Abstract
Schizophrenia has often been conceived as a disorder of connectivity between components of large-scale brain networks. We tested this hypothesis by measuring aspects of both functional connectivity and functional network topology derived from resting state fMRI time series acquired at 72 cerebral regions over 17 minutes from 15 healthy volunteers (14 male, 1 female) and 12 people diagnosed with schizophrenia (10 male, 2 female). We investigated between-group differences in strength and diversity of functional connectivity in the 0.06–0.125 Hz frequency interval, and some topological properties of undirected graphs constructed from thresholded inter-regional correlation matrices. In people with schizophrenia, strength of functional connectivity was significantly decreased; whereas diversity of functional connections was increased. Topologically, functional brain networks had reduced clustering and small-worldness, reduced probability of high degree hubs and increased robustness in the schizophrenic group. Reduced degree and clustering were locally significant in medial parietal, premotor and cingulate, and right orbitofrontal cortical nodes of functional networks in schizophrenia. Functional connectivity and topological metrics were correlated with each other and with behavioural performance on a verbal fluency task. We conclude that people with schizophrenia tend to have a less strongly integrated, more diverse profile of brain functional connectivity, associated with a less hub-dominated configuration of complex brain functional networks. Alongside these behaviourally disadvantageous differences, however, brain networks in the schizophrenic group also showed a greater robustness to random attack, pointing to a possible benefit of the schizophrenia connectome, if less extremely expressed.
Keywords: psychosis, functional magnetic resonance imaging, brain systems, small-world, graph theory, verbal fluency
Introduction
It was first proposed by 19th century pioneers such as Theodor Meynert (1833–1892) and Carl Wernicke (1848–1905) that psychotic disorders might arise from abnormal axonal connectivity between anatomically dissected cortical regions. This seminal hypothesis of disconnection or cortical mis-wiring in psychosis (Catani and ffytche, 2005; Catani and Mesulam, 2008), based on morbid anatomy and clinical intuition, has more recently been generalized to the concept of dysconnectivity: abnormal relationships between neurons, at multiple scales of space and time, compatible with - but not necessarily implying - anatomical disconnection (Volkow et al., 1988; Weinberger et al., 1992; Friston and Frith, 1995; Friston, 1996; Bullmore et al., 1997). Dysconnectivity in schizophrenia is considered an intermediate disease phenotype, conceivably attributable to various degenerative, developmental and/or genetic mechanisms (Meyer-Lindenberg and Weinberger, 2006). One distinctive and mechanistically plausible hypothesis links functional dysconnectivity at the macro-scale of neuroimaging to abnormal synaptic modulation at the micro-scale of cellular signaling (Stephan et al., 2009).
Meta-analytic reviews of MRI studies of schizophrenia have provided strong evidence for abnormal grey matter density increases in basal ganglia, and decreases in bilateral frontal, cingulate, temporal and insular cortex, and thalamus (Ellison-Wright et al., 2008; Glahn et al., 2008). Diffusion tensor imaging (DTI) studies of white matter organization have replicably found reduced anisotropy of diffusion in left frontal and temporal lobes (Ellison-Wright and Bullmore, 2009). Convergent evidence across diverse cognitive task conditions also indicates abnormal fMRI activation of dorsal and ventral prefrontal, anterior cingulate, and posterior cortical regions (Minzenberg et al., 2009). The most parsimonious explanation of this pattern of multiple local structural and functional abnormalities is that schizophrenia is represented at the scale of neuroimaging by a disconnected configuration of these grey matter regions and their interconnecting white matter tracts in MRI and DTI data, which is somehow reflected in abnormal functional connectivity in fMRI data.
More direct support for the functional dysconnectivity hypothesis comes from resting state fMRI studies of disorder-related differences in inter-regional functional connectivity (Liang et al., 2006; Bluhm et al., 2007; Zhou et al., 2007b; Zhou et al., 2007a; Jafri et al., 2008; Whitfield-Gabrieli et al., 2009; Salvador et al., 2010; Fornito and Bullmore, 2010), defined as the statistical association between spatially distributed neurophysiological time-series (Friston, 1994). In parallel, graph theoretic measurements of the topological properties of complex brain networks have found that they are less hierarchical, less small-world, less clustered and less efficiently wired in schizophrenia (Liu et al., 2008; Bassett et al., 2008; Bullmore and Sporns, 2009). These differences might be expected to impair higher-order cognitive functions demanding access to large, integrated neuronal workspaces (Dehaene and Naccache, 2001). Working memory impairments have been linked to reduced cost-efficiency of magnetoencephalographic networks in schizophrenia (Bassett et al., 2009). However, if the functional consequences of altered topology in schizophrenia are entirely negative, why have evolutionary processes not selected against risk genes for this highly heritable disorder?
We measured functional connectivity and network metrics in no-task fMRI data recorded from 15 healthy volunteers and 12 people with schizophrenia, and we investigated how brain functional organization was expressed in terms of these various, inter-dependent metrics, and how it related to cognitive function; see Figure 1 for schematic overview.
Figure 1. Schematic of fMRI data analysis pipeline.
Regional mean fMRI time series were estimated by applying a prior anatomical template image to each individual fMRI dataset after its co-registration with the template in standard space; wavelet analysis was used to bandpass filter the regional time series and to estimate frequency-specific measures of functional connectivity between regions; functional connectivity matrices were thresholded to generate binary undirected graphs or brain functional networks; between-group differences in functional connectivity, principal components and network topological metrics were assessed by permutation testing.
Methods and Materials
Sample
We recruited 15 healthy (non-psychotic) volunteers (14 male, 1 female) and 12 people with chronic schizophrenia (10 male, 2 female), diagnosed according to standard operational criteria in the Diagnostic and Statistical Manual of Mental Disorders IV (American Psychiatric Association, 2000). The two groups were matched for age, premorbid IQ estimated using the National Adult Reading Test (Nelson, 1992), and years of education. Symptom severity scores were measured using the PANSS scale (Kay et al., 1987). For subject details see Table 1. All patients were receiving antipsychotic drugs and four were receiving additional psychotropic medication. The average (± standard deviation) dose, in chlorpromazine equivalents (Woods, 2003; Bazire, 2005), was 487 ± 433 mg/day. To mitigate acute drug effects on fMRI data, patients did not receive their usual medication on the day of scanning. Healthy volunteers were screened for major psychiatric disorders using the Mini International Neuropsychiatric Interview (Sheehan et al., 1998); none were taking psychoactive medication. All subjects provided informed consent in writing and the protocol was approved by the Addenbrooke’s NHS Trust Local Research Ethics Committee.
