Abstract
Radiation therapy for head and neck cancer causes significant secondary side-effects in normal salivary glands, resulting in diminished quality of life for these individuals. Salivary glands are exquisitely sensitive to radiation and display acute and chronic responses to radiotherapy. This review will discuss clinical implications of radiosensitivity in normal salivary glands, compare animal models used to investigate radiation-induced salivary gland damage, address therapeutic advances, and project future directions in the field.
Keywords: radiation, salivary gland dysfunction, salivary glands, animal models, therapy, xerostomia
INTRODUCTION
Salivary gland function plays an important role in oral health by aiding in food digestion, protecting oral mucosa, facilitating remineralization of dental hard tissues, and moistening the palate for articulation (Grisius and Fox, 1998). Saliva is composed of water, electrolytes, proteins, and carbohydrates, which interact to accomplish diverse tasks in the oral cavity (reviewed in de Almeida et al., 2008). The major salivary glands consist of paired submandibular, sublingual, and parotid glands that work in concert with hundreds of minor salivary glands located throughout the region. Each gland has a unique combination of mucous or serous acinar cells, which are responsible for synthesizing protein components of saliva and transporting water and electrolytes (Pinkstaff, 1993). Branching ducts within major salivary glands finalize electrolyte composition of saliva and deliver it to the mouth (Pinkstaff, 1993). Submandibular glands contribute approximately two-thirds of unstimulated saliva volume, whereas parotid glands contribute the majority of stimulated saliva volume (Ship et al., 1991; de Almeida et al., 2008). The autonomic nervous system predominantly regulates salivary gland secretion and may have a role in glandular regeneration (Proctor and Carpenter, 2007). Radiation-induced xerostomia is hypothesized to be multi-factorial (Eisbruch et al., 1999), involving damage to major and minor salivary glands and associated nerves and endothelium.
CLINICAL RELEVANCE OF RADIATION-INDUCED SALIVARY GLAND DAMAGE
Each year, roughly 50,000 cases of head and neck cancer are diagnosed in the United States (St John et al., 2006). Worldwide, the problem is more significant, with head and neck cancer ranking as the 5th most common malignancy (Seiwert et al., 2007). Upon diagnosis, the standard of care is dictated by tumor stage and, for locally advanced tumors, entails surgical resection followed by radiotherapy. Due to the positioning of many oral tumors, non-diseased tissues, such as the salivary glands, are often exposed to therapeutic radiation. This results in several adverse secondary side-effects, including xerostomia, difficulty swallowing (dysphagia), oral discomfort, malnutrition, oral mucositis, changes in taste, and increased oral infections (Hancock et al., 2003; Cady, 2007; Nguyen et al., 2007). Xerostomia is the most common complication of radiotherapy for head and neck cancer (Dirix et al., 2006), and significant reductions in salivary gland function may contribute to a high frequency of mucositis and dysphagia (Kaplan et al., 2008). Destruction of the oral tissues following therapeutic radiation results in significant morbidity, diminished quality of life, and, in some cases, interruptions in treatment schedules (Malouf et al., 2003; Trotti et al., 2003; Dirix et al., 2006).
Radiosensitivity
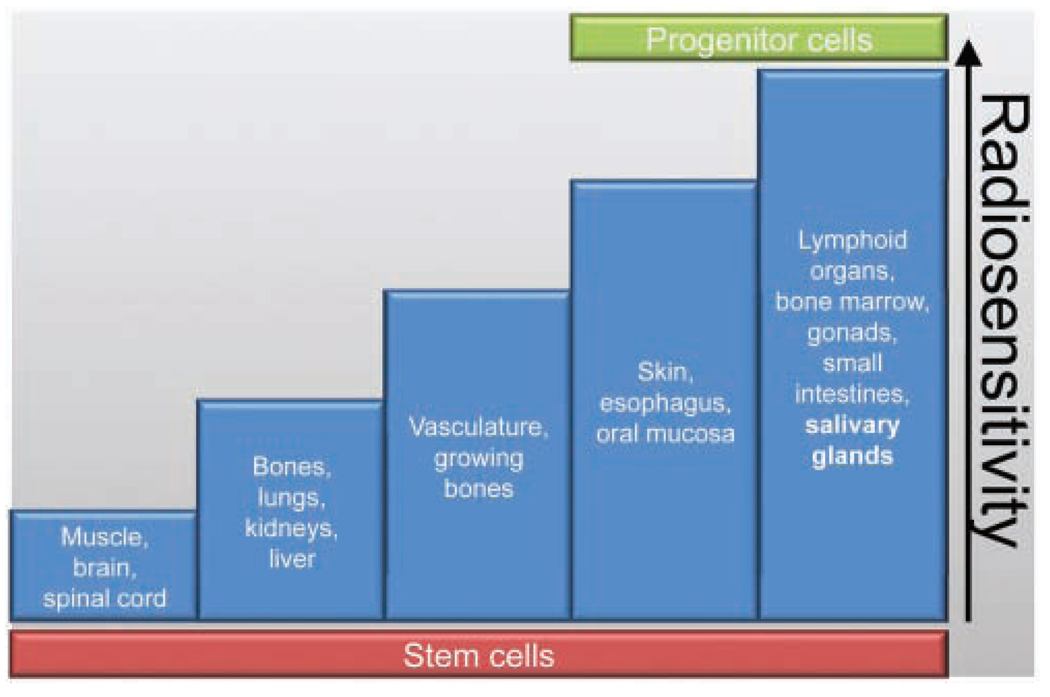
Salivary glands are exquisitely sensitive to radiation, yet, unlike classically radiosensitive tissues (Fig. 1), they proliferate slowly and are made up of highly differentiated cells (summarized in Hall, 2000). Early and late effects of radiation generally correlate with a tissue’s rate of proliferation. Early (acute) effects occur within a few days or weeks of irradiation, due to high levels of cell death. Late effects occur months or years after irradiation and may be affected by vascular damage and loss of parenchymal cells. Interestingly, there is a third type, termed ‘consequential late effects’, which are hypothesized to result from persistent severe early effects (summarized in Hall, 2000).
Figure 1.
Radiosensitivity of various tissues. Radiosensitivity of different tissues classified by early cell death and proposed distribution of stem and progenitor cells. Modified from Rubin and Casarett, 1968 (with permission).
Acute and Chronic Response in Affected Individuals
Affected individuals display a 50–60% loss of salivary flow within the first week of radiotherapy (Henson et al., 1999; Eisbruch et al., 2001; Dirix et al., 2006). The degree of salivary gland hypofunction (% of baseline) correlates with lower quality of life, as assessed by questionnaires (Malouf et al., 2003). Loss of acinar cells and glandular shrinkage also occurs during the acute phase (Robar et al., 2007; Hoebers et al., 2008), which affects the composition and volume of saliva (Henson et al., 1999; Dirix et al., 2006). The acute reduction in saliva flow and changes in saliva composition have been attributed to an impairment of the gland tissue to produce sufficient saliva volume, as well as to reduced secretion of certain components of normal saliva (Makkonen et al., 1986). Within three weeks of radiotherapy, a majority of individuals present with mucositis, which can be life-threatening (Hancock et al., 2003). Many studies have suggested that chronic effects of radiation may be the consequence of acute damage to salivary glands (Stephens et al., 1986b; Li et al., 2007). Chronically, affected individuals continue to display significant decreases in unstimulated and stimulated salivary flow for several months or years following radiotherapy (Eisbruch et al., 2001; Dirix et al., 2006; Li et al., 2007). In a subset of persons whose salivary glands received lower doses of radiation (< 25 Gy), there is recovery of salivary function within 12–24 months (Braam et al., 2006; Dirix et al., 2006; Li et al., 2007). However, many individuals have permanent salivary gland hypofunction (Li et al., 2007), which has been attributed to attrition of acinar cells followed by replacement with fibrotic tissue (Radfar and Sirois, 2003). Due to the chronic loss of salivary function, long-term dietary adaptations are required if the individuals are to maintain adequate nutrition and hydration (Hancock et al., 2003; Cady, 2007), which have a significant impact on their quality of life.
Improvements in Radiation Physics
In recent years, tremendous improvements have been made in targeting radiation to spare surrounding tissues. One of these technologies, intensity modulated radiotherapy (IMRT), allows for maximal treatment of a tumor while sparing normal tissues and reducing side-effects (Braam et al., 2006). However, one study evaluated tumor recurrence in three persons who underwent salivary gland sparing close to the treatment site and concluded that caution should be used when delineating treatment parameters (Cannon and Lee, 2008). Alternatively, hyperfractionated radiotherapy, accelerated fractionated radiotherapy, or combined chemoradiotherapy has been used to treat head and neck cancer, with side-effects similar to those associated with radiotherapy alone (Seiwert and Cohen, 2005; St John et al., 2006). The major limitations of these technologies include availability of equipment, distance to experienced treatment centers, tumor location in relation to other tissues, and anatomic changes that occur during treatment (St John et al., 2006; Robar et al., 2007; Seiwert et al., 2007). Solutions will likely involve both novel biological interventions for sparing normal tissues and improved technology.
