Abstract
Motor learning is required for the reacquisition of skills that have been compromised as a result of brain lesion or disease, as well as for the acquisition of new skills. Behaviors with well-characterized anatomy and physiology are required to yield significant insight into changes that occur in the brain during motor learning. The vestibulo-ocular-reflex (VOR) is well suited to establish connections between neurons, neural circuits, and motor performance during learning. Here we examined the linkage between neuronal and behavioural VOR responses in alert behaving monkeys (macaca mulatta) during the impressive recovery that occurs after unilateral vestibular loss. We show, for the first time, that motor learning is characterized by the dynamic reweighting of inputs from different modalities (i.e., vestibular versus extra-vestibular) at the level of the single neurons which constitute the first central stage of vestibular processing. Specifically, two types of information, which did not influence neuronal responses prior to the lesion, had an important role during compensation. First, unmasked neck proprioceptive inputs played a critical role in the early stages of this process demonstrated by faster and more substantial recovery of vestibular responses in proprioceptive sensitive neurons. Second, neuronal and VOR responses were significantly enhanced during active relative to passive head motion later in the compensation process (>3 weeks). Taken together, our findings provide evidence linking the dynamic regulation of multimodal integration at the level of single neurons and behavioural recovery, suggesting a role for homeostatic mechanisms in VOR motor learning.
Keywords: learning, plasticity, homeostatic, multisensory, self-motion, VOR
INTRODUCTION
Motor learning is essential not only for the acquisition of new skills but also the reacquisition of formerly mastered skills that have been compromised as a result of brain lesion or disease. Understanding the changes that occur during learning is a fundamental problem in neuroscience, and the relative simplicity of the vestibulo-ocular reflex (VOR) is particularly well suited to establishing links between neurons, neural circuits, and motor performance. The VOR is mediated by a 3 neuron pathway: the vestibular nerve transmits sensory information to neurons in the vestibular nuclei, which directly project to motoneurons that drive eye motion (Lorente De No', 1933). The compensatory eye movements produced by the VOR stabilize images on the retina to prevent blurred vision during the head movements made in everyday activities.
Changes in environmental requirements, such as those brought about by the magnifying lens worn to correct myopia, lead to impressive VOR adaptation (Shelhamer et al., 1992). Similarly, the VOR shows remarkable plasticity in response to the effects of aging, disease and trauma to the nervous system (reviewed in: Cullen, 2008). Long-term potentiation (LTP) and depression (LTD) are widely viewed as playing critical roles in recalibrating the efficacy of vestibular transmission through VOR pathways (Caria et al., 2001; Grassi et al., 2001). However, recent studies have emphasized that learning can be mediated by multiple processes, including homeostatic mechanisms that operate over longer time scales, in addition to rapid Hebbian mechanisms (reviewed in: Feldman, 2009). Notably, experimentally induced changes in network activity (ranging from hours to days) produce long term changes in the strength of sensory neocortical synapses (e.g., Kotak et al., 2005; Maffei and Turrigiano, 2008). Correspondingly, chronic peripheral vestibular loss can induce changes in synaptic strength onto vestibular nuclei neurons (Goto et al., 2000, 2001) as well as alterations in neuronal membrane properties (Beraneck et al., 2003; Beraneck et al., 2004).
To date, homeostatic plasticity has been primarily characterized in slice cultures (in vitro) or reduced preparations. Thus, the question of how homeostatic plasticity contributes to motor learning remains open. We hypothesized that activity-dependent synaptic scaling would drive the relative reweighting of inputs from different modalities (i.e., vestibular versus extra-vestibular) to restore network activity to a set point level after vestibular loss. To test this, we recorded from single VOR interneurons in the contralesional vestibular nuclei and determined whether sensitivities to vestibular and/or extra-vestibular signals changed in parallel with improvements in motor performance. We found that while vestibular sensitivities were markedly reduced immediately following lesion, extra-vestibular signals - not present prior to the lesion - were unmasked. Initially, neck proprioceptive inputs played a key role in the compensation process. At later stages, enhanced neuronal responses during active compared to passive head motion paralleled improvements in motor performance, consistent with the integration of a motor efference copy information at the first central stage of vestibular processing. Thus our results show that multimodal integration can be dynamically regulated in the vestibular system, and strongly favor a causal role for homeostatic plasticity in motor learning.
METHODS
Subjects and Surgery
Experiments were performed on two male rhesus macaque monkeys (Macaca mulata) weighing ~8 Kg. The animals were chronically implanted with a post for head restraint, recording chamber, and scleral search coils for high resolution eye movement recording as described previously (Sadeghi et al., 2007a). Following the surgery, the animals were administered buprenorphine (0.01 mg/kg IM) for post-operative analgesia, and the antibiotic Cephazolin (Ancef®; 25 mg/kg IM, for 5 days). Animals were trained using standard operant conditioning to fixate visual targets for a juice reward. In both animals, we recorded from single units directly after training as well as following unilateral labyrinthectomy. Labyrinthectomy was performed through the mastoid bone to remove the ampulla of the three semicircular canals, the utricle and saccule, and the distal ends of the ampullary nerve branches (Sadeghi et al., 2006). All procedures were approved by the McGill University Animal Care Committee and Johns Hopkins University Animal Care and Use Committee and were in compliance with the guidelines of the Canadian Council on Animal Care and the National Institutes of Health.
Experimental Design and Data Acquisition
Monkeys were initially head restrained during experiments and yaw rotations about the earth vertical axis were applied using a motion stimulator, located within a 1-m3 magnetic field coil (CNC Engineering). A visual target (HeNe laser) was projected, via a system of two galvanometer controlled mirrors, onto a cylindrical screen located 60 cm away from the monkey's head. Monkeys were trained to follow the visual target and neuronal sensitivities to saccades, ocular fixation, and pursuit were characterized by having the monkey follow target motion that 1) stepped between horizontal positions over a range of ±30° and 2) moved sinusoidally (0.5 Hz, ± 40 °/sec peak velocity). Target and turntable motion were controlled by a UNIX-based real-time data acquisition system (REX, Hayes et al., 1982).
The experimental design consisted of four stimulus conditions. First to stimulate the vestibular system, monkeys were rotated about an earth vertical axis with their heads restrained (0.5 Hz, peak velocity of ±40 °/sec) both in the dark (whole-body rotation) and while they suppressed their VOR by fixating a visual target that moved with the vestibular turntable (i.e., VOR cancellation condition). Second, to stimulate neck proprioceptors the monkey's head was held stationary relative to the earth while its body was sinusoidally (0.5Hz, ±40 or ±80 °/sec) rotated beneath. Third, combined stimulation of the vestibular system and neck proprioceptors was induced by passively rotating the monkey's head on its body using a torque motor (Kollmorgen) attached to the head (Huterer and Cullen, 2002; Sadeghi et al., 2006; Sadeghi et al., 2007b; Sadeghi et al., 2007a; Sadeghi et al., 2009). The applied stimulation produced horizontal sinusoidal head rotations about the vertical axis, relative to a stationary body (1 Hz, ±40 °/sec) as well as passive rotations of the head relative to the body that had trajectories comparable to those produced during actively generated orienting gaze shifts. Finally, the monkey's head was slowly and carefully released so that it was free to make active head movements (i.e., horizontal rotations about the earth vertical axis) so that the responses of the same neuron could then be recorded during voluntary (i.e., active) horizontal gaze shifts toward targets, as described previously (Roy and Cullen, 2002).
Electrophysiology
Extracellular single unit recordings were performed using enamel-insulated tungsten microelectrodes (7–10 MΩ impedance, Frederick-Haer) advanced into the brainstem through a transdural guidetube using a lightweight microdrive (Narishige). Single neurons were isolated using a conventional amplifier system and bandpass 8 pole filter (400 Hz to 10 kHz). The abducens nucleus was first identified based on its stereotypical discharge patterns during eye movements (Cullen et al., 1993; Sylvestre and Cullen, 1999) and then used as a landmark to determine the location of the medial and lateral vestibular nuclei. Position-vestibular-pause neurons were then identified on the basis of their characteristic physiological response properties including oppositely directed sensitivities to vestibular stimulation and eye position, and cessation of firing (pause) during rapid saccadic eye movements (Roy and Cullen, 1998). We made our recordings in the contralesional vestibular nuclei, since the results of prior in-vitro studies had suggested greater compensation as compared to lesioned side (reviewed in: Straka et al., 2005). In addition, we specifically focused on neurons that mainly receive inputs from the horizontal canals and divided them into two groups (Duensing and Schaefer, 1958): type I neurons (that receive excitatory inputs from the ipsilateral horizontal canal) and type II neurons. Notably, this latter class of neurons is known to receive excitatory input from contralateral type I neurons and in turn provides the major inhibitory input to ipsilateral type I neurons (Shimazu and Precht, 1966; Malinvaud et al., 2010).
