Abstract
We previously found that sustained ERK activation contributes to toxicity elicited by the parkinsonian neurotoxin 6-hydroxydopamine. In addition, substantia nigra neurons from patients with incidental Lewy body disease, Parkinson’s disease (PD), and diffuse Lewy body dementia (DLB) display abnormal phospho-ERK accumulations in the form of discrete cytoplasmic granules. In this study, we investigated the subcellular localization of phospho-ERK immunoreactive granules using double label confocal microscopy and immuno-electron microscopy. A small percentage of phospho-ERK granules co-localized with the early endosome marker Rab5, but not with cathepsin D, 20S proteasome β-subunit, or cytochrome P450 reductase. Phospho-ERK immunoreactivity was often associated with mitochondrial proteins (MnSOD, 60 kDa and 110 kDa mitochondrial antigens), and some vesicular-appearing phospho-ERK granules appeared to envelop enlarged mitochondria by confocal laser scanning microscopy. Ultrastructural immuno-gold studies revealed phospho-ERK labeling in mitochondria and in association with bundles of ~10 nm fibrils. Heavily labeled mitochondria were observed within autophagosomes. As mitochondrial pathology may play a pivotal role in Parkinson’s and other related neurodegenerative diseases, these studies suggest a potential interaction between dysfunctional mitochondria, autophagy, and ERK signaling pathways.
Introduction
Parkinson’s disease (PD), PD with dementia/diffuse Lewy body disease (DLB), and syndromes with features of both DLB and Alzheimer’s disease (AD) represent an important group of clinically and pathologically overlapping neurodegenerative disorders (14, 18, 19). They share in common the presence of α-synucle-in-rich cytoplasmic inclusions called Lewy bodies in degenerating populations of neurons. In addition, common pathogenic mechanisms implicated in these diseases include oxidative stress, mitochondrial pathology, disordered protein degradation and abnormalities in kinase signaling (4, 5, 8, 16, 20, 29, 34, 39, 53).
The mitogen activated protein (MAP) kinase superfamily includes three major branches, which have been implicated in PD and AD pathogenesis (23, 37, 50, 52, 53). The extracellular signal-regulated kinases (ERK) are involved in regulating neuronal survival, differentiation and plasticity. A growing number of recent studies also indicate that ERK activation plays a detrimental role in oxidative neuronal injury (9, 23, 31, 32, 43). The neurotoxin 6-hydroxydopamine elicits an abnormally sustained pattern of ERK activation that contributes to neuronal cell death (23). Sustained ERK activation can be blocked with neuroprotective doses of catalase and metalloporphyrin antioxidants (22), implicating redox mechanisms in neurotoxic kinase signaling.
Cytoplasmic accumulations of phosphorylated ERK (phospho-ERK) have been noted in human PD and DLB substantia nigra tissues and in 6-hydroxydopamine treated neuronal cell cultures (17, 52). While phospho-ERK elicited by trophic stimuli or in ischemic brain tissues typically display diffuse cytoplasmic staining and nuclear translocation, a lack of significant nuclear localization was noted in both human PD/DLB brains and in the 6-hydroxydopamine model (52). As the biological effects of ERK phosphorylation are critically dependent upon subcellular localization and access to downstream targets (27, 36, 38), we investigated the subcellular distribution of phospho-ERK in Lewy body diseases using double label confocal microscopy and immunogold electron microscopy.
Methods
Human tissues
Midbrain tissues from PD, DLB and control subjects were obtained from the University of Pittsburgh Brain Bank under a protocol approved by the University of Pittsburgh Institutional Review Board. Clinical and neuropathological characteristics of this set have been previously described (52). A subset of cases with appropriately fixed tissues was used in this study (Table 1). Post-mortem intervals ranged from 4 to 10 hours. We have previously reported preliminary immunofluorescence data from a single patient (13).
Table 1. Clinical and pathologic characteristics of cases examined.
Lewy body (LB) score for cortical regions, Braak stage, and CERAD plaque scores were determined using established consensus criteria (11, 28).
| Clinical Dx | Age/Gender | Pathologic Dx | LB score | Braak | CERAD Plaque |
|---|---|---|---|---|---|
| Normal | 65 year/M | Normal* | 0 | II | 0 |
| PD | 87 year/F | PD | 2/10 | I | 0 |
| PD | 79 year/M | PD | 0/10 | I | 0 |
| PD with depression | 83 year/M | DLB* | 8/10 | III | A–B |
| AD | 79 year/F | DLB, AD | 5/10 | V | C |
| PD with dementia vs. AD | 74 year/M | DLB, AD* | 5/10 | V | C |
Cases used in immuno-electron microscopy studies. As we have previously shown that midbrain tissue from age-matched control subjects exhibit virtually no P-ERK immunoreactivity (52), only one additional control case was examined by immuno-EM.
