Abstract
Multifunctional motorneurons and muscles, which are active during forward and backward locomotion and driven by common central pattern generators, are ubiquitous in animal models. However, studies in the nematode Caenorhabditis elegans suggest that some locomotor motorneurons are necessary only for forward locomotion (dorsal B-motorneurons, DB) while others (dorsal A-motorneurons, DA) are necessary only for backward locomotion. We tested this hypothesis directly by recording the activity of these motorneurons during semi-restrained locomotion. For this purpose, we used epifluorescence imaging of the genetically encoded calcium sensor cameleon, expressed in specific motorneurons, while monitoring locomotor behavior through the microscope condenser using a second camera. We found that ventral and dorsal B-motorneurons (DB and VB) were co-active during forward locomotion while ventral A-motorneurons (VA) were only active during backward locomotion. The signals we recorded correlated with the direction of locomotion but not with the faster undulatory cycles. To our knowledge, these are the first recordings of motorneuron activity in C. elegans and the only direction-dedicated motorneurons described to date.
Keywords: C. elegans, locomotion, motorneurons, cameleon, backward, forward
Introduction
The neural basis of locomotor behavior in the nematode Caenorhabditis elegans is a particularly attractive system for study because of the simplicity of its neuromuscular system. C. elegans propels itself forward or backward by the sequential contraction of 95 muscle cells arranged in 4 quadrants along the body (Altun and Hall, 2009). In adult hermaphrodites, muscle cells of the body wall are innervated by 75 motorneurons comprising 8 distinct classes (Fig. 1a; White et al., 1986; Altun and Hall, 2009; Chen et al., 2006). Four classes innervate ventral muscle (VA, VB, VD, VC) and four innervate dorsal muscle (DA, DB, DD, AS). The involvement of specific classes of motorneurons in locomotion was explored in earlier neuron-ablation studies in young larvae when only three classes of locomotor motorneuron are present: DA, DB, DD. Ablation of most DB motorneurons impairs forward, but not backward locomotion. Conversely, ablation of most DA motorneurons impairs backward but not forward locomotion. Ablation of most DD motorneurons produces animals that are severely uncoordinated when moving in either direction (Chalfie et al., 1985). These experiments and others (Harbinder et al., 1997), along with neuronal morphology and connectivity (White et al., 1976), predicted that B-motorneurons (DB, VB) would be active during forward and A-motorneurons (DA, VA) during backward locomotion (Niebur and Erdos, 1993; Bryden and Cohen, 2008; Karbowski et al., 2008). Herein, we directly test this hypothesis by imaging motorneuron activity with a genetically encoded calcium sensor and correlating it to the direction of semi restrained locomotion.
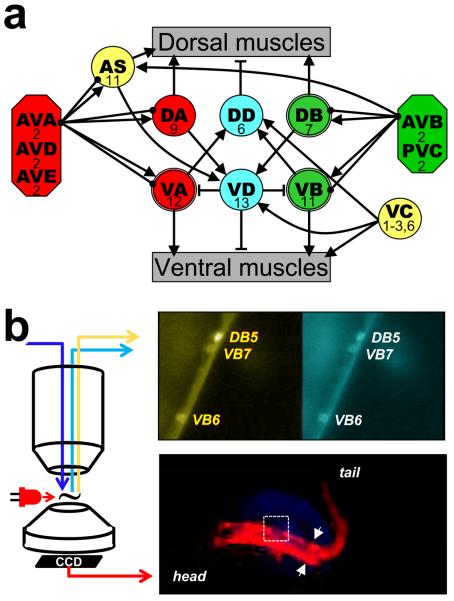
Figure 1.
Locomotor neuronal circuit and recording set up. a) Schematic of connectivity of the locomotor circuit (base on Chalfie and White (1988), Von Stetina et al. (2006) with additional connections extracted from (Altun and Hall, 2009) and supplementary material of Chen et al. (2006)): five pairs of interneurons (octagons) innervate eight groups of motorneurons (circles) that innervate either dorsal or ventral muscle of the body wall. Connecting lines represent excitatory (arrows) or inhibitory (T lines) synapses and gap junctions (solid circles). The number of neurons in each group is indicated. DB, VB and VA, which were recorded from in this study and have a double-line frame. For simplicity, connectivity among interneurons is not presented and interneurons are grouped based on targets. b) Recording set-up. Epifluorescence calcium imaging of motorneurons was done through a water immersion objective on an upright microscope. An example of pseudocolored yellow and cyan channels is shown in the upper right panel. The nematode's behavior was imaged through the condenser using a modified webcam. The animal was illuminated with a red LED positioned close to the recording chamber. An image of the nematode viewed this way is shown in the lower right hand panel. The arrows indicate the caudal limit of the glued region and the field of view of the calcium imaging is shown by the white rectangle.
