Abstract
Microglia, the resident innate immune cells in the brain, have long been implicated in the pathology of neurodegenerative diseases. Accumulating evidence points to activated microglia as a chronic source of multiple neurotoxic factors, including TNFα, NO, IL1-β, and reactive oxygen species (ROS), driving progressive neuron damage. Microglia can become chronically activated by either a single stimulus (ex. LPS or neuron damage) or multiple stimuli exposures to result in cumulative neuronal loss over time. While the mechanisms driving these phenomena are just beginning to be understood, reactive microgliosis (the microglial response to neuron damage) and ROS have been implicated as key mechanisms of chronic and neurotoxic microglial activation, particularly in the case of Parkinson’s Disease. Here, we review the mechanisms of neurotoxicity associated with chronic microglial activation and discuss the role of neuronal death and microglial ROS driving the chronic and toxic microglial phenotype.
Keywords: Microglia, inflammation-mediated neurodegeneration, oxidative stress, chronic neurotoxicity, reactive microgliosis
Neurodegenerative diseases (ex. Alzheimer’s Disease, Parkinson’s Disease, Huntington’s Disease, ALS, etc.) share many common characteristics, such as changes in microglial number and morphlogy, elevated cytokine levels, oxidative stress, and progressive neuronal loss. Increasing evidence reports that microglia can become a chronic source of cytokines and reactive oxygen (ROS) to drive progressive neuron damage and are implicated in the chronic nature of neurodegenerative diseases.1 Here, while predominantly focusing on Parkinson’s disease (PD), we discuss mechanisms through which microglia can become neurotoxic, explain current views on why the microglial response is chronic, and discuss the meaning of these findings in respect to the progressive nature of neurodegenerative disease.
Microglial Origin & Maintenance in the Central Nervous System (CNS)
Microglia reside in the CNS, comprise approximately 12% of the brain2 (depending on brain region, health or pathology), and serve as the brain’s immune defense. Microglia are unique from neurons, oligodendrocytes, and astrocytes in that they are not derived from the neuroectoderm. Instead, it is generally accepted that the original microglial population in the CNS differentiates from cells of the myeloid lineage that originate in the bone marrow2, which occurs early in embryonic development.3 These myeloid cells can be found within the CNS by embryonic day 8 in rodents4, and by the 12th week of gestation in humans.5 At this stage, the cells are referred to as fetal macrophages, and are the earliest form of microglial precursor cells present in the embryonic CNS.
Once fetal macrophages come to reside in the developing CNS, they begin the differentiation process that will result in the formation of fully-matured microglia. Although the course of this differentiation is not fully understood, one of the early steps is the formation of rounded ‘ameboid’ microglia that cluster within distinct anatomical regions in the developing brain and may act as a source of microglial progenitors.6 Later in embryonic and early fetal development, these progenitors will follow a path of migration and differentiation leading to the mature microglia, a process that extends into neonatal development. Differentiation first involves the formation of partially ramified microglia followed by the development of fully ramified, or branched, microglia that express cell surface molecules characteristic of resting microglia (discussed below).7
While the origin of initial microglia populations within the CNS has been well supported, the replacement of microglial populations is matter of more debate. Due to the presence of the blood brain barrier, it was originally perceived that the circulating immune cells did not have immediate access to the CNS, keeping populations of microglia distinct from similar, circulating blood cells. However, there is increasing evidence that bone-marrow derived cells are capable of entering the CNS and differentiating into microglia in adults.8 For example, studies using bone marrow-chimeras have shown that circulating monocytes infiltrate the CNS under different conditions of injury, inflammation, or disease.9–11 This has been shown to be possible even when the blood brain barrier remains intact, suggesting a mechanism for entry into the CNS.9 A possible mechanism for this could be replenishment from perivascular cells, which are bone marrow derived and have been shown to enter the CNS and differentiate into microglia.12, 13 The mechanisms through which circulating cells are recruited to the CNS, and whether they enter the CNS under normal, resting conditions, however, are poorly understood. At present, there is also ongoing debate regarding the function of infiltrating cells vs. resident microglia, where it has been suggested that infiltrating precursor cells may be predominantly beneficial cellular actors of wound healing14 and an under-utilized therapeutic resource.8
What also remains unclear is whether microglia are capable of a level of self-renewal that is sufficient to support the population of microglia in both resting and activated states. Microglia have a low mitotic rate when at rest, but are capable of high rates of proliferation when activated, suggesting that they have at least a partial ability to counteract cell-turnover.15, 16 In addition, populations of microglial progenitors have been proposed to persist in the adult brain that are capable of proliferating to replace microglial populations.4, 17 At present, we are just beginning to understand these basic principles regarding the origin and replacement of microglia and further research is needed to fully elucidate the mechanisms involved in the complicated life-cycle of the immune system of the CNS.
Microglial Activation & Function
Resting Microglia
Analogous to the role of macrophages and lymphocytes in the periphery, one role of microglia is to act as the brain’s immune defense against disease and injury. In addition to these duties, however, microglia are involved in number of processes in the normal, healthy CNS. In a normal brain, microglia are said to be resting, and can be distinguished by both their morphology and pattern of gene expression. In this state, microglia take on a ramified appearance, where long thin processes extend from the cell body and into the surrounding milieu. The immunological phenotype of this state is characterized by low expression of major histocompatability complex (MHC) proteins and other antigen presenting surface receptors.18 This phenotype is in stark contrast to that of other macrophages, which exist in a more readily activated state. One of the reasons for this may be the absence of serum proteins in the brain that have been shown to cause macrophage activation.19 In addition, the expression of certain receptors, such as CD200 and CX3CR1 on the microglia cell surface, may interact with ligands that keep microglia in a resting state.20, 21 Resting, ramified, microglia cell bodies are spaced throughout the CNS to avoid cell body overlap, but have been shown to be present with variable density in different brain regions.22 Notably, this ramified morphology occurs only in vivo and is relatively absent from microglia in cell cultures.
Despite the fact that these ramified microglia are referred to as resting, they are constantly surveying the surrounding environment by extending and retracting their processes.23, 24 In doing this, microglia are able to sample the microenvironment, maintain homeostasis, and identify signals that require a response. When reacting to extracellular signals, such as the presence of pathogens, foreign material and dead or dying cells, microglia may undergo a morphological change into an ameboid shape with short or non-existent processes.25 This morphological change is also accompanied by changes in signaling and gene expression that can result in changes in surface receptor expression, the release of pro- or anti-inflammatory factors, recruitment molecules, and ROS, among others.26–29 The cumulative effect of these changes in morphology and phenotype is a shift from resting to activated microglia.
Microglial Activation & Function in the Healthy CNS
Microglia are activated in response to brain injuries and immunological stimuli25, 30–32 to undergo dramatic alterations in morphology, changing from resting, ramified microglia into activated, amoeboid microglia25, which is thought to favor phagocytosis and mobility. Unfortunately, changes in morphology are unlikely to differentiate between benign and toxic microglial activation.33
In non-pathological states, microglia respond to extracellular stimuli in a number of ways. In the developing brain, and in areas of remodeling, microglia are responsible for the phagocytosis of cellular debris resulting from apoptosis and normal cell death.18 Microglia are also responsible for the phagocytosis of other debris present in the extracellular space, including damaged cells, plaques, and foreign matter. For microglia surrounding neurons, subtypes of microglia can provide trophic support to neurons through the release of nerve growth factors, neurotrophins, and other neurotrophic factors.34 These microglia are also capable of assisting in synaptic plasticity, an observation that was first made in the mid-20th century.35 Along these lines, microglia have been implicated as the “brains electricians”,36 where the release of neurotrophic factors and anti-inflammatory cytokines from microglia has been shown to promote synaptic plasticity.37–39 Specifically in response to injury, activated microglia have been shown to surround damaged neurons and participate in synaptic stripping, a process of removing branches from damaged neurons to promote repair and regrowth,35, 40, 41 although recent evidence shows that microglia may play an indirect, anti-inflammatory role in this process.42 Notably, these beneficial microglial functions often involve changes resembling an activated morphology and protein expression, yet the function is distinct from a classic pro-inflammatory response.
