Abstract
Background
Gene x environment (GxE) interactions mediating depressive symptoms have been separately identified in the stress-sensitive serotonergic (5-HTTLPR) and corticotropin-releasing hormone (CRHR1) systems. Our objective was to examine whether the effects of child abuse are moderated by gene x gene (GxG) interactions between CRHR1 and 5-HTTLPR polymorphisms.
Methods
We used an association study examining GxGxE interactions of CRHR1 and 5-HTTLPR polymorphisms and measures of child abuse on adult depressive symptomatology. The participant population (N=1392) was African-American, of low socioeconomic status (60% with <$1000/month family income), and with high rates of childhood and lifetime trauma. Depressive symptoms were measured with Beck Depression Inventory (BDI) and history of Major Depression by Structure Clinical Interview based on DSM-IV (SCID).
Results
We first replicated an interaction of child abuse and 5-HTTLPR on lifetime SCID diagnosis of major depression in a subsample (N= 236) of the study population – the largest African American 5-HTTLPR cohort reported to date. We then extended our previously reported interaction with both a CRHR1 SNP (rs110402) and TCA haplotype interacting with child abuse to predict current symptoms (N=1059; p = 0.0089). We found that the 5-HTTLPR S allele interacted with CRHR1 haplotypes and child abuse to predict current depressive symptoms (N = 856, p = 0.016).
Conclusions
These data suggest that GxE interactions predictive of depressive symptoms may be differentially sensitive to levels of childhood trauma, and the effects of child abuse are moderated by genetic variation at both the CRHR1 and 5-HTTLPR loci and by their GxG interaction.
Keywords: Child Abuse, Childhood Maltreatmet, Trauma, Depression, PTSD, Genetic, risk factor
INTRODUCTION
Epidemiological and molecular genetic data have established that both environmental and genetic factors contribute to the risk of major depression, with heritability estimates ranging between 30% and 40%, with the remaining variance influenced by mostly individual specific environmental factors (Kendler 1995; Levinson 2006). Twin and family studies have suggested that the genetic contribution is most likely oligo- to polygenetic, with many genetic variations of smaller effect contributing in an additive or interactive manner(Levinson 2006). Recently, two large genome-wide association studies for unipolar depression have been published, which reported more or less negative results for larger main genetic effects(Muglia and others 2008; Sullivan and others 2008). Both studies were case-control associations, and neither included environmental exposure as a factor nor investigated gene × gene (G × G) interactions. Inclusion of gene × environment (G × E) and G × G interactions in such analyses might, however, be critical to detect genetic effects in unipolar depression. Genome-wide G × E or gene × gene × environment (G × G × E) studies for unipolar depression have, to the best of our knowledge, not yet been conducted and would require very large sample sizes to attain sufficient power. In this study, we therefore focus on a candidate G × G × E interaction.
The most established environmental risk factors for major depression are stressful life events, with early exposure to trauma being a particularly strong risk factor in conferring risk for the development of depression. Specifically, a history of adverse childhood experience (e.g. child abuse or early separation from parents) increases the risk for developing major depression as an adult by two to three fold, and there is a highly significant dose response relationship between the degree of early adverse experience and depressive disorders as adults (Anda and others 2006; Bradley and others 2008; Chapman and others 2004) . Early trauma has been associated with long-lasting abnormalities in several biological systems that have also directly been implicated in the pathophysiology of major depression, including the serotonergic (5HT) and the corticotropin-releasing factor (CRF) systems (Arborelius and others 1999; Edwards and others 2003; Heim and Nemeroff 2002; Nemeroff 2004).
In fact, both rodents and non-human primates exposed to early adverse experiences exhibit evidence of hyperactivity of the CRF system as adults, as measured by cerebrospinal fluid (CSF) CRF concentrations, CRF and CRF receptor type 1 (CRF1) mRNA expression and CRF receptor binding (Coplan and others 1996), (Ladd and others 2000; Plotsky and Meaney 1993; Plotsky and others 2005). These observations in laboratory animals are consistent with findings in humans where early life trauma is a strong predictor of CSF CRF concentration in adults (Carpenter and others 2004; Lee and others 2005; Lee and others 2006). It is also associated with an enhanced stress response to psychosocial stressors, such as the Trier Social Stress Test, as well as to endocrine challenge tests, including the CRF stimulation test and the combined dexamethasone suppression/CRF stimulation test (Heim and others 2008; Heim and others 2001; Heim and others 2000; Tyrka and others 2008).
In rodents, maternal separation in the early postnatal period also leads to persistent alterations in serotonergic systems, including changes in cell firing of serotonergic raphe neurons, frontal cortical 5-HT concentration and expression levels of certain serotonin receptors in cortex and hippocampus (Arborelius and Eklund 2007; Arborelius and others 1999; Gardner and others 2005; Lambas-Senas and others 2008; Matthews and others 2001; Vicentic and others 2006). In primates, stressful rearing conditions are associated with altered CSF concentrations of the major serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) (Mathew and others 2002) and in humans, child abuse was shown to negatively correlate with CSF 5-HIAA concentrations (Roy 2002) and to be associated with dysregulation in serotonergic function (Rinne and others 2000; Steiger and others 2001; Tiihonen and others 1997).
The fact that early adverse experience impacts both CRF and 5-HT systems is likely due, in part, to the strong interconnection between the two neural circuits. CRF has been reported to inhibit serotonergic neurons in the dorsal raphe nuclei (Kirby and others 2000; Price and others 1998) and may thus modulate serotonin release in terminal areas (Price and Lucki 2001). Very recent data suggests that CRF leads to inhibition of raphe 5-HT neurons via increased inhibitory action of GABA (Kirby and others 2008). Brain regions central to the stress response such as the hippocampus and prefrontal cortex in turn receive dense serotonergic innervation, and serotonin release from the raphe leads to inhibition within the basolateral amygdala (Rainnie 1999).
Maternal deprivation in rats is associated with an increase in CRF binding sites in the raphe nuclei (Ladd and others 1996). In primates exposed to early adverse environment, there is a negative correlation between CSF CRF and 5-HIAA concentrations(Mathew and others 2002), supporting an interacting effect of these two systems in the biology of early adverse experience. Consistent with these data, a number of studies have demonstrated that serotonin reuptake inhibitor (SRI) antidepressants reverse the stress-related effects of CRF administration (Lowry and others 2008), and human depressed patients treated with SRIs show decreased concentrations of CSF CRF (De Bellis and others 1993; Nikisch and others 2005). Furthermore, dysregulation in both systems has been implicated in the pathophysiology of major depression. Child abuse-induced alteration of these systems could thus be causally involved in the increased risk for depression conferred by early life trauma.
In light of these data, it is thus not surprising that genetic variants impacting the function of genes within these systems have been shown to moderate the effects of early trauma on depression. The serotonin transporter-linked polymorphic region (Lesch and others 1996) (5-HTTLPR) has repeatedly been shown to interact with life stress and childhood maltreatment to predict adult depression. While a meta-analysis could not validate this interaction for number of life events (Risch et al., 2009), no meta-analysis so far has been reported for the interaction with childhood maltreatment. A number of studies report a significant moderation of the effects of childhood maltreatment on adult depressive or psychiatric disorders (Caspi and others, 2003; Kaufmann et al., 2004, Roy et al.2007, Gibb et al., 2006, Richardson et al., 2008), in that the short allele of this polymorphism is associated with an increase in symptom severity with early trauma severity. Interaction of the 5-HTTLPR may thus be more robust with early trauma then with life-events in general. We have recently reported an interaction of child abuse with polymorphisms in the type 1 CRF receptor gene, CRHR1, to predict adult depression in two independent cohorts (Bradley and others 2008). This finding has now been replicated in an independent, Caucasian cohort (Polanczyk et al., 2009), supporting the trans-ethnic relevance of the investigated interaction. This gene × environment interaction has also been shown to have endocrine repercussions, with the CRHR1 polymorphisms moderating childhood maltreatment related HPA-axis hyperactivity (Tyrka et al., 2009).
