Abstract
Fast synaptic inhibition in the forebrain is mediated primarily by GABA acting on GABAA receptors (GABARs). GABARs are regulated by numerous positive (barbiturates, benzodiazepines, and neurosteroids) and negative (picrotoxin, bicuculline, and Zn2+) allosteric modulators. The sensitivity of GABARs to GABA and to allosteric modulators changes gradually during normal development, during development of chronic epilepsy, and after prolonged exposure to GABAR agonists. Here we report the development of rapid functional plasticity of GABARs occurring over 45 min of continuous seizures (status epilepticus) in rats. Seizures induced in rats by administration of lithium followed by pilocarpine were readily terminated by the benzodiazepine diazepam when administered early during the seizures (after 10 min of seizures). However, during status epilepticus, there was a substantial reduction of diazepam potency for termination of the seizures. To determine whether the loss of sensitivity of the animals to diazepam was caused by an alteration of GABAR functional properties, we obtained whole-cell GABAR currents from hippocampal dentate granule cells isolated acutely from control rats and from rats undergoing status epilepticus. GABAR properties were characterized by determining GABA sensitivity and the sensitivity of GABARs to regulation by benzodiazepines, barbiturates, and Zn2+. When compared with those from naive controls, GABAR currents from rats undergoing status epilepticus were less sensitive to diazepam and Zn2+ but retained their sensitivity to GABA and pentobarbital. We conclude that the prolonged seizures of status epilepticus rapidly altered the functional properties of hippocampal dentate granule cell GABARs.
Keywords: status epilepticus, seizures, GABA, diazepam, benzodiazepines, zinc, GABAA receptors, pentobarbital, barbiturates, dentate gyrus, granule cells, hippocampus
Fast inhibitory synaptic transmission in the CNS is mediated primarily by the neurotransmitter GABA interacting with postsynaptic GABAA receptors (GABARs). GABARs form chloride ion channels, and GABAR currents are regulated by numerous positive and negative allosteric modulators, including barbiturates, benzodiazepines, neurosteroids, penicillin, picrotoxin, and bicuculline, and Zn2+. GABARs are pentameric combinations of several different families of GABAR subunits with multiple subunit subtypes of α (1–6), β (1–4), γ (1–4), and δ. GABAR pharmacological properties depend on their subunit subtype composition (Macdonald and Olsen, 1994). For example, the sensitivity of GABARs to benzodiazepines requires the presence of a γ subunit, and the relative affinity for many benzodiazepine receptor agonists depends on the α subtype incorporated into the receptor. In contrast, sensitivity to Zn 2+ is reduced by the presence of a γ subunit, but relative sensitivity to Zn2+ also depends on the α subtype incorporated into the receptor. GABAR functional properties have been shown to be modified under normal and pathological conditions. GABAR properties change gradually during normal development, during development of chronic epilepsy, and after prolonged exposure to GABAR agonists and GABAR regulatory drugs (Rabow et al., 1995).
Status epilepticus is a rapidly developing, prolonged epileptic state that occurs when a single seizure lasts at least 30 min or intermittent seizures last at least 30 min between which the patient does not regain consciousness (Commission on Classification and Terminology of the International League against Epilepsy, 1981; DeLorenzo, 1990). During status epilepticus, seizures rapidly alter brain function, allowing continuation of seizures. The hippocampal–parahippocampal circuit contains neurons and synaptic connections that are well suited to the occurrence of rapid plasticity that facilitates the self-sustaining nature of prolonged status epilepticus (Lothman et al., 1991). Prolonged hippocampal seizures reduce GABAR inhibition (Kapur et al., 1989, 1994; Sloviter, 1991), and this reduction of inhibition is correlated with the development of status epilepticus (Kapur and Lothman, 1989). The cellular mechanisms underlying this loss of GABAergic inhibition during status epilepticus seem to be postsynaptic, including reduced potency and efficacy of GABA in activating chloride channels and diminished driving force for the GABAR currents (Kapur and Coulter, 1995). Thus one hypothesis explaining the self-sustaining nature of status epilepticus is that prolonged seizures produce a progressive reduction of GABAR inhibition in the hippocampus that leads to the development of status epilepticus.
In humans, status epilepticus is treated with benzodiazepines, including diazepam, lorazepam, and midazolam and with barbiturates, including phenobarbital and pentobarbital, all of which exert an anticonvulsant effect by acting on the GABARs (Macdonald and Kelly, 1995). Interestingly, both in humans and in experimental animals, benzodiazepines are efficacious in early but not late status epilepticus, which in humans paradoxically responds to barbiturates (Yaffe and Lowenstein, 1993). Thus both experimental animal and human data suggest that the functional properties of hippocampal GABARs are rapidly modified during status epilepticus. We tested this hypothesis directly. The potency and efficacy of benzodiazepines in treating the early and late phases of status epilepticus in rats were compared. Whole-cell GABAR currents in hippocampal dentate granule cells acutely isolated from rats undergoing status epilepticus were less sensitive to diazepam and Zn2+ compared with currents in naive litter mates but retained their GABA and pentobarbital sensitivity. We conclude that prolonged seizures rapidly alter the functional properties of hippocampal GABARs.
MATERIALS AND METHODS
Whole animal experiments
Status epilepticus was induced in Sprague Dawley rats of both sexes (230–255 gm; Harlan Sprague Dawley, Indianapolis, IN) by intraperitoneal injection of LiCl at 3 mEq/kg followed 20 hr later by pilocarpine at 50 mg/kg (Honchar et al., 1983). After pilocarpine injection, the rats were observed continuously for occurrence of behavioral seizures. The time to onset of behavioral seizures was recorded, and behavioral seizures were observed. Behavioral seizures evoked by lithium and pilocarpine were as described by Racine (1972). Seizure termination was defined as the absence of forelimb clonus or falling, facial twitching, and stop-and-stare activity. Additionally, resumption of normal behavior within 30 min of drug injection was assessed. Diazepam was administered 10 or 45 min after pilocarpine injection. The fraction of rats that stopped having seizures within 5 min of diazepam injection was plotted against the log of diazepam dose. The data were fitted to a sigmoidal dose–response curve with the maximum fixed to 100% and the minimum to 0%. The ED50 values were derived from the equation that best fit the data.
Cell isolation
Cells were isolated from rats undergoing 45 min of seizures or from controls consisting of naive rats or saline-injected animals of the same age. All experiments were performed on dentate granule cells isolated according to the method described originally by Kay and Wong (1986) and later modified by Coulter et al. (1990). The brain was dissected free, and the region containing the hippocampus was blocked and chilled in an oxygenated 1,4-piperazinediethanesulfonic acid (PIPES)-buffered medium (4°C) for 1 min. The PIPES buffer solution contained (in mm) NaCl, 120; KCL, 2.5; CaCl2 , 1.5; MgCl2 ,1; d-glucose, 25; and PIPES, 20, pH 7.0. After blot drying, the brain was mounted on a vibratome stage, and 500 μm coronal sections containing the hippocampus were cut. The sections were allowed to recover in oxygenated (95% 02 /5% CO2) PIPES buffer for 30–60 min. Hippocampal sections were then incubated with oxygenated Sigma type XXIII (Sigma, St. Louis, MO) protease enzyme in the buffer at 32°C for 30–45 min. The dentate gyrus was dissected out and cut into 0.5 mm cubes that were triturated in a cold (4°C) PIPES-buffered medium in fire-polished glass pipettes to isolate neurons. The isolated neurons were plated on poly-l-lysine-coated 35 mm polystyrene petri dishes (Corning, Corning, NY), and the recordings were made within 1 hr of isolation.