In fact, the majority of microglial functions are beneficial and necessary for a healthy CNS, as activated microglia are critical for CNS wound healing.36 For example, ablation of infiltrating bone marrow derived cells (that become microglia) in spinal cord injury at specific times has been shown to have disastrous neurotoxic consequences.14 In addition, microglia have also been shown to release anti-inflammatory and trophic molecules to enhance the survival of surrounding neurons.43, 44 Thus, evidence supports that when microglia become neurotoxic, this is due to both the loss of the beneficial functions36 and/or a shift to a pro-inflammatory phenotype.1, 45, 46
Pro-inflammatory Microglial Activation
Microglia detect and respond to pro-inflammatory triggers by changing to an activated phenotype, resulting in a shift of cellular function to releasing cytotoxic factors (ex. TNFα, NO, & ROS) aimed at destroying the invading pathogens. Surface molecules associated with the innate immune response, such as complement receptors and MHC molecules, are also upregulated upon microglial activation.47, 48 For example, upregulation of MHC proteins enable microglia to act as antigen-presenting cells to T-cells that will enter the brain during active infections.18
Microglia are readily activated by an extensive list of pro-inflammatory stimuli, such as lipopolysaccharide (LPS),49–51 pesticides (ex. Paraquat,52 dieldrin,53, 54 lindane,54 and rotenone55), disease proteins (ex. Beta-amyloid (Aβ),56 α synuclein, 57 and HIV-Tat58), air pollution,59 and even neuron damage.46 In fact, it has been proposed that many disease proteins and environmental toxicants trigger a toxic microglial response because they are misinterpreted as a pathogen.1 In response to these triggers, microglia can produce of a large array of cytotoxic factors, such as superoxide (O2•−),27 nitric oxide (NO),60, 61 tumor necrosis factor alpha (TNFα)62, 63 and inflammatory prostaglandins.64 While the detailed components of the microglial response can be stimulus specific, there are common factors of microglial activation.46
LPS is a cell wall component of gram negative bacteria and is a potent stimulus of the microglial innate immune response. The microglial response to LPS has been well characterized and provides excellent insight into the timing of the multiple factors produced in a pro-inflammatory response. Notably, extracellular superoxide (O2•−) is an immediate toxic factor released by microglia in response to LPS. Interestingly, unique to microglia, LPS-induced production of O2•− is not mediated through the traditional LPS receptor, the toll-like 4 receptor (TLR4).50 In fact, recent work has indicated that the MAC-1 receptor is responsible for the LPS-induced activation of NADPH oxidase and the consequent production of O2•− in microglia.49 Thus, the microglial (O2•−) response is typical (although less robust) when compared to other professional phagocytes, but the mechanisms mediating this response may be cell-specific.
While the microglial superoxide response is immediate, microglia also respond to LPS with a later pulse of pro-inflammatory factors, such as TNFα, IL1-β, and NO, both in vivo and in vitro.65 Specifically, there is a delay in the production of TNFα, NO, prostaglandin E2 (PGE2) and IL-1β, where TNF-α peaks at 6 hours, NO peaks at 12 hours and both PGE2 and IL-1β peak at approximately 24 hours.65 Thus, one mechanism through which microglia are thought to cause neuron damage is through the excessive and inappropriate release of toxic factors.
Microglia in Disease
Neuroinflammation is characteristic of many neurodegenerative diseases and microglia have been implicated to play both causative and exacerbating roles. In fact, microglia and inflammation-mediated neurodegeneration has been implicated in numerous other diseases and conditions, such as hypoxia,66 stroke,67 and neuropathic pain.68 Neurodegenerative diseases are characterized by chronic and progressive neuronal loss, pathological levels of cytotoxic substances such as extracellular debris, elevated levels of pro-inflammatory factors, and production of reactive oxygen species, resulting in oxidative stress. These factors, in addition to the release of others that can activate and recruit microglia, support a role for microglia in diseases such as Alzheimer’s disease,69 Parkinson’s disease,69 multiple sclerosis,70 amyotrophic lateral sclerosis,71, 72 and HIV-associated neurocognitive disorder (HAND).73
Although microglia undergo changes as a result of normal aging, including increases in activation or decreases in function and proliferation, 74 the activation of microglia in age-dependent neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease, seems to be a distinct process.75 One of the hallmarks of Alzheimer’s disease pathology is the existence of β-amyloid plaques, an extracellular protein aggregate that is normally cleared by microglia. Activated microglia and their toxic effects have been associated with Alzheimer’s disease for decades.76–78 This has lead to research showing that not only is β-amyloid directly toxic to neurons,79 but it also causes microglia to cluster around plaques and become activated, which may perpetuate neuronal damage and death.80, 81 Similarly, activated microglia are associated with damaged neurons in patients with Parkinson’s disease,69 which is discussed in more detail below.
In contrast to diseases specifically associated with aging, microglial activation can also play a role in diseases not related to age and may involve unique properties of microglial cells. A strong example of this is in multiple sclerosis, a disease associated with severe inflammation and demyelination of axons. Usually considered an autoimmune disease, multiple sclerosis is associated with lesions within the white and gray matter of the CNS that have increased levels of activated microglia.82, 83 In addition to increases in microglia-released ROS and pro-inflammatory cytokines,84–86 microglia may play a large part in the initiation of disease by acting as antigen-presenting cells targeting myelin.87 In HAND, microglia play a strong part in harboring the HIV virus and acting as a site of viral replication.88, 89 During this process, microglia become activated and release pro-inflammatory cytokines.90 In addition, microglia are activated by HIV proteins, such as Tat,91 to produce ROS. Thus, the interaction between microglia, viral replication/proteins and the production of cytotoxic factors in HIV-associated neurocognitive disorder have strong implications for disease progression.
These neurodegenerative diseases, as well as others, such as amyotrophic lateral sclerosis, Huntington’s disease and prion diseases, highlight the role that activated microglia can play in cell damage and death. In cases of these pathologies, however, the exact role of microglia remains controversial. Ongoing research seeks to answer questions pertaining to microglial activation in both the development and progression of neurodegeneration.
Microglia Activation and Parkinson’s Disease
In contrast to the beneficial housekeeping duties of resting and moderately activated microglia, over-activation of microglia resulting in excess production of inflammatory mediators is in fact neurotoxic 92–94 and microglial activation has been strongly linked to pathology in PD.1, 95 The term over-activation describes the state in which microglia continually produce inflammatory mediators which accumulate to levels that are harmful to neurons,96–98 and often in combination, lead to neurodegeneration.94, 98, 99
Pioneering work by McGeer and colleagues69 discovered increased HLD-DR staining in the substantia nigra (SN) of post mortem PD patient brains, indicating the presence of activated microglia, and first implicating these cells may have an active pathological role in disease. Since then, many inflammatory mediators such as TNFα, IL-1β, and interleukin 6 (IL-6) have been identified in the striatum in human PD post-mortem brains100–104 in addition to an upregulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX2) in ameboid microglia located in the SN of PD patients,105 further suggesting a link between activated microglial cells and neuronal damage in disease. Research has identified a critical role for neuroinflammation in dopaminergic (DA) neuron damage, as cytokines such as TNFα106–108 have been shown to exert DA neuron damage. In fact, continuous expression of low levels of TNFα in the SN induced by an adenoviral vector will cause chronic microglia/macrophage activation, progressive neurodegeneration, and delayed motor symptoms.109 Thus, it is not surprising that anti-inflammatory approaches,95, 110–112 including those focusing on TNFα,99, 113, 114 have been targeted as therapeutic strategies.
Subsequent studies provide a wealth of evidence confirming the presence of activated microglia in PD and PD-like brains. For instance, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is an illicit drug contaminant linked to human Parkinsonism cases115 that is commonly used as an animal model of Parkinson’s disease.116 After MPTP administration, microglial activation in the SN of mouse brain has been confirmed by an increase in cell number, changed cell morphology, increased lectin staining, larger cell bodies, and thicker processes.117,118 Activated microglia are also present in the brains of MPTP intoxicated monkeys.119, 120 Using PET imaging, a 6-hydroxydomapine (6-OHDA) model of DA neuron damage showed decreased binding of C-PK11195 in the striatum, indicating increased activation of microglia.121 Importantly, these findings parallel what is seen in the human PD post-mortem brain.100, 122, 123 More recently, PET imaging has shown that over-activated microglia are also present in the SN of living PD patients and are associated with DA neuron damage.124, 125 Thus, evidence strongly supports that microglia may play a role in the active pathology driving Parkinson’s disease.