Because of the above described interaction between these two systems on a biological level, it is also possible that G × G interactions across these systems moderate the impact of early adverse experience. In fact, such G × G × E interactions have been reported for the BDNF Val66Met polymorphism as well as a functional Interleukin-6 (IL6) polymorphism with the 5-HTTLPR for example (Bull and others 2008; Kaufman and others 2006; Kim and others 2007; Wichers and others 2008). The aim of the current study was to examine the combined interaction of the 5-HTTLPR and CRHR1 polymorphisms with childhood abuse in predicting current symptoms of depression in a sample of over 1000 African American men and women.
METHODS
Sample and Sample Recruitment
The data from this study were collected as part of a larger study investigating the roles of genetic and environmental factors in predicting response to stressful life events in a predominantly African American, urban population of low socioeconomic status (SES). This is an expanded study from our original publication demonstrating CRHR1 polymorphisms × child abuse interactions with depression (Bradley and others 2008). Research participants were approached while either waiting for their medical appointments or while waiting with others who were scheduled for medical appointments, in the waiting rooms of the primary care clinic or obstetrical-gynecological clinic of a large urban, public hospital. Subjects who indicated willingness to participate provided written informed consent, participated in a verbal interview, and provided a salivary sample for DNA extraction (described below). The data presented in this paper are from the first 1492 subjects interviewed of which only data from the 1392 individuals self-identifying as African American were used in this report (93.3%) to decrease any potential confounds due to ethnic stratifications. All procedures in this study were approved by the Institutional Review Boards of Emory University School of Medicine and Grady Memorial Hospital. Demographic data on gender, self-identified race/ethnicity, education, employment, disability status and monthly household income are presented in Table 1.
Table 1.
Sample Demographics
| Demographic All self-identified African-American / Black |
Total Sample (N), % |
Male (N), % |
Female (N), % |
|---|---|---|---|
| Age (N=1385) | |||
| 18-24 | (260), 19% | (58), 11% | (198), 23% |
| 25-34 | (261), 19% | (74), 14% | (186), 22% |
| 35-44 | (250), 18% | (99), 19% | (149), 17% |
| 45-54 | (377), 27% | (182), 35% | (193), 23% |
| 55-64 | (185), 13% | (86), 17% | (98), 11% |
|
≥65
|
(52), 4% |
(21), 4% |
(31), 4% |
| Education (N=1378) | |||
| <12th grade | (354), 26% | (127), 24% | (227), 26% |
| High School Graduate | (527), 38% | (201), 39% | (326), 38% |
| Graduate equivalency diploma | (71), 5% | (27), 5 % | (44), 5% |
| Some college/technical school | (274), 20% | (100), 19% | (174), 20% |
| Technical school graduate | (52), 4% | (16), 3% | (36), 4% |
| College graduate | (82), 6% | (39), 8% | (43), 5% |
| Graduate School |
(18), 1% |
(10), 2% |
(8), 1% |
| Employment (N=1380) | |||
| Currently unemployed | (912), 66% | (358), 69% | (554), 65% |
| Currently employed |
(468), 34% |
(164) 31% |
(304) 35% |
| Disability (N=1374) | |||
| Currently receiving disability | (308), 22% | (142), 27% | (166), 19% |
| Not receiving disability |
(1066), 78% |
(376) 73% |
(690), 81% |
| Household Monthly Income, US$ (N=1351) | |||
| 0-499 | (508), 38% | (199), 38% | (309), 37% |
| 500-999 | (363), 27% | (134), 26% | (229), 27% |
| 1000-1999 | (331), 25% | (118), 23% | (213), 26% |
| ≥2000 | (149), 11 % | (66), 13% | (83), 10% |
Procedure
Participants verbally completed a battery of self-report measures, including the Beck Depression Inventory and the brief Child Trauma Questionnaire, which took 45 to 75 minutes to complete (dependent in large part on the extent of the participant's trauma history and symptoms). We read the instruments to participants to guard against relatively high rates of impaired literacy. Each person was paid $15.00 for participation in this phase of the study. The measures used in the current study are described below.
Beck Depression Inventory (BDI)
Depressed mood was assessed with the 21-item BDI (Beck and others 1961) a well validated, commonly used continuous measure of level of depressive symptoms. In this sample, the BDI had a standardized alpha coefficient of .99 (M = 14.4, SD = 13.2).
Additionally, because these analyses were performed in subjects identified in a general medical clinic, it is possible that the BDI is a proxy for medical-related depressive symptomatology separate from Major Depressive Disorder (MDD). To examine this, we have previously used history of prescribed medicine as a proxy-variable for medical illness category (Bradley and others 2008). There were no significant effects found for any of the 13 available classes of medicines examined (p's >.1). This demonstrates that the moderation effects of child abuse and CRHR1 genotype were unlikely to be affecting medical condition as a possible contributor to depressive symptoms.
Structured Clinical Interview according to DSMIV (SCID)
297 individuals underwent more extensive psychopathological evaluations including the Structured Clinical Interview for DSM-IV (SCID-DSMIV 64). This is a validated interview assessment of DSM-IV mood disorders and was used to assess the presence or absence of MDD as well as other mood disorders within our study population.
Childhood Trauma Questionnaire (CTQ)
The CTQ (Bernstein and Fink 1998) is a self-report inventory assessing three types of childhood abuse: sexual, physical and emotional. Studies have established the internal consistency, stability over time and criterion validity of both the original 70-item CTQ and the current brief version(Bernstein and others 2003). The CTQ yields a total score and subscale scores for each of the types of child abuse. Our CTQ data demonstrated high internal reliability (α = .99 for physical abuse; .94 for sexual abuse; .93 for emotional abuse; .98 for the total of these three scales). Bernstein and Fink (Bernstein and Fink 1998) established scores for none, mild, moderate and severe for each type of abuse. The data from the CTQ were used to classify participants into two categories for each type of abuse (physical, sexual, emotional). For each type of abuse (sexual, physical and emotional) those participants with CTQ scale scores in the none-mild range were classified as negative/absent and those with CTQ scores in the moderate to severe range were classified as positive/present. We then created a composite variable across all three types of abuse. We first created a two-level abuse variable dividing participants into two groups with respect to numbers of types of abuse on which they fell into the moderate to severe range: 1) those with no type of abuse in the moderate to severe range and 2) those with moderate-severe on at least one type of abuse. We also created a three level abuse variable: 1) those with no type of abuse in the moderate to severe range, 2) those with moderate-severe abuse in one type of abuse, and 3) those with moderate-severe abuse in 2 or more types of abuse.
DNA extraction
DNA was extracted from saliva collected into Scope mouthwash (N = 46) or into Oragene saliva kits (DNAGenotek, Inc.) for the rest of the sample using the Qiagen M48 system and the Purelink 96 Genomic DNA Kit by Invitrogen (Cat # K1821-04). High quality DNA was available for 1234 individuals. The 158 individuals with no genotype information consist of individuals for which DNA was not collected or for which we attempted to collect DNA using Oragene-saliva samples but DNA extraction failed completely in two separate extraction trials (these failures were either due to non-compliance of the study participants or failure to correctly break the seal that releases the stabilizing solution in the Oragene kits). When comparing the individuals with genotypes to those without, we found no significant difference on any parameters relevant for the study as described before in (Binder and others 2008).
Single Nucleotide Polymorphisms (SNP) and genotyping
The genetic methodology for SNP genotyping is described more extensively in our previous publications from this sample (Binder and others 2008; Bradley and others 2008). We examined the CRHR1 SNPs (rs7209436, rs4792887 and rs110402) and the resulting haplotypes based on our previous examination of this gene and depression (Bradley and others 2008). All SNPs were genotyped using a TaqMan allelic discrimination assay (Livak 1999) developed for use on the 7900HT instrument (Applied Biosystems, Forster City, CA, USA) using pre-designed and validated TaqMan assay reagent kits containing one pair of PCR primers and one pair of fluorescently labeled probes (Applied Biosystems; http://www.appliedbiosystems.com/). PCRs were performed in 5 μL reaction volumes in 384-well plates and contained 5 ng of DNA. The standard protocol provided with the kit was followed. Thermal cycler conditions were 95°C for 10 min, 40 cycles of 95°C for 15 s and 60°C for 1 min. The SDS 2.3 software was use for allelic discrimination. For quality control, 45% of the samples were genotyped as duplicates across and within a 384 well plate. 10 discordances were recorded for 4685 *non-duplicate genotypes (0.20%) and excluded from the analysis. *This number includes genotypes at other loci, which contributed to the finding of discordant samples. Call rates for the 3 SNPs ranged form 90.7 to 99.2%. All assay IDs, SNP position and Hardy Weinberg Equilibrium are available on request from the authors.
