Abstract
Recent clinical studies demonstrate that serum levels of brain-derived neurotrophic factor (BDNF) are significantly decreased in patients with major depressive disorder (MDD) and that antidepressant treatments reverse this effect, indicating that serum BDNF is a biomarker of MDD. These findings raise the possibility that serum BDNF may also have effects on neuronal activity and behavior, but the functional significance of altered serum BDNF is unknown. To address this issue, we determined the influence of peripheral BDNF administration on depression- and anxiety-like behavior, including the forced swim test (FST), chronic unpredictable stress (CUS)/anhedonia, novelty-induced hypophagia (NIH) test, and elevated-plus maze (EPM). Furthermore, we examined adult hippocampal neurogenesis as well as hippocampal and striatal expression of BDNF, extracellular signal-regulated kinase (ERK) and cAMP response element binding protein (CREB), in order to determine whether peripherally administered BDNF produces antidepressant-like cellular responses in the brain. Peripheral BDNF administration increased mobility in the FST, attenuated the effects of CUS on sucrose consumption, decreased latency in the NIH test, and increased time spent in the open arms of an EPM. Moreover, adult hippocampal neurogenesis was increased following chronic, peripheral BDNF administration. We also found that BDNF levels as well as expression of pCREB and pERK were elevated in the hippocampus of adult mice receiving peripheral BDNF. Taken together, these results indicate that peripheral/serum BDNF may not only represent a biomarker of MDD, but also have functional consequences on molecular signaling substrates, neurogenesis and behavior.
Keywords: antidepressant, dentate gyrus, neurogenesis, depression, neurotrophic factor, serum
Introduction
A growing body of evidence indicates that alterations of neurotrophic factor expression in limbic brain regions, including the hippocampus, play a critical role in the pathophysiology and/or treatment of major depressive disorder (MDD) (Castren et al, 2007; Duman and Monteggia, 2006; Schmidt and Duman, 2007). Exposure to stress, which is associated with, but not required for, the onset of MDD (Kendler et al, 1999) in humans and precipitates or exacerbates depressive episodes (Gold and Chrousos, 2002), has consistently been shown to decrease hippocampal neurotrophic factor expression, most notably brain derived neurotrophic factor (BDNF), while chronic antidepressant administration increases BDNF expression in the hippocampus (Castren et al, 2007; Duman et al, 2006; Schmidt et al, 2007). Stress and antidepressant administration also exert opposing effects on neurogenesis in the adult hippocampus, and this has been linked with some behavioral actions of antidepressant treatment (Saarelainen et al, 2003; Sairanen et al, 2005; Shirayama et al, 2002). Thus, altered expression of BDNF, as well as other neurotrophic factors, and aberrant regulation of neurogenesis in the hippocampus and other limbic nuclei may result in maladaptive changes in neural networks that underlie the pathophysiology of MDD.
Recent studies have also demonstrated that increased BDNF expression plays a critical role in the behavioral and cellular efficacy of antidepressants (Li et al, 2008; Saarelainen et al, 2003; Sairanen et al, 2005; Shirayama et al, 2002). Infusion of BDNF directly into the hippocampus is sufficient to produce an antidepressant-like effect in behavioral models of depression (Shirayama et al, 2002). Furthermore, the behavioral response to antidepressant administration is blocked in conditional BDNF knockout mice, transgenic mice expressing a variant BDNF allele (Val66Met), or transgenic mice expressing a dominant negative form of the BDNF receptor (TrkB), indicating that BDNF signaling is required for an antidepressant response (Chen et al, 2006; Monteggia et al, 2004; Saarelainen et al, 2003; Sairanen et al, 2005). BDNF deletion mutants also display a depressive phenotype when exposed to mild stress (Duman et al, 2007), even though there is no difference under non-stressed conditions (Chen et al, 2006; Monteggia et al, 2004; Saarelainen et al, 2003), suggesting that decreased BDNF promotes a state of increased vulnerability to stress and/or depression. Studies in humans have reported a similar increase in vulnerability to stress in subjects carrying a BDNF polymorphism (Val66/Met), which decreases the processing and release of BDNF (Egan et al, 2003; Gatt et al, 2009).
Analyses of postmortem hippocampal tissue also support a role for BDNF in MDD, demonstrating that BDNF expression is decreased in depressed suicide patients and increased in patients receiving antidepressant medication at the time of death (Chen et al, 2001b; Dwivedi et al, 2003; Karege et al, 2005b). Surprisingly, a large number of clinical studies have consistently demonstrated that serum (Aydemir et al, 2006; Gervasoni et al, 2005; Karege et al, 2002; Shimizu et al, 2003) and plasma (Kim et al, 2007; Lee et al, 2006) BDNF levels are significantly decreased in depressed patients, and that chemical (Aydemir et al, 2005; Gervasoni et al, 2005; Gonul et al, 2005; Huang et al, 2008b; Yoshimura et al, 2007) and nonchemical (Bocchio-Chiavetto et al, 2006; Okamoto et al, 2008; Zanardini et al, 2006) antidepressants reverse or normalize this effect. Meta-analysis provides further evidence that serum BDNF levels are differentially regulated by stress and antidepressants in MDD patients (Brunoni et al, 2008; Sen et al, 2008). These findings indicate that serum BDNF is a biomarker for MDD and antidepressant efficacy. Moreover, these results also suggest that serum BDNF could have functional significance in the pathophysiology and/or treatment of mood disorders. The possibility that peripheral growth factors can enter the brain and produce both behavioral and cellular responses is supported by studies of insulin like growth factor-1 (IGF-1) (Aberg et al, 2000; Duman et al, 2008) and vascular endothelial growth factor (VEGF) (Fabel et al, 2003). In addition, despite the name, BDNF is also expressed at relatively high levels in peripheral tissues, including lung, heart, and spleen (Braun et al, 1999; Nassenstein et al, 2003; Scarisbrick et al, 1993; Timmusk et al, 1993; Yamamoto et al, 1996), and BDNF in serum is likely to be derived from these tissues as well as from brain. However, there have been no studies to date examining the effects of peripheral BDNF on behavioral or neuronal responses.
The present study was conducted in order to determine the potential functional significance of peripheral BDNF in behavioral and cellular models of antidepressant-like responses. The results demonstrate that chronic, peripheral administration of BDNF produces antidepressant and anxiolytic behavioral responses in animal models, increases the survival rate of newborn neurons and increases BDNF-mediated signaling in the adult hippocampus. These findings provide the first evidence that peripheral BDNF has functional actions within the brain and on behavior, and, thus, further supports a role for serum BDNF as a relevant biomarker for depression and treatment response.
Materials and Methods
Animals and Housing
Male C57Bl/6 and BALB/c mice, 8–10 weeks of age, were obtained from Jackson Laboratories (Bar Harbor, ME) and were group-housed (4–5/cage) with water and food available ad libitum. C57Bl/6 mice were used in all of the studies except the CUS experiments, for which BALB/c mice were used. Mice were maintained on a 12 h light/dark cycle with the lights on at 7:00 AM. All experimental procedures were performed during the light cycle. For all experiments, mice were randomly assigned to experimental and control groups and tested in a counterbalanced order. All experimental protocols were consistent with the guidelines issued by the U.S. National Institutes of Health and were approved by the Yale University Institutional Animal Care and Use Committee.
Surgery
Prior to and during surgery, mice were anesthetized with isoflurane gas. Briefly, an incision in the skin was made between the scapulae, and a chronic, indwelling osmotic mini-pump (Alzet Model 1002; Durect Corp., Cupertino, CA) containing either recombinant BDNF (4.0, 8.0, or 12.0 µg/24 hr; Regeneron Pharmaceuticals) or saline was implanted subcutaneously. The incision was closed using nylon sutures. The osmotic mini-pump administered recombinant BDNF or saline subcutaneously over a period of 14 days. Recombinant BDNF was diluted in phosphate-buffered saline (PBS) to the appropriate concentrations and supplemented with 0.1% bovine serum albumin (BSA) (protease-free, Sigma Aldrich, St. Louis, MO) as described in a previous report (Altar et al, 1992). Previous studies have demonstrated that recombinant BDNF is stable in subcutaneous osmotic minipumps and retains its biological activity for 28 days (Altar et al, 1992; Mufson et al, 1996; Mufson et al, 1994).
Behavioral Tests
The behavioral response to peripheral BDNF or saline administration was measured during the last week of treatment (Figure 1A). Behavioral tests were counterbalanced and assayed across multiple cohorts of animals. Eight groups of animals were used to study peripheral BDNF administration in the FST, NIH, EPM, and OFT. Two behavioral assays were conducted randomly per group. Separate groups of animals were used to study the effects of peripheral BDNF administration and CUS exposure on sucrose consumption. Moreover, separate cohorts of animals that did not undergo behavioral testing were used for the molecular studies presented. All behavioral tests were conducted between 9:00 AM and 12:00 PM. For more detailed information regarding protocols for the behavioral tests used in this study, please refer to the Supplementary Materials.
Figure 1.