Immunofluorescence
Immunofluorescence for phospho-ERK was performed as previously described (52). In brief, an activation-specific polyclonal rabbit antibody that specifically recognizes the dually phosphorylated forms of ERK1 and ERK2 (1:10000; Sigma Immunochemicals) was used in conjunction with a tyramide amplification system (1:100; TSA™ Plus Fluorescence system; PerkinElmer Life Sciences ). Double-label immunofluorescence was conducted using a two-step protocol (48). After staining for phospho-ERK1/2, the sections were rinsed with PBST and then incubated with the following antibodies at 4°C for 24 to 48 hours, followed by Cy™ 3-conjugated 2°antibodies (Jackson Immunoresearch): Rab5 (1:50; Oncogene); 20S proteasome, β-subunit (1:400; Calbiochem); cathepsin D (1:20; Santa Cruz); cytochrome P450 reductase (1:25; Santa Cruz); MnSOD (1:50; Upstate); mitochondrial antigen 60KD (clone 113-1, 1:25; BioGenex); P110 mitochondrial protein (clone 2G2, 1:5; Oncogene). For negative controls, the phospho-ERK1/2 antibody or primary organelle-specific antibodies were replaced with nonimmune rabbit or mouse IgG.
The slides were observed using a Zeiss Axioplan 2 confocal imaging system. An Ar 488/HeNEL 543 laser provided the excitation light beam, and a neutral density filter (T 0.01) was used to uniformly attenuate the intensity of laser light. The excitation light (488 and 543 nm) was passed through a dichroic beam splitter (HFT 488/543/635) that allows the capture of images in both green and red channels simultaneously. The Z interval was adjusted to 0.25 μm. Co-localization was confirmed using Z-sectioning and orthogonal image analysis.
Immunoelectron microscopy
Substantia nigra tissues were fixed with 2% paraformaldehyde, 0.01% glutaraldehyde in 0.1 M PBS and stored at 4°C for one hour. The tissue was minced to pieces less than one mm3 in size, and then infused with 2.3 M sucrose in 0.1 M PBS overnight at 4°C. Tissue was frozen on ultra-cryotome stubs under liquid nitrogen and stored in liquid nitrogen until use. Ultrathin sections (70–100 nm) were cut using a Reichert Ultracut U ultramicrotome with a FC4S cryo-attachment, lifted on a small drop of 2.3 M sucrose and mounted on Formvar-coated copper grids. Sections were washed 3 times with PBS, then 3 times with PBS containing 0.5% bovine serum albumin and 0.15% glycine (PBG buffer) followed by a 30-minute incubation with 5% normal goat serum in PBG. Sections were incubated in the phospho-ERK1/2 antibody (1:50) for one hour then labeled with 10 nm colloidal gold-conjugated anti-rabbit antibody (1:25; Amersham Biosciences) at room temperature for one hour (Amersham, Piscataway, NJ). Sections were washed 3 times in PBG, 3 times in PBS, then fixed in 2.5% glutaraldehyde in PBS for 5 minutes, washed 2 times in PBS then washed 6 times in ddH2O. Sections were post-stained in 2% neutral uranyl acetate, for 7 minutes, washed 3 times in ddH2O, stained 2 minutes in 4% uranyl acetate, then embedded in 1.25% methyl cellulose. Labeling was observed on a JEOL JEM 1210 electron microscope (Peabody, Mass) at 80 kV.
Results
Characterization of cytoplasmic phospho-ERK expression
Immunofluorescence studies demonstrated discrete, coarse, granular cytoplasmic accumulations of phospho-ERK in the substantia nigra of all PD and DLB patient brains (Figure 1A, D, H, I). Our previous studies indicated that coarse phospho-ERK granules were limited to the cytoplasm of large substantia nigra neurons, and were not seen in glial cells or other non-pigmented midbrain nuclei (52). This staining pattern was virtually absent in 7 age-matched control cases (52), and from 3 Alzheimer’s disease brains lacking co-morbid α-synuclein pathology (CTC, unpublished data). The phospho-ERK granules sometimes had a vesicular appearance by confocal microscopy, and a central particle could occasionally be discerned (Figure 1D, G, H).
Figure 1.