Genotyping of the 5-HTTLPR utilized the following primers (forward: 5’ -Hex-TGAATGCCAGCACCTAACC -3’; reverse: 5′- ATACTGCGAGGGGTGCAG -3′). PCR was carried out in 384 well plates in a 10 ul volume with 10 ng DNA. Each PCR reaction contained 0.5 μM of each primer, 0.08 μM of dATP, dCTP and dTTP and 0.04 uM of dGTP, 0.2 uM of 7-deaza GTP (Amersham Biosciences), 5% DMSO and 1.25 units of AmpliTaq Gold (Applied Biosystems). The cycling parameters were as follows: 95°C for 5 min, then 94°C for 30sec, 63°C for 30 sec and 72°C for 1 min for 1 cycle, then the annealing temperature reduced to 62°C for one more cycles and then to 59.5°C for 38 cycles. 5 ul of the resulting PCR products was then digested with 5 U MspI (New England Biolabs) in a total volume of 10 ul for 90 minutes at 37°C to detect the A/G SNP rs25531 shown to influence the functional effects of the long and short alleles(Hu and others 2006). The digested PCR products were then separated using an Applied Biosystems 3100 genetic analyzer and analyzed with Applied Biosystems Genemapper 4.0 software. Fragment lengths for the L-A-allele are 291 bp, 148 for the L-G and 247 bp for the S-allele. The VL fragment 335 bp and the XL fragment 375 bp.
We genotyped 506 samples in duplicates and out of these only 3 discordant genotypes were observed and the call rate was 94.6%.
N in analysis
Of the 1234 samples with sufficient DNA, all entered CRHR1 SNP genotyping and 1133 entered 5-HTTLPR genotyping. Of the 1392 total samples, 1213 had a valid entry for both the BDI as well as the CTQ. Of these, all 1213 had attempted CRHR1 genotyping, 990 had attempted 5-HTTLPR genotyping and 964 had overlapping CRHR1 and 5-HTTLPR genotyping. Due to less then 100% call rates the final N in the gene × environment analyses was smaller than the attempted genotypes and is specified for each analysis. Power:
Variable Coding and Regression and Permutation Analyses for Interaction Effects
We used linear regression to assess whether the genotypes itself or in interaction with child abuse predicted current depressive symptoms. Predictors: 5-HTTLPR was coded as SS, SL, LL, extra-long alleles (VL and XL), or as S allele carriers or using functional classification as previously described (Hu and others 2006). In the latter, low function genotypes are SS, SLG and LGLG. Intermediate function alleles are SLA and LALG and the high function genotype is LALA. In this classification very or extra long allele carriers were excluded as were for the S-allele carrier status. For CRHR1 variants, we investigated the effects of the best SNP from Bradley et al 2008 (Bradley and others 2008), rs110402 using a protective allele carrier model (AA and AG vs. GG – see table 2) and a carrier model of the protective haplotype described in this paper. The SNPs rs7209436, rs4792887 and rs110402 formed three major haplotypes – CCG with 35.3%, CTG with 32.9% and TCA with 30.4%. All other haplotypes were less than 1% in this sample. In our previous study, we had identified the TCA haplotype as protective, so that the absence of any TCA haplotypes was coded as CRHR1 risk = 1 and the presence of at least one copy of this haplotype as CRHR1 risk = 0 (see table 2). Child abuse was coded into the two (none-mild and moderate to severe overall) or three levels (no moderate or severe type of abuse, 1 type of moderate or severe abuse of 2 types) described above. We further adjusted all regression models for potential confounders by including age and gender. For linear regression, we additionally examined the significance of genotype-child abuse interaction effects using permutation-based procedures (Epstein and Satten 2003; Li and others 2000) that randomly assigned the sample BDI scores to subjects (sampled without replacement), while holding each subject's genotype and or haplotypes and environmental variables fixed. This permutation method is robust against non-normal distribution of the outcome variable as we observe with the BDI in this population (Bradley and others 2008). For each analysis, the empirical p-value was based on 10,000 permutations. We conducted these analyses using appropriate components of the SAS software system (version 9.1, SAS Institute, Cary, NC). Haplotypes were estimated using SNPHAP (Adkins 2004; Sham and others 2004). All haplotypes with an estimation probability less than 95% were excluded from the analysis (4.1% excluded). For effects on SCID-based life time diagnosis, we used logistic regression in a sub-sample (N = 236-278) using 5-HTTLPR or CRHR1 rs11402 A-carrier status and the two level child abuse variable and their interaction terms as predictors, adjusting for age and sex. Due to the much smaller N with available SCID data, we did not analyze gene × gene × environment interaction analyses for this outcome.
Table 2.
Frequency of 5-HTTLPR and CRHR1 genotypes.
| 5-HTTLPR genotype | 5-HTTLPR genotype and rs25531 | Functional classification Hu et al | ||||
|---|---|---|---|---|---|---|
| N | Percent | N | Percent | |||
| LL | 598 | 55.78 | LALA | 292 | 27.2 | high |
| LALG | 269 | 25.1 | intermediate | |||
| LGLG | 37 | 3.5 | low | |||
| SL | 359 | 33.49 | SLA | 243 | 22.7 | intermediate |
| SLG | 116 | 10.8 | low | |||
| SS | 66 | 6.16 | 66 | 6.2 | low | |
| LXL | 35 | 3.26 | LAXL | 20 | 1.9 | |
| LGXL | 15 | 1.4 | ||||
| SXL | 8 | 0.75 | 8 | 0.8 | ||
| LVL | 6 | 0.56 | LAVL | 3 | 0.3 | |
| LGVL | 3 | 0.3 | ||||
| N total | 1072 | 1072 | ||||
| CRHR1 rs110402 | CRHR1 TCA Haplotype | ||||
| AA | 87 | 7.2 | 0 Alleles | 577 | 48.8 |
| AG | 544 | 44.8 | 1 Alleles | 519 | 43.9 |
| GG | 582 | 48.0 | 2 Alleles | 87 | 7.3 |
| N total | 1234 | ||||
| SERT * TCA Haplotype Risk | |||||
| No Risk Alleles | 286 | 24.2 | |||
| S-allele only | 214 | 18.1 | |||
| CRHR1 risk only | 293 | 24.8 | |||
| Both Risk Alleles | 195 | 16.5 | |||
Results
Frequency of 5-HTTLPR genotypes in African American sample
As shown in Table 2, the frequency of the 5-HTTLPR within this sample is 6.2% SS, 33.5% SL, and 55.8% LL with 4.7% carrying a longer form such as the VL-17 repeats (LVL 0.6%) and the XL – 18 repeats (LXL = 3.3%, SXL = 0.7%) genotypes. For rs25531, the frequency of G-allele carriers in individuals carrying at least one L allele was 43.9% and using the functional classification proposed by Hu et al., 2006 (Hu and others 2006), 21.4% were low-functioning, 50.0% intermediate function and 28.6% high function genotype carriers. See Table 2.
