Abstract
Background and Purpose
Mild cognitive impairment (MCI) is a risk factor for Alzheimer's disease (AD) and can be difficult to diagnose due to the subtlety of symptoms. This work attempted to examine gray and white matter changes with cortical thickness analysis and diffusion tensor imaging (DTI) in MCI patients and demographically-matched comparison subjects in order to test these measurements as possible imaging markers for diagnosis.
Materials and Methods
Subjects with amnestic MCI (n=10; age 72.2±7.1) and normal cognition (n=10; age 70.1±7.7) underwent DTI and T1 weighted MRI at 3T. Fractional anisotropy, apparent diffusion coefficient and cortical thickness were measured and compared between MCI and control groups. The diagnostic accuracy of two methods, either in combination or separately, was evaluated using binary logistic regression and nonparametric statistical analyses for sensitivity, specificity and accuracy.
Results
Decreased FA and increased ADC in white matter regions of frontal and temporal lobes and corpus callosum were observed in MCI patients. Cortical thickness was decreased in gray matter regions of the frontal, temporal, parietal lobes in MCI patients. Changes in white matter and cortical thickness appeared to be more pronounced in the left hemisphere than in the right hemisphere. Furthermore the combination of cortical thickness and DTI measurements in left temporal areas improved the accuracy of differentiating MCI patients from controls compared to either measure alone.
Conclusion
DTI and cortical thickness analyses may both serve imaging markers for differentiating MCI from normal aging. Combined use of two methods may improve the accuracy of MCI diagnosis.
Keywords: Magnetic Resonance Imaging, Cortical Thickness, Diffusion Tensor, Mild Cognitive Impairment, White Matter, Gray Matter, Alzheimer's Disease
Introduction
Mild cognitive impairment (MCI) is often a precursor to dementias such as Alzheimer's disease (AD), with rates of progression estimated between 12 - 15% a year (1-4). MCI can be difficult to diagnose due to the subtlety of the cognitive impairments, especially in very high functioning individuals. Studies have demonstrated that the cognitive cutoff scores used to define MCI affect whether or not it is diagnosed, and some patients who are thought to have MCI at their initial visit may actually revert to a normal aging profile at follow-up (5, 6). Therefore, non-invasive neuroimaging approaches that are sensitive and specific in distinguishing MCI from normal aging and predicting its progression to AD are desirable (7). Magnetic resonance imaging (MRI) has high spatial resolution and exquisite tissue contrast, providing an advanced clinical tool. Earlier studies on MCI and AD using MRI typically focused on investigating cortical structural changes in medial temporal gray matter (GM) such as volume reduction in the entorhinal cortex and hippocampus (8-13). A method of whole brain cortical thickness analysis (14, 15) has been employed to study MCI patients in relation to healthy elderly and AD subjects (16-18). Another technique, diffusion tensor imaging (DTI), is capable of analyzing white matter (WM) integrity using measurements such as fractional anisotropy (FA) and apparent diffusion coefficient (ADC). Previous DTI studies have reported decreased FA and increased ADC or mean diffusivity values in the hippocampus (19-21), corpus callosum (22, 23), and the frontal and temporal lobes in AD patients when compared to normal control subjects (24-28). In addition, DTI studies of MCI patients have also demonstrated white matter (WM) changes, especially in the medial temporal lobes (19, 26, 29-32). Since GM and WM structures are both critical but play different roles in brain functioning, it is of great interest to determine whether GM or WM is affected and if they contribute differently to the development of MCI or AD. In conjunction with the routine neuropsychological evaluation, structural abnormalities elucidated by non-invasive imaging may help us to better understand the neurodegenerative processes and to develop adequate diagnostic imaging approaches for MCI.
The current study applied DTI and cortical thickness analyses to patients and controls to determine the diagnostic value of either measure alone versus in combination. To our knowledge, only one previous study investigated the combined use of DTI and structural MRI in AD and MCI patients (32), but it did not compare cortical thickness and DTI measurements in the same cohort. Here we report observations of changes in cortical structures of GM and WM integrity in the same group of MCI patients. Results of our study suggest that the combined use of these two imaging tools may improve the accuracy of diagnosis.
Methods and Subjects
Subject Recruitment and Assessment
Study participants were recruited from the Wesley Woods Center on Aging and from the Emory University Alzheimer's Disease Research Center. The study was approved by the Emory University Institutional Review Board. Written informed consent was obtained from all participants. The patient group included 10 right handed subjects who met the criteria for amnestic MCI (5 men, 5 women; age 72.2±7.1 years; education =15.2±4.1 years). The diagnosis of amnestic MCI was made by experienced neurologists (AL, JL) based on Petersen Criteria (2, 3) including a subjective cognitive complaint (corroborated by an informant), impairment of memory with formal neuropsychological testing described below (> -1.5 SD below the performance of age and education controls), normal general cognitive functioning, and preserved instrumental activities of daily living. Ten cognitively intact subjects were recruited from the community or from the Emory Alzheimer's Disease Research Center (3 men, 7 women; age = 70.1±7.7 years, education = 14.0±4.0 years).