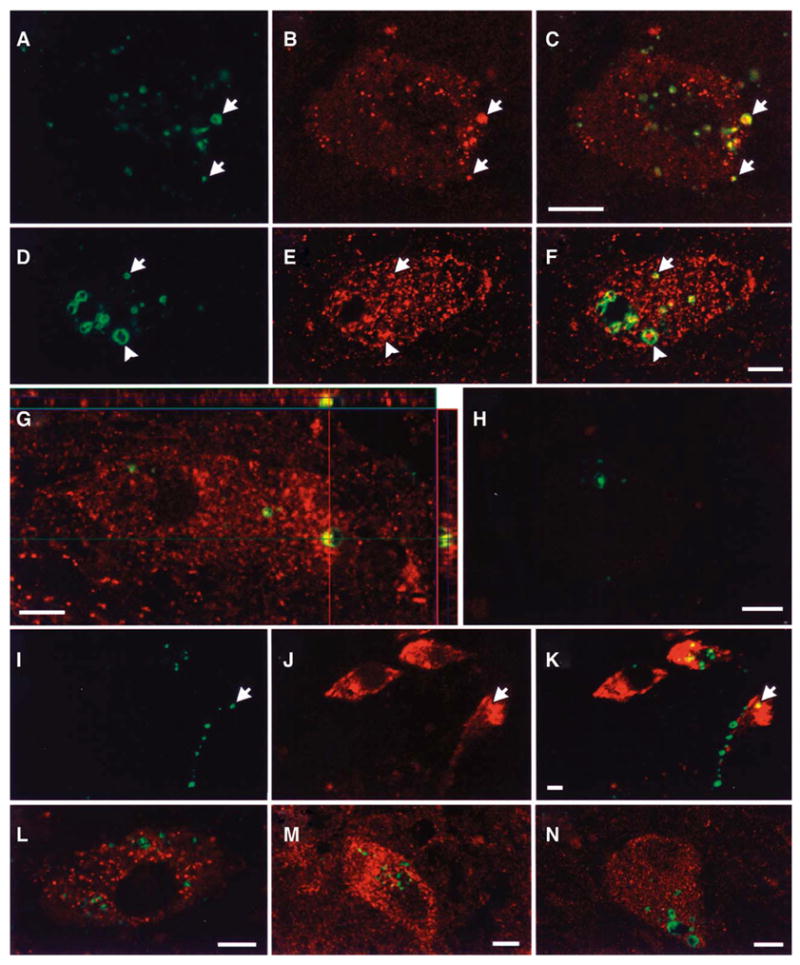
Double immunofluorescence confocal laser scanning micrographs of human PD and DLB cases stained for phospho-ERK and organellar markers. Phospho-ERK was labeled with fluorescein (green), and the organellar markers were labeled with Cy3 (red). There was colocalization (arrows) of phospho-ERK granules with three different mitochondrial markers: (A–C) the P110 mitochondrial protein, (D–F) the 60 kDa mitochondrial antigen, and (G) MnSOD, the mitochondrial isoform of SOD. Occasionally enlarged mitochondria appeared to be present within vesicular phospho-ERK structures (D–F, arrowhead). Z-sectioning and orthogonal analysis was used to confirm colocalization (Shown for example for MnSOD in panel G). H. A merged image of phospho-ERK (green) double labeled with nonimmune mouse serum (red) to show specificity of the mitochondrial antibodies. Nonimmune rabbit serum likewise showed appropriate lack of staining. I–K. The majority of phospho-ERK granules did not co-localize with the early endosome marker Rab 5, although in a few cases there was some overlap of rare phospho-ERK granules with Rab 5 rich regions. No colocalization of phospho-ERK was observed with (L) cathepsin D, a marker of mature lysosomes, (M) the 20S proteasome, β-subunit, or (N) cytochrome P450 reductase of the endoplasmic reticulum. Scale bars = 10 μm
Association of phospho-ERK with mitochondrial markers
Three selective antibodies for mitochondria were used in this study. Manganese superoxide dismutase (MnSOD) is an important antioxidant enzyme that is exclusively located in mitochondria (49). The other 2 antibodies specifically recognize protein components of human mitochondria (35). Double immunofluorescence revealed that there was an association between many phospho-ERK granules and each of the 3 mitochondrial markers (Figure 1A-G). Phospho-ERK granules were often larger than the mitochondria. Vesicular-appearing phospho-ERK immunoreactivity sometimes appeared to surround enlarged mitochondrial profiles (Figure 1F, arrowhead) (13), and sometimes enlarged mitochondria colocalized with the central particle within vesicular phospho-ERK granules (Figure 1G). Co-localization of phospho-ERK immunofluorescence with mitochondrial markers was confirmed using Z-sectioning and orthogonal image analysis for each antigen (For example, MnSOD in Figure 1G). A full set of negative controls was performed by systematically substituting each of the primary antibodies with the appropriate nonimmune sera in double fluorescence studies. For example, see Figure 1H for positive phospho-ERK1/2 stain (green) merged with negative nonimmune serum control (red) to show specificity of the mitochondrial staining.
Interaction between phospho-ERK and endosomal-lysosomal system
There is evidence that ERK signaling modules may assemble on the cytoplasmic side of signaling endosomes (40). Rab5, a small GTPase, has been localized to the early endosome, and is involved in the regulation of the endocytosis (15). We found that Rab5 was mainly present in the submembranous and perinuclear regions. In occasional neurons, rare phospho-ERK granules were associated with the early endosome marker Rab5 (Figure 1K). We also investigated whether phospho-ERK accumulations may be targeted to the lysosomal system. Cathepsin D, a proteolytic enzyme present in mature lysosomes showed no association with phospho-ERK granules (Figure 1L).
Relationship of phospho-ERK with other organelles
Because dysfunction of the intracellular degradation pathway (30) or abnormal protein folding in the endoplasmic reticulum (41) have been proposed as potential mechanistic factors in neurodegeneration associated with Parkinson’s disease, we examined potential co-localization of phospho-ERK with proteasome and endoplasmic reticulum components. There was no association between phospho-ERK granules and the β-subunit of 20S proteasome (Figure 1M). Likewise, phospho-ERK granules were not associated with cytochrome P450 reductase, an enzyme located in the endoplasmic reticulum (Figure 1N).