5-HTTLPR genotype, Child Abuse and Current Adult Depression Symptoms
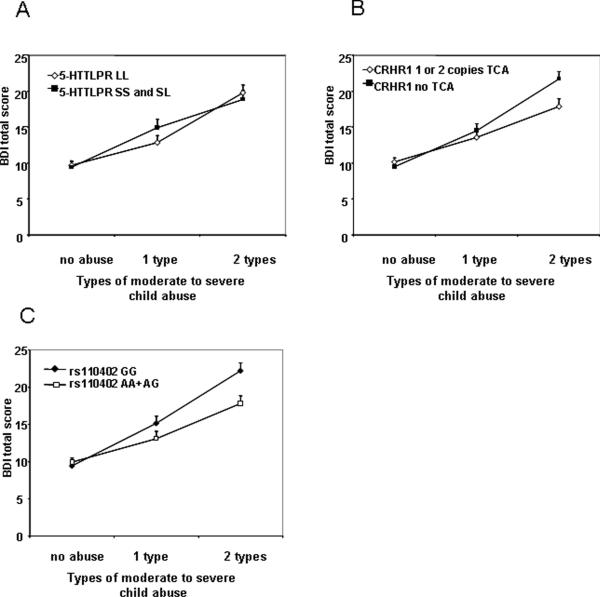
We first evaluated whether the 5-HTTLPR genotype, S-allele carrier status or functional classification together with child abuse (2 level or 3 level category) would predict current depressive symptoms, using the continuous total score from the Beck Depression Inventory as outcome, and co-varying for sex and age (N = 926 for all genotypes and N = 885 for S-allele carrier status and functional classification). We found that although there was a highly significant effect of level of child abuse history (two level) on BDI (p < 0.0001), there was no significant main effect of the 5-HTTLPR nor interaction between the 5-HTTLPR and 2 level child abuse on BDI total score, for all types of 5-HTTLPRs coding. The same outcome was observed when using a three level categorization of child abuse (Figure 1A – 5-HTTLPR S-allele carrier status).
Figure 1. Interaction of 5-HTTLLPR S-allele carrier status and CRHR1 TCA haplotype with child abuse to predict adult depression.
A) Depression symptoms using the Beck Depression Inventory (BDI), are graphed as a function of 5-HTTLPR SS, SL vs. LL carrier status, as a function of level of moderate to severe child abuse. We find no significant main or interaction effects. B) Depression symptoms using the BDI are graphed as a function of TCA haplotype (rs7209436, rs4792887, and rs110402) of the CRHR1 gene, as a function of level of moderate to severe child abuse, demonstrating a significant interaction effect (p<.05). C) Depression symptoms using the BDI, are graphed as a function of CRHR1 rs110402 (A-carrier model), as a function of level of moderate to severe child abuse, demonstrating a significant interaction effect (p=.009).
5-HTTLPR genotype, Child Abuse and lifetime history of major depression
We then tested the interaction of the 5-HTTLPR genotype, S-allele carrier status and 5-HTTLPR functional classification with child abuse (none to mild vs. moderate to severe) to predict lifetime diagnosis of depression as measured with SCID-DSMIV, with age and sex as covariates (N = 236). In these analyses, childhood abuse (p < 0.0001, B+SD = 2.21±0.59) as well as its interaction term with the 5-HTTLPR genotype (p = 0.016, B = -0.82±0.33) were included in the model. When using the S-allele carrier status, only its interaction term with child abuse remained a significant predictor in the model (p = 0.00017, B = 1.36±0.36). As previously reported (Caspi and others 2003), we also see an over-representation of S-allele carriers in the group with child abuse and life time major depression (see Table 3). No significant 5-HTTLPR × child abuse interaction was observed using the functional allele classification.
Table 3.
Distribution of 5-HTTLPR genotype by child abuse and lifetime history of major depression diagnosed with SCID-DSMIV, total N = 236. The S-allele carrier group is the combination of the cells from SS and SL.
| 5-HTTLPR genotype | N total | |||||
|---|---|---|---|---|---|---|
| SS | SL | LL | ||||
| no to mild abuse | no lifetime MDD | N | 6 | 30 | 62 | 100 |
| % | 6.0 | 30.0 | 64.0 | |||
| lifetime MDD | N | 1 | 13 | 21 | 35 | |
| % | 2.9 | 37.1 | 60.0 | |||
| moderate to severe abuse | no lifetime MDD | N | 2 | 13 | 37 | 52 |
| % | 3.8 | 25.0 | 71.2 | |||
| lifetime MDD | N | 6 | 20 | 23 | 49 | |
| % | 12.2 | 40.8 | 46.9 | |||
CRHR1, Child Abuse and Current Adult Depression Symptoms
In this analysis, we first re-examined our previously reported finding of CRHR1 genotype interaction with level of child abuse to predict adult depression with the most significant SNP rs110402 and the TCA haplotype previously described (Bradley and others 2008). The analysis was performed using both the two-level and three-level child abuse variables. The N for this gene × environment interaction in the current study is more than double (N = 1059) our prior study (N~480), with the former sample pool included in the current study. We find a significant gene × environment interaction (co-varying for age and sex) with the two level child abuse variable with the A allele of rs110402 (B = 3.53±1.35, p = 0.0089, empirical p = 0.0095) and the presence of the protective CRHR1-TCA haplotype (B = 1.59±1.66, p = 0.039) but no genetic main effects. Similar results were observed for the 3 level child abuse variable with a significant interaction of child abuse and rs110402 A carrier status (B = 4.88±1.7, p = 0.012, empirical p = 0.011) and a main effect CRHR1 A carrier status (B = 4.38±1.5, p = 0.0071) as well as a significant interaction of TCA risk haplotype carriers with child abuse (B = 1.86±0.85, p = 0.033) (see Figure 1B and 1C). In all models, child abuse (both as the two as well as three level variable) was a highly significant predictor (p < 0.0001), as were sex and age (p < 0.05 in all models).
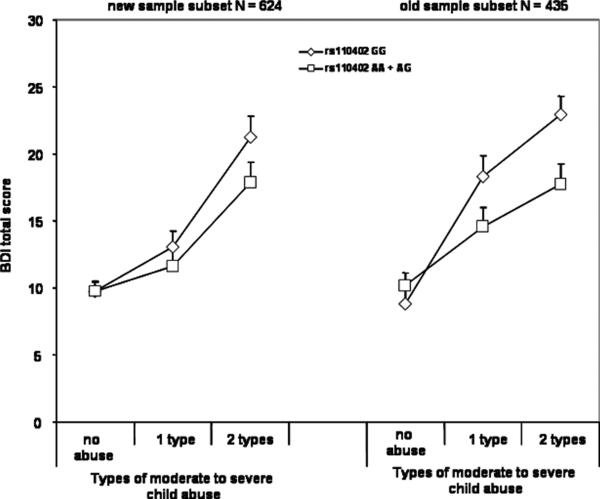
Because data from a fraction of this sample (N = 435) had been previously reported, we investigated whether similar gene × environment interactions were observed in the first and the second data sets that comprise the present sample. We added ‘presence in the first report’ coded as 0 or 1 as a predictor in the analysis in addition to its interaction term with child abuse and with child abuse × rs110402 A carrier status. We re-reran the analyses with both the two level and the three level abuse categorizations. In both analyses, we found a significant main effect of subsample on BDI total score and interaction between child abuse and presence in first paper (p < 0.05) in addition to age, sex and child abuse. No analyses indicated a significant presence in analysis × rs110402 × child abuse interaction, indicating that the interaction effect was not different in the two sets of samples (see Figure 2). The two sample subsets had, however, significant differences in environmental exposure and depression scores, possibly explaining slight differences in the interaction. The later samples had significantly lower BDI total scores (11.05±10.5(SD) vs. 13.5±12.2), F1233,1=8.6, p = 0.003), lower CTQ total scores (38.9±15.4 vs. 44.2±17.2, F1297,1=32.2, p < 0.001) and were older (41.2+13.9 years vs. 39.2±14.2). There was no difference in gender distribution (62.3% female vs. 62.9%, p = 0.81) and CRHR1 genotype distribution (36.9% rs110402 A allele carriers vs. 38.7%, p = 0.53).
Figure 2. Interaction of CRHR1 rs110402 with replicate samples.
Depression symptoms using the BDI, are graphed as a function of theCRHR1 rs110402 SNP, GG vs AA,AG as a function of level of moderate to severe child abuse in the sample reported in Bradley et al,. 2008 (old) and additional samples reported in this manuscript (new).