Materials and Methods
Strains
C. elegans were cultivated at 15 or 25°C on NGM agar plates containing Escherichia coli (strain: OP50) according to standard techniques (Stiernagle, 2006). Transgenic wild type (N2 Bristol) animals expressing the calcium indicator yellow cameleon YC2.60 or YC3.60 (Nagai et al., 2004) in a subset of motorneuron classes were used in all studies. HA1722 (rtIs29 [acr-5p::YC2.60]) animals express cameleon in all VB and DB motorneurons and in some head and tail neurons; the latter were out of the field of view during calcium imaging. HA1706 and HA1708 (pha-1(e2123); rtEx630 or rtEx631 [pha-1(+); del-1p::YC2.60]), express cameleon in all VB and VA motorneurons. TOL4 (aatIs4 [unc-17p::YC3.60]) express cameleon in all excitatory motorneurons.
Semi-restrained preparation
The anterior portion of young adults or L4 larvae (between head and vulva) was semi-restrained to a 1.5% agarose pad (saline as below, pH 9.0) with veterinary grade cyanoacrylate glue (Nexaband, Fisher). This stabilizes motorneurons within the glued region for imaging while the posterior body is free to move. This type of restraint has been used successfully to study leech (Baader and Kristan, 1992) and bullfrog tadpole (Rock et al., 1981) motor behaviors and assumes that the activity of neurons in the restrained region is similar to that in the moving part of the animal (see below for discussion). A coverslip with an agarose pad was placed on a cooling block (0-5°C), to minimize animal movements during the gluing process. The coverslip with the glued animal was placed in a perfusion chamber (RC-21BDW, Warner Instruments) and covered with saline solution (modified M13 saline (100mM NaCl, 10mM KCl, 10mM HEPES, 2mM MgCl2, 2mM CaCl2; pH 7.4 (NaOH); 350mOsm (glycerol)). Imaging sessions terminated at 30 minutes from tethering to avoid starvation (Sawin et al., 2000).
Calcium imaging
Two versions of the genetically encoded calcium indicator, Cameleon, were tested. Compared with Cameleon YC2.60, Cameleon YC3.60 has higher affinity for calcium (250 vs 40 nM (Nagai et al., 2004) and shorter time constant for decay (0.73 vs 5.24 sec; (Hendel et al., 2008). The signals acquired from YC3.60 were smaller and less reproducible than those acquired from YC2.60 (n=33 paired recording of VB and DB; and as observed before to a lesser extent in Hendel et al., (2008); see Supp. Fig. 1). Because switching between forward and backward locomotion is in the time scale of several seconds the data presented was recorded with YC2.60.
Wide-field epifluorescence imaging was performed on a fixed stage upright microscope (Axioskop2FLplus) through a 63x, 0.95 numerical aperture (NA) water immersion Achroplan objective (Zeiss). For dual-emission imaging of yellow cameleon an X-cite light source (EXFO), an excitation filter (D420/40x; all filters and mirrors from Chroma), a dichroic mirror (455dclp) and an image-splitter (W-view, Hamamatsu) were used. Two dichroic mirrors (500dcxr) in the image-splitter separate the emitted light into a cyan and a yellow image that were further filtered (470/30 and 535/30) and then projected side by side onto a cooled charge-coupled device (CCD) camera (Orca II ERG, Hamamatsu). Image stacks were acquired at 10Hz using MetaVue software (Molecular Devices). A custom program (Jmalyze, written by R. Kerr and previously described (Kerr et al., 2000)) was used to track movement of individual motorneurons in the yellow channel and to measure the intensity of four regions of interest (ROI: cyan neuron and background and yellow neuron and background). IgorPro (ver. 5.03, WaveMetrics) and Matlab (ver. 7.4.0R2007a, MathWorks) were used for additional analyses. The average intensity of a background ROI of each channel was subtracted from the average intensity of the neuron ROI. The emission ratio was calculated as the ratio of yellow and cyan values and was divided by a fitted single exponent (correcting for photobleaching) to give ΔR/R. The ΔR/R traces were denoised with a boxcar filter with a window of 3 time points prior to subsequent analysis. Signal to noise ratio (SNR) was calculated as the amplitude of the signal (filtered trace) divided by three times the root mean square of the noise (filtered trace subtracted from unfiltered). Traces with SNRs less than two were discarded. The mean SNR for traces included in the study was 5.2 (range: 2.0-15.8). Traces are presented in the figures without filtering. Signal sizes are presented as average ± standard error of the mean ΔR/R % throughout text. Mean duration and interval for the imaging signals and for behavioral scoring were restricted to complete sequences within the analysis period. Incomplete sequences at the beginning and the end of the analysis period were discarded.