Radiation Dose Delivered to Salivary Glands
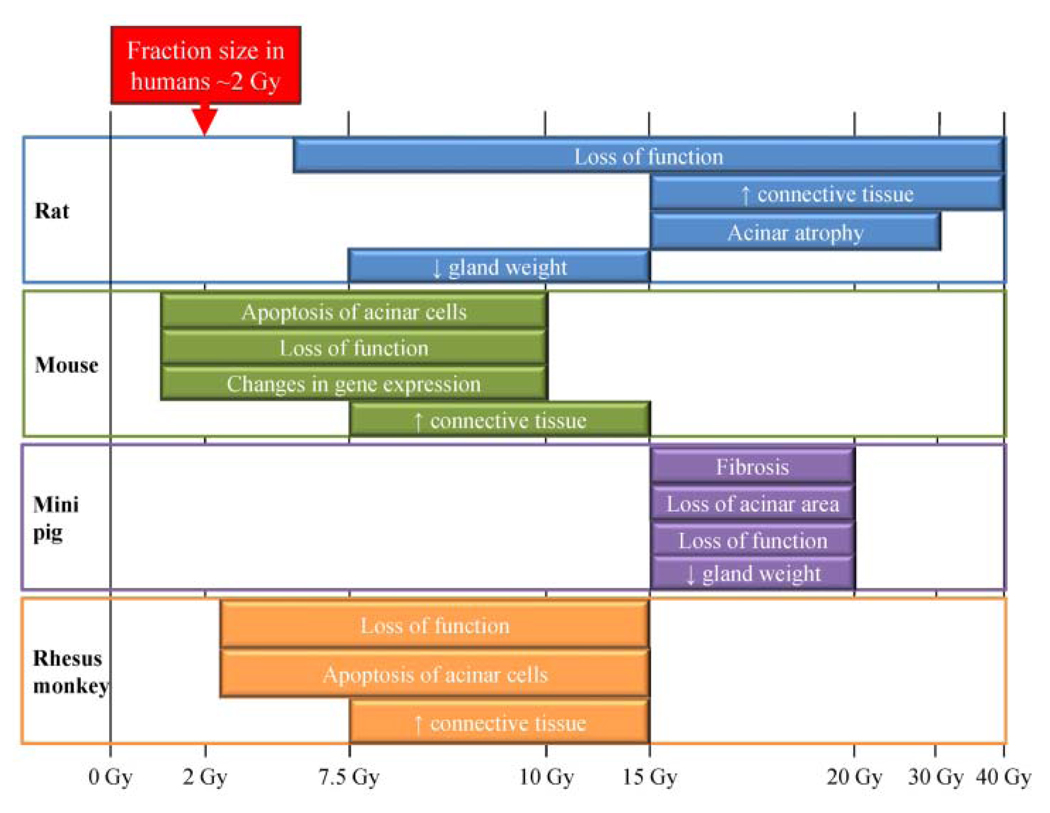
Numerous studies have defined maximal dose calculations for salivary gland exposure to minimize side-effects. Clinically, radiation exposure of parotid salivary glands is kept below 2 Gy/day and a cumulative dose of 24–26 Gy, to allow for recovery of salivary function (Eisbruch et al., 1999; Li et al., 2007). In general, there does not seem to be compensation by the contralateral gland to improve total saliva production (Li et al., 2007). Using planar salivary gland scintigraphy combined with single-photon-emission computed tomography (SPECT), Bussels et al. have determined the amount of salivary excretion fraction (SEF) lost in different anatomical slices within the parotid gland following conformal radiotherapy (Bussels et al., 2004). They estimated that the mean dose resulting in 50% loss of salivary excretion fraction (dSEF) was 22.5 Gy. Interestingly, in 7 out of 15 individuals, there was a significant loss of function at lower mean doses (10–15 Gy), which corresponded to the caudal part of the spared parotid gland (Bussels et al., 2004). Maximum cumulative exposure of the submandibular glands has recently been set at 39 Gy (Murdoch-Kinch et al., 2008), suggesting that these glands may be less radiosensitive than parotid glands. It should be noted that radiation doses are delivered 5 days/week over a total period of 35–50 days (Henson et al., 1999; Braam et al., 2006; Li et al., 2007); therefore, a dose of 26 Gy delivered to the salivary glands over multiple fractions is not equivalent to a single megadose of 26 Gy (Radfar and Sirois, 2003). This is an important consideration when the different animal models are evaluated, since the range of radiation doses used in these models varies drastically (summarized in Fig. 2).
Figure 2.
Comparison of radiation responses in animal models following a single dose. The typical fraction size (~ 2 Gy) in affected individuals is provided at the top as a reference for the other models. Physiological, molecular, and histological changes are graphed relative to the single radiation dose evaluated in the rat, mouse, miniature pig, and rhesus monkey models (Stephens et al., 1986a,b, 1991; Vissink et al., 1990; Nagler, 1998; Nagler et al., 1998; Paardekooper et al., 1998; Bralic et al., 2005; Konings et al., 2005a, 2006; Li et al., 2005; Humphries et al., 2006; Limesand et al., 2006; Muhvic-Urek et al., 2006;Takakura et al., 2007; Avila et al., 2009).
MODELS OF RADIATION-INDUCED SALIVARY GLAND DAMAGE
Elucidation of potential mechanisms underlying salivary gland radiosensitivity has focused on functional animal studies and, in recent years, on molecular approaches. While it is perceivable that no animal model will directly recapitulate human biology, significant advancements in our understanding of radiation-induced DNA damage to the salivary glands have been discovered through these models. This section serves to define and compare data gathered from the 4 major models: rat, mouse, miniature pig, and rhesus monkey.
Acute Physiological Response following Single-dose Irradiation
The most consistent observations in all animal models have been significant reductions in flow rate, loss of glandular weight, and loss of acinar area (Stephens et al., 1986b; Vissink et al., 1990; Nagler et al., 1998; Li et al., 2005). For example, studies in rats reported a 40% reduction in salivary flow rates with single doses of 5 or 10 Gy, and ~ 60% reduction following 15 or 20 Gy three days after treatment (Vissink et al., 1990). The major difference between the models involves the radiation dose administered for a significant loss of function to be observed (Fig. 2). Studies in rats have used a singledose range of 5–40 Gy (Vissink et al., 1990; Nagler et al., 1998; Konings et al., 2005a, 2006) compared with 1–15 Gy in the mouse, 2.5–15 Gy in the rhesus monkey, and 15–20 Gy in the miniature pig (Stephens et al., 1986b; Bralic et al., 2005; Li et al., 2005; Humphries et al., 2006; Limesand et al., 2006; Muhvic-Urek et al., 2006). Loss of serous acinar cells has been linked to reductions in salivary flow, since these cells account for about 80% of parotid gland volume and are responsible for water and protein secretion (Stephens et al., 1986a; Turner, 1993).
Chronic Physiological Response following Single-dose Irradiation
Persistent reductions in salivary flow rate, which suggest lifelong glandular dysfunction, have also been reported in all models (Stephens et al., 1986a; Nagler, 1998; Nagler et al., 1998; Li et al., 2005; Konings et al., 2006; Takakura et al., 2007). For example, single doses of 15 or 20 Gy to miniature pigs resulted in a 50% decrease in parotid flow rates after 16 wks and ~ 50% reduction in acinar cell area at 4 and 16 wks post-irradiation (Li et al., 2005). Another study in rats reported a higher loss of acinar cells when the cranial part of the gland was irradiated vs. the caudal part, which was replaced by connective tissue 1 yr after exposure to a single 30-Gy dose (Konings et al., 2006). In general, chronic dysfunction has been attributed to loss or impairment of serous acinar cells and replacement by connective tissue and fibrosis (Stephens et al., 1986b; Li et al., 2005; Muhvic-Urek et al., 2006). Many studies have reported significant acinar cell atrophy at chronic time-points (Stephens et al., 1986b; Nagler, 1998; Li et al., 2005; Muhvic-Urek et al., 2006), which has been postulated to be due to lack of regeneration (Stephens et al., 1986a). Inflammatory infiltration has also been observed in miniature pig and rhesus monkey models (Stephens et al., 1986b; Li et al., 2005), but not consistently in rodent models, which may indicate species differences in the responses of salivary glands to radiation (Nagler et al., 1998; Nagler, 1998; O’Connell et al., 1999b; Muhvic-Urek et al., 2006).
Radiation Targeting
It has been proposed that radiation delivered to the entire head and neck region of the rat results in indirect damage to the salivary glands, and studies should be designed targeting radiation only to the parotid and submandibular glands (Konings et al., 2005b). The authors ascertained that irradiation of non-salivary gland tissue within a head and neck treatment portal can influence radiation-induced reductions in salivary function. Importantly in affected individuals, the head and neck tumor is the primary direct target, and salivary glands are indirect targets. The functioning of the parotid and submandibular glands is only one factor contributing to the symptom of xerostomia (Kaplan et al., 2008). Therefore, studies with head and neck treatment portals in animal models provide vital information on the potential interplay between salivary and non-salivary gland tissue as it relates to radiation-induced loss of function and evaluation of protective therapies.
