Abstract
Background
The CDC et al. reported methicillin-resistant S. aureus (MRSA) are significant causes of serious human infections, including pulmonary illnesses. We investigated the role of superantigens (SAgs) in lung-associated lethal illness in rabbits.
Methods
A rabbit model was established to investigate the potential role of SAgs, staphylococcal enterotoxin (SE) B and SEC, and toxic shock syndrome toxin-1 (TSST-1). Rabbits received intra-bronchial community-associated (CA) MRSA strains USA200 (TSST-1+), MW2 (SEC+), or c99-529 (SEB+), or purified SAgs. Some rabbits were pre-immunized against SAgs or treated with soluble high-affinity T cell receptors (Vβ-TCR) to neutralize SEB and then challenged intra-bronchially with CA-MRSA or SAgs.
Results
Rabbits challenged with CA-MRSA or SAgs developed fatal, pulmonary illnesses. Animals pre-immunized against purified SAgs, or treated passively with Vβ-TCRs, and then challenged with CA-MRSA or SAgs, survived. Lung histology indicated non-immune animals developed lesions consistent with necrotizing pneumonia after challenge with CA-MRSA or purified SAgs. SAg immune animals or animals treated with soluble Vβ-TCRs did not develop pulmonary lesions.
Conclusions
SAgs contribute to lethal pulmonary illneses due to CA-MRSA; pre-existing immunity to SAgs prevents lethality. Administration of high-affinity Vβ-TCR with specificity for SEB to non-immune animals protects from lethal pulmonary illness due to SEB+ CA-MRSA and SEB.
Keywords: Community-associated methicillin-resistant Staphylococcus aureus, superantigen, pulmonary infection, toxic shock syndrome
INTRODUCTION
Staphylococcus aureus is a significant human pathogen that causes multiple illnesses [1]. In recent years, there has been a rapid emergence of severe soft tissue and pulmonary infections caused by community-associated methicillin-resistant S. aureus (CA-MRSA) [2, 3]. These potentially fatal infections, including toxic shock syndrome (TSS), purpura fulminans, and necrotizing pneumonia occur in individuals lacking predisposing risk factors, although the majority may have had prior upper respiratory viral infections [2–4].
Staphylococcal superantigens (SAgs) are exotoxins that stimulate massive cytokine production by both T lymphocytes and macrophages [5, 6]. These cytokines include TNF-α and β, IL-1β, IL-2, and IFN-γ [7], and cause many of the clinical features of TSS. SAgs bind to and crosslink variable regions of certain β-chains of T cell receptors (Vβ-TCRs) and either or both of the α- or β-chains of major histocompatibility complex (MHC) II molecules on macrophages [8, 9].
SAgs, such as TSS toxin-1 (TSST-1) made by CA-MRSA USA200 strains (CDC designation based on pulsed-field gel electrophoresis) and staphylococcal enterotoxins (SEs) B and C made by CA-MRSA USA400 strains [4], are associated with TSS and other serious illnesses in humans [5, 6]. TSST-1 is associated with nearly all cases of menstrual TSS (mTSS), and 50% of non-menstrual cases. SEB and SEC are associated with most of the remaining cases of non-menstrual TSS [10, 11].
In the present study, we investigated the role of these three SAgs produced by CA-MRSA in rabbit models of lethal pulmonary illness.
MATERIALS AND METHODS
CA-MRSA Strains
USA200 strains included MNPA (mTSS isolate), MN1021, and MN128. These organisms appear to be highly related, MN1021 and MN128 coming from the same outbreak, and data presented are accumulated from use of all three organisms. These isolates produce elevated amounts of TSST-1 compared to USA200 Methicillin-sensitive S. aureus (MSSA), but do not produce α, β, γ, or Panton-Valentine leukocidin (PVL) cytotoxins, and the organisms are non-pigmented [12]. The strains have mutations in the α- and γ-toxin genes as determined by nucleotide sequencing and lack PVL genes as determined by PCR [13, 14]. USA400 strains were MW2 and c99-529, isolated from children who succumbed to necrotizing pneumonia [4]. MW2 produces SEC, while CA-MRSA c99-529 produces SEB. Both of the USA400 strains also produce α-and γ-toxins and PVL, but not β-toxin.
Rabbits
Dutch belted rabbits (1.5 to 2 kg) were used in accordance with guidelines established by the University of Minnesota IACUC.
Superantigens
All reagents used for preparation of purified SAgs were maintained lipopolysaccharide (LPS)-free. TSST-1 was purified to homogeneity from S. aureus clone RN4220 (pCE107); this strain does not produce other SAgs. SEB was purified from S. aureus MNHo and SEC from MW2. SAgs were purified after growth of organisms in dialyzable beef heart media [15]. SAgs were precipitated from culture fluids with four volumes of absolute ethanol, resolubilized in distilled water, and purified by thin-layer isoelectric focusing [15, 16]. Initial isoelectric focusing pH gradients were 3.5–10, followed by second gradients of pH 6–8 for TSST-1 and pH 7–9 for SEB and SEC; the isoelectric point for TSST-1 is 7.2 and for SEB and SEC is 8.3 [17, 18]. Purified SAgs were quantified using the BioRad Protein assay (BioRad Co., Hercules, CA), with SEB as the protein standard. Purity was confirmed by SAg migration as single bands when subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis [19] with silver staining and reversed phase HPLC [20] [also confirming lack of contaminating lipoteichoic acid], Limulus assay to confirm lack of detectable LPS, and bioassays to confirm absence of detectable cytolysins, lipase, and protease [21]. Purified SAgs were free of detectable peptidoglycan and LPS also as demonstrated by lack of pyrogenicity with two and three hour fever peaks [22].