Behavior imaging
In some experiments, the behavior of the nematode was monitored and recorded during calcium imaging with a small CCD (extracted from a USB webcam) placed under the microscope condenser using the condenser as a low magnification objective (Fig. 1b). A piece of filter-gel (#27 Roscolux; Rosco) was positioned over the CCD and the nematode was illuminated by a red LED whose light was blocked by the filters used for the calcium imaging. The effective frame rate was 2.5Hz (30 Hz refreshed every 12 frames). The webcam also recorded the excitation light used for calcium imaging, and this signal was used to synchronize the two sets of data. This set up is similar to that described by Faumont and Lockery (2006) which utilized an inverted microscope and an additional objective. Each behavior video was scored, blind to the calcium imaging data, at least twice for transitions between backward and forward semi-restrained locomotion.
Results
VB and DB motorneurons are co-active while VB and VA are never co-active during semi-restrained locomotion
In an aqueous environment, C. elegans engage in nearly continuous, spontaneous locomotion when the anterior of their body is restrained. Neuronal activity in motorneuron cell bodies can be followed using the genetically encoded calcium indicator Yellow Cameleon that produces altered fluorescence ratio levels (ΔR/R) in response to changes in calcium levels (Nagai et al., 2004). To follow activity during semi-restrained locomotion we used two different transgenic lines expressing YC2.60: either in VA and VB motorneurons or alternatively in VB and DB motorneurons. We found that neighboring ventral A and B motorneurons (VA, VB) exhibited antagonistic changes in activity during periods of semi-restrained locomotion. Averaged peak values of the ratio changes were 16.8 ± 2.4 % (VA2-VA7, n=20 neurons) and 13.6 ± 1.1 % (VB3-VB7, n=23 neurons) respectively (Fig. 2a; 12 animals). In nematodes expressing the calcium sensor in ventral and dorsal B-motorneurons (VB, DB), activity increased at the same time in both classes of motorneuron. Averaged peak values of the ratio changes were 14.5 ± 1.0 % (VB3-VB8, n=35 neurons) and 16.4 ± 1.6 % (DB3-DB5, n=18 neurons) respectively (Fig. 2b; 17 animals).
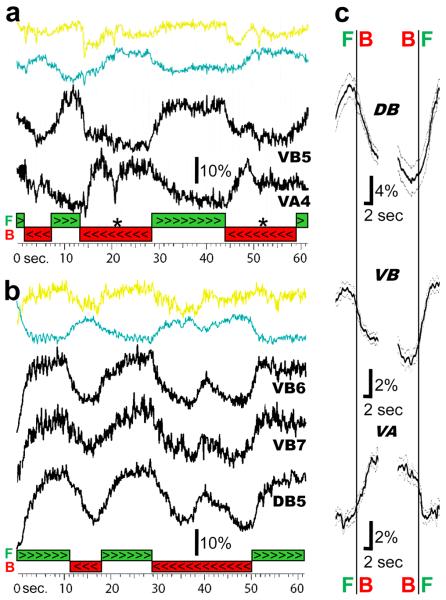
Figure 2.
B-motorneurons are active during forward semi-restrained locomotion while A-motorneurons are active during backward. a) The top two colored traces are the signals from the yellow and cyan channels recorded from a VB5 motorneuron. The ratio of the yellow/cyan signal is shown underneath these traces together with the ratio signal from a VA4 motorneuron during forward (green bar >>>) and backward (red bar <<<) locomotion. The asterisk (*) marks a period when locomotion stopped. b) Dorsal and ventral B-motorneurons: The raw yellow and cyan traces and their ratio recorded from a VB6 motorneuron (yellow and cyan and uppermost black traces) together with the activity of a VB7 motorneuron (middle black trace) and a DB5 motorneuron (bottom black trace) during forward (green bar >>>) and backward (red bar <<<) locomotion. c) Mean ratio change during the switch from forward to backward (left traces) and backward to forward (right traces) locomotion. All ratiometric recordings were aligned by the time of the independently scored changes in direction determined from the behavior video. The number of transitions used to compute the averages were: forward to backward- 12 (DB), 60 (VB), 23 (VA); backward to forward- 13 (DB), 55 (VB), 18 (VA). Grey traces are one standard error of the mean.