Uniform evaluations included screening for other types of dementia or for coexisting conditions that could affect cognition. Participants did not have histories or findings suggestive of stroke as determined by a review of their medical records and radiological and neurological exams.
MRI Data Acquisition
DTI and structural MRI were performed using a 3T whole body scanner (Phillips Intera, Philips Medical Systems, Best, The Netherlands) with a phased array head coil. High-resolution anatomic 3D T1-weighted multi-plane gradient echo (MPGR) with TR/TE=45/15 ms and T2 weighted fast spin-echo imaging with TR/TE=4900/110 ms were collected using the parallel imaging acquisition with an accelerate factor of 2. All T1 weighted and T2 weighted imaging sequences were performed in the axial direction with 60 slices, 2 mm thickness and no gap at the same slice location. A field of view (FOV) of 240 mm and matrix of 256× 256 were used.
For DTI, images were recorded in the axial direction with 60 slices and 2 mm thickness without gap. Directional sensitized diffusion weighting single-shot spin echo echo-planar imaging (EPI) sequence with 16 gradient directions was used with imaging parameters: TR/TE=9800/74 ms, b-values of 0 or 1,000 s/mm2 using the b=0 image as a reference. The same FOV and slice locations used in the structural MRI were applied so that the DT images could be aligned co-plane with structural images. DT images were collected with matrix of 128× 128 and then reconstructed to 256× 256.
Image and Data Analysis
All images were examined by the study radiologists (L.W. and C.H.) for possible abnormalities. The diffusion tensor eigen values (λ1, λ2, λ3) and eigen vectors (ε1, ε2, ε3) were calculated for each voxel. FA and ADC maps were generated using the FSL program (FMRIB Center, University of Oxford, UK). Motion and Eddy current corrections were made before calculating DTI indices. The image analyses were carried out independently by investigators (L.W., H.M.) who were blinded to the clinical diagnoses. FA and ADC measurements were obtained for the selected areas using region of interest (ROI) analyses. ROIs were typically a rectangular shape and were selected in frontal and temporal WM areas bilaterally, including inferior frontal gyrus (IFG) and the medial frontal gyrus (mFG) in the frontal lobe; and from the superior temporal gyrus and middle temporal gyrus (mTG) in the temporal lobe. ROIs were selected based on the procedure used by Head et al. (25) and atlas-based rules with anatomic landmarks by Mori et al. (33). For frontal and temporal lobes, ROIs were placed at selected slices from the beginning of the mammillary bodies and continuing on the next 4 slices for a total of 5 slices as determined on the FA maps. For the corpus callosum (CC), the genu and splenium were outlined separately on sagittal images. The regions were defined as the anterior 25% of the callosum and splenium as the posterior 25% from FA maps. Measurement of DTI indices was made on the midsagittal slice and continued on the next 2 lateral slices in both hemispheres for a total 5 slices. Examples of ROIs are shown in Figure 1. FA and ADC values of each ROI from each subject were measured and then averaged within the group. To assess the interrater reliability, two raters (L.W. and H.M.) were asked to select ROIs in the left and right frontal and temporal areas of four subjects independently. Comparison of FA values from a given ROI selected by two raters yielded <10% standard deviations.
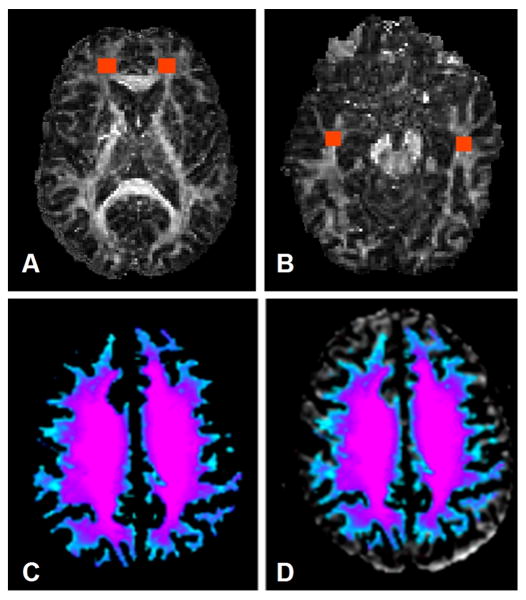
Figure 1.
Examples of Regions of Interest (ROI) placed in temporal (A), prefrontal (B) areas and corpus callosum (C) of the FA map.