Table 1.
Demographic and clinical characteristics of the sample.
| Healthy volunteers (N=15) | People with schizophrenia (N=12) | |
|---|---|---|
| Mean ± SD | Mean ± SD | |
| Age (years) | 33.3 ±9.2 | 32.8 ±9.2 |
| Premorbid NART IQ | 113 ±6 | 112 ±9 |
| Years of education | 13.3 ±6.4 | 12.8 ±2.4 |
| Gender | 14 male, 1 female | 10 male, 2 female |
| Symptom severity (PANSS scale) | - | Positive: 4.0 ±4.2 Negative: 8.3 ±5.4 General: 15.4 ±9.8 |
Cognitive testing
The FAS version of the Controlled Oral Word Association Test (Benton et al., 1976) was used to assess verbal fluency. The participant was asked to say as many words as possible beginning with the letter F, A or S within 1 minute and the test score was simply the total number of words generated. Forward and backward digit span was assessed by Wechsler Memory Scale-III Digit Span (Wechsler, 1997).
Acquisition and preprocessing of fMRI data
Functional MRI data were acquired while subjects were lying quietly in the scanner with eyes closed for 17 mins 12 s. We used a GE Signa system (General Electric, Milwaukee WI) operating at 1.5T at the BUPA Lea Hospital, Cambridge, UK. In each session, 516 gradient-echo T2*-weighted echo planar images depicting blood oxygenation level dependent contrast were acquired from 16 non-contiguous near axial planes: repetition time = 2 s, echo time = 40 ms, flip angle = 70°, voxel size = 3.05 × 3.05 × 7.00 mm, section skip = 0.7 mm, matrix size = 64 × 64, field of view = 240 × 240 × 123 mm. The first 4 volumes were discarded to allow for T1 equilibration effects leaving 512 volumes per session. Each dataset was corrected for head movement by realignment and regression (Suckling et al., 2006) and subsequently registered to MNI stereotactic standard space by a 12 parameter affine transform maximizing normalized correlation with a customised EPI template image (within-modality). Registered images were spatially smoothed with a Gaussian kernel (6mm at full width half-maximum) and the time-series were high-pass filtered (cut-off frequency: 1/120 ~0.008 Hz).
Anatomical parcellation and wavelet decomposition
For each individual dataset, up to 90 regional mean time series were estimated by averaging voxel time series within each of the 90 anatomically defined regions (excluding the cerebellum) comprising the Automated Anatomical Labelling (AAL) template image (Tzourio-Mazoyer et al., 2002). Owing to the limited FOV size in the z-dimension, cerebellar regions had to be omitted in order to ensure sufficient coverage at the top of the brain. Regional time series were only included in further analysis if good quality fMRI data were available for more than 50% of subjects; due to susceptibility artefacts at the base of the brain, this criterion excluded 18 regions from consideration (Supplementary Table 1), leaving a complete dataset of 72 regions (Supplementary Table 2) for all participants.
The maximal overlap discrete wavelet transform (MODWT; (Percival and Walden, 2000)) was used to decompose each individual regional mean fMRI time series into the following scales or frequency intervals: scale 1, 0.125–0.250 Hz; scale 2, 0.060–0.125 Hz; scale 3, 0.030–0.060 Hz; and scale 4, 0.015–0.030 Hz. Following initial analyses of functional connectivity at all scales (Table 2), subsequent analysis focused on data at scale 2, which is compatible with prior studies indicating that endogenous fMRI dynamics of neuronal origin are most salient at frequencies < 0.1 Hz.
Table 2.
Global functional connectivity measures and associated group differences at different frequency intervals.
| Wavelet Correlation | ||||
|---|---|---|---|---|
| Repeated measures ANOVA | Group effect: F = 3.944, p = 0.0581, df = 1 Freq. band effect: F = 81.15, p < 0.0001, df = 3 Group*freq. band interaction: F = 0.6754, p = 0.5704, df = 3 |
|||
| Frequency band (Hz) | Healthy | Schizophrenia | t-test (df = 25) | Permutation test |
| 0.125–0.250 | 0.3213 | 0.2752 | p = 0.1899, t = 1.3473 | p = 0.1045 |
| 0.060–0.125 | 0.4238 | 0.3289 | p = 0.0123, t = 2.6974 | p = 0.0070 |
| 0.030–0.060 | 0.5063 | 0.4382 | p = 0.1235, t = 1.5938 | p = 0.0610 |
| 0.015–0.030 | 0.5806 | 0.5119 | p = 0.1761, t = 1.3924 | p = 0.0905 |
| Wavelet Mutual Information | ||||
|---|---|---|---|---|
| Repeated measures ANOVA | Group effect: F = 2.229, p = 0.1479, df = 1 Freq. band effect: F = 79.83, p < 0.0001, df = 3 Group*freq. band interaction: F = 0.5857, p = 0.6262, df = 3 |
|||
| Frequency band (Hz) | Healthy | Schizophrenia | t-test (df = 25) | Permutation test |
| 0.125–0.250 | 0.0383 | 0.0344 | p = 0.3812, t = 0.8914 | p = 0.2130 |
| 0.060–0.125 | 0.0528 | 0.0407 | p = 0.0305, t = 2.2933 | p = 0.0130 |
| 0.030–0.060 | 0.0720 | 0.0625 | p = 0.3065, t = 1.0440 | p = 0.1580 |
| 0.015–0.030 | 0.0983 | 0.0849 | p = 0.2543, t = 1.1668 | p = 0.1345 |
Functional connectivity metrics
At each scale, the wavelet correlation, −1 ≤ ri,j ≤ +1, and mutual information, mi,j ≥ 0, were estimated between each possible {i, j} pair of regions. Although wavelet correlations can be negative in these (and other) fMRI data, we have found that they are almost always positive (Supplementary Figure 1). This is an indication that connections between regions are not predominantly conferred through anti-correlation which we would have had to treat separately otherwise. Connectivity strength, R̄, and average mutual information, M̄, were defined for each subject as the mean of all pairwise correlations or mutual informations respectively.
Connectivity strength is a global measure of connectivity. The regional strength of connectivity R̄ (i) was likewise defined for the ith region as the average of the correlations between it and all other regions in the brain:
| (1) |
The regional diversity of connections, Var(R(i)), was defined as the variance of the correlations between the ith index region and all other regions:
| (2) |
Globally, connectivity diversity was defined as the average regional diversity across the 72 brain regions.
Principal component analysis (PCA) was performed on the scale 2 wavelet coefficients and a measure of global integration (Tononi et al., 1994; Friston, 1996) was estimated by the ratio of the first eigenvalue to the sum of all other eigenvalues: .