The mean duration and interval between these signals were 12.0 ± 0.7 sec and 6.7 ± 0.4 sec, respectively, corresponding to the duration and interval between forward and backward movements, but were considerably slower than the undulation frequency (0.74 Hz± 0.03). In the next section, we compare the fluorescence changes in motorneurons with the behaviorally recorded forward and backward sequences and quantify the temporal relationships between the behavior and the activity of the A and B motorneurons.
VB and DB motorneurons are active during forward and VA during backward locomotion
In 14 animals, we monitored locomotor behavior at the same time as the calcium imaging. This allowed us to relate the fluorescence changes in specific motorneurons to the direction of movement (see Methods and Fig. 1b). As described previously (Faumont et al., 2005), two locomotor states occur during semi-restrained locomotion: a fast and shallow dorsoventral bending (0.74 ± 0.03 Hz range: 0.38-1.25 Hz) associated with forward locomotion and a slower, deeper bending (0.20 ± 0.01 Hz range: 0.08-0.44 Hz) associated with backward locomotion. Each bout of directional locomotion consisted of several dorsoventral undulations. Some bouts were interrupted by a period in which the animal would remain bent either dorsally or ventrally for a few seconds (e.g. Fig. 2a). These stops could correspond to slow backward locomotion, omega turns (Faumont et al., 2005) or to rest (Wakabayashi et al., 2004) and were excluded from this study. We found that B-motorneurons were active during forward locomotion while A-motorneurons were active during backward locomotion.
We scored the behavior movies for changes in the direction of semi-restrained locomotion and used the times when a change of direction occurred to select sections of the calcium imaging data (5 sec before and 5 sec after) for further analysis. This was done blind to the calcium imaging data.
Averaged responses (Fig. 2c) demonstrate that the decrease in calcium levels in DB (n=12 changes in direction) and VB motorneurons (n= 60) coincided with an increase in VA motorneurons (n=23) and these changes preceded a switch from backward to forward locomotion. A corresponding increase in calcium levels in DB (n=13) and VB motorneurons (n=55) coincided with a decrease in VA motorneurons (n=18) and preceded a switch from backward to forward locomotion. The change in direction of locomotion did not require the calcium level to reach maximal levels (Fig. 2c; e.g. 2a, 2b). However, the slow time constant of YC2.60 might affect the shape of the calcium signals (Hendel et al., 2008) as we discuss below.
B-motorneurons of the same class are connected by gap junctions and could sequentially activate each other along the ventral cord to signal the change in direction. However, when we examined two adjacent VAs or two VBs (e.g. VB6 and VB7 in Fig. 2b), there was no apparent delay in the onset of the calcium rise at the beginning of a bout of locomotion in 14 out of 18 animals; the remaining 4 cases, either the anterior (3) or posterior (1) VB motorneuron was relatively delayed during both backward and forward locomotion.
No dorsoventral alternating activity was recorded in B-motorneurons
Dorsal and ventral B-motorneurons innervate antagonistic muscles and were hypothesized to exhibit alternating activity correlated with the body undulations during forward locomotion (Niebur and Erdos, 1993; Bryden and Cohen, 2008; Karbowski et al., 2008). Both classes of motorneuron possess long undifferentiated posterior processes that may function as a stretch receptor (White et al., 1986). The frequency of sinusoidal movements during semi-restrained forward locomotion was 0.74 ± 0.03 Hz which is slower than reported for free locomotion (1.46Hz; Tsechpenakis et al. (2008), 0.8/ 2.1 Hz crawl/swim; Pierce-Shimomura et al. (2008)). We did not observe cameleon ratio changes that correlated with undulatory rhythms. To maximize the detection of signal at these undulation frequencies, we filtered (0.25–4 Hz) the ratiometric signal recorded in a dorsal B-motorneurons and their ventral counterparts (namely DB3/VB3, DB4/VB5 and DB5/VB7), during bouts of forward locomotion before cross correlation analysis (Fig. 3a). ). The processes of DB4, VB5, DB5 and VB7 extend beyond the vulva (D. Hall pers. comm.) and are therefore subject to the movements of the unrestrained posterior part of the body. As a result, their putative sensory feedback should be similar to that occurring in free locomotion. If ventral and dorsal neurons alternated their activity, we expected an anti-phase (negative) cross correlation of neuronal activity. Instead, only 5 of 32 pairs of recordings exhibited any cross correlation of activity in the 0.25–4 Hz frequency range and those were weakly in-phase (Fig. 3b). Moreover, the amplitude of these synchronous signals was much smaller than the signal associated with the direction of locomotion discussed above. In addition, we cannot exclude the possibility that these small signals are artifacts, resulting from uncompensated movement of the motorneurons in the imaged field. Nonetheless, in none of our recordings could we detect alternating activity between the dorsal and ventral motorneurons (n=32 pairs). Therefore, given the limitations of calcium imaging (see below), our results do not support or contradict models in which ventral and dorsal B-motorneurons alternate in activity during forward locomotion.
