Abstract
Drugs that potentiate transmission at gamma-aminobutyric acidA (GABAA) receptors are widely used to enhance sleep and to cause general anesthesia. The mechanisms underlying these effects are unknown. This study tested the hypothesis that GABAA receptors in the pontine reticular nucleus, oral part (PnO) of mouse modulate five phenotypes of arousal: sleep and wakefulness, cortical electroencephalogram (EEG) activity, acetylcholine (ACh) release in the PnO, breathing, and recovery time from general anesthesia. Microinjections into the PnO of saline (vehicle control), the GABAA receptor agonist muscimol, muscimol with the GABAA receptor antagonist bicuculline, and bicuculline alone were performed in male C57BL/6J mice (n = 33) implanted with EEG recording electrodes. Muscimol caused a significant increase in wakefulness and decrease in rapid eye movement (REM) sleep and non-REM (NREM) sleep. These effects were reversed by co-administration of bicuculline. Bicuculline administered alone caused a significant decrease in wakefulness and increase in NREM sleep and REM sleep. Muscimol significantly increased EEG power in the delta range (0.5–4 Hz) during wakefulness and in the theta range (4–9 Hz) during REM sleep. Dialysis delivery of bicuculline to the PnO of male mice (n = 18) anesthetized with isoflurane significantly increased ACh release in the PnO, decreased breathing rate, and increased anesthesia recovery time. All drug effects were concentration-dependent. The effects on phenotypes of arousal support the conclusion that GABAA receptors in the PnO promote wakefulness, and suggest that increasing GABAergic transmission in the PnO may be one mechanism underlying the phenomenon of paradoxical behavioral activation by some benzodiazepines.
Keywords: REM sleep, EEG delta power, acetylcholine release, wakefulness stimulus for breathing, recovery of righting response
Introduction
Drugs that enhance actions of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) cause sleep (Winsky-Sommerer, 2009), sedation, or general anesthesia (Franks, 2008). Preclinical studies using intracranial drug administration demonstrate that GABAergic drugs promote either sleep or wakefulness, depending upon site of drug administration within the brain (Vanini et al., 2010). The oral part of the pontine reticular formation (PnO) is a component of the ascending reticular activating system and contributes to the generation of cortical electroencephalogram (EEG) activation and rapid eye movement (REM) sleep (Lydic and Baghdoyan, 2005; Steriade and McCarley, 2005). Pharmacologically enhancing GABAergic transmission in the PnO of cat and rat increases wakefulness and decreases sleep (Camacho-Arroyo et al., 1991; Xi et al., 1999; Sanford et al., 2003; Marks et al., 2008; Watson et al., 2008), whereas blocking GABAA receptors in the PnO decreases time spent in wakefulness and increases time spent in REM sleep (Xi et al., 1999; Sanford et al., 2003; Marks et al., 2008). Direct administration of the arousal-promoting peptide hypocretin-1 to the PnO increases wakefulness and increases the concentration of GABA in the PnO (Watson et al., 2008). This hypocretin-1-induced increase in wakefulness is blocked by co-administration of the GABAA receptor antagonist bicuculline (Brevig et al., 2010), demonstrating that in the PnO hypocretin-1 increases wakefulness via a GABAergic mechanism. Increasing GABAergic transmission in the PnO increases the time needed for induction of general anesthesia, and the concentration of endogenous GABA in the PnO is greater during wakefulness than during anesthesia (Vanini et al., 2008) or sleep (Vanini et al., 2009). GABAergic transmission also modulates the release of other neurotransmitters in the PnO that regulate sleep and wakefulness. For example, acetylcholine (ACh) in the PnO promotes REM sleep (Lydic and Baghdoyan, 2008), and direct administration of bicuculline to PnO of cat increases ACh release and triggers the onset of REM sleep (Vazquez and Baghdoyan, 2004). Taken together, these pharmacological data support the interpretation that GABAergic transmission within the PnO promotes wakefulness, inhibits ACh release, and inhibits REM sleep.
No previous studies have determined whether GABAergic transmission in the PnO of mouse modulates states of behavioral arousal or traits that characterize these states. The present study used in vivo microinjection and microdialysis to test the hypothesis that GABAA receptors in the PnO of C57BL/6J (B6) mouse modulate five phenotypes of arousal: sleep and wakefulness, cortical EEG activity, ACh release in the PnO, rate of breathing, and recovery time from general anesthesia. All responses were predicted to be concentration-dependent.
Materials and Methods
Animals
Experiments were approved by the University of Michigan Committee on Use and Care of Animals and conducted in accordance with the U.S. Department of Agriculture Animal Welfare Act and the Public Health Service Policy on Humane Care and Use of Laboratory Animals (National Institutes of Health Publication 80-23, National Academy of Sciences Press, Washington DC, 1996). Adult, male, B6 mice (25–30 g, n = 51) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and housed in a humidity-controlled facility under constant light. Mice had ad libitum access to food and water, and were kept for a minimum of one week before being used for experiments.
Surgical procedures for implantation of microinjection guide tubes and recording electrodes
Mice were anesthetized with 2–3% isoflurane (Abbott Laboratories, North Chicago, IL, USA) delivered in 100% oxygen at a flow rate of 1 l/min. Once unconscious, mice were placed in a stereotaxic frame (Model 962, David Kopf, Tujunga, CA, USA) fitted with a mouse adaptor (Model 921) and a mouse anesthesia mask (Model 907). Isoflurane concentration was reduced to 1.5% and flow rate was decreased to 0.5 l/min. The concentration of isoflurane delivered to the anesthesia mask was measured continuously by spectrophotometry (Cardiocap/5, Datex-Ohmeda, Louisville, CO, USA). Core body temperature was maintained at 36–37°C using a heating pad filled with continuously circulating hot water (TP400 T/Pump Heat Therapy System, Gaymar, Orchard Park, NY, USA). One 26 gauge stainless steel guide tube (Cannula Guide # C315GS-4-SPC, Plastics One, Roanoke, VA, USA) occluded by a stylet (Dummy Cannula # C315DCS-4-SPC, Plastics One) was implanted 3 mm dorsal to either the right or left side of the PnO at stereotaxic coordinates 4.24 mm caudal to bregma, 0.8 mm lateral to the midline, and 1.5 mm ventral to bregma (Paxinos and Franklin, 2001). Three electrodes (H-Formvar Wire, 0.005″ diameter, California Fine Wire Company, Grover City, CA, USA) for recording the cortical EEG were placed directly under the skull approximately 0.5 mm caudal and 1.5 mm lateral to bregma, 2.0 mm caudal and 1.5 mm lateral to bregma, and 1.0 mm rostral and 1.5 mm lateral to bregma. One pair of electrodes for recording the electromyogram (EMG) (Biomed Bare Braided Wire, 0.12″ overall diameter, Cooner Wire, Chatsworth, CA, USA) was inserted into the neck muscle. Recording electrodes terminated in gold pins (# E363-0, Plastics One) that were gathered together and placed into a plastic connector (6-pin Electrode Pedestal # MS363, Plastics One). Two stainless steel anchor screws (# 39052, Plastics One) were inserted into the skull and the plastic connector, screws, and guide tube all were secured to the skull with dental acrylic (Jet Acrylic Self Curing Resin and Liquid, Lang Dental Manufacturing Co., Inc., Wheeling, IL, USA). Isoflurane delivery was discontinued and mice were kept warm and under continuous observation until ambulatory. Mice were housed individually and allowed one week to recover from surgery.
