigen presenting cells (APCs) in a final volume 200 μl and co-cultured with autologous T cells (5 × 105) for 3 to 5 days. Supernatants were removed from each well and stored at −80 °C, for batch cytokine analysis using Luminex-100 (BioRad).
Generation and cell surface marker expression of human moDCs
Monocyte-derived (mo)DCs were generated as previously described 13. CD14+ cells were positively selected from PBMC isolated from subjects with no evidence of AE-COPD using anti-CD14 microbeads and were separated using autoMACS (Miltenyi Biotec). To induce DC differentiation, 2 × 106 CD14+ cells were cultured in complete medium (RPMI 1640-10% FBS), with 500 IU/ml human rGM-CSF (R&D Systems), and 400 IU/ml human rIL-4 (R&D Systems) at 37°C in 5% CO2 for six days. Nonadherent, immature DCs were harvested by gentle pipetting and after two washes with PBS, and in some experiments moDC were cultured with 1 μg/ml LPS, 10 μg/ml recombinant rhinovirus 2A, or 3C proteinases, or buffer (vehicle control) for 24 hrs. Surface expression of DC maturation markers (CD-80, HLA-DR and CD86) were detected with FITC-, PE-, and APC- conjugated anti-CD80, -HLA-DR and -CD86 mAbs respectively (BD Biosciences) and were analyzed with a multicolor FACSCalibur flow cytometer (BD Biosciences) with FlowJo software as described above.
moDC induced T cell activation
Rhinoviral proteinases 2A and 3C were obtained using recombinant proteins as previously described 16, 17. CD4 T cells were positively selected using anti-CD4 microbeads (Miltenyi Biotec) using autoMACS according to the manufacturer’s instructions, and were co-cultured with autologous moDCs that had been conditioned with proteinase 2A, 3C, LPS, or buffer at 10:1 ratio for six days in a 96-well plate. Expanded T cells were then washed and stimulated with immobilized anti-CD3 and anti-CD28 mAbs for 48 h. Culture supernatants were collected and cytokine production was assessed by Beadlyte multiplex assays (Millipore).
Mouse model of rhinovirus proteinase induced inflammation
Wild type (C57BL/6) mice were purchased from the Baylor Center for Comparative Medicine facility and were kept in the Association for Assessment and Accreditation of Laboratory Animal Care (AALAC)-accredited transgenic animal facility at Baylor College of Medicine. All experiments were preformed in accordance with protocols approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. Mice were given 25 ug of chicken egg ovalbumin (OVA) and 7ug intranasal rhinoviral proteinase 2A, 3C, vehicle (buffer), or PBS every 2 days for a total of 16 days. Twenty four hrs following the last challenge, mice were anesthetized, intubated, a tail intravenous (IV) started, and respiratory system resistance was measured using the provocative concentration of acetylcholine in milligrams per gram that caused a 200 percent increase in airway resistance over the baseline (PC200), as we have described before 11, 18. The lungs were washed using two, 1.0 ml aliquots of sterile PBS through the tracheal cannula and the bronchoalveolar lavage (BAL) fluid total and differential cell count were measured using the standard hemocytometer and Giemsa stain of cytospin slides. Lung-derived single cells (1×106) were used for ELISpot 19 or were cultured; supernatants were collected after 3 days at 37°C in 5% CO2 and stored at −80 °C for batch analysis using ELISA and/or BioRad multiplex bead-based cytokine detection kit (BioRad) as previously described 11. All recombinant protein, capture antibodies, and their corresponding biotin-conjugated detection antibodies were purchased from R&D system and BD Biosciences. IL-4, IL-5, IFN-γ, IL-17, CXCL-10, and TNF concentrations were measured by Lincoplex (Millipore). Glycoprotein were measured in lung lavage fluid as described previously 11.
Statistical analysis
For the comparison of control and COPD BAL, CD4 T cell cytokine and chemokine concentration analysis, we used the one-way ANOVA test, or Wilcoxon matched pairs test (non-parametric, two-tailed). For the comparison of unpaired data, Mann-Whitney test (non-parametric, two-tailed) was used.
