Abstract
All neuronal networks are modulated by multiple neuropeptides and biogenic amines. Yet, few studies investigate how different modulators interact to regulate network activity. Here we explored the state-dependent functional interactions between three excitatory neuromodulators acting on neurokinin1 (NK1), alpha1 noradrenergic (α1 NE) and 5-HT2 serotonin receptors (5-HT2) within the Pre-Bötzinger complex (pre-BötC), an area critical for the generation of breathing. In anesthetized, in vivo mice the reliance on endogenous NK1 activation depended on spontaneous breathing frequency and the modulatory state of the animal. Endogenous NK1 activation had no significant respiratory effect when stimulating raphe magnus and/or locus ceruleus, but became critical, when α1 NE and 5-HT2 receptors were pharmacologically blocked. The dependence of the centrally generated respiratory rhythm on NK1 activation was blunted in the presence of α1 NE and 5-HT2 agonists as demonstrated in slices containing the pre-BötC. We conclude that a modulators’ action is determined by the concurrent modulation and interaction with other neuromodulators. Deficiencies in one neuromodulator are immediately compensated by the action of other neuromodulators. This interplay could play a role in the state-dependency of certain breathing disorders.
Keywords: NK1 receptor, α1 NE receptor, 5-HT2 receptor, interaction, pre-BötC, respiratory rhythm
Introduction
Neuromodulators play critical roles in regulating every aspect of neuronal activity. Their functional roles are typically identified by employing a variety of pharmacological and genetic approaches aimed at activating or blocking the action of a specific neuromodulator and characterizing the resulting functional consequences (Ptak et al., 2000; Ptak et al., 2002; Pena and Ramirez, 2004; Duangdao et al., 2009; Hodges et al., 2009; Jansen et al., 2009; Ward and Walker, 2009). However, the functional outcome of a given manipulation, be it the activation or the deletion of a neuromodulator or ion channel is often more complex than expected. This is most evident in genetically manipulated animals in which unexpected results are often attributed to compensatory mechanisms (Telgkamp et al., 2002; Hilaire et al., 2003; Swensen and Bean, 2005; Ortinski et al., 2006; Higuchi et al., 2008). The introduction of conditional gene knockouts (Benavides et al., 2007; Djukic et al., 2007) or the recent development of approaches such as the use of allatostatin in genetically modified mammalian networks helped to overcome some of the difficulties in interpreting the potential influence of long-term compensatory mechanisms (Tan et al., 2008; Wehr et al., 2009; Zhou et al., 2009). However, a major conceptual problem in assessing the role of specific modulators or specific ion channel is the fact that neurons and networks are never controlled by single types of ion channels, receptors and neuromodulators. Instead, neuronal activity is typically the result of a complex orchestration involving multiple ion channels, multiple receptors and multiple neuropeptides and biogenic amines (Thoby-Brisson and Simmers, 1998; Doi and Ramirez, 2008; Grashow et al., 2009) and this orchestration is state-dependent (Nadim et al., 2008). Thus, manipulating or lesioning a single type of ion channel, receptor or neuromodulator will have unexpected effects, due to the interference with multiple regulatory systems and the state of the animal. In fact, it may be wrong to assume that any neuronal function depends on a single modulator, receptor or ion channel. The multitude of regulatory processes is a fundamental biological property and understanding how multiple receptors, ion channels and neuromodulators are functionally integrated is of great basic scientific and clinical relevance.
Unfortunately, only few studies directly investigate the role and interaction between different modulators and receptor subtypes (Peck et al., 2006; Stein et al., 2007; Fadok et al., 2009; Grashow et al., 2009). Here, we explored the converging influence of three excitatory neuromodulators on the mammalian respiratory network. The respiratory rhythm is generated within the ventrolateral side of the brainstem distributed between the pontine respiratory group (PRG), pre-Botzinger complex (pre-BötC) and rostral ventral respiratory group (rVRG) (Onimaru and Homma, 2003; Feldman and Del Negro, 2006; Smith et al., 2007). The pre-Bötc is essential for generating rhythmic breathing activity (Achard et al., 2005), and is thought to play a role in generating different forms of inspiratory activities (Lieske et al., 2000).
Respiratory rhythm generation depends on classical neurotransmitters, such as amino acids, and acetylcholine, biogenic amines (Pena and Ramirez, 2002; Pagnotta et al., 2005; Shao and Feldman, 2005; Viemari and Ramirez, 2006), and neuropeptides (Bianchi et al., 1995). The release of these neuromodulators varies by awake/sleep/anesthetized states (Dringenberg and Vanderwolf, 1998; Brown et al., 2001; Ursin, 2002; Berridge and Waterhouse, 2003). Here, we investigate the role of Substance P (SP) in relation to norepinephrine and serotonin. Our study suggests that the relative contribution of these modulators depends on network state, and the state-dependency of the network is partly determined by the interplay between these neuromodulators.
Methods
The transverse slice preparation
Brain stem transverse slice preparations from CD1 mice (P6–P10, Charles River laboratories)were obtained using a technique described in detail previously (Ramirez et al., 1996). The most important steps are summarizedhere. Experimental procedures were approved by the Institutional Animal Care and Use at the Seattle Children’s Research Institute. Mice were deeply anesthetized with ether and decapitated. Isolated brainstems were then placed in ice-cold artificial cerebrospinalfluid (aCSF) bubbled with carbogen (95% O2-5% CO2). The aCSFcontained (in mM): 128 NaCl, 3 KCl, 1.5 CaCl2, 1 MgCl2, 24 NaHCO3, 0.5 NaH2PO4, and 30 D-glucose (pH 7.4). Brainstems were glued rostral end-up onto an agar block for mounting into a vibratome (Leica Microsystems, Waukegan, IL). Brainstems were serially sliced untilthe rostral boundary of the pre-Bötc (Smith et al., 1991) was identified by anatomical landmarks suchas disappearance of the facial nucleus, and the first appearance of theinferior olive, the nucleus ambiguous, and hypoglossal nucleus. A single 500–680 μm-thick slice was then taken. Because theslices also contained regions caudal to the pre-Bötc, we refer tothe region encompassed in the slice as the ventral respiratory group (VRG). Slices were transferred into a recording chamber, continuously superfused with oxygenated aCSF, and maintained at a temperature of 30 ± 0.5°C. To initiate and maintainfictive respiratory rhythmic activity the potassium concentration of the perfusate was raised from 3 to 8 mM over 30 min (Ramirez et al., 1996; Tryba et al., 2003).
In vivo anesthetized mouse preparation
CD1 mice (P15–23) were anesthetized with urethane (1.5 g/kg). The electrical signal of the hypoglossal nerve and/or the electromyography (EMG) of intercostal muscles were recorded. Mice were placed in a supine position and the head was fixed with a stereotaxic apparatus. The neck of the mice was opened from the ventral side, the trachea was cut and plastic Y-shaped tubing for supplying oxygen was inserted into the proximal end of the trachea (cannulation). The bone of the skull covering the ventral brainstem was partially removed with small scissors and forceps. In this preparation, the reverse Y-shaped basilar artery and the branches of the hypoglossal nerve could be observed. The dura and arachnoid membrane were removed to expose the ventral medulla. The surface of the ventral medulla was continuously perfused with 95% O2–5% CO2 equilibrated aCSF solution at 30 ± 0.5°C. In all cases, 100% oxygen was supplied through cannulation without artificial ventilation.
Electrical measurements
Extracellular recordings
In the transverse slice preparation, population activity recordings were obtained with suction electrodes positioned on the surface of the ventrolateral region containing the pre-Bötc. Signals were amplified 2,000×, filtered (low-pass, 1.5 kHz; high-pass, 250 Hz), rectified, and integrated using an electronic filter (time constant of 30–50 ms). Integrated population activity from the VRG was always in phase with integrated inspiratory activity of the hypoglossal motor nucleus (Telgkamp and Ramirez, 1999). All recordings were stored on a personal computer using pClamp software (Version 9.2, Molecular Devices Corporation, Sunnyvale, CA) and analyzed off-line using customized analysis software written with IGOR Pro and Mini Analysis (Wavemetrics, Lake Oswego, OR and Synaptosoft Inc, FortLee, NJ) (Viemari and Ramirez, 2006). Burst amplitude, frequency and duration were detected and calculated automatically and manually. An irregularity score was determined for each cycle by applying the following formula for consecutive cycle length value: Sn = 100*ABS (Pn – Pn-1)/Pn-1 with Sn = score of the nth cycle, Pn = period of the nth cycle, Pn-1 = period of the cycle preceding the nth cycle, ABS = the absolute value (Barthe and Clarac, 1997). Detected values were automatically sorted into tables that were imported into Excel and graphics programs (Corel Draw). These values were also used to analyze regularity of the VRG population.
Extracellular hypoglossal nerve or electromyography (EMG) recordings from intercostal muscles in anesthetized mice
A glass monopolar suction electrode (tip diameter 100–200 μm) held in a micromanipulator was used to record from the hypoglossal nerve. Without cutting the hypoglossal nerve, the suction electrode was placed on the nerve. Extracellular signals were recorded using a patch-clamp amplifier (HEKA EPC8, HEKA Electronic, Lambrecht-Pfalz, Germany). A teflon covered Ag bipolar electrode was used for EMG recordings. The skin over the abdominal and intercostal area on the right side was partially removed, and the bipolar electrode was placed on the surface of the intercostal muscle. Signals were AC amplified and band pass filtered (8Hz to 3kHz)
Electrical stimulation of Raphe magnus and Locus ceruleus
After mice were full anesthetized and immobilized, concentric bipolar stimulation electrodes (TM53CCINS, World Precision Instruments, FL) were inserted to both Raphe magnus (RM) and Locus ceruleus (LC) from the ventral side of the medulla. Electrical stimulation for both nuclei employed repetitive rectangular pulses (duration, 1ms; intensity, 10–100μA). Because of the large electrical stimulus artifacts created while RM and/or the LC were stimulated, it was impossible to record activity from the hypoglossal nerve during electrical stimulation. EMG activity was very stable and much less affected by the artifacts caused by electrical stimulation. We therefore recorded EMG from intercostal muscle as a measure of breathing activity for these stimulation experiments.