Intracranial microinjection procedure
Mice were conditioned to being housed in a Raturn recording chamber (Bioanalytical Systems Inc., West Lafayette, IN, USA) where they had ad libitum access to food and water. The conditioning period included handling the mice, removing the stylet from the guide tube, reinserting the stylet to simulate a microinjection, and tethering the mice to a recording cable (Connector Cable System # 363-441/6-150cm-6TC, Plastics One). After one week of conditioning, mice entered the microinjection protocol. Before each microinjection and recording session, mice spent 18 h in the recording environment (Tang et al., 2005).
Solutions of the GABAA receptor agonist muscimol (Sigma Chemical Co., St. Louis, MO, USA) and the GABAA receptor antagonist bicuculline methiodide (Sigma Chemical Co.) were prepared immediately prior to use. Unilateral microinjections (50 nl) were made using a 1-μl Hamilton syringe (Fisher Scientific, Pittsburgh, PA, USA) mounted in a manual microdrive and connected to a 33-gauge microinjector (Internal Cannula # C315IS-4-SPC, Plastics One) via PE-20 tubing (Fisher Scientific). Microinjections occurred between 8:00 and 10:00 a.m. and were followed immediately by 4 h of continuous recording. Mice used in the present study were housed in constant light and therefore were not entrained to a light-dark cycle. Combining a design that used a free-running sleep-wake cycle with a consistent time for drug administration made it possible to avoid a systematic error (i.e., a confound) due to the circadian distribution of sleep and wakefulness. This follows from the fact that the endogenously generated free-running rhythm of sleep and wakefulness was randomly distributed relative to the constant microinjection time.
Each mouse received one microinjection of saline (0.9%, vehicle control) and either four concentrations of muscimol (0.5, 5, 50, and 500 pmol/50 nl, corresponding to 0.057, 0.571, 5.71, and 57.1 ng/50 nl) or three concentrations of bicuculline (0.5, 5, and 50 pmol/50 nl, corresponding to 0.25, 2.5, and 25 ng/50 nl) for a total of five or four microinjections, respectively, per mouse. A second group of mice each received one microinjection of saline, muscimol (50 pmol), and muscimol (50 pmol) co-administered with bicuculline (5 pmol), for a total of three microinjections per mouse. Microinjection duration was one min. The order of the microinjections was randomized and microinjections in the same mouse were separated by one week.
Recording and objectively identifying states of sleep and wakefulness
EEG and EMG signals were amplified, filtered, digitized at 128 Hz, and scored as previously described (Watson et al., 2007) using Icelus Data Acquisition and Analysis software (Opp, 1998). States of sleep and wakefulness for each 4-h recording were scored in 10-s epochs according to the following criteria. Wakefulness was characterized by a mixed frequency EEG (0.5–25 Hz) and a relatively high amplitude EMG containing periods of movement artifact. If an epoch of wakefulness contained movement artifact in the EEG, that epoch was coded differently from artifact-free wakefulness and was excluded from the epochs used for power spectral analysis. Non-REM (NREM) sleep was scored according to the presence of slow waves in the EEG (0.5–4 Hz), lower EMG amplitude than during wakefulness, and lack of movement artifact. REM sleep was identified by a dominant EEG theta rhythm (6–8 Hz) and EMG hypotonia. Sixty five percent of all sleep records were scored by two investigators, one of which was blinded to the treatment condition. Inter-rater reliability between the two scorers was 93%. To construct plots of average EEG power (VRMS), five 1-min intervals of recording time were analyzed for each of the three behavioral states from selected recordings. Fast Fourier Transform analysis identified dominant EEG frequencies in 2-s bins. Frequencies ranging from 0.5 to 25 Hz were analyzed in increments of 0.5 Hz, and five consecutive 2-s bins were averaged for each 10-s epoch.
In vivo microdialysis experiments to determine the effects of bicuculline on ACh release, breathing rate, and time to recovery from general anesthesia
Microdialysis experiments were performed using mice that were anesthetized with isoflurane (one mouse per experiment) and procedures that have been described in detail (Van Dort et al., 2009). Briefly, mice were placed in a Kopf stereotaxic frame and a CMA/7 microdialysis probe (CMA Microdialysis, Chelmsford, MA) was aimed for either the left or right side of the PnO at coordinates 4.7 mm posterior, 0.8 mm lateral, and 5.4 mm ventral to bregma (Paxinos and Franklin, 2001). The probe was perfused continuously (2 μl/min) with Ringer’s solution (147 mM NaCl, 2.4 mM CaCl2, 4 mM KCl, 10 μM neostigmine) followed by Ringer’s containing bicuculline methiodide (0, 0.1, 0.3, 1, 3, or 10 mM). Dialysis samples (25 μl) were collected sequentially every 12.5 min. Core body temperature and breathing rate were recorded each time a dialysis sample was collected. The delivered concentration of isoflurane and body temperature were maintained at 1.3% and 36–37°C, respectively, as described above.
For each experiment, five dialysis samples were obtained during 62.5 min of dialysis with Ringer’s and five samples were acquired during 62.5 min of dialysis administration of bicuculline. For each mouse, ACh release during dialysis with Ringer’s (first five samples) was averaged and became the baseline measure for that mouse. ACh release during dialysis with bicuculline (second five samples) was averaged and the percent change from baseline ACh release was calculated. Rate of breathing was also measured every time a dialysis sample was collected. Only one concentration of bicuculline was tested per experiment, and each concentration of bicuculline was tested in three mice. Control experiments were performed in three mice during which the first 62.5 min period of dialysis with Ringer’s was followed by a second 62.5 min period of dialysis with Ringer’s containing no bicuculline (0 mM). For each of these experiments, ACh release and breathing were calculated for the first and second dialysis periods. Using this design, it was possible to determine whether keeping the mice anesthetized for 125 min without administration of bicuculline significantly altered the dependent measures of ACh release or rate of breathing. These data also provided baseline measures for quantifying effects of bicuculline on anesthesia recovery time. Schematics showing the design used for these experiments can be found in Fig. 1A of Van Dort et al., (2009) and Fig. 1A of Hambrecht-Wiedbusch et al., (2010).