Microglia-mediated Dopaminergic Neuron Damage
Several studies using animal models have demonstrated that the presence of microglia and the production of neurotoxic factors can initiate and amplify neuron damage. Interestingly, multiple factors and toxins are shown to selectively damage DA neurons through microglial activation, such as rotenone,126 diesel exhaust particles (DEP),127 paraquat (PQ),52 α-synuclein,57 and the beta amyloid peptide (Aβ).128 However, early studies using the immunogen LPS, demonstrate that a pro-inflammatory trigger such as LPS is toxic to DA neurons only in the presence of microglia,127, 129, 130 where LPS was one of the first microglia-mediated selective DA toxins identified. At present, it is unclear why DA neurons are more vulnerable to microglial activation when compared to other cells types, but inherent vulnerability to oxidative stress has been implicated.1
Microglial NADPH Oxidase, ROS, and Neurotoxicity
Redox Signaling
Unregulated, excessive ROS can indiscriminately damage biomolecules (ex. protein carbonyls) to impair cellular function, a mechanism through which ROS is thought to contribute to neurodegenerative disease.131 However, in addition to permanent damage, ROS is capable of a more elegant and selective modification of proteins, where ROS is known to target thiol functional groups on cysteine amino acid residues.131 These reversible modifications can be likened to phosphorylation, and can regulate protein function, acting as an important process of signal transduction in multiple cell types, including microglia.
Microglial ROS Production & NADPH Oxidase
The production of ROS in phagocytes is derived from multiple sources, such as peroxidases inside the cell, NADPH oxidase on the membrane surface, or the oxidative processes of mitochondria.132 NADPH oxidase is a multi-subunit enzyme that catalyzes the production of O2•− from molecular oxygen and is the predominant source of extracellular ROS in phagocytes, such as microglia.129, 130 The enzyme complex is dormant in resting phagocytes and can be activated upon exposure to specific stimuli, such as bacteria and inflammatory peptides133 In resting cells, the cytosolic subunits of NADPH oxidase (p47, p67, p40, and Rac2) are distributed between the cytosol and the membranes of intracellular vesicles and organelles.133 However, activation induces the cytosolic subunits to translocate to the membranes, where they bind to the membrane-associated subunits (p22 and gp91), assembling the active oxidase that produces O2•−.133
NADPH oxidase and intracellular ROS have been implicated in several cellular functions such as migration134 and phagocytosis.135 Specific to microglia, ROS produced from NADPH oxidase has been shown to mediate changes in microglia morphology, 130 pro-inflammatory gene expression,130 and upregulation of markers in response to immunological stimuli.136
Interestingly, NADPH oxidase is upregulated in neurodegenerative diseases such as PD137 and Alzheimer’s disease,138 indicating a potential role for microglial NADPH oxidase activation in disease and neuron death. In fact, the critical role of NADPH oxidase in mediating inflammation-related neurotoxicity has been well documented,130 where the LPS-induced loss of nigral DA neurons in vivo and in vitro was significantly less pronounced in NADPH oxidase-deficient mice, when compared to control mice. NADPH oxidase has also been linked to microglia-derived oxidative stress from a variety of neurotoxic insults, such as rotenone, 126 DEP,127 α-synuclein,57 Aβ,128 PQ,139 dieldrin,54 DA neuronal injury,137, 140 prothrombin kringle-2,141 β2 adrenergic receptor activation,142 angiotensin,143 and cerebral ischemia-reperfusion injury,144, 145 indicating that microglial NADPH oxidase activation may also be a common denominator of microglial activation associated with neurotoxicity. Currently, the precise species of ROS responsible for NADPH oxidase-induced neurotoxicity is unknown. However, SOD and catalase mimetics, which remove O2•− and hydrogen peroxide (H2O2), respectively, reduce LPS-induced DA toxicity146 indicating the critical importance of H2O2 and O2•− in microglia-mediated neurotoxicity. Thus, microglial-derived ROS may be an essential and common factor of toxic microglial activation.
NADPH Oxidase & Redox Signaling
The phagocytic oxidase (PHOX)-ROS signaling pathway is the signaling mechanism induced by the increase in intracellular ROS in phagocytes as a response to NADPH oxidase activation (pro-inflammatory redox signaling in phaogocytes). The increase in intracellular ROS in phagocytes, such as microglia, includes a number of oxygen species, such as O2•−, hydroxyl radical (OH•−), lipid hydroperoxides, and their by-products (e.g., H2O2).147 While multiple cellular organelles and enzymes contribute to intracellular ROS, it is not surprising that the amount of intracellular ROS produced by NADPH oxidase is dependent upon the stimuli. For example, NADPH oxidase contributes to approximately 50% of the LPS-induced intracellular ROS increase130 in microglia, but substance P-induced intracellular ROS is nearly completely dependent upon NADPH oxidase.148 In the traditional phagocyte, the PHOX-ROS pathway has been shown to amplify pro-inflammatory gene expression through their function as second messengers to regulate several downstream signaling molecules, including protein kinase C, mitogen activated protein kinase, and nuclear factor-κB (NFκB),135, 149–151 through redox signaling.
Using neuron-glia cultures from NADPH oxidase-deficient mice, studies have shown that NADPH oxidase initiates an intracellular ROS signaling pathway152 that can activate microglia and amplify the production of pro-inflammatory cytokines, such as TNFα130 or PGE2.153 Additionally, Min and colleagues154 demonstrated that ganglioside induces the activation of microglia, where the production of IL-1β, TNF-α, and iNOS are attenuated by the addition of the NADPH oxidase inhibitor, diphenylene iodonium (DPI). Furthermore, NADPH oxidase inhibitors and catalase are shown to suppress LPS-induced expression of cytokines (IL-1, IL-6, and TNFα), iNOS, mitogen activated protein kinase (MAPK), and NFκB phosphorylation.155 Thus, both microglial cellular function and signaling pathways are modified by NADPH oxidase ROS production.
Accumulating evidence supports that NADPH oxidase contributes to microglia-mediated neurotoxicity through two mechanisms. First, activation of NADPH oxidase results in the production of extracellular ROS that is toxic to neurons. Second, activation of NADPH oxidase causes an increase in the microglial intracellular ROS, which enhances the production of pro-inflammatory factors that are toxic to neurons. Given the dual impact of NADPH oxidase activation on neurotoxicity, the role of NADPH oxidase in generating toxic ROS, and the prevalence of NADPH oxidase activation upon microglial activation, this suggests that microglial NADPH oxidase is a critical mechanism of neuronal death across multiple neurodegenerative diseases. However, the specific mechanisms defining how ROS causes this toxic microglial phenotype through redox-signaling is unknown.
Chronic Microglial Activation - Propagation of Disease
Microglia are unique when compared to differentiated myeloid immune cells in the periphery for multiple reasons. In addition to the obvious morphological differences and unique resting profiles characterized in microglia, these cells are also more likely to establish chronic pro-inflammatory responses, rather than demonstrate resolution of the innate immune response, as is common in the peripheral immune system.156 While the mechanisms are unclear, we believe that this microglial tendency for a chronic pro-inflammatory response is a key factor driving progressive neuron damage, contributing to the chronic nature of neurodegenerative diseases.
Reactive Microgliosis-Chronic Response to a Single Stimulus
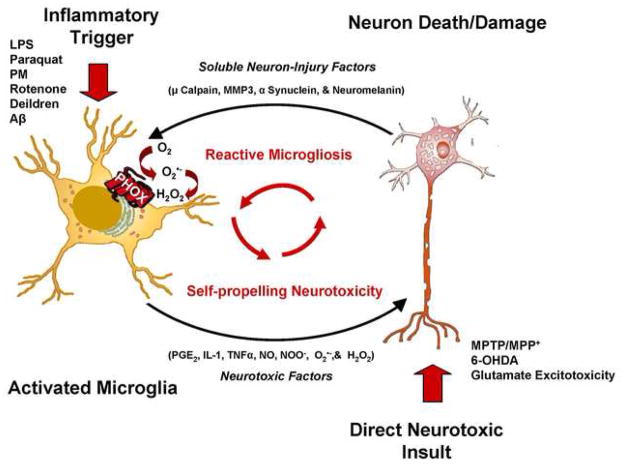
While microglial activation was initially perceived as a transient event, 31 it is now thought to be chronic and culpable in the propagation of disease.1, 94, 157 Reports have shown that microglia can remain chronically activated140, 158, 159 in a process that has been termed reactive microgliosis. Reactive microgliosis can be defined as microglial activation that occurs in response to neuronal damage, which is then perpetuated by further microglial activation and neurotoxicity (Figure 1). Thus, a self-propelling and progressive cycle of microglial activation and neuron damage ensues.1
Figure 1. Reactive Microgliosis Drives Chronic Neuron Damage.