Cortical thickness data were calculated from high resolution 3D T1-weighted gradient echo images using FreeSurfer software (http://surfer.nmr.mgh.harvard.edu). For each subject, original T1 images with optimized gray and the white matter contrast were normalized first. Next, the boundary between the gray and the white matter, and the outer surface of the cortex (the pial surface) were segmented. The cortical surface was demarcated into different regions, such as entorhinal, fusiform, parahippocampal and superior temporal (Figure 2). Mean thickness of these cortical regions was calculated based on the boundary of the gray and white matter and the outline of the pial surface, respectively. All of these processes were carried out in FreeSurfer (34-38). Final segmentation of the gray and white matter boundary and parcellation of cortical and sub-cortical structures were verified by experienced radiologists (LW, CH). Any inaccurate segmentation of the gray and white matter boundary or the outline of the pial surface was corrected manually. Figure 2 shows selected cortical structures of the left hemisphere from a representative subject.
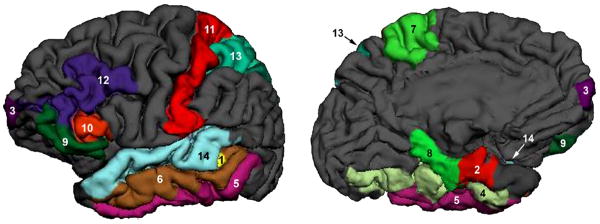
Figure 2.
Cortical thickness was measured in cortical structures shown in this 3D rendering view of the brain from outside (A) and inside (B). The cortical structures, where statistically significant changes were observed in MCI groups, are highlighted in the different colors in order to distinguish each cortical region, which is numbered as: 1: bankssts, 2: entorhinal, 3: frontal pole, 4: fusiform, 5: inferior temporal, 6: middle temporal, 7: paracentral, 8: parahippocampal, 9: parsorbitalis, 10: parstriangularis, 11: postcentral, 12: rostral middle frontal, 13: superior parietal, 14: superior temporal.
Cognitive Evaluations
Verbal memory was assessed using the CERAD Word List (39) which required participants to recall 10 words over three trials, followed by short-delay recall. Story A of Logical Memory (40) was also administered during which participants recalled a story both immediately after hearing it and then after 30 minutes. The dependent variables included the number of words or story units recalled. Visual memory was evaluated by having participants learn and recall the patterns and locations of six designs both immediately and after 30 minutes (Brief Visuospatial Memory Test-Revised) (41). The dependent variable included the number of total points for correct reproduction and placement of the designs.
Statistical Analysis
Data analysis was performed using the SAS 9.1.3 statistical package (SAS Institute, Cary, NC). An independent-sample T-test was used to analyze differences between the MCI and control groups in the measurements of FA, ADC and averaged cortical thickness in the ROIs of the frontal and temporal lobes and other selected areas. Results were expressed as mean ± standard deviation (SD). Pearson correlation was used to examine relationships between measurements of FA, ADC and averaged cortical thickness in different cortical structures. Binary logistic regression with receiver operator characteristics (ROC) analysis was performed to evaluate the sensitivity, specificity and accuracy of each measurement for detecting MCI. Area under the curve (AUC) was used to evaluate the Optimal Cutoff Point, which is given by the maximum of the Youden index. The Youden index J returns the maximum value of the expression (for inverted models, it returns the minimum):
where SE(t) and SP(t) are, respectively, the sensitivity and specificity over all possible threshold values t of the model. Thus, the Optimal Model Threshold corresponds to the model output at the Optimal Cutoff Point. A result with p<0.05 was considered statistically significant. The specificity and sensitivity of each measurement or combined measurements were indicated by areas under the ROC curve.
Results
There were no statistically significant differences between the MCI and control groups in age, education, or the distribution of race and gender (p > 0.05) (Table 1). Mini-Mental State Exam (MMSE) scores of the MCI group (mean = 26.6 points, SD = 2.1) were significantly lower than those of the control group (mean = 29.7, SD = 0.5, p < 0.001).
Table 1.
Demographic and clinical features of the participants
| MCI (n=10) |
Normal Controls (n=10) | P value | |
|---|---|---|---|
| Mean (SD) Age | 72.2 (7.7) | 70.1 (7.7) | 0.50 |
| Mean (SD) Education | 14.0 (4.0) | 15.2 (4.1) | 0.20 |
| Race | 10/10 Caucasian | 9/10 Caucasian; 1 African American |
---- |
| Gender | 7 Female, 3 Male | 5 Female, 5 Male | 0.62 |
| Mean (SD) MMSE | 26.4 (2.2) | 29.7 (0.5) | 0.0007 |
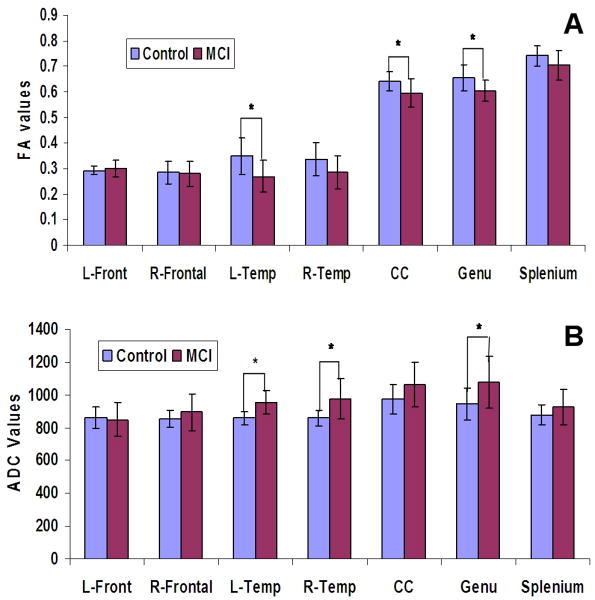
Analysis of the DTI data showed that the MCI group had decreases in FA values compared to the control group in all of ROIs except in the left frontal areas (Figure 3, A). Furthermore, statistically significant decreases in FA values were observed in the left temporal regions (p = 0.016), in the post CC (p = 0.043) and genu of the CC (p = 0.025).