Drug application
Slice preparation
In slice preparations, a perfusion system was employed which allowed rapid drug applications within 5 sec, using a modification of the “Y-tube technique” (Murase et al., 1990). The Y-tube technique uses both negative vacuum pressure and gravity. The Y-shaped tube is created from polyethylene tubing bent into a V-shape, with a small hole made at the curve end, into which a short segment of thinner polyethylene tubing was inserted and affixed with silicon-based glue, forming the tip of the Y-tube. One end of the V-shaped tubing was connected to the test aCSF reservoir by silicon tubing. Separate control and test solution reservoirs were held 50 cm above the level of the Y-tube tip, so that solution flowed from the Y-tube tip by gravity. The other end of the V-shaped tubing was connected to a drain bottle through an electrical valve (Series 075T, Bio-Chem Inc, Boonton, NJ). Constant negative pressure (6.77PSI) was maintained in the drain bottle by the vacuum pump (VPO435A, MEDO USA Inc, Hanover, IL). Closing the valve to the drain bottle allowed the flow of perfusate by gravity (10ml/3min), whereas opening the valve closed this flow. The system was used as follows. The tip of the Y-tube was positioned at a distance 1–2 mm from the pre-Bötc. For control perfusions, the input end of the Y-tubing was placed in the control aCSF reservoir, with the drain valve closed. To apply drugs, the input end of the Y-tubing was removed from the control aCSF reservoir, wiped on tissue paper and transferred to the test aCSF reservoir. The difference of temperature between control and test aCSF was measured to be constant within ± 0.3 °C
Microinjection studies in in vivo anesthetized mice
Micro syringes (Hamilton micro-syringe No. 80330) with 33-gauge needles contained neurokinin1 (NK1) receptor antagonist (100μM RP67580 1μL) or α1 noradrenergic (α1 NE) receptor antagonist (60μM prazosin 0.5μL) or cocktail of NE receptor antagonist (α1 NE antagonist 30μM prazosin, α2 NE antagonist 1μM yohimbine, βNE antagonist 30μM alprenelol total 0.6μL) and/or 5-HT2 serotonin (5-HT2) receptor antagonist (ketanserin 80μM 0.5μL) were positioned with micro manipulators (KITE, World Precision Instruments, FL). The needles of the micro syringes were inserted into the right pre-Bötc from the ventral side. During hypoglossal nerve recording or intercostal EMG recordings, these antagonists were microinjected into the right pre-Bötc at a rate of 0.3 μL/min. We did not attempt to perform bilateral needle injections to limit the damage to the pre-Bötc which would have compromised respiratory rhythm generating mechanisms.
Identification of drug injection and electrical stimulation sites in in vivo preparations
Anodal electrolytic lesions were made by DC currents (500μA) to mark the stimulation sites. Evans blue (Sigma 0.1μL) was injected into the pre-Bötc to label NK1 antagonist injection sites. After electrophysiological recordings were completed, mice were deeply anesthetized with supplemental urethane. Then, transcardial perfusions with 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PBS), pH 7.4, were performed. The medulla was removed and immersed overnight in 4% PFA with 30% sucrose. The medulla was subsequently cut into 50μm sections on a cryostat and stained with cresyl violet.
Drugs
The following drugs were used in this study: SP (Tocris Cookson), [Sar9, Met(O2)11]-SP (Sar, Met-SP, Tocris Cookson), (3aR,7aR)-Octahydro-2-[1-imino-2-(2-methoxyphenyl) ethyl]-7, 7-diphenyl-4H-isoindol (RP67580, Tocris Cookson), GR82334 (Tocris Cookson), (2S,3S)-3-[[3,5-bis (Trifluoromethyl) phenyl] methoxy]-2-phenylpiperidine hydrochloride (L733060, Tocris Cookson), N-Acetyl-L-tryptophan 3,5-bis (trifluoromethyl) benzyl ester (L732138, Tocris Cookson), 1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylca rbonyl) piperazine hydrochloride (prazosin, Tocris Cookson), 3-(2-[4-(4-Fluorobenzoyl)-1-piperidinyl (ketanserin, Sigma-RBI, St. Louis, MO), β NE antagonist alprenolol 30μM 2-[(2-Cyclopropylphenoxy) methyl (cirazoline hydrochloride, Sigma-RBI, St. Louis, MO), (±)-2,5-Dimethoxy-4-iodoamphetamine hydrochloride, (±)-D O I, ( ± )-1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI hydrochloride, Sigma-RBI, St. Louis, MO). All drugs were initially dissolved in dimethylsulfoxide (DMSO, Sigma-RBI) or distilled water and were mixed to final concentrations in aCSF. The final concentration of DMSO was below 0.3%in the aCSF.
Statistical analysis
Data were analyzed with SPSS and StatView software (SPSS 8.0, SPSS Inc, Chicago, IL) and StatView-J5.0 (Adept Scientific Inc, Acton, MA). Burst frequency and irregularity score were assessed by Wilcoxon signed rank test as a paired non-parametric statistical test. The Mann-Whitney U-test was utilized for unpaired non-parametric statistical analysis. In other cases, a one-way ANOVA was used forrepeated measurements on the same subjects, followed by Tukey’sor Bonferroni tests for multiple-comparison analysis. Statistical significance was assumed to be significant if p < 0.05. Deviations from the mean are given in SE.
Results
Endogenous SP release consistently activates respiratory activity in slice preparations containing the pre-Bötc
The endogenous SP release and tonic NK1 receptor activation were characterized in isolated transverse slice preparations containing the pre-Bötc. SP and the NK1 agonist Sar, Met-SP, increased extracellularly recorded rhythmic population activity in a dose dependent manner (Fig. 1), while NK1 receptor-specific antagonists, RP67580, L733060, L732138 and GR82334 produced an inhibitory effect on respiratory frequency in a dose dependent manner (Fig. 2 and supplemental fig. 1). The inhibitory effects of RP67580 on population activity was confirmed in reduced pre-Bötc island preparations (Fig. 3), suggesting that the source for endogenous SP release is contained within the pre-Bötc region.
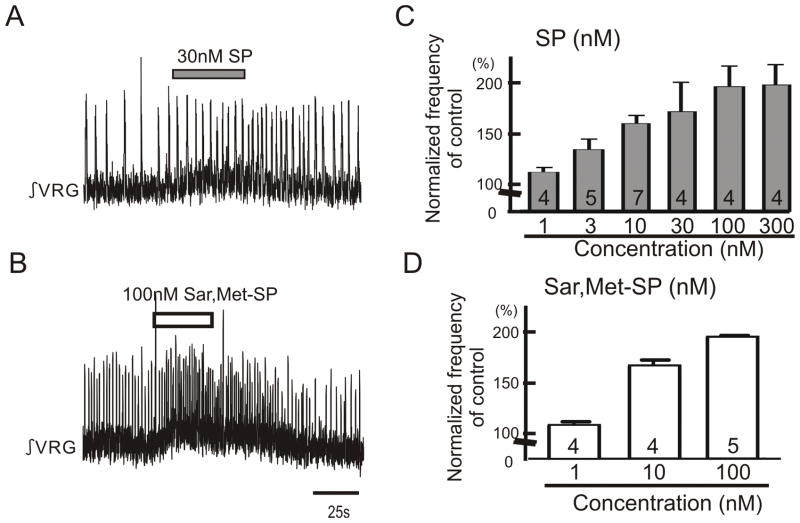
Figure 1. Exogenous application of either SP or NK1 agonist facilitates respiratory population activity recorded in medullary slice preparation containing the pre-Bötc.
Effects of substance P (SP) and NK1 agonist, Sar, Met-SP on respiratory rhythmic activity recorded in a portion of the VRG containing the pre-Bötc in vitro. A, B: Two traces of integrated activity obtained from extracellular population activity recorded from the surface of a portion of the VRG that contains the pre-Bötc. Upward deflections reflect bursts of fictive inspiratory activity. Note the application of 30nM SP (A) and 100nM Sar, Met-SP (B) facilitates respiratory activity. C, D: Graphs indicating that SP (C) and Sar, Met-SP (D) enhance respiratory rhythmic activity in a dose dependent manner (Collected number is shown in each bar graph).
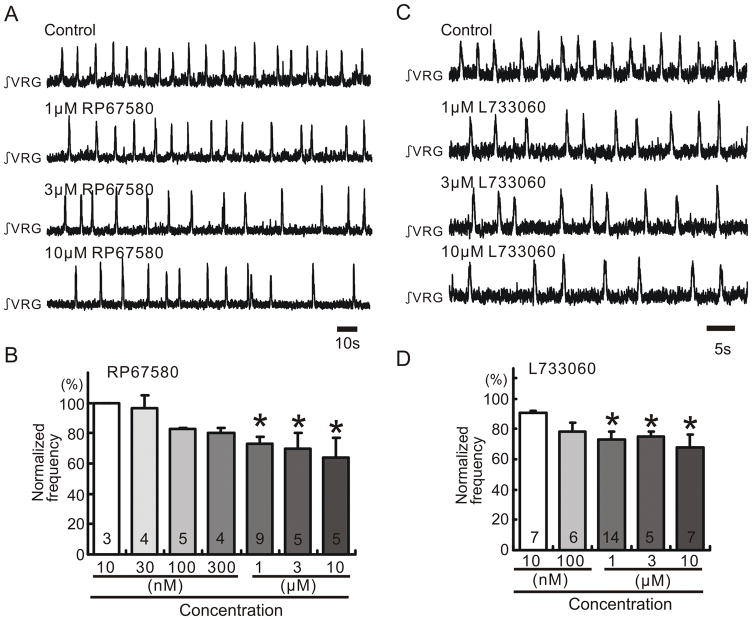
Figure 2. Endogenous SP release activates NK1 receptors in medullary slice preparation containing the pre-Bötc.