Ultrastructural characterization of phospho-ERK immunoreactivity
Substantia nigra neurons were identified at screening power by size and abundant neuromelanin particles. Immunogold labeling studies revealed two discrete patterns of phospho-ERK immunoreactivity (Figure 2). Intense immunolabeling was observed in association with bundles of ~10 nm thick fibrils (Figure 2A), or in membrane-delimited cytoplasmic structures (Figure 2B). No significant labeling was detected within nearby neuromelanin granules or in association with lipofuscin. Diffuse labeling was sometimes observed in rare glial nuclei (not illustrated), but not in neuronal nuclei. No immuno-gold labeling for phospho-ERK was observed in the one control case examined by immuno-electron microscopy, consistent with our negative immunohistochemical and immunofluorescence studies of 7 other age-matched control cases (52).
Figure 2.
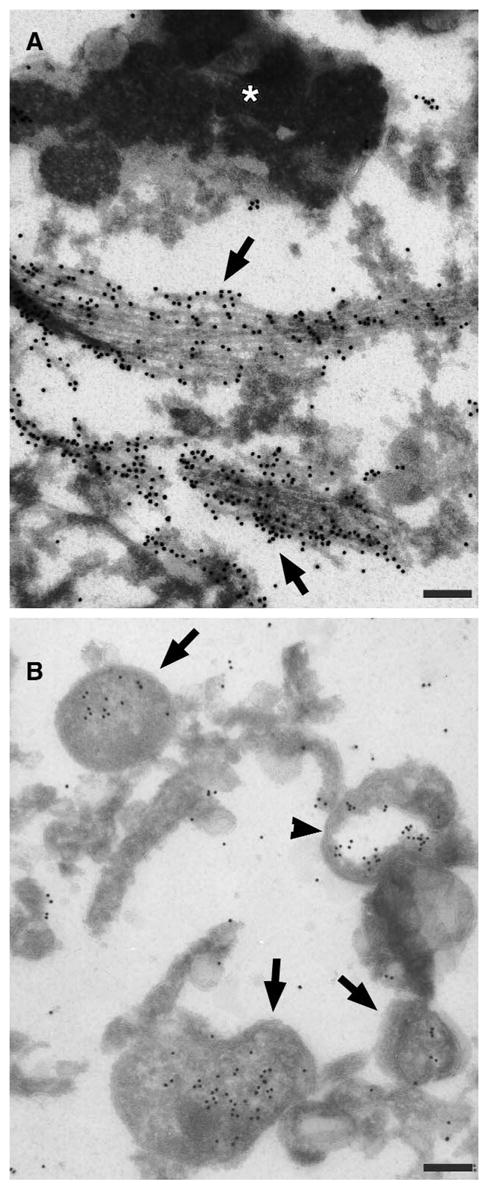
Immuno-gold localization of phospho-ERK to cytoplasmic structures in substantia nigra neurons. A. There was intense phospho-ERK labeling of ~10 nm filamentous or fibrillar bundles (arrows), but not of adjacent neuromelanin and lipofuscin (asterisk). B. Phospho-ERK labeling of discrete cytoplasmic organelles (arrows) was also observed. Some of these were delimited by multiple negatively-stained membrane layers (arrowhead). Scale bars = 200 nm.
Selective labeling was observed within membrane delimited cytoplasmic organelles consistent in size, electron density and shape with mitochondria (Figures 2B, 3). Negatively stained internal cristae could be discerned but were usually disorganized (Figure 3, asterisks). The majority of more normal appearing mitochondria were unlabeled (Figure 3E). In contrast, heavily labeled mitochondria were often delimited from the cytoplasm by an additional single (Figure 3B) or double outer membrane (Figure 3C), indicative of mitochondrial autophagocytosis.
Figure 3.
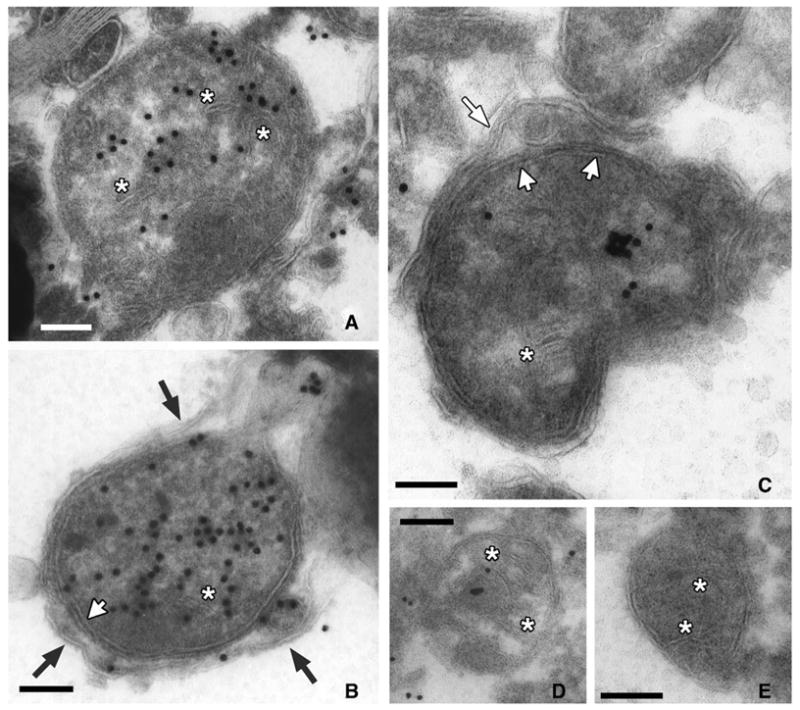
Immuno-gold localization of phospho-ERK to abnormal mitochondria. A–D. Gold particles were concentrated within double membrane delimited cytoplasmic structures (short white arrows), most of which showed internal membranes (asterisks) consistent with mitochondria. Membranes appear as negatively stained structures in these uranyl-stained sections. At higher magnification, a distinct single (B, black arrows) or double (C, long white arrow) membrane can be seen extending around many of the mitochondria, indicative of autophagocytosis. Gold labeling was also observed within the autophagosome membrane (B, lower right). The most heavily labeled mitochondria showed disorganized internal membranes (A, B). E. The majority of normal appearing mitochondria with organized parallel cristae (asterisks) showed little to no labeling. Scale bars = 100 nm.