After collecting the last sample, the dialysis probe was removed from the brain, the scalp incision was closed, isoflurane delivery was discontinued, and mice were placed on their backs under a heating lamp. The time (min) from cessation of isoflurane delivery until the mice righted themselves was recorded. Recovery of righting response is a measure of time to wakefulness following general anesthesia (Van Dort et al., 2009). Total anesthesia time (min) was defined as the time from onset of induction to cessation of isoflurane delivery and was recorded for each experiment.
Quantification of ACh
Each 25-μl sample obtained by dialysis was injected into a high performance liquid chromatography system (Bioanalytical Systems) immediately after collection. ACh was separated and quantified as previously described (Van Dort et al., 2009). Chromatograms were digitized using ChromGraph software (Bioanalytical Systems) and the amount of ACh in each dialysis sample was calculated using a standard curve based on seven known amounts of ACh ranging from 0.05 to 1.0 pmol.
Recovery of ACh by all dialysis probes was determined in vitro before and after each experiment by placing the probe in a known concentration of ACh and collecting five dialysis samples. There was no significant difference in pre- and post-experimental probe recoveries. This information is important for two reasons. First, experiments that satisfy this criterion ensure that changes in ACh measured in vivo can be ascribed to the effects of bicuculline and did not result from intra-experimental changes in the dialysis membrane. Second, the percent probe recovery can be used to estimate the amount of drug delivered to the brain (Watson et al., 2006). The average ACh recovery by the dialysis membranes used for this study was 5.5%, indicating that the concentrations of bicuculline delivered to the PnO ranged from approximately 5.5 to 550 μM.
Histological analysis of microinjection and microdialysis sites
Following completion of the last experiment, mice were deeply anesthetized and decapitated. Brains were rapidly removed, frozen, and sectioned coronally at 40 μm in thickness. Slide mounted tissue sections were dried, fixed in paraformaldehyde vapor (80°C), and stained with cresyl violet. Tissue sections were digitized and compared with a mouse brain atlas (Paxinos and Franklin, 2001) to assign stereotaxic coordinates to each microinjection or microdialysis site. Only data obtained from sites localized to the PnO were included in the statistical analyses.
Quantitative analysis of drug effects
Data were analyzed using descriptive and inferential statistics. Dependent measures of sleep and wakefulness included the percent of total recording time spent in wakefulness, NREM sleep, and REM sleep, the average duration and number of episodes of each state, the number of transitions between states, and the latency to onset of NREM sleep and REM sleep. Sleep latency was defined as the time in min from the end of drug delivery to the beginning of the first NREM sleep or REM sleep episode. Drug effects on dependent measures of sleep and wakefulness were analyzed by repeated measures one-way analysis of variance (ANOVA) or ANOVA using a linear mixed model adjusted for incomplete block design. Each ANOVA was followed by a Dunnett’s multiple comparisons test. ACh release during dialysis administration of bicuculline is reported as the percent of ACh measured during dialysis with Ringer’s (control). The effect of bicuculline on ACh release in the PnO was analyzed using ANOVA and a post hoc Dunnett’s test. Drug effects on EEG power were assessed using repeated measures two-way ANOVA and post hoc comparison of treatment at each frequency. The effects of bicuculline on recovery of righting response, core body temperature, and rate of breathing were analyzed by ANOVA and Dunnett’s test. Statistical programs used included GBStat v.6.5.6 for Macintosh, GraphPad Prism 5.0a for Windows, and SAS 9.1.2 for Windows. A probability (p) value of ≤ 0.05 was considered significant. Data are reported as mean ± standard error of the mean (SEM).
Results
Muscimol microinjected into the PnO increased EEG power and caused a concentration-dependent increase in wakefulness and decrease in sleep
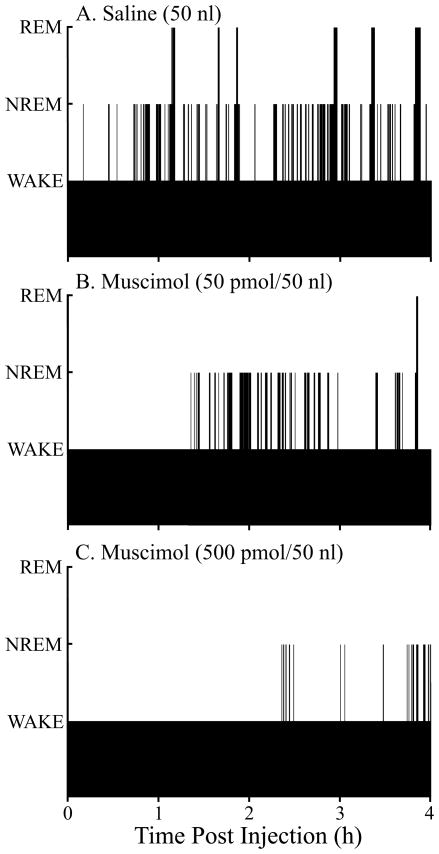
The effects of muscimol on sleep and wakefulness are summarized by Figures 1, 2, and 3. Figure 1 plots the temporal distribution of sleep and wakefulness from the same mouse following microinjection of saline and muscimol. Figure 1 is representative of the group data and illustrates that muscimol increased the time spent in wakefulness, decreased the time spent in NREM sleep, and decreased (Fig. 1B) or abolished (Fig. 1C) REM sleep compared to control (Fig. 1A).
Figure 1.
Microinjection of muscimol into the pontine reticular nucleus, oral part (PnO) alters the distribution of sleep and wakefulness. Black bars plot the time course of sleep and wakefulness recorded from the same mouse for 4 hours after microinjection of saline (vehicle control) and two concentrations of muscimol. The height of the bars corresponds to arousal state, with the lowest bars indicating wakefulness, intermediate bars representing NREM sleep, and highest bars showing the occurrence of REM sleep. Latency to onset of the first episode of NREM sleep and REM sleep was measured from Time 0, which marks the end of the 1-min period during which saline or muscimol was delivered to the PnO.
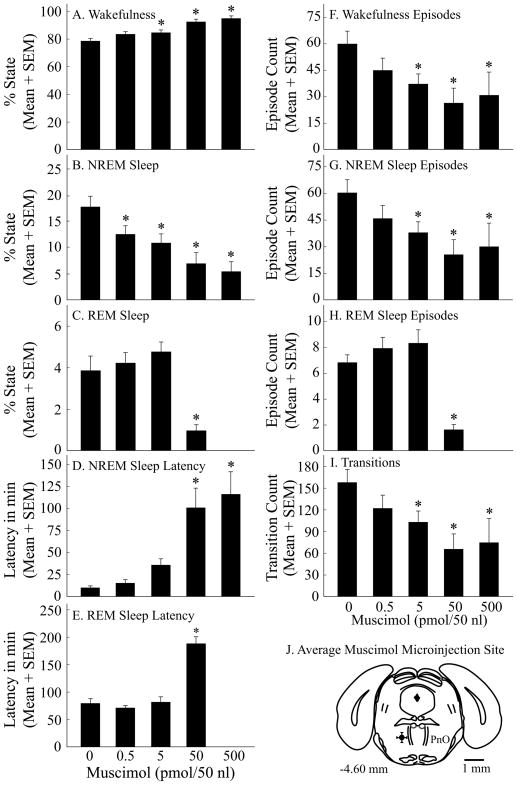
Figure 2.