Results
The etiology of COPD exacerbation; detection of viral causes
Respiratory viruses are among the most important triggers of acute exacerbation of COPD (AE-COPD). Therefore, we determined the viral etiology of upper and lower respiratory infections prospectively in the volunteers participating in the LES-COPD study. We collected nasal and throat swab samples from our volunteers during initial enrollment and at the time of AE-COPD (see Methods for the definition of AE-COPD). Respiratory viral infections were identified using a high throughput, multiplex-based assay that simultaneously identifies up to 17 respiratory viruses and subtypes (MultiCode-Plx, Eragen biosciences Madison, WI). A total of 45 illness episodes in a 24-month period (including a few during which the subject was not seen during acute illness) were identified. We found that, consistent with others and our prior observations 4, a majority of exacerbations occurred in those with more advanced COPD (GOLD criteria III–IV; 29 cases) as compared to subjects with mild (COPD I–II; 10 cases) or no disease (control; 6 cases). Samples from volunteers at baseline (at the time of entry into LES COPD) failed to yield PCR evidence of viral infection. In contrast, thirteen of 45 (29 %) samples collected during the return visit for AE-COPD were PCR-positive for viruses, the majority of which (10/13; 77%) were rhinovirus (Online Repository Table E2). In addition, 3 out of 8 (37%) of bronchoalveolar (BAL) fluids samples from intubated subjects with AE-COPD were positive for respiratory virus infection (Online Repository Table E2). Thus, common respiratory viruses, especially HRVs, were specifically linked to the presence of AE-COPD.
Th1 and Th2 cytokine bias in BAL fluid in AE-COPD
Under stable disease conditions, lymphocytes extracted from the lung parenchyma of patients with moderate to severe COPD spontaneously secrete predominant Th1-specific cytokines and chemokines 20. To understand the lung immune profile during AE-COPD, we determined the cytokine profile of BAL fluid (normalized for total protein concentration) of COPD patients under stable and exacerbated conditions as compared to control subjects (Fig. 1). In contrast to findings from peripheral lung lymphocytes studied in vitro, we found that more than half of the subjects in each group had no detectable IFN-γ in their BAL fluid. Although higher concentrations of this cytokine were detected in subjects with AE-COPD, it did not reach statistical significance (Fig. 1A). Similarly, no differences in the concentration of BAL fluid IL-13 were found (Fig. 1B). However, we detected significantly higher concentrations of IL-4, the canonical Th2 cytokine, in the BAL fluid of subjects with AE-COPD while very few subjects in the other groups produced this cytokine (Fig. 1C).
Figure 1. Detection of cytokines in the BAL fluid of subjects with COPD.
BAL fluids were collected from three groups; Control (n=25), COPD Stable (n=18) and COPD exacerbation that required mechanical ventilation (n=8), and were stored in −80°C and were batch analyzed using multiplex quantitative cytokine bead assay (R&D systems) and verified by quantitative ELISA for IFN-γ (A), IL-13 (B), IL-4 (C), CXCL-9 (MIG, D) and CXCL-10 (IP-10, E). More than half of subjects from each group did not show any detectable levels of IFN-γ, or IL-13, but IL-4 was consistently detected in the BAL fluid of COPD exacerbation subjects. Each group was compared to the control subjects and statistical analysis was performed using the Mann-Whitney test.
The latter finding was unexpected as we and others have previously shown that the lung immune phenotype of subjects with stable COPD and emphysema is highly Th1 biased, and that T cells extracted from the lung of patients with COPD and emphysema specifically do not secrete IL-4 20, 21. Nonetheless, higher concentrations of CXCL9 (MIG) and CXCL10 (IP-10), two Th1 specific chemokines that are induced by IFN-γ, were present in the BAL of subjects with AE-COPD (Fig. 1D, E). Together these data reconcile the lack of IFN-γ in BAL with our previous finding that COPD patients have a Th1 biased immune response during AE-COPD, but our findings further suggest that Th2 immune responses may arise in the setting of AE-COPD and possibly influence respiratory function.