Gaze and head position were monitored using the magnetic search coil technique, and turntable velocity was measured using an angular velocity sensor (Watson). Single unit responses, horizontal and vertical gaze and head positions, target position, and table velocity were recorded on a DAT tape for later playback. Action potentials were discriminated during playback using a windowing circuit (BAK) that was manually set to generate a pulse coincident with the rising phase of each action potential. In addition, gaze position, head position, target position, and table velocity signals were low-pass filtered at 250 Hz (8 pole Bessel filter) and sampled at 1 kHz.
Data Analysis
Data were imported into the Matlab (The MathWorks, Natick MA) programming environment for analysis. Recorded gaze and head position signals were digitally filtered with zero-phase at 125 Hz using a 51st order finite-impulse-response (FIR) filter with a Hamming window. Eye position was calculated from the difference between gaze and head position signals. Gaze, eye, and head position signals were digitally differentiated to produce velocity signals. Neuronal responses were represented using a spike density function in which a Gaussian was convolved with the spike train (SD = 10 msec for sinusoidal rotations and SD=5 msec for gaze shifts) (Cullen et al., 1996; Sylvestre and Cullen, 2006). Statistical significance was determined using Student's t-tests.
Control data was obtained by recording eye, head, and table rotations, as well as neuronal responses in the vestibular nuclei of each animal before labyrinthectomy. A labyrinthectomy was then preformed on the contralateral side and postlesion data was collected during experimental sessions in which recordings were made in the contralesional vestibular nuclei, starting from day 1 (i.e., 15–28 hours) post-lesion. Later recordings were made on a weekly basis up to 2 months post-lesion.
To quantify behavioral performance, we calculated the gain of the VOR response during sinusoidal rotations for full cycles as well as for ipsilateral and contralateral half-cycles of rotation. For the latter analysis, the head velocity signal was divided into right and left half cycles based upon zero crossings of the stimulus. At least ten cycles of rotations were analyzed for each measurement. VOR and cervico-ocular reflex (COR) gains were calculated as the resultant slow phase (i.e., desaccaded) eye velocity divided by the turntable velocity after accounting for the phase difference (Sadeghi et al., 2006). In addition, to compute the gain of VOR during active head movements, eye responses were characterized during head motion made both prior to the period during which gaze was redirected, as well as during the 10–80 ms period following the end of the gaze shift (i.e., post-gaze shift period), where the gaze was stable but the head continued to move.
The recordings of neural responses concentrated on the functionally distinct group of cells in the vestibular nuclei classified in prior experiments (Roy and Cullen, 1998) as position-vestibular-pause (PVP) neurons. To identify PVP cells, neuronal eye-position sensitivities [k, (spikes/sec)/°] were computed from periods of fixation using a multiple regression analysis (Roy and Cullen, 1998). Spike trains were also assessed to verify that neurons paused during saccades. In addition, a least-squared regression analysis was used to determine each unit's response to vestibular stimulation during passive whole-body rotations:
| (1) |
where is the estimated firing rate, Svvest and Savest are coefficients representing sensitivities to head velocity and acceleration, b is a bias term, E is eye position, and and are head velocity and head acceleration, respectively. Only unit data from periods of slow-phase eye velocity that occurred between quick phases of vestibular nystagmus and/or saccades were included in the analysis. The estimated coefficients Svvest and Savest were then used to calculate each unit's modulation sensitivity ((spikes/sec)/(°/sec)) and phase shift (deg) relative to head velocity (Sadeghi et al., 2009).
A comparable approach was next used to describe each unit's response to neck proprioceptive stimulation during passive rotation of the body under a stationary head. To quantify neuronal responses, we determined the best estimate of each neuron's sensitivity to neck rotation using the equation:
| (2) |
Where Svneck and Saneck are coefficients representing sensitivities to neck (=body or equivalently the vestibular turntable) velocity and acceleration, and and are body velocity and acceleration, respectively. Because neuronal responses typically led rather than lagged body velocity, our formalization of the model included velocity and acceleration terms. Similar to vestibular sensitivities, the estimated coefficients were then used to calculate each unit's modulation sensitivity ((spikes/sec)/(°/sec)) and phase shift (deg) relative to velocity of body rotation (Sadeghi et al., 2009).
Finally, we used a similar approach for the characterization of responses during combined vestibular and proprioceptive stimulation evoked by passive sinusoidal head-on-body rotations (i.e., the combined condition). Neuronal responses were estimated using the following equation:
| (3) |
where Svhob and Sahob are coefficients representing sensitivities to head-on-body velocity and acceleration, and are head-on-body velocity and acceleration, respectively. Estimated sensitivities (Svhob and Sahob) were then compared to those predicted based on the linear summation of the vestibular and proprioceptive sensitivities estimated for the same neuron during whole-body rotations (equation 1) and body-under-head rotations (equation 2), respectively.
To quantify the ability of the linear regression analyses to model neuronal discharges during each paradigm, we computed the variance-accounted-for (VAF) provided by each regression equation (Cullen et al., 1996). The VAF was defined as , where represents the modeled firing rate (i.e., regression equation estimate) and fr represents the actual firing rate.
RESULTS
In order to investigate the contributions of different modalities (i.e., vestibular versus extra-vestibular) to re-establishing network function after vestibular loss, we not only need to separately assess the contribution of each signal to neuronal responses, but also determine whether the observed changes lead to improvements in motor performance (i.e. VOR compensation). We begin by considering the linkage between changes in neuronal vestibular sensitivities and simultaneously measured VOR responses. We then determine whether sensitivities to extra-vestibular signals are unmasked in parallel with modality-specific improvements in motor performance. Finally, we address whether dynamic regulation of multimodal integration is associated with increases in the recovery of vestibular sensitivity by individual neurons during this motor learning.
Neural correlates of compensation: vestibular responses
We directly measured the efficacy of VOR pathways during vestibular stimulation by simultaneously recording motor performance and the single unit responses of the individual neurons in the vestibular nuclei (Fig. 1, input 1), which constitute the intermediate leg of the direct VOR pathway (McCrea et al., 1987; Scudder and Fuchs, 1992; Cullen and McCrea, 1993). These VOR neurons receive a strong monosynaptic drive from the ipsilateral nerve and, in turn, project directly to contralateral extraocular motoneurons. They can be easily identified by their characteristic responses, (i.e., an increase in their activity as a function of contralateral eye position and ipsilateral head velocities) and are called type I position-vestibular-pause (PVP) neuron. Recordings were also made from type II PVP neurons, which contribute to the commissural pathways that interconnect the vestibular nuclei on each side (Shimazu and Precht, 1966; Malinvaud et al., 2010). These neurons are characterized by oppositely directed head and eye movement sensitivities to those of type I PVP neurons (Roy and Cullen, 2002).
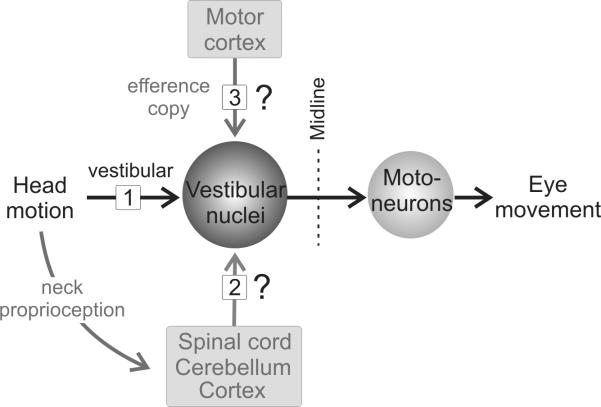
Figure 1.
Schematic diagram of the main direct pathway that mediates the vestibulo-ocular reflex (VOR). Primary sensory afferents (input 1) send vestibular signals to the PVP neurons in the vestibular nuclei. These neurons in turn project to the extraocular motoneurons on the contralateral side to produce VOR eye movements. In addition, self motion information arising from the activation of proprioceptors (input 2) and/or motor efference copy information (input 3) could help drive compensation at this site.