Muscimol caused a concentration-dependent increase in wakefulness and decrease in sleep. A–I, asterisks indicate a significant (p < 0.05) difference from control (0 pmol muscimol). Data summarize the results from four hours of recordings. J, a coronal diagram modified from a mouse brain atlas (Paxinos and Franklin, 2001) illustrates the mean ± SEM location of the 10 microinjection sites (black dot and bars in left PnO).
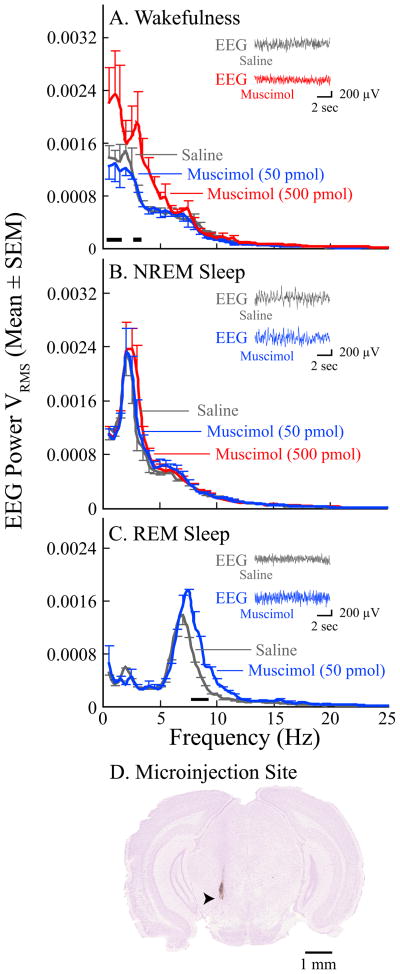
Figure 3.
Microinjection of muscimol into the PnO increased EEG power during wakefulness and REM sleep. Data are from four mice. Black bars directly above the abscissa in parts A and C indicate the frequencies at which muscimol caused a significant increase in EEG power relative to saline. A, during wakefulness muscimol (500 pmol) significantly increased cortical EEG power between 0.5–1.5 Hz and at 3 Hz. B, muscimol did not alter EEG power in NREM sleep. C, during REM sleep muscimol (50 pmol) caused a significant frequency-by-treatment interaction. Post hoc analyses comparing the means at each frequency revealed that muscimol increased EEG power between 7.5–9.5 Hz. The EEG power functions are based on 57 min of recording during wakefulness (A), 54 min of recording during NREM sleep (B), and 21 min of recording during REM sleep (C). The insets in parts A–C show representative pairs of 10-s EEG recordings obtained from the same mouse following microinjection of saline and muscimol. D, this digitized image of a cresyl violet-stained coronal section shows a representative microinjection site (arrowhead) in the PnO, located approximately 4.2 mm posterior to bregma.
Figure 2 summarizes the group data for the quantitative effects of muscimol on sleep and wakefulness in 10 mice. Because the highest concentration of muscimol (500 pmol/50 nl) eliminated REM sleep, each ANOVA for dependent measures of REM sleep was based on four rather than five concentrations of muscimol. Muscimol increased the percent of time spent in wakefulness (Fig. 2A; F = 27.4; df = 4, 36; p < 0.0001) and decreased the time spent in both NREM sleep (Fig. 2B; F = 18.9; df = 4, 36; p < 0.0001) and REM sleep (Fig. 2C; F = 16.2; df = 3, 27; p < 0.0001). Microinjection of muscimol increased the latency to onset of NREM sleep (Fig. 2D; F = 15.5; df = 4, 36; p < 0.0001) and REM sleep (Fig. 2E; F = 39.0; df = 3, 27; p < 0.0001), and decreased the number of episodes of wakefulness (Fig. 2F; F = 6.4; df = 4, 36; p = 0.0005), NREM sleep (Fig. 2G; F = 7.2; df = 4, 36; p = 0.0002), and REM sleep (Fig. 2H; F = 14.9; df = 3, 27; p < 0.0001). Muscimol also decreased the number of transitions between states of wakefulness, NREM sleep, and REM sleep (Fig. 2I; F = 8.4; df = 4, 36; p < 0.0001). Muscimol significantly increased the average duration of wakefulness (F = 7.04; df = 4, 36; p = 0.0003) and had no effect on the average duration of NREM sleep or REM sleep (data not shown). All microinjection sites were localized to the PnO (Fig. 2J). Black circle with variance bars summarizes mean ± SEM stereotaxic coordinates for the PnO microinjection sites as 4.6 ± 0.1 mm posterior to bregma, 0.9 ± 0.1 mm lateral to the midline, and 4.6 ± 0.1 mm below the skull surface (Paxinos and Franklin, 2001).
Power spectral analyses shown in Figure 3 revealed that microinjection of muscimol into the PnO (Fig. 3D) significantly increased EEG power during wakefulness (Fig. 3A; F = 20.54; df = 2, 6; p = 0.0021). During REM sleep there was a significant treatment by EEG frequency interaction (Fig. 3C; F = 4.72; df = 12, 12; p = 0.0059). Muscimol did not alter EEG power during NREM sleep (Fig. 3B).