Fractionated Radiation
In contrast to single megadoses of 15–40 Gy reported in many studies, fractionated radiation schedules resemble clinical practice. Following a clinically relevant fractionated radiation schedule (30 × 2 Gy over 6 wks, cumulative dose of 60 Gy), there is a progressive deterioration of function, loss of serous acinar cells, and development of fibrosis and inflammation 6 mos following radiation to the head and neck in rats (Friedrich et al., 2002; Sagowski et al., 2003). Another study in miniature pigs (Radfar and Sirois, 2003) found significant reductions in flow rates, with pronounced acinar atrophy, fibrosis, and parenchymal loss that could be detected as early as 30 days after a fractionated radiation treatment (35×2 Gy, cumulative dose of 70 Gy).
The most consistent observations in all animal models have been significant reductions in flow rate, loss of glandular weight, and loss of acinar area or cells. However, not all animal models have been described in the same amount of detail (i.e., methods, radiation doses, time-points, etc.), so it is premature to decide the best animal model. Our ability to decipher mechanisms involved in salivary gland dysfunction is limited in fractionated radiation studies; therefore, studies using an appropriate single therapeutic dose of radiation can contribute greatly.
MECHANISMS OF SENSITIVITY
The complexity of salivary gland morphology suggests involvement of multiple pathways leading to dysfunction following irradiation. Therefore, stable cell lines and primary or organ cultures from salivary glands are valuable tools for uncovering regulatory events in the cellular response of salivary acinar cells to radiation (Stephens et al., 1989; O’Connell et al., 1998; Limesand et al., 2003b, 2006). In recent years, several genetically engineered mouse models have been evaluated, which provide considerable insight into the molecules involved.
Cellular Attrition
Three major pathways are involved in maintaining tissue homeostasis via cell death: apoptosis, necrosis, and autophagy. Apoptosis is a highly conserved, tightly regulated process that has been defined morphologically and biochemically (Riedl and Shi, 2004). Many methods for the detection of apoptotic cells have been reported, based on early (activation of caspase-3, keratin 18 cleavage) or late events (nuclear condensation, DNA fragmentation) in the pathway (reviewed in Krysko et al., 2008). It has been noted in some tissues that apoptotic cells are usually taken up by phagocytes before chromosomal fragmentation (Savill et al., 1993). In contrast to apoptosis, no standard biochemical markers exist for necrosis, but rather the absence of apoptotic markers, together with morphological changes detected by electron microscopy, is indicative of necrosis. Autophagy is characterized by the presence of double-membrane autophagic vacuoles, detected by transmission electron microscopy, or lipidation of LC3, as detected by immunofluorescence (Martinet et al., 2006). An alternative method of autophagy detection is quantification of LC3-II processing by Western blot (Ogata et al., 2006); however, this would not provide cell-type-specific information. Depending on the radiation dose, it is likely that all 3 modes of cell death are present and not mutually exclusive. Although apoptosis has been studied to some extent in the salivary glands, contributions from necrosis and autophagy to radio-sensitivity and persistent loss of function in salivary glands remain elusive.
The major cause for significant acinar cell loss across animal models following irradiation (Fig. 2) has been widely debated. Earlier work in the rat quantified radiation-induced apoptosis by counting condensed nuclei and reported 2–3% apoptotic cells 6 hrs after treatment within a broad range of doses (2.5–25 Gy) (Paardekooper et al., 1998). The extent of apoptosis was not dose-dependent, and the authors concluded that the magnitude of apoptosis could not explain the significant loss of function (Paardekooper et al., 1998). In contrast to rats, radiation-induced apoptosis is dose-dependent in parotid glands of mice, with significantly higher levels detected by immunohistochemistry against activated caspase-3 (Humphries et al., 2006; Limesand et al., 2006). Twenty-four hours after radiation exposure, mouse parotid glands have ~ 30% apoptosis after a single 5-Gy exposure, compared with ~ 15% after a single 1-Gy dose (Humphries et al., 2006; Limesand et al., 2006). One group reported that 5–8% of murine submandibular acinar cells are apoptotic 3 days after 7.5 and 15 Gy (Bralic et al., 2005), while another group observed only 2% apoptosis 24 hrs after 5 Gy (Humphries et al., 2006). In rhesus monkeys, loss of acinar cells in the first 24 hrs is also dose-dependent (range, 2.5 to 15 Gy) and attributed to interphase cell death caused by apoptosis (Stephens et al., 1991). While decreases in acinar cell area have been reported in miniature pigs (Li et al., 2005), the mode of cell death was not reported.
Considerable debate has emerged regarding the ratio of acinar cells lost following radiation and the extent of salivary gland dysfunction (Konings et al., 2005b; Limesand et al., 2006). Logically, there are at least two possible discussion positions. The first position maintains a 1:1 ratio in the percentage of acinar cells lost and percent decrease in function. This position relies on the assumption that all acinar cells contribute equally to saliva production. The second position maintains that a certain percentage of cells are lost, and these cells affect the function of the cells around them. For example, in the process of activating a cell death program, factors could be secreted that communicate an adverse environment to the surrounding cells (Gudkov and Komarova, 2003). In this case, a low percentage of acinar cells lost acutely could signal surrounding cells to decrease function that potentially contributes to a chronic 50% loss of function. At this point, there is no definitive evidence for either position, further emphasizing the need for molecular and biochemical studies on the mechanisms of radiosensitivity in salivary glands.
Signaling Pathways Involved in Apoptosis of Salivary Acinar Cells
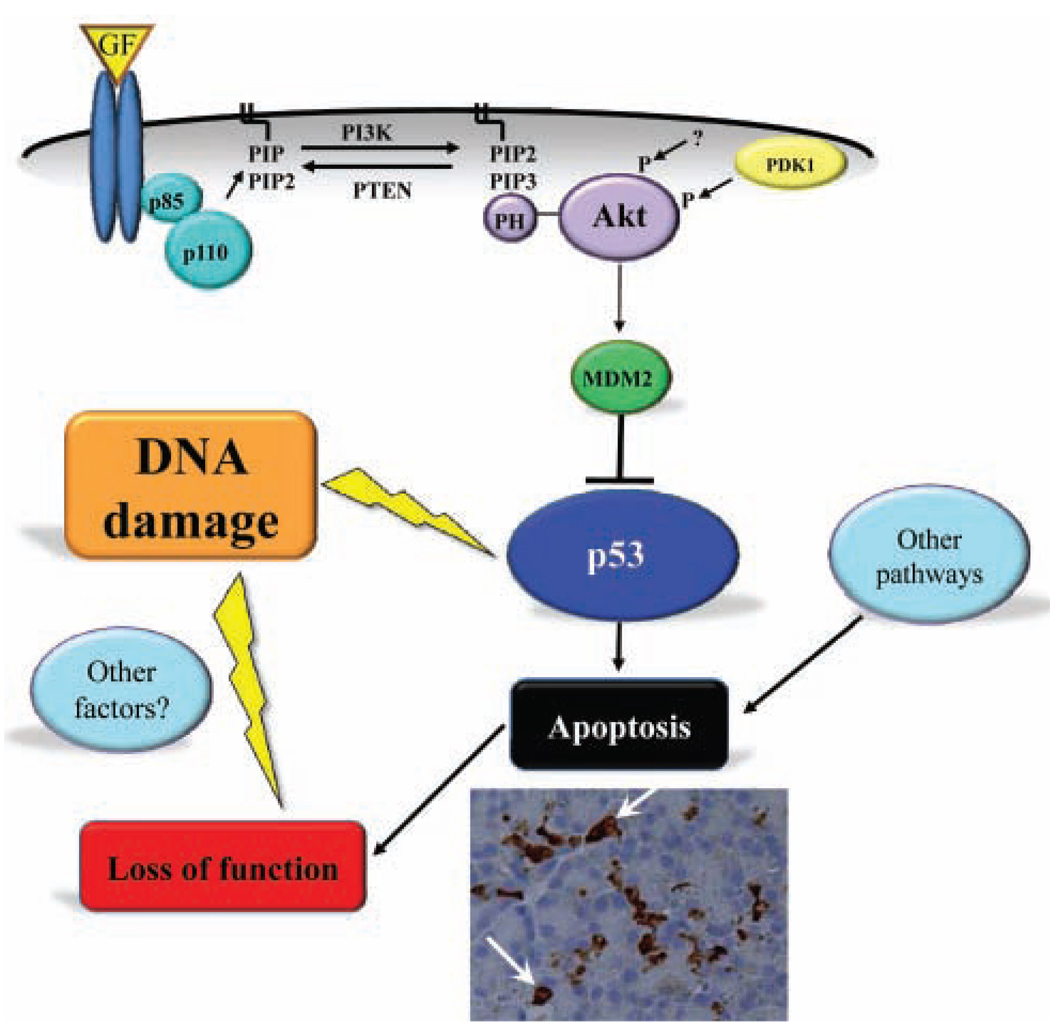
Although many intracellular signaling steps in the regulation of apoptosis are similar, there are cell-type- and tissue-specific differences that may, in part, account for the radiosensitivity of salivary glands. An important molecule that regulates radiosensitivity in other tissues is p53. Classically, p53 is known as a tumor suppressor, due to its response to oncogene activation (Horn and Vousden, 2007). It is intricately involved in regulating DNA damage repair, cell cycle arrest, and apoptosis (Riley et al., 2008). Ionizing radiation (5 Gy) induced p53 transcriptional activation and apoptosis of mouse salivary acinar cells in vitro and in vivo (Limesand et al., 2006). In transgenic mice expressing a constitutively activated mutant of the anti-apoptotic kinase Akt (myr-Akt1), p53 activation was reduced and the induction of apoptosis was abrogated in vitro and in vivo (Limesand et al., 2006). This negative regulation of p53 protein levels by myr-Akt1 was mediated via the activation of Murine Double Minute Clone 2 (MDM2) (Limesand et al., 2006) (Fig. 3), which binds to p53 and targets it for degradation by the proteasome (Ogawara et al., 2002). These findings are supported by a recent investigation from our research group in irradiated p53-null mice, which had no apoptosis and preserved salivary flow 3 and 30 days following a single dose of 2 or 5 Gy (Avila et al., 2009). These studies suggest that in vivo p53 expression is required for acute and chronic salivary gland dysfunction following irradiation.