Pulmonary illness model
Rabbits were administered CA-MRSA or purified SAg through intra-bronchial inoculation (2 × 109 colony-forming units [CFU] in 200 µl dialyzable beef heart medium, 100–200 µg purified SAg in 200 µl PBS. Rabbits were anesthetized by subcutaneous injections of ketamine (25 mg/kg) and xylazine (25 mg/kg) (Phoenix Pharmaceuticals Inc., St. Joseph, MO) [23]. Once under anesthesia, their necks were shaved, and small incisions were made along the tracheas. Incisions (3 mm) were made in the tracheas, and polyethylene catheters (1 mm diameter, Fisher Scientific, Hampton, NH) were inserted and threaded into the left bronchi. Bacteria or purified SAgs were administered through the catheters. Once exposed to CA-MRSA or SAgs, rabbits were monitored for up to seven days for development of respiratory distress and lethal illness (This point is defined as death, or in agreement with the University of Minnesota IACUC and 28 years research experience, rabbit failure to exhibit both escape behavior and ability to right themselves). At this point the animals were euthanized by intravenous [iv] administration of 1 ml/kg Beuthanasia D, [Schering—Plough Animal Health Corp., Union, NJ]). Rabbits that did not develop respiratory distress and lethal illness were euthanized after seven days.
Immunizations
Dutch belted rabbits were hyperimmunized against either purified TSST-1 or SEC prior to receiving intra-bronchial CA-MRSA or purified SAgs. SAgs (in phosphate buffered saline, PBS, 0.005M NaPO4, pH 7.2, 0.15M NaCl) were mixed with equal volumes of incomplete Freund’s adjuvant (Difco Laboratories, Detroit, MI). Final concentrations of 50 µg/ml of SAgs were used for injections, with each rabbit receiving 1.0 ml, injected subcutaneously into four sites on nape of the neck. Animals received initial injections, followed by booster injections every two weeks until antibody titers were >10,000 as determined by ELISA; antibody titers of >10,000 were considered hyperimmune (humans who do not develop menstrual TSS typically have IgG titers of 160). For ELISA, wells of flat bottom 96-well plates were coated with 1.0 µg/well purified TSST-1 or SEC [24] and washed. Rabbit sera were diluted serially 2-fold in the wells beginning with 1/10 dilutions, the plates were incubated for 2 hr at room temperature, and then wells were washed. Horseradish peroxidase-conjugated anti-rabbit IgG (Sigma-Aldrich, St. Louis, MO) were added to wells, the plates were incubated for 2 hr at room temperature, and the wells were again washed. The relative levels of IgG were determined by the addition color substrate containing o-phenylenediamine and H2O2 (100 µl/well). Reactions were stopped by addition of 12.5% sulfuric acid (50 µl), and then absorbances at 490 nm wavelength were measured spectophotometrically.
Soluble high affinity Vβ-T cell receptor (G5–8)
Some rabbits received soluble high-affinity Vβ-TCR (100 ug iv daily) G5-8 in addition to CA-MRSA or SAgs. Soluble high affinity Vβ-TCR G5-8 with 48pM specificity for SEB was generated by Vβ-TCR mutagenesis and selection by flow cytometry [25]. This 12,000 molecular weight molecule is highly specific for reactivity to SEB, but not SEC and TSST-1, and effectively neutralizes SEB superantigenicity, and thus neutralizes lethal activity, through competition with Vβ-TCR.
Histology
Rabbit lung tissue samples were excised immediately upon death of animals or at the termination of experimentation (7 days), fixed in 10% formalin, and embedded in paraffin wax. Thick tissue sections (10 µm) were obtained using a microtome (Leica RM2235, Wetzlar, Germany). Sections were stained with hematoxylin (Fisher Scientific, Fair Lawn, NJ) and eosin (Sigma-Aldrich, St. Louis, MO) (H & E) following standard protocols at the University of Minnesota Veterinary Animal Pathology Laboratory.
Statistical analyses
Data were analyzed by Fisher’s Exact test. P values of ≤0.05 were considered significant. The Reed and Muench method was used to calculate the lethal dose 50% endpoint (LD50) for pulmonary exposure to purified SEC [26].