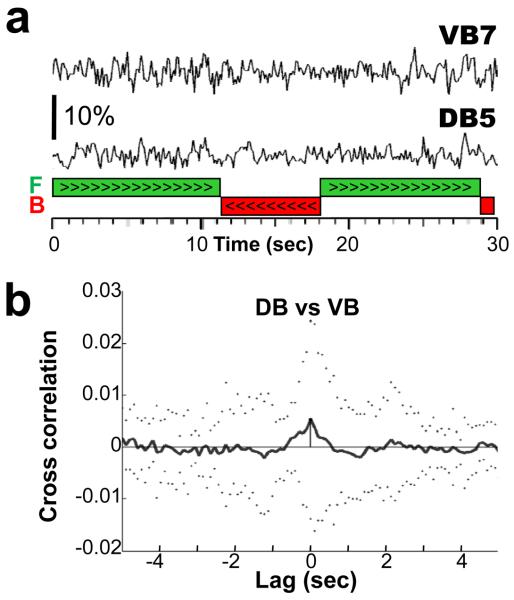
Figure 3.
No alternating activity associated with the body undulation was recorded in neighboring VB and DB motorneurons. a) The traces are the first 30 seconds of the band-pass filtered (0.25-4 Hz) middle (VB7) and bottom (DB5) ratio traces shown in figure 2b. No alternating activity between VB7 and DB5 is evident during the two bouts of forward locomotion (green bars) which comprised 8 and 11 undulations respectively. b) Mean (solid line) and S.D. (dotted line) cross correlation of ratio changes in all adjacent pairs of VB and DB motorneurons (n=32 DB/VB pairs). Ratio traces were band-pass filtered (0.25-4Hz) before cross correlation.
Discussion
We found that tonic fluorescence changes in the B-motorneurons (VB and DB) and A-motorneurons (VA) were mutually exclusive and correlated with the direction of locomotion. B-motorneurons were coactive during forward locomotion while A-motorneurons were active during backward locomotion. To our knowledge, this is the first demonstration of a specialized function for motorneurons in forward and backward locomotion. Furthermore, it is the first recording of motorneuron activity driving locomotion in C. elegans.
Our results demonstrate that DB, VB and VA classes of motorneurons are active during one direction of locomotion but not the other, consistent with earlier lesion studies showing that DA and DB were necessary for backward or forward locomotion respectively (Chalfie et al., 1985). The other classes of motorneurons have not yet been recorded from. This suggests that at least 39 (7 DB, 11 VB, 12 VA and 9 DA) of the 75 motorneurons that innervate locomotor muscles are dedicated to one direction of locomotion or another. In contrast to motorneurons, locomotor muscles are multifunctional and are active during both directions of locomotion (Pierce-Shimomura et al., 2008). This is because each muscle cell is innervated by four to eight motorneurons of different classes (White et al., 1976; Chen et al., 2006).
Failure to detect sinusoidal alternating activity between VB and DB motorneurons
Existing models for C. elegans locomotion (Niebur and Erdos, 1993; Bryden and Cohen, 2008; Karbowski et al., 2008) address only forward locomotion during crawling. In all of these models VB and DB are the sources of alternating activity to ventral and dorsal muscle. We were not able to discern any such alternating activity between pairs of VB and DB motorneurons that innervate contralateral muscle cells. If alternating motorneuron activity does occur, then one of several reasons may account for our failure to detect it. First, the activity of motorneurons at the recording site might be affected by the tethering. In addition, the decay time constant of YC2.60 we used to detect calcium changes accompanying forward and backward locomotion was too slow (5.2 seconds; Hendel et al., 2008) to allow us to resolve the faster oscillatory signals. Although YC3.60 has a faster decay time constant, the signals it generated were consistently smaller than YC2.60 and fast rhythmic signals accompanying sinusoidal movements could not be detected (Supp. Fig. 1). Finally, we cannot exclude the possibility that changes in calcium levels recorded in the cell body are decoupled from the activity of neuronal processes and the neuromuscular junction. The relationship between motorneuronal calcium levels and membrane potential is not known in C. elegans. As a result, calcium signals may not reflect changes in membrane potential. This is consistent with earlier work indicating that genetically encoded calcium indicators cannot monitor fast, sub-threshold or hyperpolarizing activity (Jayaraman and Laurent, 2007; Hendel et al., 2008).