Discussion
Abnormally sustained activation of ERK contributes to 6-OHDA toxicity in B65 (23) and SH-SY5Y cell lines (C. T. Chu, unpublished data). Immunocytochemistry reveals that phospho-ERK staining is cytoplasmic, attaining a punctate or vesicular appearance at time points corresponding with commitment to cell death (52). Moreover, substantia nigra neurons in patients with the full spectrum of Lewy body diseases display unusual, discrete cytoplasmic accumulations of phospho-ERK immunoreactivity (52). In this study, we found that the phospho-ERK granules in human PD and DLB substantia nigra neurons were rarely associated with an endosomal marker, but were commonly associated with three different mitochondrial markers. Ultrastructurally, phospho-ERK immunogold labeling was associated with filamentous bundles as well as accumulating in mitochondria and autophagosomes.
Mitochondria are vulnerable to various insults and can undergo enlargement and structural disorganization, associated with decreased membrane potential and reduced ATP production. Not only are mitochondria important for energy homeostasis, but also they appear to play pivotal roles in the active regulation of cell death. Moreover, a variety of human and experimental studies implicate mitochondrial dysfunction as a key element in PD pathogenesis (5, 8, 45).
Phospho-ERK can regulate cell survival/death through phosphorylation of Bcl-2 family members (3, 6). In addition, MAP kinase signaling modules have been reported in mitochondrial fractions isolated from cardiac myocytes (3). While it is unclear whether the MAP kinases are located within cardiac mitochondria or are assembled cytoplasmically in association with the outer membrane, there is biochemical evidence supporting a role for other kinases in mitochondrial inner membrane and matrix fractions (46). To our knowledge, this study of Lewy body disease neurons presents the first ultra-structural demonstration of an activated kinase inside mitochondria. While it is unclear whether ERK is normally present within mitochondria or gains entry following mitochondrial injury, the association of phospho-ERK granules with a subset of mitochondria in PD and DLB brains suggest a potential interaction between mitochondrial function and ERK signaling pathways in parkinsonian neurodegeneration.
In addition to potential effects upon mitochondrial biology and turnover, altered patterns of phospho-ERK subcellular localization could influence neuronal viability and function through transcriptional effects as well. ERK signaling is typically initiated by receptor-mediated activation of Ras family GTPases, followed by sequential activation of cytoplasmic kinases (12, 36). Activated ERK then classically translocates to the nucleus and regulates trophic or pro-survival transcription (6, 38). Thus, cytoplasmic retention or diversion of ERK and/or its downstream targets may serve to inhibit neuroprotective transcriptional responses to injury (E. M. Chalovich and C. T. Chu, unpublished data). It is interesting to note that, in our cell culture model, persistent, cytoplasmic ERK phosphorylation can be inhibited by antioxidant treatment (22, 52). This suggests potential differences in the mechanism and downstream effects of oxidatively activated ERK compared to ERK activated in response to trophic stimuli.
Dysfunction of cellular degradation has been implicated in PD pathogenesis. Macroautophagy is the primary means of degrading long-lived proteins and damaged organelles. The autophagic process begins with the remodeling of subcellular membranes into double-membrane vesicles that sequester cytoplasm and organelles in autophagic vacuoles. Shortly thereafter, the inner membrane is lost, resulting in single-membrane vesicles containing recognizable organelles (42). The contents of the autophagolysosome are ultimately degraded by lysosomal degradative enzymes (21). While autophagocytosis has been studied largely in the context of starvation or large scale tissue remodeling, recent evidence suggests that autophagy may also characterize a form of programmed cell death (7).
Increased autophagosomes have been observed in human PD nigral neurons (2), but not in nigral neurons during normal aging (1). Recent experimental evidence also implicates autophagy in mechanisms of dopamine neuron toxicity (24, 25, 44). PC12 cells transfected with the mutant A53T α-synuclein undergo an autophagosome-rich form of cell death distinct from apoptosis (44). There was little evidence of colocalization of α-synuclein with cathepsin D, suggesting that deleterious effects of mutant α-synuclein may occur at the level of endosomes or early autophagosomes (44). Likewise, we found accumulation of phospho-ERK in human substantia nigra neurons in association with early autophagosomes by immuno-EM, but did not observe significant colocalization of phospho-ERK granules with mature lysosomal structures containing cathepsin D or lipofuscin. The absence of phospho-ERK immunoreactivity in mature lysosomes may reflect dephosphorylation or degradation within lysosomes, or dissociation from autophagosomes prior to formation of secondary lysosomes.