To assess sensitivities to vestibular inputs, we first quantified the behavioral performance recorded during passive whole-body rotations (0.5 Hz, 40 °/s) before and following unilateral labyrinthectomy. Before unilateral labyrinthectomy, eye movement responses were fully compensatory (Fig. 2A1, top row). However, immediately following lesion, VOR responses were reduced and asymmetric, characterized by diminished responses to rotations towards the side of the lesion (Fig. 2A1, middle row). Finally when measured 4 weeks following lesion, VOR responses evoked by rotations towards the side of the lesion remained defective, while responses evoked by rotations in the opposite direction appeared normal (Fig. 2A1, bottom row).
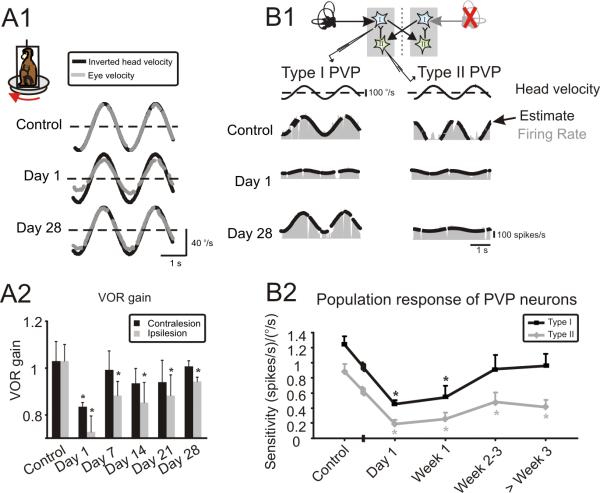
Figure 2.
Changes in simultaneously measured VOR and neuronal responses after unilateral labyrinthectomy. (A1) Example VOR responses show reduced and asymmetric gains immediately following lesion (day 1), and impressive compensation, recovering to nearly normal values by day 28. Note, head velocity traces have been inverted to facilitate comparison with the evoked eye velocities. (A2) VOR gains averaged across both animals. On day 1, responses were reduced for both ipsi and contralaterally directed rotations. Over the next 2–3 weeks, contralesional gains improved to normal values and ipsilesional gains were nearly compensatory. (B1) Examples of type I and type II PVP responses before and at different time points after contralateral labyrinthectomy. Response of both cell types decreased significantly immediately following lesion (day 1). While the sensitivity of type I neurons improved over time reaching normal values by day 28, that of type II neurons did not show significant improvement (P> 0.1). Inset shows that type I neurons receive indirect inputs from contralateral labyrinth through inhibitory type II neurons. (B2) Summary of the analysis of i) the population of 57 neurons (40 type I and 17 type II) recorded under control conditions and ii) the population of 109 neurons recorded after lesion (56 type I and 53 type II). Of the latter group, 44 were recorded on the first day (i.e., 15–28 hours) post-lesion, 32 in the period of 7–21 days post-lesion, and 33 in the 1–2 months post-lesion. * represent significant difference re. control (i.e., before lesion), P<0.05
To quantify behavioral performance, we computed the average gain of the VOR eye movement response (see Methods) for both animals before and after lesion (Fig. 2A2). On the day following lesion, gains of responses evoked by rotations in either direction were dramatically reduced to 70–80% relative to control values. However, responses to both ipsilesionally and contralesionally-directed rotations improved over the next month, with contralesional responses fully recovering to normal levels by day 7 (p < 0.01).
Having established that the VOR shows robust compensation following vestibular unilateral labyrinthectomy, we next quantified the corresponding responses of vestibular nuclei neurons. Based on the circuitry of the VOR pathways (Fig. 2B1), we predicted that type II as well as type I PVPs in the contralesional vestibular nuclei should show a decreased sensitivity to vestibular stimulation. Both neurons lose inputs that they normally receive from the lesioned nerve via the commissural connections between the two vestibular nuclei; for type II neurons this input is direct, while for type I neurons it is mediated mostly via type II neurons (Shimazu and Precht, 1966; Malinvaud et al., 2010).
Figure 2B1 (control) shows the robust responses of an example type I PVP neuron before lesion. Consistent with our prediction, the sensitivity of neurons decreased dramatically immediately following contralateral labyrinthectomy (Fig. 2B1, Day 1). Strikingly, however, this diminished response nearly recovered to normal values over the following weeks (Fig. 2B1, Day 28). Sensitivity of type II neurons similarly decreased immediately following lesion. However, in contrast to type I neurons, the responses of type II neurons never fully recovered. This is illustrated for the example neuron shown in Fig. 2B1 (right column). Even 4 weeks following lesion, this type of neuron was typical in that its response to vestibular stimulation was far less robust than observed before the lesion.
Figure 2B2 shows the time course of the change in vestibular sensitivity of the population of neurons before (n = 57) and on different days following lesion (n = 109). The average sensitivities of neurons recorded in control animals were 1.2±0.1 and 0.9±0.1 (spikes/s)/(°/s), for type I and II neurons respectively, similar to those reported in previous studies (Roy and Cullen, 1998, 2002). Immediately following contralateral labyrinthectomy (day 1), the average sensitivity of both type I and type II neurons dramatically decreased (>50% reduction; P<0.0001) reaching values of 0.45±0.05 and 0.19±0.06 (spikes/s)/(°/s), respectively. In the following weeks, the responses of type I neurons improved, so that their average vestibular sensitivity reached normal values by week 2–3 post-lesion (0.92±0.19 (spikes/s)/(°/s), P=0.12). Although type II neurons showed a slight improvement in their responses, their sensitivities never reached normal values, even 60 days after lesion (>50% reduction in sensitivity; 0.41±0.1 (spikes/s)/(°/s), P=0.0002). Together, these results from lesioned animals indicate that increased weighting of the excitatory input to type I neurons (i.e., via direct input from the intact contralesional vestibular nerve) provides a robust substrate to mediate compensation. In contrast, type II neurons show little recovery consistent with the fact that the source of the excitatory inputs to these cells is the contralateral nerve which had been lesioned.
Neural correlates of compensation: the unmasking of extra-vestibular inputs
The results shown so far demonstrate a strong relationship between changes in the vestibular sensitivity of single neurons and the recovery of motor performance after lesion. However, these findings consider the VOR as a unimodal pathway, since changes in neuronal sensitivities and behavioral performance were only characterized for vestibular stimulation. Because the vestibular system, unlike other senses, is multisensory and multimodal immediately at the first central stage of processing, we next tested whether information about self motion derived from sources other than the vestibular sensors also plays an important role in compensation. Notably, when head movements are made in a natural context, the brain has access to proprioceptive and motor-related signals as well as vestibular information. If neuronal sensitivities to stimulation of proprioceptive inputs (Fig. 1, input 2) and/or the production of motor commands resulting in self motion (Fig. 1, input 3) showed changes that parallel improvements in motor performance during compensation, then we could conclude that the reweighting of extra-vestibular inputs at the first stage of central vestibular processing induces motor recovery.
First, to test whether proprioceptive information could potentially be used to support compensation, we recorded from single neurons before and after lesion during a paradigm in which proprioceptive stimulation was delivered in isolation (Methods). Fig. 3A illustrates the responses recorded from three typical type I PVP neurons while we sinusoidally rotated the monkey's body beneath its earth-stationary head. As previously shown by Roy and Cullen (2001), type I PVP neurons did not respond to the passive stimulation of neck proprioceptors prior to labyrinthectomy (Fig. 3A; control). Strikingly, however immediately following lesion, the majority of neurons (>70%) showed robust modulation in response to the identical stimulation (Fig. 3A; Day 1). When tested a month after lesion, the majority of neurons remained sensitive to the stimulation of neck proprioceptors, but responses were far less striking than those measured on day 1 (Fig. 3A; Day 28). Figure 3B shows the percentage of neck-sensitive neurons across the population as a function of time. Although this percentage showed little change after lesion, neck sensitivities, which were non-existent in control animals, peaked just following lesion and decreased significantly by the second week post-lesion (Fig. 3C; p < 0.05; note, population values were computed from the average of absolute values since the polarity of the responses differed across individual neurons).