Co-administration of bicuculline and muscimol to the PnO blocked the increase in wakefulness and decrease in sleep caused by muscimol
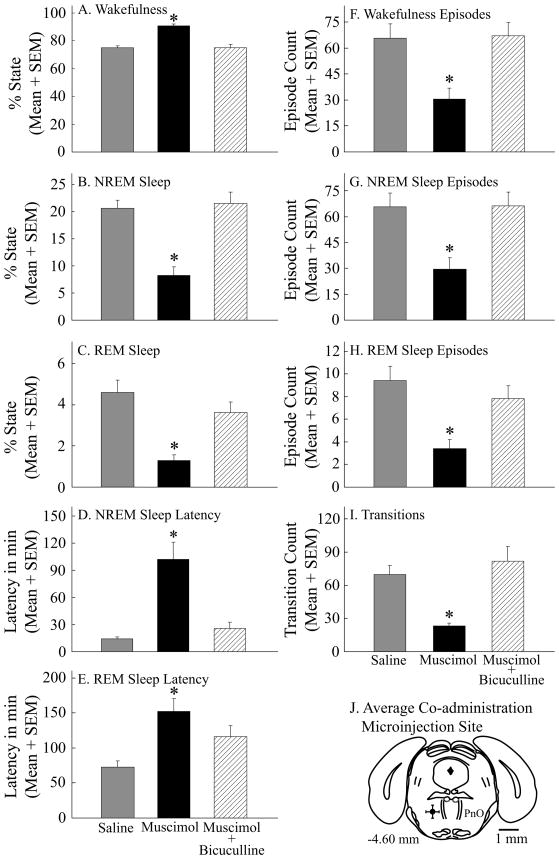
Figure 4 summarizes the effects on sleep and wakefulness of co-administering muscimol and bicuculline to the PnO of 12 mice. ANOVA revealed a significant drug main effect on wakefulness (Fig. 4A; F = 87.2; df = 2, 22; p < 0.0001), NREM sleep (Fig. 4B; F = 85.8; df = 2, 22; p < 0.0001), REM sleep (Fig. 4C; F = 12.0; df = 2, 22; p = 0.0003), NREM sleep latency (Fig. 4D; F = 20.0; df = 2, 22; p < 0.0001), REM sleep latency (Fig. 4E; F = 7.6; df = 2, 22; p = 0.003), and the number of episodes of wakefulness (Fig. 4F; F = 30.3; df = 2, 22; p < 0.0001) and NREM sleep (Fig. 4G; F = 30.6; df = 2, 22; p < 0.0001). There was also a drug main effect on the number of REM sleep episodes (Fig. 4H; F = 10.8; df = 2, 22; p = 0.0005), and a significant treatment effect on the number of transitions (Fig. 4I; F = 19.8; df = 2, 22; p < 0.0001) between states of wakefulness, NREM sleep, and REM sleep. Mean ± SEM stereotaxic coordinates (Paxinos and Franklin, 2001) for the antagonist blocking study microinjection sites (Fig. 4J) were 4.6 ± 0.1 mm posterior to bregma, 1.0 ± 0.1 mm lateral to the midline, and 4.6 ± 0.1 mm below the skull surface.
Figure 4.
Microinjection of bicuculline into the PnO blocked the increase in wakefulness, decrease in NREM sleep, and decrease in REM sleep caused by muscimol. A–I, asterisks indicate a significant (p < 0.05) difference from control (saline). Data summarize the results from four hours of recordings. J, a coronal diagram at 4.60 mm posterior to bregma was modified from a mouse brain atlas (Paxinos and Franklin, 2001) to indicate the mean ± SEM location of 12 histologically confirmed microinjection sites (black dot and bars in left PnO).
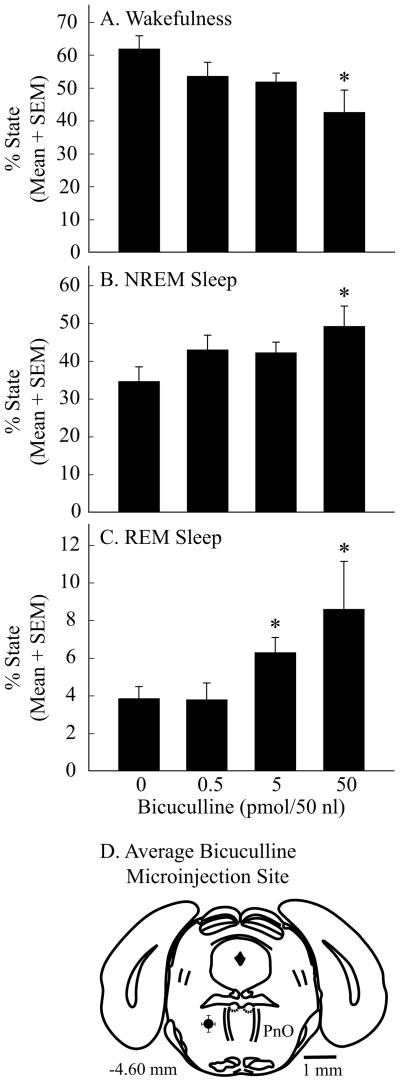
Microinjection of bicuculline into the PnO significantly decreased wakefulness and increased sleep
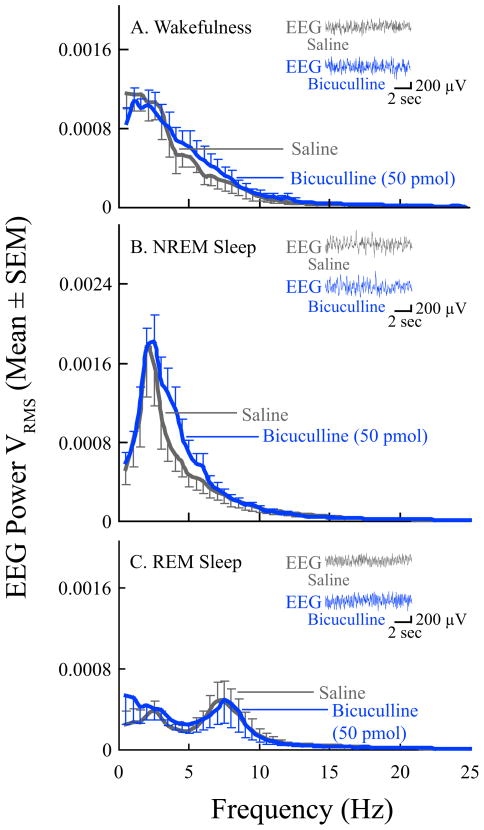
Having demonstrated that muscimol administered directly into the PnO causes a concentration-dependent and reversible increase in wakefulness and decrease in sleep, this study next aimed to determine whether endogenous GABA in the PnO of B6 mouse modulates sleep and wakefulness. The results are summarized by Figures 5 and 6. Bicuculline (n = 11 mice) caused a significant, concentration-dependent decrease in the amount of wakefulness (Fig. 5A; F = 3.0; df = 3, 27; p = 0.05) and increase in the amount of NREM sleep (Fig. 5B; F = 2.9; df = 3, 27; p = 0.05) and REM sleep (Fig. 5C; F = 7.9; df = 3, 27; p = 0.0006). Histological analyses revealed that all bicuculline microinjection sites were within the PnO (Fig. 5D), with mean ± SEM stereotaxic coordinates of 4.6 ± 0.1 mm posterior to bregma, 0.9 ± 0.04 mm lateral to the midline, and 4.3 ± 0.1 mm below the skull surface (Paxinos and Franklin, 2001). Bicuculline did not alter EEG power at any frequency during wakefulness (Fig. 6A), NREM sleep (Fig. 6B), or REM sleep (Fig. 6C). The Figure 6 insets show typical EEG recordings obtained after microinjection of saline and bicuculline (50 pmol/50 nl). Visual inspection of EEG recordings from each mouse confirmed that EEG activity during sleep and wakefulness appeared normal after microinjection of all concentrations of bicuculline.
Figure 5.
Microinjection of bicuculline into the PnO caused a concentration-dependent decrease in wakefulness and increase in NREM sleep and REM sleep. A–C, asterisks indicate a significant (p < 0.05) difference from control (0 pmol bicuculline). Data summarize the results from four hours of recordings. D, a coronal diagram at 4.60 mm posterior to bregma was modified from a mouse brain atlas (Paxinos and Franklin, 2001) with a black dot and bars in left PnO indicating the mean ± SEM location of the 11 histologically confirmed microinjection sites.