Figure 3.
FA (A) and ADC (B) values in different ROIs of the MCI and control groups.
Statistically significant increases in ADC values were found in the ROIs of the left temporal region (p = 0.002), right temporal region (p = 0.016) and in the genu of CC (p = 0.038) of MCI patients (Figure 3, B) when compared to the controls. The increase in ADC was more noticeable in the genu than in the splenium.
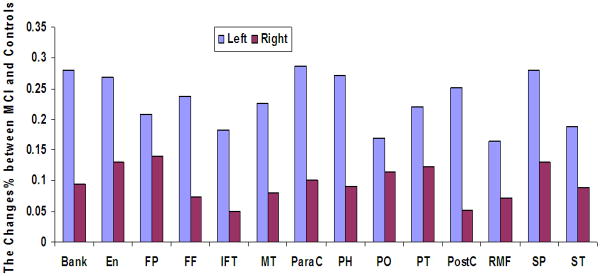
Changes in GM cortical thickness values were also observed in the MCI group. There was a reduction in cortical thickness in the superior temporal and medial temporal lobe areas of MCI patients when compared to the controls (Figure 2). The affected areas included entorhinal, fusiform, inferior temporal, middle temporal and parahippocampal cortical structures. Reductions in cortical thickness were also observed in the frontal and parietal lobes, including the frontal pole, paracentral, pars orbitalis, pars triangularis, postcentral, rostral middle frontal, superior parietal and superior temporal areas (Figure 4). Changes in cortical thickness appeared to be more pronounced in the left hemisphere than in the right hemisphere of MCI patients except in the regions of superior frontal and superior temporal cortices.
Figure 4.
Decreases in cortical thickness were observed in MCI patients in several cortical structures (colored) of the left and right hemisphere. Bank: bankssts (i.e., cortical areas around superior temporal sulcus), En: entorhinal, FP: frontal pole, FF: fusiform, IFT: inferior temporal, MT: middle temporal, ParaC: paracentral, PH: para-hippocampal, PO: parsorbitalis; PT: parstriangularis, PostC: postcentral, RMF: rostral middle frontal, SF: superior frontal, SP: superior parietal, ST: superior temporal areas
We further examined the correlation between DTI measured WM changes and cortical thickness analysis of GM changes in both the MCI patients and controls. Because changes in both the WM and GM of MCI patients were more evident in the left than in the right hemisphere, only data from the left temporal area were used in the analysis. In the selected regions, such as middle temporal, parahippocampal and superior temporal cortices, we observed that FA values were positively correlated with the cortical thickness measurements while ADC values were negatively correlated with the cortical thickness measurements in the control group. However, FA values in the same regions were negatively correlated with the cortical thickness values in the MCI group, suggesting that a reduction in WM integrity may not couple with a reduction in GM cortical thickness. More detailed results are summarized in Table 2.
TABLE 2.
Pearson correlations between changes in cortical thickness in the GM and changes of WM integrity in ROIs in the left temporal lobes of MCI patients.
| DTI Measurements* | Subject Groups | Cortical Thickness ** | |||||
|---|---|---|---|---|---|---|---|
| Fusiform | Middle temporal | Infer-temporal | Para hippocampal | Parstri-angularis | Superior temporal | ||
| FA (r) | Control | 0.30 | 0.11 | 0.43 | 0.03 | 0.30 | 0.28 |
| MCI | -0.18 | -0.34 | -0.15 | -0.30 | -0.73 | -0.28 | |
| ADC (r) | Control | -0.52 | -0.49 | -059 | -0.70 | -0.47 | -0.55 |
| MCI | -0.19 | 0.19 | 0.01 | -0.55 | -0.04 | 0.12 | |
FA and ADC were averaged;
Only cortical structures with statistically significant changes are shown;
Logistic regression was applied to evaluate the cortical thickness and DTI measurements. Our results suggested that taking into account both measurements improved the accuracy of discriminating MCI patients from controls. In other words, measurements of FA and ADC of the left temporal lobe and the cortical thickness of left entorhinal, middle temporal, parahippocampal and superior temporal regions together gave higher accuracy in distinguishing MCI patients from controls than either measure alone. For example, the accuracy of determining MCI was 80% if only the change in the temporal FA value or the reduction of the parahippocampal cortical thickness in the left hemisphere was considered. However, the accuracy rose to 95% when we considered both the GM and WM measures. The AUC, an indicator of accuracy, was 0.98 as shown in the ROC plot (Figure 5). More detailed results from the ROC analysis of different brain areas are presented in Table 3.