CRHR1, Child Abuse and lifetime history of major depression
We then tested the interaction of the rs110402 A carrier status with child abuse (none to mild vs. moderate to severe) to predict lifetime diagnosis of depression as measured with SCID-DSMIV, with age and sex as covariates (N = 275). In these analyses only childhood abuse (p = 0.00022) was included in the model. While rs110402 carrier status and its interaction term were not significant, we found a nominal over-representation of protective A-allele carriers in the abuse but not major depression group (see Table 4).
Table 4.
Distribution of rs110402 A-allele carriers by child abuse and lifetime history of major depression diagnosed with SCID-DSMIV, total N = 278.
| rs110402 GG | rs110402 AA + AG | Total N/group | |||
|---|---|---|---|---|---|
| no to mild abuse | no lifetime MDD | N | 59 | 60 | 119 |
| % | 49.6 | 50.4 | |||
| lifetime MDD | N | 22 | 19 | 41 | |
| % | 53.7 | 46.3 | |||
| moderate or severe abuse | no lifetime MDD | N | 25 | 36 | 61 |
| % | 41.0 | 59.0 | |||
| lifetime MDD | N | 30 | 27 | 57 | |
| % | 52.6 | 47.4 |
5HTLLPR, CRHR1, Child Abuse and Current Adult Depression Symptoms
We next examined the data for a gene × gene × environment interaction between the 5HTLLPR (S-allele carrier) and CRHR1 haplotype (protective haplotype carrier) and level of child abuse history. Given the number of predictors in the model with the 856 subjects for whom we have all genotype and phenotype data, we found that we would have >95% power to detect a p-value at 0.01 that accounts for more than 5% of the variance in a G×G×E model.
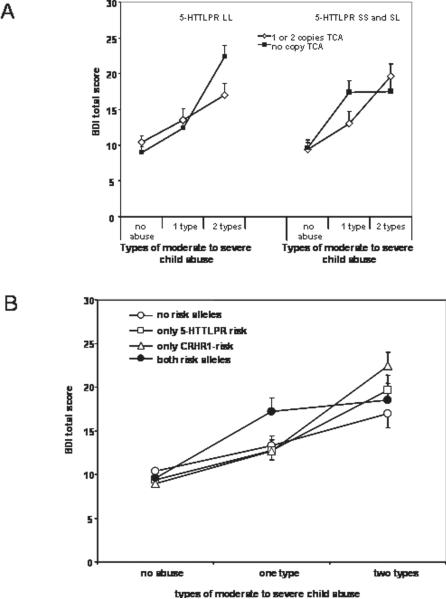
Although no significant gene × gene × environment interactions were observed with the 2 level child abuse variable, we find a significant gene × gene × environment interaction (covarying for age and sex) when using the three level child abuse variable. In this model, we observe significant effects of age, sex and child abuse level (all p values < 0.05), as well as a significant gene × gene × environment interaction (N = 856, B = 9.26±3.95, p = 0.0161, empirical 0.0164) (see Figure 3A). Note that after log transforming the BDI score to account for any possible skewness, the gene × gene × environment interaction remains (p<.01). The protective effect of the CRHR1 TCA haplotype is only seen in absence of the 5-HTTLPR S-allele (Figure 3 left panel) and only in absence of the TCA protective haplotype, the S-allele increases current depression with moderate levels of child abuse (Figure 3A right panel). Using a combined coding of presence of risk alleles (no risk group = 5-HTTLPR LL and 1 or 2 copies of CRHR1 TCA; CRHR1 risk only = 5-HTTLPR LL and 0 copies CRHR1 TCA; 5-HTTLPR risk only = 5-HTTLPR SS or SL and 1 or 2 copies CRHR1 TCA; and combined risk group = 5-HTTLPR SS or SL and 0 copies CRHR1 TCA), lower levels of child abuse severity are sufficient to elicit clinically relevant symptoms of depression in individuals with both genetic risk factors, as compared to the no risk or single genetic risk groups (see Figure 3B).
Figure 3. Interaction between 5-HTTLPR S-allele carrier status and CRHR1 TCA haplotype carriers and child abuse on adult depressive symptoms.
Panel A: Depression symptoms using the BDI are graphed as a function of 5-HTTLPR and CRHR1 genotype. Left panel, individuals with 5-HTTLPR LL genotypes are represented with lines representing 1 or copies vs. no copies of the protective TCA CRHR1 haplotype. Right panel, individuals with 5-HTTLPR SS or SL genotypes are represented with lines representing 1 or copies vs. no copies of the protective TCA CRHR1 haplotype. An overall gene × gene × environment interaction is seen (N=856, p=.016). Panel B: Representation of the same interaction but grouping the individuals by number of risk variants. No abuse group: N = 159 no risk alleles, N = 115 only 5-HTTLPR s-allele, N = 148 only CRHR1 risk, N = 102 both risk alleles. 1 type of abuse group: N = 44 no risk alleles, N = 39 only 5-HTTLPR s-allele, N = 69 only CRHR1 risk, N = 44 both risk alleles. 2 types of abuse: N = 42 no risk alleles, N = 36 only 5-HTTLPR s-allele, N = 46 only CRHR1 risk, N = 30 both risk alleles.
Discussion
This paper presents three primary findings. First, we did not find a main effect of 5-HTTPLR genotype or an interaction effect of 5-HTTPLR and child abuse on current level of depression symptoms in a sample of over 900 African American men and women but did replicate an interaction of child abuse and 5-HTTLPR on lifetime diagnosis of major depression in a subsample of 234 individuals. Second, using a data set of 624 more individuals, we extended our previously reported interaction of CRHR1 variants with child abuse to predict adult depressive symptoms in a sample of now over 1000 individuals. Third we found that the 5-HTTLPR interacts with CRHR1 variants and child abuse to predict current, adult depressive symptoms: individuals carrying the risk alleles in both genes exhibit clinically relevant depressive symptoms at less severe levels of child abuse than individuals with no or only one of the risk alleles.
To the best of our knowledge this the first paper to present data on 5-HTTPLR and depressive symptoms in an African American sample - though a previous study in a similar population investigated this polymorphism as predictor of suicide attempts in a sample with a history of drug abuse (Roy and others 2007). The observed allele frequencies for the 5-HTTLPR, including the VL and XL alleles are in agreement with previous publications and support that the SS genotype (6-7%) is much less frequent in individuals from African American descent that in European Americans (20-25%) (Gelernter and others 1998; Gelernter and others 1997; Hu and others 2006). The XL allele has thus far only been observed in individuals from African descent and VL allele had previously been detected in Asian populations (Gelernter and others 1997; Nakamura and others 2000). The few VL alleles observed in this sample might be related to admixture from Native American or Asian ancestry that we observe in our sample (Bradley and others 2008).
Consistent with the majority of the extant literature, we did not find a main effect of 5-HTTLPR genotype on current symptoms of depression in this sample. We replicate the previously described increased prevalence of incidence of lifetime major depression in individuals carrying the 5-HTTLPR S-allele and exposed to child abuse (Caspi and others 2003), but this interaction was not seen when using the functional classification of Hu and colleagues (Hu and others 2006). Possibly, the higher prevalence of the low-function rs25531 G-allele in African American compared to Caucasians as well as additional genetic differences of this locus depending on ethnic background might lead to this discrepancy. We however failed to find a significant interaction of childhood abuse and 5-HTTLPR on current depression symptoms in this sample. To resolve the conflicting data on life-time vs. current depression for this gene × environment interaction in our sample, we are currently gathering more comprehensive data on factors such as age of onset of abuse, age of onset and recurrence of depression symptoms in the current sample which will allow us to more closely analyze the roles of these factors in the current sample. While a recent meta-analysis concluded that the observed interaction of the 5-HTTLPR with life events to predict adult depression is likely attributable to chance (Risch, 2009), these controversial results might be due to sample-related differences in such factors including age, gender, type of environmental exposure (early life trauma vs. recent life stressors) and type of depression (recent onset adult vs. chronic) (Brown and Harris 2008).