Intense labeling of phospho-ERK was also observed in association with ~10 nm fibrils. These may represent intermediate filaments such as neurofilament, which is a component of Lewy bodies. In our immunofluorescence studies, we noted association of P-ERK with the halo region of Lewy bodies, and in the periphery of α-synuclein immunoreactive deposits (52). Interestingly, α-synuclein itself appears to associate with signaling proteins including ERK (34). A subset of substantia nigra neurons also exhibited colocalization of P-ERK with the endosomal marker Rab5. Indeed, P-ERK signaling modules have been shown to assemble on the cytoplasmic surface of early endosomes (40), particularly in association with β-arrestin scaffolds (27). Moreover, phospho-ERK1 is able to phosphorylate Rab5a, suggesting a role in regulating membrane trafficking (10). A possible role for ERK2 in regulating colon cancer cell autophagy has also been reported (33).
It has been proposed that membrane potential alterations involving increasing numbers of mitochondria links autophagy, apoptosis, and necrosis into a mechanistic continuum (26). Selective mitochondrial removal from cells has been noted with many forms of injury, and may correspond with commitment to cell death (47). Autophagy has been suggested as an alternative mechanism of death execution in post-mitotic sympathetic neurons (51). Alternatively, increased autophagocytosis may represent a protective, compensatory response to neuronal injury, potentially sequestering harmful products of damaged mitochondria from the cytosol. Further studies to elucidate the role of mitochondrial autophagy in Parkinsonian pathogenesis will serve to clarify these issues.
To summarize, discrete phospho-ERK accumulations are associated with abnormal mitochondria, autophagosomes, and fibrillar cellular elements within substantia nigra neurons of patients with PD and DLB. While the mechanisms of cell death in Parkinson’s and related Lewy body diseases are still unclear, altered kinetics and subcellular localization of activated kinases may serve to regulate mitochondrial and autophagic responses during the neurodegenerative process.
Acknowledgments
Supported by grants from the National Institutes of Health (R01 NS40817) and the Rockefeller Brothers Fund (CTC is a Charles E. Culpeper Scholar in Medical Science).
References
- 1.Anglade P, Vyas S, Hirsch EC, Agid Y. Apoptosis in dopaminergic neurons of the human substantia nigra during normal aging. Histol Histopathol. 1997;12:603–610. [PubMed] [Google Scholar]
- 2.Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson’s disease. Histol Histopathol. 1997;12:25–31. [PubMed] [Google Scholar]
- 3.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Card-well EM, Bolli R, Ping P. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 4.Beal MF. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995;38:357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- 5.Betarbet R, Sherer TB, Di Monte DA, Greenamyre JT. Mechanistic approaches to Parkinson’s disease pathogenesis. Brain Pathol. 2002;12:499–510. doi: 10.1111/j.1750-3639.2002.tb00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- 7.Bursch W. The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ. 2001;8:569–581. doi: 10.1038/sj.cdd.4400852. [DOI] [PubMed] [Google Scholar]
- 8.Cassarino DS, Halvorsen EM, Swerdlow RH, Abramova NN, Parker WD, Jr, Sturgill TW, Bennett JP., Jr Interaction among mitochondria, mitogen-activated protein kinases, and nuclear factor-kappaB in cellular models of Parkinson’s disease. J Neurochem. 2000;74:1384–1392. doi: 10.1046/j.1471-4159.2000.0741384.x. [DOI] [PubMed] [Google Scholar]
- 9.Cha YK, Kim YH, Ahn YH, Koh JY. Epidermal growth factor induces oxidative neuronal injury in cortical culture. J Neurochem. 2000;75:298–303. doi: 10.1046/j.1471-4159.2000.0750298.x. [DOI] [PubMed] [Google Scholar]
- 10.Chiariello M, Bruni CB, Bucci C. The small GTPases Rab5a, Rab5b and Rab5c are differentially phosphorylated in vitro. FEBS Lett. 1999;453:20–24. doi: 10.1016/s0014-5793(99)00686-9. [DOI] [PubMed] [Google Scholar]
- 11.Chu CT, Caruso JL, Cummings TJ, Ervin J, Rosenberg C, Hulette CM. Ubiquitin immunochemistry as a diagnostic aid for community pathologists evaluating patients who have dementia. Modern Pathology. 2000;13:420–426. doi: 10.1038/modpathol.3880072. [DOI] [PubMed] [Google Scholar]
- 12.Chu CT, Everiss KD, Batra S, Wikstrand CJ, Kung H-J, Bigner DD. Receptor dimerization is not a factor in the signalling activity of a transforming variant epidermal growth factor receptor (EGFRvIII) Biochem J. 1997;324:855–861. doi: 10.1042/bj3240855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chu CT, Zhu J-H. Subcellular compartmentalization of P-ERK in the Lewy body disease substantia nigra. Ann NY Acad Sci. 2003;991:288–290. [Google Scholar]