A, C: Integrated activity obtained from extracellular population activity recorded from the surface of a portion of the VRG containing the pre-Bötc. Increasing concentrations of the NK1 antagonists RP67580 (A) and L733060 (C) cause an increased inhibitory effect on rhythmic population activity (traces). B, D: Graphs indicating that RP67580 (B) and L733060 (D) inhibit the frequency of respiratory rhythmic activity (ordinate) in a dose dependent manner (abscissa; * p < 0.05, Number is shown in each bar graph). The frequency values were normalized to the control frequency obtained before the application of the NK1 antagonists (= 100%).
Figure 3. Endogenous SP activation persists in pre-Bötc island preparation.
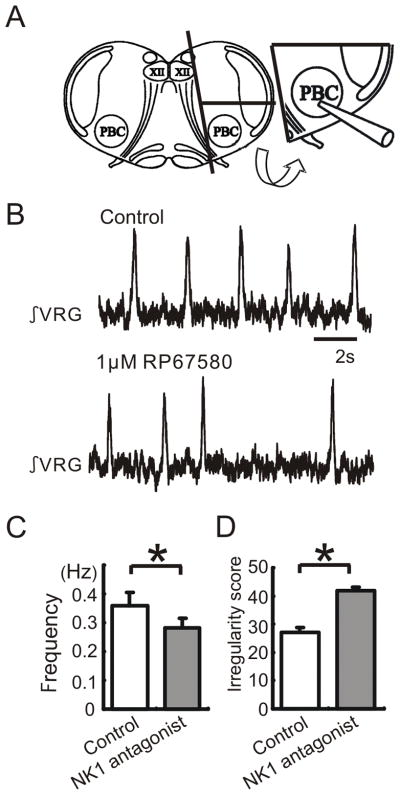
The effect of the NK1 antagonist on respiratory population activity recorded from a pre-Bötc island slice. A: Schematic of a transverse slice (left schematic) which is used to isolate an island slice (right schematic) that contains the pre-Bötc and a minimal amount of surrounding tissue. B: Integrated population activity obtained from extracellular activity recorded from the surface of a portion of the VRG containing the pre-Bötc under control conditions (upper trace) and following the application of the NK1 antagonist RP67580 (lower trace). C, D: The application of the NK1 antagonist RP67580 decreases significantly the frequency (C) and increases the irregularity of respiratory rhythmic activity (D). The irregularity is expressed as the “irregularity score” as explained in Materials and Methods (white bar: control, grey bar: 1μM RP67580, n = 5).
Inhibition of respiratory rhythm by NK1 antagonist in hemilateral pre-Bötc depends on the breathing rate in anesthetized whole animals
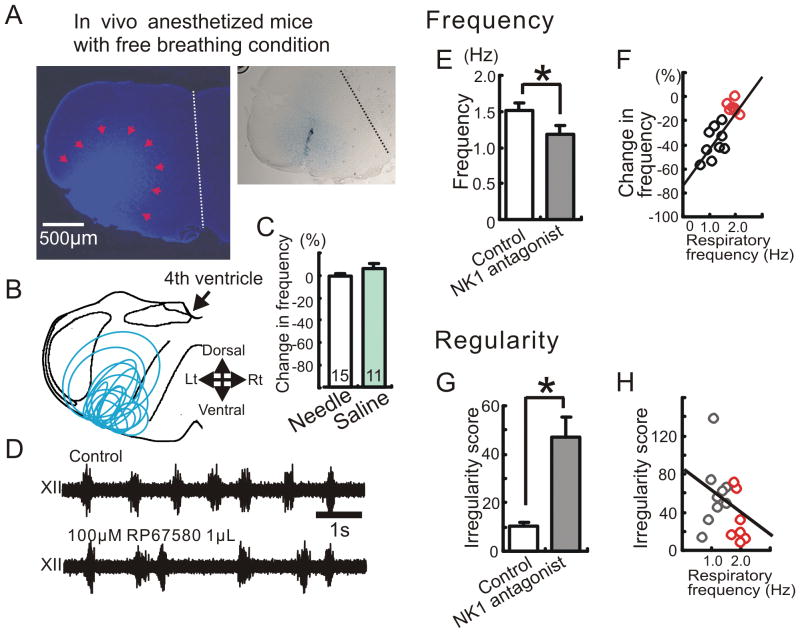
We next investigated the effects of endogenous NK1 receptor activation within the pre-Bötc on spontaneous breathing rhythms using anesthetized in vivo mice. In a series of experiments, we directly delivered the specific NK1 antagonist RP67580 by focal hemilateral injection into the pre-Bötc region (Fig. 4A and B), while monitoring respiration in a hypoglossal nerve recording. Control needle insertions and aCSF injections (1μL) into the right pre-Bötc had no significant effect on respiratory activity (Fig. 4C). Injection of the NK1 antagonist RP67580 (100μM, 1μL) in the same location significantly decreased both the rate (Fig. 4D, E and supplemental fig. 2) and regularity of respiratory rhythm (Fig. 4G). However, in contrast to the in vitro experiments, the effect on the respiratory rhythm was not as consistent and we observed that blocking NK1 receptor had striking effects in some, but not all animals. Indeed, we found a significant correlation between the degree of inhibition on respiratory rate and initial breathing rate (Fig. 4F). This effect was most notable for slow initial breathing rates less than 2 Hz. We observed no significant correlation between NK1 antagonist-dependent decreases in irregularity score and initial breathing rate (Fig. 4H). We hypothesize that spontaneous breathing rates of ~2 Hz and those below 2Hz reflect different modulatory states of the respiratory network, determined by other excitatory neuromodulators.
Figure 4. The inhibitory effect of NK1 antagonist on respiratory activity depends on the breathing frequency in the anesthetized animal.
The effect of NK1 antagonist on respiratory activity in in vivo mice. A: Identification of the injection site by Evans blue, which was injected together with the NK1 antagonist (left panel). Right panel shows the tract of a single needle that was inserted into the pre-Bötc from the ventral side. B: Schematic of the medulla indicating that all injection sites were located in the ventral medulla in the area of the pre-Bötc. C: Graph indicating that the needle insertion (white bar) and the injection of saline (green bar) have no significant effect on respiratory activity. D: Extracellularly recorded XII activity in control (upper trace) and following the injection of NK1 antagonist into the hemilateral pre-Bötc (lower trace). E: The injection of the NK1 antagonist (RP67580, 100μM 1μL) modulates the respiratory frequency (* p < 0.05, n = 16). F: Scatter plot illustrates a direct relationship between the percent change in respiratory frequency (ordinate) and the baseline breathing frequency of the animal before the injection of the antagonist (abscissa). The percent change in respiratory frequency describes the percentage frequency decrease caused by the NK1 antagonist compared to the control frequency before the antagonist injection. Note: the inhibitory effect of the NK1 antagonist depends significantly on the control breathing frequency in the anesthetized animal (y = 31.96 × −74.11, R2 = 0.633). G: The injection of NK1 antagonist increases the irregularity score (* p < 0.05). H: Scatter plot illustrates that there is no significant relationship between the irregularity score (ordinate) and the baseline respiratory frequency (abscissa) (y = −24.4 × −83.5, R2 = 0.11).
To address this hypothesis, we manipulated the modulatory state of the respiratory network using various pharmacological and electrophysiological approaches targeted at the pre-Bötc.
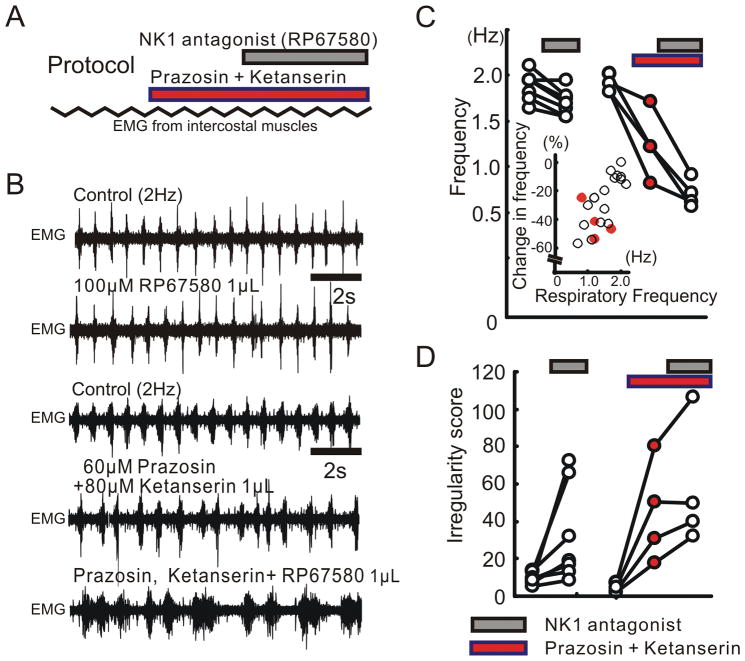
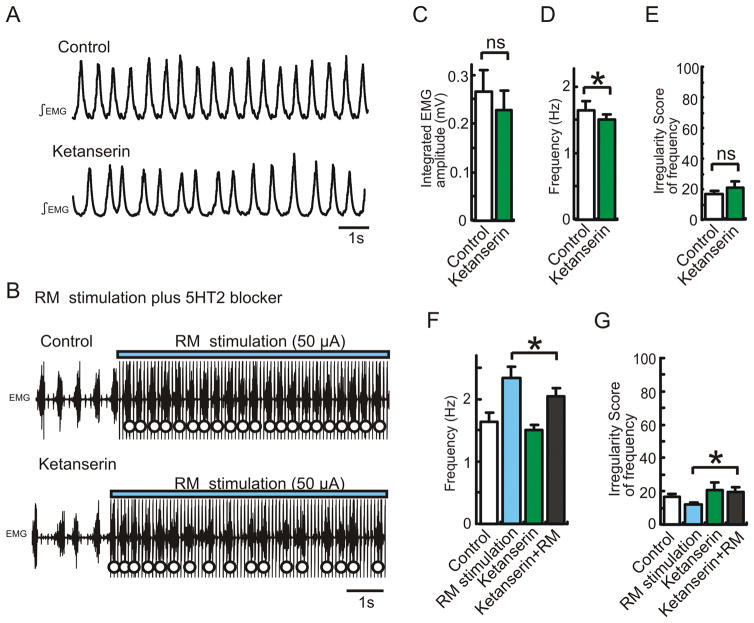
In one series of experiments, we only used anesthetized mice with an initial spontaneous respiratory rate of 2 Hz. Prior to injecting NK1 antagonist into the pre-Bötc, we pre-injected antagonists of both α1 NE receptors (prazosin, 60μM 0.5μL), and 5-HT2 receptors (ketanserin, 80μM 0.5μL) into the same hemilateral site in the pre-Bötc (Fig. 5A and B). The combined injection of both prazosin and ketanserin decreased both the frequency and regularity of respiratory rhythms. These results suggest that tonic activation of both ⍰1 NE receptors and 5-HT2 receptors within the pre-Bötc is required for the maintenance of both high respiratory frequency and regularity. In the absence of tonic ⍰1 NE and 5-HT2 receptor activation, subsequent injection of NK1 receptor antagonist further reduced respiratory rate (Fig. 5B, C and D). This shows that endogenous activation of NK1 receptors becomes critical for respiratory rhythm generation when ⍰1 NE and 5-HT2 receptor activation is decreased and baseline respiratory rate is low.