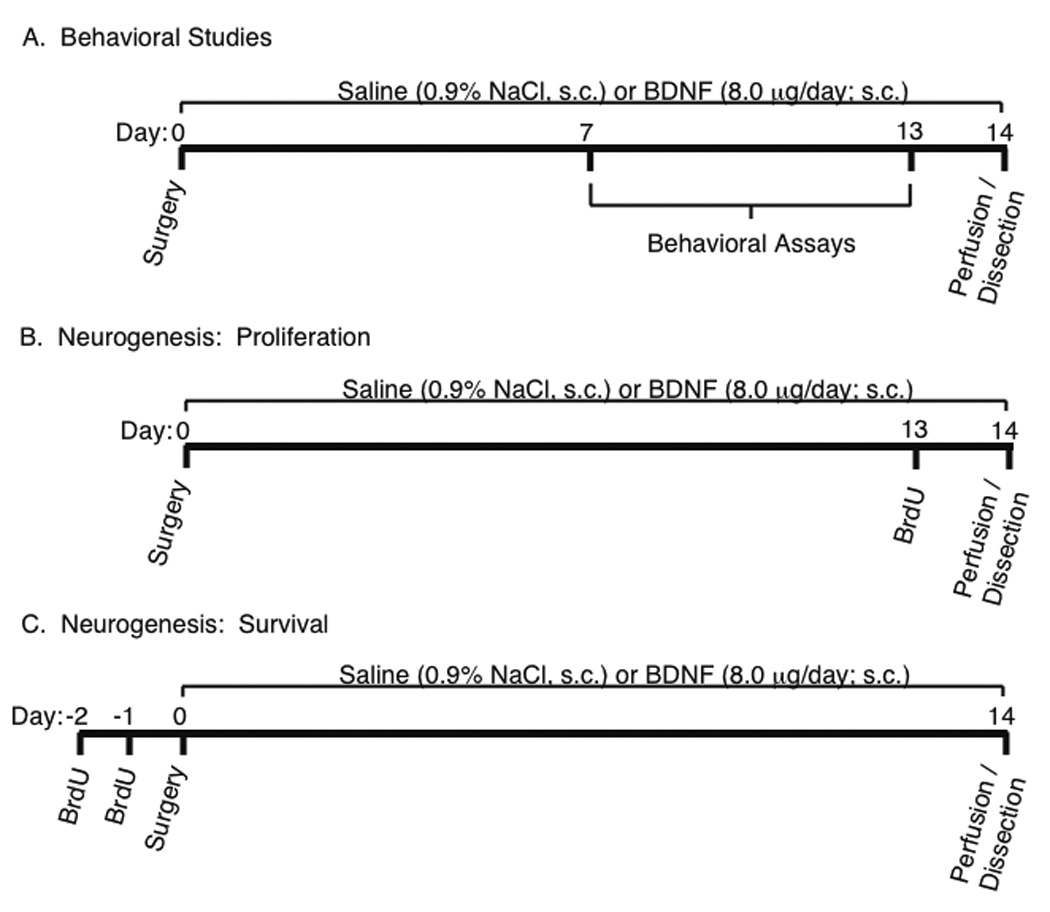
Experimental designs used for assaying the behavioral and cellular responses to peripheral BDNF administration in separate cohorts of mice. (A) Osmotic mini-pumps filled with either saline or BDNF (4.0, 8.0, or 12.0 µg/24 hr) were subcutaneously implanted dorsal to the scapulae in mice. Saline or BDNF was administered over 14 days at a rate of 25 µl/hr. Behavioral tests were counterbalanced, run across multiple cohorts of animals and conducted during the second week of peripheral saline or BDNF administration. (B) On day 14 of chronic, peripheral saline or BDNF administration, mice that did not undergo behavioral testing were perfused and brain tissue was isolated and processed for cellular and molecular studies. In order to study rates of cell proliferation in the hippocampus and prefrontal cortex of adult mice, a single injection of 5-bromo-2-deoxyuridine (BrdU; 100 m/kg, i.p.), a synthetic analogue of thymidine, was administered on the 13th consecutive day of chronic, peripheral saline or BDNF administration. Animals were sacrificed and brains removed 24 hrs following injection of BrdU. (C) In order to study the survival rates of neural progenitor cells in the adult hippocampus and prefrontal cortex, BrdU (100 mg/kg, i.p.) was injected twice daily over two consecutive days prior to surgical implantation of osmotic mini-pumps containing saline or recombinant BDNF. Survival of BrdU-positive cells was measured following 14 days of chronic, peripheral saline or BDNF.
Forced Swim Test (FST)
The FST was performed according to previously published methods from our laboratory (Duman et al, 2007) and others (Caldarone et al, 2003). Briefly, C57Bl/6 mice were placed in a glass cylinder (12 cm diameter) filled to a depth of 10 cm with water (23–25°C). A 10 minute swim test session was videotaped, and the time spent immobile during the swim session was recorded by an observer blinded to treatment groups.
Novelty-Induced Hypophagia (NIH)
C57Bl/6 mice were single-housed and trained daily to drink a diluted solution of sweetened condensed milk for 30 min/day. On the fourth consecutive day, the mice were tested in their home cage for latency to drink the milk solution. On the fifth day, latency to drink the milk solution was tested in a novel environment.
Chronic Unpredictable Stress (CUS)
BALB/c mice were used in this experiment because antidepressant administration has been shown to reverse behavioral deficits in this strain following CUS exposure (Yalcin et al, 2008). Briefly, animals receiving peripheral BDNF or saline administration were exposed to a variable, semi-random sequence of mild, unpredictable stressors. The exact stressors, duration of stress exposure, and sequence of exposure is shown in Supplementary Table 1. Three stressors were applied daily: once in the morning (beginning at 9:00AM), once in the afternoon (beginning at 2:00 PM), and overnight. Stress exposure began one day after implantation of the mini-osmotic pump and lasted for the duration of the experiment. Sucrose consumption was evaluated following 12 days of CUS exposure in order to determine the long-term consequences of chronic, peripheral BDNF administration on stress-induced anhedonia (adapted from Gourley et al, 2008a). Briefly, mice were habituated to a 1% w/v sucrose solution for 2 days and then modest (4, 14, then 19 hrs) water restriction to habituate animals to water restriction (adapted from Gourley et al, 2008a; Willner et al, 1996) and prevent neophobia during testing. On the test day, mice were allowed access to sucrose solution in the home cage for 1 hr in the absence of cagemates. Testing was repeated the following day, except that mice were given access to water instead of sucrose solution in order to verify that peripheral BDNF did not influence thirst levels.
Elevated Plus Maze (EPM)
Mice were placed in the center of the maze facing an open arm and were allowed to explore the maze for 5 minutes. A blinded observer recorded the total time spent in each arm as well as the number of entries into each arm. Entries were operational defined as occurring when all four paws crossed into a particular arm (Pellow et al, 1985).
Open Field Test (OFT)
In this paradigm, a mouse was placed into the center of an open field (76.5 cm × 76.5 cm × 40 cm) constructed of Plexiglas and allowed to explore for 10 minutes. Mice were videotaped and activity was measured using the Noldus (Alexandria, VA) EthoVisionPro Video Tracking System in the absence of the observer. Total locomotor activity was measured for one hour in standard mouse cages. Locomotor activity was videotaped in the absence of the observer.
BDNF Enzyme-linked Immunosorbent Assays (ELISAs)
Hippocampus and ventral striatum tissue homogenates or blood plasma were prepared and assayed using the ChemiKine BDNF Sandwich ELISA (Chemicon, Temecula, CA) according to the manufacturers’ instructions (for more detail see Supplementary Materials).
In Situ Hybridization (ISH) Analysis
Briefly, coronal sections of frozen brain (16 µm) were cut on a cryostat and stored at ~70°C. Tissue sections were thaw-mounted on RNase-free Probon slides (Fisher), fixed, and dried. ISH was performed using radiolabeled riboprobes according to conventional protocols with minor modifications (for more detail see Supplementary Materials).
5-Bromo-2-deoxyuridine (BrdU) Immunohistochemistry and Quantification
Immunostaining was performed on free-floating sections (35 µm) according to previously published procedures (for more detail see Supplementary Materials). The influence of chronic, peripheral BDNF administration on the rate of proliferation of progenitor cells in the SGZ and prelimbic cortex was examined in animals injected with BrdU (Sigma, St. Louis, MO; 100 mg/kg, i.p.) 24 hours prior to perfusion (Figure 1B). In a separate cohort of animals, the influence of chronic, peripheral BDNF administration on the survival rate of newborn cells in the SGZ and prelimbic cortex was examined. In this study, animals were injected with BrdU (100 mg/kg, i.p.) twice daily for two consecutive days prior to surgical implantation of the osmotic mini-pumps and allowed to survive for 14 days (Figure 1C).
Immunoblotting
Standard Western blotting techniques were used to quantify CREB, pCREB (ser133), ERK1/2, and pERK1/2 (for more detail see Supplementary Materials). Proteins were transferred onto nitrocellulose membranes (0.2 µM; Invitrogen) and immunoprobed with anti-CREB (Ms; 1:500; Cell Signaling Technology, Danvers, MA), anti-phospho-CREB (Rb; 1:500; Cell Signaling Technology), anti-ERK1/2 (Rb; 1:2000; Cell Signaling Technology), and anti-phospho-ERK1/2 (Ms; 1:1000; Cell Signaling Technology) at 4°C overnight. Uniformity of loading was confirmed by re-probing with an antibody against β-actin (Rb; 1:1000; Cell Signaling Technology). Membranes were incubated at room temperature for 1 hour with IRDye700 Dx Anti-Rb IgG and IRDye800 Dx Anti-Ms IgG (1:5000; Rockland Immunochemicals, Gilbertsville, PA). The Odyssey imaging system (LI-COR, Lincoln, NE) was used to scan blots and bands were analyzed using fluorescent densitometry analysis.
Statistical Analyses
Immobility in the FST was analyzed using one-factor (BDNF) analysis of variance (ANOVA). NIH latencies to drink data were analyzed using a two-factor (BDNF × condition) ANOVA. Furthermore, sucrose and water consumption data following CUS were analyzed using a two-factor (BDNF × stress) ANOVA. Pairwise comparisons of group means were done using Tukey’s HSD (P <0.05). Student’s t tests were used to analyze data from all other behavioral tests, neurogenesis assays, BDNF ELISAs, BDNF ISH analysis, and immunoblotting experiments. Fluorescence scores were square-root transformed to preserve required homogeneity of variance. In all cases comparisons were considered statistically significant for P < 0.05.