Whole-cell recording
Whole-cell GABAR currents were recorded from hippocampal dentate granule cells acutely isolated from 28- to 35-d-old rats using the technique described by Hamill et al. (1981). The extracellular recording solution consisted of (in mm) NaCl, 142; CaCl2 , 1.0; KCl, 8; MgCl2 , 6; glucose, 10; and HEPES, 10, pH-adjusted to 7.4; osmolarity was 310–320 mOsm (all reagents from Sigma). Glass recording patch pipettes were filled with a solution consisting of the following (in mm): Trizma phosphate (dibasic), 115; Trizma base, 30; EGTA, 11; MgCl2 , 2; and CaCl2 , 0.5, pH 7.35. Recording pipettes also contained ATP (2 mm) unless otherwise specified. All recordings were obtained at room temperature (24°C). With a bathing solution containing a chloride concentration of 164 mm and whole-cell recording pipettes containing a 5 mm chloride ion solution, the chloride ion concentration gradient produced a chloride ion equilibrium potential (ECl–) of –76 mV. Granule cells were voltage-clamped to 0 mV, and thus, application of GABA produced outward currents. Patch pipettes (resistance of 6–10 MΩ) were pulled on a Flaming–Brown P-87 puller by a four-stage pull. Currents were recorded with an Axopatch 1-D amplifier and low-pass filtered at 2 kHz with an eight pole Bessel filter before digitization, storage, and display. Currents were displayed on a Gould 2400S chart recorder, and peak whole-cell currents were measured manually from the chart paper. Currents were also recorded on a hard disk using the Axotape program (Axon Instruments) (digitized at 208 Hz) and on a videocassette tape recorder (Sony SL-HF360) via a digital audio processor (Sony PCM-501 ES; 14 bit, 44 kHz).
Drug application
GABA, pentobarbital, and ZnCl2 dissolved in extracellular solution were applied to neurons using a modified U-tube “multipuffer” rapid application system (Greenfield and Macdonald, 1996) with the tip of the application pipette placed 100–200 μm from the cell. Diazepam was dissolved first in dimethylsulfoxide (DMSO) and then diluted in extracellular buffer with the final DMSO dilution being at least 1:50,000. GABA, diazepam, pentobarbital, and ZnCl2 were obtained from Sigma.
Data analysis
The magnitude of the enhancement or inhibition of GABAR current by a drug was measured by dividing the peak amplitude of GABAR current elicited in the presence of a given concentration of the drug and GABA by the peak amplitude of control current elicited by GABA alone and by multiplying the fraction by 100 to express it as percent control. Thus the control response was 100%. Peak GABAR currents at various drug concentrations were fitted to a sigmoidal function using a four parameter logistic equation (sigmoidal concentration–response) with a variable slope. The equation used to fit the concentration–response relationship was:
where I was the GABAR current at a given GABA concentration, and I(max) was the maximal GABAR current. Maximal current and concentration–response curves were obtained after pooling data from all neurons tested for GABA and for all drugs. The curve-fitting algorithm minimized the sum of the squares of the actual distance of points from the curve. Convergence was reached when two consecutive iterations changed the sum of squares by <0.01%. The curve fit was performed on an IBM-compatible personal computer using the program Prism (Graph Pad, San Diego, CA). All data are presented as mean ± SEM.
RESULTS
Benzodiazepine treatment of brief and prolonged seizures
Behavioral seizures began 3–5 min after the injection of pilocarpine. Behavioral seizures during lithium- and pilocarpine-induced status epilepticus were characterized by immobility, repetitive chewing, head nodding, vibrisal twitching, forelimb clonus with or without rearing, and falling as described previously (Racine, 1972; Walton and Treiman, 1988). Four rats were not treated with an anticonvulsant drug, and they continued to have seizures for 2 hr. After 10 min of seizures, diazepam (20 mg/kg) terminated seizures in all treated animals (n = 3). However, after 45 min of seizures (status epilepticus), diazepam (20 mg/kg) terminated the seizures in none of the animals (n = 3). Seizure termination was defined as the absence of behavioral convulsion, facial twitching, and stop-and-stare activity. Additionally, resumption of normal behavior within 30 min of drug injection was assessed.
A detailed diazepam dose–response (fraction of animals becoming seizure free) analysis was performed using a total of 30 rats. Increasing doses of diazepam from 2 to 20 mg/kg were administered after 10 min of seizures; five rats were treated with diazepam at 2 mg/kg, and three rats each were treated with diazepam at 7.5, 10, and 20 mg/kg. After 45 min of seizures, three rats each were treated with diazepam at 20, 30, 50, and 100 mg/kg. At high doses of diazepam (50 and 100 mg/kg), behavioral seizures seemed terminated, but rats were extremely sedated, and resumption of normal activity did not occur. The dose–response data were fit to a sigmoidal dose–response relationship, and the ED50 values for diazepam control of behavioral seizures after 10 and 45 min of seizures were derived. The dose–response curve showed that the ED50 for diazepam-induced termination of seizures shifted from 4.2 mg/kg when administered after 10 min of continuous seizures to 40 mg/kg when administered after 45 min of continuous seizures (Fig. 1).
Figure 1.
Diazepam was effective in controlling brief (10 min) seizures but lost efficacy after prolonged (45 min) seizures. Seizures were induced in 70–150 gm rats by intraperitoneal injection of LiCl at 3 mEq/kg followed 16–24 hr later by intraperitoneal injection of pilocarpine at 50 mg/kg. Behavioral seizures started within 1–5 min in all rats. Diazepam was administered 10 min (filled boxes, solid line; n = 14) or 45 min (filled circles, dashed line; n = 12) after pilocarpine injection. The percent of rats that stopped having seizures within 5 min of diazepam injection was plotted against the log of the diazepam dose. The data were fitted to a sigmoidal dose–response curve with the maximum fixed to 100% and the minimum to 0%. The ED50 values were derived from the equation that best fit the data.
Because diazepam exerts its anticonvulsant effect primarily by enhancing GABAergic inhibition by acting on GABARs (Macdonald et al., 1992), we hypothesized that seizures altered the functional properties of GABARs. The seizures could potentially alter the modulation of GABAR by various drugs, such as enhancement by benzodiazepines, barbiturates, and neurosteroids and antagonism by penicillin, picrotoxin, bicuculline, and Zn2+. We characterized GABAR currents recorded from acutely isolated hippocampal dentate granule cells, their potentiation by benzodiazepines and barbiturates, and their inhibition by Zn2+.