In a sample expanded to 1059 individuals, we were able to extend our previously reported interaction of CRHR1 variants and child abuse to predict adult depressive symptoms (Bradley and others 2008). Using more graded levels of child abuse as the environmental variables, we could show that the protective effects of CRHR1 variants are more pronounced with the presence of at least one type of severe abuse. However, it is important to note, as in figure 3, that sufficiently high levels of environmental exposure to trauma may overcome any genetic effects. The additional samples not reported in the initial manuscript show an interaction of CRHR1 variants and child abuse in the same direction, but less pronounced than in the first sample. This might be due to differences of the initial sample vs. the second set of recruited individuals. The more recently recruited individuals had lower BDI and CTQ scores and were on average two years older, while gender and CRHR1 haplotype and 5-HTTLPR genotype distribution was the same. These differences in environmental exposure and depressive symptoms might, in part, explain the differences in effect size between the first and second set of samples (see figure 2). We did not see a significant interaction of CRHR1 variants and child abuse on lifetime diagnosis of major depression; however, the distribution of the protective alleles in the different groups was consistent with an over-representation of the protective CRHR1 alleles in individuals with child abuse but no life time major depression. This analysis was further limited by a small sample size, prohibiting analysis with a more graded child abuse variable due to very small Ns/cell (see Table 4).
The main finding of this manuscript is that the 5-HTTLPR interacts with CRHR1 variants and child abuse to predict adult depressive symptoms. In fact, the protective effects of the CRHR1 TCA haplotype were only observed in individuals carrying the 5-HTTLPR LL genotype. On the other hand, the 5-HTTLPR S-allele appeared to enhance the effects of lower levels of child abuse only in the additional presence of the CRHR1 risk haplotypes. Overall, individuals carrying the risk alleles in both genes, exhibit more severe depressive symptoms already at lower “doses” of child abuse than individuals with no or only one of the risk alleles (see Figure 3B). The finding that this interaction is present when child abuse is divided across three levels but not across two levels is consistent with research suggesting a dose-response relationship between number of types of adverse experiences in early life and increased risk for a number of health and behavioral problems, including major depression, over the lifespan (Anda and others 2006; Chapman and others 2004; Edwards and others 2003). Note, however, that with sufficient levels of childhood abuse, any amount of genetic ‘protection’ may be overcome.
Although this gene × gene × environment interaction needs further independent replication to be confirmed, it is well in agreement with the previously reported interactions between the serotonergic and CRF system on a cellular and systems level as described in the introduction (De Bellis and others 1993; Kirby and others 2008; Kirby and others 2000; Lowry and others 2008; Nikisch and others 2005; Price and others 1998; Price and Lucki 2001; Rainnie 1999). This gene × gene interaction could also help to clarify the meaning of gene × environment interaction findings with the 5-HTTLPR or CRHR1 variants alone. In fact, different frequencies in the combination of 5-HTTLPR and CRHR1 risk or protective variants could alter the effect size of the individual gene × environment interaction, if the second variant is not considered. Likewise our finding that this interaction is present when child abuse is divided across three levels but not across two levels points to the importance of paying attention to the ways in which broad constructs such as “stressful life events” or even more specific constructs such as “child abuse” are defined in evaluating the meaning of gene × environment research. Within these broad categories are varying stressful experiences for which the underlying neurobiological processes contributing to risk/resilience are likely to be different.
Our study has several limitations such as the use of a non-diagnostic, self report measure of current depression symptoms for the main analysis. A subset of our data now has lifetime and current psychiatric diagnoses according to the SCID-DSMIV (First and others 1995) and we have previously reported good agreement of BDI scores > 16 and current major depressive episode (see (Bradley and others 2008)). Our interaction results might thus be extended at least to the presence of a current major depressive episode. Unfortunately, the sample with data on current major depression does not have sufficient power yet for meaningful gene × environment and even less for gene × gene × environment interaction analyses. Likewise, our sample with data on lifetime diagnosis of major depression is still being collected and our gene × environment interaction analyses using this data should be considered preliminary. As with diagnosis of current depression, our current sample size for lifetime diagnosis of depression does not allow for gene × gene × environment analyses. As with the majority of gene × environment studies to date, our data are cross-sectional and rely on adult self-reported history of childhood abuse and are thus likely confounded to a certain degree by recall bias. The fact that our data are gathered in sample of low income, African American men and women presenting for primary care in public hospital also limits the generalizability of the results. However, this limitation is mitigated by the facts that exposure to traumatic events including childhood abuse occurs at a notably higher rate among low income, urban dwelling African American populations (Alim and others 2006; Bierut and others 2007) with a high percentage of this exposure occurring during youth (Fitzpatrick 1993; Shakoor and Chalmers 1991) . This level of trauma exposure is associated with increased levels of risk for physical and mental health problems and associated healthcare costs (Fullilove and others 1998; Schwartz and others 2005; Schwartz and others 2006). Additionally, the limitation of self report of history of abuse is a well-known one, which is important to attend to, but which does not alter the primary findings. Certainly prospective studies are indicated in the future. Understanding the complex relationship between risk and resilience for disease among adults with a history of childhood abuse, particularly in highly traumatized high risk populations is of high public health relevance.
By design, gene × gene × environment interactions require large sample sizes, so that independent replication and extension in a larger sample will be needed to confirm our finding. Nonetheless, our sample represents one of the larger samples available for gene × environment interaction analyses and suggests that more complex analyses than case-control associations are necessary to identify the genetic variants relevant in the genetics of depression.
Acknowledgements
This work was primarily supported by National Institutes of Mental Health (MH071537). Support was also received from NIMH, MH069884 to KJR and MH082256 to CFG, National Institute of Drug Abuse (DA015766 to JFC), Emory and Grady Memorial Hospital General Clinical Research Center, NIH National Centers for Research Resources (M01 RR00039), the Doris Duke Foundation (EBB) and the Burroughs Wellcome Fund (KJR). We thank Allen Graham, BS, Mark Gapen, PhD, Daniel Crain, BS, and Arnita Ross for excellent technical support and Wei Liu, PhD for statistical support.
Footnotes
Financial Disclosure Statement:
There were no commercial sponsors or commercial relationships related to the current work. All additional past and present financial ties of the investigators are disclosed herein.
Dr. Binder receives grant support or has received awards from NARSAD, the Doris Duke foundation and NIMH.
Dr. Bradley receives grant support or has received awards from AFSP and the American Psychoanalytic Association Psychoanalytic Research Fund.
Dr. Gillespie receives grant support or has received awards from APIRE, NARSAD, NIDA and NIMH.
Dr. Ressler has received awards and/or funding support related to other studies from Lundbeck, Burroughs Wellcome Foundation, Pfizer, NARSAD, NIMH, NIDA, and has a consulting agreement with Tikvah Therapeutics for NMDA-based therapeutics.
In the past, Dr. Nemeroff consulted to, served on the Scientific Advisory Board and/or Board of Directors, has been a grant recipient, and/or owned equity in one or more of the following: Abbott Laboratories, Acadia Pharmaceuticals, American Foundation for Suicide Prevention( AFSP), American Psychiatric Institute for Research and Educations(APIRE), AstraZeneca, BMC-JR LLC, Bristol-Myers-Squibb, CeNeRx, Corcept, Cypress Biosciences, Cyberonics, Eli Lilly, Forest Laboratories, George West Mental Health Foundation, GlaxoSmithKline, i3 DLN, Janssen Pharmaceutica, Lundbeck, National Alliance for Research on Schizophrenia and Depression( NARSAD), Neuronetics, NIMH, NFMH, NovaDel Pharma, Otsuka, Pfizer Pharmaceuticals, Quintiles, Reevax, UCB Pharma, Wyeth-Ayerst. Currently, Dr. Nemeroff serves on the Scientific Advisory Board for Astra-Zeneca, Johnson & Johnson, Pharma Neuroboost, and NARSAD. He serves on the Board of Directors of AFSP, NovaDel Pharmaceuticals, Mt. Cook Pharma, Inc., and the George West Mental Health Foundation. He owns equity in CeNeRx and Reevax. He owns stock or stock options in Corcept and NovaDel.
Dr. Smith has received an award from AFSP and grant support from Schering-Plough Pharmaceuticals.