Results
Peripheral BDNF administration produces antidepressant-like and anxiolytic behavioral responses
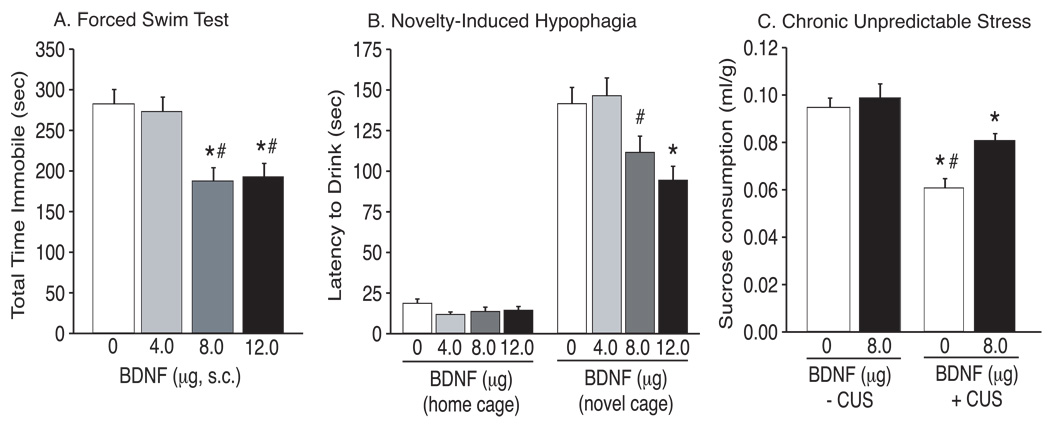
The efficacy of antidepressants in the FST is measured as a decrease in immobility during the swim test session. The total time spent immobile by mice receiving peripheral saline or different doses of BDNF in the FST is plotted in Figure 2A. Immobility data from Figure 2A were analyzed using a one-way ANOVA, which revealed a significant main effect of treatment (F3,59 = 9.643, P < 0.0001). Further pairwise analyses revealed a significant difference in total time spent immobile between the saline and 8.0 or 12.0 µg/24 hr BDNF, and between 4.0 µg/24 hr BDNF and 8.0 or 12.0 µg/24hr BDNF administration (Tukey’s HSD, P < 0.05).
Figure 2.
Peripheral administration of BDNF produces antidepressant-like behavioral responses. (A) Peripheral BDNF administration dose-dependently decreased immobility of mice in the FST. Total time (mean±SEM) spent immobile following peripheral administration of saline (n=22), or 4.0 (n=14), 8.0 (n=13) or 12.0 µg/24 hr BDNF (n=14) are shown (*P<0.05,compared to saline-treated controls; #P<0.05, compared to 4.0 µg BDNF; Tukey’s HSD). (B) Chronic, peripheral BDNF administration dose-dependently decreased latencies to drink a sweetened milk solution in the novel, but not home, cage environment. Latencies (mean±SEM) to consume milk in the home and novel cages are plotted for mice treated with saline (n=22), or 4.0 (n=13), 8.0 (n=13) or 12.0 µg/24 hr BDNF (n=14) (*P<0.05 compared to control group, Tukey’s HSD). (C) Peripheral BDNF partially attenuated the behavioral deficits induced by CUS. Sucrose consumption normalized to bodyweight (mean±SEM) is displayed for stressed and non-stressed animals receiving saline or 8.0 µg/24 hr BDNF. *P<0.05 compared to non-stressed control groups; #P<0.05 compared to stressed mice receiving 8.0 µg/24 hr BDNF; n = 12–16/group (Tukey’s HSD).
Latency to drink in the NIH paradigm is reduced by chronic, but not acute, antidepressant administration, and is thereby used as a measure of chronic antidepressant response (Dulawa et al, 2004). Latencies to drink a sweetened milk solution in the home and novel cage environments are plotted in Figure 2B. Two-way ANOVA analysis revealed significant main effects of treatment (F3,58 = 4.749, P < 0.01) and condition (F3,58 = 425.22, P < 0.0001), as well as a significant interaction between treatment and condition (F3,58 = 5.178, P < 0.01). Subsequent pairwise analyses showed that there was a significant difference in latencies to drink between all treatments in the home versus novel cage environments as well as in the novel cage environment between animals receiving chronic, peripheral saline (n = 22) or 4.0 µg/24 hr BDNF (n = 13) and animals receiving 8.0 (n = 13) or 12.0 µg/24 hr BDNF (n = 14) (Tukey’s HSD, P < 0.05). Latencies to drink in the home cage were not significantly different between treatments (P > 0.05), nor was total volume consumed in the home or novel cage (data not shown). Total locomotor activity was not significantly different between treatments when measured in standard mouse cages (Figure 2B).
The CUS model induces behavioral deficits that are reversed by chronic, but not acute, administration of antidepressants (Willner, 1997). Total sucrose consumption for stressed and non-stressed mice receiving saline or 8.0 µg/24 hr BDNF is plotted in Figure 2C. Two-way ANOVA analysis revealed significant main effects of treatment (F1,46 = 12.295, P = 0.001) and stress (F1,46 = 52.877, P < 0.0001), as well as a significant interaction between treatment and stress (F1,46 = 13.321, P < 0.001). Pairwise comparisons revealed that stressed animals consumed significantly less sucrose than non-stressed controls, indicating an anhedonic-like behavioral phenotype (Tukey’s HSD, P < 0.05). Stressed mice receiving chronic, peripheral BDNF consumed significantly more sucrose than stressed mice receiving saline indicating a partial attenuation of CUS-induced behavioral deficits (Tukey’s HSD, P < 0.05). There were no significant effects of consumption during habituation or bodyweights (data not shown). Furthermore, two-way ANOVA analysis did not reveal a significant main effect of treatment (F1,46 = 0.644, P = 0.43) or stress (F1,46 = 0.011, P = 0.92) on total water consumption in stressed and non-stressed mice receiving saline or 8.0 µg/24 hr BDNF (see Supplementary Figure 1).
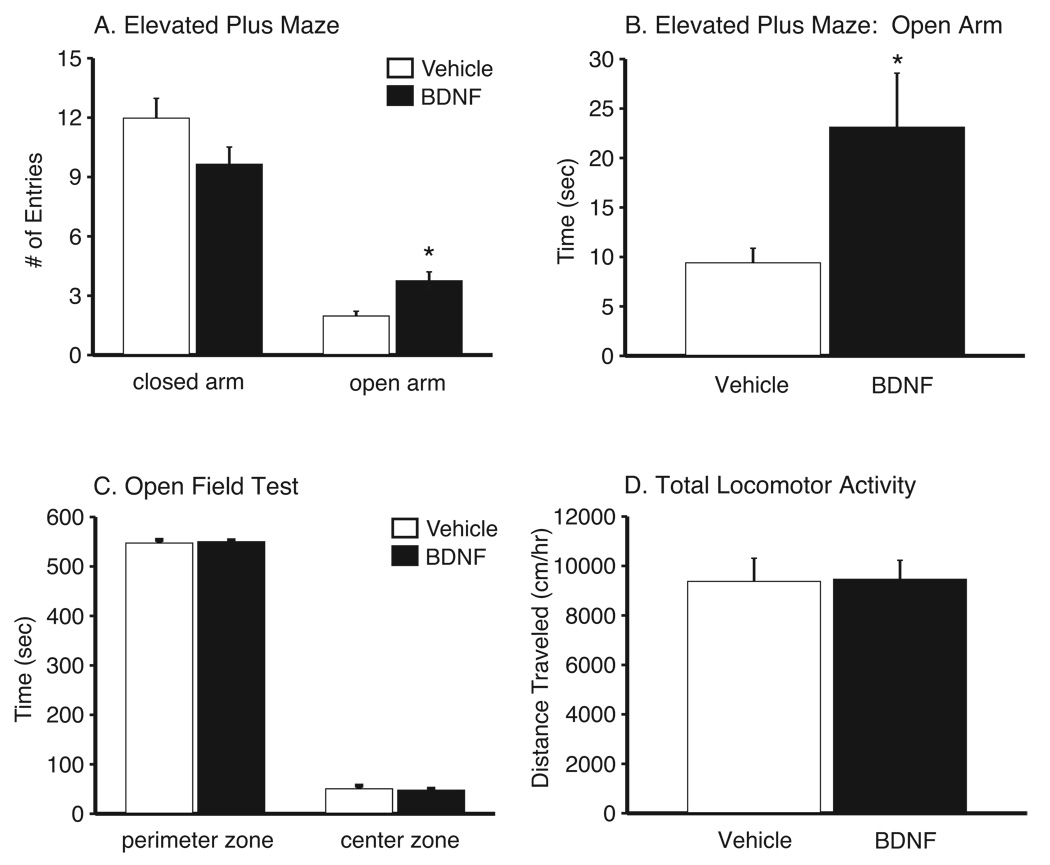
The EPM is an animal model of anxiety-like behavior in which the efficacy of anxiolytic and anxiogenic compounds can be screened (Lister, 1987). Figure 3A shows the cumulative number of entries (mean±SEM) into both the closed and open arms during the 5 minute EPM test. Peripheral BDNF administration significantly increased the number of entries into the open arms relative to saline controls (t(20) = −3.63, P < 0.01) as well as total time spent in the open arms (Figure 3B) (t(20) = −2.82, P = 0.01). There was no difference in the total number of entries into the closed arms (P = 0.12). In the OFT (Figure 3C) there was no significant difference between treatments in total time spent in the perimeter (P = 0.31) or center (P = 0.31) zones of a novel, open-field environment. Furthermore, there was no difference in the total distance traveled in the center or perimeter zones (see Supplementary Figure 2). Also, total locomotor activity was not significantly different between treatments when measured in standard mouse cages (Figure 3D).
Figure 3.