Figure 3.
Putative signaling pathway involved in radiation-induced salivary gland dysfunction. In general, activation of Akt occurs through the binding of growth factors (GF) to their specific receptor (reviewed in (Manning and Cantley, 2007) and, in salivary acinar cells, requires the activation of PI-3 kinase (combined p85 and p110 subunits) (Limesand et al., 2003a). An important molecule that regulates the DNA damage response is p53, and negative regulation of p53 protein levels by Akt is mediated via activation of the Murine Double Minute Clone 2 (MDM2). p53 activation following radiation leads to apoptosis of salivary acinar cells (arrows mark activated caspase-3-positive cells) and subsequent loss of function. It is probable that other pathways (right-side icon) regulating the apoptotic response, discussed in the section “Other Pathways Involved in Radiation Damage”, could play a role in radiation-induced salivary gland dysfunction. In addition, other factors (left-side icon), such as autophagy, necrosis, senescence, etc., could be involved in DNA damage, leading to loss of function.
Other Pathways Involved in Radiation Damage to Salivary Acinar Cells
Potentially, multiple pathways could lead to salivary gland dysfunction following irradiation (Fig. 3). Other relevant pathways in the salivary glands reported in the literature include: de-granulation hypothesis, aquaporin expression, calcium signaling, and protein kinase C δ (PKCδ) regulation of apoptosis. An earlier hypothesis suggested that the secretory granules of acinar cells are damaged by radiation-induced lipid peroxidation, which leads to the generation of free radicals and lysis of these cells (Nagler et al., 1997). While the administration of muscarinic or adrenergic agonists, which trigger degranulation of secretory cells, prior to radiation (15 Gy) has been shown to maintain partial salivary flow in rats and mice (Nagler et al., 1997; Nagler and Laufer, 1998; Coppes et al., 2000; Takakura et al., 2007), not all clinical studies have corroborated these effects (Roesink et al., 2004). Down-regulation of aquaporin-5 (AQP5), a water channel present on the apical membrane of acinar cells, has been observed in rat submandibular glands on days 3 and 30 after a single 15-Gy dose (Li et al., 2006). Restoration of AQP5 levels in mouse submandibular acinar cells, with the oral administration of the muscarinic agonist cevimeline, prior to a single dose of 15 Gy resulted in partial preservation of salivary flow rates up to day 28 in vivo (Takakura et al., 2007). Reports on the impairment of calcium signaling following irradiation have been inconsistent; therefore, it is unclear whether it has a role in chronic loss of function (O’Connell et al., 1998, 1999b; Coppes et al., 2005). Expression of PKCδ has been shown to regulate apoptosis in salivary acinar cells (reviewed in Reyland, 2007), and PKCδ-deficient mice exhibited significantly lower levels of radiation-induced apoptosis (1 and 5 Gy) (Humphries et al., 2006). Since the response of the salivary glands to irradiation is complex and presumably multi-factorial, it will be important to integrate these pathways with each other and with functional studies by therapeutic doses of radiation.
TREATING RADIATION-INDUCED XEROSTOMIA
Some therapies for radiation-induced xerostomia are designed to protect salivary glands during radiotherapy. Some are intended to provide temporary relief of symptoms. Others are aimed at restoring function to previously damaged glands. It is difficult to say that any one of these approaches is better than another. Certainly, outright prevention is a lofty goal, but for more than 500,000 (Jemal et al., 2008) people in the U.S. who have undergone treatment for head and neck cancer, palliative and restorative therapies are equally important. In this section, we provide an overview of available treatments and promising new leads in each of these treatment categories.
PROTECTIVE THERAPIES
Amifostine and Tempol
During the 1950s, the U.S. Army developed the prodrug, amifostine, to protect soldiers in case of nuclear attack. Amifostine is dephosphorylated by alkaline phosphatase, yielding an active free thiol that can scavenge free radicals and limit indirect damage by ionizing radiation. Studies have indicated that accumulation of the active metabolite of amifostine, WR-1065, is selective to normal tissues including the salivary glands (Utley et al., 1976), which may be due to lower alkaline phosphatase activity in tumor vasculature than in normal vasculature (Calabro-Jones et al., 1985). In 1999, a phase III clinical trial examining the radioprotective effects of amifostine on salivary glands led the FDA to approve it as an agent for preventing radiation-induced xerostomia (Brizel et al., 2000). The study reported that amifostine administered intravenously 15 to 30 min prior to doses of fractionated radiation (~ 2 Gy/day; cumulative dose of 50–70 Gy) reduced the occurrence of acute xerostomia (grade ≥ 2) from 78% to 51%, and the occurrence of chronic xerostomia (grade ≥ 2), 1 yr after treatment, from 57% to 38%. Persons receiving amifostine also have a reduced caries incidence (Rudat et al., 2000), which is the most costly complication of salivary gland hypofunction. Importantly, overall survival after 2 yrs was not significantly affected by amifostine—a concern for any radioprotective therapy (Brizel et al., 2000). A later phase III trial ended after 41% of individuals discontinued amifostine due to severe side-effects, including hypotension, vomiting, and allergic reaction (Rades et al., 2004). After a review of several studies using a range of doses, the authors concluded that roughly 25% of persons receiving intravenous injections of amifostine discontinue treatment. It has been suggested, however, that subcutaneous injection of amifostine may reduce toxicity (Koukourakis et al., 2000; Anne, 2002).
Due to high toxicity and claims that it may protect tumors (Lindegaard and Grau, 2000), others have begun looking at alternatives to amifostine. One alternative is the nitroxide tempol. In a recent study with fractionated radiation (6 Gy/day for 5 days), mice were administered tempol (i.p. or topical) 10 min prior to each dose (Cotrim et al., 2007a). After 8 wks, these mice had significantly higher levels of stimulated salivary flow than mice treated with radiation alone. Preliminary results are promising, and tempol may soon be ready for clinical trials.
Growth Factors
Recent studies have indicated growth factors’ potential use as radioprotectants. These endocrine proteins activate cellular signaling pathways promoting cell survival, DNA repair, and growth. Our own work focuses on survival signals modulated by the protein kinase Akt (Fig. 3). One study indicated that insulinlike growth factor (IGF1) is a potent activator of Akt in salivary acinar cells cultured from rat parotid glands (Limesand et al., 2003a). In these cells, as well as primary parotid acinar cells from mice, stimulation with IGF1 prior to etoposide treatment resulted in an Akt-dependent reduction of apoptosis (Limesand et al., 2003a). In another study, constitutive activation of Akt1 in primary murine salivary acinar cells resulted in reduced apoptosis following treatment with ionizing radiation (Limesand et al., 2006). Importantly, activation of Akt1 through transgenic mice or intravenous injections of IGF1 completely prevented radiation-induced salivary gland hypofunction at acute and chronic time-points (Limesand et al., 2009). Currently, we are pursuing targets downstream of Akt that may be manipulated clinically to protect salivary glands from ionizing radiation.
One growth factor that is currently undergoing clinical trials for the prevention of radiation-induced xerostomia is keratinocyte growth factor (KGF). Unfortunately, a recent phase II trial of recombinant human KGF (palifermin) had mixed results (Brizel et al., 2008). In persons receiving standard fractionated radiotherapy (2 Gy/day; cumulative dose of 70 Gy), palifermin provided no protection against xerostomia (grade ≥ 2) up to 12 wks post-treatment. In those receiving hyper-fractionated doses of radiation (1.25 Gy twice per day to a cumulative dose of 72 Gy), however, palifermin seemed to offer some protection, although the results were not significant. Palifermin did not affect five-year survival in either group.