Figure 5. The modulatory role of NK1 receptors depends on the activation of α1 NE and 5-HT2 receptors.
A: Schematic illustrating the experimental protocol used for examining the relationship between NK1, α1 NE and 5-HT2 receptors. B: EMG activity recorded from intercostal muscles under control conditions (upper trace), following the injection of the NK1 antagonist (RP67580, second trace), control conditions of other case (3rd trace), following the injection of a cocktail containing prazosin and ketanserin (4th trace) and following the additional injection of the NK1 antagonist (lower trace). C, D: The effects of the NK1 antagonist (grey bars) on respiratory frequency (C) and irregularity score (D) of the respiratory frequency is enhanced following the concurrent injection of α1 NE and 5-HT2 antagonists (red bars). Inset in panel C illustrates that blocking of both α1 NE and 5-HT2 receptors decreases the respiratory frequency, indicated as a negative percentage change (red dots).
Electrical stimulation of Locus ceruleus (LC) and Raphe magnus (RM) blocks the effect of NK1 receptor antagonist
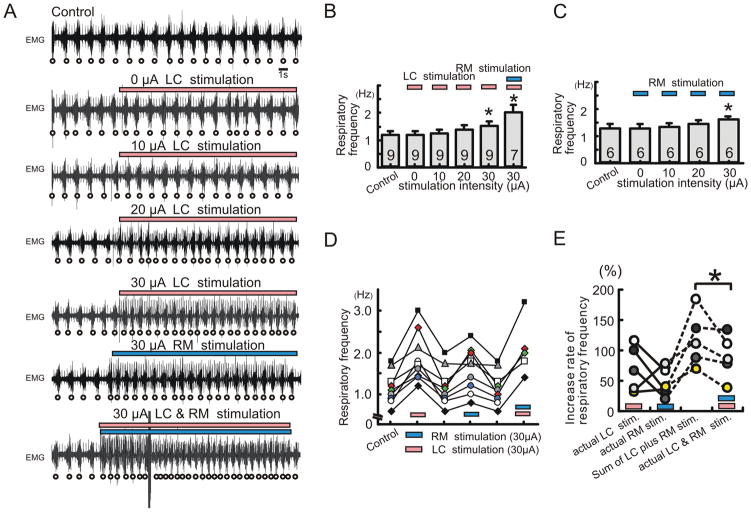
We next performed ipsilateral electrical stimulation of locus ceruleus (LC) and raphe magnus (RM) in combination with hemilateral injection of NK1 antagonist within the pre-Bötc, in semi-intact mice to investigate how increased aminergic drive influences the dependency on endogenous NK1 activation. In the central nervous system, the LC and RM provide the main sources for NE and 5-HT, respectively (Mason, 2001; VanderHorst and Ulfhake, 2006). Both these nuclei provide strong projections to the pre-Bötc (Bianchi et al., 1995). Under control conditions, electrical stimulation of either LC or RM increased the frequency of the breathing activity, in an intensity dependent manner (Fig. 6A, B and C, electrical stimulation for 10 sec). Electrical stimulation-induced facilitation of respiration was acute and reversible, suggesting that the effect was due to the neuronal stimulation and not due to a collateral structural damage (Fig. 6D). In all of the five examined cases, the actual increase in breathing rate caused by the simultaneous LC and RM stimulation was smaller than the sum of the rate increases expected from the experiments in which LC and RM were stimulated separately (Fig. 6E).
Figure 6. Electrical stimulation of LC or RM facilitates respiratory activity in an intensity dependent manner.
A: EMG activity obtained from intercostal muscles under control condition (upper trace), in the presence of 0μA LC stimulation (2nd trace), 10μA LC stimulation (3rd trace), 20μA LC stimulation (4th trace), 30μA LC stimulation (5th trace), 30μA RM stimulation (6th trace) and 30μA LC plus RM stimulation (lower trace). B, C: Bar graphs illustrating that electrical stimulation of LC (pink bar) and/or RM (blue bar) increase respiratory activity in an intensity dependent manner (B and C: * p < 0.05). D: Graph illustrating that the effect on respiratory activity is completely reversible upon termination of the stimulation (pink bar: LC, blue bar: RM stimulation). E: Graph illustrating the percent increases in respiratory frequency caused by separate LC stimulation (pink bar) and RM stimulation (blue bar), the sum calculated from the sum of each of these separate stimulations, and the actual increases caused by the simultaneous stimulation of LC and RM. Each dot represents a stimulation experiment. Experiments conducted in the same animals are connected by lines.
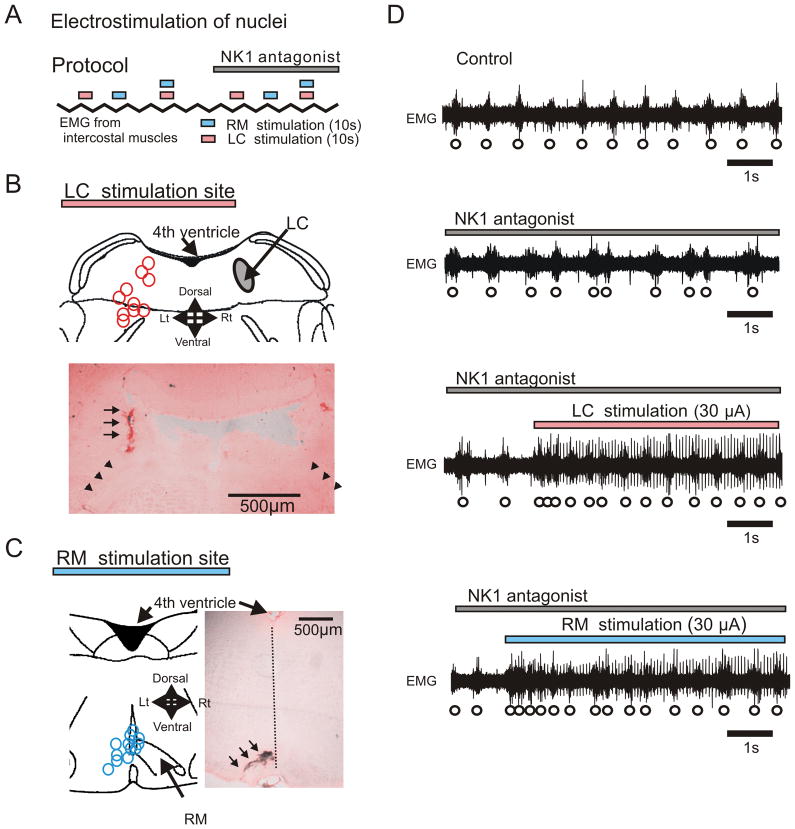
After injection of the NK1 receptor antagonist into the pre-Bötc (Fig. 7D, 2nd trace), electrical stimulation of either LC or RM nuclei facilitated the respiratory rhythm (Fig. 7D, 3rd and 4th traces).
Figure 7. Electrostimulation of locus ceruleus (LC) or raphe magnus (RM) blunts inhibitory effects of NK1 receptors.
A: Schematic illustrating the experimental protocol for the electrical stimulation of LC (pink bars) and RM (blue bars) in the absence and presence of NK1 antagonist (grey bar). B, C: Stimulation sites of LC (B, pink circles) and RM (C, blue circles) were labeled at the end of each experiment by injecting a high current amplitude into the stimulation electrode (Anatomical labeling is indicated by arrows in the histological sections shown in B and C). D: EMG activity obtained from intercostal muscles under control conditions (upper trace), during the injection of the NK1 antagonist (2nd trace), during electrical stimulation of LC while injecting the NK1 antagonist (3rd trace) and during RM electrical stimulation while injecting the NK1 antagonist (lower trace).