RESULTS
CA-MRSA rabbit pulmonary model
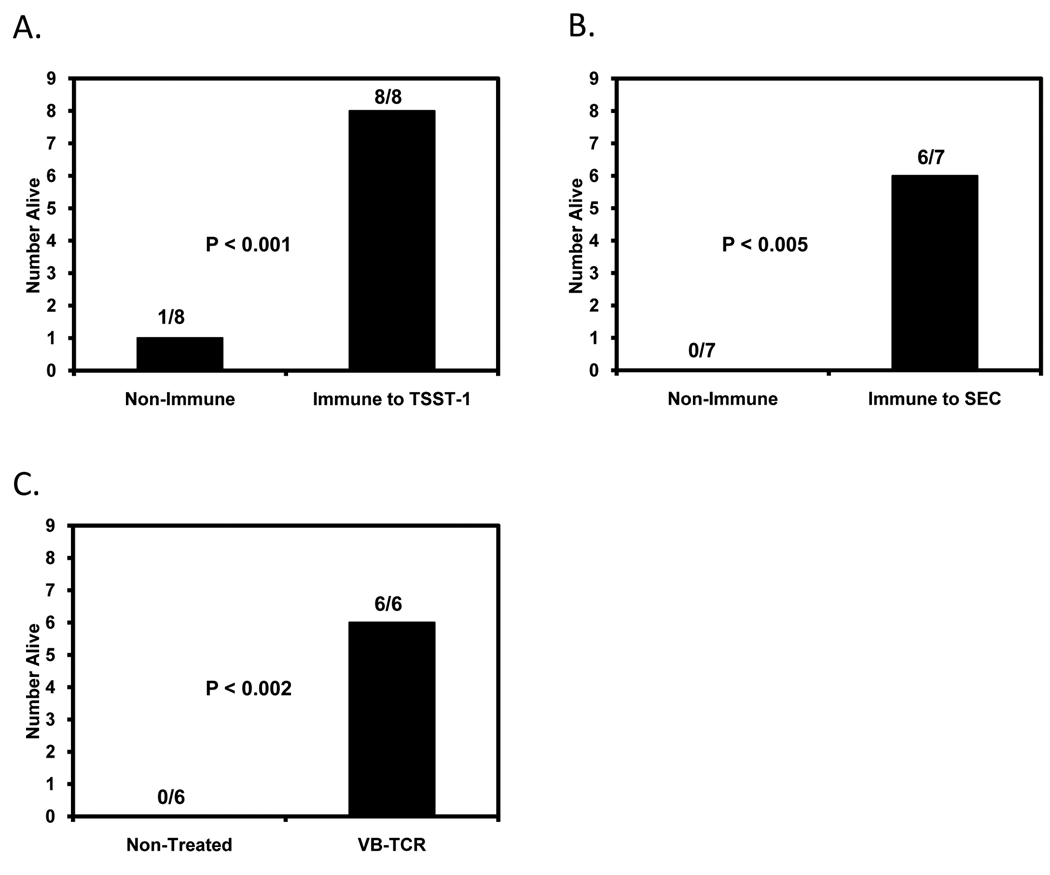
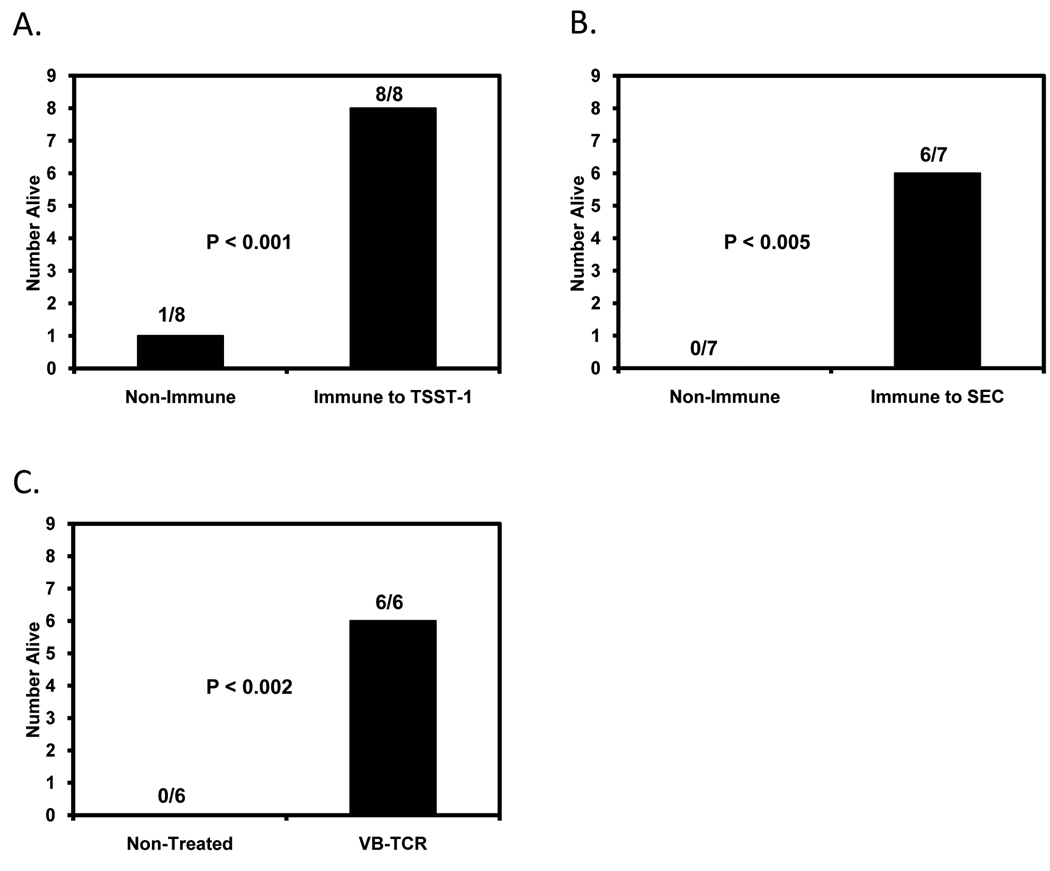
Rabbits were exposed intra-bronchial to CA-MRSA and monitored for signs of respiratory distress and lethal illness. To initiate pulmonary infections, bacteria were administered into rabbit bronchi. CA-MRSA strains tested were USA200 MNPA, MN1021 or MN128 (TSST-1+; these strains do not produce the cytotoxins α-toxin, β-toxin, γ-toxin, and PVL), and USA400 MW2 (SEC4+, α-toxin+, β-toxin−, γ-toxin+, and PVL+) [4] and USA400 c99-529 (SEB+, α-toxin+, β-toxin−, γ-toxin+, and PVL+) [4]. The animals were monitored for signs of illness for up to 7 days. Typically, rabbits exposed to CA-MRSA succumbed in 2–3 days post infection, and had high fevers on days 1 and 2 post infection. Rabbits exposed to both USA200 and USA400 CA-MRSA organisms developed illness associated with respiratory distress and lethal illness, and the animals succumbed (figure 1); only 1/8 animals exposed to the TSST-1+ USA200 organisms survived (figure 1A), and no animals survived when challenged intra-bronchial with SEC+ USA400 MW2 organisms (figure1B).