Recently, two novel methods have been proposed for the delivery of growth factors to salivary glands prior to irradiation. One study showed that rat cells treated with basic fibroblast growth factor (bFGF) 4 hrs prior to a single dose of radiation have a 44% reduction in apoptosis (Thula et al., 2005). Importantly, the study demonstrated that polymer spheres loaded with bFGF can be used for the delayed release of growth factor over 28 days—roughly the length of a radiotherapy regimen. Another proposed mechanism for the delivery of growth factors is by gene transfer with adenoviral vectors (Cotrim et al., 2007b). One study proposed that salivary gland dysfunction results from radiation-induced loss of microvasculature in salivary glands (Cotrim et al., 2007b). To test this, the investigators administered adenoviruses expressing bFGF (AdbFGF) or vascular endothelial growth factor (AdVEGF) via cannulation to the submandibular glands of mice 48 hrs prior to irradiation (15 Gy). Microvascular density of the gland assessed 4 hrs post-treatment was reduced by 50% in control mice, but by only 20% in mice treated with either AdbFGF or AdVEGF. These results corresponded with similar improvements in salivary flow rates measured after 8 wks. One concern with adenoviral-mediated gene transfer is expression of the transgene outside of target cells or tissues. However, ductal cannulation results in delivery of the vector directly to the salivary glands. One group suggests that salivary glands are well-encapsulated, thereby limiting spread of a viral vector (Baum et al., 2006). Also, it is possible to target adenoviral vectors specifically to one type of salivary cell (Zheng et al., 2001).
While work in this area has provided very promising leads, one drawback for most growth factors is the lack of specificity for normal tissues required for clinical use. Understanding survival signaling, however, may allow us to uncover an effector molecule that can be specifically modulated in affected individuals to protect normal tissues such as salivary glands.
PALLIATIVE THERAPIES
Generally, palliative treatments for radiation-induced xerostomia are muscarinic-cholinergic agonists intended to stimulate secretion from remaining salivary cells or the use of artificial saliva and mouth moisturizers. One such drug, pilocarpine, had been approved by the FDA for this purpose. Another, cevimeline, which is already approved for Sjögren’s syndrome, has undergone open-label studies for use in affected individuals following radiotherapy (Chambers et al., 2007a,b). Both drugs improve salivary flow, but are fairly short-lived and, due to a non-specific mechanism of action, can cause a variety of side-effects, including nausea, diarrhea, and excessive sweating. Overall, these treatments are not well-suited for long-term treatment; thus, an emphasis has been placed on restorative therapies.
RESTORATIVE THERAPIES
Gene Transfer
A recent review describes a clinical trial for the use of adenoviral-mediated gene transfer in treating persons with chronic radiation-induced xerostomia (Baum et al., 2006). The authors suggest that water is the crucial component protecting the upper GI tract. Therefore, they propose that increasing the water permeability of ductal cells that remain following radiotherapy may alleviate the symptoms of chronic xerostomia. To achieve this, they plan to deliver an adenoviral vector expressing the water channel protein human aquaporin-1 (AdhAQP1) to salivary glands via ductal cannulation. AQP1 was chosen because, unlike other aquaporins, it is not specific to basolateral or apical sides of the cell, thus allowing for an overall increase in cellular permeabilization.
This approach has been tested extensively in vivo. In one study, ductal cannulation of AdhAQP1 to the submandibular glands of rats resulted in a roughly five-fold increase in AQP1 present in membranes throughout the gland (Delporte et al., 1997). Four months after receiving a single dose of radiation (21 Gy), rats were infected with AdhAQP1 or a control vector, and salivary secretion was measured 3 days later. Rats treated with AdhAQP1 had salivary flow rates 2 to 3 times higher than those of rats treated with the control vector (Delporte et al., 1997). A similar study in miniature pigs, with a single dose (20 Gy) of radiation targeted to 1 parotid gland, reported that delivery of AdhAQP1 after 17 wks resulted in recovery of parotid flow to roughly 80% of pre-irradiation values, vs. 20% in animals receiving a control vector (Shan et al., 2005). Unfortunately, another study showed post-irradiation salivary flow improvements in only 2 of 3 rhesus monkeys treated (O’Connell et al., 1999a).
It is important to note that adenoviral expression of the hAQP1 transgene is transient. Recovery of parotid flow by AdAQP1 in miniature pigs fell from roughly 80% of pre-irradiation values 3 days after vector delivery to roughly 69% of pre-irradiation values 7 days after vector delivery (Shan et al., 2005). To address this issue, a recent study demonstrated that an adenoviral vector containing specific retroviral elements resulted in gene expression for at least 2 mos when delivered via ductal cannulation to rat subman-dibular glands (Zheng et al., 2008). While adenoviral gene therapy has had several experimental set-backs and may still have issues with the host immune response, there is still some optimism that it is a viable therapeutic option (Cotrim and Baum, 2008).
Artificial Salivary Gland
To benefit from gene therapy, individuals suffering from xerostomia must have some intact salivary tissue. Unfortunately, one group suggests that this therapeutic option is limited because of the massive amounts of fibrosis seen in salivary glands after radiotherapy. To address this issue, they have begun developing an artificial salivary gland (Tran et al., 2006). Their design consists of a biodegradable polymer tube covered with an extracellular matrix protein, such as collagen, on which a monolayer of polarized epithelial cells can be grown. Recently, it has been demonstrated that primary cells from rhesus monkey parotid glands can proliferate on a poly-L-lactic acid membrane coated with collagen (Tran et al., 2006). These cells, which appear to be ductal, are correctly polarized and can limit fluid movement from the basal to the apical surface. When transduced with an adeno-associated virus expressing aquaporin-1 (AAV2-hAQP1), roughly 9% of the cells became positive for AQP1 within 72 hrs, allowing for a six-fold increase in fluid movement. The percentage of transduced cells was low, and there are questions about whether these channels alone will be enough to establish proper osmotic gradients for secretion; nevertheless, the work is promising.
Stem Cell Transplantation
It has been proposed that the loss of salivary function post-irradiation is due to attrition of the salivary stem cells necessary for maintaining a healthy gland (Konings et al., 2005b). Based on this hypothesis, a 2008 study revealed that salivary stem cell transplantation post-irradiation can rescue glandular function (Lombaert et al., 2008). To isolate putative stem cells, murine submandibular glands were digested enzymatically, and cells were cultured in vitro. After 2 days, mucin-containing acinar cells were undetectable in culture. In time, many of the remaining ductal-like cells differentiated, and the culture was repopulated by acinar cells. Indeed, these ductal cells stained positive for several stem cell markers, supporting previous claims that salivary stem cells reside in the ductal compartment (Denny and Denny, 1999; Man et al., 2001).
To determine whether cultured stem cells could re-populate a damaged gland, a group of investigators grew salivary stem cells from male mice in culture for 3 days and injected them into the submandibular glands of female mice 30 days post-irradiation (15 Gy) (Lombaert et al., 2008). Remarkably, after 90 days, the glands of these mice were re-populated by donor-derived proliferating acinar cells, as determined by the presence of the Y chromosome. These mice also exhibited a marked recovery of salivary flow at the same time-point, demonstrating the first use of transferring salivary specific cells to restore glandular function.
While this work is exciting, there are still obstacles. For instance, the authors propose using autologous salivary stem cells to re-populate the glands of affected individuals after radiotherapy. Unfortunately, they show that these cells lost expression of stem cell markers after 3 days in culture, which would diminish their use after the ~ 30-day radiotherapy regimen. It is clear that the next step involves the development of methods for maintaining the pluripotency of these cells in culture.
FUTURE DIRECTIONS
In the past few years, a wealth of research has been conducted that has improved our understanding of radiation-induced salivary gland dysfunction and affected the development of new treatment strategies. The renewed national interest in quality-of-life issues coincides perfectly with the future direction of this field.
One major obstacle in the field has been identification of the salivary stem cell. Radiosensitivity of stem cells has been evaluated in numerous other systems, including cancer, intestine, bone marrow, mammary gland, and skin, with no clear consensus, since both radiosensitive and radioresistant populations have been described (Potten, 2004; Rieger et al., 2005; Zhao et al., 2005; Bao et al., 2006; Woodward et al., 2007) (Fig. 1). Rachidi et al. evaluated the radiosensitivity of keratinocyte stem cells and their direct progeny progenitor cells (Rachidi et al., 2007). Interestingly, the stem cells were radioresistent, and the progenitor cells were radiosensitive (Fig. 1). The radioresistence of the keratinocyte stem cells was correlated with the down-regulation of apoptosis and cell-death-related genes following a therapeutic dose of 2 Gy. This dichotomy of radiosensitivity within a particular tissue may have applicability to the salivary glands as well. Identification of stem cells or progenitor cells within the salivary gland and their fate following radiotherapy directly influences the type of therapy that could be beneficial.
This path to improved care for the secondary side-effects of radiotherapy on salivary glands will no doubt be challenging, and several different approaches to success could be envisioned. Importantly, new biological interventions will need to be paired with new technological advances to accomplish this goal. Advances in other areas of dental research—salivary diagnostics and nanoparticles, for instance—could be incorporated into investigations on radiation-induced injury to the salivary glands. It is also possible that collaborations with other areas of medicine could generate useful outside perspectives on this problem and may demonstrate that ideas discussed in this section are far from exhaustive. Although precise mechanisms for the exquisite sensitivity of salivary glands to therapeutic radiation have yet to be completely elucidated, important advances have improved our understanding of the pathways involved and provided substantial optimism for future studies.