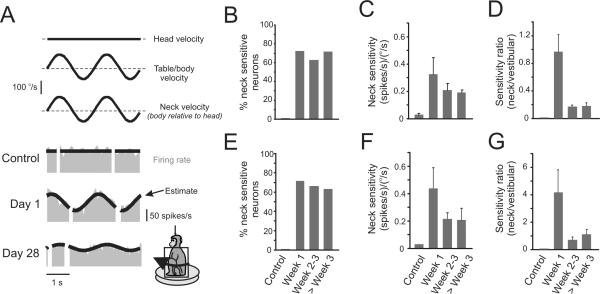
Figure 3.
The majority of PVP neurons show robust modulation to stimulation of neck proprioceptors after contralateral labyrinthectomy. (A) Examples of type I neuronal responses during stimulation of neck proprioceptors. In intact animals, neurons are not sensitive to stimulation. In contrast, the example neuron shown on day 1 after the lesion responded robustly to neck stimulation. The neuron shown on day 28 also responded to neck stimulation, but with a lower sensitivity. (B) The percentage of neck-sensitive type I neurons remained constant (60–70%) from week 1 to 8 after lesion. (C) The average of the absolute values of neck sensitivities of type I neurons decreased during compensation, but never reached control values (i.e., responses remained significant). (D) Decreases in neck sensitivity of type I neurons were temporally linked to increases in the vestibular sensitivities. Accordingly, neck sensitivities were the most robust the first week after lesion. (E) The percentage of neck sensitive type II neurons remained constant (60–70%) from week 1 to 8 after lesion (n=29). (F) The average of the absolute values of neck sensitivity in type II neurons decreased from 0.44±0.15 on week 1 post-lesion to 0.22±0.05 and 0.20±0.08 during weeks 2–3 and after week 3, respectively. (G) The ratio of neck and vestibular sensitivities shows that neck sensitivities of type II neurons were the most robust the first week after lesion.
Further analysis revealed that the observed decreases in neck sensitivities were associated with a coincident increase in the vestibular sensitivity of type I PVP neurons. To quantify this observation, we computed the ratio between neck and vestibular sensitivities for all neurons recorded after lesion that were sensitive to neck proprioceptor stimulation. The trends across time (Fig. 3D) show that neck inputs made the greatest contribution immediately after the lesion. Overall, similar findings were also obtained in the analysis of the percentages and sensitivities of neck-sensitive type II neurons following lesion (Fig. 3E–G).
Relationships between neural sensitivities to proprioception and vestibular compensation
The presence of neck proprioceptive responses on VOR interneurons was observed immediately following but never before labyrinthectomy. The fact that VOR interneurons show significant modulation in response to stimulation of proprioceptors after lesion, suggests that the unmasking of this extra-vestibular input plays a role in the compensation process. To further investigate this possibility, we examined two possible, non-mutually exclusive roles, namely that the unmasking of neck proprioceptive inputs i) results in the enhancement of neck driven ocular responses to improve gaze stabilization, and/or ii) reflects a homeostatic mechanism which ensures continued dynamic stimulation of individual neurons following lesion.
Proprioceptive driven ocular responses, such as the cervico-ocular reflex (COR), do not make significant contribution to gaze stabilization in normal subjects (Dichgans et al., 1973; Bronstein and Hood, 1986; Jurgens and Mergner, 1989; Roy and Cullen, 2002). If the unmasking of proprioceptive inputs enhanced neck driven ocular responses to compensate for the defective VOR, then we would have expected more robust behavioral responses during neck proprioceptive stimulation following vestibular lesion. This prediction was contradicted by our quantification of behavioral performance (i.e., the cervico-ocular reflex) measured during the same paradigms used to compute neuronal neck sensitivity above (i.e., as in Fig. 3). Average behavioral gains were computed by measuring eye movements evoked by sinusoidal rotation of the monkey's body beneath its earth-stationary head. Fig. 4A1 shows the average behavioral performance measured in the two monkeys, before and at different times after unilateral labyrinthectomy (dashed grey line). Neck proprioceptive driven eye movements were negligible before and remained negligible after the lesion.
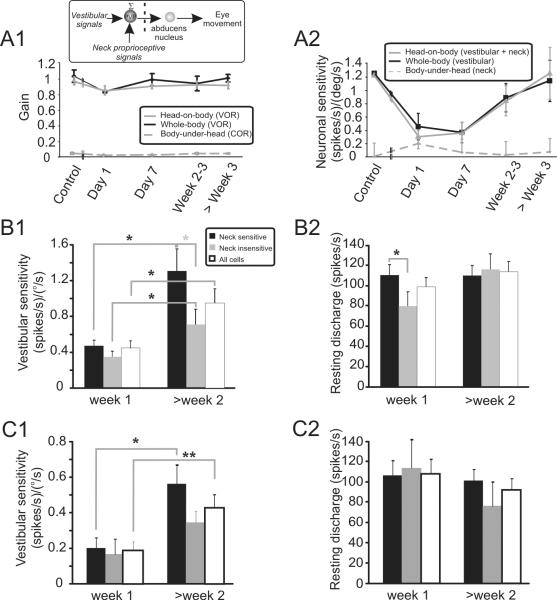
Figure 4.
The role of neck inputs in vestibular compensation of PVP neurons. (A1) The presence of neck proprioceptive responses on type I neurons did not enhance the cervico-ocular reflex (COR, dashed grey line); response gains were negligible before and after lesion. Similarly, the VOR responses evoked by combined vestibular and neck stimulation (head-on-body rotation, grey line) and vestibular stimulation alone (whole-body, black line) were comparable. (A2) Average neuronal sensitivities computed for the population of type I neurons during the same paradigms as in A1. Note that the direction of neck sensitivities was accounted for in the calculation (i.e., sensitivities to rightward versus leftward stimulation were considered as positive and negative values, respectively). As can be seen by the average sensitivities to body-under-head rotation (dashed line) and the lack of difference between head-on-body (black line) and whole-body rotations (gray line), average responses were minimal even immediately after lesion. (B1) The vestibular sensitivities of neck-sensitive type I neurons showed more substantial (grey asterisk) recovery over time than neck-insensitive neurons. (B2) The resting discharge of neck-sensitive type I neurons initially showed better recovery over time, compared to neck-insensitive neurons. However, later in the compensation process (> week 2) both groups showed comparable resting discharges. (C1) The vestibular sensitivities of neck-sensitive type II neurons (n=29) showed more substantial recovery over time, than neck insensitive neurons (n=21). As a result, the entire population of neurons also showed a significant increase in their sensitivities >2 weeks post-lesion. (C2) The resting discharges of neck sensitive and insensitive neurons were similar at all times after lesion and did not differ from control values (t-test, p > 0.2). * and ** show significant differences (t-test) at p < 0.05 and p < 0.01, respectively.
Given that neck proprioceptive driven ocular reflexes were not enhanced following labyrinthectomy, it follows that eye movements evoked by combined stimulation of the vestibular system and neck proprioceptors should be comparable to those evoked by vestibular stimulation alone. To test this proposal, we also recorded eye movements during head movements made by rotating the head relative to a stationary body. In this condition, PVP neurons in the vestibular nuclei would receive information from neck proprioceptive inputs in addition to their primary input from the vestibular nerve (Fig. 4A1, inset). As expected, the eye movement responses evoked by combined vestibular/proprioceptive stimulation and vestibular stimulation alone (i.e., passive whole-body rotations) were comparable (Fig. 4A1 compare grey and black lines, respectively). Figure 4A2 compares the simultaneously recorded neuronal sensitivities to head-in-space motion for both conditions. Consistent with our behavioral findings, neuronal sensitivities were comparable (p > 0.1).
The absence of a cervico-ocular reflex following lesion might appear surprising, considering the high neck proprioceptive sensitivity of PVP neurons in the first week after lesion, particularly of type I neurons that project to the eye motoneurons (Fig. 3B and 3C). However, when computing the net neck-related command produced by these neurons, it is essential to account for the directional sensitivity of the neck-driven response since neck-related and vestibular responses could be either agonistic or antagonistic. Accordingly, when response direction as well as magnitude was considered, our population of type I PVP neurons showed minimal neck sensitivity acutely after lesion and during the compensation process (0.19±0.06 and 0.04±0.04 (spikes/s)/(°/s), in week 1 and later, respectively). Similar qualitative findings were found for type II PVP neurons. Thus, the unmasking of neck related inputs on VOR interneurons did not result in a parallel change in proprioceptive driven ocular reflex, even though these neurons project directly to the extraocular motor nuclei.