Peripheral BDNF administration produces anxiolytic-like behavior in a rodent model of anxiety. (A) Total number of entries (mean±SEM) into the open arm, but not the closed arm, are significantly different between mice receiving saline (n= 13) or 8.0 µg/24 hr BDNF (n= 9) (*P<0.05 compared to saline, t-test). (B) Total time (mean±SEM) spent in the open arms of the EPM was significantly different between saline- (n= 13) and 8.0 µg/24 hr BDNF-treated (n= 9) animals. (*P<0.05, t-test). (C) Total duration (mean±SEM) spent in the center or the perimeter of an open field is not significantly different between mice receiving peripheral 8.0µg/24 hr BDNF (n = 12) when compared to saline-treated controls (n = 15). (D) Total locomotor activity (mean±SEM), as measured in a mouse standard cage, is not significantly different between controls (n= 10) and mice receiving peripheral 8.0 µg/24 hr BDNF (n= 12).
Peripheral BDNF administration produces antidepressant-like cellular responses
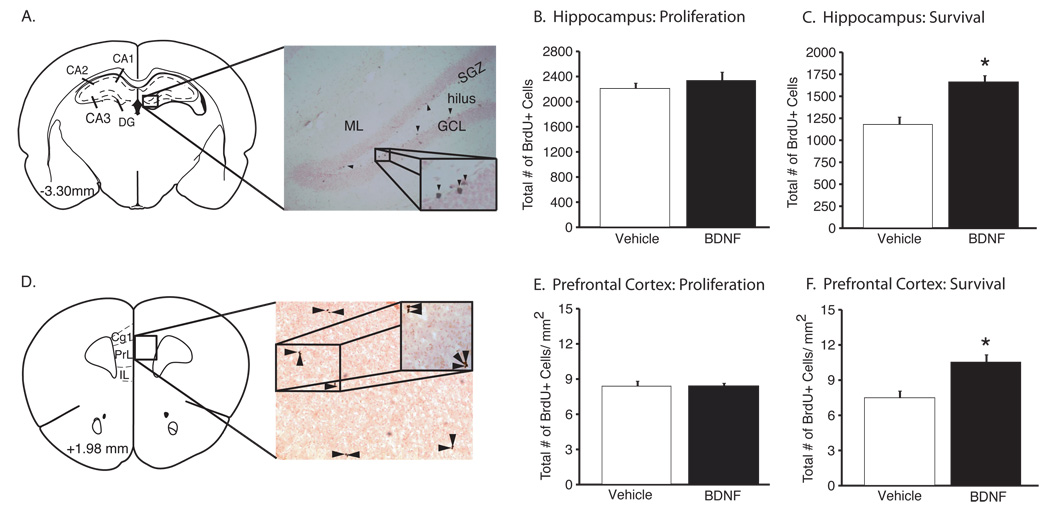
Recent studies demonstrate that BDNF-TrkB signaling is required for the increase in the proliferation (Li et al, 2008) and/or survival (Sairanen et al, 2005) of newborn neurons in the adult hippocampus in response to antidepressant administration. In order to determine the influence of peripheral BDNF on proliferation and survival of newborn cells in the dentate gyrus, BrdU was administered according to a temporal design (Figures 1B & 1C) that distinguishes these two cellular processes. We also examined the influence of peripheral BDNF on BrdU-labeled (BrdU+) cells in the prefrontal cortex, a region where proliferation of glia is increased by antidepressant administration (Banasr and Duman, 2007a).
Total numbers of Brdu+ cells were counted in the dentate gyrus (Figure 4A) and prefrontal cortex (Figure 4D) of mice receiving chronic, peripheral BDNF or saline. In the proliferation paradigm, there was no difference in the total number of BrdU+ cells in the dentate gyrus of BDNF- versus saline-treated subjects 24 hours following a single injection of BrdU (Figure 4B) (P=0.46). In contrast, the survival rate of immature neurons in the dentate gyrus (assessed 15 days following BrdU administration) was significantly increased by peripheral BDNF compared to saline controls (Figure 4C) (t(25) = −6.36, P<0.0001). Similar effects were observed in the prefrontal cortex, with no change on proliferation (Figure 4E) but a significant increase in the survival (Figure 4F) of BrdU+ cells in BDNF vs. saline-treated subjects (t(20) = −3.33, P<0.01).
Figure 4.
Chronic, peripheral BDNF increases the survival rate of neural progenitor cells in the adult hippocampus and prefrontal cortex. (A) Coronal sections were taken at the level of the dorsal hippocampus (number indicates distance from bregma in the anteroposterior direction (2001). The insert depicts BrdU+ cells (indicated by arrows) in the subgranular zone (SGZ), which borders the hilus and granular cell layer (GCL) of the dentate gyrus. The influence of BDNF (8 µg/24 hr, 14 d) administration on the total number of Brdu+ cells (mean±SEM) was determined at different time points after BrdU administration to examine cell proliferation (24 hr, B) or survival (14 d, C). There was no significant effect on cell proliferation (n = 6 per group, B), but peripheral BDNF administration significantly increased the number of BrDU+ cells following 14 d of BDNF administration compared to saline controls (*P<0.0001, n = 12–15, C). (D) Coronal section taken at the level of the medial prefrontal cortex, including the anterior cingulated (Cg1), prelimbic (PL) and infralimbic (IL) cortices. The insert depicts BrdU+ cells (indicated by arrows) in the PL cortex. There was no significant effect of BDNF administration on the proliferation of BrdU+ cells in the PL (E, n = 6), but there was a significant increase in the survival of newborn cells when compared to saline-treated controls (*P<0.01, n = 10–12, F).
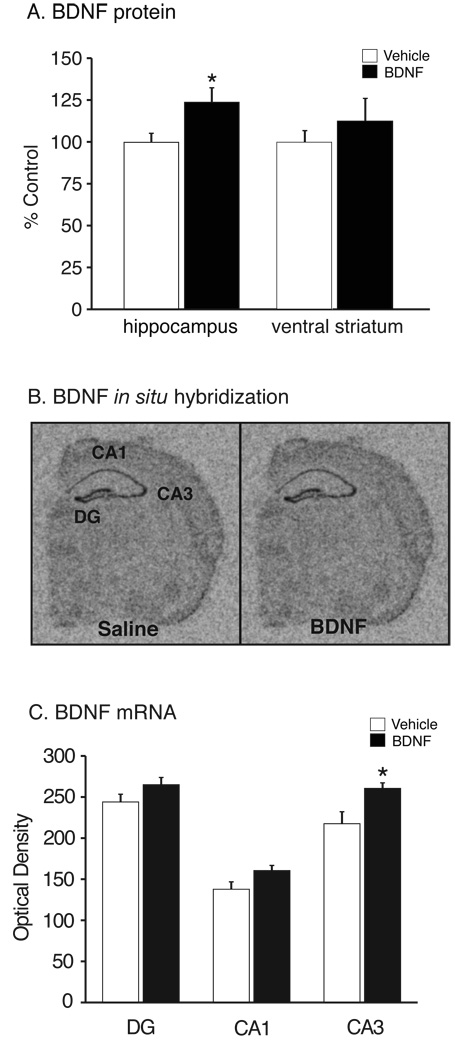
BDNF is increased in the hippocampus following peripheral administration
Studies were conducted in order to determine if peripheral BDNF administration alters central levels of BDNF in the hippocampus and striatum, regions implicated in the actions of antidepressants (Nestler and Carlezon, 2006; Shirayama et al, 2002). ELISA and in situ hybridization (ISH) techniques were used to measure BDNF protein and mRNA levels, respectively. BDNF protein levels were significantly increased in the hippocampus of animals receiving peripheral BDNF when compared to saline-treated controls (Figure 5A)( t(25) = −2.265, P=0.03). ELISA analysis of tissue homogenates prepared from the ventral striatum revealed no significant difference in BDNF protein levels between treatments (Figure 5A) (P=0.43). Representative coronal sections of dorsal hippocampus from saline- and BDNF-treated mice are shown in Figure 5B. Peripheral BDNF administration significantly increased BDNF mRNA expression in the CA3 subregion of the hippocampus compared to saline-treated controls (Figure 5C) (t(10) = −2.64, P<0.05). Despite trends, BDNF mRNA expression was not altered in the CA1 (P=0.07) or dentate gyrus (P=0.14) of mice receiving peripheral BDNF (Figure 5C).
Figure 5.
BDNF protein and mRNA expression is increased in the hippocampus following 14 days of chronic, peripheral BDNF administration. (A) Hippocampal BDNF protein levels (mean±SEM) determined by ELISA were significantly increased in animals treated with 8.0 µg/24 hr BDNF when compared to saline-treated controls (*P=0.03, n = 13). BDNF protein levels in the ventral striatum were not significantly different (P=0.43, n = 13). (B) Representative autoradiographs are shown for a saline- and BDNF-treated (8.0 µg/24 hr) mice. Levels of BDNF mRNA in different subregions of the hippocampus were determined by quantitative densitometry. (C) Total BDNF mRNA levels (mean±SEM) were significantly different in the CA3 subregion of the hippocampus in response to BDNF (n=6) when compared to saline-controls (*P=0.02, n = 6 per group, t-test). No significant differences were observed in the CA1 (t-test, P=0.07) or DG (t-test, P=0.14) (n=6) mice. CA1, cornu ammonis 1; CA3, cornu ammonis 3; DG, dentate gyrus.