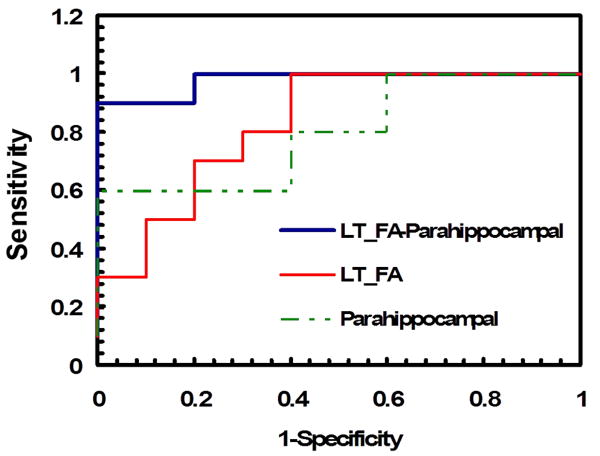
Figure 5.
The levels of sensitivity and specificity for predicting MCI are shown in the plot of the ROC analysis. Combining the FA measurement of WM changes and the cortical thickness measurement of GM changes in the left hemisphere resulted in the increased AUC (blue line), i.e., improved sensitivity and specificity of differentiating MCI from the controls when compared to those of the FA measurement (red) or cortical thickness analysis (green) alone.
TABLE 3.
The sensitivity and specificity of predicting MCI with different imaging measurements of WM and GM changes. The accuracy and the quality of prediction are shown as increases in AUC when concurrently using DTI and cortical thickness measurements.
| Modal | Cortical Regions | Sensitivity (%) | Specificity (%) | Accuracy (%) | AUC |
|---|---|---|---|---|---|
| DTI Indices | Left temporal FA | 1.00 | 0.60 | 0.80 | 0.83 |
| Left temporal ADC | 0.80 | 0.80 | 0.80 | 0.86 | |
| Cortical Thickness | Entorhinal | 0.90 | 0.70 | 0.80 | 0.84 |
| Middle temporal | 1.00 | 0.60 | 0.80 | 0.81 | |
| Parahippocampal | 0.60 | 1.00 | 0.80 | 0.80 | |
| Superior temporal | 0.70 | 0.80 | 0.75 | 0.78 | |
| FA + Cortical thickness | Left temporal FA + Entorhinal | 0.80 | 0.80 | 0.80 | 0.87 |
| Left temporal FA + Middle temporal | 0.80 | 0.90 | 0.85 | 0.92 | |
| Left temporal FA + Parahippocampal | 0.90 | 1.00 | 0.95 | 0.98 | |
| Left temporal FA + Superior temporal | 1.00 | 0.60 | 0.80 | 0.90 | |
| ADC + Cortical thickness | Left temporal ADC + Entorhinal | 0.90 | 0.90 | 0.90 | 0.92 |
| Left temporal ADC + Middle temporal | 0.90 | 0.90 | 0.90 | 0.94 | |
| Left temporal ADC + Parahippocampal | 1.00 | 0.60 | 0.80 | 0.87 | |
| Left temporal ADC + Superior temporal | 0.90 | 0.90 | 0.90 | 0.92 |
Discussion
We found that the combination of DTI measurements of left temporal WM with cortical thickness measurements in the temporal GM significantly improved the accuracy of diagnosing MCI compared to either measurement alone. It appears that the most effective combination is the FA measurement of the left temporal lobe and cortical thickness of the parahippocampal cortex. Other useful combined measurements included left temporal FA and cortical thickness of the middle and superior temporal cortices, left temporal ADC, and cortical thickness of the entorhinal and middle and superior temporal cortices.
This study revealed decreased FA and increased ADC values in the temporal lobes of the MCI patients compared to healthy controls. This finding is consistent with previous reports (19-21, 26-28). However, our present study further investigated changes in both GM and WM in the same individuals, allowing us to concurrently analyze the two together. Our results indicated that reductions of WM integrity and cortical thickness occur together in several regions, especially in the temporal lobe areas such as entorhinal, middle temporal, parahippocampal and superior temporal cortex. Furthermore, stronger correlations between WM changes as measured by ADC and GM changes as measured by cortical thickness were observed in normal controls but were weaker in the MCI group. To our knowledge this observation has not been previously reported. Although disease progression in AD and MCI patients varies among individuals, pathology typically starts in the transentorhinal cortex and quickly spreads to the entorhinal cortex and the hippocampus (42). It is likely that patients with MCI may already have the pathological hallmarks of AD including neocortical senile plaques, neurofibrillary tangles, atrophy, and neuronal loss in layer II of the entorhinal cortex (43, 44). Therefore, our observations of loss of WM integrity and cortical thickness in entorhinal and hippocampal cortex may reflect such pathological changes. These data suggested DTI and cortical thickness measurements of changes in WM and GM may serve as functional markers for diagnosis and monitoring of MCI.