GABAR currents recorded from acutely isolated hippocampal dentate granule cells
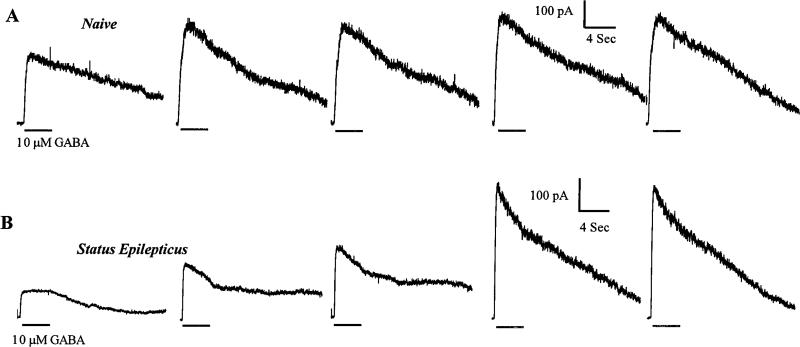
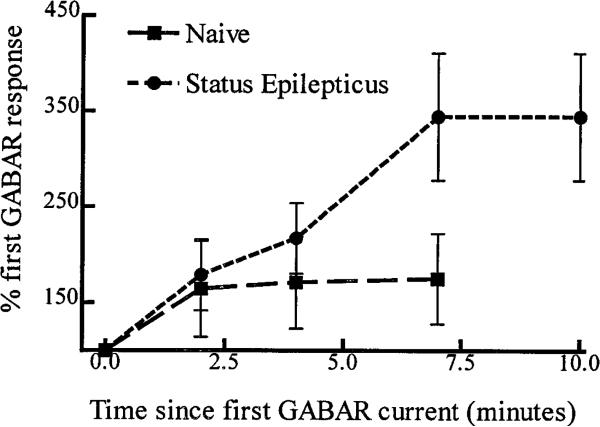
Whole-cell voltage-clamp recordings were made from dentate granule cells (Kay and Wong, 1986; Oh et al., 1995) acutely isolated from control rats or from same-age rats who had 45 min of continuous seizures (status epilepticus). When access was initially established in granule cells from control rats, GABAR currents evoked by 10 μm GABA increased slightly and became stable in 2–4 min (run-up) (Fig. 2A). The stable response compared with the first response increased 174 ± 47% (n = 4) (Fig. 3). In contrast, GABAR currents evoked from hippocampal neurons from animals undergoing status epilepticus required 10 min to stabilize (Fig. 2B), and the run-up was substantially larger (374 ± 66%; n = 5; p < 0.05) (Fig. 3).
Figure 2.
Stabilization of GABAR currents after access. GABAR currents elicited immediately on access from dentate granule cells. Traces were from two neurons, the top from a cell isolated from a control animal and the bottom from an animal undergoing status epilepticus. The durations of GABA application were indicated by horizontal bars. Two minutes elapsed between each GABA application. A, GABAR currents elicited from hippocampal dentate granule cells isolated from control animals rapidly increased to a relatively stable amplitude. B, GABAR currents elicited from hippocampal dentate granule cells isolated from animals undergoing status epilepticus took longer to stabilize and showed a greater increase in amplitude.
Figure 3.
Run-up of GABAR currents after access. Granule cell GABAR peak currents were normalized to the initial current evoked by 10 μm GABA after access. Means ± SEMs of peak normalized GABAR currents from five neurons from animals undergoing status epilepticus and four neurons from control animals were plotted.
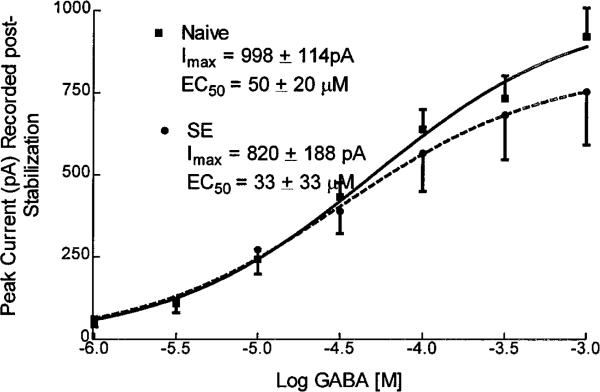
Once stable responses to 10 μm GABA were obtained, GABA was applied to granule cells at concentrations ranging from 1 to 1000 μm (Fig. 4). For each of the groups, data from individual cells were pooled and fitted to a sigmoidal logistic equation. In neurons from control animals, the mean GABA EC50 for GABARs was 50 ± 20 μm (n = 17), similar to that in neurons from animals undergoing status epilepticus, 33 ± 14 μm (n = 9; p > 0.05). The maximal GABAR current in cells from control animals was 962 ± 109 pA (n = 19), similar to that in cells from animals undergoing status epilepticus, 820 ± 188 pA (n = 9). Thus after status epilepticus, there was increased run-up of GABAR currents after initial access, but once stable currents had been obtained, the potency and efficacy of GABA on dentate granule cell GABARs was similar to those in neurons from control animals. Modulation of GABAR currents was studied in dentate granule cells isolated from control rats and from those undergoing status epilepticus after stabilization of currents.
Figure 4.
GABA concentration dependency. GABA concentration and normalized GABAR peak current relationships were plotted for 17 neurons isolated from control animals and for 9 neurons isolated from animals undergoing status epilepticus. Concentration–response data were obtained after stabilization of currents. Each point represented the mean of normalized peak currents, and the error bars showed SEMs. The line was the best fit of data to a sigmoidal function. The EC50 and Imax were derived from the equation for the sigmoidal function that best fitted the data.
Diazepam enhancement of GABAR currents
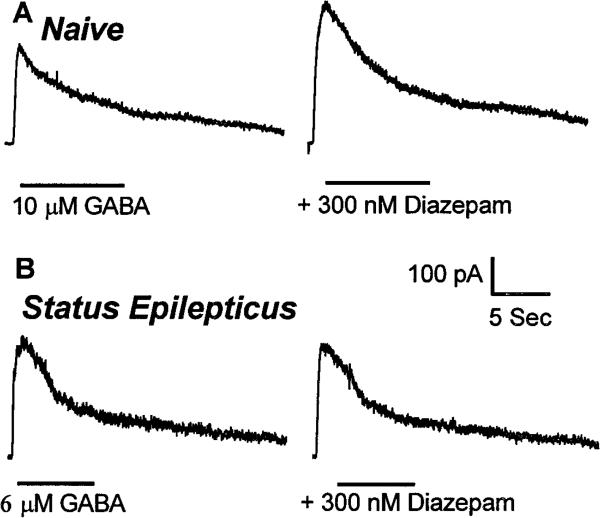
In hippocampal dentate granule cells from control animals, when 10 μm GABA was coapplied with 300 nm diazepam, GABAR currents were enhanced in all neurons by 68 ± 10% (n = 6) (Fig. 5A). In contrast, in dentate granule cells from animals undergoing status epilepticus, 300 nm diazepam inconsistently enhanced 6 or 10 μm GABA-evoked GABAR currents by 10 ± 6% (n = 5; p < 0.001; grouped t test) (Fig. 5B).
Figure 5.
Diazepam enhancement of GABAR currents in dentate granule cells from control animals and in cells isolated from rats after 45 min of seizures. Diazepam at 300 nm enhanced GABAR current in dentate granule cells from control animals but did not enhance current in cells isolated from rats after 45 min of seizures. The traces are from two different neurons. Horizontal bars showed the duration of application of the drug. A, Diazepam (300 nm) was applied with 10 μm GABA to a dentate granule cell from a control animal. B, Diazepam (300 nm) was applied with 6 μm GABA to a granule cell isolated from a rat after status epilepticus. A lower concentration of GABA was used to compensate for a small leftward shift of the GABA concentration–response curve in cells from animals undergoing status epilepticus (equipotent GABA concentration).