Peripheral BDNF increases pERK and/or pCREB in the adult hippocampus and ventral striatum
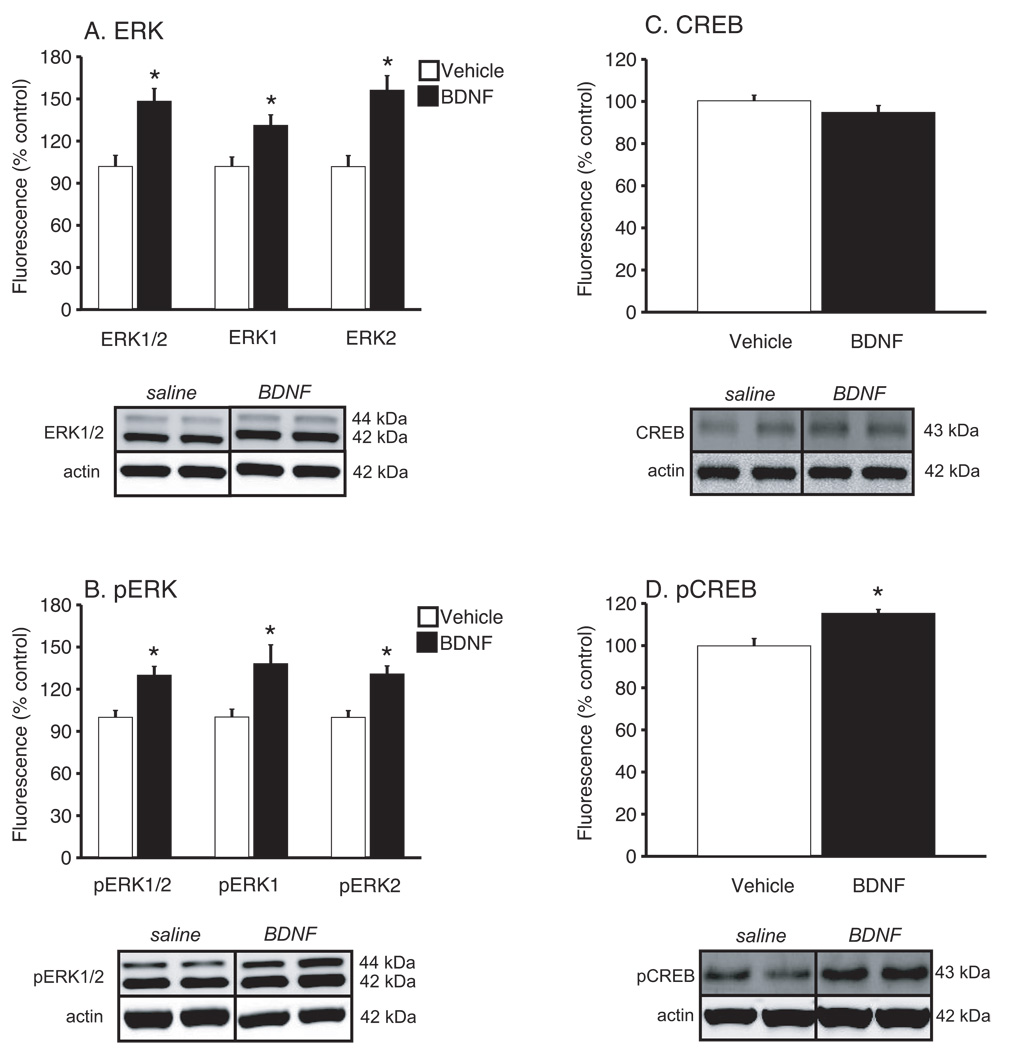
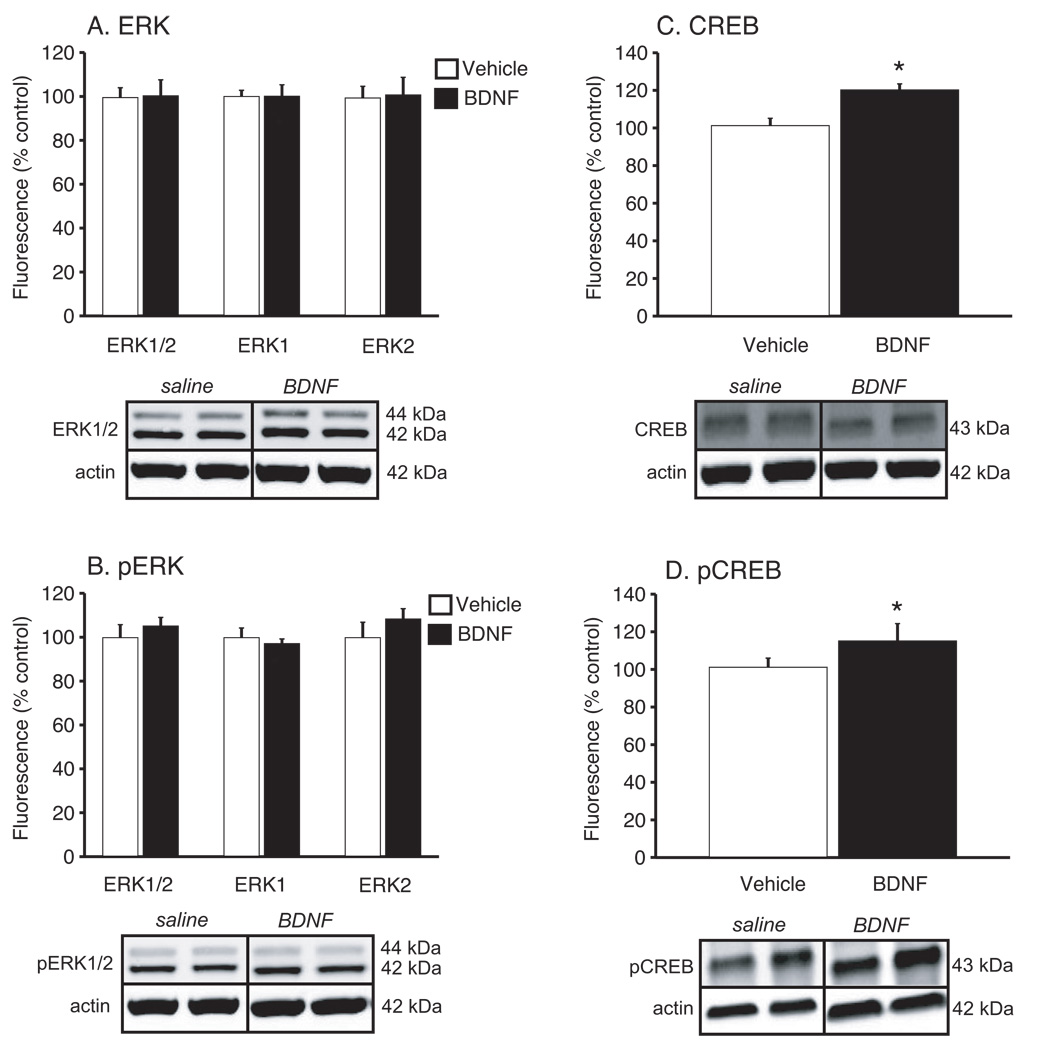
Levels of ERK and CREB, as well as the activated forms of these proteins (pERK and pCREB) in hippocampus and ventral striatum were determined by Western blot analysis. There were no significant differences in the expression of β-actin, measured as a control protein. The results are expressed as a ratio of each signaling protein to β-actin and presented as percentage of the saline group. In hippocampus (Figure 6), unpaired t-tests revealed significant differences in total ERK1/2 (t(12) = −3.81, P<0.01), ERK1 (t(12) = −2.85, P=0.014) and ERK2 (t(12) = −4.13, P<0.01) between animals receiving chronic saline or BDNF administration (Figure 6A). There were also significant differences in levels of pERK1/2 (t(12) = −3.66, P<0.01), pERK1 (t(12) = −2.57, P=0.02) and pERK2 (t(12) = −4.02, P<0.01) (Figure 6B). Analysis of total CREB levels in the hippocampus (Figure 6C) revealed no significant difference between treatments (P=0.2), but a significant difference in pCREB levels (Figure 6D) (t(12) = −3.78, P<0.01). In the ventral striatum there were no significant differences in total or phosphorylated ERK1/2, ERK1, or ERK2 (Figures 7A & 7B). However, peripheral BDNF administration significantly increased levels of CREB (t(11) = −3.48, P=0.005) and pCREB (t(12) = −2.77, P=0.02) (Figures 7C & 7D).
Figure 6.
Chronic, peripheral BDNF administration is associated with increased pERK and pCREB in the adult hippocampus. Representative Western blots are shown for total ERK, pERK, CREB, pCREB and β-actin (loading control) from the saline and 8.0 µg/24 hr BDNF treatments. Densitometric values from all Western blots were normalized to β-actin and then expressed as percentage control. (A) Total ERK1/2, ERK1 and ERK2 (A) and total pERK1/2, ERK1 and ERK2 (B) levels were significantly increased in animals treated with BDNF when compared to saline controls (unpaired t-test, *P<0.05). While there was no significant difference in CREB levels (C) between groups, there was a significant increase in pCREB levels in the BDNF treated group (unpaired t-test, *P<0.05). There were 7 animals per treatment.
Figure 7.
Total CREB and pCREB levels are increased in the ventral striatum following 14 days of chronic, peripheral BDNF administration. The inserts represent some of the Western blots for total ERK, pERK, CREB, pCREB and β-actin (loading control) in the hippocampus from the saline and 8.0 µg BDNF treatments. Densitometric values from all Western blots were normalized to β-actin and then expressed as percentage control. (A) When analyzed with unpaired t-tests, total ERK1/2 (P=0/93), ERK1 (P=0.99), and ERK2 (P=0.89) levels were not significantly different between treatments. (B) No significant difference in levels of phosphorylated ERK1/2 (P=0.49), ERK1 (P=0.57), or ERK2 (P=0.34) in the ventral striatum were found between mice receiving saline or 8.0 µg BDNF (unpaired t-tests). Significant increases in total CREB (C) and pCREB (D) levels in the ventral striatum were revealed by unpaired t-tests (*P<0.05 when compared to control group). There were a total of 7 subjects per treatment.