To our knowledge, the difference in FA between the left and right hemisphere in MCI has not been closely investigated, although several studies of other cerebral diseases have demonstrated that cognitive performances mediated by the left hemisphere correlates significantly with changes in diffusion anisotropy and diffusivity of selected WM regions (45). Our data showed that decreased FA and increased ADC values were more apparent in the genu of the CC than in the splenium, which is different from what has been reported by Zhang et al (32). Finally we did not find significant differences between frontal WM and GM in the MCI versus the control group, which supports the hypothesis that observed changes in the frontal region are more associated with normal aging (25).
The current study allowed us to directly correlate changes between GM and WM in controls and MCI subjects. The observed positive correlation between FA and cortical thickness in structures of the temporal lobes in the normal controls suggests that changes in WM and GM are comparable and are not preferentially affected by the normal aging process. However, this positive relationship is not seen or becomes negative in the MCI group. One possible explanation could be that the change of either GM or WM is more pronounced in those cortical structures. In other words, the brain degeneration occurs preferentially. It has been reported that GM structure are more vulnerable and affected in AD (46, 47). It is likely that MCI may share a similar path.
It should be noted that there are several limitations to the present study. For example, the number of subjects included in the study was small. We used the ROI method to analyze data which, in turn, is subject to inherent subjectivity even though our raters were blinded to the clinical findings during the analysis. Reproducibility of manually drawing and placing ROI may also vary with raters and brain anatomy, which may contribute to our interrater deviation (<10%) in selecting ROI for DTI data analysis. Moreover, the ROIs of WM and GM were selected in close proximity but not in the identical areas, and therefore, correlations between the changes of GM and WM only represent a fairly large region. In addition, DTI used in this study only applied 16 gradient directions for diffusion encoding. More accurate FA measurement can be achieved with most advanced and latest MRI systems and high angular resolution diffusion imaging (HARDI) approach that are capable of high resolution DTI with gradient directions ranging from 30-64 in the clinically feasible time, or even higher in the research settings. It is also noticed that the results from the current study were derived from the groups of subjects with a number of imaging measurements included in the analysis. Although we have provided a proof-of-principle example of using multi-modal approach to identify subtle differences between MCI and normal aging, it is important to further investigate prospectively in a larger study whether our findings are reproducible, and more importantly, to test if this approach can be applied in clinical settings when the imaging diagnosis is done at the individual level with single patient.
Currently diagnosis of MCI is still mostly based on the cognitive and behavioral tests in neurology clinic. However, cognitive impairments can be subtle in patients with high functions and may be fluctuating and even reverted at the follow-up (5, 6). Neuroimaging with MCI specific imaging markers, such as WM integrity and cortical thickness, thus can assist neurological diagnosis with objective and quantitative measurements of brain structural changes. Furthermore, diffusion properties of brain tissue and cortical thickness measured by MRI may provide critical information on primary neurodegenerative etiology of the cognitive decline in MCI patients, as opposed to other causes, such as psychiatric problems. The ability of quantitative measurement of cortical changes can be particularly useful in monitoring and even predicting the progression of MCI. As estimated 12 - 15% MCI patients may progress to Alzheimer's disease each year (4), one of the most important questions in clinical management of MCI patients is to identify or predict whether MCI progresses to AD in a patient. It is expected that DTI and cortical thickness analysis may provide potential quantitative imaging measurements in evaluation of MCI patients for their prognosis. It is of great interest for us to follow the MCI subjects enrolled in this study to determine whether there will be differences in changes of cortical thickness and DTI measured WM integrity between patients who eventually progress to AD and who remain with MCI.
Conclusion
DTI and cortical thickness analyses provide measurements of respective white and gray matter changes that may be associated with MCI. The changes in WM integrity and GM cortical structure were found mostly in the temporal areas of the left hemisphere in MCI patients. Using both DTI and cortical thickness measurements of the temporal lobe yielded high accuracy in distinguishing MCI patients from normal controls. Therefore, the combined use of these two techniques may provide a clinically applicable approach to improve the accuracy of diagnosing MCI. However, our preliminary findings as diagnostic imaging markers for MCI will need to be further investigated with a larger future study. The utility of such imaging markers will need to be tested, confirmed and further improved for its sensitivity in the situation of individual patient.
Acknowledgments
The authors thank Xiangchuan Chen and Longchuan Li (Department of Biomedical Engineering, Emory University) for their help in analyzing cortical thickness and DTI, Yu Zhang (MR Unit, VA Medical Center San Francisco, CA 94121) for her help on the ROC analysis and Eric Jablonowski (Department of Radiology, Emory University) for his help with illustrations.