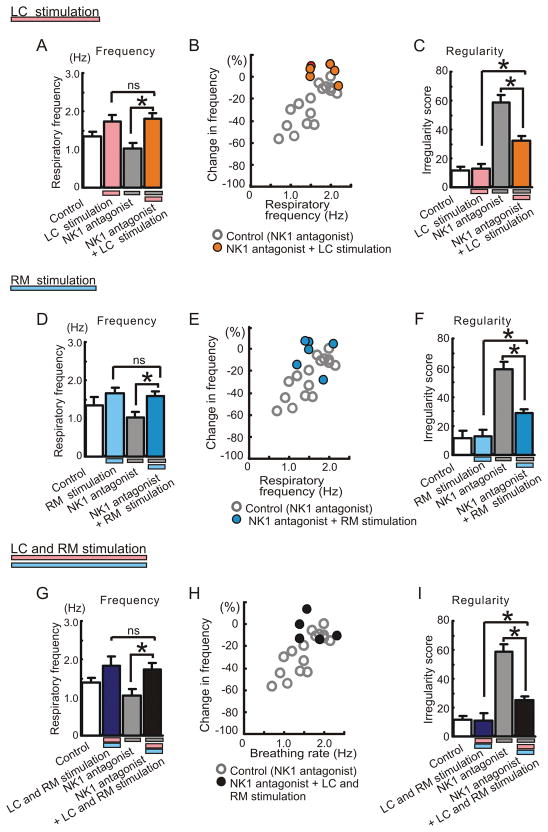
The respiratory frequency reduced by the NK1 receptor antagonist returned to control levels following stimulation of LC and/or RM (Fig. 8A, B, D, E, G and H). Stimulation of LC and/or RM significantly reduced also the breathing irregularity caused by the NK1 antagonist. However, the regularity of the respiratory rhythm did not fully return to control levels (Fig. 8C, F and I).
Figure 8. Effects of electrical stimulation of LC and/or RM on the frequency and regularity changes caused by NK1 antagonists.
A, D and G: The inhibitory effect of the NK1 antagonist on respiratory frequency is abolished by electrical stimulation of LC (A, * p < 0.05 and ns; no significance, n = 6), RM (D, * p < 0.05 and ns, n = 6) or both (G, * p < 0.05 and ns; no significance, n = 5). B, E and H: Scatter plots illustrating the relationship between percent change in respiratory frequency (ordinate) and baseline respiratory frequency (abscissa) before manipulating the preparations. The ordinate reflects the percentage change in frequency compared to the control frequency (= 100%) in any given preparation. Note the electrical stimulation of LC and/or RM significantly reduces the irregularity score caused by the NK1 antagonist, but does not fully recover the regularity when compared to control conditions (C, F and I: * p < 0.05).
Electrical stimulation of LC induces endogenous release of NE and acts on NE receptors presumably in the pre-Bötc
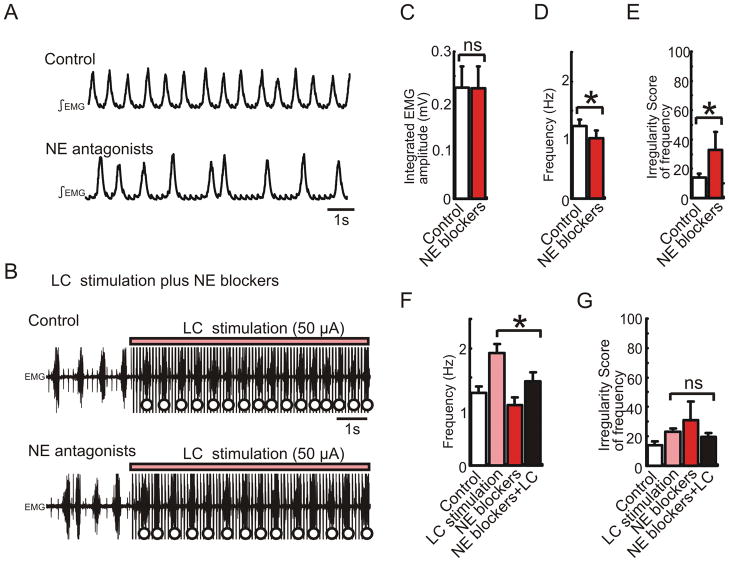
To investigate the mechanisms underlying the facilitation of the respiratory rhythm by LC electrical stimulation (Fig. 6), we compared the increase of the respiratory frequency in the presence and absence of NE receptor antagonists. The cocktail injection of NE receptor antagonists (α1 NE antagonist prazosin 30μM, α2 NE antagonist yohimbine 1μM and β NE antagonist alprenolol 30μM, total 0.6μL) into the hemilateral pre-Bötc decreased the frequency and increased the irregularity score (Fig. 9A, D and E * p < 0.05, n = 5). Under this condition, further LC stimulation enhanced the respiratory frequency (Fig. 9B). However, the facilitation induced by LC electrical stimulation in the presence of NE antagonists was significantly lower than the facilitation induced in the absence of NE antagonists (Fig. 9F, * p < 0.05, n = 5). These results suggest that tonic activation of α1, α2 and β NE receptors contributes to an increase in respiratory frequency and regularity, that LC stimulation induces endogenous release of NE, and that the endogenous NE release seems to activate NE receptors within the pre-Bötc.
Figure 9. Electrical stimulation of LC induces endogenous release of NE and acts on NE receptors presumably within the pre-Bötc.
A: Integrated EMG activity obtained from intercostal muscle under control conditions (upper trace) and following the injection of a cocktail of NE antagonists containing prazosin (30μM), yohimbine (1μM) and alprenolol (30μM, total 0.6μL, lower trace). B: EMG activity recorded during LC stimulation under control conditions (upper trace) and following the injection of the cocktail containing NE antagonists (lower trace). C–E: Bar graphs illustrating the effects of NE antagonists on integrated respiratory amplitude (C, ns, n = 5), frequency (D, * p < 0.05) and regularity of respiratory frequency (E, * p < 0.05). F, G: Bar graphs illustrating that LC stimulation (pink bars) affects only respiratory frequency (F, * p < 0.05, n = 5), but not the regularity of the frequency (G, irregularity score, ns) under control conditions (white bars) and following the injection of the cocktail containing NE antagonists (red bars).
Electrical stimulation of RM facilitates endogenous release of 5-HT and activates 5-HT2 receptor in the pre-Bötc
To explore the mechanisms underlying the increase of the respiratory rhythm by RM electrical stimulation (Fig. 6), we compared the RM-induced frequency increase in the absence and presence of a 5-HT2 receptor antagonist. The injection of the 5-HT2 receptor antagonist ketanserin (80μM, 0.6μL) into the hemilateral pre-Bötc decreased the frequency, but did not significantly increase the irregularity score (Fig. 10A, D and E * p < 0.05, n = 8). Under these conditions, further RM stimulation enhanced the respiratory frequency (Fig. 10B). However, the RM-evoked respiratory facilitation in the presence of the 5-HT2 receptor antagonist was significantly lower than the facilitation evoked in the absence of the antagonist (Fig. 10F, * p < 0.05, n = 8). These results suggest that tonic activation of 5–HT2 receptor increases respiratory rhythm, that RM stimulation induces the endogenous release of 5-HT, and that endogenous 5-HT release seems to activate 5-HT2 receptors within the pre-Bötc.
Figure 10. Electrical stimulation of RM facilitates endogenous release of 5-HT and activates 5-HT2 receptor presumably in the pre-Bötc.
A: Integrated EMG activity obtained from intercostal muscle under control conditions (upper trace) and following the injection of the 5-HT2 antagonist (ketanserin 80μM, 0.6μL, lower trace). B: EMG activity recorded during RM stimulation under control conditions (upper trace) and following the injection of ketanserin (lower trace). C–E: Bar graphs illustrating the effects of ketanserin on integrated EMG amplitude (C, ns, n = 8), frequency (D, * p < 0.05) and regularity of respiratory frequency (E, ns). F, G: Bar graphs illustrating that RM stimulation (blue bars) affects respiratory frequency (F, * p < 0.05, n = 8) and the regularity of the frequency (G, irregularity score, * p < 0.05) under control conditions (white bars) and following the injection of ketanserin (green bars).
Activation of α1 NE receptors or 5-HT2 receptors masks the inhibitory effect of NK1 antagonists on respiratory activity in the slice preparation
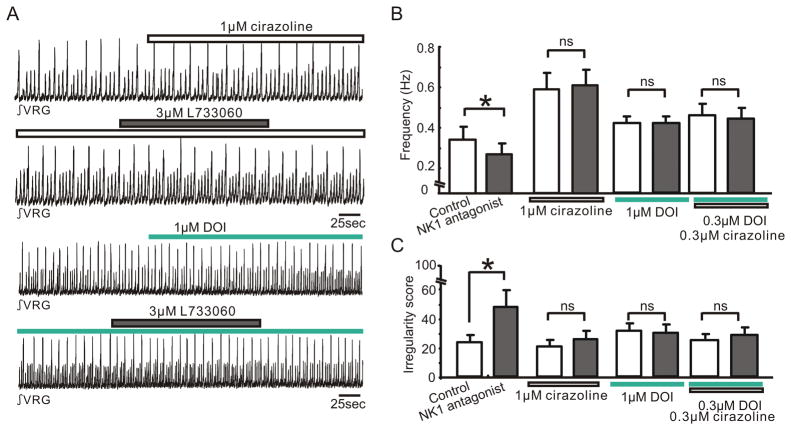
Our in vivo data suggested that the modulation of respiratory frequency by endogenous NK1 receptor activation is dependent on the interaction with ⍰1 NE and 5-HT2 receptors (Fig. 5). We next examined whether this is also the case under in vitro conditions. We pharmacologically manipulated these receptors using the NK1 receptor antagonist L733060, the ⍰1 NE receptor agonist cirazoline, and the 5-HT2 receptor agonist DOI. Pretreatment with either of these drugs, suppressed the inhibitory effects of the NK1 antagonist (3 ⍰M L733060) on frequency and regularity of the respiratory rhythm (Fig. 11B and C, ns, n = 5). These results suggest that, also in the reduced slice preparation the activation of ⍰1 NE and 5-HT2 receptors can mask the effects of endogenous NK1 receptor activation.
Figure 11. Interactions between NK1, α1 NE and 5-HT2 receptors in slice preparations.