Both stimulation of microglia with pro-inflammatory triggers (ex. LPS) and direct neuron damage (ex. Glutamate excitability) result in microglial activation causing the release of neurotoxic factors, such as IL-1β, NO, TNFα, ONOO−, O2•− and H2O2.. Subsequently, following damage with either a pro-inflammatory trigger or a direct neurotoxin, the neuron releases microglial activators (Soluble Neuron-Injury Signals) such as μ calpain, MMP3, α-synuclein, and neuromelanin which activate microglial cells and propagate the cycle. This self-perpetuating cycle of neurotoxicity is known as reactive microgliosis. Abbreviations: Hydrogen peroxide (H2O2); Interleukin 1 beta (IL-1β); Lipopolysaccharide (LPS); Matrix Metalloproteinase 3 (MMP3); 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine (MPTP); 1-methyl-4-phenylpyridinium ion (MPP+); nitric oxide (NO); peroxynitrite (ONOO−); prostaglandin E2 (PGE2); superoxide (O2•−); tumor necrosis factor alpha (TNFα). This figure was slightly modified from Block & Hong (2007)1.
For example, to damage DA neurons, MPTP is metabolized to 1-methyl-4-phenylpyridinium (MPP+), which is then selectively taken up by the dopamine transporter (DAT), resulting in inhibition of the mitochondrial electron transport chain complex I.160 In addition to this mechanism of direct neurotoxicity, MPTP-induced neurotoxicity is also clearly linked with microglial activation in vivo and in vitro.137, 140, 159
It is particularly interesting that chronic microglial activation can continue years after MPTP exposure in humans122 and primates,159 despite the fact that the exposure to MPTP was brief, indicating an incessant, active pathologic process. Importantly, several studies have shown that microglia have an active role in the process of neuronal death in MPTP/MPP+-induced neurotoxicity.137, 140, 159 Specifically, in vitro studies show that while MPTP directly damages DA neurons, both MPTP and MPP+ fail to directly activate microglia.140 Rather, microglial activation in response to MPTP or MPP+ occurs only when neurons are present and this response takes time (days) to accumulate.140 Further, the addition of microglia to enriched neuron cultures greatly enhances MPTP-induced DA toxicity,140 demonstrating that microglia cause DA neuron damage in addition to the direct toxic effects of MPTP/MPP+ on the neuron.
In vivo studies also emphasize the important role of inflammation as a toxic component of MPTP/MPP+ neurotoxicity,161 where DA neuron damage in response to MPTP is significantly reduced in mutant mice with deficient production of pro-inflammatory factors, such as nitric oxide,162 superoxide,137, 163 prostaglandins,164, 165 and TNFα.108 Thus, several lines of evidence suggest that microglial activation initiated by neuronal damage may be toxic and persistent, continuing long after the initiating damaging/toxic stimulus is gone.
Recently, we employed an in vitro approach to separate neuron injury factors from the cellular actors of reactive microgliosis in an attempt to begin to discover molecular signals (soluble neuron injury factors) responsible for chronic and toxic microglial activation.166 We found that upon injury with the DA neurotoxin MPP+, DA neurons released soluble neuron injury factors that activated microglia and were selectively toxic to DA neurons in mixed mesencephalic neuron-glia cultures through NADPH oxidase.166 This is consistent with other studies that have identified other soluble neuron injury factors that signal toxic microglial activation such as matrix metalloproteinase 3,167 α synuclein,57 and neuromelanin.168 In addition, we identified μ calpain as a key soluble neuron-injury signal released from damaged neurons, causing selective DA neuron death through activation of microglial NADPH oxidase and superoxide production, converging on the common mechanism of toxic microglial activation through ROS production.166 Together, these findings support that DA neurons may be inherently susceptible to reactive microgliosis, providing much needed insight into the chronic nature of Parkinson’s disease. Notably, while DA neurons may be more vulnerable to reactive microgliosis, it is likely that reactive microgliosis is a contributing factor to most neurodegenerative diseases.
LPS-Chronic Response to a Single Stimulus
LPSis reported to activate microglia both in vivo and in vitro causing the progressive and cumulative loss of DA neurons over time.129, 169, 170 While traditionally thought of as a model of infectious insult, a recently developed PD animal model uses a systemic LPS administration, and shows that a single pro-inflammatory stimulus can persistently activate microglia to cause neuron death.156 Specifically, we have recently shown that systemic LPS administration activates cells in the liver to produce TNFα, which is distributed in the blood and transferred to the brain through TNFα receptors to induce the synthesis of additional TNFα and other pro-inflammatory factors, creating a persistent and self-propelling neuroinflammation that induces delayed and progressive loss of DA neurons in the SN of adult animals.156 This work is the first to support that a single pro-inflammatory stimulus (whether a pathogen or environmental in origin) in the adult animal can cause progressive neuron damage later in life, suggesting a wide therapeutic window for the effective use of anti-inflammatory therapy in neurodegenerative disease.
However, earlier reports have already shown that exposure to LPS early in life can induce and enhance DA neuron damage later in life. Studies show that during critical periods of embryonic development (E 10.5), maternal exposure to low concentrations of LPS in mice impacts microglial activation and DA neuron survival in offspring that persists into adulthood.170, 171 Interestingly, LPS has been implicated in the potential etiology of sporadic PD through sepsis and early life exposure during pregnancy.169, 172, 173 Further, diagnosed parkinsonian symptoms, brain inflammation, and damage to the SN have been described in a patient who had received accidental systemic administration of LPS.174 However, the most important implication of these findings is that not only can microglia induce neuron damage, but that microglia can become persistently activated to produce continuous and uncontrolled neurotoxicity that fails to resolve, long after the instigating stimulus has dissipated.
Priming: Lowering the Threshold to Initiate Neurotoxicity
The phenomenon of microglial priming offers valuable insight into why microglia continue to respond to additional stimuli in the chronic cycle of neuroinflammation (Figure 1). In the case of priming, microglia are not just exhibiting and enhanced toxic microglial response. Rather, in the case of priming, the microglial phenotype shifts, where a much lower stimulus is needed to exact a toxic microglial response, enhancing the probability that the chronic cycle of toxic reactive microgliosis will continue.
Systemic LPS has been widely explored for the ability to amplify ongoing neuropathology in adults. Systemic LPS administration has been shown to enhance prion-induced cognitive deficits, neuroinflammation, and neuropathology.175 Further, systemic neonatal exposure to LPS is shown to significantly amplify neuronal death associated with ischemic insult.176 Finally, immunological perturbation during critical periods of development170 or aging177 and sentience,178 can prime microglia, where additional stimuli result in an exaggerated and prolonged pro-inflammatory response that enhances neuron damage.
By altering concentrations of intracellular ROS and consequent redox-signaling, NADPH oxidase is reported to prime the microglial response to further insult. Triggers of microglia activation, such as rotenone55 and neuronal death,179 are shown to prime microglia through NADPH oxidase and result in synergistic microglial activation that is associated with neurotoxicity upon additional insult with LPS. This has particular importance given that a multiple hit hypothesis180 has been proposed as a potential mechanism through which environmental toxicants induce neurodegenerative disease over an in individual’s lifetime and may provide significant insight into the progressive nature of neuroinflammation.
Conclusions & Implications
Microglia can be continuously activated to produce toxic factors (cytokines and reactive oxygen species) by either single or chronic exposure to disease proteins, environmental toxins, cytokines, and neuronal damage (reactive microgliosis), resulting in the progressive loss of neurons over time, a fundamental component of neurodegenerative disease. Recent work suggests that DA neurons may be inherently vulnerable to reactive microgliosis, providing much needed insight into the progressive nature of Parkinson’s disease. Current research also suggests that redox signaling in microglia may be a critical mechanism of chronic neuroinflammation that propagates the microglial pro-inflammatory response through amplification of cytokine production and lowering of the stimulus threshold to cause a neurotoxic toxic response. Future research will need to focus on why the microglial response fails to resolve when compared to the peripheral immune system, the mechanisms through which ROS is signaling toxic microglial activation, and harness this information for the identification of novel therapeutic approaches.
Acknowledgments
This work was supported by the NIEHS/NIH Pathway to Independence Award R00ES01549, the NIEHS/NIH ONES Award R01ES016951 and the Institute for the Study of Aging/Alzheimer’s Drug Discovery Foundation.