A second possible role for the unmasking of neck proprioceptive inputs is that it supports a homeostatic mechanism that ensures continued dynamic stimulation of individual neurons following lesion. If this were the case, then we would expect that neck-sensitive neurons should show better and/ or faster compensation following lesion. Figures 4B1 and 4B2 verify this prediction. The recovery of vestibular sensitivities is compared for neck-sensitive and neck-insensitive neurons during compensation (Fig. 4B1; black and grey bars respectively versus all neurons (white bars). While the vestibular sensitivities of both groups of neurons improved significantly over time (black stars, p < 0.04), those of the neck-sensitive neurons showed significantly greater improvement (grey star, p < 0.04). Similarly, the resting discharges of neck-sensitive neurons recovered faster compared to those of neck-insensitive neurons, reaching control values (105 ± 11 spikes/s) during the first week following lesion (Fig. 4B2, p < 0.05). The situation was similar for type II PVPs in that neck-sensitive neurons (n=29) showed significantly better improvement in their vestibular sensitivities compared to neck-insensitive neurons (n=21, Fig. 4C1). However, the resting discharge was not different between the two groups of neurons (Fig. 4C2) and control value (89 ± 18 spikes/s).
Unmasking of extra-vestibular inputs: motor efference copy
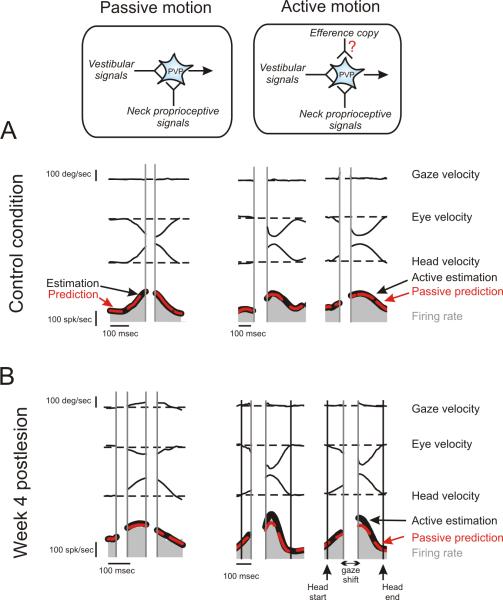
In natural conditions, head movements are commonly self generated. During these active movements, the brain has access to information about self motion as a result of both the motor command it produces and the resultant stimulation of vestibular and proprioceptive inputs (Fig. 5, compare schematics in the top row). Having established above the relationship between VOR compensation and the unmasking of proprioceptive inputs and changes in vestibular sensitivity of single VOR interneurons, we next asked whether an efferent copy of the head motor command could also contribute to compensation during active head movements (Fig. 1, input 3). Specifically, we quantified the linkage between changes in neuronal response sensitivities and simultaneously measured VOR responses during passive and active head-on-body rotations with comparable trajectories.
Figure 5.
The response of an example type I PVP neurons during passive versus active head-on-body rotations before and 4 weeks after contralateral labyrinthectomy. As illustrated in the schematic, the brain produces a motor command to move the head during active motion and as a result, additional self motion information is potentially available in this condition. For example, this can be a copy of the motor command from cortex to neck muscles or alternatively, a modified version of neck proprioceptive signals during active head movements vs. passive movements (e.g., as a result of the fusimotor drive accompanying active motion). (A) In control animals, there was excellent correspondence between the optimal neuron's response (black line) and the prediction based on the sum of the neuron's vestibular and neck sensitivities (red) during both passive and active motion. (B) In contrast, after labyrinthectomy, there was only excellent correspondence between the optimal neuron's response (black line) and the prediction based on the sum of the neuron's vestibular and neck sensitivities (red) during passive motion. Notably, neuronal responses were enhanced during active head movements on week 4 post-lesion.
Figure 5 shows the responses of two typical type I PVP neurons, one recorded before (Fig. 5A) and the other recorded 4 weeks after lesion (Fig. 5B). As was the case during passive sinusoidal stimulation, there was excellent correspondence between the optimal fit to the neuron's response (black line) during passive motion and the prediction computed from the sum of the neuron's individual vestibular and proprioceptive response sensitivities. This was the case both before and after lesion (left panels; dashed red lines). Similarly, before lesion the same linear summation prediction well estimated neuronal responses during active head movements (Fig. 5A; right panels; dashed red lines). In contrast, following lesion neuronal responses were underestimated by the linear summation of the neuron's sensitivities to vestibular and proprioceptive stimulation (Fig. 5B, right panels; dashed red lines). Thus, following labyrinthectomy neurons showed more robust modulation in response to active head than passive head movements. Note that fits were restricted to the neuronal firing associated with active head motion produced before and after the shift in gaze in both conditions, since PVP neurons show a marked pause in firing during the gaze shift portion of active head movements (Roy and Cullen, 1998, 2002).
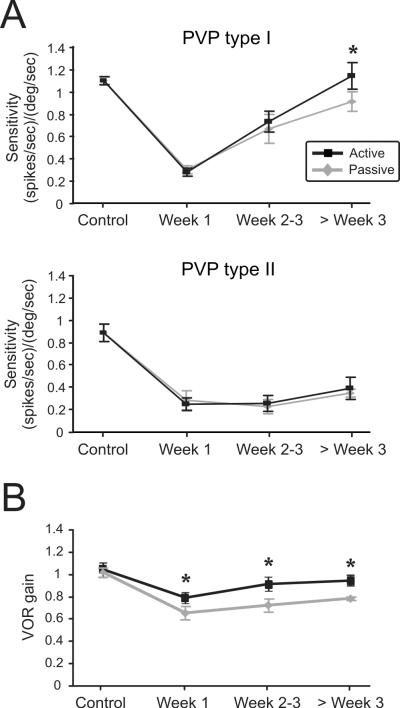
Figure 6A (top panel) summarizes the sensitivities of the population of type I PVP neurons (n=34) recorded before and following lesion during passive (grey) and active (black) movements. Neuronal sensitivities to active and passive head motion were not significantly different prior to the lesion (p>0.9). Similarly, head motion sensitivities remained comparable in both conditions during the first 3 weeks after lesion. Note, however, that neuronal head motion sensitivities were significantly elevated 4 weeks after lesion. The mean neuronal sensitivity for our population of type I PVP neurons was ~20% higher during active as compared to passive movements (paired t-test, p < 0.05). This difference was consistent across all neurons tested, regardless of the presence or absence of neck sensitivity. In contrast, type II neurons (n=20) did not show a significant change in their sensitivities during active head rotations compared to passive rotations at any time following lesion (Fig. 6A, bottom panel; paired t-test, p>0.2 at each time point). Thus, Type I, but not type II, PVP neurons generally fire more robustly in response to active versus passive head movements during the later (i.e., > 3 weeks) stages of vestibular compensation.
Figure 6.
Comparison of average neuronal and behavioural responses during passive and active head-on-body rotations. (A) Average sensitivities of the population of type I (n=34) and type II (n=20) PVP neurons during passive (grey) and active (black) movements before (n=16) and after (n=38) contralateral labyrinthectomy. The difference in the sensitivity of type I PVP neurons (top panel) during active versus passive movements reached significance 3 weeks post-lesion (20% difference; paired t-test, p = 0.04). In contrast, there was no significant difference between the responses of Type II PVP neurons during active and passive movements even 2 month after lesion (paired t-test, p > 0.3). (B) After lesion, VOR gains averaged across both animals were significantly higher during active head movements compared to similar passive rotations (paired t-test, p < 0.03). * indicates significant difference, paired t-test, p < 0.05
Finally, we asked whether the preferential enhancement of VOR interneuron responses (i.e. type I PVP neurons) contributes to improved behavioral performance during active movements. To address this question, we quantified performance by computing the average gain of the VOR eye movement response evoked by passive head rotations for both animals before and after lesion (METHODS). There was no significant difference in VOR gains measured in each condition before the lesion (p > 0.9). However, when we compared the VOR gains measured 4 weeks after lesion, gains were significantly elevated during active (Fig. 6B, black) as compared to passive (Fig. 6B, grey) rotations reaching enhancements of >14% by week 4 (paired t-test, p <0.0001). Thus, our data show that an increase in VOR interneuron sensitivity is associated with improved VOR compensation and are consistent with the proposal that the measured changes in VOR responses were driven by the enhanced neuronal response sensitivities during active motion.