Functional network metrics
Undirected graphs were constructed from the scale 2 wavelet correlation matrices (Achard et al., 2006; Achard and Bullmore, 2007; Meunier et al., 2009); see (Bullmore and Sporns, 2009) for a general review of graph theory in relation to neuroscience. Any correlation ri,j in the functional connectivity matrix  greater than a given threshold, τ, was retained as an edge connecting regions i and j in the adjacency matrix
greater than a given threshold, τ, was retained as an edge connecting regions i and j in the adjacency matrix  ; if ri;j < τ no edge connects regions i and j. Graphs of different connection densities or costs are produced by thresholding at different values of τ; the connection density is the number of edges in a graph comprising N nodes divided by the maximum number of possible edges (
). When studying the topological properties of such graphs across a number of individuals, we prefer to consider graphs that are fully connected for all subjects (i.e. degree k(i) > 1 for all nodes) and that have non-random topological organizational properties. These criteria defined a regime of cost or connection densities in the range 37–50%: below a connection density of 37% some graphs began to fragment and above a connection density of 50% graph topology becomes increasingly random (Humphries et al., 2006) and less small-world. Work on brain connectivity in macaques (Kaiser and Hilgetag, 2006) suggests that connections at higher costs are likely to be non-biological. All network results reported in this study are thus averages of the various metrics estimated for each individual network over a range of connection densities 37%–50% (14 values, 1% increments) (Bassett et al., 2008). The following graph metrics were estimated:
; if ri;j < τ no edge connects regions i and j. Graphs of different connection densities or costs are produced by thresholding at different values of τ; the connection density is the number of edges in a graph comprising N nodes divided by the maximum number of possible edges (
). When studying the topological properties of such graphs across a number of individuals, we prefer to consider graphs that are fully connected for all subjects (i.e. degree k(i) > 1 for all nodes) and that have non-random topological organizational properties. These criteria defined a regime of cost or connection densities in the range 37–50%: below a connection density of 37% some graphs began to fragment and above a connection density of 50% graph topology becomes increasingly random (Humphries et al., 2006) and less small-world. Work on brain connectivity in macaques (Kaiser and Hilgetag, 2006) suggests that connections at higher costs are likely to be non-biological. All network results reported in this study are thus averages of the various metrics estimated for each individual network over a range of connection densities 37%–50% (14 values, 1% increments) (Bassett et al., 2008). The following graph metrics were estimated:
Degree, k(i), is simply equal to the number of edges connecting the ith region to the rest of the network:
| (3) |
where  is the binary adjacency matrix obtained by thresholding the functional connectivity matrix,
is the binary adjacency matrix obtained by thresholding the functional connectivity matrix,  .
.
Regional efficiency, E(i), (Achard and Bullmore, 2007; Latora and Marchiori, 2001) is computed for each node in a graph, G, as:
| (4) |
Here Li,j is the minimum path length between regions i and j. Global efficiency, E(G), is the mean regional efficiency over all nodes.
Clustering coefficient, C(i), of a node v is the ratio of connected triangles, δv to connected triples τv The clustering coefficient of a graph is:
| (5) |
where V′ is the set of nodes with degree > 2 (Watts and Strogatz, 1998; Schank and Wagner, 2005). Small-worldness, σ, is a property of a network with high clustering, C, but low characteristic path length, L, compared to the clustering, CR, and path length, LR, of a comparable random graph (Watts and Strogatz, 1998; Humphries et al., 2006). Path length can be estimated as the inverse of global efficiency (Latora and Marchiori, 2001), allowing the following formulation (Achard and Bullmore, 2007) of small-worldness:
| (6) |
where E(G)R is the global efficiency of a comparable random graph. A network is said to be “small-world” when σ > 1.
Robustness, ρ, indicates the network’s resilience to either targeted, ρt, or random, ρr, attack. In a targeted attack, hubs are removed one by one in order of degree, k, while in a random attack, nodes are removed at random independent of their degree. Each time a node was removed from the network, we re-calculated the size of the largest connected component, s. Robustness is then usually visualized by a plot of the size of the largest connected component, s, versus the number of nodes removed, n (Achard et al., 2006); Supplementary Figure 2. The robustness parameter, ρ, is defined as the area under this s versus n curve. More robust networks retain a larger connected component even when several nodes have been knocked out, as represented by a larger area under the curve or higher values of ρ.
Degree distribution parameters for graphs at a cost of 37% were estimated using the nonlinear fitting function in “Brainwaver” software (http://cran.r-project.org/(Achard, 2007)). For each subject, goodness of fit of the degree distribution to three laws (exponential, P(k) ~ e−αk; power, P(k) ~ k−α; and truncated power, P(k) ~ kα−1ek/kc) was estimated using Akaike’s information criterion. The exponentially-truncated power law was the best fit for all subjects and the parameters of this distribution (the power exponent, α, and the lower exponential degree cut-off, kc) were estimated for each subject.
Correlations between variables
We explored associations between all the functional connectivity, PCA-based and graph theoretical metrics considered in the analysis of fMRI data (12 in total; see Table 3), simply using Pearson’s correlation coefficient to estimate the association between each pair of variables over all subjects in the study (N= 27); see Figure 5. In general, all the fMRI metrics were (positively or negatively) correlated with each other, see Supplemental Table 3 for details. As reported in more detail below, many of the brain functional metrics were also significantly correlated with behavioural variability in terms of verbal fluency scores. In an effort to isolate more specific associations between behavioural variability and brain functional metrics, we also estimated the partial correlations between each pair of variables and tested each of them for significance. We found that partial correlations were generally small and not significant, indicating that we cannot disambiguate any specific associations between behavioural variability and any one of the highly inter-correlated connectivity, PCA or graph metrics considered in analysis of the fMRI data. We also used multivariate analysis of covariance (MANCOVA) to estimate the effects of all functional connectivity and network metrics on the dependent variable of verbal fluency. This analysis also demonstrated that no single metric demonstrated a specific relationship with cognitive performance when the effects of all other connectivity and network metrics were simultaneously considered. These results are reported in full in Supplementary Tables 4 and 5.
Table 3.