Abbreviations
- 6-OHDA
6-hydroxy dopamine
- Aβ
Beta amyloid peptide
- ALS
amyotrophic lateral sclerosis
- CNS
central nervous system
- COX2
cyclooxygenase 2
- DA
dopamine
- DAT
dopamine trasporter
- DEP
Diesel Exhaust Particles
- DPI
diphenylene iodonium
- HAND
HIV associated neurocognitive disorder
- H2O2
hydrogen peroxide
- IL-1β
interleukin 1-β
- IL-6
interleukin 6
- iNOS
inducible nitric oxide synthase
- LPS
lipopolysaccharide
- MHC
major histocompatability complex
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NADPH
nicotinamide adenine dinucleotide phosphate
- NFκB
nuclear factor-κB
- NO
nitric oxide
- O2•−
superoxide
- PD
Parkinson’s disease
- PGE2
prostaglandin E2
- PHOX
phagocytic oxidase
- PQ
paraquat
- ROS
reactive oxygen species
- SN
substantia nigra
- TLR4
toll-like receptor 4
- TNFα
tumor necrosis factor-alpha
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007;35:1127–1132. doi: 10.1042/BST0351127. [DOI] [PubMed] [Google Scholar]
- 2.del Rio-Hortega P. Cytology and cellular pathology of the nervous system. New York: Penfeild Wed; 1932. [Google Scholar]
- 3.Morris L, Graham CF, Gordon S. Macrophages in haemopoietic and other tissues of the developing mouse detected by the monoclonal antibody F4/80. Development. 1991;112:517–526. doi: 10.1242/dev.112.2.517. [DOI] [PubMed] [Google Scholar]
- 4.Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- 5.Rezaie P, Trillo-Pazos G, Greenwood J, Everall IP, Male DK. Motility and ramification of human fetal microglia in culture: an investigation using time-lapse video microscopy and image analysis. Exp Cell Res. 2002;274:68–82. doi: 10.1006/excr.2001.5431. [DOI] [PubMed] [Google Scholar]
- 6.Ling EA, Wong WC. The origin and nature of ramified and amoeboid microglia: a historical review and current concepts. Glia. 1993;7:9–18. doi: 10.1002/glia.440070105. [DOI] [PubMed] [Google Scholar]
- 7.Dalmau I, Vela JM, Gonzalez B, Finsen B, Castellano B. Dynamics of microglia in the developing rat brain. J Comp Neurol. 2003;458:144–157. doi: 10.1002/cne.10572. [DOI] [PubMed] [Google Scholar]
- 8.Soulet D, Rivest S. Bone-marrow-derived microglia: myth or reality? Curr Opin Pharmacol. 2008;8:508–518. doi: 10.1016/j.coph.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Mildner A, Schmidt H, Nitsche M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 10.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 11.Flugel A, Bradl M, Kreutzberg GW, Graeber MB. Transformation of donor-derived bone marrow precursors into host microglia during autoimmune CNS inflammation and during the retrograde response to axotomy. J Neurosci Res. 2001;66:74–82. doi: 10.1002/jnr.1198. [DOI] [PubMed] [Google Scholar]
- 12.Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 13.Streit WJ, Graeber MB, Kreutzberg GW. Expression of Ia antigen on perivascular and microglial cells after sublethal and lethal motor neuron injury. Exp Neurol. 1989;105:115–126. doi: 10.1016/0014-4886(89)90111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shechter R, London A, Varol C, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- 16.Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 17.Alliot F, Lecain E, Grima B, Pessac B. Microglial progenitors with a high proliferative potential in the embryonic and adult mouse brain. Proc Natl Acad Sci U S A. 1991;88:1541–1545. doi: 10.1073/pnas.88.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 19.Adams RA, Bauer J, Flick MJ, et al. The fibrin-derived gamma377–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007;204:571–582. doi: 10.1084/jem.20061931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoek RM, Ruuls SR, Murphy CA, et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 21.Harrison JK, Jiang Y, Chen S, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 23.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 24.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science. 2005 doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 25.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 26.Graeber MB, Streit WJ, Kreutzberg GW. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J Neurosci Res. 1988;21:18–24. doi: 10.1002/jnr.490210104. [DOI] [PubMed] [Google Scholar]
- 27.Colton CA, Gilbert DL. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987;223:284–288. doi: 10.1016/0014-5793(87)80305-8. [DOI] [PubMed] [Google Scholar]
- 28.Hurley SD, Walter SA, Semple-Rowland SL, Streit WJ. Cytokine transcripts expressed by microglia in vitro are not expressed by ameboid microglia of the developing rat central nervous system. Glia. 1999;25:304–309. doi: 10.1002/(sici)1098-1136(19990201)25:3<304::aid-glia10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 29.Si Q, Kim MO, Zhao ML, Landau NR, Goldstein H, Lee S. Vpr- and Nef-dependent induction of RANTES/CCL5 in microglial cells. Virology. 2002;301:342–353. doi: 10.1006/viro.2002.1613. [DOI] [PubMed] [Google Scholar]
- 30.Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 31.Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- 32.Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 33.Lynch MA. The multifaceted profile of activated microglia. Mol Neurobiol. 2009;40:139–156. doi: 10.1007/s12035-009-8077-9. [DOI] [PubMed] [Google Scholar]
- 34.Elkabes S, DiCicco-Bloom EM, Black IB. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J Neurosci. 1996;16:2508–2521. doi: 10.1523/JNEUROSCI.16-08-02508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85:145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- 36.Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 37.Zhong Y, Zhou LJ, Ren WJ, et al. The direction of synaptic plasticity mediated by C-fibers in spinal dorsal horn is decided by Src-family kinases in microglia: The role of tumor necrosis factor-alpha. Brain Behav Immun. doi: 10.1016/j.bbi.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Batchelor PE, Liberatore GT, Wong JY, et al. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batchelor PE, Porritt MJ, Martinello P, et al. Macrophages and Microglia Produce Local Trophic Gradients That Stimulate Axonal Sprouting Toward but Not beyond the Wound Edge. Mol Cell Neurosci. 2002;21:436–453. doi: 10.1006/mcne.2002.1185. [DOI] [PubMed] [Google Scholar]
- 40.Yamada J, Hayashi Y, Jinno S, et al. Reduced synaptic activity precedes synaptic stripping in vagal motoneurons after axotomy. Glia. 2008;56:1448–1462. doi: 10.1002/glia.20711. [DOI] [PubMed] [Google Scholar]
- 41.Trapp BD, Wujek JR, Criste GA, et al. Evidence for synaptic stripping by cortical microglia. Glia. 2007;55:360–368. doi: 10.1002/glia.20462. [DOI] [PubMed] [Google Scholar]
- 42.Siskova Z, Page A, O’Connor V, Perry VH. Degenerating synaptic boutons in prion disease: microglia activation without synaptic stripping. Am J Pathol. 2009;175:1610–1621. doi: 10.2353/ajpath.2009.090372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao H, Bu WY, Wang TH, Ahmed S, Xiao ZC. Tenascin-R plays a role in neuroprotection via its distinct domains coordinately modulating the microglia function. J Biol Chem. 2004 doi: 10.1074/jbc.M412730200. [DOI] [PubMed] [Google Scholar]
- 44.Morgan SC, Taylor DL, Pocock JM. Microglia release activators of neuronal proliferation mediated by activation of mitogen-activated protein kinase, phosphatidylinositol-3-kinase/Akt and delta-Notch signalling cascades. J Neurochem. 2004;90:89–101. doi: 10.1111/j.1471-4159.2004.02461.x. [DOI] [PubMed] [Google Scholar]
- 45.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–145. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 46.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 47.Graeber MB, Streit WJ, Kreutzberg GW. The microglial cytoskeleton: vimentin is localized within activated cells in situ. J Neurocytol. 1988;17:573–580. doi: 10.1007/BF01189811. [DOI] [PubMed] [Google Scholar]
- 48.Oehmichen W, Gencic M. Experimental studies on kinetics and functions of monuclear phagozytes of the central nervous system. Acta Neuropathol Suppl (Berl) 1975;(Suppl 6):285–290. doi: 10.1007/978-3-662-08456-4_50. [DOI] [PubMed] [Google Scholar]
- 49.Pei Z, Pang H, Qian L, et al. MAC1 mediates LPS-induced production of superoxide by microglia: the role of pattern recognition receptors in dopaminergic neurotoxicity. Glia. 2007;55:1362–1373. doi: 10.1002/glia.20545. [DOI] [PubMed] [Google Scholar]
- 50.Qin L, Li G, Qian X, et al. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 2005;52:78–84. doi: 10.1002/glia.20225. [DOI] [PubMed] [Google Scholar]