SUMMARY
Salivary glands are exquisitely sensitive to radiation and display acute and chronic responses to radiotherapy. Maximum cumulative exposure to the parotid and submandibular glands in affected individuals has been set at 24–26 Gy and 39 Gy, respectively, to limit sideeffects. In recent years, elucidation of the potential mechanisms underlying salivary gland radiosensitivity has been approached by functional animal studies as well as from a molecular perspective. The most consistent observations in all animal models have been significant reductions in flow rate, loss of glandular weight, and loss of acinar area or cells. However, the major difference among the models is the radiation dose necessary for significant loss of function to be observed. While several pathways may be involved in the radiosensitivity of salivary glands, studies suggest that p53 expression plays a major role in acute and chronic salivary gland dysfunction following irradiation. Some of the most exciting advances in therapeutic options involve preventive measures to preserve salivary function and restore function to previously damaged glands. Future directions in molecular mechanisms and stem cell biology might lead to new therapeutic interventions to improve the quality of life for persons undergoing radiation therapy for head and neck malignancies.
ACKNOWLEDGMENTS
The authors thank Drs. Randy Burd (University of Arizona), Nam Nguyen (University of Arizona), and Carol-Anne Murdoch-Kinch (University of Michigan) for their critical review of this manuscript. KHL is supported in part by the NIH (K22 DE16096, R03 DE017918, R01 DE18888) and by start-up funds from the University of Arizona. GCM is supported in part by an NSF GK-12 Graduate Student Fellowship (NSF 0338247) through the University of Arizona CATTS program and by the Cancer Biology Training Grant (T32 CA09213-30).
REFERENCES
- Anne PR. Phase II trial of subcutaneous amifostine in patients undergoing radiation therapy for head and neck cancer. Semin Oncol. 2002;29(6 Suppl19):80–83. doi: 10.1053/sonc.2002.37350b. erratum in Semin Oncol 30:417, 2003. [DOI] [PubMed] [Google Scholar]
- Avila JL, Grundmann O, Burd R, Limesand KH. Radiation-induced salivary gland dysfunction results from p53-dependent apoptosis. Int J Radiat Oncol Biol Phys. 2009;73:523–529. doi: 10.1016/j.ijrobp.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Zheng C, Cotrim AP, Goldsmith CM, Atkinson JC, Brahim JS, et al. Transfer of the AQP1 cDNA for the correction of radiation-induced salivary hypofunction. Biochim Biophys Acta. 2006;1758:1071–1077. doi: 10.1016/j.bbamem.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Braam PM, Terhaard CH, Roesink JM, Raaijmakers CP. Intensity-modulated radiotherapy significantly reduces xerostomia compared with conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:975–980. doi: 10.1016/j.ijrobp.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Bralic M, Muhvic-Urek M, Stemberga V, Golemac M, Jurkovic S, Borcic J, et al. Cell death and cell proliferation in mouse submandibular gland during early post-irradiation phase. Acta Med Okayama. 2005;59:153–159. doi: 10.18926/AMO/31948. [DOI] [PubMed] [Google Scholar]
- Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. J Clin Oncol. 2000;18:3339–3345. doi: 10.1200/JCO.2000.18.19.3339. [DOI] [PubMed] [Google Scholar]
- Brizel DM, Murphy BA, Rosenthal DI, Pandya KJ, Gluck S, Brizel HE, et al. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. J Clin Oncol. 2008;26:2489–2496. doi: 10.1200/JCO.2007.13.7349. [DOI] [PubMed] [Google Scholar]
- Bussels B, Maes A, Flamen P, Lambin P, Erven K, Hermans R, et al. Dose-response relationships within the parotid gland after radiotherapy for head and neck cancer. Radiother Oncol. 2004;73:297–306. doi: 10.1016/j.radonc.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Cady J. Nutritional support during radiotherapy for head and neck cancer: the role of prophylactic feeding tube placement. Clin J Oncol Nurs. 2007;11:875–880. doi: 10.1188/07.CJON.875-880. [DOI] [PubMed] [Google Scholar]
- Calabro-Jones PM, Fahey RC, Smoluk GD, Ward JF. Alkaline phosphatase promotes radioprotection and accumulation of WR-1065 in V79-171 cells incubated in medium containing WR-2721. Int J Radiat Biol Relat Stud Phys Chem Med. 1985;47:23–27. doi: 10.1080/09553008514550041. [DOI] [PubMed] [Google Scholar]
- Cannon DM, Lee NY. Recurrence in region of spared parotid gland after definitive intensity-modulated radiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2008;70:660–665. doi: 10.1016/j.ijrobp.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Posner M, Jones CU, Biel MA, Hodge KM, Vitti R, et al. Cevimeline for the treatment of postirradiation xerostomia in patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 2007a;68:1102–1109. doi: 10.1016/j.ijrobp.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Jones CU, Biel MA, Weber RS, Hodge KM, Chen Y, et al. Open-label, long-term safety study of cevimeline in the treatment of postirradiation xerostomia. Int J Radiat Oncol Biol Phys. 2007b;69:1369–1376. doi: 10.1016/j.ijrobp.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Coppes RP, Roffel AF, Zeilstra LJ, Vissink A, Konings AW. Early radiation effects on muscarinic receptor-induced secretory responsiveness of the parotid gland in the freely moving rat. Radiat Res. 2000;153:339–346. doi: 10.1667/0033-7587(2000)153[0339:ereomr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Coppes RP, Meter A, Latumalea SP, Roffel AF, Kampinga HH. Defects in muscarinic receptor-coupled signal transduction in isolated parotid gland cells after in vivo irradiation: evidence for a non-DNA target of radiation. Br J Cancer. 2005;92:539–546. doi: 10.1038/sj.bjc.6602365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrim AP, Baum BJ. Gene therapy: some history, applications, problems, and prospects. Toxicol Pathol. 2008;36:97–103. doi: 10.1177/0192623307309925. [DOI] [PubMed] [Google Scholar]
- Cotrim AP, Hyodo F, Matsumoto K, Sowers AL, Cook JA, Baum BJ, et al. Differential radiation protection of salivary glands versus tumor by Tempol with accompanying tissue assessment of Tempol by magnetic resonance imaging. Clin Cancer Res. 2007a;13:4928–4933. doi: 10.1158/1078-0432.CCR-07-0662. [DOI] [PubMed] [Google Scholar]
- Cotrim AP, Sowers A, Mitchell JB, Baum BJ. Prevention of irradiation-induced salivary hypofunction by microvessel protection in mouse salivary glands. Mol Ther. 2007b;15:2101–2106. doi: 10.1038/sj.mt.6300296. [DOI] [PubMed] [Google Scholar]
- de Almeida PdelV, Grégio AM, Machado MÂ, Soares de Lima AA, Azevedo LR. Saliva composition and functions: a comprehensive review. J Contemp Dent Pract. 2008;9(3):72–80. [PubMed] [Google Scholar]
- Delporte C, O’Connell BC, He X, Lancaster HE, O’Connell AC, Agre P, et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci USA. 1997;94:3268–3273. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny PC, Denny PA. Dynamics of parenchymal cell division, differentiation, and apoptosis in the young adult female mouse subman-dibular gland. Anat Rec. 1999;254:408–417. doi: 10.1002/(SICI)1097-0185(19990301)254:3<408::AID-AR12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Dirix P, Nuyts S, Van den Bogaert W. Radiation-induced xerostomia in patients with head and neck cancer: a literature review. Cancer. 2006;107:2525–2534. doi: 10.1002/cncr.22302. [DOI] [PubMed] [Google Scholar]
- Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–587. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- Eisbruch A, Kim HM, Terrell JE, Marsh LH, Dawson LA, Ship JA. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2001;50:695–704. doi: 10.1016/s0360-3016(01)01512-7. [DOI] [PubMed] [Google Scholar]
- Friedrich RE, Bartel-Friedrich S, Holzhausen HJ, Lautenschlager C. The effect of external fractionated irradiation on the distribution pattern of extracellular matrix proteins in submandibular salivary glands of the rat. J Craniomaxillofac Surg. 2002;30:246–254. doi: 10.1054/jcms.2002.0318. [DOI] [PubMed] [Google Scholar]
- Grisius MM, Fox PC. Salivary gland dysfunction and xerostomia. Front Oral Biol. 1998;9:156–167. [Google Scholar]
- Gudkov AV, Komarova EA. The role of p53 in determining sensitivity to radiotherapy. Nat Rev Cancer. 2003;3:117–129. doi: 10.1038/nrc992. [DOI] [PubMed] [Google Scholar]
- Hall E. Radiobiology for the radiologist. 5th ed. Philadelphia: Lippincott, Williams and Wilkins; 2000. [Google Scholar]