- 14.Dickson DW. Alpha-synuclein and the Lewy body disorders. Curr Opin Neurol. 2001;14:423–432. doi: 10.1097/00019052-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Duclos S, Corsini R, Desjardins M. Remodeling of endosomes during lysosome biogenesis involves ‘kiss and run’ fusion events regulated by rab5. J Cell Sci. 2003;116:907–918. doi: 10.1242/jcs.00259. [DOI] [PubMed] [Google Scholar]
- 16.Duda JE, Giasson BI, Chen Q, Gur TL, Hurtig HI, Stern MB, Gollomp SM, Ischiropoulos H, Lee VM, Trojanowski JQ. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am J Pathol. 2000;157:1439–1445. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrer I, Blanco R, Carmona M, Puig B, Barrachina M, Gomez C, Ambrosio S. Active, phosphorylation-dependent mitogen-activated protein kinase (MAPK/ERK), stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK), and p38 kinase expression in Parkinson’s disease and Dementia with Lewy bodies. J Neural Transm. 2001;108:1383–1396. doi: 10.1007/s007020100015. [DOI] [PubMed] [Google Scholar]
- 18.Galvin JE, Lee VM, Trojanowski JQ. Synucleinopathies: clinical and pathological implications. Arch Neurol. 2001;58:186–190. doi: 10.1001/archneur.58.2.186. [DOI] [PubMed] [Google Scholar]
- 19.Goedert M. Alpha-synuclein and neurodegenerative diseases. Nat Rev Neurosci. 2001;2:492–501. doi: 10.1038/35081564. [DOI] [PubMed] [Google Scholar]
- 20.Jenner P, Olanow CW. Understanding cell death in Parkinson’s disease. Ann Neurol. 1998;44(Suppl 1):S72–S84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- 21.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290 :1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kulich SM, Chu CT. Role of reactive oxygen species in ERK phosphorylation and 6-hydroxydopamine cytotoxicity. J Biosci. 2003;28:83–89. doi: 10.1007/BF02970136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kulich SM, Chu CT. Sustained extracellular signal-regulated kinase activation by 6-hydroxydopamine: Implications for Parkinson’s disease. J Neurochem. 2001;77:1058–1066. doi: 10.1046/j.1471-4159.2001.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci. 2002;22:8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen KE, Sulzer D. Autophagy in neurons: a review. Histol Histopathol. 2002;17:897–908. doi: 10.14670/HH-17.897. [DOI] [PubMed] [Google Scholar]
- 26.Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, Crowe RA, Cascio WE, Bradham CA, Brenner DA, Herman B. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–196. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- 27.Luttrell LM. ‘Location, location, location’: activation and targeting of MAP kinases by G protein-coupled receptors. J Mol Endocrinol. 2003;30:117–126. doi: 10.1677/jme.0.0300117. [DOI] [PubMed] [Google Scholar]
- 28.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 29.McNaught KS, Jenner P. Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci Lett. 2001;297:191–194. doi: 10.1016/s0304-3940(00)01701-8. [DOI] [PubMed] [Google Scholar]
- 30.McNaught KS, Olanow CW, Halliwell B, Isacson O, Jenner P. Failure of the ubiquitin-proteasome system in Parkinson’s disease. Nat Rev Neurosci. 2001;2:589–594. doi: 10.1038/35086067. [DOI] [PubMed] [Google Scholar]
- 31.Namura S, Iihara K, Takami S, Nagata I, Kikuchi H, Matsushita K, Moskowitz MA, Bonventre JV, Alessandrini A. Intravenous administration of MEK inhibitor U0126 affords brain protection against forebrain ischemia and focal cerebral ischemia. Proc Natl Acad Sci U S A. 2001;98:11569–11574. doi: 10.1073/pnas.181213498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noshita N, Sugawara T, Hayashi T, Lewen A, Omar G, Chan PH. Copper/zinc superoxide dismutase attenuates neuronal cell death by preventing extracellular signal-regulated kinase activation after transient focal cerebral ischemia in mice. J Neurosci. 2002;22:7923–7930. doi: 10.1523/JNEUROSCI.22-18-07923.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogier-Denis E, Pattingre S, El Benna J, Codogno P. Erk1/2-dependent phosphorylation of Galpha-interacting protein stimulates its GTPase accelerating activity and autophagy in human colon cancer cells. J Biol Chem. 2000;275:39090–39095. doi: 10.1074/jbc.M006198200. [DOI] [PubMed] [Google Scholar]
- 34.Ostrerova N, Petrucelli L, Farrer M, Mehta N, Choi P, Hardy J, Wolozin B. alpha-Synuclein shares physical and functional homology with 14-3-3 proteins. J Neurosci. 1999;19:5782–5791. doi: 10.1523/JNEUROSCI.19-14-05782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulin-Levasseur M, Chen G, Lariviere C. The 2G2 antibody recognizes an acidic 110-kDa human mitochondrial protein. Histochem J. 1998;30:617–625. doi: 10.1023/a:1003577609799. [DOI] [PubMed] [Google Scholar]