- 51.Qin L, Liu Y, Qian X, Hong JS, Block ML. Microglial NADPH oxidase mediates leucine enkephalin dopaminergic neuroprotection. Ann N Y Acad Sci. 2005;1053:107–120. doi: 10.1196/annals.1344.009. [DOI] [PubMed] [Google Scholar]
- 52.Wu X, Block ML, Zhang W, et al. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal. 2005;7:654–661. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- 53.Mao H, Fang X, Floyd KM, Polcz JE, Zhang P, Liu B. Induction of microglial reactive oxygen species production by the organochlorinated pesticide dieldrin. Brain Res. 2007;1186:267–274. doi: 10.1016/j.brainres.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 54.Mao H, Liu B. Synergistic microglial reactive oxygen species generation induced by pesticides lindane and dieldrin. Neuroreport. 2008;19:1317–1320. doi: 10.1097/WNR.0b013e32830b3677. [DOI] [PubMed] [Google Scholar]
- 55.Gao HM, Hong JS, Zhang W, Liu B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. J Neurosci. 2003;23:1228–1236. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bamberger ME, Landreth GE. Microglial interaction with beta-amyloid: implications for the pathogenesis of Alzheimer’s disease. Microsc Res Tech. 2001;54:59–70. doi: 10.1002/jemt.1121. [DOI] [PubMed] [Google Scholar]
- 57.Zhang W, Wang T, Pei Z, et al. Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. Faseb J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 58.Turchan-Cholewo J, Dimayuga FO, Gupta S, et al. Morphine and HIV-Tat increase microglial-free radical production and oxidative stress: possible role in cytokine regulation. J Neurochem. 2009;108:202–215. doi: 10.1111/j.1471-4159.2008.05756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Block ML, Calderon-Garciduenas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moss DW, Bates TE. Activation of murine microglial cell lines by lipopolysaccharide and interferon-gamma causes NO-mediated decreases in mitochondrial and cellular function. Eur J Neurosci. 2001;13:529–538. doi: 10.1046/j.1460-9568.2001.01418.x. [DOI] [PubMed] [Google Scholar]
- 61.Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci. 2002;962:318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 62.Sawada M, Kondo N, Suzumura A, Marunouchi T. Production of tumor necrosis factor-alpha by microglia and astrocytes in culture. Brain Res. 1989;491:394–397. doi: 10.1016/0006-8993(89)90078-4. [DOI] [PubMed] [Google Scholar]
- 63.Lee SC, Liu W, Dickson DW, Brosnan CF, Berman JW. Cytokine production by human fetal microglia and astrocytes. Differential induction by lipopolysaccharide and IL-1 beta. J Immunol. 1993;150:2659–2667. [PubMed] [Google Scholar]
- 64.Wang T, Pei Z, Zhang W, et al. MPP+-induced COX-2 activation and subsequent dopaminergic neurodegeneration. Faseb J. 2005 doi: 10.1096/fj.04-2457fje. [DOI] [PubMed] [Google Scholar]
- 65.Liu B, Gao HM, Hong JS. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ Health Perspect. 2003;111:1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olson EE, McKeon RJ. Characterization of cellular and neurological damage following unilateral hypoxia/ischemia. J Neurol Sci. 2004;227:7–19. doi: 10.1016/j.jns.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 67.Morioka T, Kalehua AN, Streit WJ. Characterization of microglial reaction after middle cerebral artery occlusion in rat brain. J Comp Neurol. 1993;327:123–132. doi: 10.1002/cne.903270110. [DOI] [PubMed] [Google Scholar]
- 68.Tsuda M, Mizokoshi A, Shigemoto-Mogami Y, Koizumi S, Inoue K. Activation of p38 mitogen-activated protein kinase in spinal hyperactive microglia contributes to pain hypersensitivity following peripheral nerve injury. Glia. 2004;45:89–95. doi: 10.1002/glia.10308. [DOI] [PubMed] [Google Scholar]
- 69.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 70.Ziehn MO, Avedisian AA, Tiwari-Woodruff S, Voskuhl RR. Hippocampal CA1 atrophy and synaptic loss during experimental autoimmune encephalomyelitis, EAE. Lab Invest. doi: 10.1038/labinvest.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Banati RB, Gehrmann J, Kellner M, Holsboer F. Antibodies against microglia/brain macrophages in the cerebrospinal fluid of a patient with acute amyotrophic lateral sclerosis and presenile dementia. Clin Neuropathol. 1995;14:197–200. [PubMed] [Google Scholar]
- 72.Hall ED, Oostveen JA, Gurney ME. Relationship of microglial and astrocytic activation to disease onset and progression in a transgenic model of familial ALS. Glia. 1998;23:249–256. doi: 10.1002/(sici)1098-1136(199807)23:3<249::aid-glia7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 73.Bell JE, Arango JC, Robertson R, Brettle RP, Leen C, Simmonds P. HIV and drug misuse in the Edinburgh cohort. J Acquir Immune Defic Syndr. 2002;31 (Suppl 2):S35–42. doi: 10.1097/00126334-200210012-00003. [DOI] [PubMed] [Google Scholar]
- 74.Miller KR, Streit WJ. The effects of aging, injury and disease on microglial function: a case for cellular senescence. Neuron Glia Biol. 2007;3:245–253. doi: 10.1017/S1740925X08000136. [DOI] [PubMed] [Google Scholar]
- 75.Finch GL, Hobbs CH, Blair LF, et al. Effects of subchronic inhalation exposure of rats to emissions from a diesel engine burning soybean oil-derived biodiesel fuel. Inhal Toxicol. 2002;14:1017–1048. doi: 10.1080/08958370290084764. [DOI] [PubMed] [Google Scholar]
- 76.Yankner BA. Amyloid and Alzheimer’s disease--cause or effect? Neurobiol Aging. 1989;10:470–471. doi: 10.1016/0197-4580(89)90101-2. discussion 477–478. [DOI] [PubMed] [Google Scholar]
- 77.Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol Aging. 1988;9:339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- 78.McGeer PL, Itagaki S, Tago H, McGeer EG. Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neurosci Lett. 1987;79:195–200. doi: 10.1016/0304-3940(87)90696-3. [DOI] [PubMed] [Google Scholar]
- 79.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 80.Sasaki A, Yamaguchi H, Ogawa A, Sugihara S, Nakazato Y. Microglial activation in early stages of amyloid beta protein deposition. Acta Neuropathol (Berl) 1997;94:316–322. doi: 10.1007/s004010050713. [DOI] [PubMed] [Google Scholar]
- 81.Meda L, Cassatella MA, Szendrei GI, et al. Activation of microglial cells by beta-amyloid protein and interferon-gamma. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- 82.Banati RB, Newcombe J, Gunn RN, et al. The peripheral benzodiazepine binding site in the brain in multiple sclerosis: quantitative in vivo imaging of microglia as a measure of disease activity. Brain. 2000;123 (Pt 11):2321–2337. doi: 10.1093/brain/123.11.2321. [DOI] [PubMed] [Google Scholar]
- 83.Peterson JW, Bo L, Mork S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50:389–400. doi: 10.1002/ana.1123. [DOI] [PubMed] [Google Scholar]
- 84.Takeuchi H, Wang J, Kawanokuchi J, Mitsuma N, Mizuno T, Suzumura A. Interferon-gamma induces microglial-activation-induced cell death: A hypothetical mechanism of relapse and remission in multiple sclerosis. Neurobiol Dis. 2006;22:33–39. doi: 10.1016/j.nbd.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 85.Murphy AC, Lalor SJ, Lynch MA, Mills KH. Infiltration of Th1 and Th17 cells and activation of microglia in the CNS during the course of experimental autoimmune encephalomyelitis. Brain Behav Immun. doi: 10.1016/j.bbi.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 86.Liu Y, Hao W, Letiembre M, et al. Suppression of microglial inflammatory activity by myelin phagocytosis: role of p47-PHOX-mediated generation of reactive oxygen species. J Neurosci. 2006;26:12904–12913. doi: 10.1523/JNEUROSCI.2531-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mack CL, Vanderlugt-Castaneda CL, Neville KL, Miller SD. Microglia are activated to become competent antigen presenting and effector cells in the inflammatory environment of the Theiler’s virus model of multiple sclerosis. J Neuroimmunol. 2003;144:68–79. doi: 10.1016/j.jneuroim.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 88.Jordan CA, Watkins BA, Kufta C, Dubois-Dalcq M. Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J Virol. 1991;65:736–742. doi: 10.1128/jvi.65.2.736-742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Speth C, Dierich MP, Sopper S. HIV-infection of the central nervous system: the tightrope walk of innate immunity. Mol Immunol. 2005;42:213–228. doi: 10.1016/j.molimm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 90.Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. Eur J Clin Invest. 2006;36:447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- 91.Turchan-Cholewo J, Dimayuga VM, Gupta S, Gorospe RM, Keller JN, Bruce-Keller AJ. NADPH oxidase drives cytokine and neurotoxin release from microglia and macrophages in response to HIV-Tat. Antioxid Redox Signal. 2009;11:193–204. doi: 10.1089/ars.2008.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Polazzi E, Contestabile A. Neuron-conditioned media differentially affect the survival of activated or unstimulated microglia: evidence for neuronal control on apoptotic elimination of activated microglia. J Neuropathol Exp Neurol. 2003;62:351–362. doi: 10.1093/jnen/62.4.351. [DOI] [PubMed] [Google Scholar]