The movements generated during semi-restrained locomotion are not identical to those occurring in free locomotion. For example, during semi-restrained locomotion, backward cycles are larger in amplitude and longer than those of free locomotion. At present it is not possible to image motorneuron activity during free locomotion, although recent technical developments may allow this goal to be accomplished (Chronis et al., 2007; Ben Arous et al., 2010). Despite the differences between semi-restrained and free locomotion, work in other systems suggests that it is unlikely that the partial restraint employed in these experiments results in a completely novel mode of operation of the motorneuronal network.
The results presented here cannot establish the presence or absence of alternating activity. It is possible, for example, that A and B class of motorneurons remain tonically active during backward and forward locomotion respectively and do not contribute to sinusoidal oscillations. Other cells may be the source of alternating activity. For example, alternating activity could be carved out of tonically activated muscle by phasic activity of inhibitory D class motorneurons (VD and DD). Consistent with this idea, laser ablation of DD motorneurons prevented both forward and backward locomotion in newly hatched larvae (Chalfie et al., 1985). Rhythmic activity has been described in Ascaris suum inhibitory motorneurons (Angstadt and Stretton, 1989; Davis and Stretton, 1989) but was not found to correlate with body undulation in a semi-intact preparation (Stretton et al., 1992). However, adult mutants that lack functional GABAergic transmission (e.g. unc-25/GAD mutants (McIntire et al., 1993)) and nematodes in which GABAergic transmission was genetically blocked in the adult (Davis et al., 2008) do exhibit both forward and backward sinusoidal locomotion which is at odds with this hypothesis. Alternatively, muscle cells produce the oscillations while the motorneurons modulate their amplitude. This model was suggested by White and colleagues (1976) but lacks experimental support. Rhythmic activity of muscle cells is not predicted by an electrophysiological model (Boyle and Cohen, 2008) and has not been reported in dissected preparations (Richmond and Jorgensen, 1999), nor in primary cultures (Christensen et al., 2002). Lastly, an alternative source of rhythmic activity might be excitatory motorneurons classes AS, VC or both, but their role in C. elegans locomotion has not been explored.
Evolutionary considerations and comparison with other species
Backward, as well as forward, locomotion is exhibited by many animal species. However, only a few studies have addressed the mechanism of backward locomotion directly. In cat, human and lamprey, it has been suggested that common spinal central pattern generators are used for forward and backward locomotion (Buford and Smith, 1990; Matsushima and Grillner, 1992; Lamb and Yang, 2000; Zehr and Hundza, 2005). In the stick insect, backward walking appears to be produced not by reversing the forward locomotion pattern but by overriding the forward pattern (Graham and Epstein, 1985). In all of these preparations, the motorneurons are multifunctional and are active during both forward and backward locomotion.
By contrast, it appears that specific motorneurons are dedicated to a particular direction of movement in the nematode. Interneurons in the nematode appear to release one pattern or another rather than participate directly in pattern generation, as in the other animals described. This is supported by tonic activation of AVA interneurons during backward locomotion (Chronis et al., 2007; Faumont, Thiele, and Lockery, pers. comm.), by the common innervation of A-motorneurons by AVA, AVD and AVE (anterior only) interneurons and of B-motorneurons by AVB and PVC interneurons. Furthermore, the synchronous onset of calcium transients in neighboring VB or neighboring VA motorneurons at the beginning of locomotion suggests a common source of activation for motorneurons of the same class along the ventral cord. While the relatively slow decay time constant of YC2.60 might interfere with resolving sequential activity during a bout locomotion, it should not affect the onset of the signal at the onset of a bout.
A neuronal network comprised of direction-specific classes of motorneurons, might be an ancestral form of locomotor control to which dedicated and multifunctional interneurons were subsequently added and which then assumed the role of pattern generation. This would have freed motorneurons to become multifunctional, thereby greatly extending the repertoire of motor behaviors. In the nematode, a motoneuronal pattern generating mechanism might suffice because the behavioral repertoire is limited, and the total number of motorneurons and muscle cells is relatively small.
Supplementary Material
Acknowledgements
We thank S. Lockery and S. Faumont (University of Oregon) for their help and comments. YC2.60 and YC3.60 plasmids were a generous gift from A. Miyawaki (RIKEN, Japan). Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the NIH National Center for Research Resources. This work was supported by funding from NIH NIGMS and NINDS (ACH). GH was supported by The International Human Frontier Science Program Organization. The work was supported in part by the intramural program of the NIH National Institute of Neurological Disorders and Stroke.