Figure 6.
EEG power was not altered by microinjection of bicuculline into the PnO. Graphs plot average (n = 6 mice) EEG power during A, wakefulness, B, NREM sleep, and C, REM sleep after microinjection of saline (gray line) and bicuculline (blue line). The EEG power functions are based on 50 min of recording during wakefulness (A), 60 min of recording during NREM sleep (B), and 50 min of recording during REM sleep (C). The insets show representative pairs of 10-s EEG recordings from the same mouse and illustrate the similarity of the raw signals after microinjection of bicuculline and saline.
Microdialysis delivery of bicuculline to the PnO caused a concentration-dependent increase in ACh release within the PnO, decrease in respiratory rate, and increase in anesthesia recovery time
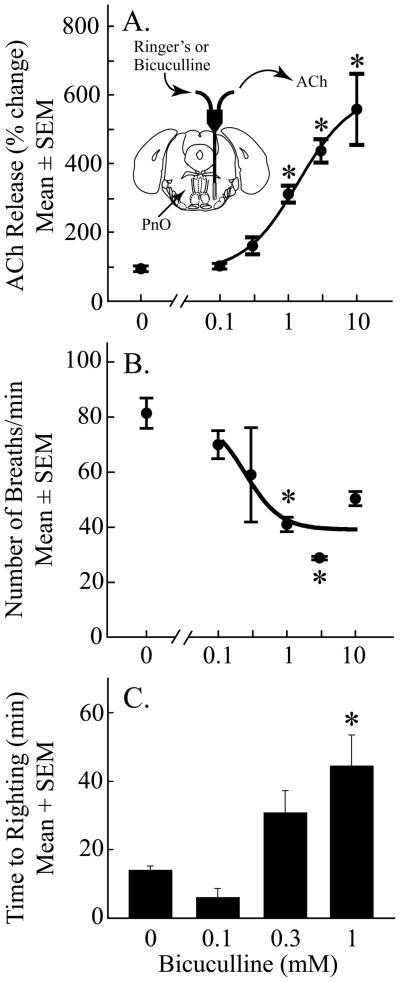
ACh release in PnO of cat changes significantly across states of sleep and wakefulness (Leonard and Lydic, 1997). Therefore, behavioral state was held constant with isoflurane anesthesia in order to determine whether bicuculline alters ACh release in the PnO of B6 mouse. Figure 7A shows that bicuculline caused a significant, concentration-dependent increase in ACh release within the PnO (F = 16.73; df = 5, 12; p < 0.0001). Figure 7B shows that bicuculline administered to the PnO also caused a significant, concentration-dependent decrease in rate of breathing (F = 6.04; df = 5, 12; p = 0.005). Dialysis with 0.1, 0.3, 1, 3, and 10 mM bicuculline decreased rate of breathing by 14, 16, 50, 55, and 40%, respectively. During control experiments, when the dialysis probe was perfused with Ringer’s containing no bicuculline (Fig. 7B; 0 mM), breathing decreased by 7% relative to baseline. There was no significant effect of bicuculline on core body temperature, which averaged 37.3 ± 0.06 degrees C across all dialysis experiments. Upon completion of dialysis sample collection, the effect of bicuculline on time to recovery from isoflurane anesthesia was determined. Figure 7C shows that blocking GABAA receptors in the PnO caused a significant, concentration-dependent increase in anesthesia recovery time (F = 8.67; df = 3, 8; p = 0.007). The two highest concentrations of bicuculline caused seizures followed by death, thus measures of recovery time following dialysis of the PnO with 3 mM (n = 3 mice) or 10 mM (n = 3 mice) bicuculline were not included in the final data set. Duration of exposure to anesthetics can influence recovery time, therefore the duration of isoflurane administration was held constant at 234 ± 1 min across all experiments. Mean ± SEM stereotaxic coordinates of the dialysis sites in the PnO were 4.8 ± 0.1 mm posterior to bregma, 1.0 ± 0.1 mm lateral to the midline, and 4.9 ± 0.1 mm below the skull surface (Paxinos and Franklin, 2001).
Figure 7.
Blocking GABAA receptors in the pontine reticular nucleus, oral part (PnO) caused a concentration-dependent change in three phenotypes of wakefulness. Asterisks indicate a significant difference from control (0 mM bicuculline). A, bicuculline increased ACh release in the PnO. The inset shows a coronal diagram modified from a mouse brain atlas (Paxinos and Franklin, 2001) to schematize a microdialysis probe in the PnO. The probe was perfused with Ringer’s or Ringer’s containing one concentration of bicuculline, and acetylcholine (ACh) was collected from the outlet tubing. The dialysis membrane is drawn to scale (1 mm length and 0.24 mm diameter). The concentration of bicuculline accounted for 87% of the variance in ACh release. Data are from 3 mice per concentration for a total of 18 mice. B, dialysis administration of bicuculline to the PnO decreased respiratory rate. The concentration of bicuculline accounted for 76% of the variance in rate of breathing. Data are from 3 mice per concentration, for a total of 18 mice. C, bicuculline administered to the PnO increased time to righting after cessation of isoflurane delivery. The concentration of bicuculline accounted for 76% of the variance in anesthesia recovery time. Data are from 3 mice per concentration, for a total of 12 mice.
Discussion
The present study is the first to characterize the effects of GABAergic transmission in mouse PnO on states of arousal and traits characterizing those states. The findings demonstrate that GABAA receptors in PnO of B6 mouse modulate occurrence of sleep/wake states, activity of the cortical EEG, release of ACh in the PnO, breathing rate, and time needed to regain wakefulness after isoflurane anesthesia. The ensuing discussion considers the results in relation to clinical implications of brain region-specific effects of GABAergic transmission on arousal states, and evidence that endogenous GABA in the PnO promotes wakefulness.
GABAergic transmission causes brain-region-specific effects on sleep and wakefulness
This study shows for the first time that microinjection of muscimol into the PnO of B6 mouse causes a concentration-dependent increase in wakefulness and decrease in sleep (Figs. 1 & 2) that is blocked by co-administration of bicuculline (Fig. 4). The data indicate that these changes in sleep and wakefulness are mediated by GABAA receptors localized to the PnO. Additionally, by showing that microinjection of bicuculline alone causes a concentration-dependent decrease in wakefulness and increase in sleep (Fig. 5), this study provides the first in vivo evidence that endogenous GABA in the PnO of B6 mouse promotes wakefulness and inhibits sleep.