Functional connectivity and network topology metrics in the frequency interval 0.06–0.125 Hz for healthy volunteers and people with schizophrenia. P-values refer to the probability of the observed between-group difference under the null hypothesis estimated by a permutation test. SD denotes standard deviation.
| Healthy volunteers Mean ± SD |
People with schizophrenia Mean ± SD |
P-value, permutation test | |
|---|---|---|---|
| Connectivity strength | 0.4238 ±0.0811 | 0.3289 ±0.1018 | p = 0.007 |
| Connectivity diversity | 0.0240 ±0.0047 | 0.0282 ±0.0046 | p = 0.016 |
| Variance 1st PC | 43.1 ±8.4% | 32.6 ±11% | p = 0.005 |
| Global efficiency | 0.7439 ±0.0044 | 0.7475 ±0.0030 | p = 0.009 |
| Average clustering | 0.7423 ±0.0364 | 0.6917 ±0.0562 | p = 0.005 |
| Hierarchy | 0.0371 ±0.0086 | 0.1013 ±0.0107 | p = 0.010 |
| Degree distr. (variance) | 183 ±38 | 120 ±43 | p < 0.0001 |
| Degree distr. (power exponent) | 3.259 ±1.919 | 6.116 ±3.427 | p = 0.005 |
| Degree distr. (degree cut-off) | 9.450 ±2.896 | 5.373 ±2.383 | p = 0.0005 |
| Small-worldness | 1.6144 ±0.0745 | 1.5300 ±0.1184 | p = 0.015 |
| Robustness (random attack) | 2.534e3 ±11 | 2.544e3 ±5 | p = 0.001 |
| Robustness (targeted attack) | 2.454e3 ±44 | 2.480e3 ±39 | p = 0.065 |
| Verbal fluency | 15.27 ±3.86 | 13.25 ±5.26 | p = 0.1065 |
Figure 5. Matrix of correlations between global functional connectivity metrics, topological metrics and verbal fluency score across all participants.
Non-significant correlations (p < 0.05) are left blank. The inset shows a scatterplot of verbal fluency versus connectivity strength, where the lines indicate the best linear fits for the data within each group (red = people with schizophrenia; black = healthy volunteers) and for the data pooled over both groups (green). For details, see Supplementary Table 3.
Cortical surface rendering
Caret v5.61 software (Van Essen et al., 2001) (with Atlas map (Van Essen, 2005)) was used to make cortical surface representations of the distributions of regional strength, regional diversity, degree, and clustering. The value plotted at a given point is the value of the AAL volume at a point below the surface at the level of cortical layer 4. We tested the significance of the group differences in these metrics at each region using two-sample t-tests with a false positive correction P < (1/N) = 0.014; which is equivalent to saying that we expect less than one false positive regional result per cortical map at this threshold. We note that this correction for multiple comparisons is not as conservative as a Bonferroni or false discovery rate correction and therefore we do not claim strong type I error control for these multiple exploratory analyses at a regional level of network organization.
Results
Functional connectivity: strength, diversity and global integration
We measured the statistical association between spatially distributed pairs of regional fMRI time series using two metrics of frequency-specific functional connectivity. The wavelet correlation is a measure of the linear association between processes in a wavelet scale-specific frequency interval; the wavelet mutual information is a scale-specific measure of linear and non-linear dependencies between processes. By both metrics, we found that the magnitude or strength of functional connectivity was greater at lower frequencies; see Table 2 and Figure 2. This trend for bivariate correlations to be greater at lower frequencies is typical of the broad class of multivariate long memory time series models and is linked to the colored noise or persistent autocorrelation structure of a single fMRI time series (Achard et al., 2008). As anticipated by previous studies on resting state networks in fMRI (Achard et al., 2006), the difference between schizophrenic and comparison groups was most salient by both metrics in the frequency interval 0.06–0.125 Hz (Table 2). Subsequent analysis focused in more detail on functional connectivity and networks based on the wavelet correlation matrices at this scale.
Figure 2. Functional connectivity matrices and group differences in global connectivity.
Matrices of pairwise correlations at 0.060–0.125 Hz for individual participants: (A) healthy controls and (B) people with schizophrenia. Both axes represent the 72 regions used in the analysis, ordered by average strength in healthy subjects, and pixel color represents the level of correlation. (C) Connectivity strength, R̄, and (D) average mutual information, M̄, at four different wavelet scales for healthy volunteers (black) and people with schizophrenia (red). The group differences were significant at the wavelet scale 0.060–0.125 Hz for connectivity strength and mutual information (Table 2). Error bars indicate standard deviation.
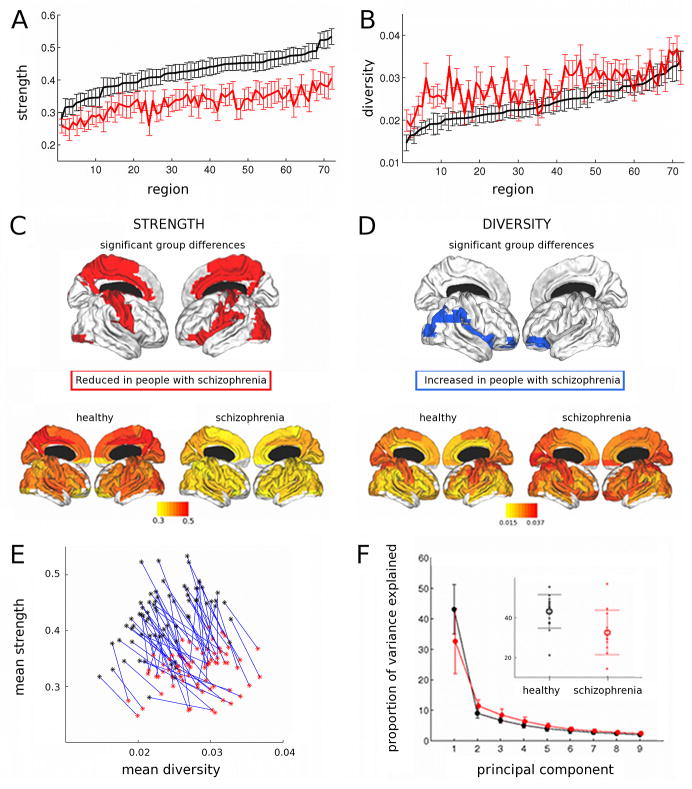
For each of 72 anatomically-defined brain regions, we estimated the strength and diversity (or variability (Campbell et al., 1986)) of its functional connectivity to the rest of the brain in each individual dataset. Functional connectivity strength was generally greater, and ranged more widely over different brain regions, in healthy volunteers than in people with schizophrenia; see Table 3 and Figure 3(A). Connectivity strength was significantly reduced in the schizophrenic group at a regional level in medial premotor, cingulate and parietal cortex, pre- and post-central cortex, occipital association cortex, and left inferior frontal, superior temporal and insular cortex; see Figure 3(B) and Supplementary Table 6. In contrast, the diversity of functional connections was significantly increased, on average over all regions, in the schizophrenic group (see Table 3, rFigure 3(C) and Supplementary Table 7). This difference was also significant at a regional level in orbitofrontal, insular and parietal association cortex; see Figure 3(D) and Supplementary Table 6. These two aspects of regional connectivity were negatively correlated over all subjects ( = −0.4; df = 25; p = 0.04; see Figure 3(E), Figure 5 and Supplementary Table 3). In other words, greater strength of connectivity was associated with reduced diversity of functional connections.