Peripheral blood CD4 T cells in AE-COPD show mixed Th1/Th2 bias
We next determined whether the immune phenotype of peripheral blood T cells isolated during AE-COPD was altered when compared to the same individual under baseline conditions. Spontaneous cytokine release from PBMC-derived CD4 T cells was determined during co-culture with autologous antigen presenting cells under two distinct conditions: 1) baseline (free of respiratory symptoms) and 2) during exacerbation episodes. In order to determine the CD4 T cell immune response at the time of respiratory infection, we compared cytokines at baseline and during upper or lower respiratory infection (URI/LRI) in control (no COPD), or exacerbation in mild to moderate (COPD I–II), and in advanced disease (COPD III–IV) groups as defined by the revised GOLD criteria 22. Cultured CD4 T cell at baseline and at the time of URI/LRI in subjects with no (Control) or during AE-COPD in mild to moderate COPD (I–II) showed no significant change in the production of IFN-γ, IL-13 (Fig 2A, B), while IL-10 production was significantly increased at the time of respiratory infection in those with mild to moderate disease (Fig 2B; p=0.04). Despite the lack of detectable IFN-γ in the BAL fluid of AE-COPD subjects, PBMC-derived CD4 T cells from those with advanced lung disease (COPD III–IV) secreted significantly higher amounts of IFN-γ and IL-10 when compared to stable conditions (Fig 2C; p=0.04, and p=0.02 respectively). In agreement with the Th2 cytokine findings from BAL fluid analyses of AE-COPD subjects, PBMC-derived CD4 T cells during AE-COPD in COPD III–IV, secreted significantly higher amounts of IL-13 when compared to the same group during stable periods (Fig. 2C; p=0.04). We could not detect expression of IL-4 in any of our in vitro co-culture conditions, indicating the extremely low concentration of this cyokine under physiological conditions. These findings indicate that two Th2-specific cytokines, namely IL-4 and IL-13, and Th1 cyttokine (IFN-γ) are associated with local and systemic immune responses in those with advanced COPD that present with upper or lower respiratory tract infection.
Fig 2. Peripheral blood T cell responses in AE-COPD.
CD4 T cell were isolated from PBMC of volunteers at baseline (free of symptoms) and during upper or lower respiratory infection/exacerbation and co-cultured with autologous irradiated CD1a APCs (1:10 ratio). Supernatant from co-culture assays were collected and were stored in −80°C and was batch analyzed using multiplex quantitative cytokine bead assay and verified by quantitative cytokines. Data show individual responses at baseline ( A: Control n=4; B: COPD I–II n=10; C: COPD III–IV n=14) and the time of upper respiratory infection/exacerbation with ELISA for IFN-γ (top), IL-13 (middle), IL-10 (lower) panels. Baseline cytokine level in each group was compared to exacerbation data point in the same cohort and statistical analysis was performed using the Mann-Whitney test.
Rhinovirus proteinases 2A and 3C prime monocyte derived DCs
We next sought to examine the mechanism(s) underlying the induction of Th1 and Th2 cells during viral infection. We chose to study rhinovirus proteinases 2A and 3C because of the allergenic potential of other microbial proteinases and the established link between AE-COPD cases and rhinovirus infection 16, 23. Conditioned monocyte (mo) derived DCs (moDCs) stimulated for 24 hrs in response to 2A, but not 3C, rhinovirus proteinases resulted in significant up-regulation of MHC class II (HLA-DR), and CD86, when compared to control (vehicle) or LPS stimulation (Fig. 3A, B; p=0.004 and p=0.017 respectively). Analysis of cytokine secretion under these conditions revealed a significant increase in TNF production in moDCs activated by 2A proteinase, but no significant differences were found in secretion of IL-12, IL-10 and IL-6 (Fig. 3C, D, and data not shown). These findings indicate that viral proteinase 2A induces maturation of moDCs and potentially alters their function.
Fig 3. Effect of rhinovirus proteinase (2A and 3C) on DC maturation and activation.
Monocytes (CD14+) from peripheral blood of COPD patients and healthy controls were cultured with GM-CSF and IL-4 for 6 days to induce monocyte derived DCs (moDCs). moDCs were treated with indicated stimuli (buffer, rhinovirus proteinases 2A or 3C or lipopolysaccharide (LPS)) for 24 hrs and analyzed for surface DC maturation marker expression and cytokine production. Up-regulation of HLA-DR (A), and CD80 (B), were measured by flow cytometry. Indicated values (n=10) in scatter plots show mean fluorescence intensity of expression of HLA-DR, CD80 on DC after treatment. Data represent mean ± SD of ten independent experiments. DC culture supernatants were analyzed for the presence of TNF (C), IL-10 (D), IFN-γ and IL-12 by multiplex cytokine assay system (undetectable levels of IFN-γ and IL-12 were found). Statistical analysis was performed using the two-tailed Wilcoxon signed rank test.