A: Integrated activity obtained from population activity recorded extracellularly from the surface of a portion of the VRG containing the pre-Bötc. Note the slice exhibited spontaneously normal respiratory activity (small upward deflections) as well as large amplitude deflections that reflect fictive sigh activity (Lieske et al. 2000). Integrated activity was recorded in the presence of the α1NE agonist cirazoline before (upper trace, white bar) and during the application of the NK1 antagonist L733060 (2nd trace, grey bar), as well as in the presence of the 5-HT2 agonist DOI before (3rd trace, green bar) and during the application of the NK1 antagonist L733060 (4th trace, grey bar). B, C: Bar graphs quantifying the effects on frequency (B) and irregularity score (C) under the conditions before (white bars) and during the application of the NK1 antagonist (3μM L733060; black bars). The bar graphs from left to right illustrate the effects of the NK1 antagonist under control conditions, in the presence of 1μM cirazoline, 1μM DOI, and 0.3μM DOI plus 0.3μM cirazoline (bar graphs from left to right, * p < 0.05, n = 5).
Discussion
Our study indicates that the dependency of the respiratory rhythm on endogenous SP release and tonic NK1 receptor activation is partly determined by the interaction with other excitatory neuromodulators. Respiration depends on endogenous NK1 receptor activation only at low breathing frequency (below 1.5Hz) or at decreased α1 NE and/or 5-HT2 receptor activation. At high breathing rates (about 2Hz) or when locus ceruleus or raphe magnus are stimulated, NK1 activation is not essential for regulating breathing frequency.
However, it must be emphasized that respiratory rhythm generation will depend also on numerous other neuromodulators. Endogenously released NE from the A5 region and LC bind both α1 and α2 NE receptors that contribute to respiratory rhythm generation (Hilaire et al., 2004). These nuclei also contain acetylcholine acting on m3 Ach receptors in the pre-Bötc (Shao and Feldman, 2005). Serotonin released from RM will also activate 5HT4 and 5HT7 receptors known to exert Gs protein mediated excitatory effects on the respiratory rhythm (Manzke et al., 2003; Kvachnina et al., 2005). RM stimulation might also release CCK and TRH (Ellenberger and Smith, 1999; Hodges and Richerson, 2008). Moreover, orexin originating from hypothalamus might enhance LC and RM activity (Bernard et al., 2003; Tao et al., 2006; Dias et al., 2009). Thus, it is likely that these and many other yet unidentified modulatory actions will contribute to respiratory rhythm generation. To appreciate the complexity of neuromodulation, it is insightful to learn from the elegant studies performed in a small crustacean neuronal network (Nusbaum, 2002; Thirumalai and Marder, 2002; Birmingham et al., 2003; Peck et al., 2006).
We focused on the concurrent modulation of NK1, α1 NE and 5HT2 receptors, because all three receptors presumably act on Gq/11 proteins, and possibly converge on common downstream targeted channels. Here, we demonstrated that stimulating LC and RM facilitates release of NE and 5HT respectively. These neuromodulators bind to NE and 5HT2 receptors within the pre-Bötc (Fig. 9 and 10). The effects caused by stimulating LC and RM were not additive (Fig. 6E), suggesting the involvement of common downstream targets. These targets could include e.g. TRPM4/5(Crowder et al., 2007), TRPC3/7 (Ben-Mabrouk and Tryba, 2010), NALCN (leak Na+) (Lu et al., 2009) and TASK (leak K+) channels (Hodges and Richerson, 2008).
The localization, physiological and pharmacological properties of NK1 receptors in the pre-Bötc are well established and NK1 receptor abundance defines the pre-Bötc (Gray et al., 1999; Guyenet et al., 2002; Liu et al., 2004; Morgado-Valle and Feldman, 2004; Hayes and Del Negro, 2007; Fong and Potts, 2008). However, in the brainstem SP and NK1 receptors are also expressed in the nucleus of solitary tracts (NTS), raphe nucleus and nucleus ambiguus (Colin et al., 2002; Guyenet et al., 2002; Leger et al., 2002). These nuclei have connections within the ventral respiratory group (VRG) which includes not only the pre-Bötc (Bianchi et al., 1995; Zec and Kinney, 2003). To reduce the possibility of endogenous SP diffusing from NTS and raphe, we characterized the NK1 effects also in pre-Bötc island preparations (Fig. 3). Although, suggestive for a direct pre-Bötc effect, SP may be released also from SP-containing active presynaptic terminals from areas including NTS, raphe, or nucleus ambiguus (Guyenet et al., 2002). This applies not only to the island but to all in vitro slices irrespective of size and thickness; pre-synaptic terminals from other areas may still be active and effective. Thus, we can not exclude the possibility that some effects caused in this study (in vivo and in vitro) were mediated by areas other than the pre-Bötc.
Blocking endogenous NK1 activation affected respiratory frequency and regularity. The frequency decreased by approximately 30% in response to NK1 antagonists, suggesting that endogenous NK1 activation contributes only partly to the regulation of the respiratory frequency. LC and RM stimulation was capable of restoring the respiratory frequency and also reducing respiratory irregularity in vivo. But, some irregularity remained. By contrast, exogenously applied α1 NE and 5-HT2 agonists were capable of fully restoring respiratory frequency and regularity in vitro. A possible explanation is that LC and RM stimulation caused more complex modulatory effects and the release of different modulators that together could partly oppose the full recovery of the regularity. It could also be that the electrical stimulation of LC and RM was not sufficiently strong to cause sufficient neuromodulator release to fully compensate for the irregularity. However, to avoid unspecific effects, we did not attempt to further increase the current injected into LC and RM. Hence some irregularity remained in the stimulation experiments, while the pharmacological application of specific agonists in the more reduced in vitro network sufficed to fully restore frequency and regularity.
Although, pacemaker and non-pacemaker neurons were not intracellularly recorded in the present study, it is known that substance P (Pena and Ramirez, 2004), serotonin (Pena and Ramirez, 2002), and NE (Viemari and Ramirez, 2006) endogenously modulate respiratory neurons and differentially alter two types of bursting mechanisms described within the pre-Botzinger complex: bursting that depends on the activation of the calcium-activated non-specific cation current (ICAN, TRPM4,5, Pena et al., 2004; Crowder et al., 2007) and bursting that relies on the persistent sodium current (INap) (Del Negro et al., 2002; Pena et al., 2004). These inward currents as well as some outward currents such as the TASK channel are present not only in pacemaker neurons, but also in many non-pacemaker neurons and are thought to contribute to the amplification of synaptic transmission and rhythm generation (Ramirez et al., 1996; Del Negro et al., 2002; Crowder et al., 2007; Koizumi et al., 2010). Following blockade of INap with riluzole, NE causes irregularities and a break-down of respiratory rhythm generation. By contrast, the respiratory rhythm remains stable following blockade of ICAN (Viemari and Ramirez, 2006). This finding suggests that INap is critical for the modulatory effect on the regularity of the respiratory rhythm. Given that INap-dependent bursting is also modulated by NK1 and 5-HT2A receptor activation (Pena and Ramirez, 2002, 2004; Ramirez et al., 2004) this mechanism could be involved in some of the modulatory effects described in the present study. However, to substantiate this hypothesis, further studies will be necessary that specifically address the convergent endogenous modulation of respiratory nonpacemakers, pacemakers and their bursting mechanisms. But, it is important to emphasize that the modulatory effects described in the present study are likely more complex.
The electrical stimulation of LC and RM will also activate fibers of passage. Moreover, neurons in LC and RM release not only NE and serotonin, but also other neuromodulators that are co-localized in these neurons. Serotonergic neurons from raphe pallidus, raphe magnus and raphe obscurus project to the pre-Bötc (Paterson et al., 2009), and serotonin co-localizes with SP in presynaptic terminals within the pre-Bötc (Hodges and Richerson, 2008; Ptak et al., 2009). 5-HT- and SP-containing neurons in raphe obscurus project and excite neurons in the pre-Bötc (Ptak et al., 2009). Indeed, large proportions of 5-HT cell bodies in the raphe region of the lower medulla contain SP (Hokfelt et al., 2000). Thus, if both 5-HT and SP co-exist within the broad area of raphe nuclei, it is likely that both molecules may be released not only from raphe obscurus, but also raphe magnus and raphe pallidus. Immunohistological evidence suggests that some substance P in the pre-Bötc appears to co-localize with glutamatergic, serotonergic and also GABAergic presynaptic terminals (Ribeiro-da-Silva and Hokfelt, 2000; Liu et al., 2004).
While generally accepted that NK1 receptor activation is involved in breathing, its relative importance remained uncertain. Newborn NK1 receptor knockout mice do not exhibit significant changes in the respiratory rhythm (Ptak et al., 2000). In light of the present study, these results are not surprising, since the other excitatory neuromodulators remained presumably active in these mutant mice and were thus able to compensate for the loss of NK1 activation.
Injections aimed at selectively destroying NK1 receptor-immunoreactive (NK1R-ir) neurons using SP-saporin (SP-SAP) had dramatic breathing effects (Gray et al., 2001; Wang et al., 2003; McKay et al., 2005). But this approach lesions NK1R-ir neurons which eliminates not only NK1R activation, but presumably also the very neurons that receive convergent noradrenergic and serotonergic inputs. Interestingly, when these animals fell asleep they stopped breathing altogether (McKay et al., 2005; McKay and Feldman, 2008). Our results provide a possible explanation for this state-dependency: during sleep NE and 5HT activation is low, thus rendering the network more sensitive to the loss of neuromodulatory drive.
Our studies may have also important clinical implications. For example mutations of the Mafb gene in mice cause respiratory arrest at birth, and result in the loss of about one-third of the NK1 positive cells in the pre-Bötc (Blanchi et al., 2003). Similar human mutations may be discovered that link respiratory insufficiencies and susceptibilities to sudden infant death syndrome (SIDS) and sleep apnea. In case of SIDS, there is increasing evidence for a role for 5-HT (Paterson et al., 2006; Rand et al., 2007; Broadbelt et al., 2009; Kinney et al., 2009; Duncan et al., 2010). SIDS (and also sleep apnea) occurs predominantly during sleep, when aminergic and presumably also SP levels are reduced. Only in such a reduced modulatory state is the respiratory network critically dependent on an individual neuromodulator, as suggested here for the dependency on SP. In the awake state, it is unlikely that a disruption of a single excitatory neuromodulator will have much of a critical effect, since the modulatory state is relatively high. This may explain why children that later died of SIDS have apparently no breathing problems during the day. Thus, understanding the convergence of different neuromodulators is not only an interesting basic scientific issue, but will also be important for gaining insights into the neurobiology of breathing disorders.