Studies using intracranial drug administration have revealed that the effects of GABAA receptor agonists and antagonists on sleep and wakefulness vary significantly as a function of brain region. Within cat hypothalamus the GABAA receptor agonist muscimol induces sleep onset with short latency and increases sleep time when microinjected into posterior hypothalamus, but increases wakefulness and decreases sleep time when microinjected into preoptic and anterior hypothalamic areas (Lin et al., 1989). Opposite effects of muscimol on sleep and wakefulness also can be evoked from within the pontine portion of the brainstem. REM sleep is increased by microinjecting muscimol into cat dorsal raphé nucleus (Nitz and Siegel, 1997) and inhibited by delivering muscimol to the PnO of cat (Xi et al., 1999), rat (Camacho-Arroyo et al., 1991), and mouse (Figs. 1 & 2). Studies using intracranial administration of drugs that block transmission at GABAA receptors, such as bicuculline (Figs. 4 & 5) (Camacho-Arroyo et al., 1991; Xi et al., 1999; Sanford et al., 2003), gabazine (Marks et al., 2008), and picrotoxin (Kaur et al., 1997; Nitz and Siegel, 1997; Ali et al., 1999; Pal and Mallick, 2009), provide convincing evidence that endogenous GABA differentially alters sleep and wakefulness depending upon brain site of action. One unifying explanation for these findings is that in brain regions that function to generate wakefulness GABAergic inhibition causes sleep, whereas in brain regions that generate sleep GABAergic transmission increases wakefulness.
The discovery that GABAergic effects on arousal states are anatomically site-specific within the brain provides insight into how systemically administered benzodiazepines produce sleep architecture that differs from that of spontaneously occurring sleep. Benzodiazepine hypnotics increase total sleep time and shorten sleep latency, but some of these drugs decrease deep, slow wave sleep and increase REM sleep latency (Feinberg et al., 1979; Borbély and Achermann, 1991). The present results suggest that drug actions at the level of the PnO may account for the increased amount of lighter NREM sleep and increased REM latency caused by some benzodiazepine hypnotics. This interpretation is supported by the demonstration that intravenous administration of the benzodiazepine receptor agonist eszopiclone to rat decreases the release of ACh in the PnO and prevents REM sleep (Hambrecht-Wiedbusch et al., 2010).
Muscimol causes state-trait dissociations
States of wakefulness, NREM sleep, and REM sleep can occur in a humbling complexity of admixtures that combine traits characteristic of one state with traits typical of a completely different state. These mixed states are referred to as dissociated states and represent a significant clinical concern (Mahowald and Schenck, 1992, 2001; Schiff, 2010). The present study found that direct administration of muscimol into the PnO caused dissociation between behavior during wakefulness and EEG activity. During the increase in wakefulness caused by muscimol (500 pmol) mice showed a marked increase in running activity yet significantly greater EEG power in the delta range (0.5–5 Hz) (Fig. 3). Increased delta activity normally occurs during NREM sleep, which is characterized by decreased motor activity and a loss of consciousness. A previous study that administered muscimol to B6 mice by intraperitoneal injection reported periods of vigorous wheel running activity during which the frontal EEG was dominated by high amplitude slow waves characteristic of NREM sleep (Vyazovskiy et al., 2007). The finding that the slowing of the EEG caused by systemic administration of muscimol (Vyazovskiy et al., 2007) also occurs after muscimol is delivered directly into the PnO (Fig. 3) supports the interpretation that the increase in delta power caused by intraperitoneal muscimol administration is mediated, at least in part, by GABAA receptors in the PnO. This interpretation is consistent with data showing that the benzodiazepine receptor agonists zolpidem and diazepam increase EEG delta power when administered to the PnO of isoflurane anesthetized rat (Hambrecht-Wiedbusch et al., 2010).
Microinjection of muscimol into the PnO significantly decreased the amount of time spent in REM sleep, and the REM sleep that did occur following muscimol (50 pmol) administration was characterized by increased EEG power in the theta range (4–9 Hz) (Fig. 3). Theta activity normally is prominent during REM sleep and increases during wakefulness when animals are exploring their environment. Although the mechanisms underlying GABA-induced state-trait dissociations are not known, the phenomenon of paradoxical activation by benzodiazepines has been documented by multiple case reports (Paton, 2002; Brefel-Courbon et al., 2007; Schiff and Posner, 2007; Hall et al., 2008). Efforts to synthesize these case reports have led to a network model by which the benzodiazepine receptor agonist zolpidem might produce EEG and behavioral activation (Schiff, 2008, 2010). The present results expand this network model by showing that increasing GABAergic transmission in the PnO may be another mechanism through which hypnotics produce state dissociations.
GABAA receptors in the PnO function to inhibit local ACh release, stimulate breathing, and decrease anesthesia recovery time: implications for arousal state control
Dialysis delivery of bicuculline to the PnO caused a concentration-dependent increase in ACh release (Fig. 7A). Bicuculline also increases ACh release in PnO of cat (Vazquez and Baghdoyan, 2004), and these findings support the conclusion that one functional role of endogenous GABA in the PnO is to inhibit ACh release. Extensive evidence demonstrates that cholinergic transmission within the PnO promotes REM sleep (Lydic and Baghdoyan, 2008), and the increase in REM sleep caused by blocking GABAA receptors in the PnO can be prevented by pre-treating the PnO with the muscarinic receptor antagonist atropine (Marks et al., 2008). Considered together, these findings suggest that GABAergic tone in the PnO normally suppresses REM sleep, in part by inhibiting ACh release in the PnO.
The finding that human rate of breathing declines significantly during the loss of wakefulness caused by anesthesia (Fink, 1961) or sleep (Phillipson and Sullivan, 1978) initiated ongoing efforts to identify cellular and molecular substrates comprising a wakefulness stimulus for breathing. Although the PnO does not contain neurons with respiratory-related discharge patterns, breathing is significantly altered by PnO neurons that regulate EEG and behavioral arousal (Lydic and Baghdoyan, 1993). Blocking GABAA receptors in the PnO with bicuculline decreased the rate of breathing (Fig. 7B). This decrease was not observed during control experiments during which the PnO was dialyzed with Ringer’s containing no bicuculline. Thus, breathing rate did not decrease due to changes in arousal that may occur during general anesthesia. The bicuculline-induced decrease in rate of breathing implies that endogenous GABA in the PnO stimulates breathing, and suggests that GABAergic transmission in the PnO contributes to the wakefulness stimulus for breathing. Consistent with this interpretation is the finding that bicuculline delivered to the PnO delayed recovery time from isoflurane anesthesia (Fig. 7C). In rat, administering drugs that decrease or increase GABAergic transmission in the PnO shortens or lengthens, respectively, the time required for isoflurane to induce loss of consciousness (Vanini et al., 2008). Induction of and emergence from general anesthesia are mechanistically distinct processes (Kelz et al., 2008), and the present data are the first in any species to show that blocking GABAA receptors in the PnO increases anesthesia recovery time. Taken together, the findings of increased ACh release, decreased breathing rate, and increased anesthesia recovery time caused by blocking GABAA receptors in the PnO provide novel evidence that GABAergic transmission in the PnO promotes wakefulness.