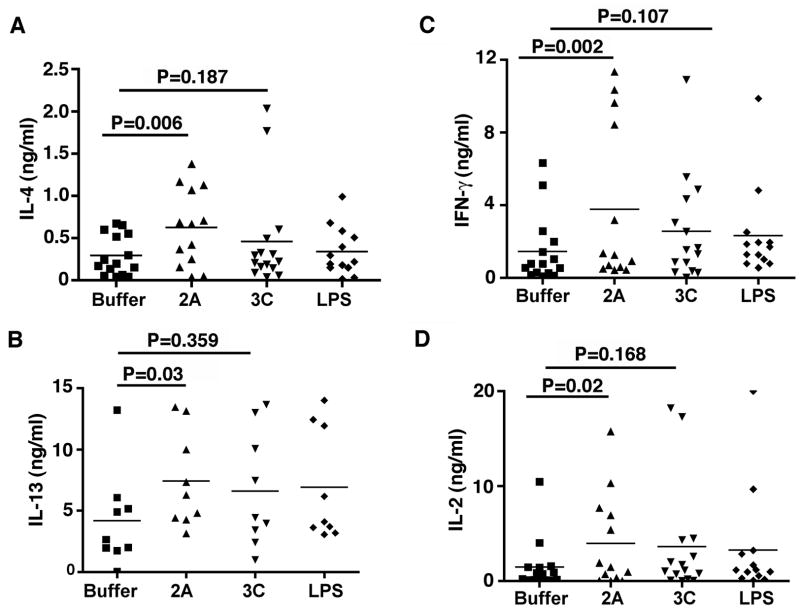
Rhinovirus proteinase 2A induces Th1 and Th2 cell differentiation
We next determined the functional significance of rhinovirus 2A proteinase activated moDCs on T cell activation. Proteinase 2A, but not 3C or LPS, activated moDCs to induce greater secretion of IL-13, IL-4, IFN-γ, and IL-2 from co-cultured CD4 T cells (Fig. 4A–D). These finding are in agreement with the BAL fluid analysis of patients with AE-COPD that showed in addition to Th1, a significant Th2 immune bias as well (Fig. 1). Together, our data suggest a mechanism by which rhinovirus proteinase 2A induces the maturation and activation of human moDCs and promotes Th1, and Th2 cell differentiation.
Fig 4. Analysis of Th1 and Th2 cytokines production by DC primed CD4 T cells.
moDCs (n=15) were treated with 2A, 3C, LPS or vehicle for 24 hrs and washed to remove any residual cytokines. Treated moDC were then co-cultured with autologous CD4 T cells (1:10 ratio), for 6 additional days. The expanded cells were collected and stimulated with anti-CD3/CD28 mAbs for 48h and culture supernatants were analyzed for the presence of IL-4 (A), IL-13 (B), IFNγ (C), and IL-2 (D), by Luminex. Statistical analysis was performed using the two-tailed Wilcoxon signed rank test.
Intranasal challenge with rhinovirus 2A induces Th2 responses in mice
Our data thus far points to a direct contribution of the purified HRV proteianse 2A in induction of the skewed Th1 and Th2 immune responses in human PBMC. Therefore, we next sought to determine whether HRV 2A and or 3C proteinases could induce physiological changes in the lung that are consistent with Th2 response in mice. Therefore, to confirm that HRV 2A and 3C proteinases could induce Th1 and or Th2 immune responses in vivo, we administered ovalbumin (OVA) PBS, OVA vehicle, or proteinases and OVA (OVA was used to ensure detection of antigen specific IgG1, a marker of Th2 immunity) to mice over a three-week period using a well-established model of active proteinase induced allergic lung disease 11. Proteinases were delivered intranasally to mimic conditions likely to be present during active human rhinovirus upper respiratory tract infection (Fig 5A). Compared to OVA or vehicle challenge, intranasal challenge with 2A and 3C resulted in airway hyperreactivity (AHR) as assessed by both an increase in respiratory system resistance (RRS), in response to multiple acetylcholine (Ach) doses and a decrease in the provocative concentration of Ach that was required to elicit a 200% increase in RRS from baseline (PC200) (Fig. 5B–D and data not shown). Rhinovirus 2A proteinase further induced more robust lung inflammation as assessed by both enhanced eosinophila and neutrophilia. Whole lung single cell analysis by ELISpot technique also showed predominant spontaneous IL-4 production from mice challenged with rhinovirus 2A proteinase when compared to the other treatment groups (Fig. 5C–D). Interestingly, despite the more robust Th2 response to 2A proteinase, B cell response, as determined by detection of OVA-specific IgG1, was present in mice treated with 2A and 3C (Fig. 5E). Analysis of the lung lavage fluid in the same group of mice revealed increase glycoprotein secretion in mice treated with 2A proteinase (Fig. 5F).