This research was supported by the Emory Alzheimer's Disease Research Center (NIH-NIA P50 AG025688) and presented at: 16th International Society for Magnetic Resonance in Medicine (ISMRM), Toronto, Ontario, Canada. May 3-9, 2008
Abbreviations
- MRI
Magnetic resonance imaging
- DTI
Diffusion Tensor Imaging
- MCI
Mild Cognitive Impairment
- AD
Alzheimer's disease
- WM
White matter
- GM
Gray matter
- FA
Fractional anisotropy
- ADC
Apparent diffusion coefficient
- ROI
Region of interest
- ROC
Receiver operator characteristics
- AUC
Area under curve
- MMSE
Mini-Mental State Exam
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease
- IFG
inferior frontal gyrus
- mFG
medial frontal gyrus
- mTG
medial temporal gyrus
References
- 1.Kelley BJ, Petersen RC. Alzheimer's disease and mild cognitive impairment. Neurologic clinics. 2007;25:577–609. doi: 10.1016/j.ncl.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Archives of neurology. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review) Report of the Quality Standards Subcommittee of the American Academy of Neurology Neurology. 2001;56:1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 4.Petersen RC. Mild Cognitive Impairment: Aging to Alzheimer's disease. New York: Oxford University Press; 2003. [Google Scholar]
- 5.Rasquin SM, Lodder J, Visser PJ, Lousberg R, Verhey FR. Predictive accuracy of MCI subtypes for Alzheimer's disease and vascular dementia in subjects with mild cognitive impairment: a 2-year follow-up study. Dementia and geriatric cognitive disorders. 2005;19:113–119. doi: 10.1159/000082662. [DOI] [PubMed] [Google Scholar]
- 6.Matthews FE, Stephan BC, McKeith IG, Bond J, Brayne C. Two-year progression from mild cognitive impairment to dementia: to what extent do different definitions agree? J Am Geriatr Soc. 2008;56:1424–1433. doi: 10.1111/j.1532-5415.2008.01820.x. [DOI] [PubMed] [Google Scholar]
- 7.Becker JT, Davis SW, Hayashi KM, et al. Three-dimensional patterns of hippocampal atrophy in mild cognitive impairment. Archives of neurology. 2006;63:97–101. doi: 10.1001/archneur.63.1.97. [DOI] [PubMed] [Google Scholar]
- 8.deToledo-Morrell L, Stoub TR, Bulgakova M, et al. MRI-derived entorhinal volume is a good predictor of conversion from MCI to AD. Neurobiology of aging. 2004;25:1197–1203. doi: 10.1016/j.neurobiolaging.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 9.Du AT, Schuff N, Amend D, et al. Magnetic resonance imaging of the entorhinal cortex and hippocampus in mild cognitive impairment and Alzheimer's disease. Journal of neurology, neurosurgery, and psychiatry. 2001;71:441–447. doi: 10.1136/jnnp.71.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Killiany RJ, Hyman BT, Gomez-Isla T, et al. MRI measures of entorhinal cortex vs hippocampus in preclinical AD. Neurology. 2002;58:1188–1196. doi: 10.1212/wnl.58.8.1188. [DOI] [PubMed] [Google Scholar]
- 11.Pennanen C, Kivipelto M, Tuomainen S, et al. Hippocampus and entorhinal cortex in mild cognitive impairment and early AD. Neurobiology of aging. 2004;25:303–310. doi: 10.1016/S0197-4580(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 12.Rusinek H, Endo Y, De Santi S, et al. Atrophy rate in medial temporal lobe during progression of Alzheimer disease. Neurology. 2004;63:2354–2359. doi: 10.1212/01.wnl.0000148602.30175.ac. [DOI] [PubMed] [Google Scholar]
- 13.Stoub TR, Bulgakova M, Leurgans S, et al. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology. 2005;64:1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- 14.Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC. Focal decline of cortical thickness in Alzheimer's disease identified by computational neuroanatomy. Cereb Cortex. 2005;15:995–1001. doi: 10.1093/cercor/bhh200. [DOI] [PubMed] [Google Scholar]
- 15.Thompson PM, Hayashi KM, de Zubicaray G, et al. Dynamics of gray matter loss in Alzheimer's disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lerch JP, Evans AC. Cortical thickness analysis examined through power analysis and a population simulation. NeuroImage. 2005;24:163–173. doi: 10.1016/j.neuroimage.2004.07.045. [DOI] [PubMed] [Google Scholar]
- 17.Kim JS, Singh V, Lee JK, et al. Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. NeuroImage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Singh V, Chertkow H, Lerch JP, Evans AC, Dorr AE, Kabani NJ. Spatial patterns of cortical thinning in mild cognitive impairment and Alzheimer's disease. Brain. 2006;129:2885–2893. doi: 10.1093/brain/awl256. [DOI] [PubMed] [Google Scholar]
- 19.Fellgiebel A, Wille P, Muller MJ, et al. Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dementia and geriatric cognitive disorders. 2004;18:101–108. doi: 10.1159/000077817. [DOI] [PubMed] [Google Scholar]