Figure 3. Group differences in regional connectivity metrics and global integration.
(A) Group mean connectivity strength for each of the 72 regions, ordered by mean regional strength in healthy volunteers; error bars indicate standard error of the mean. (B) Regional diversity of correlations, ordered by mean diversity in healthy volunteers; error bars indicate standard error of the mean. (C) Cortical surface renderings of strength. (D) Cortical surface renderings of diversity. Regions showing a significant group difference in the metric when corrected for multiple comparisons using false positive correction (P < .014) are indicated. (E) Graph to show link between group differences in strength and diversity for individual regions. Lines connect equivalent anatomical regions in healthy volunteers (black) and people with schizophrenia (red). (F) Principal components analysis: scree plot of the proportion of variance explained by successive principal components in people with schizophrenia (red) and healthy volunteers (black). Inset shows the group difference in the proportion of variance explained by the first principal component. Error bars indicate standard deviation. For details, see Table 3 and Supplementary Tables 6 and 7.
Using principal component (PC) analysis to provide a measure of the global integration of functional activity in each dataset, we found that the percentage of variance accounted for by the first PC was significantly reduced in people with schizophrenia (33%) compared to healthy volunteers (43%); Figure 3(F) and Table 3.
Taken overall, these results indicate that strength of brain functional connectivity is reduced, and that individual regions have a more diverse or less globally coordinated mode of connectivity to the rest of the brain, in people with schizophrenia.
Functional networks: topology, degree distributions and robustness
To complement these results based on analysis of continuous measures of association between regions, we also measured the topological properties of binary (unweighted and undirected) graphs derived by thresholding the individual functional connectivity matrices.
At a global level, functional networks expressed some key organizational properties consistently across both groups. All individual networks had economical small-world properties, i.e., high local and global efficiency, and broad scale degree distributions consistent with the existence of “hubs”. However, the quantitative values of many of these topological metrics were significantly different between groups; see Table 3 and Figure 4.
Figure 4. Group differences in topological properties of brain functional networks.
(A) Pooled degree distributions and (B) cumulative degree distributions for healthy volunteers (black) and people with schizophrenia (red), showing lower probability of high-degree network hubs in schizophrenia. Cortical surface renderings of (C) degree, and (D) clustering. Regions showing a significant group difference in the metric when corrected for multiple comparisons using false positive correction (P < .014) are indicated. For details, see Table 3 and Supplementary Table 8.
Clustering and small-worldness were significantly reduced by about 5%, and global efficiency was significantly increased by < 1%, in the schizophrenic group. It was also notable that although the degree distributions of both groups were broadly similar, there were visible differences between them (Figure 4(A) and 4 (B)): both higher degree hubs, and lower degree nodes, were more probable in the healthy brain networks; whereas a greater proportion of nodes had modal degree in the schizophrenic brain networks. This was reflected by significantly higher brain-wide variance of regional degree in healthy volunteers; Table 3.
At a regional level of analysis, we mapped clustering and degree for each cortical node of the network and compared nodal clustering and degree between groups; Figure 4. Consistent with the between-group differences in global topology, clustering was reduced for most cortical nodes in the schizophrenic group although this difference was only significant for medial posterior parietal and anterior cingulate regions. Degree was also significantly reduced in medial posterior parietal and premotor cortex, and significantly increased in right orbitofrontal cortex, in the schizophrenic group; Supplementary Table 8.
Possible forms of the degree distribution were evaluated more rigorously using Akaike’s information criterion (AIC) as a measure of comparative goodness of fit for three possible degree distributions: a power law, P(k) ~ k−α; an exponential, P(k) ~ e−αk; and an exponentially truncated power law, P(k) ~ kα −1ek/kc. Of these, the exponentially truncated power law was the best-fitting model for the degree distribution for all subjects in both groups. We compared the parameters of this distribution between the two groups (Table 3) and found that the exponential cut-off degree (kc) was significantly lower in the schizophrenic group. This indicates that the transition from the scaling regimen to the exponential fall-off occurred at a lower degree in the schizophrenic group, which corresponds to a relative loss of hubs. We also found that the power exponent (α) was significantly higher in the schizophrenic group. Together with the lower exponential cut-off degree, this reflects the narrower degree distribution in the schizophrenic group.
We also investigated the robustness of the networks to random error (removal of nodes in random order) and targeted attack (removal of nodes in descending order of degree). Under both conditions, schizophrenic networks demonstrated greater robustness and this was statistically significant for robustness to random error (Table 3, Supplementary Figure 1). Robustness was significantly negatively correlated with connectivity strength, global integration and degree distribution parameters (Figure 5, Supplementary Table 3).
Relationships between functional connectivity, functional networks and behaviour
Functional connectivity and network topology metrics were generally highly correlated (Figure 5, Supplementary Table 3). For example, greater strength and global integration of functional connectivity were positively correlated with greater small-worldness, greater clustering and changes in degree distribution parameters indicating a higher probability of high degree hubs.
Moreover, both connectivity and topological metrics were related to variability of behavioural performance on a test of verbal fluency. Greater fluency was positively correlated with greater connectivity strength and integration, greater small-worldness and clustering, and a more hub-dominated degree distribution. These associations were statistically significant when the correlations were tested pooling data from both groups (Figure 5, Supplementary Table 3) and the pattern of results was conserved when testing each group separately although many of the within-group correlations were not statistically significant due to smaller sample size. Partial correlations, which estimate the component of covariation specifically attributable to the direct interaction between each pair of variables, were generally smaller than Pearson’s correlations, and not statistically significant; see Supplemental Table 4 for details.
There were no significant associations between any connectivity or topological metrics and either forward or backward digit span scores. There were also no significant associations, within the patient group, between clinical symptom severity (measured using the PANSS scale (Kay et al., 1987)) or current dose of atypical antipsychotic medication, and any of the brain functional measures.
Discussion
These results corroborate and extend prior studies indicating that brain systems measured by resting state fMRI are abnormally organized in schizophrenia, as anticipated by theories of schizophrenia as a functional dysconnectivity syndrome.