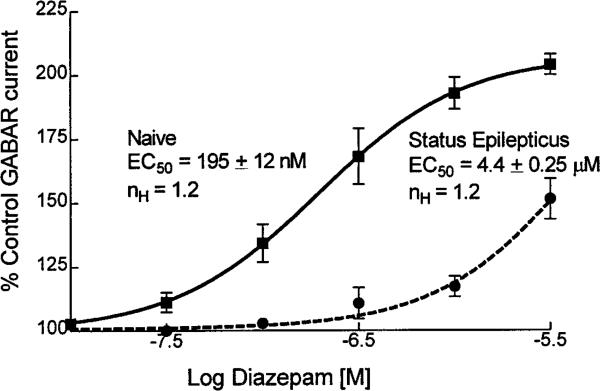
Diazepam concentration–response curves were obtained for enhancement of GABAR currents from neurons from both naive animals and from animals subjected to status epilepticus. In neurons from naive animals, 1 or 3 μm diazepam elicited maximal enhancement of GABAR currents, whereas in neurons from rats undergoing status epilepticus, 3 μm diazepam elicited more enhancement of GABAR currents than did 1 μm diazepam. Because diazepam causes a leftward shift of the GABA concentration–response curve, the same amount of diazepam will cause more enhancement of GABAR currents if applied with a lower GABA concentration. Additionally, the GABA EC50 was slightly (but not statistically significantly) left-shifted in granule cells acutely isolated from rats undergoing status epilepticus when compared with the value in controls. In this situation, it was important to use equipotent and not equal GABA concentrations. In four neurons from rats undergoing status epilepticus, varying concentrations of diazepam were coapplied with 6 μm (instead of 10 μm) GABA; however the diazepam EC50 and maximal enhancement in these experiments were similar to those with diazepam coapplied with 10 μm GABA. The data from these experiments were pooled. In neurons from control animals, 1 μm diazepam enhanced GABAR currents by 92 ± 6% (n = 6), but in neurons from animals undergoing status epilepticus, 3 μm diazepam only enhanced GABAR currents by 51 ± 8% (n = 5; p < 0.05; grouped t test) (Fig. 6). The EC50 for diazepam enhancement of GABAR currents in neurons from control animals was 195 ± 12 nm, and the EC50 in neurons from animals undergoing status epilepticus was 4.4 ± 0.25 μm (Fig. 6). Thus the prolonged seizures of status epilepticus reduced the potency and efficacy of diazepam for enhancement of granule cell GABAR currents.
Figure 6.
Diazepam concentration–dentate granule cell GABAR current enhancement relationships. Diazepam concentration–response curves were obtained for neurons isolated from control animals (filled boxes, solid line; n = 9) and for neurons isolated from animals undergoing status epilepticus (filled circles, dashed line; n = 12). Higher concentrations of diazepam inhibited GABAR current as reported previously (De Deyn and Macdonald, 1988).
Zn2+ inhibition of GABAR currents
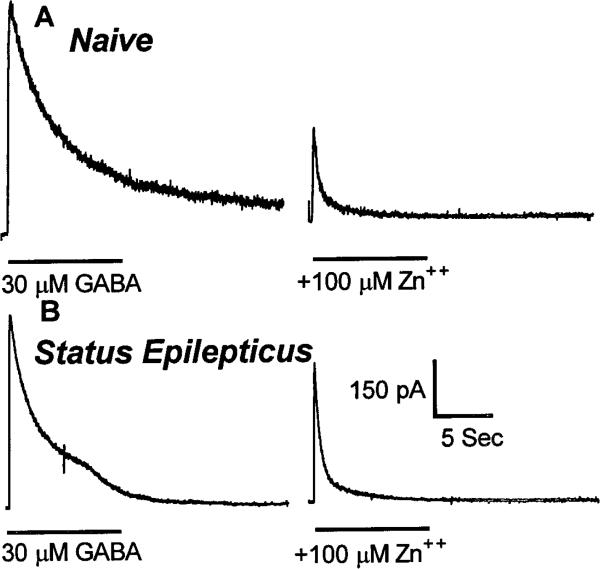
Because Zn2+ modulation of recombinant GABAR currents varies inversely with benzodiazepine sensitivity (Draguhn et al., 1990; Smart et al., 1991), Zn2+ inhibition of granule cell GABAR currents was studied. Zn2+ was less potent in inhibiting GABAR currents recorded from granule cells isolated from animals undergoing status epilepticus than from control granule cells. In neurons from control animals, GABAR currents were inhibited 59 ± 4% (n = 8) by 100 μm Zn2+ (Fig. 7A), but in neurons isolated from animals undergoing status epilepticus, the inhibition was reduced to 39 ± 6% (n = 6; p < 0.05; grouped t test) (Fig. 7B).
Figure 7.
Zn 2+ inhibition of GABAR currents in dentate granule cells from control animals and from animals undergoing status epilepticus. Zn 2+ (100 μm) inhibited GABAR currents in dentate granule cells from control animals more than it did in granule cells from animals undergoing status epilepticus. The traces are from two different neurons. Zn 2+ (100 μm) was coapplied with 30 μm GABA. Horizontal bars show the duration of application of the drug. A, Traces from a dentate granule cell isolated from a control animal. B, Traces from a granule cell isolated from an animal undergoing status epilepticus.
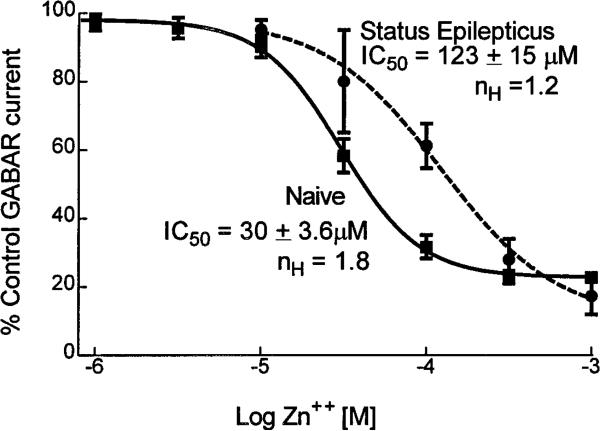
Zn2+, ranging in concentration from 1 to 1000 μm, was coapplied with GABA to define the mechanism of the reduced Zn2+ block (Fig. 8). In dentate granule cells from control rats, GABAR currents were reduced by Zn 2+ in a concentration-dependent manner with an IC50 of 30 ± 3.6 μm (n = 12). In dentate granule cells isolated from animals undergoing status epilepticus, the IC50 of Zn2+ inhibition of GABAR currents was 123 ± 15 μm (n = 10; p < 0.01; grouped t test). The maximal inhibition of GABAR currents by Zn2+ was unchanged (78 ± 3% in neurons from control animals and 90 ± 16% in neurons from animals undergoing status epilepticus). Thus the prolonged seizures of status epilepticus reduced the potency of Zn2+ without altering the efficacy of inhibition of granule cell GABAR currents.
Figure 8.
Zn 2+ concentration–dentate granule cell GABAR current reduction relationships. Zn 2+ concentration–dentate granule cell GABAR current inhibition relationships were obtained from neurons isolated from control animals (filled boxes, solid line; n = 12) and from neurons isolated from animals undergoing status epilepticus (filled circles, dashed line; n = 12). The lines were the best fit of the data to a sigmoidal function. The IC50 and Hill slope (nH) were derived from the equation for the sigmoidal function that best fitted the data.