Dr. Ressler and Dr. Binder had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
None of the above funding agencies had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
References
- Adkins RM. Comparison of the accuracy of methods of computational haplotype inference using a large empirical dataset. BMC Genet. 2004;5:22. doi: 10.1186/1471-2156-5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alim TN, Graves E, Mellman TA, Aigbogun N, Gray E, Lawson W, Charney DS. Trauma exposure, posttraumatic stress disorder and depression in an African-American primary care population. J Natl Med Assoc. 2006;98(10):1630–6. [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremner JD, Walker JD, Whitfield C, Perry BD, Dube SR, Giles WH. The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174–86. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arborelius L, Eklund MB. Both long and brief maternal separation produces persistent changes in tissue levels of brain monoamines in middle-aged female rats. Neuroscience. 2007;145(2):738–50. doi: 10.1016/j.neuroscience.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol. 1999;160(1):1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood trauma questionnaire manual. Psychological Corporation; San Antoinio, TX: 1998. [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27(2):169–90. doi: 10.1016/s0145-2134(02)00541-0. others. [DOI] [PubMed] [Google Scholar]
- Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet. 2007;16(1):24–35. doi: 10.1093/hmg/ddl441. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB. Association of FKBP5 Polymorphisms and Childhood Abuse with Risk of Posttraumatic Stress Disorder Symptoms in Adults. Jama. 2008;299(11):1–15. doi: 10.1001/jama.299.11.1291. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, Liu W, Gillespie CF, Berg T, Evces M, Newport DJ. Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry. 2008;65(2):190–200. doi: 10.1001/archgenpsychiatry.2007.26. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Depression and the serotonin transporter 5-HTTLPR polymorphism: a review and a hypothesis concerning gene-environment interaction. J Affect Disord. 2008;111(1):1–12. doi: 10.1016/j.jad.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, Miyazaki C, Alexander N, Hotopf M, Cleare AJ. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.48. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter LL, Tyrka AR, McDougle CJ, Malison RT, Owens MJ, Nemeroff CB, Price LH. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsychopharmacology. 2004;29(4):777–84. doi: 10.1038/sj.npp.1300375. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–9. doi: 10.1126/science.1083968. others. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82(2):217–25. doi: 10.1016/j.jad.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci U S A. 1996;93(4):1619–23. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Geracioti TD, Jr., Altemus M, Kling MA. Cerebrospinal fluid monoamine metabolites in fluoxetine-treated patients with major depression and in healthy volunteers. Biol Psychiatry. 1993;33(8-9):636–41. doi: 10.1016/0006-3223(93)90103-k. [DOI] [PubMed] [Google Scholar]
- Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160(8):1453–60. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- Eley TC, Sugden K, Corsico A, Gregory AM, Sham P, McGuffin P, Plomin R, Craig IW. Gene-environment interaction analysis of serotonin system markers with adolescent depression. Mol Psychiatry. 2004;9(10):908–15. doi: 10.1038/sj.mp.4001546. [DOI] [PubMed] [Google Scholar]
- Epstein MP, Satten GA. Inference on haplotype effects in case-control studies using unphased genotype data. Am J Hum Genet. 2003;73:1316–1329. doi: 10.1086/380204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer R, Williams J. Structured clinical interview for DSM-IV (SCID-I) (User's guide and Interview) Research Version. Biometrics Research Department, New York Psychiatric Institute; New York: 1995. [Google Scholar]
- Fitzpatrick KM. Exposure to violence and presence of depression among low-income, African-American youth. J Consult Clin Psychol. 1993;61(3):528–31. doi: 10.1037//0022-006x.61.3.528. [DOI] [PubMed] [Google Scholar]
- Fullilove MT, Heon V, Jimenez W, Parsons C, Green LL, Fullilove RE. Injury and anomie: effects of violence on an inner-city community. Am J Public Health. 1998;88(6):924–7. doi: 10.2105/ajph.88.6.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KL, Thrivikraman KV, Lightman SL, Plotsky PM, Lowry CA. Early life experience alters behavior during social defeat: focus on serotonergic systems. Neuroscience. 2005;136(1):181–91. doi: 10.1016/j.neuroscience.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Coccaro EF, Siever LJ, New AS. Serotonin transporter protein gene polymorphism and personality measures in African American and European American subjects. Am J Psychiatry. 1998;155(10):1332–8. doi: 10.1176/ajp.155.10.1332. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101(2):243–6. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Gibb BE, McGeary JE, Beevers CG, Miller IW. Serotonin transporter (5-HTTLPR) genotype, childhood abuse, and suicide attempts in adult psychiatric inpatients. Suicide Life Threat Behav. 2006;36(6):687–93. doi: 10.1521/suli.2006.36.6.687. [DOI] [PubMed] [Google Scholar]
- Gillespie NA, Whitfield JB, Williams B, Heath AC, Martin NG. The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression. Psychol Med. 2005;35(1):101–11. doi: 10.1017/s0033291704002727. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Lange M, Wolff B, Volzke H, Lucht M, Freyberger HJ, John U, Cascorbi I. Mental and physical distress is modulated by a polymorphism in the 5-HT transporter gene interacting with social stressors and chronic disease burden. Mol Psychiatry. 2005;10(2):220–4. doi: 10.1038/sj.mp.4001555. [DOI] [PubMed] [Google Scholar]
- Heim C, Mletzko T, Purselle D, Musselman DL, Nemeroff CB. The dexamethasone/corticotropin-releasing factor test in men with major depression: role of childhood trauma. Biol Psychiatry. 2008;63(4):398–405. doi: 10.1016/j.biopsych.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. Neurobiology of early life stress: Clinical studies. Semin Clin Neuropsychiatry. 2002;7(2):147–59. doi: 10.1053/scnp.2002.33127. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am J Psychiatry. 2001;158(4):575–81. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592–7. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–26. doi: 10.1086/503850. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal JH, Gelernter J. Social supports and serotonin transporter gene moderate depression in maltreated children. Proc Natl Acad Sci U S A. 2004;101(49):17316–21. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Grasso D, Lipschitz D, Houshyar S, Krystal JH, Gelernter J. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59(8):673–80. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Genetic epidemiology in psychiatry. Taking both genes and environment seriously. Arch Gen Psychiatry. 1995;52(11):895–9. doi: 10.1001/archpsyc.1995.03950230009003. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication. Arch Gen Psychiatry. 2005;62(5):529–35. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Kim YH, Yoon JS. Interactions between life stressors and susceptibility genes (5-HTTLPR and BDNF) on depression in Korean elders. Biol Psychiatry. 2007;62(5):423–8. doi: 10.1016/j.biopsych.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Freeman-Daniels E, Lemos JC, Nunan JD, Lamy C, Akanwa A, Beck SG. Corticotropin-releasing factor increases GABA synaptic activity and induces inward current in 5-hydroxytryptamine dorsal raphe neurons. J Neurosci. 2008;28(48):12927–37. doi: 10.1523/JNEUROSCI.2887-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby LG, Rice KC, Valentino RJ. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology. 2000;22(2):148–62. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137(4):1212–8. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- Lambas-Senas L, Mnie-Filali O, Certin V, Faure C, Lemoine L, Zimmer L, Haddjeri N. Functional correlates for 5-HT(1A) receptors in maternally deprived rats displaying anxiety and depression-like behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2008 doi: 10.1016/j.pnpbp.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Lee R, Geracioti TD, Jr., Kasckow JW, Coccaro EF. Childhood trauma and personality disorder: positive correlation with adult CSF corticotropin-releasing factor concentrations. Am J Psychiatry. 2005;162(5):995–7. doi: 10.1176/appi.ajp.162.5.995. [DOI] [PubMed] [Google Scholar]