Fig 5. Intranasal recombinant 2A and 3C proteinase challenged mice show increased airway allergic phenotype.
Age and sex matched C57/BL6 mice, (n = 5 in each group) were immunized intranasally with OVA and recombinant 2A, 3C, buffer or PBS every 2 days for a total of 16 days and 24 h after the last immunization (A), mice were assessed for (B) AHR is shown as dose response to acetylcholine and using PC 200, (C) BAL and differential cell, and (D) lung specific IL-4 and IFN-γ ELISpot (E) OVA-specific IgG1 in serum, (F) lung lavage glycoprotein were determined from the same groups of mice. *P < 0.05 relative to PBS, and buffer and **P < 0.05 relative to 3C and PBS and buffer using one way ANOVA and t-test. Data represent mean values ± SD. Concentration of IL-4 (G), IL-5 (H), IFN-γ (I), IL-17 (J), CXCL-10 (K), and TNF (L), were measured in supernatant of single cell culture of lung cells using Luminex. *p < 0.05 relative to buffer and **p < 0.05 relative to 3C, buffer using one way ANOVA and t-test. Data is representative of three independent experiments; values shown are mean values ± SD.
To more comprehensively assess cytokine profiles elicited by viral proteinases, lung cell cultures were established ex vivo and luminex-based multiplex technology was used to quantify secreted cytokines from cell supernatants. These studies showed selective increased secretion of the Th2 cytokines (IL-4, IL5), and IL-17, that were induced by rhinovirus 2A, while the Th1 chemokine CXCL10, and IFN-γ were induced by both 2A, and 3C-stimulation (Fig. 5G–K). Only TNF, and CXCL1 were exclusively upregulated by 3C proteinase (Fig. 5L and data not shown). Thus, rhinovirus proteinase 2A elicits secretion of Th1 and Th2 cytokines and allergic lung disease when given intranasally to mice.
Discussion
In this study, we prospectively investigated the association between respiratory tract viral infection and COPD exacerbation and the likely mechanism by which common respiratory tract viruses could induce aberrant immune responses in the lung. Using a well-characterized cohort of former, current or never smokers with and without advanced lung disease, we have previously shown that AE-COPD occurs in smokers with severe and very severe COPD (%FEV1 <50) and that HRVs are among the most common viral isolates linked to respiratory infection 25–27. In this work, we show that BAL fluid from subjects with COPD and respiratory failure show significantly higher concentrations of the Th1 associated chemokines CXCL10 and CXCL9. However the unexpected detection of IL-4, albeit low levels of the canonical Th2 cytokine 28, provided initial evidence that mixed Th1 and Th2 responses elicited by viruses might be contributing to respiratory failure seen in these patients.
Rhinoviral proteinase 2A, further induced Th1 and Th2 cell development from naïve human CD4 T cells through effects on moDC and powerfully induced Th1 and Th2 cytokines, increase in neutrophil and esosinophil in the lung, and increase airway resistance in mice, confirming the highly immune altering nature of this proteinase. Together, these findings suggest that proteinases produced by HRVs elicit Th1 and Th2 responses that may exacerbate chronic airway obstruction that is characteristic of advanced COPD and potentially trigger respiratory failure.
HRVs, members of the family Picornaviridae, are among the most common causes of human respiratory infections 29. HRV infection normally occurs by direct inoculation of the virus into the ciliated epithelial cells of the upper airway where binding to host intercellular adhesion molecule-1 (ICAM-1; CD54), results in the release of the single stranded viral RNA genome into the cytoplasm 27, 29, 30. Th1 immune responses are well described as an effective host immune response to eradicate viral infection 31. However, in asthmatic individuals, a weak Th1 immune response and a robust Th2 immunity to rhinovirus infection has been postulated as a mechanism to exacerbate disease in susceptible asthmatics 32.
Although type 2 immunity has long been linked to allergic diseases and has occasionally been associated with COPD 33, 34, a consistent link between type 2 cytokines and airway obstruction in smokers has been elusive. Moreover, type 1 immune mechanisms are generally required to eradicate intracellular pathogens such as viruses. Indeed, pre-existing allergic inflammation has the potential to impair requisite viral clearance mechanisms and essentially convert respiratory viruses into self-replicating allergens 32.