- 93.McGeer EG, Klegeris A, McGeer PL. Inflammation, the complement system and the diseases of aging. Neurobiol Aging. 2005;26 (Suppl 1):94–97. doi: 10.1016/j.neurobiolaging.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 94.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 95.Long-Smith CM, Sullivan AM, Nolan YM. The influence of microglia on the pathogenesis of Parkinson’s disease. Prog Neurobiol. 2009;89:277–287. doi: 10.1016/j.pneurobio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 96.Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- 97.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 98.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- 101.Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1 beta and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci Lett. 1995;202:17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 102.Muller T, Blum-Degen D, Przuntek H, Kuhn W. Interleukin-6 levels in cerebrospinal fluid inversely correlate to severity of Parkinson’s disease. Acta Neurol Scand. 1998;98:142–144. doi: 10.1111/j.1600-0404.1998.tb01736.x. [DOI] [PubMed] [Google Scholar]
- 103.Nagatsu T, Mogi M, Ichinose H, Togari A. Changes in cytokines and neurotrophins in Parkinson’s disease. J Neural Transm Suppl. 2000:277–290. doi: 10.1007/978-3-7091-6301-6_19. [DOI] [PubMed] [Google Scholar]
- 104.Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson’s disease. J Neural Transm Suppl. 2000:143–151. [PubMed] [Google Scholar]
- 105.Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol Cell Neurosci. 2000;16:724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- 106.Sriram K, O’Callaghan JP. Divergent roles for tumor necrosis factor-alpha in the brain. J Neuroimmune Pharmacol. 2007;2:140–153. doi: 10.1007/s11481-007-9070-6. [DOI] [PubMed] [Google Scholar]
- 107.Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP. Deficiency of TNF receptors suppresses microglial activation and alters the susceptibility of brain regions to MPTP-induced neurotoxicity: role of TNF-alpha. Faseb J. 2006;20:670–682. doi: 10.1096/fj.05-5106com. [DOI] [PubMed] [Google Scholar]
- 108.Sriram K, Matheson JM, Benkovic SA, Miller DB, Luster MI, O’Callaghan JP. Mice deficient in TNF receptors are protected against dopaminergic neurotoxicity: implications for Parkinson’s disease. Faseb J. 2002;16:1474–1476. doi: 10.1096/fj.02-0216fje. [DOI] [PubMed] [Google Scholar]
- 109.De Lella Ezcurra AL, Chertoff M, Ferrari C, Graciarena M, Pitossi F. Chronic expression of low levels of tumor necrosis factor-alpha in the substantia nigra elicits progressive neurodegeneration, delayed motor symptoms and microglia/macrophage activation. Neurobiol Dis. 37:630–640. doi: 10.1016/j.nbd.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 110.Lee JK, Tran T, Tansey MG. Neuroinflammation in Parkinson’s disease. J Neuroimmune Pharmacol. 2009;4:419–429. doi: 10.1007/s11481-009-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nimmo AJ, Vink R. Recent patents in CNS drug discovery: the management of inflammation in the central nervous system. Recent Pat CNS Drug Discov. 2009;4:86–95. doi: 10.2174/157488909788452997. [DOI] [PubMed] [Google Scholar]
- 112.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 113.Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee JK, McCoy MK, Harms AS, Ruhn KA, Gold SJ, Tansey MG. Regulator of G-protein signaling 10 promotes dopaminergic neuron survival via regulation of the microglial inflammatory response. J Neurosci. 2008;28:8517–8528. doi: 10.1523/JNEUROSCI.1806-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 116.Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat Protoc. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- 117.Czlonkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Czlonkowski A. Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson’s disease mice model. Neurodegeneration. 1996;5:137–143. doi: 10.1006/neur.1996.0020. [DOI] [PubMed] [Google Scholar]
- 118.Kurkowska-Jastrzebska I, Wronska A, Kohutnicka M, Czlonkowski A, Czlonkowska A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine intoxication in mouse. Exp Neurol. 1999;156:50–61. doi: 10.1006/exnr.1998.6993. [DOI] [PubMed] [Google Scholar]
- 119.Hurley SD, O’Banion MK, Song DD, Arana FS, Olschowka JA, Haber SN. Microglial response is poorly correlated with neurodegeneration following chronic, low-dose MPTP administration in monkeys. Exp Neurol. 2003;184:659–668. doi: 10.1016/S0014-4886(03)00273-5. [DOI] [PubMed] [Google Scholar]
- 120.Barcia C, Sanchez Bahillo A, Fernandez-Villalba E, et al. Evidence of active microglia in substantia nigra pars compacta of parkinsonian monkeys 1 year after MPTP exposure. Glia. 2004;46:402–409. doi: 10.1002/glia.20015. [DOI] [PubMed] [Google Scholar]
- 121.Cicchetti F, Brownell AL, Williams K, Chen YI, Livni E, Isacson O. Neuroinflammation of the nigrostriatal pathway during progressive 6-OHDA dopamine degeneration in rats monitored by immunohistochemistry and PET imaging. Eur J Neurosci. 2002;15:991–998. doi: 10.1046/j.1460-9568.2002.01938.x. [DOI] [PubMed] [Google Scholar]
- 122.Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 123.Hunot S, Brugg B, Ricard D, et al. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc Natl Acad Sci U S A. 1997;94:7531–7536. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ouchi Y, Yagi S, Yokokura M, Sakamoto M. Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat Disord. 2009;15 (Suppl 3):S200–204. doi: 10.1016/S1353-8020(09)70814-4. [DOI] [PubMed] [Google Scholar]
- 125.Ouchi Y, Yoshikawa E, Sekine Y, et al. Microglial activation and dopamine terminal loss in early Parkinson’s disease. Ann Neurol. 2005;57:168–175. doi: 10.1002/ana.20338. [DOI] [PubMed] [Google Scholar]
- 126.Gao HM, Liu B, Hong JS. Critical role for microglial NADPH oxidase in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2003;23:6181–6187. doi: 10.1523/JNEUROSCI.23-15-06181.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Block ML, Wu X, Pei Z, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. Faseb J. 2004;18:1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- 128.Qin L, Liu Y, Cooper C, Liu B, Wilson B, Hong JS. Microglia enhance beta-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. J Neurochem. 2002;83:973–983. doi: 10.1046/j.1471-4159.2002.01210.x. [DOI] [PubMed] [Google Scholar]
- 129.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 130.Qin L, Liu Y, Wang T, et al. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J Biol Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 131.Paulsen CE, Carroll KS. Orchestrating redox signaling networks through regulatory cysteine switches. ACS Chem Biol. 5:47–62. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 134.Nam HJ, Park YY, Yoon G, Cho H, Lee JH. Co-treatment with hepatocyte growth factor and TGF-b1 enhances migration of HaCaT cells through NADPH oxidase-dependent ROS generation. Exp Mol Med. doi: 10.3858/emm.2010.42.4.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sun HN, Kim SU, Lee MS, et al. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent activation of phosphoinositide 3-kinase and p38 mitogen-activated protein kinase signal pathways is required for lipopolysaccharide-induced microglial phagocytosis. Biol Pharm Bull. 2008;31:1711–1715. doi: 10.1248/bpb.31.1711. [DOI] [PubMed] [Google Scholar]