It is well established that gasping critically depends on persistent sodium dependent pacemaker neurons. This was originally shown in vitro (Pena et al 2004) and subsequently confirmed in vivo (Paton and St-John, 2007; Pena and Aguileta, 2007). However, whether gasping, depends on a single neuromodulator (in this case 5-HT) as suggested by in vitro experiments (Tryba et al., 2006) or on multiple modulators as suggested by an in situ preparation (Toppin et al., 2007) remains unknown. Further studies will be necessary to investigate how the state-dependency of these neuromodulatory systems is affected under hypoxic conditions both in vitro and in vivo. Moreover, it will also be important to unravel how gasping, sighs and apneas, are simultaneously controlled by neuropeptides (SP), noradrenergic and serotonergic systems in the intact animal, and how they change during postnatal development (Pena et al., 2008), interesting questions that go beyond the scope of the present study.
Another interesting observation is that conditional KO mice for 5-HT exhibit dramatic respiratory disturbance and excessive mortality when lesioned in neonates (Ptak et al., 2009), while the same manipulation had much less dramatic effects in more mature mice (Ptak et al., 2009). This difference could be explained by the differential maturation of other modulatory systems (Murrin et al., 2007) and/or the respiratory rhythm generating network (Pena et al., 2008).
Thus, our study emphasizes the importance of considering the convergence of different excitatory neuromodulators when interpreting the outcome of gene knock-outs or when considering the consequences in a patient that is missing one critical neuromodulator. Patients may appear normal during wakefulness, but reveal irregularities at sleep, when modulatory tone is low.
Supplementary Material
Acknowledgments
We would like to thank Dr. Aguan Wei for his wonderful suggestions and editorial comments. This study was supported by the National Institute of Health (RO1 HL/NS60120; P01 HL090554-01; RO1 HL/68860).
References
- Achard P, Zanella S, Rodriguez R, Hilaire G. Perinatal maturation of the respiratory rhythm generator in mammals: from experimental results to computational simulation. Respir Physiol Neurobiol. 2005;149:17–27. doi: 10.1016/j.resp.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Barthe JY, Clarac F. Modulation of the spinal network for locomotion by substance P in the neonatal rat. Exp Brain Res. 1997;115:485–492. doi: 10.1007/pl00005718. [DOI] [PubMed] [Google Scholar]
- Ben-Mabrouk F, Tryba AK. Substance P modulation of TRPC3/7 channels improves respiratory rhythm regularity and ICAN-dependent pacemaker activity. Eur J Neurosci. 2010;31:1219–1232. doi: 10.1111/j.1460-9568.2010.07156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benavides DR, Quinn JJ, Zhong P, Hawasli AH, DiLeone RJ, Kansy JW, Olausson P, Yan Z, Taylor JR, Bibb JA. Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J Neurosci. 2007;27:12967–12976. doi: 10.1523/JNEUROSCI.4061-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard R, Lydic R, Baghdoyan HA. Hypocretin-1 causes G protein activation and increases ACh release in rat pons. Eur J Neurosci. 2003;18:1775–1785. doi: 10.1046/j.1460-9568.2003.02905.x. [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Birmingham JT, Billimoria CP, DeKlotz TR, Stewart RA, Marder E. Differential and history-dependent modulation of a stretch receptor in the stomatogastric system of the crab, Cancer borealis. J Neurophysiol. 2003;90:3608–3616. doi: 10.1152/jn.00397.2003. [DOI] [PubMed] [Google Scholar]
- Blanchi B, Kelly LM, Viemari JC, Lafon I, Burnet H, Bevengut M, Tillmanns S, Daniel L, Graf T, Hilaire G, Sieweke MH. MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nat Neurosci. 2003;6:1091–1100. doi: 10.1038/nn1129. [DOI] [PubMed] [Google Scholar]
- Broadbelt KG, Barger MA, Paterson DS, Holm IA, Haas EA, Krous HF, Kinney HC, Markianos K, Beggs AH. Serotonin-related FEV gene variant in the sudden infant death syndrome is a common polymorphism in the African-American population. Pediatr Res. 2009;66:631–635. doi: 10.1203/PDR.0b013e3181bd5a31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Colin I, Blondeau C, Baude A. Neurokinin release in the rat nucleus of the solitary tract via NMDA and AMPA receptors. Neuroscience. 2002;115:1023–1033. doi: 10.1016/s0306-4522(02)00541-9. [DOI] [PubMed] [Google Scholar]
- Crowder EA, Saha MS, Pace RW, Zhang H, Prestwich GD, Del Negro CA. Phosphatidylinositol 4,5-bisphosphate regulates inspiratory burst activity in the neonatal mouse preBotzinger complex. J Physiol. 2007;582:1047–1058. doi: 10.1113/jphysiol.2007.134577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-botzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002;88:2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- Dias MB, Li A, Nattie E. The orexin receptor 1 (OX(1)R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle. Respir Physiol Neurobiol. 2009 doi: 10.1016/j.resp.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD. Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci. 2007;27:11354–11365. doi: 10.1523/JNEUROSCI.0723-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol. 2008;164:96–104. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dringenberg HC, Vanderwolf CH. Involvement of direct and indirect pathways in electrocorticographic activation. Neurosci Biobehav Rev. 1998;22:243–257. doi: 10.1016/s0149-7634(97)00012-2. [DOI] [PubMed] [Google Scholar]
- Duangdao DM, Clark SD, Okamura N, Reinscheid RK. Behavioral phenotyping of neuropeptide S receptor knockout mice. Behav Brain Res. 2009;205:1–9. doi: 10.1016/j.bbr.2009.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Paterson DS, Hoffman JM, Mokler DJ, Borenstein NS, Belliveau RA, Krous HF, Haas EA, Stanley C, Nattie EE, Trachtenberg FL, Kinney HC. Brainstem serotonergic deficiency in sudden infant death syndrome. JAMA. 2010;303:430–437. doi: 10.1001/jama.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH, Smith FM. Sulfated cholecystokinin octapeptide in the rat: pontomedullary distribution and modulation of the respiratory pattern. Can J Physiol Pharmacol. 1999;77:490–504. [PubMed] [Google Scholar]
- Fadok JP, Dickerson TM, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. J Neurosci. 2009;29:11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–242. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong AY, Potts JT. Neurokinin-1 receptors modulate the excitability of expiratory neurons in the ventral respiratory group. J Neurophysiol. 2008;99:900–914. doi: 10.1152/jn.00864.2007. [DOI] [PubMed] [Google Scholar]
- Grashow R, Brookings T, Marder E. Reliable neuromodulation from circuits with variable underlying structure. Proc Natl Acad Sci U S A. 2009;106:11742–11746. doi: 10.1073/pnas.0905614106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBotzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBotzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Sevigny CP, Weston MC, Stornetta RL. Neurokinin-1 receptor-expressing cells of the ventral respiratory group are functionally heterogeneous and predominantly glutamatergic. J Neurosci. 2002;22:3806–3816. doi: 10.1523/JNEUROSCI.22-09-03806.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JA, Del Negro CA. Neurokinin receptor-expressing pre-botzinger complex neurons in neonatal mice studied in vitro. J Neurophysiol. 2007;97:4215–4224. doi: 10.1152/jn.00228.2007. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Niki T, Shiiya T. Feeding behavior and gene expression of appetite-related neuropeptides in mice lacking for neuropeptide Y Y5 receptor subclass. World J Gastroenterol. 2008;14:6312–6317. doi: 10.3748/wjg.14.6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilaire G, Viemari JC, Coulon P, Simonneau M, Bevengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respir Physiol Neurobiol. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Burnet H, Ptak K, Sieweke M, Blanchi B, De Felipe C, Hunt S, Monteau R. Deletion of tachykinin NK1 receptor gene in mice does not alter respiratory network maturation but alters respiratory responses to hypoxia. Adv Exp Med Biol. 2003;536:497–504. doi: 10.1007/978-1-4419-9280-2_63. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir Physiol Neurobiol. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci. 2009;29:10341–10349. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokfelt T, Arvidsson U, Cullheim S, Millhorn D, Nicholas AP, Pieribone V, Seroogy K, Ulfhake B. Multiple messengers in descending serotonin neurons: localization and functional implications. J Chem Neuroanat. 2000;18:75–86. doi: 10.1016/s0891-0618(99)00037-x. [DOI] [PubMed] [Google Scholar]
- Jansen F, Heiming RS, Lewejohann L, Touma C, Palme R, Schmitt A, Lesch KP, Sachser N. Modulation of behavioural profile and stress response by 5-HTT genotype and social experience in adulthood. Behav Brain Res. 2009 doi: 10.1016/j.bbr.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–550. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi H, Smerin SE, Yamanishi T, Moorjani BR, Zhang R, Smith JC. TASK channels contribute to the K+-dominated leak current regulating respiratory rhythm generation in vitro. J Neurosci. 2010;30:4273–4284. doi: 10.1523/JNEUROSCI.4017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvachnina E, Liu G, Dityatev A, Renner U, Dumuis A, Richter DW, Dityateva G, Schachner M, Voyno-Yasenetskaya TA, Ponimaskin EG. 5-HT7 receptor is coupled to G alpha subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J Neurosci. 2005;25:7821–7830. doi: 10.1523/JNEUROSCI.1790-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leger L, Gay N, Cespuglio R. Neurokinin NK1- and NK3-immunoreactive neurons in serotonergic cell groups in the rat brain. Neurosci Lett. 2002;323:146–150. doi: 10.1016/s0304-3940(01)02543-5. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nat Neurosci. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Liu YY, Wong-Riley MT, Liu JP, Wei XY, Jia Y, Liu HL, Fujiyama F, Ju G. Substance P and enkephalinergic synapses onto neurokinin-1 receptor-immunoreactive neurons in the pre-Botzinger complex of rats. Eur J Neurosci. 2004;19:65–75. doi: 10.1111/j.1460-9568.2004.03099.x. [DOI] [PubMed] [Google Scholar]