Pentobarbital enhancement of GABAR currents
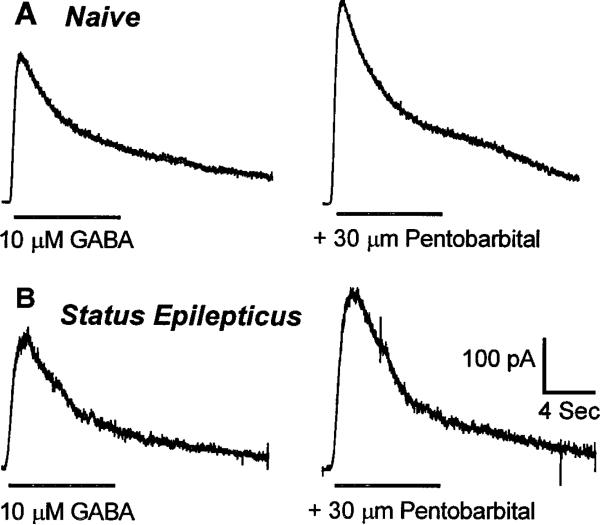
In neurons from control animals, GABAR currents elicited by 10 μm GABA were enhanced 77 ± 7% (n = 6) by 30 μm pentobarbital (Fig. 9A), whereas in neurons from animals undergoing status epilepticus, GABAR currents elicited by 10 μm GABA were enhanced 62 ± 11% (n = 3) by 30 μm pentobarbital (Fig. 9B) (p > 0.05; grouped t test).
Figure 9.
Pentobarbital enhancement of GABAR currents from dentate granule cells from control animals and from cells isolated from animals undergoing status epilepticus. Pentobarbital (30 μm) equally enhanced GABAR currents in dentate granule cells from control animals and in granule cells from animals undergoing status epilepticus. The traces are from two different neurons. Pentobarbital (30 μm) was coapplied with 10 μm GABA. Horizontal bars show the duration of application of the drug. A, Traces from a dentate granule cell isolated from a control animal. B, Traces from a granule cell isolated from an animal undergoing status epilepticus.
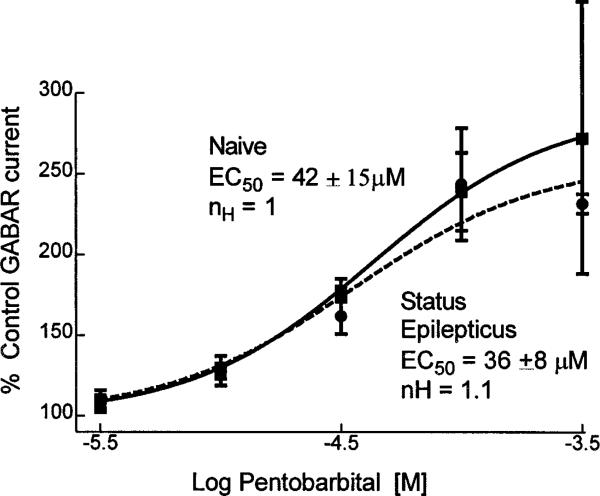
Concentration–response curves were obtained by coapplying 1–300 μm pentobarbital with 10 μm GABA to neurons obtained from control animals and from animals undergoing status epilepticus (Fig. 10). In dentate granule cells from control animals, the pentobarbital EC50 was 42 ± 15 μm (n = 6), and in neurons from animals undergoing status epilepticus, the pentobarbital EC50 was not significantly different (36 ± 8 μm; n = 6) (Fig. 10). Maximal enhancement of GABAR currents by pentobarbital in neurons from control rats (190 ± 55%) and in neurons from animals undergoing status epilepticus (158 ± 20%) were not significantly different (p > 0.05; grouped t test). Thus, the prolonged seizures of status epilepticus did not alter the efficacy or potency of pentobarbital enhancement of GABAR currents in dentate granule cells.
Figure 10.
Pentobarbital concentration–dentate granule cell GABAR current enhancement relationships. Pentobarbital concentration–dentate granule cell GABAR current enhancement relationships were obtained for neurons isolated from control animals (filled boxes, solid line; n = 7) and for neurons isolated from animals undergoing status epilepticus (filled circles, dashed line; n = 6). The lines were the best fit of the data to a sigmoidal function. The EC50 and Hill slope were derived from the equation for the sigmoidal function that best fitted the data.
DISCUSSION
Conclusions
This study demonstrated that the prolonged seizures of status epilepticus reduced the potency but not the efficacy of the benzodiazepine diazepam in terminating seizures. Diazepam and Zn2+ sensitivity of hippocampal dentate granule cell GABARs were rapidly and selectively altered by the prolonged seizures. In contrast, the GABA and barbiturate sensitivities of GABARs were unaffected. These findings demonstrate a novel form of rapidly developing functional plasticity of GABARs and may explain in part the observation that status epilepticus becomes more difficult to treat the longer its duration at the time treatment begins.
Diazepam loses effectiveness in the treatment of status epilepticus
This study demonstrated that the prolonged seizures of status epilepticus reduced the ability of diazepam to terminate status epilepticus. This refractoriness to diazepam resulted from the loss of diazepam potency but not of diazepam efficacy. This phenomenon of refractoriness to diazepam has been reported previously in humans (Yaffe and Lowenstein, 1993) and rats (Walton and Treiman, 1988). Several possible mechanisms can be hypothesized to explain the loss of diazepam effectiveness in the treatment of prolonged seizures of status epilepticus; seizures may become more intense, there may be enhanced excitatory transmission, or there may be altered inhibition. Past studies indicate that the hippocampus is involved in the generation of status epilepticus (Lothman et al., 1991; VanLandingham and Lothman, 1991; Kapur and Macdonald, 1996), and hippocampal GABAergic inhibition is altered during status epilepticus (Kapur and Lothman, 1989; Kapur et al., 1989; Kapur and Coulter, 1995). These studies suggested that refractoriness of seizures to diazepam may result from altered GABAR function in the hippocampus. The experiments reported here support the altered GABAR function hypothesis.
The principal limitation of the whole-animal experiments was that no electroencephalogram (EEG) was recorded during the induction and treatment of status epilepticus. This was of concern because brief limbic seizures may occur in rats without behavioral change (Walton and Treiman, 1988; Lothman et al., 1989). Thus behaviorally a rat may appear seizure free but may still be having electrographic seizures after treatment. To circumvent this difficulty, we used an additional criterion, resumption of normal behavior within 30 min, to define termination of status epilepticus. Our conclusion that status epilepticus in rats can be controlled with diazepam after brief seizures but that after prolonged seizures the rats become refractory to the same dose of diazepam was supported by several previous studies (Morrisett et al., 1987; Walton and Treiman, 1988) that used combined EEG and behavioral observations. These published studies documented that diazepam at 20 mg/kg terminates lithium- and pilocarpine-induced status epilepticus 10 min after the onset of seizures but not 45 min after the onset. We wanted to determine whether this loss of effectiveness of diazepam in terminating prolonged seizures represented a loss of efficacy or a loss of potency of diazepam. The current study suggests that this was caused by a loss of potency.