DISCUSSION
To understand the neuronal basis of the impressive recovery in the VOR that occurs after vestibular loss, we examined the linkage between neuronal and behavioural responses in alert behaving monkeys. We provide the first evidence that motor learning is mediated by the dynamic reweighting of inputs from different modalities (i.e., vestibular versus extra-vestibular) on the single neurons which constitute the first stage of vestibular processing in the brain. Notably, two types of signals, not present prior to the lesion, were shown to have an important role in re-establishing network function. Early in the course of this process, unmasked neck proprioceptive inputs played a critical role, demonstrated by faster and more substantial recovery of vestibular sensitivities in neck proprioceptive sensitive neurons. At later stages of recovery, neurons showed enhanced responses during active head movements, as a result of the unmasking of a motor efference copy signal. Our study of the linkage between changes in neuronal response sensitivities and simultaneously measured VOR responses during passive and active head-on-body rotations with comparable trajectories demonstrated that dynamic regulation of multimodal integration (i.e., an efference copy signal) was associated with increases in the recovery of vestibular sensitivity by individual neurons. Taken together, our findings provide evidence, at the single neuron level, for a functional linkage between the dynamic reweighting of extra-vestibular inputs and behavioural recovery, and suggest that homeostatic mechanisms underlie the unmasking of extra-vestibular signals at the level of VOR interneurons during motor learning.
Compensatory changes in vestibular sensitivities
The vestibular sensitivities of VOR interneurons (i.e., type I PVP neurons) showed robust recovery within one month (Fig. 7, blue line) consistent with the observed behavioral compensation. Previous experiments performed in anesthetized preparations (reviewed in: Straka et al., 2005) have reported far less neuronal recovery. However, it is important to note that synaptic inputs were likely suppressed and/or VOR interneurons could not be identified in these latter studies. Interestingly, we further found that neuronal recovery trailed behavioural recovery by ~1 week. One intriguing possibility is that PVP neurons consolidate adjustments previously computed elsewhere. Notably, floccular target neurons in the vestibular nuclei contribute to a parallel drive to the VOR (Broussard and Lisberger, 1992; Scudder and Fuchs, 1992). Further studies will be required to determine whether these neurons predominantly drive the earliest phases of VOR compensation.
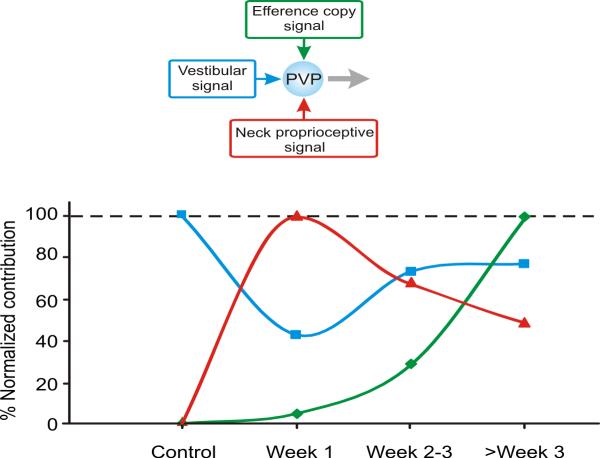
Figure 7.
The time course of dynamic regulation of multimodal integration in the direct VOR pathway (i.e., type I PVP neurons) following contralateral labyrinthectomy. Responses are normalized relative to the maximum response to each of the 3 inputs: vestibular (blue, measured by whole-body rotation), neck proprioceptive (red, measured by body-under-head rotation), and efference copy signal (green, measured by the difference between active and passive head-on-body rotation). All values are normalized relative to the maximum response (i.e., 100%) for each input. The relative contribution of the vestibular input decreased during the first week post-lesion by >50%, while during this same period the contribution of neck signals increased to its maximum value. Over the next weeks as the vestibular contribution returned to pre-lesion levels, the contribution of neck inputs decreased. Moreover, by week 3 the response of PVP neurons were enhanced during active relative to passive head-on-body movements suggesting the further integration of an efference copy of the neck motor command at the level of the VOR interneurons.
The unmasking of extra-vestibular inputs
Neurons in alert rhesus monkeys with intact vestibular function do not respond to neck proprioceptive stimulation (Roy and Cullen 2001). Following labyrinthectomy, proprioceptive responses were unmasked and were most enhanced immediately after lesion (Fig. 7, red line). Because the vestibular nuclei receive neck proprioceptive information via direct projections from the central cervical nucleus (Sato et al., 1997) and cerebellum (Eccles et al., 1974; Furuya et al., 1975; Akaike, 1983; Noda et al., 1990; Robinson et al., 1994) this suggests that the synapses mediating neck inputs are either normally silent (Kerchner and Nicoll, 2008) or, given that silent synapses are not normally abundant in the developed brain, cancelled by gating in an additional input (Keuroghlian and Knudsen, 2007). Previous studies in isolated, in vitro whole brain preparations have characterized the synaptic efficacy of spinal inputs to the vestibular nucleus. Notably, a progressive asymmetry develops during compensation in which the synaptic efficacy decreases on the intact side and increases on the lesioned one (Vibert et al., 1999). This reorganization could potentially have more beneficial results at the cellular than network level (see Rohregger and Dieringer, 2003). Our paper is the first to directly measure the functional implications of the dynamic reweighting of spinal inputs to individual VOR interneurons. While our results are also consistent with a change in the efficacy of spinal inputs, the intact side showed an increased, not decreased, sensitivity. Even more importantly, the increased response to neck proprioception, measured at the level of single neurons, was not accompanied by a parallel modality-specific improvement in motor performance. By combining neuronal and behavioural measurements, our experiments firmly establish that changes in the efficacy of spinal inputs to vestibular nucleus neurons are not linked to changes in spinal driven ocular performance (i.e., the compensatory cervico-ocular reflex).
What role does the increased efficacy of spinal inputs to vestibular nucleus neurons have in mediating vestibular compensation? As discussed above, our findings clearly show that proprioceptive inputs do not drive a compensatory eye movement to enhance motor performance. Rather, they suggest the unmasking of neck proprioceptive inputs supports a homeostatic mechanism that ensures continued dynamic stimulation of the reflex network following lesion. Evidence for a causal role of the unmasking of neck inputs in the recovery of neuronal responses was provided by two key findings. First, the resting firing rates of neck-sensitive type-I PVP neurons were normal even on the first day after lesion, whereas it took more than two weeks for the resting discharge of neck-insensitive neurons to attain normal values. Second, we found that the recovery of neuronal sensitivity to vestibular stimulation was more rapid for our population of neck-sensitive versus neck-insensitive type-I PVP neurons.
Our paper is also the first to compare neuronal response sensitivities and simultaneously measured VOR responses during passive and active head-on-body rotations during vestibular compensation (Fig. 7, green line). By evaluating how neuronal responses change during actively generated versus passively applied movements, we show that the dynamic regulation of multimodal integration (in this case, an efference copy signal) can be associated with behavioural recovery. Thus, our results establish a neural correlate for the improvement in gaze stability that is observed during active motion following vestibular loss in patients and rhesus monkeys (Dichgans et al., 1973; Newlands et al., 2001; Della Santina et al., 2002). It is possible that PVP neurons might show even further enhancement for active head motion at later stages of compensation (see trend in Fig. 7).
Mechanisms for compensatory changes
Previous studies of vestibular processing have focused on how correlation-based mechanisms (e.g., long-term potentiation (LTP) and long-term depression (LTD)) contribute to VOR plasticity. High frequency stimulation of the vestibular nerve evokes both LTD and LTP in the vestibular nuclei (Caria et al., 1996; Caria et al., 2001; Grassi and Pettorossi, 2001) and the induction of either form of synaptic plasticity can be mediated through the activation of NMDA receptors (Capocchi et al., 1992; Grassi et al., 1995). The results of more recent studies have provided evidence that compensation also involves longer term changes in the vestibular nuclei including the modification of neuronal pacemaker activity (Him and Dutia, 2001) and response dynamics (Beraneck et al., 2003; Beraneck et al., 2004), as well as changes in the balance of excitatory and inhibitory inputs (Goto et al., 2000, 2001). In addition to these central compensatory mechanisms, we have described long term changes at the level of vestibular periphery that could contribute to compensation (Sadeghi et al., 2007a).