- 20.Kantarci K, Jack CR, Jr, Xu YC, et al. Mild cognitive impairment and Alzheimer disease: regional diffusivity of water. Radiology. 2001;219:101–107. doi: 10.1148/radiology.219.1.r01ap14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller MJ, Greverus D, Dellani PR, et al. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. NeuroImage. 2005;28:1033–1042. doi: 10.1016/j.neuroimage.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 22.Rose SE, Chen F, Chalk JB, et al. Loss of connectivity in Alzheimer's disease: an evaluation of white matter tract integrity with colour coded MR diffusion tensor imaging. Journal of neurology, neurosurgery, and psychiatry. 2000;69:528–530. doi: 10.1136/jnnp.69.4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi S, Yonezawa H, Takahashi J, Kudo M, Inoue T, Tohgi H. Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neuroscience letters. 2002;332:45–48. doi: 10.1016/s0304-3940(02)00914-x. [DOI] [PubMed] [Google Scholar]
- 24.Choi SJ, Lim KO, Monteiro I, Reisberg B. Diffusion tensor imaging of frontal white matter microstructure in early Alzheimer's disease: a preliminary study. Journal of geriatric psychiatry and neurology. 2005;18:12–19. doi: 10.1177/0891988704271763. [DOI] [PubMed] [Google Scholar]
- 25.Head D, Buckner RL, Shimony JS, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14:410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- 26.Medina D, DeToledo-Morrell L, Urresta F, et al. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiology of aging. 2006;27:663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Naggara O, Oppenheim C, Rieu D, et al. Diffusion tensor imaging in early Alzheimer's disease. Psychiatry research. 2006;146:243–249. doi: 10.1016/j.pscychresns.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Xie S, Xiao JX, Gong GL, et al. Voxel-based detection of white matter abnormalities in mild Alzheimer disease. Neurology. 2006;66:1845–1849. doi: 10.1212/01.wnl.0000219625.77625.aa. [DOI] [PubMed] [Google Scholar]
- 29.Fellgiebel A, Muller MJ, Wille P, et al. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiology of aging. 2005;26:1193–1198. doi: 10.1016/j.neurobiolaging.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Huang J, Auchus AP. Diffusion tensor imaging of normal appearing white matter and its correlation with cognitive functioning in mild cognitive impairment and Alzheimer's disease. Annals of the New York Academy of Sciences. 2007;1097:259–264. doi: 10.1196/annals.1379.021. [DOI] [PubMed] [Google Scholar]
- 31.Stahl R, Dietrich O, Teipel SJ, Hampel H, Reiser MF, Schoenberg SO. White matter damage in Alzheimer disease and mild cognitive impairment: assessment with diffusion-tensor MR imaging and parallel imaging techniques. Radiology. 2007;243:483–492. doi: 10.1148/radiol.2432051714. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Schuff N, Jahng GH, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori Susumu WS, van Zijl Peter CM, Nagae-Poetscher Lidia M. MRI Atlas Of Human White Matter. Elsevier Science Ltd; 2005. [Google Scholar]
- 34.Dickerson BC, Feczko E, Augustinack JC, et al. Differential effects of aging and Alzheimer's disease on medial temporal lobe cortical thickness and surface area. Neurobiology of aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 36.Feczko E, Augustinack JC, Fischl B, Dickerson BC. An MRI-based method for measuring volume, thickness and surface area of entorhinal, perirhinal, and posterior parahippocampal cortex. Neurobiology of aging. 2007 doi: 10.1016/j.neurobiolaging.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 39.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 40.Wechsler D. Wechsler Memory Scale-Revised manual. San Antonio, Texas: The Psychological Corporation; 1987. [Google Scholar]
- 41.Benedict R. Brief Visuospatial Memory Test-revised. Odessa Florida: Psychological Assessment Resources, Inc; 1997. [Google Scholar]
- 42.Braak H, Braak E. Evolution of neuronal changes in the course of Alzheimer's disease. J Neural Transm Suppl. 1998;53:127–140. doi: 10.1007/978-3-7091-6467-9_11. [DOI] [PubMed] [Google Scholar]
- 43.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Annals of neurology. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 44.Kordower JH, Chu Y, Stebbins GT, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Annals of neurology. 2001;49:202–213. [PubMed] [Google Scholar]
- 45.Seo SW, Im K, Lee JM, et al. Cortical thickness in single- versus multiple-domain amnestic mild cognitive impairment. NeuroImage. 2007;36:289–297. doi: 10.1016/j.neuroimage.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 46.Villain N, Desgranges B, Viader F, et al. Relationships between hippocampal atrophy, white matter disruption, and gray matter hypometabolism in Alzheimer's disease. J Neurosci. 2008;28:6174–6181. doi: 10.1523/JNEUROSCI.1392-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karas GB, Scheltens P, Rombouts SA, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer's disease. NeuroImage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]