- 36.Pearson G, Robinson F, Gibson TB, Xu B–E, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocrine Reviews. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 37.Perry G, Roder H, Nunomura A, Takeda A, Friedlich AL, Zhu X, Raina AK, Holbrook N, Siedlak SL, Harris PL, Smith MA. Activation of neuronal extracellular receptor kinase (ERK) in Alzheimer disease links oxidative stress to abnormal phosphorylation. Neuroreport. 1999;10:2411–2415. doi: 10.1097/00001756-199908020-00035. [DOI] [PubMed] [Google Scholar]
- 38.Pouyssegur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem Pharmacol. 2002;64:755–763. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- 39.Rideout HJ, Larsen KE, Sulzer D, Stefanis L. Proteasomal inhibition leads to formation of ubiquitin/alpha-synuclein-immunoreactive inclusions in PC12 cells. J Neurochem. 2001;78:899–908. doi: 10.1046/j.1471-4159.2001.00474.x. [DOI] [PubMed] [Google Scholar]
- 40.Rizzo MA, Shome K, Watkins SC, Romero G. The recruitment of Raf-1 to membranes is mediated by direct interaction with phosphatidic acid and is independent of association with Ras. J Biol Chem. 2000;275:23911–23918. doi: 10.1074/jbc.M001553200. [DOI] [PubMed] [Google Scholar]
- 41.Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. J Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shelburne JD, Arstila AU, Trump BF. Studies on cellular autophagocytosis. The relationship of autophagocytosis to protein synthesis and to energy metabolism in rat liver and flounder kidney tubules in vitro. Am J Pathol. 1973;73:641–670. [PMC free article] [PubMed] [Google Scholar]
- 43.Stanciu M, Wang Y, Kentor R, Burke N, Watkins S, Kress G, Reynolds I, Klann E, Angiolieri M, Johnson J, DeFranco DB. Persistent activation of ERK contributes to glutamate-induced oxidative toxicity in a neuronal cell line and primary cortical neuron cultures. J Biol Chem. 2000;275:12200–12206. doi: 10.1074/jbc.275.16.12200. [DOI] [PubMed] [Google Scholar]
- 44.Stefanis L, Larsen KE, Rideout HJ, Sulzer D, Greene LA. Expression of A53T mutant but not wild-type alpha-synuclein in PC12 cells induces alterations of the ubiquitin-dependent degradation system, loss of dopamine release, and autophagic cell death. J Neurosci. 2001;21:9549–9560. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swerdlow RH, Parks JK, Davis JN, 2nd, Cassarino DS, Trimmer PA, Currie LJ, Dougherty J, Bridges WS, Bennett JP, Jr, Wooten GF, Parker WD. Matrilineal inheritance of complex I dysfunction in a multigenerational Parkinson’s disease family. Ann Neurol. 1998;44:873–881. doi: 10.1002/ana.410440605. [DOI] [PubMed] [Google Scholar]
- 46.Technikova-Dobrova Z, Sardanelli AM, Speranza F, Scacco S, Signorile A, Lorusso V, Papa S. Cyclic adenosine monophosphate-dependent phosphorylation of mammalian mitochondrial proteins: enzyme and substrate characterization and functional role. Biochemistry. 2001;40:13941–13947. doi: 10.1021/bi011066p. [DOI] [PubMed] [Google Scholar]
- 47.Tolkovsky AM, Xue L, Fletcher GC, Borutaite V. Mitochondrial disappearance from cells: a clue to the role of autophagy in programmed cell death and disease? Biochimie. 2002;84:233–240. doi: 10.1016/s0300-9084(02)01371-8. [DOI] [PubMed] [Google Scholar]
- 48.Wang G, Achim CL, Hamilton RL, Wiley CA, Soontornniyomkij V. Tyramide signal amplification method in multiple-label immunofluorescence confocal microscopy. Methods. 1999;18:459–464. doi: 10.1006/meth.1999.0813. [DOI] [PubMed] [Google Scholar]
- 49.Weisiger RA, Fridovich I. Mitochondrial superoxide dismutase. Site of synthesis and intramitochondrial localization. J Biol Chem. 1973;248:4793–4796. [PubMed] [Google Scholar]
- 50.Xia XG, Harding T, Weller M, Bieneman A, Uney JB, Schulz JB. Gene transfer of the JNK interacting protein-1 protects dopaminergic neurons in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:10433–10438. doi: 10.1073/pnas.181182298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xue L, Fletcher GC, Tolkovsky AM. Autophagy is activated by apoptotic signalling in sympathetic neurons: an alternative mechanism of death execution. Mol Cell Neurosci. 1999;14:180–198. doi: 10.1006/mcne.1999.0780. [DOI] [PubMed] [Google Scholar]
- 52.Zhu J–H, Kulich SM, Oury TD, Chu CT. Cytoplasmic aggregates of phosphorylated extracellular signal-regulated kinase in Lewy body diseases. Am J Pathol. 2002;161:2087–2098. doi: 10.1016/S0002-9440(10)64487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu X, Raina AK, Rottkamp CA, Aliev G, Perry G, Boux H, Smith MA. Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer’s disease. J Neurochem. 2001;76:435–441. doi: 10.1046/j.1471-4159.2001.00046.x. [DOI] [PubMed] [Google Scholar]