Figure 1.
Immunization against SAg or administration of soluble high affinity Vβ-TCR G5-8 protects rabbits from lethal CA-MRSA pulmonary illness. A, non-immunized and TSST-1-immunized rabbits alive after challenge with TSST-1+ USA200 strain; B, non-immunized and SEC-immunized rabbits alive after challenge with SEC+ USA400 strain; and C, PBS- and high affinity Vβ-TCR (G5-8)-treated rabbits alive after challenge with SEB+ USA400 strain.
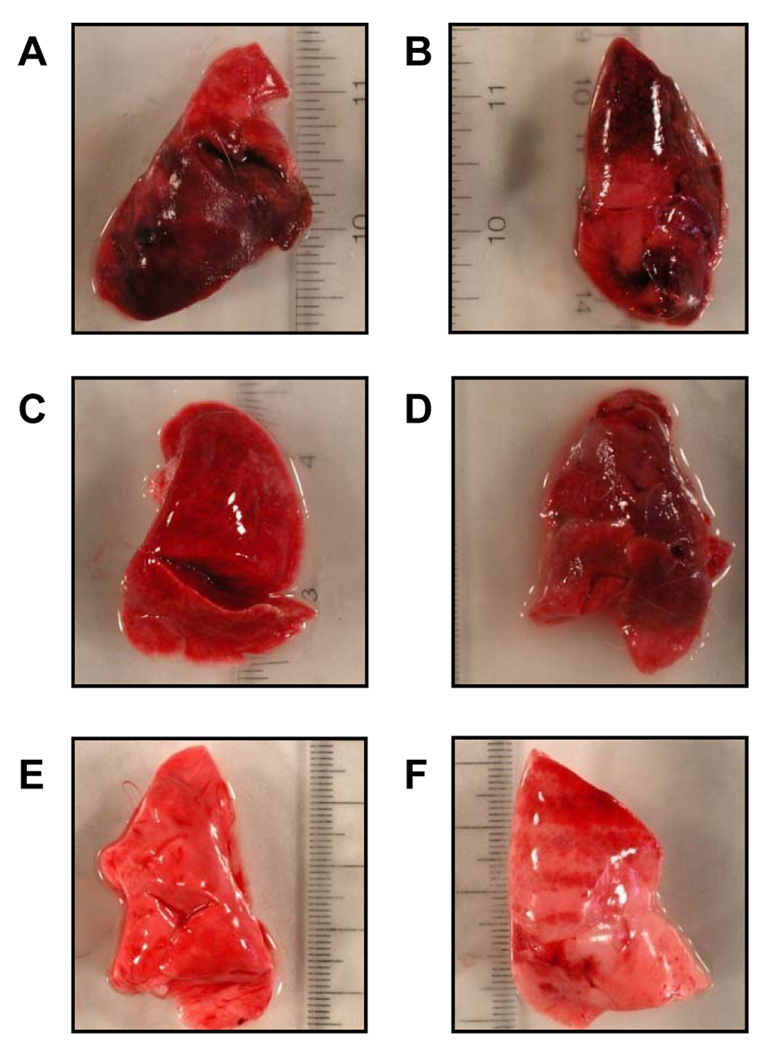
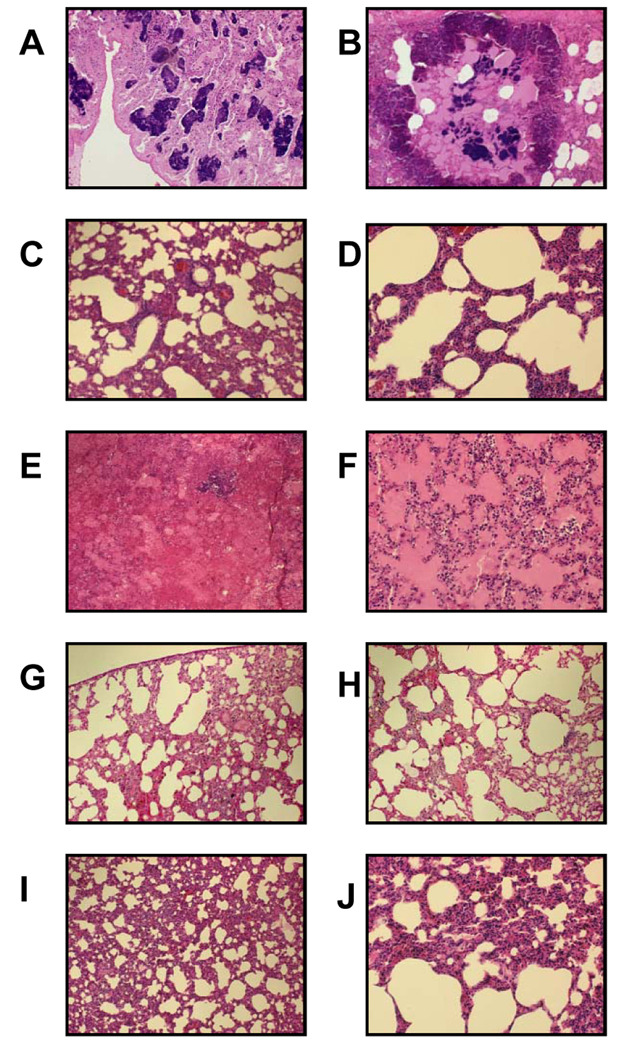
Excised lungs from rabbits exposed to CA-MRSA USA200 bacteria and USA400 MW2 were severely hemorrhagic (figures 2A–B and 3A–B), compared to rabbits challenged with PBS (figures 2E–F and 3I–J). Histology of representative lung sections confirmed the presence of hemorrhagic tissue (figures 4A–B and 5A–B), compared to rabbits challenged with PBS (figures 4E–F and 5I–J).