References
- Altun ZF, Hall DH. Nervous System. WormAtlas. 2009 http://www.webcitation.org/5qMVQWHl2.
- Angstadt JD, Stretton AO. Slow active potentials in ventral inhibitory motor neurons of the nematode Ascaris. J Comp Physiol [A] 1989;166:165–177. doi: 10.1007/BF00193461. [DOI] [PubMed] [Google Scholar]
- Baader AP, Kristan WB., Jr. Monitoring neuronal activity during discrete behaviors: a crawling, swimming and shortening device for tethered leeches. J Neurosci Meth. 1992;43:215–223. doi: 10.1016/0165-0270(92)90031-8. [DOI] [PubMed] [Google Scholar]
- Ben Arous J, Tanizawa Y, Rabinowitch I, Chatenay D, Schafer WR. Automated imaging of neuronal activity in freely behaving Caenorhabditis elegans. Journal of Neuroscience Methods. 2010;187:229–234. doi: 10.1016/j.jneumeth.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Boyle JH, Cohen N. Caenorhabditis elegans body wall muscles are simple actuators. Biosystems. 2008 doi: 10.1016/j.biosystems.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Bryden J, Cohen N. Neural control of Caenorhabditis elegans forward locomotion: the role of sensory feedback. Biol Cybern. 2008;98:339–351. doi: 10.1007/s00422-008-0212-6. [DOI] [PubMed] [Google Scholar]
- Buford JA, Smith JL. Adaptive control for backward quadrupedal walking. II. Hindlimb muscle synergies. J Neurophysiol. 1990;64:756–766. doi: 10.1152/jn.1990.64.3.756. [DOI] [PubMed] [Google Scholar]
- Chalfie M, White JG. The nervous system. In: Wood WB, editor. The nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1988. pp. 337–391. [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S. The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci. 1985;5:956–964. doi: 10.1523/JNEUROSCI.05-04-00956.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BL, Hall DH, Chklovskii DB. Wiring optimization can relate neuronal structure and function. PNAS. 2006;103:4723–4728. doi: 10.1073/pnas.0506806103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen M, Estevez A, Yin X, Fox R, Morrison R, McDonnell M, Gleason C, Miller DM, 3rd, Strange K. A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron. 2002;33:503–514. doi: 10.1016/s0896-6273(02)00591-3. [DOI] [PubMed] [Google Scholar]
- Chronis N, Zimmer M, Bargmann CI. Microfluidics for in vivo imaging of neuronal and behavioral activity in Caenorhabditis elegans. Nat Methods. 2007;4:727–731. doi: 10.1038/nmeth1075. [DOI] [PubMed] [Google Scholar]
- Davis MW, Morton JJ, Carroll D, Jorgensen EM. Gene activation using FLP recombinase in C. elegans. PLoS Genet. 2008;4:e1000028. doi: 10.1371/journal.pgen.1000028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Stretton AO. Signaling properties of Ascaris motorneurons: graded active responses, graded synaptic transmission, and tonic transmitter release. J Neurosci. 1989;9:415–425. doi: 10.1523/JNEUROSCI.09-02-00415.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faumont S, Lockery SR. The awake behaving worm: simultaneous imaging of neuronal activity and behavior in intact animals at millimeter scale. J Neurophysiol. 2006;95:1976–1981. doi: 10.1152/jn.01050.2005. [DOI] [PubMed] [Google Scholar]
- Faumont S, Miller AC, Lockery SR. Chemosensory behavior of semi-restrained Caenorhabditis elegans. J Neurobiol. 2005;65:171–178. doi: 10.1002/neu.20196. [DOI] [PubMed] [Google Scholar]
- Graham D, Epstein S. Behaviour and Motor Output for an Insect Walking on a Slippery Surface: II. Backward Walking. J Exp Biol. 1985;118:287–296. [Google Scholar]
- Harbinder S, Tavernarakis N, Herndon LA, Kinnell M, Xu SQ, Fire A, Driscoll M. Genetically targeted cell disruption in Caenorhabditis elegans. PNAS. 1997;94:13128–13133. doi: 10.1073/pnas.94.24.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendel T, Mank M, Schnell B, Griesbeck O, Borst A, Reiff DF. Fluorescence Changes of Genetic Calcium Indicators and OGB-1 Correlated with Neural Activity and Calcium In Vivo and In Vitro. J Neurosci. 2008;28:7399–7411. doi: 10.1523/JNEUROSCI.1038-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman V, Laurent G. Evaluating a genetically encoded optical sensor of neural activity using electrophysiology in intact adult fruit flies. Frontiers in Neural Circuits. 2007:1. doi: 10.3389/neuro.04.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski J, Schindelman G, Cronin CJ, Seah A, Sternberg PW. Systems level circuit model of C. elegans undulatory locomotion: mathematical modeling and molecular genetics. J Comput Neurosci. 2008;24:253–276. doi: 10.1007/s10827-007-0054-6. [DOI] [PubMed] [Google Scholar]