- Lu B, Su Y, Das S, Wang H, Wang Y, Liu J, Ren D. Peptide neurotransmitters activate a cation channel complex of NALCN and UNC-80. Nature. 2009;457:741–744. doi: 10.1038/nature07579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- Mason P. Contributions of the medullary raphe and ventromedial reticular region to pain modulation and other homeostatic functions. Annu Rev Neurosci. 2001;24:737–777. doi: 10.1146/annurev.neuro.24.1.737. [DOI] [PubMed] [Google Scholar]
- McKay LC, Feldman JL. Unilateral ablation of pre-Botzinger complex disrupts breathing during sleep but not wakefulness. Am J Respir Crit Care Med. 2008;178:89–95. doi: 10.1164/rccm.200712-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBotzinger complex neurons. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgado-Valle C, Feldman JL. Depletion of substance P and glutamate by capsaicin blocks respiratory rhythm in neonatal rat in vitro. J Physiol. 2004;555:783–792. doi: 10.1113/jphysiol.2003.060350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K, Randic M, Shirasaki T, Nakagawa T, Akaike N. Serotonin suppresses N-methyl-D-aspartate responses in acutely isolated spinal dorsal horn neurons of the rat. Brain Res. 1990;525:84–91. doi: 10.1016/0006-8993(90)91323-9. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Sanders JD, Bylund DB. Comparison of the maturation of the adrenergic and serotonergic neurotransmitter systems in the brain: implications for differential drug effects on juveniles and adults. Biochem Pharmacol. 2007;73:1225–1236. doi: 10.1016/j.bcp.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadim F, Brezina V, Destexhe A, Linster C. State dependence of network output: modeling and experiments. J Neurosci. 2008;28:11806–11813. doi: 10.1523/JNEUROSCI.3796-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum MP. Regulating peptidergic modulation of rhythmically active neural circuits. Brain Behav Evol. 2002;60:378–387. doi: 10.1159/000067791. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Turner JR, Barberis A, Motamedi G, Yasuda RP, Wolfe BB, Kellar KJ, Vicini S. Deletion of the GABA(A) receptor alpha1 subunit increases tonic GABA(A) receptor current: a role for GABA uptake transporters. J Neurosci. 2006;26:9323–9331. doi: 10.1523/JNEUROSCI.2610-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnotta SE, Lape R, Quitadamo C, Nistri A. Pre- and postsynaptic modulation of glycinergic and gabaergic transmission by muscarinic receptors on rat hypoglossal motoneurons in vitro. Neuroscience. 2005;130:783–795. doi: 10.1016/j.neuroscience.2004.09.046. [DOI] [PubMed] [Google Scholar]
- Paterson DS, Hilaire G, Weese-Mayer DE. Medullary serotonin defects and respiratory dysfunction in sudden infant death syndrome. Respir Physiol Neurobiol. 2009;168:133–143. doi: 10.1016/j.resp.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA. 2006;296:2124–2132. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- Paton JF, St-John WM. Counterpoint: Medullary pacemaker neurons are essential for gasping, but not eupnea, in mammals. J Appl Physiol. 2007;103:718–720. doi: 10.1152/japplphysiol.00003.2007a. discussion 721–712. [DOI] [PubMed] [Google Scholar]
- Peck JH, Gaier E, Stevens E, Repicky S, Harris-Warrick RM. Amine modulation of Ih in a small neural network. J Neurophysiol. 2006;96:2931–2940. doi: 10.1152/jn.00423.2005. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Aguileta MA. Effects of riluzole and flufenamic acid on eupnea and gasping of neonatal mice in vivo. Neurosci Lett. 2007;415:288–293. doi: 10.1016/j.neulet.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105–117. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Pena F, Meza-Andrade R, Paez-Zayas V, Gonzalez-Marin MC. Gasping generation in developing Swiss-Webster mice in vitro and in vivo. Neurochem Res. 2008;33:1492–1500. doi: 10.1007/s11064-008-9616-x. [DOI] [PubMed] [Google Scholar]
- Ptak K, Hunt SP, Monteau R. Substance P and central respiratory activity: a comparative in vitro study in NK1 receptor knockout and wild-type mice. Pflugers Arch. 2000;440:446–451. doi: 10.1007/s004240000300. [DOI] [PubMed] [Google Scholar]
- Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci. 2009;29:3720–3737. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, Burnet H, Blanchi B, Sieweke M, De Felipe C, Hunt SP, Monteau R, Hilaire G. The murine neurokinin NK1 receptor gene contributes to the adult hypoxic facilitation of ventilation. Eur J Neurosci. 2002;16:2245–2252. doi: 10.1046/j.1460-9568.2002.02305.x. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Quellmalz UJ, Richter DW. Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. J Physiol. 1996;491 ( Pt 3):799–812. doi: 10.1113/jphysiol.1996.sp021258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Tryba AK, Pena F. Pacemaker neurons and neuronal networks: an integrative view. Curr Opin Neurobiol. 2004;14:665–674. doi: 10.1016/j.conb.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Rand CM, Berry-Kravis EM, Zhou L, Fan W, Weese-Mayer DE. Sudden infant death syndrome: rare mutation in the serotonin system FEV gene. Pediatr Res. 2007;62:180–182. doi: 10.1203/PDR.0b013e3180a725a0. [DOI] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A, Hokfelt T. Neuroanatomical localisation of Substance P in the CNS and sensory neurons. Neuropeptides. 2000;34:256–271. doi: 10.1054/npep.2000.0834. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Cholinergic neurotransmission in the preBotzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neuroscience. 2005;130:1069–1081. doi: 10.1016/j.neuroscience.2004.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Koizumi H, Rybak IA, Paton JF. Spatial and functional architecture of the Mammalian brain stem respiratory network: a hierarchy of three oscillatory mechanisms. J Neurophysiol. 2007;98:3370–3387. doi: 10.1152/jn.00985.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein W, DeLong ND, Wood DE, Nusbaum MP. Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur J Neurosci. 2007;26:1148–1165. doi: 10.1111/j.1460-9568.2007.05744.x. [DOI] [PubMed] [Google Scholar]
- Swensen AM, Bean BP. Robustness of burst firing in dissociated purkinje neurons with acute or long-term reductions in sodium conductance. J Neurosci. 2005;25:3509–3520. doi: 10.1523/JNEUROSCI.3929-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBotzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Ma Z, McKenna JT, Thakkar MM, Winston S, Strecker RE, McCarley RW. Differential effect of orexins (hypocretins) on serotonin release in the dorsal and median raphe nuclei of freely behaving rats. Neuroscience. 2006;141:1101–1105. doi: 10.1016/j.neuroscience.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Ramirez JM. Differential responses of respiratory nuclei to anoxia in rhythmic brain stem slices of mice. J Neurophysiol. 1999;82:2163–2170. doi: 10.1152/jn.1999.82.5.2163. [DOI] [PubMed] [Google Scholar]
- Telgkamp P, Cao YQ, Basbaum AI, Ramirez JM. Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol. 2002;88:206–213. doi: 10.1152/jn.2002.88.1.206. [DOI] [PubMed] [Google Scholar]
- Thirumalai V, Marder E. Colocalized neuropeptides activate a central pattern generator by acting on different circuit targets. J Neurosci. 2002;22:1874–1882. doi: 10.1523/JNEUROSCI.22-05-01874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson M, Simmers J. Neuromodulatory inputs maintain expression of a lobster motor pattern-generating network in a modulation-dependent state: evidence from long-term decentralization in vitro. J Neurosci. 1998;18:2212–2225. doi: 10.1523/JNEUROSCI.18-06-02212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toppin VA, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol. 2007;103:220–227. doi: 10.1152/japplphysiol.00071.2007. [DOI] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Stabilization of bursting in respiratory pacemaker neurons. J Neurosci. 2003;23:3538–3546. doi: 10.1523/JNEUROSCI.23-08-03538.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tryba AK, Pena F, Ramirez JM. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci. 2006;26:2623–2634. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursin R. Serotonin and sleep. Sleep Med Rev. 2002;6:55–69. doi: 10.1053/smrv.2001.0174. [DOI] [PubMed] [Google Scholar]
- VanderHorst VG, Ulfhake B. The organization of the brainstem and spinal cord of the mouse: relationships between monoaminergic, cholinergic, and spinal projection systems. J Chem Neuroanat. 2006;31:2–36. doi: 10.1016/j.jchemneu.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol. 2006;95:2070–2082. doi: 10.1152/jn.01308.2005. [DOI] [PubMed] [Google Scholar]
- Wang H, Weston MC, McQuiston TJ, Stornetta RL, Guyenet PG. Neurokinin-1 receptor-expressing cells regulate depressor region of rat ventrolateral medulla. Am J Physiol Heart Circ Physiol. 2003;285:H2757–2769. doi: 10.1152/ajpheart.00528.2003. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Walker EA. Sex and cannabinoid CB1 genotype differentiate palatable food and cocaine self-administration behaviors in mice. Behav Pharmacol. 2009 doi: 10.1097/FBP.0b013e328331ba30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr M, Hostick U, Kyweriga M, Tan A, Weible AP, Wu H, Wu W, Callaway EM, Kentros C. Transgenic silencing of neurons in the mammalian brain by expression of the allatostatin receptor (AlstR) J Neurophysiol. 2009;102:2554–2562. doi: 10.1152/jn.00480.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zec N, Kinney HC. Anatomic relationships of the human nucleus of the solitary tract in the medulla oblongata: a DiI labeling study. Auton Neurosci. 2003;105:131–144. doi: 10.1016/S1566-0702(03)00027-4. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Won J, Karlsson MG, Zhou M, Rogerson T, Balaji J, Neve R, Poirazi P, Silva AJ. CREB regulates excitability and the allocation of memory to subsets of neurons in the amygdala. Nat Neurosci. 2009 doi: 10.1038/nn.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.