- Hancock PJ, Epstein JB, Sadler GR. Oral and dental management related to radiation therapy for head and neck cancer. J Can Dent Assoc. 2003;69:585–590. [PubMed] [Google Scholar]
- Henson BS, Eisbruch A, D’Hondt E, Ship JA. Two-year longitudinal study of parotid salivary flow rates in head and neck cancer patients receiving unilateral neck parotid-sparing radiotherapy treatment. Oral Oncol. 1999;35:234–241. doi: 10.1016/s1368-8375(98)00104-3. [DOI] [PubMed] [Google Scholar]
- Hoebers FJ, Kartachova M, de Bois J, van den Brekel MW, van Tinteren H, van Herk M, et al. 99mTc Hynic-rh-Annexin V scintigraphy for in vivo imaging of apoptosis in patients with head and neck cancer treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging. 2008;35:509–518. doi: 10.1007/s00259-007-0624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn HF, Vousden KH. Coping with stress: multiple ways to activate p53. Oncogene. 2007;26:1306–1316. doi: 10.1038/sj.onc.1210263. [DOI] [PubMed] [Google Scholar]
- Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME. Suppression of apoptosis in the PKCdelta null mouse in vivo. J Biol Chem. 2006;281:9728–9737. doi: 10.1074/jbc.M507851200. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Kaplan I, Zuk-Paz L, Wolff A. Association between salivary flow rates, oral symptoms, and oral mucosal status. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:235–241. doi: 10.1016/j.tripleo.2007.11.029. [DOI] [PubMed] [Google Scholar]
- Konings AW, Cotteleer F, Faber H, van Luijk P, Meertens H, Coppes RP. Volume effects and region-dependent radiosensitivity of the parotid gland. Int J Radiat Oncol Biol Phys. 2005a;62:1090–1095. doi: 10.1016/j.ijrobp.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Konings AW, Coppes RP, Vissink A. On the mechanism of salivary gland radiosensitivity. Int J Radiat Oncol Biol Phys. 2005b;62:1187–1194. doi: 10.1016/j.ijrobp.2004.12.051. erratum in Int J Radiat Oncol Biol Phys 64:330, 2006. [DOI] [PubMed] [Google Scholar]
- Konings AW, Faber H, Cotteleer F, Vissink A, Coppes RP. Secondary radiation damage as the main cause for unexpected volume effects: a histopathologic study of the parotid gland. Int J Radiat Oncol Biol Phys. 2006;64:98–105. doi: 10.1016/j.ijrobp.2005.06.042. [DOI] [PubMed] [Google Scholar]
- Koukourakis MI, Kyrias G, Kakolyris S, Kouroussis C, Frangiadaki C, Giatromanolaki A, et al. Subcutaneous administration of amifostine during fractionated radiotherapy: a randomized phase II study. J Clin Oncol. 2000;18:2226–2233. doi: 10.1200/JCO.2000.18.11.2226. [DOI] [PubMed] [Google Scholar]
- Krysko DV, Vanden Berghe T, D’Herde K, Vandenabeele P. Apoptosis and necrosis: detection, discrimination and phagocytosis. Methods. 2008;44:205–221. doi: 10.1016/j.ymeth.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Li J, Shan Z, Ou G, Liu X, Zhang C, Baum BJ, et al. Structural and functional characteristics of irradiation damage to parotid glands in the miniature pig. Int J Radiat Oncol Biol Phys. 2005;62:1510–1516. doi: 10.1016/j.ijrobp.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Li Y, Taylor JM, Ten Haken RK, Eisbruch A. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int J Radiat Oncol Biol Phys. 2007;67:660–669. doi: 10.1016/j.ijrobp.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhao D, Gong B, Xu Y, Sun H, Yang B, et al. Decreased saliva secretion and down-regulation of AQP5 in submandibular gland in irradiated rats. Radiat Res. 2006;165:678–687. doi: 10.1667/RR3569.1. [DOI] [PubMed] [Google Scholar]
- Limesand KH, Barzen KA, Quissell DO, Anderson SM. Synergistic suppression of apoptosis in salivary acinar cells by IGF1 and EGF. Cell Death Differ. 2003a;10:345–355. doi: 10.1038/sj.cdd.4401153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand KH, Barzen KA, Sanders LA, Sclafani RA, Raynolds MV, Reyland ME, et al. Characterization of rat parotid and sub-mandibular acinar cell apoptosis in primary culture. In Vitro Cell Dev BiolAnim. 2003b;39:170–177. doi: 10.1007/s11626-003-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand KH, Schwertfeger KL, Anderson SM. MDM2 is required for suppression of apoptosis by activated Akt1 in salivary acinar cells. Mol Cell Biol. 2006;26:8840–8856. doi: 10.1128/MCB.01846-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limesand KH, Siad S, Anderson SM. Suppression of radiation-induced salivary gland dysfunction by IGF-1. PLoS One. 2009;4(3):e4663. doi: 10.1371/journal.pone.0004663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegaard JC, Grau C. Has the outlook improved for amifostine as a clinical radioprotector? Radiother Oncol. 2000;57:113–118. doi: 10.1016/s0167-8140(00)00235-8. [DOI] [PubMed] [Google Scholar]
- Lombaert IM, Brunsting JF, Wierenga PK, Faber H, Stokman MA, Kok T, et al. Rescue of salivary gland function after stem cell transplantation in irradiated glands. PLoS ONE. 2008;3(4):e2063. doi: 10.1371/journal.pone.0002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makkonen TA, Tenovuo J, Vilja P, Heimdahl A. Changes in the protein composition of whole saliva during radiotherapy in patients with oral or pharyngeal cancer. Oral Surg Oral Med Oral Pathol. 1986;62:270–275. doi: 10.1016/0030-4220(86)90007-1. [DOI] [PubMed] [Google Scholar]
- Malouf JG, Aragon C, Henson BS, Eisbruch A, Ship JA. Influence of parotid-sparing radiotherapy on xerostomia in head and neck cancer patients. Cancer Detect Prev. 2003;27:305–310. doi: 10.1016/s0361-090x(03)00095-3. [DOI] [PubMed] [Google Scholar]
- Man YG, Ball WD, Marchetti L, Hand AR. Contributions of intercalated duct cells to the normal parenchyma of submandibular glands of adult rats. Anat Rec. 2001;263:202–214. doi: 10.1002/ar.1098. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet W, De Meyer GR, Andries L, Herman AG, Kockx MM. Detection of autophagy in tissue by standard immunohistochemistry: possibilities and limitations. Autophagy. 2006;2:55–57. doi: 10.4161/auto.2217. [DOI] [PubMed] [Google Scholar]
- Muhvic-Urek M, Bralic M, Curic S, Pezelj-Ribaric S, Borcic J, Tomac J. Imbalance between apoptosis and proliferation causes late radiation damage of salivary gland in mouse. Physiol Res. 2006;55:89–95. doi: 10.33549/physiolres.930739. [DOI] [PubMed] [Google Scholar]
- Murdoch-Kinch CA, Kim HM, Vineberg KA, Ship JA, Eisbruch A. Dose-effect relationships for the submandibular salivary glands and implications for their sparing by intensity modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:373–382. doi: 10.1016/j.ijrobp.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagler RM. Short- and long-term functional vs morphometrical salivary effects of irradiation in a rodent model. Anticancer Res. 1998;18(1A):315–320. [PubMed] [Google Scholar]
- Nagler RM, Laufer D. Protection against irradiation-induced damage to salivary glands by adrenergic agonist administration. Int J Radiat Oncol Biol Phys. 1998;40:477–481. doi: 10.1016/s0360-3016(97)00574-9. [DOI] [PubMed] [Google Scholar]
- Nagler R, Marmary Y, Fox PC, Baum BJ, Har-El R, Chevion M. Irradiation-induced damage to the salivary glands: the role of redox-active iron and copper. Radiat Res. 1997;147:468–476. [PubMed] [Google Scholar]
- Nagler RM, Baum BJ, Miller G, Fox PC. Long-term salivary effects of single-dose head and neck irradiation in the rat. Arch Oral Biol. 1998;43:297–303. doi: 10.1016/s0003-9969(97)00120-9. [DOI] [PubMed] [Google Scholar]
- Nguyen NP, Vos P, Karlsson U, Nguyen P, Dutta S, Lemanski C, et al. Quality of life following chemoradiation and postoperative radiation for locally advanced head and neck cancer. ORL J Otorhinolaryngol Relat Spec. 2007;69:271–276. doi: 10.1159/000103870. [DOI] [PubMed] [Google Scholar]
- O’Connell AC, Lillibridge CD, Zheng C, Baum BJ, O’Connell BC, Ambudkar IS. Gamma-irradiation-induced cell cycle arrest and cell death in a human submandibular gland cell line: effect of E2F1 expression. J Cell Physiol. 1998;177:264–273. doi: 10.1002/(SICI)1097-4652(199811)177:2<264::AID-JCP8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- O’Connell AC, Baccaglini L, Fox PC, O’Connell BC, Kenshalo D, Oweisy H, et al. Safety and efficacy of adenovirus-mediated transfer of the human aquaporin-1 cDNA to irradiated parotid glands of non-human primates. Cancer Gene Ther. 1999a;6:505–513. doi: 10.1038/sj.cgt.7700078. [DOI] [PubMed] [Google Scholar]