Plasticity of GABAR function during status epilepticus
During status epilepticus, GABAR-mediated inhibition in the hippocampus is reduced both in the CA1 region and in the dentate gyrus (Kapur and Lothman, 1989; Sloviter, 1991; Kapur and Coulter, 1995). One proposed mechanism for the reduction in inhibition is a specific alteration in the functional properties of GABARs (Kapur and Coulter, 1995). This study demonstrates directly that two functional properties of GABARs, diazepam enhancement and Zn2+ inhibition of GABAR currents, were altered by the prolonged seizures. This plasticity of GABARs in the hippocampus may play a role in the pathogenesis and treatment of status epilepticus. Seizures in the hippocampus reduce GABAergic inhibition, and these findings demonstrate that this is in part because of changes in GABAR function. The reduction of diazepam sensitivity of dentate granule cell GABARs parallels the loss of effectiveness of diazepam in the treatment of experimental status epilepticus. It is possible that changes in the diazepam sensitivity of dentate granule cell GABARs reflect reduction of diazepam sensitivity in the treatment of status epilepticus. Additionally, pentobarbital sensitivity of GABARs on dentate granule cells isolated from animals undergoing status epilepticus was preserved. This suggested that status epilepticus alters specific properties of GABARs rather than causing a generalized dysfunction of the receptor.
In the present study, we wanted to study allosteric modulation of GABARs by diazepam, Zn2+, and pentobarbital. To study the effect of the prolonged seizures of status epilepticus on allosteric modulation of GABAR currents, it was necessary to obtain comparable baseline GABAR currents in granule cells from controls and from animals undergoing status epilepticus. This was achieved by using the conventional patch-clamp technique used to record whole-cell GABAR currents, which allowed dialysis of intracellular contents including chloride ions with the contents of the pipette. Increased run-up observed in cells isolated from rats undergoing status epilepticus was consistent with the previous report of intracellular chloride loading in neurons after status epilepticus (Kapur and Coulter, 1995). Presumably in the present study, after access was established, the high intracellular chloride ion concentration was replaced by the pipette solution containing lower chloride ion and permeant phosphate anion concentrations that caused the currents to increase.
A potential confounding factor in the study using acutely isolated neurons was that the procedure itself may alter the functional properties of GABARs. It was also possible that neurons in animals undergoing status epilepticus were selectively vulnerable to the isolation procedure. Additionally, inclusion of EGTA in the recording pipette would buffer any Ca2+-dependent changes in GABAR function (Chen and Wong, 1991; De Koninck and Mody, 1996). However, excluding EGTA from the recording pipette resulted in rapid run-down of GABAR currents, and therefore, it was necessary to include EGTA in the recording pipettes to perform these experiments (Chen and Wong, 1991). This cell isolation procedure has been used repeatedly in the past to study GABARs (Akaike et al., 1985; Coulter et al., 1990; Oh et al., 1995) and epileptic neurons (Mody et al., 1992), and the results of the study were similar to those obtained in more intact preparations (Walton and Treiman, 1988; Kapur and Lothman, 1989).
Status epilepticus and chronic temporal lobe epilepsy have distinct effects on hippocampal GABARs
Studies investigating the role of GABAR-mediated inhibition in the hippocampus in kindling and other models of temporal lobe epilepsy are the most comparable with the current study. However, brief seizures of temporal lobe epilepsy and prolonged seizures of status epilepticus are distinct phenomena. Close to 50% of those having an episode of status epilepticus have not previously experienced a seizure (DeLorenzo et al., 1996). Epileptic seizures are brief, and data from epilepsy monitoring units indicate that the majority of seizures spontaneously terminate within 10 min (Ramsay, 1993). In contrast, status epilepticus is a syndrome consisting of a very prolonged seizure with continuous evolution of neurological state, worsening cerebral metabolism, a steady rise in core temperature, a rise in blood pressure, lactic acidosis, hyperglycemia (Meldrum and Horton, 1973), and increased catecholamine levels (Simon et al., 1984). Hippocampal injury and neuronal loss occur because of status epilepticus in humans (DeGiorgio et al., 1992; Nohria et al., 1994) and in most animal models of status epilepticus (Meldrum et al., 1973; Clifford et al., 1987; Bertram and Lothman, 1993; Fujikawa et al., 1994; Sloviter et al., 1996), but whether individual brief seizures cause neuronal loss remains controversial (Bertram and Lothman, 1993; Cavazos et al., 1994; Watanabe et al., 1996). It is thus expected that status epilepticus and chronic temporal lobe epilepsy have different effects on hippocampal dentate granule cell GABARs.
In kindling, subconvulsive, electrical stimulation applied repeatedly to various regions of the brain evokes progressively prolonged behavioral and electrographic seizures that terminate in generalized tonic–clonic seizures. However there are important differences between the gradual plasticity occurring during the kindling process and the rapidly evolving changes of status epilepticus reported here. Several studies have reported enhanced [3H]muscimol and [3H]benzodiazepine binding in hippocampal membranes (Shin et al., 1985) and specifically in the hippocampal dentate gyrus (McNamara et al., 1980; Valdes et al., 1982). This increase in the hippocampal dentate granule cell GABARs after kindling was associated with an increase in the amplitude of miniature IPSCs and enhancement of paired pulse depression of kindled dentate gyrus (Otis et al., 1994). These long-term changes in GABAR-mediated inhibition in the dentate gyrus were likely to be antiepileptic in nature. The findings of this study, however, do not contradict studies on the kindling model. Although inhibitory neurotransmission in the dentate gyrus was enhanced during kindling and diminished during status epilepticus, the changes in kindling were slower to develop compared with the rapid changes occurring during status epilepticus.
In electrical stimulation models of epilepsy, GABAR-mediated inhibition in the dentate gyrus was chronically reduced, but this reduction was hypothesized to be caused by circuit rearrangement and dormancy of basket cells (Sloviter, 1991). Recently, Buhl et al. (1996) demonstrated enhanced Zn2+ sensitivity of hippocampal dentate granule cell GABARs after kindling and suggested that this increased sensitivity resulted in a collapse of the augmented inhibition during seizures. Gibbs et al. (1997) found increased GABAR density and enhanced GABAR Zn2+ sensitivity in another model of chronic temporal lobe epilepsy. Several important distinctions between these studies and this report pertain. First, the reduced diazepam sensitivity demonstrated here has not been reported in the past. Second, the changes observed here were acute, occurring over minutes, whereas previous reports documented changes that were chronic, occurring over several weeks. Finally, previous studies reported increased Zn2+ sensitivity of hippocampal dentate granule cell GABARs, whereas the current study reports diminished Zn2+ sensitivity of granule cell GABARs.
Possible molecular mechanisms for altered GABAR function
This rapid selective loss of benzodiazepine and Zn 2+ sensitivity is a novel form of GABAR plasticity, and the underlying molecular basis is unclear. Diminished benzodiazepine sensitivity with development of benzodiazepine tolerance occurred over a prolonged period of time (Rabow et al., 1995). During development of cerebellar granule cells, benzodiazepine sensitivity of GABARs is lost during maturation in parallel with increasing expression of the α6 subtype in the GABAR. Similarly, development of tolerance to benzodiazepines requires chronic benzodiazepine administration.