Functional connectivity and networks in schizophrenia
One novel aspect of the study is that it is the first, we believe, to report a pathophysiological profile for schizophrenia in terms of both connectivity and topological metrics. Given that the topological metrics are estimated on a binary adjacency matrix constructed by thresholding the continuous association matrix of inter-regional connectivity measures, one would expect these two sets of metrics to be related, and indeed they were. For example, both strength and global integration of connectivity were positively correlated with small-worldness and clustering; whereas diversity of connections was negatively correlated with clustering. Functional networks in both groups consistently demonstrated small-world and other topological properties that have previously been described in normal human and non-human brain networks, and are likely to represent highly conserved principles of brain network architecture (Bassett and Bullmore, 2009; Bullmore and Sporns, 2009). Here, the shift to reduced strength and greater diversity of functional connectivity in the schizophrenic group was associated with a less clustered and hub-dominated network topology.
Some of these findings directly replicate prior fMRI and EEG reports of reduced functional connectivity, globally or regionally (Liang et al., 2006; Bluhm et al., 2007; Liu et al., 2008), or reduced clustering and small-worldness of functional networks (Micheloyannis et al., 2006; Liu et al., 2008; Rubinov et al., 2009) in schizophrenia. Notably, however, other studies have reported regionally increased functional connectivity (Zhou et al., 2007b; Zhou et al., 2007a; Whitfield-Gabrieli et al., 2009; Salvador et al., 2010). It is unclear why some studies should report predominantly decreased connectivity, and others increased connectivity. However, between-study differences in defining regions of interest or network nodes, differences in pre-processing strategies and connectivity metrics, and the inherent variability in small-medium sized patient samples, may all play a role. Larger and methodologically more comparable future studies will be useful.
Some of our other findings are consistent with, rather than directly replicable of, prior observations based on somewhat different metrics. For example, our finding of reduced probability of high degree hubs is compatible with previous observations of reduced degree and centrality of network hubs in schizophrenia (Rubinov et al., 2009). Likewise, our observation of greater diversity of connectivity between a single region and the rest of the brain (Figure 3) seems compatible with prior observations of reduced homogeneity of neural activity within a single region in schizophrenia (Liu et al., 2006), given that adjacent subregions with dissimilar activity will likely show dissimilar connectivity, contributing to regional diversity. Connectivity diversity in brain networks has not been previously investigated, although studies of social interactions have used analogous metrics (Knoke and Yang, 2008). In fact, while high between-subject variability in candidate traits of schizophrenia is common (Preston and Weinberger, 2005), previous reports of any disorder-related differences in within-subject variability in fMRI are few (Manoach et al., 2001; Barch et al., 2003; Jafri et al., 2008).
Broadly speaking, many of our results are compatible with the idea that there is a “subtle randomization” of the functional network architecture in schizophrenia (Rubinov et al., 2009). Given that similar shifts to randomness, or de-differentiation, have been described as characteristic of network architectural changes with normal aging (Cabeza, 2001) and in a wide range of other disorders (including brain tumours, epilepsy and Alzheimer’s disease (Stam et al., 2009)), we need to understand more clearly which aspects of a less centralised, more robust network configuration are specific to schizophrenia and which might be common to a group of clinically distinct randomized or de-differentiated network syndromes.
Relationship to other aspects of schizophrenia
We now more speculatively consider how our analytical metrics might relate to the behavioural phenotype in schizophrenia. In this study, all subjects’ performance on a verbal fluency task was correlated with many of the analytical metrics. Verbal fluency tasks test the ability to generate multiple words with a given starting letter, or in a given sematic category, in limited time. Verbal fluency performance in schizophrenia has been shown to predict functional outcomes for independent living (Jaeger et al., 2003) and daily problem solving skills (Revheim et al., 2006; Rempfer et al., 2003). In schizophrenia, verbal fluency is best predicted by psychomotor speed, rather than executive functioning or memory (van Beilen et al., 2004) and processing speed seems to mediate the link between verbal fluency performance and functional outcome in schizophrenia (Ojeda et al., 2008). Our results suggest that impaired verbal fluency, presumably reflecting slower processing speed, is associated with a less strongly connected, less globally integrated, less clustered and hub-dominated brain functional organization. However, we did not find a significant association between task performance and global network efficiency, despite prior data and theory indicating that topological efficiency and cost-efficiency correlate with intelligence and executive task performance (Dehaene and Naccache, 2001; van den Heuvel et al., 2009; Bassett et al., 2009; Li et al., 2009).
Prior evidence suggests that low frequency functional networks are constrained by the topology of underlying anatomical networks (Honey et al., 2007). Thus we would expect the functional dysconnectivity of schizophrenia to be at least partly explicable in terms of anatomical disconnection, as reported in MRI or DTI studies of white matter anatomy (Bassett et al., 2008; Zhou et al., 2008); but this remains to be compellingly demonstrated. Future studies could also further test the contrasting mechanistic hypothesis that functional dysconnectivity and related network metrics are attributable to underlying abnormalities of synaptic plasticity in schizophrenia (Stephan et al., 2009).
These results, and most prior studies, have tended to focus on intuitively or demonstrably disadvantageous aspects of the schizophrenia connectome - such as lower integration of connectivity (Liu et al., 2006), lower clustering and small-worldness (Liu et al., 2008), reduced hierarchy and inefficiently increased wiring distance (Bassett et al., 2008), or reduced cost-efficiency (Bassett et al., 2009). Given the high heritability of schizophrenia, and the theoretically predicted frequency of risk genes in the general population, might there be aspects of the schizophrenia connectome which confer advantages, if expressed less extremely? This could help explain persistence of risk genes despite selection pressures acting against the adverse aspects of the schizophrenia connectome.
One possible advantage identified here is the greater robustness to random attack of functional networks in schizophrenia. This means simply that the whole brain network is less likely to fragment into disconnected islands as regional nodes are removed at random. This could conceivably offer the survival advantage of greater resilience of global brain function in the face of multifocal brain lesions due to disease or injury. We might predict decreased incidence or severity of distributed brain disorders such as Alzheimer’s disease in first-degree relatives of people with schizophrenia. We know of no prior data that can immediately test this hypothesis directly; but it seems intuitively convergent with prior theory that risk for Alzheimer’s disease may be relatively increased in individuals with greater capacity for higher brain functions (Arendt, 2001). In any case, hub-dominated networks are less robust to random attack (Figure 5); thus, disadvantages to integrated workspace functions due to reduced hub dominance will generally be offset by greater network robustness.