- 136.Roy A, Jana A, Yatish K, et al. Reactive oxygen species up-regulate CD11b in microglia via nitric oxide: Implications for neurodegenerative diseases. Free Radic Biol Med. 2008;45:686–699. doi: 10.1016/j.freeradbiomed.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wu DC, Teismann P, Tieu K, et al. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Block ML. NADPH oxidase as a therapeutic target in Alzheimer’s disease. BMC Neurosci. 2008;9 (Suppl 2):S8. doi: 10.1186/1471-2202-9-S2-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wu XF, Block ML, Zhang W, et al. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxid Redox Signal. 2005;7:654–661. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- 140.Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson’s disease. Faseb J. 2003 doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- 141.Won SY, Choi SH, Jin BK. Prothrombin kringle-2-induced oxidative stress contributes to the death of cortical neurons in vivo and in vitro: role of microglial NADPH oxidase. J Neuroimmunol. 2009;214:83–92. doi: 10.1016/j.jneuroim.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 142.Qian L, Hu X, Zhang D, et al. beta2 Adrenergic receptor activation induces microglial NADPH oxidase activation and dopaminergic neurotoxicity through an ERK-dependent/protein kinase A- independent pathway. Glia. 2009;57:1600–1609. doi: 10.1002/glia.20873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Rodriguez-Pallares J, Rey P, Parga JA, Munoz A, Guerra MJ, Labandeira-Garcia JL. Brain angiotensin enhances dopaminergic cell death via microglial activation and NADPH-derived ROS. Neurobiol Dis. 2008;31:58–73. doi: 10.1016/j.nbd.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 144.Hur J, Lee P, Kim MJ, Kim Y, Cho YW. Ischemia-activated microglia induces neuronal injury via activation of gp91phox NADPH oxidase. Biochem Biophys Res Commun. 391:1526–1530. doi: 10.1016/j.bbrc.2009.12.114. [DOI] [PubMed] [Google Scholar]
- 145.Green SP, Cairns B, Rae J, et al. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. J Cereb Blood Flow Metab. 2001;21:374–384. doi: 10.1097/00004647-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 146.Wang T, Liu B, Qin L, Wilson B, Hong JS. Protective effect of the SOD/catalase mimetic MnTMPyP on inflammation-mediated dopaminergic neurodegeneration in mesencephalic neuronal-glial cultures. J Neuroimmunol. 2004;147:68–72. doi: 10.1016/j.jneuroim.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 147.Li Y, Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- 148.Block ML, Li G, Qin L, et al. Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin Faseb J. 2006;20:251–258. doi: 10.1096/fj.05-4553com. [DOI] [PubMed] [Google Scholar]
- 149.Konishi H, Tanaka M, Takemura Y, et al. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci U S A. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Guyton KZ, Gorospe M, Kensler TW, Holbrook NJ. Mitogen-activated protein kinase (MAPK) activation by butylated hydroxytoluene hydroperoxide: implications for cellular survival and tumor promotion. Cancer Res. 1996;56:3480–3485. [PubMed] [Google Scholar]
- 151.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. Embo J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166:S4–8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 153.Wang T, Qin L, Liu B, et al. Role of reactive oxygen species in LPS-induced production of prostaglandin E2 in microglia. J Neurochem. 2004;88:939–947. doi: 10.1046/j.1471-4159.2003.02242.x. [DOI] [PubMed] [Google Scholar]
- 154.Min KJ, Pyo HK, Yang MS, Ji KA, Jou I, Joe EH. Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia. 2004;48:197–206. doi: 10.1002/glia.20069. [DOI] [PubMed] [Google Scholar]
- 155.Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77:540–551. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- 156.Qin L, Wu X, Block ML, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gao HK, Yin Z, Zhou N, Feng XY, Gao F, Wang HC. Glycogen synthase kinase 3 inhibition protects the heart from acute ischemia-reperfusion injury via inhibition of inflammation and apoptosis. J Cardiovasc Pharmacol. 2008;52:286–292. doi: 10.1097/FJC.0b013e318186a84d. [DOI] [PubMed] [Google Scholar]
- 158.Huh Y, Jung JW, Park C, et al. Microglial activation and tyrosine hydroxylase immunoreactivity in the substantia nigral region following transient focal ischemia in rats. Neurosci Lett. 2003;349:63–67. doi: 10.1016/s0304-3940(03)00743-2. [DOI] [PubMed] [Google Scholar]
- 159.McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann Neurol. 2003;54:599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- 160.Przedborski S, Tieu K, Perier C, Vila M. MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J Bioenerg Biomembr. 2004;36:375–379. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- 161.Wu DC, Jackson-Lewis V, Vila M, et al. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liberatore GT, Jackson-Lewis V, Vukosavic S, et al. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- 163.Zhang W, Wang T, Qin L, et al. Neuroprotective effect of dextromethorphan in the MPTP Parkinson’s disease model: role of NADPH oxidase. Faseb J. 2004;18:589–591. doi: 10.1096/fj.03-0983fje. [DOI] [PubMed] [Google Scholar]
- 164.Feng ZH, Wang TG, Li DD, et al. Cyclooxygenase-2-deficient mice are resistant to 1-methyl-4-phenyl1, 2, 3, 6-tetrahydropyridine-induced damage of dopaminergic neurons in the substantia nigra. Neurosci Lett. 2002;329:354–358. doi: 10.1016/s0304-3940(02)00704-8. [DOI] [PubMed] [Google Scholar]
- 165.Teismann P, Vila M, Choi DK, et al. COX-2 and neurodegeneration in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:272–277. doi: 10.1111/j.1749-6632.2003.tb07482.x. [DOI] [PubMed] [Google Scholar]
- 166.Levesque S, Wilson B, Gregoria V, et al. Reactive Microgliosis: Extracellular Calpain & Microglia-mediated Dopaminergic Neurotoxicity. Brain. doi: 10.1093/brain/awp333. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kim YS, Choi DH, Block ML, et al. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. Faseb J. 2007;21:179–187. doi: 10.1096/fj.06-5865com. [DOI] [PubMed] [Google Scholar]
- 168.Zecca L, Zucca FA, Wilms H, Sulzer D. Neuromelanin of the substantia nigra: a neuronal black hole with protective and toxic characteristics. Trends Neurosci. 2003;26:578–580. doi: 10.1016/j.tins.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 169.Ling Z, Gayle DA, Ma SY, et al. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov Disord. 2002;17:116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- 170.Ling Z, Zhu Y, Tong CW, Snyder JA, Lipton JW, Carvey PM. Progressive dopamine neuron loss following supra-nigral lipopolysaccharide (LPS) infusion into rats exposed to LPS prenatally. Exp Neurol. 2006 doi: 10.1016/j.expneurol.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 171.Carvey PM, Chang Q, Lipton JW, Ling Z. Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: a potential, new model of Parkinson’s disease. Front Biosci. 2003;8:s826–837. doi: 10.2741/1158. [DOI] [PubMed] [Google Scholar]
- 172.Gayle DA, Ling Z, Tong C, Landers T, Lipton JW, Carvey PM. Lipopolysaccharide (LPS)-induced dopamine cell loss in culture: roles of tumor necrosis factor-alpha, interleukin-1beta, and nitric oxide. Brain Res Dev Brain Res. 2002;133:27–35. doi: 10.1016/s0165-3806(01)00315-7. [DOI] [PubMed] [Google Scholar]
- 173.Ling ZD, Chang Q, Lipton JW, Tong CW, Landers TM, Carvey PM. Combined toxicity of prenatalbacterial endotoxin exposure and postnatal 6-hydroxydopamine in the adult rat midbrain. Neuroscience. 2004;124:619–628. doi: 10.1016/j.neuroscience.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 174.Niehaus I. Lippopolysaccharides induce inflammation-mediated neurodeheneration in the substantia nigra and the cerebral cortex (A case report). AD/PD 6th International Conference; 2003. pp. 1–38. [Google Scholar]
- 175.Cunningham C, Campion S, Lunnon K, et al. Systemic inflammation induces acute behavioral and cognitive changes and accelerates neurodegenerative disease. Biol Psychiatry. 2009;65:304–312. doi: 10.1016/j.biopsych.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lehnardt S, Massillon L, Follett P, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23:309–317. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Godbout JP, Chen J, Abraham J, et al. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. Faseb J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- 179.Gao HM, Liu B, Zhang W, Hong JS. Synergistic dopaminergic neurotoxicity of MPTP and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson’s disease. Faseb J. 2003;17:1957–1959. doi: 10.1096/fj.03-0203fje. [DOI] [PubMed] [Google Scholar]
- 180.Carvey PM, Punati A, Newman MB. Progressive dopamine neuron loss in Parkinson’s disease: the multiple hit hypothesis. Cell Transplant. 2006;15:239–250. doi: 10.3727/000000006783981990. [DOI] [PubMed] [Google Scholar]