This selective loss of benzodiazepine and Zn2+ sensitivity may result from altered structural composition or an altered state of phosphorylation of GABARs. Diazepam sensitivity of GABARs requires the presence of the γ2 subtype with a β subtype and either α1, α2, α3, or α5 subtypes (Pritchett et al., 1989; Macdonald and Olsen, 1994). Recombinant GABARs expressed without the γ2 subtype are highly sensitive to Zn2+ (IC50 < 10 μm), whereas GABARs expressed with the γ2 subtype were relatively insensitive to Zn2+ (Draguhn et al., 1990; Smart et al., 1991). Thus one explanation for acute reduction of diazepam sensitivity of hippocampal dentate granule cell GABARs after seizures would be a loss of the γ2 subtype from the receptor; however this would not explain diminished Zn2+ sensitivity of these receptors. Another potential explanation for diminished diazepam and Zn2+ sensitivity would be an altered α subtype expression, because α subtypes are known to alter both Zn2+ and diazepam sensitivity of the GABARs. For example, recombinant GABARs with the α4or α6 subtype with a β subtype and a γ2 subtype have low diazepam and Zn2+ sensitivities (Saxena and Macdonald, 1996).
Seizures may alter GABAR function by other mechanisms such as posttranslational modification of GABARs or release of endogenous benzodiazepine-like substances. Modification of GABARs by phoshorylation is well demonstrated (Lin et al., 1994; Macdonald and Olsen, 1994; Macdonald, 1995), and seizures are known to modulate activities of cAMP-dependent kinase, calcium- and calmodulin-dependent kinase, and calcium- and phospholipid-dependent kinase (Jope et al., 1992; Perlin et al., 1992). However, it remains to be shown that posttranslational modification can alter benzodiazepine and Zn2+ sensitivity of GABARs.
Acknowledgments
This work was supported by United States Public Health Service Grants RO1 33300 (R.L.M.) and KO8 NS01748 (J.K.) and by a grant from the Epilepsy Foundation of America (J.K.). We thank Nadia Esmaiel and Eric M. Ortwig for assistance with the experiments.
REFERENCES
- Akaike N, Hattori K, Inomata N, Oomura Y. Gamma-Aminobutyric-acid- and pentobarbitone-gated chloride currents in internally perfused frog sensory neurones. J Physiol (Lond) 1985;360:367–386. doi: 10.1113/jphysiol.1985.sp015622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram EH, Lothman EW. Morphometric effects of intermittent kindled seizures and limbic status epilepticus in the dentate gyrus of the rat. Brain Res. 1993;603:25–31. doi: 10.1016/0006-8993(93)91295-4. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Otis TS, Mody I. Zinc-induced collapse of augmented inhibition by GABA in a temporal lobe epilepsy model. Science. 1996;271:369–373. doi: 10.1126/science.271.5247.369. [DOI] [PubMed] [Google Scholar]
- Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci. 1994;14:3106–3121. doi: 10.1523/JNEUROSCI.14-05-03106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QX, Wong RK. Intracellular Ca2+ suppressed a transient potassium current in hippocampal neurons. J Neurosci. 1991;11:337–343. doi: 10.1523/JNEUROSCI.11-02-00337.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Olney JW, Maniotis A, Collins RC, Zorumski CF. The functional anatomy and pathology of lithium-pilocarpine and high-dose pilocarpine seizures. Neuroscience. 1987;23:953–968. doi: 10.1016/0306-4522(87)90171-0. [DOI] [PubMed] [Google Scholar]
- Commission on Classification and Terminology of the International League against Epilepsy Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- Coulter DA, Huguenard JR, Prince DA. Differential effects of petit mal anticonvulsants and convulsants on thalamic neurones: GABA current blockade. Br J Pharmacol. 1990;100:807–813. doi: 10.1111/j.1476-5381.1990.tb14096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deyn PP, Macdonald RL. Effects of non-sedative anxiolytic drugs on responses to GABA and on diazepam-induced enhancement of these responses on mouse neurones in cell culture. Br J Pharmacol. 1988;95:109–120. doi: 10.1111/j.1476-5381.1988.tb16554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgio CM, Tomiyasu U, Gott PS, Treiman DM. Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia. 1992;33:23–27. doi: 10.1111/j.1528-1157.1992.tb02278.x. [DOI] [PubMed] [Google Scholar]
- De Koninck Y, Mody I. The effects of raising intracellular calcium on synaptic GABAA receptor-channels. Neuropharmacology. 1996;35:1365–1374. doi: 10.1016/s0028-3908(96)00063-9. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ. Status epilepticus: concepts in diagnosis and treatment. Semin Neurol. 1990;10:396–405. doi: 10.1055/s-2008-1063984. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ, Hauser WA, Towne AR, Boggs JG, Pellock JM, Penberthy L, Garnett L, Ko D. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–1035. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Verdorn TA, Ewert M, Seeburg PH, Sakmann B. Functional and molecular distinction between recombinant rat GABAA receptor subtypes by Zn 2+. Neuron. 1990;5:781–788. doi: 10.1016/0896-6273(90)90337-f. [DOI] [PubMed] [Google Scholar]
- Fujikawa DG, Daniels AH, Kim JS. The competitive NMDA receptor antagonist CGP 40116 protects against status epilepticus-induced neuronal damage. Epilepsy Res. 1994;17:207–219. doi: 10.1016/0920-1211(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Gibbs JW, Shumate MD, Coulter DA. Differential epilepsy-associated alterations in postsynaptic GABAA receptor function in dentate granule and CA1 neurons. J Neurophysiol. 1997;77:1924–1938. doi: 10.1152/jn.1997.77.4.1924. [DOI] [PubMed] [Google Scholar]
- Greenfield LJ, Jr, Macdonald RL. Whole-cell and single-channel alpha1 beta1 gamma2S-GABAA receptor currents elicited by a “multipuffer” drug application device. Pflügers Arch. 1996;432:1080–1090. doi: 10.1007/s004240050238. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Honchar MP, Olney JW, Sherman WR. Systemic cholinergic agents induce seizures and brain damage in lithium-treated rats. Science. 1983;220:323–325. doi: 10.1126/science.6301005. [DOI] [PubMed] [Google Scholar]
- Jope RS, Song L, Kolasa K. Inositol trisphosphate, cyclic AMP, and cyclic GMP in rat brain regions after lithium and seizures. Biol Psychiatry. 1992;31:505–514. doi: 10.1016/0006-3223(92)90261-w. [DOI] [PubMed] [Google Scholar]
- Kapur J, Coulter DA. Experimental status epilepticus alters GABAA receptor function in CA1 pyramidal neurons. Ann Neurol. 1995;38:893–900. doi: 10.1002/ana.410380609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur J, Lothman EW. Loss of inhibition precedes delayed spontaneous seizures in the hippocampus after tetanic electrical stimulation. J Neurophysiol. 1989;61:427–434. doi: 10.1152/jn.1989.61.2.427. [DOI] [PubMed] [Google Scholar]
- Kapur J, Macdonald RL. Status epilepticus: a proposed patho-physiology. In: Shorvon S, Dreyfuss F, Fish D, Thomas D, editors. The treatment of epilepsy. Blackwell Science; Oxford: 1996. pp. 258–268. [Google Scholar]