Methodological limitations
The main limitation of the study is the modest sample size (N=27), limiting the power of comparative analysis between groups and justifying a multiple comparisons correction that effected less than maximal strength of type 1 error control at the regional level of analysis. The modest sample size, especially in relation to the number of variables considered (13), also impacted adversely on the capacity of this dataset to elucidate bivariate or multivariate associations between variables. The long acquisition time of the datasets (17 mins) will have benefited the precision of estimation of correlations and networks derived from them (Achard et al., 2008). Conversely, longer time series are less likely to represent a stable brain functional state; future studies might profitably measure behavioural arousal prospectively and/or model non-stationary or time-resolved changes in functional connectivity over the course of the scanning period (Chang and Glover, 2010); see Supplementary Figure 3 and Table 9. Head motion may confound fMRI data, but this was individually corrected, and realignment parameters showed no between-group differences. Cardiac and respiratory sources can also contribute to variance in fMRI series, but neuronal sources are usually regarded as making the major contribution to oscillations in the frequency interval (0.06–0.125 Hz) investigated here. Medication is another possible confound; dopamine receptor antagonists can alter functional connectivity and network parameters (Honey et al., 2003; Achard and Bullmore, 2007). Although people with schizophrenia were withdrawn from medication > 20 hrs prior to scanning, mitigating acute pharmacological effects, all had been treated with antipsychotics for several years. However, antipsychotic dosage (in chlorpromazine equivalents) was not significantly correlated with any of the connectivity or network metrics. Data were scrutinised for acceptable image quality and brain regions where susceptibility artefact or incomplete brain coverage had compromised image quality in > 50% of participants were excluded: analysis was thus based on a subset of 72 regions (rather than the 90 template regions); a list of excluded regions, including inferior temporal and prefrontal regions relevant to schizophrenia, is provided in Supplementary Tables 1 and 2.
Supplementary Material
Figure 6. Hypothetical schematic of group differences in functional connectivity.
People with schizophrenia show both higher diversity at each region and lower variance in connectivity strength across the brain. This can be conceptualized as a randomization or de-differentiation of functional connectivity.
Acknowledgments
This research was supported by a Human Brain Project grant from the National Institute of Mental Health and the National Institute of Biomedical Imaging & Bioengineering. Data acquisition was supported by a grant from Bristol Myers Squibb. DSB was supported by the NIH-Cambridge Graduate Partnership Program. Edith Pomarol-Clotet/Peter McKenna is supported by the Instituto de Salud Carlos III, Centro de Investigación en Red de Salud Mental, CIBERSAM. The authors wish to thank Dr Rebecca Jones for her contribution to patient recruitment, and Glyn Johnson for supervising fMRI acquisition at the BUPA Lea Hospital, Cambridge UK. This paper is dedicated to the memory of Professor Robert W. Kerwin (1955–2007), neuropharmacologist and psychiatrist.
Footnotes
Financial Disclosures
EB is employed half-time by GlaxoSmithKline.
References
- Achard S. Package ‘brainwaver’. 2007 downloaded from http://cran.r-project.org/ on 30/11/09.
- Achard S, Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26:63–72. doi: 10.1523/JNEUROSCI.3874-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achard S, Bassett DS, Meyer-Lindenberg A, Bullmore E. Fractal connectivity of long-memory networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;77:036104. doi: 10.1103/PhysRevE.77.036104. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- Arendt T. Alzheimer’s disease as a disorder of mechanisms underlying structural brain self-organization. Neuroscience. 2001;102:723–65. doi: 10.1016/s0306-4522(00)00516-9. [DOI] [PubMed] [Google Scholar]
- Barch DM, Mathews JR, Buckner RL, Maccotta L, Csernansky JG, Snyder AZ. Hemo-dynamic responses in visual, motor, and somatosensory cortices in schizophrenia. Neuroimage. 2003;20:1884–93. doi: 10.1016/s1053-8119(03)00449-x. [DOI] [PubMed] [Google Scholar]
- Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–48. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET. Human brain networks in health and disease. Curr Op Neurol. 2009;22:340–7. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett DS, Bullmore ET, Meyer-Lindenberg A, Apud JA, Weinberger DR, Coppola R. Cognitive fitness of cost-efficient brain functional networks. Proc Natl Acad Sci USA. 2009;106:11747–52. doi: 10.1073/pnas.0903641106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazire S. Psychotropic Drug Directory. Fivepin Limited; Salisbury: 2005. [Google Scholar]
- Benton A, Hamsher K, Sivan A. Multilingual aphasia examination. Iowa City, IA: 1976. [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–12. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–98. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28:143–56. doi: 10.1016/s0920-9964(97)00114-x. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Cognitive neuroscience of aging: contributions of functional neuroimaging. Scand J Psychol. 2001;42:277–86. doi: 10.1111/1467-9450.00237. [DOI] [PubMed] [Google Scholar]
- Campbell K, Marsden P, Hurlbert JS. Social resources and socioeconomic status. Social Networks. 1986;8:97–117. [Google Scholar]
- Catani M, Ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–39. doi: 10.1093/brain/awh622. [DOI] [PubMed] [Google Scholar]
- Catani M, Mesulam M. What is a disconnection syndrome? Cortex. 2008;44:911–3. doi: 10.1016/j.cortex.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Chang C, Glover GH. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. NeuroImage. 2010;50:81–98. doi: 10.1016/j.neuroimage.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition. 2001;79:1–37. doi: 10.1016/s0010-0277(00)00123-2. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–23. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Bullmore ET. What can spontaneous fluctuations of the blood oxygenation-level-dependent signal tell us about psychiatric disorders? Curr Opin Psychiatry. 2010 doi: 10.1097/YCO.0b013e328337d78d. (e-pub ahead of print) [DOI] [PubMed] [Google Scholar]
- Friston KJ. Theoretical neurobiology and schizophrenia. Br Med Bull. 1996;52:644–55. doi: 10.1093/oxfordjournals.bmb.a011573. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3:89–97. [PubMed] [Google Scholar]