Figure 2.
Rabbit lungs after exposure to CA-MRSA USA200 (TSST-1+, α-toxin−, β-toxin−, γ-toxin−, and PVL−). A–B, Left and right lungs removed from a non-immunized rabbit that received CA-MRSA USA200; C–D, Left and right lungs from a rabbit immunized against purified TSST-1 prior to administration of CA-MRSA USA200; and E–F, Left and right lungs removed from a rabbit exposed only to PBS.
Figure 3.
Rabbit lungs after exposure to CA-MRSA USA400 MW2 (SEC+, α-toxin+, β-toxin−, γ-toxin+, and PVL+) or purified SEC. A–B, Left and right lungs from a non-immune rabbit that received MW2 CA-MRSA; C–D, Left and right lungs from a rabbit immunized against purified SEC prior to administration of MW2 CA-MRSA;. E–F, Left and right lungs from a non-immune rabbit after intra-bronchial administration of 200 µg purified SEC; G–H, Left and right lungs from a rabbit immunized against purified SEC prior to administration of 200 µg purified SEC; and I–J, Left and right lungs from a rabbit administered PBS.
Figure 4.
Histology of lung sections from rabbits challenged with CA-MRSA USA200 MNPA (TSST-1+, α-toxin−, β-toxin−, γ-toxin−, and PVL−). A–B, H&E staining of sections from a rabbit (lung image shown in figure 2A–B) that received MNPA CA-MRSA; C–D, H&E staining of sections from a rabbit (lung image shown in figure 2C–D) hyperimmunized against purified TSST-1 prior to administration of MNPA CA-MRSA; and E–F, H&E staining of sections from a rabbit (lung image shown in figure 2E–F) challenged with only PBS. Images in A, C, and E: 100× magnification; images in B, D, and F: 200×.
Figure 5.
Histology of lung sections from rabbits challenged with CA-MRSA MW2 (SEC+, α-toxin+, β-toxin−, γ-toxin+, and PVL+) or purified SEC. A–B, H&E staining of sections from a non-immune rabbit (lung images shown in figure 3A–B) that received CA-MRSA MW2; C–D, H&E staining of sections from a rabbit (lung images shown in figure 3C–D) immunized against purified SEC prior to administration of CA-MRSA MW2; E–F, H&E staining of sections from a rabbit (lung images shown in figure 3E–F) that received 200 µg purified SEC; G–H, H&E staining of sections from a rabbit (lung images shown in figure 3G–H) hyperimmunized against purified SEC prior to administration of 200 µg purified SEC; and I–J, H&E staining of sections from a rabbit (lung images shown in figure 3I–J) administered only PBS. Image in A: 40× magnification; Images in B, C, E, G, and I: 100×; images in D, F, H, and J: 200×.
We attempted to construct an allelic replacement sec (gene encoding SEC) knock-out to test the role of SEC in lethality in this rabbit model. Although we were successful in constructing knock-outs, making isogenic strains, the strains were not isophenotypic. Thus, the constructed knock-outs constructed lacked production of both SEC and α-toxin (only proteins tested), despite having similar growth kinetics. Therefore, in order to assess the role of TSST-1 and SEC in lethal illness in rabbits, groups of rabbits were hyperimmunized against highly purified TSST-1 or SEC (ELISA IgG titers >10,000) [15, 16] and then challenged intra-bronchially with corresponding CA-MRSA isolates that produce TSST-1 or SEC. Rabbits pre-immunized against TSST-1 or SEC did not develop respiratory distress or lethal illness when challenged (p<0.001 and p<0.005, respectively) (figure 1A–B), and other than fever did not develop overt clinical signs.
Excised lungs removed from non-immune animals challenged with CA-MRSA USA200 or USA400 MW2 showed hemorrhagic tissue (figure 2A–B and figure 3A–B). Lungs from TSST-1 or SEC hyperimmunized rabbits challenged with USA200 bacteria or USA400 MW2 did not have visible hemorrhagic lesions (figure 2C–D and figure 3C–D), though the lungs appeared somewhat congested, consistent with staphylococcal infections. Lungs from PBS-treated rabbits did not show hemorrhagic tissue and were not congested (figure 2E–F and figure 3I–J).
Histology showed hemorrhagic lung tissue in non-immune rabbits (figure 4A–B and figure 5A–B) challenged with CA-MRSA USA200 bacteria or MW2, and normal lung tissue in TSST-1 and SEC hyperimmunized rabbits challenged with USA200 and MW2 organisms (figure 4C–D and 5C–D) or PBS challenged rabbits (figure 4E–F and figure 5I–J).