Our finding that rhinoviral proteinases could powerfully induce Th1 and Th2 inflammation thus provides potentially critical insight into the pathogenesis of both COPD exacerbation and rhinovirus infections. It is interesting to note that while we found an increase in IL-13 production during AE-COPD specifically in subjects with severe and very severe COPD (III–IV GOLD classification), we found that IL-10, a cytokine that is generally inhibitory to both Th1 and Th2 immune responses is also up regulated in those with AE-COPD irrespective of disease severity. The increase in IL-10 during the acute inflammation/infection might represent a natural regulatory response to limit T cell activation following the initial viral infection. Nonetheless, by inducing lower respiratory tract type 2 immune responses, rhinoviral proteinases likely induce a second component of airway obstruction mediated not through loss of lung structural integrity, but through the pro-obstructive effects of IL-4 and/or IL-13, i.e., airway hyperresponsiveness and airway mucus hypersecretion 35, 36. Moreover, HRVs likely gain from the immune deviation catalyzed by their proteinases. As in asthma, the type 2 immunity induced by viral proteinases may impair otherwise efficient type 1 immune clearance mechanisms, leading to prolonged infection and enhanced spread of the micro-organism. While this point is speculative, we propose that accelerated production of proteinase 2A may have evolved as a strategy not to enhance the processing of polyproteins, since interference leads to a decrease in viral titer 37, however modulation of the host immune response that may be maximal at much lower levels of proteinase production, may promote disease dissemination. While this putative viral-adaptive mechanism is of little consequence to hosts with normal respiratory function, it can, unfortunately, be perilous to those with severe COPD with respiratory compromise.
Clinical studies of COPD exacerbation have shown that inhibition of the immune response with systemic corticosteroids is effective in reducing the length of hospitalization and shortening the duration of respiratory failure 38. These findings are consistent with our observations suggesting that an aberrant Th2 immune response superimposed on the chronic type 1 immunity of COPD is responsible for further respiratory compromise. Specifically, systemic or topical corticosteroids may suppress production of IL-4 and IL-13, thereby alleviating airway hyperresponsiveness and mucus hyperproduction that are induced by these cytokines 35. Although further studies are required, corticosteroids may potentially promote elimination of respiratory viruses by inhibiting the aberrant allergic immune response to secreted viral proteinases.
Our discovery of the allergenic nature of rhinovirus proteinase 2A, in addition to Th1 responses, expands the list of highly allergenic proteinases of potential relevance to human respiratory tract disease, which includes proteinases of fungal, plant and dust mite origin 39, 40. However, we have recently shown that common dust mite proteinases isolated from human environments fail to retain the proteinase activity that is required for allergenicity 12, 41. Therefore, the environmental proteinases of greatest proximal relevance to human respiratory disease may be confined to those derived from plant and fungal sources and, as shown here, from viruses.
Interestingly, although our studies have revealed the allergenic potential of multiple proteinases, it is also clear that not all proteinases have equivalent allergenic potential. This is perhaps best exemplified by rhinoviral proteinase 3C, which relative to proteinase 2A demonstrated only weak allergenic activity. Nonetheless, our findings further support the contention that proteinases are crucial virulence factors for allergic disease elicited by diverse organisms 39, 41. Superimposition of allergic inflammation on pre-existing Th1-based disease might be expected to compound airway obstruction through multiple non-redundant mechanisms, thereby leading to clinical deterioration. In this regard, our findings of mixed Th1 and Th2 immunity in AE-COPD, may indicate the role of host antigen presenting cells in sensing the viral proteinases that can skew the immune response toward Th2. The exact mechanism involved in conditioning antigen presenting cells toward Th2 responses is currently unknown and remains an intense area of investigation.
Key Messages
COPD patients with acute respiratory failure show mixed Th1/Th2 immune responses both locally (lung) and systemically (PBMC).
Human Rhinoviruses (HRVs) are frequent isolates from the lung of those with respiratory failure.
HRV-encoded proteinase 2A activates dendritic cells to skew CD4 T cell development towards Th1 and Th2 pole.