- Lee RJ, Gollan J, Kasckow J, Geracioti T, Coccaro EF. CSF corticotropin-releasing factor in personality disorder: relationship with self-reported parental care. Neuropsychopharmacology. 2006;31(10):2289–95. doi: 10.1038/sj.npp.1301104. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274(5292):1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levinson DF. The genetics of depression: a review. Biol Psychiatry. 2006;60(2):84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Li T, Liu X, Zhu ZH, Zhao J, Hu X, Ball DM, Sham PC, Collier DA. No association between (AAT)n repeats in the cannabinoid receptor gene (CNR1) and heroin abuse in a Chinese population. Mol Psychiatry. 2000;5(2):128–30. doi: 10.1038/sj.mp.4000670. [DOI] [PubMed] [Google Scholar]
- Livak KJ. Allelic discrimination using fluorogenic probes and the 5' nuclease assay. Genet Anal. 1999;14(5-6):143–9. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Hale MW, Plant A, Windle RJ, Shanks N, Wood SA, Ingram CD, Renner KJ, Lightman SL, Summers CH. Fluoxetine inhibits corticotropin-releasing factor (CRF)-induced behavioural responses in rats. Stress. 2008:1. doi: 10.1080/10253890802309861. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Coplan JD, Smith EL, Scharf BA, Owens MJ, Nemeroff CB, Mann JJ, Gorman JM, Rosenblum LA. Cerebrospinal fluid concentrations of biogenic amines and corticotropin-releasing factor in adolescent non-human primates as a function of the timing of adverse early rearing. Stress. 2002;5(3):185–93. doi: 10.1080/1025389021000010521. [DOI] [PubMed] [Google Scholar]
- Matthews K, Dalley JW, Matthews C, Tsai TH, Robbins TW. Periodic maternal separation of neonatal rats produces region- and gender-specific effects on biogenic amine content in postmortem adult brain. Synapse. 2001;40(1):1–10. doi: 10.1002/1098-2396(200104)40:1<1::AID-SYN1020>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Muglia P, Tozzi F, Galwey NW, Francks C, Upmanyu R, Kong XQ, Antoniades A, Domenici E, Perry J, Rothen S. Genome-wide association study of recurrent major depressive disorder in two European case-control cohorts. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.131. others. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Durrant C, Lewis G, Flint J. Gene x Environment Interactions at the Serotonin Transporter Locus. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Mol Psychiatry. 2000;5(1):32–8. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- Nikisch G, Agren H, Eap CB, Czernik A, Baumann P, Mathe AA. Neuropeptide Y and corticotropin-releasing hormone in CSF mark response to antidepressive treatment with citalopram. Int J Neuropsychopharmacol. 2005;8(3):403–10. doi: 10.1017/S1461145705005158. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18(3):195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Thrivikraman KV, Nemeroff CB, Caldji C, Sharma S, Meaney MJ. Long-term consequences of neonatal rearing on central corticotropin-releasing factor systems in adult male rat offspring. Neuropsychopharmacology. 2005;30(12):2192–204. doi: 10.1038/sj.npp.1300769. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, Sugden K, Uher R, Poulton R, Moffitt TE. Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry. 2009;66(9):978–85. doi: 10.1001/archgenpsychiatry.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ML, Curtis AL, Kirby LG, Valentino RJ, Lucki I. Effects of corticotropin-releasing factor on brain serotonergic activity. Neuropsychopharmacology. 1998;18(6):492–502. doi: 10.1016/S0893-133X(97)00197-8. [DOI] [PubMed] [Google Scholar]
- Price ML, Lucki I. Regulation of serotonin release in the lateral septum and striatum by corticotropin-releasing factor. J Neurosci. 2001;21(8):2833–41. doi: 10.1523/JNEUROSCI.21-08-02833.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainnie DG. Serotonergic modulation of neurotransmission in the rat basolateral amygdala. J Neurophysiol. 1999;82(1):69–85. doi: 10.1152/jn.1999.82.1.69. [DOI] [PubMed] [Google Scholar]
- Richardson J, Steiger H, Schmitz N, Joober R, Bruce KR, Israel M, Gauvin L, Anestin AS, Dandurand C, Howard H, de Guzman R. Relevance of the 5-HTTLPR polymorphism and childhood abuse to increased psychiatric comorbidity in women with bulimia-spectrum disorders. J Clin Psychiatry. 2008;69(6):981–90. doi: 10.4088/jcp.v69n0615. [DOI] [PubMed] [Google Scholar]
- Rinne T, Westenberg HG, den Boer JA, van den Brink W. Serotonergic blunting to meta-chlorophenylpiperazine (m-CPP) highly correlates with sustained childhood abuse in impulsive and autoaggressive female borderline patients. Biol Psychiatry. 2000;47(6):548–56. doi: 10.1016/s0006-3223(99)00181-x. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis. JAMA. 2009;301(23):2462–71. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. Self-rated childhood emotional neglect and CSF monoamine indices in abstinent cocaine-abusing adults: possible implications for suicidal behavior. Psychiatry Res. 2002;112(1):69–75. doi: 10.1016/s0165-1781(02)00176-2. [DOI] [PubMed] [Google Scholar]
- Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32(9):2046–52. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- Schwartz A, Bradley RG, Sexton M, Sherry A, Ressler KJ. Posttraumatic stress disorder among African Americans in an inner city mental health clinic. Psychiatr Serv. 2005;56(2):212–5. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- Schwartz AC, Bradley R, Penza KM, Sexton M, Jay D, Haggard PJ, Garlow SJ, Ressler KJ. Pain medication use among patients with posttraumatic stress disorder. Psychosomatics. 2006;47(2):136–42. doi: 10.1176/appi.psy.47.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoor BH, Chalmers D. Co-victimization of African-American children who witness violence: effects on cognitive, emotional, and behavioral development. J Natl Med Assoc. 1991;83(3):233–8. [PMC free article] [PubMed] [Google Scholar]
- Sham PC, Rijsdijk FV, Knight J, Makoff A, North B, Curtis D. Haplotype association analysis of discrete and continuous traits using mixture of regression models. Behav Genet. 2004;34(2):207–14. doi: 10.1023/B:BEGE.0000013734.39266.a3. [DOI] [PubMed] [Google Scholar]
- Steiger H, Gauvin L, Israel M, Koerner N, Ng Ying Kin NM, Paris J, Young SN. Association of serotonin and cortisol indices with childhood abuse in bulimia nervosa. Arch Gen Psychiatry. 2001;58(9):837–43. doi: 10.1001/archpsyc.58.9.837. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, de Geus EJ, Willemsen G, James MR, Smit JH, Zandbelt T, Arolt V, Baune BT, Blackwood D, Cichon S. Genome-wide association for major depressive disorder: a possible role for the presynaptic protein piccolo. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.125. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Willis-Owen SA, Luben R, Day NE, Flint J. Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder. Biol Psychiatry. 2006;59(3):224–9. doi: 10.1016/j.biopsych.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biol Psychiatry. 2006;60(7):671–6. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Kuikka JT, Bergstrom KA, Karhu J, Viinamaki H, Lehtonen J, Hallikainen T, Yang J, Hakola P. Single-photon emission tomography imaging of monoamine transporters in impulsive violent behaviour. European Journal of Nuclear Medicine. 1997;24(10):1253–60. doi: 10.1007/s002590050149. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Gelernter J, Schepker C, Anderson GM, Carpenter LL. Interaction of childhood maltreatment with the corticotropin-releasing hormone receptor gene: effects on hypothalamic-pituitary-adrenal axis reactivity. Biol Psychiatry. 2009;66(7):681–5. doi: 10.1016/j.biopsych.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic-pituitary-adrenal function. Biol Psychiatry. 2008;63(12):1147–54. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R. The implications of gene-environment interactions in depression: will cause inform cure? Mol Psychiatry. 2008;13(12):1070–8. doi: 10.1038/mp.2008.92. [DOI] [PubMed] [Google Scholar]
- Vicentic A, Francis D, Moffett M, Lakatos A, Rogge G, Hubert GW, Harley J, Kuhar MJ. Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience. 2006;140(1):355–65. doi: 10.1016/j.neuroscience.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Wichers M, Kenis G, Jacobs N, Mengelers R, Derom C, Vlietinck R, van Os J. The BDNF Val(66)Met x 5-HTTLPR x child adversity interaction and depressive symptoms: An attempt at replication. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(1):120–3. doi: 10.1002/ajmg.b.30576. [DOI] [PubMed] [Google Scholar]