- Friston K. Functional and effective connectivity in neuroimaging: a synthesis. Hum Brain Mapp. 1994;2:56–78. [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–81. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Kotter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci U S A. 2007;104:10240–5. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey GD, Suckling J, Zelaya F, Long C, Routledge C, Jackson S, Ng V, Fletcher PC, Williams SCR, Brown J, Bullmore ET. Dopaminergic drug effects on physiological connectivity in a human cortico-striato-thalamic system. Brain. 2003;126:1767–81. doi: 10.1093/brain/awg184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD, Gurney K, Prescott TJ. The brainstem reticular formation is a small-world, not scale-free, network. Proc Biol Sci. 2006;273:503–11. doi: 10.1098/rspb.2005.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger J, Czobor P, Berns SM. Basic neuropsychological dimensions in schizophrenia. Schizophr Res. 2003;65:105–16. doi: 10.1016/s0920-9964(03)00052-5. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–81. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M, Hilgetag CC. Nonoptimal component placement, but short processing paths, due to long-distance projections in neural systems. PLoS Comput Biol. 2006;2:e95. doi: 10.1371/journal.pcbi.0020095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Knoke D, Yang S. Social network analysis. Sage Publications; 2008. [Google Scholar]
- Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Li Y, Liu Y, Li J, Qin W, Li K, Yu C, Jiang T. Brain anatomical network and intelligence. PLoS Comput Biol. 2009;5:e1000395. doi: 10.1371/journal.pcbi.1000395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–13. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Z, Liang M, Hao Y, Tan L, Kuang F, Yi Y, Xu L, Jiang T. Decreased regional homogeneity in schizophrenia: a resting state functional magnetic resonance imaging study. Neuroreport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang M, Zhou Y, He Y, Hao Y, Song M, Yu C, Liu H, Liu Z, Jiang T. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–61. doi: 10.1093/brain/awn018. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Halpern EF, Kramer TS, Chang Y, Goff DC, Rauch SL, Kennedy DN, Gollub RL. Test-retest reliability of a functional MRI working memory paradigm in normal and schizophrenic subjects. Am J Psychiatry. 2001;158:955–8. doi: 10.1176/appi.ajp.158.6.955. [DOI] [PubMed] [Google Scholar]
- Meunier D, Achard S, Morcom A, Bullmore E. Age-related changes in modular organization of human brain functional networks. Neuroimage. 2009;44:715–23. doi: 10.1016/j.neuroimage.2008.09.062. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–27. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Micheloyannis S, Pachou E, Stam CJ, Breakspear M, Bitsios P, Vourkas M, Erimaki S, Zervakis M. Small-world networks and disturbed functional connectivity in schizophrenia. Schizophr Res. 2006;87:60–6. doi: 10.1016/j.schres.2006.06.028. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–22. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H. National Adult Reading Test (NART): Test Manual. National Foundation for Educational Research; Windsor, England: 1992. [Google Scholar]
- Ojeda N, Peña J, Sánchez P, Elizagárate E, Ezcurra J. Processing speed mediates the relationship between verbal memory, verbal fluency, and functional outcome in chronic schizophrenia. Schizophr Res. 2008;101:225–33. doi: 10.1016/j.schres.2007.12.483. [DOI] [PubMed] [Google Scholar]
- Percival D, Walden A. Wavelet Methods for Time Series Analysis. Cambridge University Press; Cambridge, UK: 2000. [Google Scholar]
- Preston GA, Weinberger DR. Intermediate phenotypes in schizophrenia: a selective review. Dialogues Clin Neurosci. 2005;7:165–79. doi: 10.31887/DCNS.2005.7.2/gpreston. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempfer MV, Hamera EK, Brown CE, Cromwell RL. The relations between cognition and the independent living skill of shopping in people with schizophrenia. Psychiatry Res. 2003;117:103–12. doi: 10.1016/s0165-1781(02)00318-9. [DOI] [PubMed] [Google Scholar]
- Revheim N, Schechter I, Kim D, Silipo G, Allingham B, Butler P, Javitt DC. Neurocognitive and symptom correlates of daily problem-solving skills in schizophrenia. Schizophr Res. 2006;83:237–45. doi: 10.1016/j.schres.2005.12.849. [DOI] [PubMed] [Google Scholar]
- Rubinov M, Knock SA, Stam CJ, Micheloyannis S, Harris AW, Williams LM, Breakspear M. Small-world properties of nonlinear brain activity in schizophrenia. Hum Brain Mapp. 2009;30:403–16. doi: 10.1002/hbm.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R, Sarr’o S, Gomar J, Ortiz J, Vila F, Capdevila A, Bullmore E, McKenna P, Pomarol-Clotet E. Overall brain connectivity maps show cortico-subcortical abnormalities in schizophrenia. Hum Brain Mapp. 2010 doi: 10.1002/hbm.20993. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank T, Wagner D. Approximating clustering coefficient and transistivity. Journal of Graph Algorithms and Applications. 2005;9:265–275. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- Stam CJadHW, Daffertshofer A, Jones BF, Manshanden I, van Cappellen van Walsum AM, Montez T, Verbunt JP, de Munck JC, van Dijk BW, Berendse HW, Scheltens P. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer’s disease. Brain. 2009;132:213–24. doi: 10.1093/brain/awn262. [DOI] [PubMed] [Google Scholar]
- Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–27. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckling J, Long C, Triantafyllou C, Brammer M, Bullmore E. Variable precision registration via wavelets: optimal spatial scales for inter-subject registration of functional MRI. Neuroimage. 2006;31:197–208. doi: 10.1016/j.neuroimage.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Tononi G, Sporns O, Edelman GM. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc Natl Acad Sci USA. 1994;91:5033–7. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- van Beilen M, Pijnenborg M, van Zomeren EH, van den Bosch RJ, Withaar FK, Bouma A. What is measured by verbal fluency tests in schizophrenia? Schizophr Res. 2004;69:267–76. doi: 10.1016/j.schres.2003.09.007. [DOI] [PubMed] [Google Scholar]
- van den Heuvel MP, Stam CJaK RS, Hulshoff Pol HE. Efficiency of functional brain networks and intellectual performance. J Neurosci. 2009;29:7619–24. doi: 10.1523/JNEUROSCI.1443-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC. A population-average, landmark- and surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–62. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH. An integrated software suite for surface-based analyses of cerebral cortex. J Am Med Inform Assoc. 2001;8:443–59. doi: 10.1136/jamia.2001.0080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N, Wolf A, Brodie J, Cancro R, Overall J, Rhoades H, van Gelder P. Brain interactions in chronic schizophrenics under resting and activation conditions. Schizophr Res. 1988;1:47–53. doi: 10.1016/0920-9964(88)90039-4. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–2. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Weschler memory scale-III, UK manual. Psychological Corporation; San Antionio, TX: 1997. [Google Scholar]
- Weinberger D, Berman K, Suddath R, Torrey E. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Shenton ME, Green AI, Nieto-Castanon A, LaViolette P, Wojcik J, Gabrieli JD, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–7. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Liu H, Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007a;417:297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007b;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Shu N, Liu Y, Song M, Hao Y, Liu H, Yu C, Liu Z, Jiang T. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res. 2008;100:120–32. doi: 10.1016/j.schres.2007.11.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.