Acknowledgments
Supported by grants to F.K (HL082487; HL72419), D.B.C (HL075243, AI057696, AI070973), National Institutes of Health (NIH), M01-RR00188, General Clinical Research Center, by contract N01-AI65298 from the NIH, the American Heart Association Fellowship to S-H.L., Alpha1-AT foundation fellowship grant to M.S. We thank all the subjects who participated in this study.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- AE
Acute exacerbation
- BAL
bronchoalveolar lavage
- HRV
human rhinovirus
- Th1
type 1 T helper
- Th2
type 2 T helper
- AHR
airway hyperreactivity
- RRS
respiratory system resistance
- OVA
chicken egg ovalbumin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Senior RM. Mechanisms of COPD: conference summary. Chest. 2000;117:320S–3S. doi: 10.1378/chest.117.5_suppl_1.320s-a. [DOI] [PubMed] [Google Scholar]
- 2.Croxton TL, Weinmann GG, Senior RM, Wise RA, Crapo JD, Buist AS. Clinical research in chronic obstructive pulmonary disease: needs and opportunities. Am J Respir Crit Care Med. 2003;167:1142–9. doi: 10.1164/rccm.200207-756WS. [DOI] [PubMed] [Google Scholar]
- 3.Connors AF, Jr, Dawson NV, Thomas C, Harrell FE, Jr, Desbiens N, Fulkerson WJ, et al. Outcomes following acute exacerbation of severe chronic obstructive lung disease. The SUPPORT investigators (Study to Understand Prognoses and Preferences for Outcomes and Risks of Treatments) Am J Respir Crit Care Med. 1996;154:959–67. doi: 10.1164/ajrccm.154.4.8887592. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:167–73. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 5.Bandi V, Jakubowycz M, Kinyon C, Mason EO, Atmar RL, Greenberg SB, et al. Infectious exacerbations of chronic obstructive pulmonary disease associated with respiratory viruses and non-typeable Haemophilus influenzae. FEMS Immunol Med Microbiol. 2003;37:69–75. doi: 10.1016/S0928-8244(03)00100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. American Journal of Respiratory & Critical Care Medicine. 2006;173:1114–21. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 7.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:13562–7. doi: 10.1073/pnas.0804181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedlander SL, Busse WW. The role of rhinovirus in asthma exacerbations. Journal of Allergy & Clinical Immunology. 2005;116:267–73. doi: 10.1016/j.jaci.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Corry DB, Kiss A, Song LZ, Song L, Xu J, Lee SH, et al. Overlapping and independent contributions of MMP2 and MMP9 to lung allergic inflammatory cell egression through decreased CC chemokines. Faseb J. 2004;18:995–7. doi: 10.1096/fj.03-1412fje. Epub 2004 Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses [see comment] Nature Immunology. 2008;9:310–8. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goswami S, Angkasekwinai P, Shan M, Greenlee KJ, Barranco WT, Polikepahad S, et al. Divergent functions for airway epithelial matrix metalloproteinase 7 and retinoic acid in experimental asthma. Nature Immunology. 2009;10:496–503. doi: 10.1038/ni.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiss A, Montes M, Susarla S, Jaensson EA, Drouin SM, Wetsel RA, et al. A new mechanism regulating the initiation of allergic airway inflammation. Journal of Allergy & Clinical Immunology. 2007;120:334–42. doi: 10.1016/j.jaci.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Lamhamedi-Cherradi S-E, Martin RE, Ito T, Kheradmand F, Corry DB, Liu Y-J, et al. Fungal proteases induce Th2 polarization through limited dendritic cell maturation and reduced production of IL-12. Journal of Immunology. 2008;180:6000–9. doi: 10.4049/jimmunol.180.9.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nolte FS, Marshall DJ, Rasberry C, Schievelbein S, Banks GG, Storch GA, et al. MultiCode-PLx system for multiplexed detection of seventeen respiratory viruses. Journal of Clinical Microbiology. 2007;45:2779–86. doi: 10.1128/JCM.00669-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandi V, Apicella MA, Mason E, Murphy TF, Siddiqi A, Atmar RL, et al. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am J Respir Crit Care Med. 2001;164:2114–9. doi: 10.1164/ajrccm.164.11.2104093. [DOI] [PubMed] [Google Scholar]
- 16.Amineva SP, Aminev AG, Palmenberg AC, Gern JE. Rhinovirus 3C protease precursors 3CD and 3CD’ localize to the nuclei of infected cells. Journal of General Virology. 2004;85:2969–79. doi: 10.1099/vir.0.80164-0. [DOI] [PubMed] [Google Scholar]
- 17.Liebig HD, Ziegler E, Yan R, Hartmuth K, Klump H, Kowalski H, et al. Purification of two picornaviral 2A proteinases: interaction with eIF-4 gamma and influence on in vitro translation. Biochemistry. 1993;32:7581–8. doi: 10.1021/bi00080a033. [DOI] [PubMed] [Google Scholar]