Discussion
Response to peripheral BDNF in models of depression and anxiety
The present results demonstrate that peripheral BDNF administration produces antidepressant-like behavioral responses in animal models of depression and anxiety. These effects are similar to the actions of different classes of chemical antidepressants in the FST (Cryan et al, 2005), as well as to centrally administered BDNF, including infusions into the midbrain (Siuciak et al, 1997), hippocampus (Shirayama et al, 2002), and lateral ventricles (Hoshaw et al, 2005), and to over expression of BDNF in forebrain structures of transgenic mice (Govindarajan et al, 2006). Peripheral BDNF also partially blocks the effects of CUS, which causes behavioral deficits that are similar to those observed in MDD, most notably anhedonia. The relevance of the CUS paradigm is also highlighted by the requirement for chronic, long-term antidepressant administration, which is consistent with the time course for a therapeutic response in MDD (Willner, 2005). Attenuation of CUS-induced anhedonia provides additional support that peripheral BDNF produces antidepressant-like effects that could result from blocking stress-mediated deficits in central BDNF (Duman et al, 2006). The inability of peripheral BDNF administration to completely block CUS-induced anhedonia may be due to a requirement for a higher dose (only the 8 µg dose of BDNF was tested) or may suggest that altered expression of other neurotrophic factors and/or biochemical mechanisms contribute to this stress-mediated behavioral deficit. Further studies will be required to test these possibilities.
The NIH paradigm is considered a model of anxiety, and response time in this model is consistent with that for the therapeutic action of antidepressants (Dulawa and Hen, 2005; Dulawa et al, 2004). We found that chronic (14 d), but not shorter (6 d, data not shown) BDNF administration decreases the latency to drink in the NIH paradigm, as well as anxiety-like behaviors in the EPM, consistent with studies in mutant mice (i.e., decreased anxiety in TrkB over expressing mice (Koponen et al, 2004) and increased anxiety in mice with a BDNF mutation that decreases secretion(Chen et al, 2006)). Peripheral BDNF administration did not influence activity in either the center or periphery of an open field, consistent with our previous report that central infusions of BDNF did not influence behavior in this model (Shirayama et al, 2002). The reason for this is not clear, but could be related to the level of stress (i.e., BDNF may only be effective under conditions of elevated stress, such as in the EPM). Peripheral BDNF administration decreased anxiety-like behavior in the EPM consistent with previous reports (Cirulli et al, 2004; Shirayama et al, 2002). However, the role of intra-hippocampal BDNF is unclear as some reports suggest an anxiogenic-like effect (Branchi et al, 2006; Deltheil et al, 2009). Moreover, there was no effect on activity in locomotor chambers, providing further evidence that peripheral BDNF does not influence general activity levels.
These behavioral results demonstrate that the effects of peripheral BDNF are not specific to models of either depression or anxiety. The relatively high comorbidity of depression and anxiety (Gorman, 1996; Kaufman and Charney, 2000) suggests that these disorders are not entirely distinct conditions in humans or animals, and positive treatment responses in models of both disorders may be expected, as shown for chemical antidepressants (Gorman, 1996; Kaufman et al, 2000).
Actions of peripheral BDNF on cell proliferation and survival
The number of newborn cells was used as another functional measure, at the cellular level, of the central actions of peripheral BDNF. Chronic, peripheral BDNF administration increased the survival, but not the proliferation, of BrdU+ cells in the adult hippocampus. Central BDNF has also been linked with increased survival of newborn neurons in the adult hippocampus: basal and antidepressant induction of the survival of newborn cells in the hippocampus is decreased or blocked in BDNF heterozygous deletion mutants or dominant negative TrkB (dnTrkB) transgenic mice (Sairanen et al, 2005). Conditional deletion of TrkB is reported to also block antidepressant-induction of proliferation, although this effect is likely due to the selective deletion of TrkB in neural progenitor cells (Li et al, 2008). Based upon these previous studies, it is likely that increased Brdu+ cells in the hippocampus reflect increased neurogenesis. However, we did not phenotype these cells and firm conclusions regarding the exact ratio of neurons to glia cannot be drawn.
Peripheral BDNF administration also increased the survival, but not proliferation of BrdU+ cells in the prefrontal cortex. Previous studies have reported that antidepressant administration increases gliogenesis in the prefrontal cortex (Kodama et al, 2004; Madsen et al, 2005). In addition, stress decreases gliogenesis (Banasr et al, 2007b; Czeh et al, 2007) and this could contribute to the reduction of glia reported in postmortem prefrontal cortex of depressed subjects (Cotter et al, 2002; Cotter et al, 2001; Rajkowska et al, 1999; Uranova et al, 2004). The influence of central manipulation of BDNF or TrkB on glial proliferation and/or survival in the prefrontal cortex has not been examined, and additional studies will be required to determine if these are direct or indirect effects of BDNF. Furthermore, future studies aimed at determining whether peripheral BDNF increases gliosis and/or neurogenesis in the prefrontal cortex are critical to understanding the effects of peripheral BDNF on specific cell populations within this brain region.
Previous studies demonstrate that induction of hippocampal neurogenesis is required for actions of antidepressants in certain behavioral models (Santarelli et al, 2003; Surget et al, 2008; Wang et al, 2008) (however see, Holick et al, 2008; Huang et al, 2008a), and we have found that ablation of glia in the prefrontal cortex is sufficient to produce a depressive-like phenotype in rodents (Banasr and Duman, 2008). However, the precise relationship between increased survival of newborn cells in the hippocampus and/or prefrontal cortex and responses in behavioral models will require further investigation. Regardless of the relationship, the present findings demonstrate that peripheral BDNF administration has functional consequences on survival of cells in these two limbic structures associated with depression and antidepressant responses.
BDNF levels in brain and activation of ERK and CREB
Regulation of behavioral and cellular responses by peripheral BDNF could result from indirect actions (e.g., mechanisms that involve intermediate peripheral effectors) or from direct actions of BDNF on limbic brain structures. The results of our analyses of BDNF levels as well as downstream signal transduction pathways are consistent with the hypothesis that peripheral BDNF directly influences brain function. One limitation of the current study is that it does not identify what mechanisms (i.e., direct or indirect) underlie peripheral BDNF-mediated molecular changes in the brain. Peripheral BDNF administration significantly increased levels of BDNF in the hippocampus, and increased levels of activated/phosphorylated ERK and CREB, downstream targets of BDNF-TrkB signaling (Carlezon et al, 2005; Schmidt et al, 2008). These findings are consistent with the possibility that peripheral BDNF enters the brain and directly activates TrkB-ERK-CREB signaling. It is also possible that peripheral BDNF acts indirectly by increasing the expression of hippocampal BDNF, although induction of BDNF mRNA was only significant in the CA3 pyramidal cell layer and therefore unlikely to account for an increase in total hippocampal BDNF protein levels. Yet another possibility is that peripheral BDNF increases the expression of other growth factors (e.g., vascular endothelial growth factor and insulin-like growth factor 1), either centrally or peripherally, which also activate ERK signaling and have antidepressant-like behavioral responses (Duman et al, 2008; Warner-Schmidt and Duman, 2007). Finally, peripheral BDNF administration may influence corticosterone levels, which in turn may regulate BDNF-mediated signaling in the hippocampus (Gourley et al, 2008b), neurogenesis (Cameron and Gould, 1994) and depressive-like behaviors (Gourley et al, 2008a). While the present results do not provide an exact mechanism by which peripheral BDNF alters endogenous BDNF levels in the brain and activation of ERK and CREB, they do suggest that these central effects are mediated directly by exogenous BDNF entering the brain or indirectly by peripheral effectors that in turn cross the blood brain barrier. Future studies are required in order to determine the precise mechanism(s) underlying the cellular responses to peripheral BDNF administration.
A functional relationship between BDNF-ERK-CREB signaling in the hippocampus and behavior is supported by previous studies (Schmidt et al, 2008). ERK is required for the behavioral effects of BDNF infusions (Shirayama et al, 2002) or antidepressant treatment (Duman et al, 2007), and CREB expression and function in the hippocampus is sufficient to produce antidepressant-like behavioral and neurogenenic responses (Chen et al, 2001a; Nakagawa et al, 2002a; Nakagawa et al, 2002b). We also found that peripheral BDNF administration increases levels of phosphorylated CREB, but not ERK, in the ventral striatum. However, a relationship between striatal CREB and the antidepressant responses to peripheral BDNF is not likely as previous studies demonstrate a pro-depressive effect of CREB in the nucleus accumbens (Berton et al, 2006; Eisch et al, 2003; Nestler et al, 2006; Newton et al, 2002; Pliakas et al, 2001; Wallace et al, 2009). Together, the results of the current study indicate that the predominant effects of peripheral BDNF are antidepressant-like behavioral and cellular responses consistent with increased BDNF-ERK-CREB signaling in the hippocampus.
Peripheral BDNF: brain uptake and source
Peripheral BDNF could be actively transported into the brain, as shown for other growth factors, and have direct actions. There are reports that peripheral BDNF can cross the blood brain barrier, although this remains controversial (Pan et al, 1998; Pardridge et al, 1998; Poduslo and Curran, 1996; Zhang and Pardridge, 2001), and additional studies are required to determine if the effects observed in the current study are direct or indirect. The findings presented here also raise the possibility that the therapeutic effects of antidepressants could be mediated, in part, by increased serum BDNF, which has been reported in depressed patients that have received antidepressant treatment (Aydemir et al, 2005; Bocchio-Chiavetto et al, 2006; Gonul et al, 2005; Huang et al, 2008b; Shimizu et al, 2003; Yoshimura et al, 2007; Zanardini et al, 2006). Future studies are required in order to determine whether these effects are produced by transport of peripheral BDNF into the brain with direct effects on neuronal circuitry (e.g., hippocampus).