Additional experiments investigated the ability of soluble high affinity Vβ-TCR G5-8, specific for neutralization of SEB lethality in rabbits, through inhibition of superantigenicity [25], to provide passive protection from CA-MRSA or purified SEB intra-bronchial challenge. Rabbits that received high affinity Vβ-TCR G5-8 iv at the same time as the intra-bronchial SEB+ CA-MRSA c99-529 did not develop respiratory distress and lethal TSS (figure 1C), whereas animals that did not receive G5-8 succumbed.
Pulmonary exposure to purified SEB or SEC
Rabbits were administered purified SEC (0 µg, 50 µg, 100 µg, or 200 µg) intra-bronchially in PBS. The LD50 of SEC was 75 µg by this route. Post mortem examination of lungs from SEC-treated rabbits revealed hemorrhagic tissue (figure 3E–F), compared to normal tissues from PBS-treated animals (figure 3I–J).
Rabbits were also hyperimmunized against purified SEC and then administered 200 µg SEC intra-bronchially in PBS. SEC hyperimmunized rabbits did not develop respiratory distress and lethal illness (data not shown) over the 7 day test period. In contrast, non-immunized rabbits exposed to 200 µg purified SEC showed respiratory distress and succumbed within 24 hr (data not shown). Excised lungs from non-immunized rabbits revealed the presence of hemorrhagic lesions (figure 3E–F). Lung samples from SEC hyperimmunized rabbits did not show hemorrhagic lesions (figure 3G–H); they resembled lungs from PBS-treated animals figure 3I–J).
Histology confirmed that excised lung sections from non-immunized rabbits administered 200 µg SEC contained hemorrhagic lesions (figure 5E–F). Tissue from SEC hyperimmunized rabbits administered 200 µg SEC (figure 5G–H) and rabbits that received PBS (figure 5I–J) showed normal lung tissue.
Administration of high-affinity G5-8 iv with intra-bronchial SEB also protected rabbits from respiratory distress and lethal TSS (figure 6).
Figure 6.
Soluble high affinity Vβ-TCR (G5-8) protects rabbits from respiratory distress and lethal illness due to intra-bronchial purified SEB (100 or 200 µg). PBS or high affinity Vβ-TCR (G5-8)-treated rabbits alive after intra-bronchial administration of 100 (3 rabbits) or 200 µg SEB (3 rabbits) Note: all rabbits that received Vβ-TCR G5-8 were administered 200 µg purified SEB.
DISCUSSION
We evaluated the role of staphylococcal SAgs in serious pulmonary CA-MRSA infections and intoxications. Through TSST-1 and SEC immunization studies and use of soluble high-affinity Vβ-TCR G5-8 to neutralize SEB, we showed that these three SAgs, are critical for development of serious pulmonary illness, caused both by CA-MRSA and highly purified SAgs. We used rabbits as models since these animals are more similar to humans in susceptibility to SAgs [6, 27–31]; rabbits are also highly susceptible to cytotoxins [32]. Prior studies of CA-MRSA pulmonary infections used mice as the animal model [13, 33, 34] and have generated conflicting results regarding the roles of staphylococcal exotoxins, one group suggesting that PVL is critical to necrotizing pneumonia [34], while other groups suggesting α-toxin and phenol-soluble modulins, but not PVL, are critical [13, 33]. However, none of these studies assessed the role of SAgs in disease since mice are at least 1011 more resistant to SAgs on a weight basis than humans [35, 36]. Indeed, the presence of SAgs increases the resistance of mice to infections [37]. In contrast, young adult rabbits are only 102–103 more resistant than humans to SAgs, and rabbits ≥ 8 months of age are equally susceptible as humans to SAgs.
Prior hyperimmunization against TSST-1 protected rabbits from the lethality associated with intra-bronchial challenge with CA-MRSA USA200. Interestingly, these CA-MRSA strains do not produce α, β, γ, or PVL cytotoxins, yet cause fatal pulmonary illness. The studies suggest that cytotoxins are not required for the fatal outcomes. Our hyperimmunization of rabbits against PVL followed by challenge with USA400 MW2 (SEC+, α-toxin+, β-toxin−, γ-toxin+, PVL+) resulted in lethal pulmonary illnesses, suggesting PVL is not critical for lethality (unpublished data). It appears that these redundantly expressed cytotoxins, including α-toxin, β-toxin, γ-toxin, and PVL, when produced, contribute significantly to serious lung diseases, either through direct toxicity or induction of inflammation, but are not required for lethality in rabbits.
Presently, MRSA and their MSSA counterparts are highly significant causes of infectious disease deaths in the United States, including fatal pulmonary infections [3]. Our data suggest that SAgs are important contributors to those fatal infections. The initial report of CA-MRSA USA400 strains associated with deaths of four young children in the Upper Midwest due to necrotizing pneumonia demonstrated that 2 isolates produced SEB and the other 2 produced SEC, including MW2 and c99-529 [4]. Non-immunized rabbits used in our studies that received intra-bronchial MW2 and c99-529 developed illness that resembled necrotizing pneumonia, including by histologic examination of lung tissue. A subsequent larger study of CA-MRSA USA400 strains indicated the vast majority produce either SEB or SEC [38]. It is also our experience that some regions of the United States are experiencing emergences of CA-MRSA USA200 S. aureus that produce TSST-1. As shown in the present studies, TSST-1 is critical in fatal pulmonary illness associated with these strains.