The findings of the present study further suggest that the slower homeostatic mechanisms that promote network stability do so through the dynamic regulation of multimodal integration. One type of homeostatic plasticity that has received considerable attention is activity-dependent synaptic scaling, in which a neuron adjusts its synaptic strengths in response to changes in its own firing. Prior studies have shown that vestibular and proprioceptive inputs to the vestibular nuclei neurons are mediated by AMPA and NMDA receptors, respectively (Smith et al., 1991; Straka and Dieringer, 2004). The observation that neurons are insensitive to neck rotation before lesion suggests that these synapses are normally silent. Following lesion, the increase in the number of AMPA, but not NMDA, receptors (King et al., 2002) can lead to an increase in colocalization of NMDA and AMPA receptors (Chen et al., 2000), leading to activation of `silent' NMDA synapses (Kerchner and Nicoll, 2008). In this schema, homeostatic plasticity (i.e., activation of silent synapses) and the resultant manifestation of neuronal sensitivity to neck inputs could support the long-term reweighting of synapses from vestibular inputs required for VOR compensation.
Another possible, not mutually exclusive, explanation for why extravestibular inputs are silent under normal conditions is that they are normally gated out by additional inputs. Gating mechanisms have been proposed to explain the elimination of inputs both originating from irregular afferents (Minor and Goldberg, 1991) as well as the differential processing of active and passive motion (Roy and Cullen, 2004) in vestibular pathways. Future experiments using selective manipulations of sensory inputs from each modality will be required to evaluate these potential mechanisms.
Conclusion
In closing, our findings establish at the single unit level a functional link between the recovery of vestibular responses and the unmasking of extra-vestibular information during motor learning. Notably, the recovery of VOR motor performance involved not only the reweighting of synapses from vestibular inputs as is generally thought, but also the unmasking of inputs from other modalities. Thus, this work provides a foundation for understanding the role of multimodal convergence in learning, as well as a basis for the potential development of novel rehabilitation approaches to take advantage of the convergence of sensory inputs and motor signals that contribute to the early and late stages of compensation.
ACKNOWLEDGEMENTS
We thank Drs. Maurice Chacron, Paul Fuchs, Elisabeth Glowatzki, and Charley Della Santina for critically reading the manuscript. Marion Van Horn, Jessica Brooks, Diana Mitchel, Corentin Massot, Mohsen Jamali, and Jerome Carriot provided useful comments. Diana Mitchel and Marion Van Horn helped in preparing the figures. This work was supported by grants from the Canadian Institutes of Health Research (CIHR) and NIH R01 DC02390.
REFERENCES
- Akaike T. Electrophysiological analysis of cerebellar corticovestibular and fastigiovestibular projections to the lateral vestibular nucleus in the cat. Brain Res. 1983;272:223–235. doi: 10.1016/0006-8993(83)90568-1. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Idoux E, Uno A, Vidal PP, Moore LE, Vibert N. Unilateral labyrinthectomy modifies the membrane properties of contralesional vestibular neurons. J Neurophysiol. 2004;92:1668–1684. doi: 10.1152/jn.00158.2004. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Hachemaoui M, Idoux E, Ris L, Uno A, Godaux E, Vidal PP, Moore LE, Vibert N. Long-term plasticity of ipsilesional medial vestibular nucleus neurons after unilateral labyrinthectomy. J Neurophysiol. 2003;90:184–203. doi: 10.1152/jn.01140.2002. [DOI] [PubMed] [Google Scholar]
- Bronstein AM, Hood JD. The cervico-ocular reflex in normal subjects and patients with absent vestibular function. Brain Res. 1986;373:399–408. doi: 10.1016/0006-8993(86)90355-0. [DOI] [PubMed] [Google Scholar]
- Broussard DM, Lisberger SG. Vestibular inputs to brain stem neurons that participate in motor learning in the primate vestibuloocular reflex. J Neurophysiol. 1992;68:1906–1909. doi: 10.1152/jn.1992.68.5.1906. [DOI] [PubMed] [Google Scholar]
- Capocchi G, Della Torre G, Grassi S, Pettorossi VE, Zampolini M. NMDA receptor-mediated long term modulation of electrically evoked field potentials in the rat medial vestibular nuclei. Exp Brain Res. 1992;90:546–550. doi: 10.1007/BF00230937. [DOI] [PubMed] [Google Scholar]
- Caria MA, Melis F, Podda MV, Solinas A, Deriu F. Does long-term potentiation occur in guinea-pig Deiters' nucleus? Neuroreport. 1996;7:2303–2307. doi: 10.1097/00001756-199610020-00007. [DOI] [PubMed] [Google Scholar]
- Caria MA, Melis F, Solinas A, Tavera C, Mameli O. Frequency-dependent LTP/LTD in guinea pig Deiters' nucleus. Neuroreport. 2001;12:2353–2358. doi: 10.1097/00001756-200108080-00014. [DOI] [PubMed] [Google Scholar]
- Chen LW, Yung KK, Chan YS. Co-localization of NMDA receptors and AMPA receptors in neurons of the vestibular nuclei of rats. Brain Res. 2000;884:87–97. doi: 10.1016/s0006-8993(00)02913-9. [DOI] [PubMed] [Google Scholar]
- Cullen KE. Procedural learning. In: Roediger HL III, editor. Cognitive psychology of memory. Elsevier; Oxford: 2008. [Google Scholar]
- Cullen KE, McCrea RA. Firing behavior of brain stem neurons during voluntary cancellation of the horizontal vestibuloocular reflex. I. Secondary vestibular neurons. J Neurophysiol. 1993;70:828–843. doi: 10.1152/jn.1993.70.2.828. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Chen-Huang C, McCrea RA. Firing behavior of brain stem neurons during voluntary cancellation of the horizontal vestibuloocular reflex. II. Eye movement related neurons. J Neurophysiol. 1993;70:844–856. doi: 10.1152/jn.1993.70.2.844. [DOI] [PubMed] [Google Scholar]
- Cullen KE, Rey CG, Guitton D, Galiana HL. The use of system identification techniques in the analysis of oculomotor burst neuron spike train dynamics. J Comput Neurosci. 1996;3:347–368. doi: 10.1007/BF00161093. [DOI] [PubMed] [Google Scholar]
- Della Santina CC, Cremer PD, Carey JP, Minor LB. Comparison of head thrust test with head autorotation test reveals that the vestibulo-ocular reflex is enhanced during voluntary head movements. Arch Otolaryngol Head Neck Surg. 2002;128:1044–1054. doi: 10.1001/archotol.128.9.1044. [DOI] [PubMed] [Google Scholar]
- Dichgans J, Bizzi E, Morasso P, Tagliasco V. Mechanisms underlying recovery of eye-head coordination following bilateral labyrinthectomy in monkeys. Exp Brain Res. 1973;18:548–562. doi: 10.1007/BF00234137. [DOI] [PubMed] [Google Scholar]
- Duensing F, Schaefer KP. The activity of single neurons in the region of vestibular nuclei in horizontal acceleration, with special reference to vestibular nystagmus. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1958;198:225–252. doi: 10.1007/BF00941383. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Sabah NH, Taborikova H. The pathways responsible for excitation and inhibition of fastigial neurones. Exp Brain Res. 1974;19:78–99. doi: 10.1007/BF00233396. [DOI] [PubMed] [Google Scholar]
- Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya N, Kawano K, Shimazu H. Functional organization of vestibulofastigial projection in the horizontal semicircular canal system in the cat. Exp Brain Res. 1975;24:75–87. doi: 10.1007/BF00236018. [DOI] [PubMed] [Google Scholar]
- Goto F, Straka H, Dieringer N. Expansion of afferent vestibular signals after the section of one of the vestibular nerve branches. J Neurophysiol. 2000;84:581–584. doi: 10.1152/jn.2000.84.1.581. [DOI] [PubMed] [Google Scholar]
- Goto F, Straka H, Dieringer N. Postlesional vestibular reorganization in frogs: evidence for a basic reaction pattern after nerve injury. J Neurophysiol. 2001;85:2643–2646. doi: 10.1152/jn.2001.85.6.2643. [DOI] [PubMed] [Google Scholar]