Another major question is the source(s) of BDNF in the periphery and its contribution to serum? While BDNF mRNA and protein are expressed at relatively high levels in many peripheral tissues, including heart (Scarisbrick et al, 1993; Timmusk et al, 1993; Yamamoto et al, 1996), lung (Braun et al, 1999; Nassenstein et al, 2003; Timmusk et al, 1993), kidney (Lommatzsch et al, 2005), bladder (Kawakami et al, 2002) and blood (Lommatzsch et al, 2005; Radka et al, 1996), the functional significance of these peripheral sources of BDNF is unknown. Serum BDNF is derived from several sources including mononuclear blood cells and endothelial cells (Gielen et al, 2003; Kerschensteiner et al, 1999; Lommatzsch et al, 1999; Nakahashi et al, 2000), release from platelets (Karege et al, 2005a; Lommatzsch et al, 2007) and, to a minor extent, passage through the brain blood barrier (Pan et al, 1998). The contribution from other peripheral tissues has not been determined. Moreover, the factors underlying decreased serum BDNF in MDD have not been identified. Stress exposure decreases BDNF expression in limbic regions of the rodent brain (Schmidt et al, 2007), and it is possible that decreases in brain BDNF levels contribute to the serum decrease. However, it is also possible, and even likely, that reductions in peripheral tissue(s) also contribute to the reported reduction in serum BDNF in patients with MDD.
Detecting serum BDNF levels in naïve mice is problematic (Radka et al, 1996). A significant increase in serum BDNF protein levels was observed in mice receiving chronic, peripheral BDNF administration when compared to saline controls (data not shown). However, the baseline levels of serum BDNF detected in the control animals were close to the lower limits of detection as measured using two commercially available ELISA kits that are commonly used to measure BDNF protein levels in rat and human serum as well as rodent brain tissue homogenates. Therefore, the presence of BDNF protein in mouse hippocampus and striatum (present study) suggests that the absence of BDNF in mouse serum is not due to an assay defect that is specific to the mouse. Despite measuring a significant increase in serum BDNF with treatment, we are unable to draw any firm conclusions regarding the physiological relevance of this dose of BDNF. This caveat to measuring serum BDNF is mice has been reported previously (Radka et al, 1996).
Future Directions and Clinical Implications
One potential implication of the present findings is that peripheral BDNF may have significant clinical potential for treating neuropsychiatric disorders including MDD. However, the clinical efficacy of peripherally administered BDNF and other neurotrophic factors is limited due to unsuitable pharmacokinetic profiles (i.e. poor blood-brain barrier penetrability, short-half lives, low bioavailability, etc.) and adverse side-effects (e.g. enhanced tumor cell survival) (Pearse et al, 2005; Price et al, 2007). Moreover, it should be emphasized that it remains to be determined whether exogenous BDNF in the periphery enters the brain or whether the central effects of peripherally delivered exogenous BDNF are mediated by an indirect mechanism(s) that influences BDNF/TrkB signaling in the brain.
Collectively, these data suggest that, in addition to serving as a potential biomarker for MDD and/or a therapeutic marker for the efficacy of antidepressant treatments, peripheral BDNF has behavioral and cellular effects that are similar to antidepressants. The precise mechanism(s) underlying the effects of peripheral BDNF remain to be determined but are likely to involve, in part, increased BDNF levels and TrkB-ERK signaling in the brain. These findings indicate that measures of serum BDNF can provide a novel window into brain structure and function that is relevant to the pathophysiology and treatment of mood disorders.
Supplementary Material
Acknowledgments
We thank Regeneron Pharmaceuticals for their generous gift of recombinant hBDNF. This work is supported by USPHS grants MH45481 and 2 P01 MH25642 and by the Connecticut Mental Health Center.
Footnotes
Conflict of Interest: H.D.S. and R.S.D. have no conflicts of interest to declare with regard to the experiments and results reported in this manuscript.
References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20(8):2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Boylan CB, Jackson C, Hershenson S, Miller J, Wiegand SJ, et al. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci U S A. 1992;89(23):11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydemir C, Yalcin ES, Aksaray S, Kisa C, Yildirim SG, Uzbay T, et al. Brain-derived neurotrophic factor (BDNF) changes in the serum of depressed women. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(7):1256–1260. doi: 10.1016/j.pnpbp.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Aydemir O, Deveci A, Taneli F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(2):261–265. doi: 10.1016/j.pnpbp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Regulation of neurogenesis and gliogenesis by stress and antidepressant treatment. CNS Neurol Disord Drug Targets. 2007a;6(5):311–320. doi: 10.2174/187152707783220929. [DOI] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial Loss in the Prefrontal Cortex Is Sufficient to Induce Depressive-like Behaviors. Biol Psychiatry. 2008;64(10):863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007b;62(5):496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311(5762):864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bocchio-Chiavetto L, Zanardini R, Bortolomasi M, Abate M, Segala M, Giacopuzzi M, et al. Electroconvulsive Therapy (ECT) increases serum Brain Derived Neurotrophic Factor (BDNF) in drug resistant depressed patients. Eur Neuropsychopharmacol. 2006;16(8):620–624. doi: 10.1016/j.euroneuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Branchi I, D'Andrea I, Sietzema J, Fiore M, Di Fausto V, Aloe L, et al. Early social enrichment augments adult hippocampal BDNF levels and survival of BrdU-positive cells while increasing anxiety- and "depression"-like behavior. J Neurosci Res. 2006;83(6):965–973. doi: 10.1002/jnr.20789. [DOI] [PubMed] [Google Scholar]
- Braun A, Lommatzsch M, Mannsfeldt A, Neuhaus-Steinmetz U, Fischer A, Schnoy N, et al. Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am J Respir Cell Mol Biol. 1999;21(4):537–546. doi: 10.1165/ajrcmb.21.4.3670. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Lopes M, Fregni F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: implications for the role of neuroplasticity in depression. Int J Neuropsychopharmacol. 2008;11(8):1169–1180. doi: 10.1017/S1461145708009309. [DOI] [PubMed] [Google Scholar]
- Caldarone BJ, Karthigeyan K, Harrist A, Hunsberger JG, Wittmack E, King SL, et al. Sex differences in response to oral amitriptyline in three animal models of depression in C57BL/6J mice. Psychopharmacology (Berl) 2003;170(1):94–101. doi: 10.1007/s00213-003-1518-7. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61(2):203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28(8):436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Castren E, Voikar V, Rantamaki T. Role of neurotrophic factors in depression. Curr Opin Pharmacol. 2007;7(1):18–21. doi: 10.1016/j.coph.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Chen AC, Shirayama Y, Shin KH, Neve RL, Duman RS. Expression of the cAMP response element binding protein (CREB) in hippocampus produces an antidepressant effect. Biol Psychiatry. 2001a;49(9):753–762. doi: 10.1016/s0006-3223(00)01114-8. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 2001b;50(4):260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314(5796):140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli F, Berry A, Chiarotti F, Alleva E. Intrahippocampal administration of BDNF in adult rats affects short-term behavioral plasticity in the Morris water maze and performance in the elevated plus-maze. Hippocampus. 2004;14(7):802–807. doi: 10.1002/hipo.10220. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP. Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex. 2002;12(4):386–394. doi: 10.1093/cercor/12.4.386. [DOI] [PubMed] [Google Scholar]
- Cotter D, Mackay D, Landau S, Kerwin R, Everall I. Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry. 2001;58(6):545–553. doi: 10.1001/archpsyc.58.6.545. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Differential behavioral effects of the antidepressants reboxetine, fluoxetine, and moclobemide in a modified forced swim test following chronic treatment. Psychopharmacology (Berl) 2005;182(3):335–344. doi: 10.1007/s00213-005-0093-5. [DOI] [PubMed] [Google Scholar]
- Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, et al. Chronic social stress inhibits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32(7):1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- Deltheil T, Tanaka K, Reperant C, Hen R, David DJ, Gardier AM. Synergistic neurochemical and behavioural effects of acute intrahippocampal injection of brain-derived neurotrophic factor and antidepressants in adult mice. Int J Neuropsychopharmacol. 2009:1–11. doi: 10.1017/S1461145709000017. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev. 2005;29(4–5):771–783. doi: 10.1016/j.neubiorev.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29(7):1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A Role for MAP Kinase Signaling in Behavioral Models of Depression and Antidepressant Treatment. Biol Psychiatry. 2007;61(5):661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Terwilliger R, Russell DS, Newton SS, Duman RS. Peripheral insulin-like growth factor-I produces antidepressant-like behavior and contributes to the effect of exercise. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Bolanos CA, de Wit J, Simonak RD, Pudiak CM, Barrot M, et al. Brain-derived neurotrophic factor in the ventral midbrain-nucleus accumbens pathway: a role in depression. Biol Psychiatry. 2003;54(10):994–1005. doi: 10.1016/j.biopsych.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18(10):2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RA, Schofield PR, et al. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry. 2009;14(7):681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Gervasoni N, Aubry JM, Bondolfi G, Osiek C, Schwald M, Bertschy G, et al. Partial normalization of serum brain-derived neurotrophic factor in remitted patients after a major depressive episode. Neuropsychobiology. 2005;51(4):234–238. doi: 10.1159/000085725. [DOI] [PubMed] [Google Scholar]
- Gielen A, Khademi M, Muhallab S, Olsson T, Piehl F. Increased brain-derived neurotrophic factor expression in white blood cells of relapsing-remitting multiple sclerosis patients. Scand J Immunol. 2003;57(5):493–497. doi: 10.1046/j.1365-3083.2003.01260.x. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7(3):254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gonul AS, Akdeniz F, Taneli F, Donat O, Eker C, Vahip S. Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):381–386. doi: 10.1007/s00406-005-0578-6. [DOI] [PubMed] [Google Scholar]