- O’Connell AC, Redman RS, Evans RL, Ambudkar IS. Radiation-induced progressive decrease in fluid secretion in rat submandibular glands is related to decreased acinar volume and not impaired calcium signaling. Radiat Res. 1999b;151:150–158. [PubMed] [Google Scholar]
- Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, et al. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- Paardekooper GM, Cammelli S, Zeilstra LJ, Coppes RP, Konings AW. Radiation-induced apoptosis in relation to acute impairment of rat salivary gland function. Int J Radiat Biol. 1998;73:641–648. doi: 10.1080/095530098141898. [DOI] [PubMed] [Google Scholar]
- Pinkstaff CA. Cytology, histology, and histochemistry of salivary glands: an overview. In: Dobrosielski-Vergona K, editor. Biology of the salivary glands. Boca Raton: CRC Press; 1992. pp. 15–38. [Google Scholar]
- Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161:123–136. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- Proctor GB, Carpenter GH. Regulation of salivary gland function by autonomic nerves. Auton Neurosci. 2007;133:3–18. doi: 10.1016/j.autneu.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Rachidi W, Harfourche G, Lemaitre G, Amiot F, Vaigot P, Martin MT. Sensing radiosensitivity of human epidermal stem cells. Radiother Oncol. 2007;83:267–276. doi: 10.1016/j.radonc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother Oncol. 2004;70:261–264. doi: 10.1016/j.radonc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Radfar L, Sirois DA. Structural and functional injury in minipig salivary glands following fractionated exposure to 70 Gy of ionizing radiation: an animal model for human radiation-induced salivary gland injury. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003;96:267–274. doi: 10.1016/s1079-2104(03)00369-x. [DOI] [PubMed] [Google Scholar]
- Reyland ME. Protein kinase Cdelta and apoptosis. Biochem Soc Trans. 2007;35(Pt 5):1001–1004. doi: 10.1042/BST0351001. [DOI] [PubMed] [Google Scholar]
- Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol. 2004;5:897–907. doi: 10.1038/nrm1496. [DOI] [PubMed] [Google Scholar]
- Rieger K, Marinets O, Fietz T, Korper S, Sommer D, Mucke C, et al. Mesenchymal stem cells remain of host origin even a long time after allogeneic peripheral blood stem cell or bone marrow transplantation. Exp Hematol. 2005;33:605–611. doi: 10.1016/j.exphem.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- Robar JL, Day A, Clancey J, Kelly R, Yewondwossen M, Hollenhorst H, et al. Spatial and dosimetric variability of organs at risk in head-and-neck intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68:1121–1130. doi: 10.1016/j.ijrobp.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Roesink JM, Moerland MA, Hoekstra A, Van Rijk PP, Terhaard CH. Scintigraphic assessment of early and late parotid gland function after radiotherapy for head-and-neck cancer: a prospective study of dose-volume response relationships. Int J Radiat Oncol Biol Phys. 2004;58:1451–1460. doi: 10.1016/j.ijrobp.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Rubin P, Casarett G. Clinical radiation pathology. Philadelphia: WB Saunders; 1968. [DOI] [PubMed] [Google Scholar]
- Rudat V, Meyer J, Momm F, Bendel M, Henke M, Strnad V, et al. Protective effect of amifostine on dental health after radiotherapy of the head and neck. Int J Radiat Oncol Biol Phys. 2000;48:1339–1343. doi: 10.1016/s0360-3016(00)00768-9. [DOI] [PubMed] [Google Scholar]
- Sagowski C, Wenzel S, Tesche S, Jenicke L, Jaehne M. Investigation of radiosialadenitis during fractioned irradiation: sialoscintigraphical and histomorphological findings in rats. Eur Arch Otorhinolaryngol. 2003;260:513–517. doi: 10.1007/s00405-003-0631-x. [DOI] [PubMed] [Google Scholar]
- Savill J, Fadok V, Henson P, Haslett C. Phagocyte recognition of cells undergoing apoptosis. Immunol Today. 1993;14:131–136. doi: 10.1016/0167-5699(93)90215-7. [DOI] [PubMed] [Google Scholar]
- Seiwert TY, Cohen EE. State-of-the-art management of locally advanced head and neck cancer. Br J Cancer. 2005;92:1341–1348. doi: 10.1038/sj.bjc.6602510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiwert TY, Salama JK, Vokes EE. The chemoradiation paradigm in head and neck cancer. Nat Clin Pract Oncol. 2007;4:156–171. doi: 10.1038/ncponc0750. [DOI] [PubMed] [Google Scholar]
- Shan Z, Li J, Zheng C, Liu X, Fan Z, Zhang C, et al. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther. 2005;11:444–451. doi: 10.1016/j.ymthe.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Ship JA, Fox PC, Baum BJ. How much saliva is enough? ‘Normal’ function defined. J Am Dent Assoc. 1991;122(3):63–69. doi: 10.14219/jada.archive.1991.0098. [DOI] [PubMed] [Google Scholar]
- St John MA, Abemayor E, Wong DT. Recent new approaches to the treatment of head and neck cancer. Anticancer Drugs. 2006;17:365–375. doi: 10.1097/01.cad.0000198913.75571.13. [DOI] [PubMed] [Google Scholar]
- Stephens LC, Ang KK, Schultheiss TE, King GK, Brock WA, Peters LJ. Target cell and mode of radiation injury in rhesus salivary glands. Radiother Oncol. 1986a;7:165–174. doi: 10.1016/s0167-8140(86)80096-2. [DOI] [PubMed] [Google Scholar]
- Stephens LC, King GK, Peters LJ, Ang KK, Schultheiss TE, Jardine JH. Acute and late radiation injury in rhesus monkey parotid glands. Evidence of interphase cell death. Am J Pathol. 1986b;124:469–478. [PMC free article] [PubMed] [Google Scholar]
- Stephens LC, Schultheiss TE, Small SM, Ang KK, Peters LJ. Response of parotid gland organ culture to radiation. Radiat Res. 1989;120:140–153. [PubMed] [Google Scholar]
- Stephens LC, Schultheiss TE, Price RE, Ang KK, Peters LJ. Radiation apoptosis of serous acinar cells of salivary and lacrimal glands. Cancer. 1991;67:1539–1543. doi: 10.1002/1097-0142(19910315)67:6<1539::aid-cncr2820670613>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Takakura K, Takaki S, Takeda I, Hanaue N, Kizu Y, Tonogi M, et al. Effect of cevimeline on radiation-induced salivary gland dysfunction and AQP5 in submandibular gland in mice. Bull Tokyo Dent Coll. 2007;48(2):47–56. doi: 10.2209/tdcpublication.48.47. [DOI] [PubMed] [Google Scholar]
- Thula TT, Schultz G, Tran-Son-Tay R, Batich C. Effects of EGF and bFGF on irradiated parotid glands. Ann Biomed Eng. 2005;33:685–695. doi: 10.1007/s10956-005-1853-z. [DOI] [PubMed] [Google Scholar]
- Tran SD, Sugito T, Dipasquale G, Cotrim AP, Bandyopadhyay BC, Riddle K, et al. Re-engineering primary epithelial cells from rhesus monkey parotid glands for use in developing an artificial salivary gland. Tissue Eng. 2006;12:2939–2948. doi: 10.1089/ten.2006.12.2939. [DOI] [PubMed] [Google Scholar]
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, Gwede CK, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66:253–262. doi: 10.1016/s0167-8140(02)00404-8. [DOI] [PubMed] [Google Scholar]
- Turner RJ. Mechanisms of fluid secretion by salivary glands. Ann NY Acad Sci. 1993;694:24–35. doi: 10.1111/j.1749-6632.1993.tb18339.x. [DOI] [PubMed] [Google Scholar]
- Utley JF, Marlowe C, Waddell WJ. Distribution of 35S-labeled WR-2721 in normal and malignant tissues of the mouse1,2. Radiat Res. 1976;68:284–291. [PubMed] [Google Scholar]
- Vissink A, ‘s-Gravenmade EJ, Ligeon EE, Konings WT. A functional and chemical study of radiation effects on rat parotid and submandibular/sublingual glands. Radiat Res. 1990;124:259–265. [PubMed] [Google Scholar]
- Woodward WA, Chen MS, Behbod F, Alfaro MP, Buchholz TA, Rosen JM. WNT/beta-catenin mediates radiation resistance of mouse mammary progenitor cells. Proc Natl Acad Sci USA. 2007;104:618–623. doi: 10.1073/pnas.0606599104. erratum in Proc Natl Acad Sci USA 104:7307, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhan Y, Burke KA, Anderson WF. Soluble factor(s) from bone marrow cells can rescue lethally irradiated mice by protecting endogenous hematopoietic stem cells. Exp Hematol. 2005;33:428–434. doi: 10.1016/j.exphem.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Zheng C, Hoque AT, Braddon VR, Baum BJ, O’Connell BC. Evaluation of salivary gland acinar and ductal cell-specific promoters in vivo with recombinant adenoviral vectors. Hum Gene Ther. 2001;12:2215–2223. doi: 10.1089/10430340152710559. [DOI] [PubMed] [Google Scholar]
- Zheng C, Vitolo JM, Zhang W, Mineshiba F, Chiorini JA, Baum BJ. Extended transgene expression from a nonintegrating adenoviral vector containing retroviral elements. Mol Ther. 2008;16:1089–1097. doi: 10.1038/mt.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]