- Grassi S, Pettorossi VE. Synaptic plasticity in the medial vestibular nuclei: role of glutamate receptors and retrograde messengers in rat brainstem slices. Prog Neurobiol. 2001;64:527–553. doi: 10.1016/s0301-0082(00)00070-8. [DOI] [PubMed] [Google Scholar]
- Grassi S, Frondaroli A, Pessia M, Pettorossi VE. Exogenous glutamate induces short and long-term potentiation in the rat medial vestibular nuclei. Neuroreport. 2001;12:2329–2334. doi: 10.1097/00001756-200108080-00010. [DOI] [PubMed] [Google Scholar]
- Grassi S, Della Torre G, Capocchi G, Zampolini M, Pettorossi VE. The role of GABA in NMDA-dependent long term depression (LTD) of rat medial vestibular nuclei. Brain Res. 1995;699:183–191. doi: 10.1016/0006-8993(95)00895-w. [DOI] [PubMed] [Google Scholar]
- Hayes A, Richmond B, Optican L. A UNIX-based multiple process system for real-time data acquisition and control. WESCON Conf Proc. 1982;2:1–10. [Google Scholar]
- Him A, Dutia MB. Intrinsic excitability changes in vestibular nucleus neurons after unilateral deafferentation. Brain Res. 2001;908:58–66. doi: 10.1016/s0006-8993(01)02600-2. [DOI] [PubMed] [Google Scholar]
- Huterer M, Cullen KE. Vestibuloocular reflex dynamics during high-frequency and high-acceleration rotations of the head on body in rhesus monkey. J Neurophysiol. 2002;88:13–28. doi: 10.1152/jn.2002.88.1.13. [DOI] [PubMed] [Google Scholar]
- Jurgens R, Mergner T. Interaction between cervico-ocular and vestibulo-ocular reflexes in normal adults. Exp Brain Res. 1989;77:381–390. doi: 10.1007/BF00274995. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Nicoll RA. Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci. 2008;9:813–825. doi: 10.1038/nrn2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82:109–121. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- King J, Zheng Y, Liu P, Darlington CL, Smith PF. NMDA and AMPA receptor subunit protein expression in the rat vestibular nucleus following unilateral labyrinthectomy. Neuroreport. 2002;13:1541–1545. doi: 10.1097/00001756-200208270-00011. [DOI] [PubMed] [Google Scholar]
- Kotak VC, Fujisawa S, Lee FA, Karthikeyan O, Aoki C, Sanes DH. Hearing loss raises excitability in the auditory cortex. J Neurosci. 2005;25:3908–3918. doi: 10.1523/JNEUROSCI.5169-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente De No' R. Vestibular-ocular reflex arc. Arch Neurol Psychiatry. 1933;30:245–291. [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinvaud D, Vassias I, Reichenberger I, Rossert C, Straka H. Functional organization of vestibular commissural connections in frog. J Neurosci. 2010;30:3310–3325. doi: 10.1523/JNEUROSCI.5318-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea RA, Strassman A, Highstein SM. Anatomical and physiological characteristics of vestibular neurons mediating the vertical vestibulo-ocular reflexes of the squirrel monkey. J Comp Neurol. 1987;264:571–594. doi: 10.1002/cne.902640409. [DOI] [PubMed] [Google Scholar]
- Minor LB, Goldberg JM. Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional-ablation study in the squirrel monkey. J Neurosci. 1991;11:1636–1648. doi: 10.1523/JNEUROSCI.11-06-01636.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlands SD, Hesse SV, Haque A, Angelaki DE. Head unrestrained horizontal gaze shifts after unilateral labyrinthectomy in the rhesus monkey. Exp Brain Res. 2001;140:25–33. doi: 10.1007/s002210100810. [DOI] [PubMed] [Google Scholar]
- Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990;302:330–348. doi: 10.1002/cne.903020211. [DOI] [PubMed] [Google Scholar]
- Robinson FR, Phillips JO, Fuchs AF. Coordination of gaze shifts in primates: brainstem inputs to neck and extraocular motoneuron pools. J Comp Neurol. 1994;346:43–62. doi: 10.1002/cne.903460104. [DOI] [PubMed] [Google Scholar]
- Rohregger M, Dieringer N. Postlesional vestibular reorganization improves the gain but impairs the spatial tuning of the maculo-ocular reflex in frogs. J Neurophysiol. 2003;90:3736–3749. doi: 10.1152/jn.00561.2003. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. A neural correlate for vestibulo-ocular reflex suppression during voluntary eye-head gaze shifts. Nat Neurosci. 1998;1:404–410. doi: 10.1038/1619. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Selective processing of vestibular reafference during self-generated head motion. J Neurosci. 2001;21:2131–2142. doi: 10.1523/JNEUROSCI.21-06-02131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Vestibuloocular reflex signal modulation during voluntary and passive head movements. J Neurophysiol. 2002;87:2337–2357. doi: 10.1152/jn.2002.87.5.2337. [DOI] [PubMed] [Google Scholar]
- Roy JE, Cullen KE. Dissociating self-generated from passively applied head motion: neural mechanisms in the vestibular nuclei. J Neurosci. 2004;24:2102–2111. doi: 10.1523/JNEUROSCI.3988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Dynamics of the horizontal vestibuloocular reflex after unilateral labyrinthectomy: response to high frequency, high acceleration, and high velocity rotations. Exp Brain Res. 2006;175:471–484. doi: 10.1007/s00221-006-0567-7. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Minor LB, Cullen KE. Response of vestibular-nerve afferents to active and passive rotations under normal conditions and after unilateral labyrinthectomy. J Neurophysiol. 2007a;97:1503–1514. doi: 10.1152/jn.00829.2006. [DOI] [PubMed] [Google Scholar]
- Sadeghi SG, Mitchell DE, Cullen KE. Different neural strategies for multimodal integration: comparison of two macaque monkey species. Exp Brain Res. 2009;195:45–57. doi: 10.1007/s00221-009-1751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi SG, Chacron MJ, Taylor MC, Cullen KE. Neural variability, detection thresholds, and information transmission in the vestibular system. J Neurosci. 2007b;27:771–781. doi: 10.1523/JNEUROSCI.4690-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Ohkawa T, Uchino Y, Wilson VJ. Excitatory connections between neurons of the central cervical nucleus and vestibular neurons in the cat. Exp Brain Res. 1997;115:381–386. doi: 10.1007/pl00005708. [DOI] [PubMed] [Google Scholar]
- Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol. 1992;68:244–264. doi: 10.1152/jn.1992.68.1.244. [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Robinson DA, Tan HS. Context-specific adaptation of the gain of the vestibulo-ocular reflex in humans. J Vestib Res. 1992;2:89–96. [PubMed] [Google Scholar]
- Shimazu H, Precht W. Inhibition of central vestibular neurons from the contralateral labyrinth and its mediating pathway. J Neurophysiol. 1966;29:467–492. doi: 10.1152/jn.1966.29.3.467. [DOI] [PubMed] [Google Scholar]
- Smith PF, de Waele C, Vidal PP, Darlington CL. Excitatory amino acid receptors in normal and abnormal vestibular function. Mol Neurobiol. 1991;5:369–387. doi: 10.1007/BF02935559. [DOI] [PubMed] [Google Scholar]
- Straka H, Dieringer N. Basic organization principles of the VOR: lessons from frogs. Prog Neurobiol. 2004;73:259–309. doi: 10.1016/j.pneurobio.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Straka H, Vibert N, Vidal PP, Moore LE, Dutia MB. Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog Neurobiol. 2005;76:349–392. doi: 10.1016/j.pneurobio.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Cullen KE. Quantitative analysis of abducens neuron discharge dynamics during saccadic and slow eye movements. J Neurophysiol. 1999;82:2612–2632. doi: 10.1152/jn.1999.82.5.2612. [DOI] [PubMed] [Google Scholar]
- Sylvestre PA, Cullen KE. Premotor correlates of integrated feedback control for eye-head gaze shifts. J Neurosci. 2006;26:4922–4929. doi: 10.1523/JNEUROSCI.4099-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert N, Babalian A, Serafin M, Gasc JP, Muhlethaler M, Vidal PP. Plastic changes underlying vestibular compensation in the guinea-pig persist in isolated, in vitro whole brain preparations. Neuroscience. 1999;93:413–432. doi: 10.1016/s0306-4522(99)00172-4. [DOI] [PubMed] [Google Scholar]