- Gorman JM. Comorbid depression and anxiety spectrum disorders. Depress Anxiety. 1996;4(4):160–168. doi: 10.1002/(SICI)1520-6394(1996)4:4<160::AID-DA2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR. Acute hippocampal brain-derived neurotrophic factor restores motivational and forced swim performance after corticosterone. Biol Psychiatry. 2008a;64(10):884–890. doi: 10.1016/j.biopsych.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, et al. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psychiatry. 2008b;63(4):353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, et al. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc Natl Acad Sci U S A. 2006;103(35):13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33(2):406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effects. Brain Res. 2005;1037(1–2):204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Huang GJ, Bannerman D, Flint J. Chronic fluoxetine treatment alters behavior, but not adult hippocampal neurogenesis, in BALB/cJ mice. Mol Psychiatry. 2008a;13(2):119–121. doi: 10.1038/sj.mp.4002104. [DOI] [PubMed] [Google Scholar]
- Huang TL, Lee CT, Liu YL. Serum brain-derived neurotrophic factor levels in patients with major depression: effects of antidepressants. J Psychiatr Res. 2008b;42(7):521–525. doi: 10.1016/j.jpsychires.2007.05.007. [DOI] [PubMed] [Google Scholar]
- Karege F, Bondolfi G, Gervasoni N, Schwald M, Aubry JM, Bertschy G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol Psychiatry. 2005a;57(9):1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res. 2005b;136(1–2):29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Comorbidity of mood and anxiety disorders. Depress Anxiety. 2000;12(Suppl 1):69–76. doi: 10.1002/1520-6394(2000)12:1+<69::AID-DA9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kawakami T, Wakabayashi Y, Isono T, Aimi Y, Okada Y. Expression of neurotrophin messenger RNAs during rat urinary bladder development. Neurosci Lett. 2002;329(1):77–80. doi: 10.1016/s0304-3940(02)00598-0. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156(6):837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, Klinkert WE, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189(5):865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Lee HP, Won SD, Park EY, Lee HY, Lee BH, et al. Low plasma BDNF is associated with suicidal behavior in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):78–85. doi: 10.1016/j.pnpbp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Kodama M, Fujioka T, Duman RS. Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry. 2004;56(8):570–580. doi: 10.1016/j.biopsych.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Koponen E, Voikar V, Riekki R, Saarelainen T, Rauramaa T, Rauvala H, et al. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCgamma pathway, reduced anxiety, and facilitated learning. Mol Cell Neurosci. 2004;26(1):166–181. doi: 10.1016/j.mcn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Lee BH, Kim H, Park SH, Kim YK. Decreased plasma BDNF level in depressive patients. J Affect Disord. 2006 doi: 10.1016/j.jad.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, et al. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92(2):180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Braun A, Mannsfeldt A, Botchkarev VA, Botchkareva NV, Paus R, et al. Abundant production of brain-derived neurotrophic factor by adult visceral epithelia. Implications for paracrine and target-derived Neurotrophic functions. Am J Pathol. 1999;155(4):1183–1193. doi: 10.1016/S0002-9440(10)65221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lommatzsch M, Niewerth A, Klotz J, Schulte-Herbruggen O, Zingler C, Schuff-Werner P, et al. Platelet and plasma BDNF in lower respiratory tract infections of the adult. Respir Med. 2007;101(7):1493–1499. doi: 10.1016/j.rmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Lommatzsch M, Quarcoo D, Schulte-Herbruggen O, Weber H, Virchow JC, Renz H, et al. Neurotrophins in murine viscera: a dynamic pattern from birth to adulthood. Int J Dev Neurosci. 2005;23(6):495–500. doi: 10.1016/j.ijdevneu.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Yeh DD, Valentine GW, Duman RS. Electroconvulsive seizure treatment increases cell proliferation in rat frontal cortex. Neuropsychopharmacology. 2005;30(1):27–34. doi: 10.1038/sj.npp.1300565. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, et al. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci U S A. 2004;101(29):10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Kroin JS, Liu YT, Sobreviela T, Penn RD, Miller JA, et al. Intrastriatal and intraventricular infusion of brain-derived neurotrophic factor in the cynomologous monkey: distribution, retrograde transport and co-localization with substantia nigra dopamine-containing neurons. Neuroscience. 1996;71(1):179–191. doi: 10.1016/0306-4522(95)00431-9. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Kroin JS, Sobreviela T, Burke MA, Kordower JH, Penn RD, et al. Intrastriatal infusions of brain-derived neurotrophic factor: retrograde transport and colocalization with dopamine containing substantia nigra neurons in rat. Exp Neurol. 1994;129(1):15–26. doi: 10.1006/exnr.1994.1143. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, et al. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. J Neurosci. 2002a;22(22):9868–9876. doi: 10.1523/JNEUROSCI.22-22-09868.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, et al. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002b;22(9):3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi J, Tandon NN, et al. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470(2):113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- Nassenstein C, Braun A, Erpenbeck VJ, Lommatzsch M, Schmidt S, Krug N, et al. The neurotrophins nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 are survival and activation factors for eosinophils in patients with allergic bronchial asthma. J Exp Med. 2003;198(3):455–467. doi: 10.1084/jem.20010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, et al. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22(24):10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Yoshimura R, Ikenouchi-Sugita A, Hori H, Umene-Nakano W, Inoue Y, et al. Efficacy of electroconvulsive therapy is associated with changing blood levels of homovanillic acid and brain-derived neurotrophic factor (BDNF) in refractory depressed patients: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1185–1190. doi: 10.1016/j.pnpbp.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37(12):1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Wu D, Sakane T. Combined use of carboxyl-directed protein pegylation and vector-mediated blood-brain barrier drug delivery system optimizes brain uptake of brain-derived neurotrophic factor following intravenous administration. Pharm Res. 1998;15(4):576–582. doi: 10.1023/a:1011981927620. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. New York: Academic Press; 2001. [Google Scholar]
- Pearse RN, Swendeman SL, Li Y, Rafii D, Hempstead BL. A neurotrophin axis in myeloma: TrkB and BDNF promote tumor-cell survival. Blood. 2005;105(11):4429–4436. doi: 10.1182/blood-2004-08-3096. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21(18):7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res Mol Brain Res. 1996;36(2):280–286. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- Price RD, Milne SA, Sharkey J, Matsuoka N. Advances in small molecules promoting neurotrophic function. Pharmacol Ther. 2007;115(2):292–306. doi: 10.1016/j.pharmthera.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Radka SF, Holst PA, Fritsche M, Altar CA. Presence of brain-derived neurotrophic factor in brain and human and rat but not mouse serum detected by a sensitive and specific immunoassay. Brain Res. 1996;709(1):122–301. doi: 10.1016/0006-8993(95)01321-0. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45(9):1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Saarelainen T, Hendolin P, Lucas G, Koponen E, Sairanen M, MacDonald E, et al. Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci. 2003;23(1):349–357. doi: 10.1523/JNEUROSCI.23-01-00349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25(5):1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Scarisbrick IA, Jones EG, Isackson PJ. Coexpression of mRNAs for NGF, BDNF, and NT-3 in the cardiovascular system of the pre- and postnatal rat. J Neurosci. 1993;13(3):875–893. doi: 10.1523/JNEUROSCI.13-03-00875.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Banasr M, Duman RS. Future Antidepressant Targets: Neurotrophic Factors and Related Signaling Cascades. Drug Discov Today Ther Strateg. 2008;5(3):151–156. doi: 10.1016/j.ddstr.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol. 2007;18(5–6):391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64(6):527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Okamura N, Koike K, Komatsu N, Kumakiri C, et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol Psychiatry. 2003;54(1):70–75. doi: 10.1016/s0006-3223(03)00181-1. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56(1):131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, et al. Drug-Dependent Requirement of Hippocampal Neurogenesis in a Model of Depression and of Antidepressant Reversal. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Timmusk T, Palm K, Metsis M, Reintam T, Paalme V, Saarma M, et al. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron. 1993;10(3):475–489. doi: 10.1016/0896-6273(93)90335-o. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67(2–3):269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12(2):200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28(6):1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Duman RS. VEGF is an essential mediator of the neurogenic and behavioral actions of antidepressants. Proc Natl Acad Sci U S A. 2007;104(11):4647–4652. doi: 10.1073/pnas.0610282104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134(4):319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52(2):90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Moreau JL, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60(1):129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- Yalcin I, Belzung C, Surget A. Mouse strain differences in the unpredictable chronic mild stress: a four-antidepressant survey. Behav Brain Res. 2008;193(1):140–143. doi: 10.1016/j.bbr.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sobue G, Yamamoto K, Terao S, Mitsuma T. Expression of mRNAs for neurotrophic factors (NGF, BDNF, NT-3, and GDNF) and their receptors (p75NGFR, trkA, trkB, and trkC) in the adult human peripheral nervous system and nonneural tissues. Neurochem Res. 1996;21(8):929–938. doi: 10.1007/BF02532343. [DOI] [PubMed] [Google Scholar]
- Yoshimura R, Mitoma M, Sugita A, Hori H, Okamoto T, Umene W, et al. Effects of paroxetine or milnacipran on serum brain-derived neurotrophic factor in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(5):1034–1037. doi: 10.1016/j.pnpbp.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Zanardini R, Gazzoli A, Ventriglia M, Perez J, Bignotti S, Rossini PM, et al. Effect of repetitive transcranial magnetic stimulation on serum brain derived neurotrophic factor in drug resistant depressed patients. J Affect Disord. 2006;91(1):83–86. doi: 10.1016/j.jad.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Conjugation of brain-derived neurotrophic factor to a blood-brain barrier drug targeting system enables neuroprotection in regional brain ischemia following intravenous injection of the neurotrophin. Brain Res. 2001;889(1–2):49–56. doi: 10.1016/s0006-8993(00)03108-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.