- 18.Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, et al. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183:109–17. doi: 10.1084/jem.183.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corry DB, Rishi K, Kanellis J, Kiss A, Song LZ, Xu J, et al. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol. 2002;3:347–53. doi: 10.1038/ni773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1:e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, et al. Rules of chemokine receptor association with T cell polarization in vivo. Journal of Clinical Investigation. 2001;108:1331–9. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–76. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 23.Deszcz L, Cencic R, Sousa C, Kuechler E, Skern T. An antiviral peptide inhibitor that is active against picornavirus 2A proteinases but not cellular caspases. Journal of Virology. 2006;80:9619–27. doi: 10.1128/JVI.00612-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kheradmand F, Rishi K, Corry DB. Environmental contributions to the allergic asthma epidemic. Environ Health Perspect. 2002;110:553–6. doi: 10.1289/ehp.02110s4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respir Care. 2003;48:1204–13. discussion 13–5. [PubMed] [Google Scholar]
- 26.Seemungal TA, Wedzicha JA. Viral infections in obstructive airway diseases. Curr Opin Pulm Med. 2003;9:111–6. doi: 10.1097/00063198-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg SB. Rhinovirus and coronavirus infections. Seminars in Respiratory & Critical Care Medicine. 2007;28:182–92. doi: 10.1055/s-2007-976490. [DOI] [PubMed] [Google Scholar]
- 28.Corry DB, Kheradmand F. Biology and therapeutic potential of the interleukin-4/interleukin-13 signaling pathway in asthma. Am J Respir Med. 2002;1:185–93. doi: 10.1007/BF03256608. [DOI] [PubMed] [Google Scholar]
- 29.Bermingham A, Henrickson K, Hayden F, Zambon M. VII International Symposium on Respiratory Viral Infections. Antiviral Therapy. 2007;12:671–93. [PubMed] [Google Scholar]
- 30.van de Stolpe A, van der Saag PT. Intercellular adhesion molecule-1. J Mol Med. 1996;74:13–33. doi: 10.1007/BF00202069. [DOI] [PubMed] [Google Scholar]
- 31.Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nature Reviews Immunology. 2009;9:377–84. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 32.Coyle AJ, Erard F, Bertrand C, Walti S, Pircher H, Le Gros G. Virus-specific CD8+ cells can switch to interleukin 5 production and induce airway eosinophilia. J Exp Med. 1995;181:1229–33. doi: 10.1084/jem.181.3.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–8. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 34.Kerstjens HA, Schouten JP, Brand PL, Schoonbrood DF, Sterk PJ, Postma DS. Importance of total serum IgE for improvement in airways hyperresponsiveness with inhaled corticosteroids in asthma and chronic obstructive pulmonary disease. The Dutch CNSLD Study Group. Am J Respir Crit Care Med. 1995;151:360–8. doi: 10.1164/ajrccm.151.2.7842192. [DOI] [PubMed] [Google Scholar]
- 35.Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, et al. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–3. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corry DB, Kheradmand F. 7. Control of allergic airway inflammation through immunomodulation. Journal of Allergy & Clinical Immunology. 2006;117:S461–4. doi: 10.1016/j.jaci.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Crowder S, Kirkegaard K. Trans-dominant inhibition of RNA viral replication can slow growth of drug-resistant viruses. [see comment] Nature Genetics. 2005;37:701–9. doi: 10.1038/ng1583. [DOI] [PubMed] [Google Scholar]
- 38.Niewoehner DE, Erbland ML, Deupree RH, Collins D, Gross NJ, Light RW, et al. Effect of systemic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. Department of Veterans Affairs Cooperative Study Group. N Engl J Med. 1999;340:1941–7. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 39.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–11. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 40.Gough L, Campbell E, Bayley D, Van Heeke G, Shakib F. Proteolytic activity of the house dust mite allergen Der p 1 enhances allergenicity in a mouse inhalation model. Clinical & Experimental Allergy. 2003;33:1159–63. doi: 10.1046/j.1365-2222.2003.01716.x. [DOI] [PubMed] [Google Scholar]
- 41.Porter P, Susarla SC, Polikepahad S, Qian Y, Hampton J, Kiss A, et al. Link Between Allergic Asthma and Airway Mucosal Infection Suggested by Proteinase-Secreting Household Fungi. Mucosal Immunol. 2009;2:504–17. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]