- Kapur J, Stringer JL, Lothman EW. Evidence that repetitive seizures in the hippocampus cause a lasting reduction of GABAergic inhibition. J Neurophysiol. 1989;61:417–426. doi: 10.1152/jn.1989.61.2.417. [DOI] [PubMed] [Google Scholar]
- Kapur J, Lothman EW, DeLorenzo RJ. Loss of GABAA receptors during partial status epilepticus. Neurology. 1994;44:2407–2408. doi: 10.1212/wnl.44.12.2407. [DOI] [PubMed] [Google Scholar]
- Kay AR, Wong RK. Isolation of neurons suitable for patch-clamping from adult mammalian central nervous systems. J Neurosci Methods. 1986;16:227–238. doi: 10.1016/0165-0270(86)90040-3. [DOI] [PubMed] [Google Scholar]
- Lin Y, Browning MD, Dudek EM, Macdonald RL. Protein kinase C enhances recombinant bovine a1b1G2L GABAA receptor whole-cell currents expressed in L929 fibroblasts. Neuron. 1994;13:1421–1431. doi: 10.1016/0896-6273(94)90427-8. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, Bekenstein JW, Perlin JB. Self-sustaining limbic status epilepticus induced by “continuous” hippocampal stimulation: electrographic and behavioral characteristics. Epilepsy Res. 1989;3:107–119. doi: 10.1016/0920-1211(89)90038-7. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, III, Stringer JL. Functional anatomy of hippocampal seizures. Prog Neurobiol. 1991;37:1–82. doi: 10.1016/0301-0082(91)90011-o. [DOI] [PubMed] [Google Scholar]
- Macdonald RL. Ethanol, gamma-aminobutyrate type A receptors, and protein kinase C phosphorylation. Proc Natl Acad Sci USA. 1995;92:3633–3635. doi: 10.1073/pnas.92.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia. 1995;36:S2–S12. doi: 10.1111/j.1528-1157.1995.tb05996.x. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Twyman RE, Ryan-Jastrow T, Angelotti TP. Regulation of GABAA receptor channels by anticonvulsant and convulsant drugs and by phosphorylation. Epilepsy Res. 1992;9(Suppl):265–277. [PubMed] [Google Scholar]
- McNamara JO, Peper AM, Patrone V. Repeated seizures induce long-term increase in hippocampal benzodiazepine receptors. Proc Natl Acad Sci USA. 1980;77:3029–3032. doi: 10.1073/pnas.77.5.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum BS, Horton RW. Physiology of status epilepticus in primates. Arch Neurol. 1973;28:1–9. doi: 10.1001/archneur.1973.00490190019001. [DOI] [PubMed] [Google Scholar]
- Meldrum BS, Vigouroux RA, Brierley JB. Systemic factors and epileptic brain damage. Prolonged seizures in paralyzed, artificially ventilated baboons. Arch Neurol. 1973;29:82–87. doi: 10.1001/archneur.1973.00490260026003. [DOI] [PubMed] [Google Scholar]
- Mody I, Otis TS, Staley KJ, Kohr G. The balance between excitation and inhibition in dentate granule cells and its role in epilepsy. Epilepsy Res. 1992;9(Suppl):331–339. [PubMed] [Google Scholar]
- Morrisett RA, Jope RS, Snead OC. Effects of drugs on the initiation and maintenance of status epilepticus induced by administration of pilocarpine to lithium-pretreated rats. Exp Neurol. 1987;97:193–200. doi: 10.1016/0014-4886(87)90293-7. [DOI] [PubMed] [Google Scholar]
- Nohria V, Lee N, Tien RD, Heinz ER, Smith JS, DeLong GR, Skeen MB, Resnick TJ, Crain B, Lewis DV. Magnetic resonance imaging evidence of hippocampal sclerosis in progression: a case report. Epilepsia. 1994;35:1332–1336. doi: 10.1111/j.1528-1157.1994.tb01807.x. [DOI] [PubMed] [Google Scholar]
- Oh K-S, Lee C-J, Gibbs JW, Coulter DA. Postnatal development of GABAA receptor function in somatosensory thalamus and cortex: whole-cell voltage clamp recordings in acutely isolated rat neurons. J Neurosci. 1995;15:1341–1351. doi: 10.1523/JNEUROSCI.15-02-01341.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, De Koninck Y, Mody I. Lasting potentiation of inhibition is associated with an increased number of gamma-aminobutyric acid type A receptors activated during miniature inhibitory postsynaptic currents. Proc Natl Acad Sci USA. 1994;91:7698–7702. doi: 10.1073/pnas.91.16.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlin JB, Churn SB, Lothman EW, DeLorenzo RJ. Loss of type II calcium/calmodulin-dependent kinase activity correlates with stages of development of electrographic seizures in status epilepticus in rat. Epilepsy Res. 1992;11:111–118. doi: 10.1016/0920-1211(92)90045-u. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse. 1995;21:189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Ramsay RE. Treatment of status epilepticus. Epilepsia. 1993;34(Suppl 1):S71–S81. doi: 10.1111/j.1528-1157.1993.tb05908.x. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar GABAA receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- Shin C, Pedersen HB, McNamara JO. γ-Aminobutyric acid and benzodiazepine receptors in the kindling model of epilepsy: a quantitative radiohistochemical study. J Neurosci. 1985;5:2696–2701. doi: 10.1523/JNEUROSCI.05-10-02696.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon RP, Aminoff MJ, Benowitz NL. Changes in plasma catecholamines after tonic-clonic seizures. Neurology. 1984;34:255–257. doi: 10.1212/wnl.34.2.255. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Permanently altered hippocampal structure, excitability, and inhibition after experimental status epilepticus in the rat: the “dormant basket cell” hypothesis and its possible relevance to temporal lobe epilepsy. Hippocampus. 1991;1:41–66. doi: 10.1002/hipo.450010106. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dean E, Sollas AL, Goodman JH. Apoptosis and necrosis induced in different hippocampal neuron populations by repetitive perforant path stimulation in the rat. J Comp Neurol. 1996;366:516–533. doi: 10.1002/(SICI)1096-9861(19960311)366:3<516::AID-CNE10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Smart TG, Moss SJ, Xie X, Huganir RL. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br J Pharmacol. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes F, Dasheiff RM, Birmingham F, Crutcher KA, McNamara JO. Benzodiazepine receptor increases after repeated seizures: evidence for localization to dentate granule cells. Proc Natl Acad Sci USA. 1982;79:193–197. doi: 10.1073/pnas.79.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanLandingham KE, Lothman EW. Self-sustaining limbic status epilepticus. I. Acute and chronic cerebral metabolic studies: limbic hypermetabolism and neocortical hypometabolism. Neurology. 1991;41:1942–1949. doi: 10.1212/wnl.41.12.1942. [DOI] [PubMed] [Google Scholar]
- Walton NY, Treiman DM. Response of status epilepticus induced by lithium and pilocarpine to treatment with diazepam. Exp Neurol. 1988;101:267–275. doi: 10.1016/0014-4886(88)90010-6. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Johnson RS, Butler LS, Binder DK, Spiegelman BM, Papaioannou VE, McNamara JO. Null mutation of c-fos impairs structural and functional plasticities in the kindling model of epilepsy. J Neurosci. 1996;16:3827–3836. doi: 10.1523/JNEUROSCI.16-12-03827.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lowenstein DH. Prognostic factors of pentobarbital therapy for refractory generalized status epilepticus. Neurology. 1993;43:895–900. doi: 10.1212/wnl.43.5.895. [DOI] [PubMed] [Google Scholar]