Of great significance, our studies show that administration of soluble high-affinity Vβ-TCR G5-8 or prior hyperimmunization to raise neutralizing antibodies against SAgs dramatically increases rabbit survival. There are anecdotal studies of patients with severe S. aureus pulmonary infections being treated successfully with intravenous immunoglobulin (IVIG) [39, 40]. IVIG is highly capable of neutralizing SAgs [41]. Additionally, a study has shown that IVIG reduces the case:fatality rate of streptococcal TSS in humans [42]. However, IVIG is costly, requires large amounts of immunoglobulin to be administered, and has side effects. Our prior studies have shown that the soluble high-affinity Vβ-TCR G5-8 requires 2,200 times less than IVIG for comparable ability to neutralize SEB in rabbits. G5-8 is easy to prepare from bacterial clones, and requires only equimolar amounts to neutralize SEB, such small amounts for SEB neutralization that it may be possible to nebulize into the lungs, as well as administered iv.
Acknowledgments
Financial Support: US Public Health Service (research grants AI057153, AI074283, and AI064611) from the National Institute of Allergy and Infectious Diseases. P.M.S. and D.M.K. acknowledge membership in and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium.
Footnotes
Potential conflicts of interest: None of the authors have conflicts of interest to report.
This work was presented in part at the American Society for Microbiology 108th General Meeting; June, 2008 Boston, MA.
REFERENCES
- 1.Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 2.Daum RS, Ito T, Hiramatsu K, et al. A novel methicillin-resistance cassette in community-acquired methicillin-resistant Staphylococcus aureus isolates of diverse genetic backgrounds. J Infect Dis. 2002;186:1344–1347. doi: 10.1086/344326. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. Jama. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.From the Centers for Disease Control and Prevention. Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus--Minnesota and North Dakota, 1997–1999. Jama. 1999;282:1123–1125. [PubMed] [Google Scholar]
- 5.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- 6.McCormick JK, Yarwood JM, Schlievert PM. Toxic shock syndrome and bacterial superantigens: an update. Annu Rev Microbiol. 2001;55:77–104. doi: 10.1146/annurev.micro.55.1.77. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Llera A, Malchiodi EL, Mariuzza RA. The structural basis of T cell activation by superantigens. Annu Rev Immunol. 1999;17:435–466. doi: 10.1146/annurev.immunol.17.1.435. [DOI] [PubMed] [Google Scholar]
- 8.Wang L, Zhao Y, Li Z, et al. Crystal structure of a complete ternary complex of TCR, superantigen and peptide-MHC. Nat Struct Mol Biol. 2007;14:169–171. doi: 10.1038/nsmb1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marrack P, Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990;248:1066. [PubMed] [Google Scholar]
- 10.Schlievert PM. Staphylococcal enterotoxin B and toxic-shock syndrome toxin-1 are significantly associated with non-menstrual TSS. Lancet. 1986;1:1149–1150. doi: 10.1016/s0140-6736(86)91859-3. [DOI] [PubMed] [Google Scholar]
- 11.Schlievert PM, Tripp TJ, Peterson ML. Reemergence of staphylococcal toxic shock syndrome in Minneapolis-St. Paul, Minnesota, during the 2000–2003 surveillance period. J Clin Microbiol. 2004;42:2875–2876. doi: 10.1128/JCM.42.6.2875-2876.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlievert PM, Strandberg KL, Lin YC, Peterson ML, Leung DY. Secreted virulence factor comparison between methicillin-resistant and methicillin-sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. J Allergy Clin Immunol. 125:39–49. doi: 10.1016/j.jaci.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voyich JM, Otto M, Mathema B, et al. Is Panton-Valentine leukocidin the major virulence determinant in community-associated methicillin-resistant Staphylococcus aureus disease? J Infect Dis. 2006;194:1761–1770. doi: 10.1086/509506. [DOI] [PubMed] [Google Scholar]
- 14.O'Reilly M, Kreiswirth B, Foster TJ. Cryptic alpha-toxin gene in toxic shock syndrome and septicaemia strains of Staphylococcus aureus. Mol Microbiol. 1990;4:1947–1955. doi: 10.1111/j.1365-2958.1990.tb02044.x. [DOI] [PubMed] [Google Scholar]
- 15.Blomster-Hautamaa DA, Schlievert PM. Preparation of toxic shock syndrome toxin-1. Methods Enzymol. 1988;165:37–43. doi: 10.1016/s0076-6879(88)65009-9. [DOI] [PubMed] [Google Scholar]
- 16.Blomster-Hautamaa DA, Kreiswirth BN, Novick RP, Schlievert PM. Resolution of highly purified toxic-shock syndrome toxin 1 into two distinct proteins by isoelectric focusing. Biochemistry. 1986;25:54–59. doi: 10.1021/bi00349a009. [DOI] [PubMed] [Google Scholar]
- 17.Schlievert PM, Shands KN, Dan BB, Schmid GP, Nishimura RD. Identification and characterization of an exotoxin from Staphylococcus aureus associated with toxic-shock syndrome. J Infect Dis. 1981;143:509–516. doi: 10.1093/infdis/143.4.509. [DOI] [PubMed] [Google Scholar]