- Kerr R, Lev-Ram V, Baird G, Vincent P, Tsien RY, Schafer WR. Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans. Neuron. 2000;26:583–594. doi: 10.1016/s0896-6273(00)81196-4. [DOI] [PubMed] [Google Scholar]
- Lamb T, Yang JF. Could Different Directions of Infant Stepping Be Controlled by the Same Locomotor Central Pattern Generator? J Neurophysiol. 2000;83:2814–2824. doi: 10.1152/jn.2000.83.5.2814. [DOI] [PubMed] [Google Scholar]
- Matsushima T, Grillner S. Neural mechanisms of intersegmental coordination in lamprey: local excitability changes modify the phase coupling along the spinal cord. J Neurophysiol. 1992;67:373–388. doi: 10.1152/jn.1992.67.2.373. [DOI] [PubMed] [Google Scholar]
- McIntire SL, Jorgensen E, Horvitz HR. Genes required for GABA function in Caenorhabditis elegans. Nature. 1993;364:334–337. doi: 10.1038/364334a0. [DOI] [PubMed] [Google Scholar]
- Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. PNAS. 2004;101:10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niebur E, Erdos P. Theory of the locomotion of nematodes: control of the somatic motor neurons by interneurons. Math Biosci. 1993;118:51–82. doi: 10.1016/0025-5564(93)90033-7. [DOI] [PubMed] [Google Scholar]
- Pierce-Shimomura JT, Chen BL, Mun JJ, Ho R, Sarkis R, McIntire SL. Genetic analysis of crawling and swimming locomotory patterns in C. elegans. PNAS. 2008;105:20982–20987. doi: 10.1073/pnas.0810359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Jorgensen EM. One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat Neurosci. 1999;2:791–797. doi: 10.1038/12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock MK, Hackett JT, Les Brown D. Does the Mauthner cell conform to the criteria of the command neuron concept? Brain Research. 1981;204:21–27. doi: 10.1016/0006-8993(81)90648-x. [DOI] [PubMed] [Google Scholar]
- Sawin ER, Ranganathan R, Horvitz HR. C. elegans Locomotory Rate Is Modulated by the Environment through a Dopaminergic Pathway and by Experience through a Serotonergic Pathway. Neuron. 2000;26:619–631. doi: 10.1016/s0896-6273(00)81199-x. [DOI] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. WormBook. 2006:1–11. doi: 10.1895/wormbook.1.101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stretton A, Donmoyer J, Davis R, Meade J, Cowden C, Sithigorngul P. Motor Behavior and Motor Nervous System Function in the Nematode Ascaris suum. J Parasitol. 1992;78:206–214. [PubMed] [Google Scholar]
- Tsechpenakis G, Bianchi L, Metaxas D, Driscoll M. A novel computational approach for simultaneous tracking and feature extraction of C. elegans populations in fluid environments. IEEE. 2008;55:1539–1549. doi: 10.1109/TBME.2008.918582. [DOI] [PubMed] [Google Scholar]
- Von Stetina SE, Treinin M, Miller DM., 3rd The motor circuit. Int Rev Neurobiol. 2006;69:125–167. doi: 10.1016/S0074-7742(05)69005-8. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Kitagawa I, Shingai R. Neurons regulating the duration of forward locomotion in Caenorhabditis elegans. Neurosci Res. 2004;50:103–111. doi: 10.1016/j.neures.2004.06.005. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S. The Structure of the Ventral Nerve Cord of Caenorhabditis elegans. Philosophical Transactions of the Royal Society of London B, Biological Sciences. 1976;275:327–348. doi: 10.1098/rstb.1976.0086. [DOI] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner FRS. The Structure of the Nervous System of the Nematode C. elegans. Phil Trans B. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- Zehr EP, Hundza SR. Forward and Backward Arm Cycling Are Regulated by Equivalent Neural Mechanisms. J Neurophysiol. 2005;93:633–640. doi: 10.1152/jn.00525.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.