- 18.Bohach GA, Fast DJ, Nelson RD, Schlievert PM. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–272. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Schlievert PM, Case LC, Nemeth KA, et al. Alpha and beta chains of hemoglobin inhibit production of Staphylococcus aureus exotoxins. Biochemistry. 2007;46:14349–14358. doi: 10.1021/bi701202w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlievert PM, Osterholm MT, Kelly JA, Nishimura RD. Toxin and enzyme characterization of Staphylococcus aureus isolates from patients with and without toxic shock syndrome. Ann Intern Med. 1982;96:937–940. doi: 10.7326/0003-4819-96-6-937. [DOI] [PubMed] [Google Scholar]
- 22.Schlievert PM, Bettin KM, Watson DW. Effect of antipyretics on group A streptococcal pyrogenic exotoxin fever production and ability to enhance lethal endotoxin shock. Proc Soc Exp Biol Med. 1978;157:472–475. doi: 10.3181/00379727-157-40079. [DOI] [PubMed] [Google Scholar]
- 23.Schlievert PM, Gahr PJ, Assimacopoulos AP, et al. Aggregation and binding substances enhance pathogenicity in rabbit models of Enterococcus faecalis endocarditis. Infect Immun. 1998;66:218–223. doi: 10.1128/iai.66.1.218-223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hudson LaH. F.C. Practical Immunology. Blackwell Scientific Publications; 1980. [Google Scholar]
- 25.Buonpane RA, Churchill HR, Moza B, et al. Neutralization of staphylococcal enterotoxin B by soluble, high-affinity receptor antagonists. Nat Med. 2007;13:725–729. doi: 10.1038/nm1584. [DOI] [PubMed] [Google Scholar]
- 26.Reed LJ, Muench H. A simple method of estimating 50 per cent endpoints. American Journal of Hygiene. 1938;27 [Google Scholar]
- 27.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sriskandan S, Moyes D, Buttery LK, et al. Streptococcal pyrogenic exotoxin A release, distribution, and role in a murine model of fasciitis and multiorgan failure due to Streptococcus pyogenes. J Infect Dis. 1996;173:1399–1407. doi: 10.1093/infdis/173.6.1399. [DOI] [PubMed] [Google Scholar]
- 29.Giantonio BJ, Alpaugh RK, Schultz J, et al. Superantigen-based immunotherapy: a phase I trial of PNU-214565, a monoclonal antibody-staphylococcal enterotoxin A recombinant fusion protein, in advanced pancreatic and colorectal cancer. J Clin Oncol. 1997;15:1994–2007. doi: 10.1200/JCO.1997.15.5.1994. [DOI] [PubMed] [Google Scholar]
- 30.Dinges MM, Gregerson DS, Tripp TJ, McCormick JK, Schlievert PM. Effects of total body irradiation and cyclosporin a on the lethality of toxic shock syndrome toxin-1 in a rabbit model of toxic shock syndrome. J Infect Dis. 2003;188:1142–1145. doi: 10.1086/378514. [DOI] [PubMed] [Google Scholar]
- 31.Dinges MM, Schlievert PM. Comparative analysis of lipopolysaccharide-induced tumor necrosis factor alpha activity in serum and lethality in mice and rabbits pretreated with the staphylococcal superantigen toxic shock syndrome toxin 1. Infect Immun. 2001;69:7169–7172. doi: 10.1128/IAI.69.11.7169-7172.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat Med. 2007;13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 34.Labandeira-Rey M, Couzon F, Boisset S, et al. Staphylococcus aureus Panton-Valentine leukocidin causes necrotizing pneumonia. Science. 2007;315:1130–1133. doi: 10.1126/science.1137165. [DOI] [PubMed] [Google Scholar]
- 35.Dinges MM, Jessurun J, Schlievert PM. Comparisons of mouse and rabbit models of toxic shock syndrome. International Congress and Symposium Series. 1998;229:167–168. [Google Scholar]
- 36.Dinges MM, Schlievert PM. Comparative analysis of lipopolysaccharide-induced tumor necrosis factor alpha activity in serum and lethality in mice and rabbits pretreated with the staphylococcal superantigen toxic shock syndrome toxin 1. Infect Immun. 2001;69:7169–7172. doi: 10.1128/IAI.69.11.7169-7172.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sriskandan S, Unnikrishnan M, Krausz T, Cohen J. Molecular analysis of the role of streptococcal pyrogenic exotoxin A (SPEA) in invasive soft-tissue infection resulting from Streptococcus pyogenes. Mol Microbiol. 1999;33:778–790. doi: 10.1046/j.1365-2958.1999.01525.x. [DOI] [PubMed] [Google Scholar]
- 38.Fey PD, Said-Salim B, Rupp ME, et al. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:196–203. doi: 10.1128/AAC.47.1.196-203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlievert PM. Use of intravenous immunoglobulin in the treatment of staphylococcal and streptococcal toxic shock syndromes and related illnesses. J Allergy Clin Immunol. 2001;108:S107–S110. doi: 10.1067/mai.2001.117820. [DOI] [PubMed] [Google Scholar]
- 40.Barry W, Hudgins L, Donta ST, Pesanti EL. Intravenous immunoglobulin therapy for toxic shock syndrome. JAMA. 1992;267:3315–3316. [PubMed] [Google Scholar]
- 41.Holm SE, Kohler W, Kaplan EL, et al. Streptococcal toxic shock syndrome (STSS). An update: a roundtable presentation. Adv Exp Med Biol. 1997;418:193–199. doi: 10.1007/978-1-4899-1825-3_47. [DOI] [PubMed] [Google Scholar]
- 42.Kaul R, McGeer A, Norrby-Teglund A, et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome--a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis. 1999;28:800–807. doi: 10.1086/515199. [DOI] [PubMed] [Google Scholar]