Acknowledgments
Supported by NIH grants MH45361, HL40881, HL65272, and by the Department of Anesthesiology. We thank S. Jiang, M.A. Norat, and W. Wang from the Department of Anesthesiology for expert assistance. We are grateful to K.B. Welch of the University of Michigan Center for Statistical Consultation and Research for providing input on statistical analyses.
References
- Ali M, Jha SK, Kaur S, Mallick BN. Role of GABA-A receptor in the preoptic area in the regulation of sleep-wakefulness and rapid eye movement sleep. Neurosci Res. 1999;33:245–250. doi: 10.1016/s0168-0102(99)00013-9. [DOI] [PubMed] [Google Scholar]
- Borbély AA, Achermann P. Ultradian dynamics of sleep after a single dose of benzodiazepine hypnotics. Eur J Pharmacol. 1991;195:11–18. doi: 10.1016/0014-2999(91)90376-2. [DOI] [PubMed] [Google Scholar]
- Brefel-Courbon C, Payoux P, Ory F, Sommet A, Slaoui T, Raboyeau G, Lemesle B, Puel M, Montastruck J-L, Cardebat D. Clinical and imaging evidence of zolpidem effect in hypoxic encephalopathy. Ann Neurol. 2007;62:102–105. doi: 10.1002/ana.21110. [DOI] [PubMed] [Google Scholar]
- Brevig HN, Watson CJ, Lydic R, Baghdoyan HA. Wakefulness is increased by a GABAA-hypocretin receptor interaction in the pontine reticular formation. Sleep. 2010:33. doi: 10.1093/sleep/33.10.1285. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Arroyo I, Alvarado R, Manjarrez J, Tapia R. Microinjections of muscimol and bicuculline into the pontine reticular formation modify the sleep-waking cycle in the rat. Neurosci Lett. 1991;129:95–97. doi: 10.1016/0304-3940(91)90728-c. [DOI] [PubMed] [Google Scholar]
- Feinberg I, Fein G, Walker JM, Price LJ, Floyd TC, March JD. Flurazepam effects on sleep EEG. Arch Gen Psych. 1979;36:95–102. doi: 10.1001/archpsyc.1979.01780010101012. [DOI] [PubMed] [Google Scholar]
- Fink BR. Influence of cerebral activity in wakefulness on regulation of breathing. J Appl Physiol. 1961;16:15–20. doi: 10.1152/jappl.1961.16.1.15. [DOI] [PubMed] [Google Scholar]
- Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Hall SD, Yamawaki N, Fisher AE, Clauss RP, Woodhall GL, Stanford IM. Desynchronization of pathological low-frequency brain activity by the hypnotic drug zolpidem. Nature Precedings. 2008 hdl:10101/npre.2008.1966.1. [Google Scholar]
- Hambrecht-Wiedbusch VS, Gauthier EA, Baghdoyan HA, Lydic R. Benzodiazepine receptor agonists cause drug-specific and state-specific alterations in EEG power and acetylcholine release in rat pontine reticular formation. Sleep. 2010;33:909–918. doi: 10.1093/sleep/33.7.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Saxena RN, Mallick BN. GABA in locus coeruleus regulates spontaneous rapid eye movement sleep by acting on GABAA receptors in freely moving rats. Neurosci Lett. 1997;223:105–108. doi: 10.1016/s0304-3940(97)13410-3. [DOI] [PubMed] [Google Scholar]
- Kelz MB, Sun Y, Chen J, Cheng Meng Q, Moore JT, Veasey SC, Dixon S, Thornton M, Funato H, Yanagisawa M. An essential role for orexins in emergence from general anesthesia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:1309–1314. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard TO, Lydic R. Pontine nitric oxide modulates acetylcholine release, rapid eye movement sleep generation, and respiratory rate. J Neurosci. 1997;17:774–785. doi: 10.1523/JNEUROSCI.17-02-00774.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JS, Sakai K, Vanni-Mercier G, Jouvet M. A critical role of the posterior hypothalamus in the mechanisms of wakefulness determined by microinjection of muscimol in freely moving cats. Brain Res. 1989;479:225–240. doi: 10.1016/0006-8993(89)91623-5. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Pedunculopontine stimulation alters respiration and increases ACh release in the pontine reticular formation. Am J Physiol. 1993;264:R544–554. doi: 10.1152/ajpregu.1993.264.3.R544. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–1295. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Acetylcholine modulates sleep and wakefulness: A synaptic perspective. In: Monti JM, Pandi-Perumal SR, Sinton CM, editors. Neurochemistry of Sleep and Wakefulness. New York: Cambridge University Press; 2008. pp. 109–143. [Google Scholar]
- Mahowald MW, Schenck CH. Dissociated states of wakefulness and sleep. Neurology. 1992;42:44–51. discussion 52. [PubMed] [Google Scholar]
- Mahowald MW, Schenck CH. Evolving concepts of human state dissociation. Arch Ital Biol. 2001;139:269–300. [PubMed] [Google Scholar]
- Marks GA, Sachs OW, Birabil CG. Blockade of GABA, type A, receptors in the rat pontine reticular formation induces rapid eye movement sleep that is dependent upon the cholinergic system. Neuroscience. 2008;156:1–10. doi: 10.1016/j.neuroscience.2008.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitz D, Siegel J. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Physiol. 1997;273:R451–455. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opp MR. Rat strain differences suggest a role for corticotropin-releasing hormone in modulating sleep. Physiol Behav. 1998;63:67–74. doi: 10.1016/s0031-9384(97)00390-9. [DOI] [PubMed] [Google Scholar]
- Pal D, Mallick BN. GABA in pedunculopontine tregmentum increases rapid eye movement sleep in freely moving rats: possible role of GABA-ergic inputs from substania nigra pars reticulata. Neuroscience. 2009;164:404–414. doi: 10.1016/j.neuroscience.2009.08.025. [DOI] [PubMed] [Google Scholar]
- Paton C. Benzodiazepines and disinhibition: a review. Psychiatric Bulletin. 2002;26:460–462. [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
- Phillipson EA, Sullivan C. Arousal: The forgotten response to respirator stimuli. Am Rev Resp Dis. 1978;118:807–809. doi: 10.1164/arrd.1978.118.5.807. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Tang X, Xiao J, Ross RJ, Morrison AR. GABAergic regulation of REM sleep in reticularis pontis oralis and caudalis in rats. J Neurophysiol. 2003;90:938–945. doi: 10.1152/jn.00993.2002. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann NY Acad Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- Schiff ND. Recovery of consciousness after brain injury: a mesocircuit hypothesis. Trends Neurosci. 2010;33:1–9. doi: 10.1016/j.tins.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND, Posner JB. Another “Awakenings”. Ann Neurol. 2007;62:5–6. doi: 10.1002/ana.21158. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brain Control of Wakefulness and Sleep. 2. New York: Plenum Press; 2005. [Google Scholar]
- Tang X, Xiao J, Parris BS, Fang J, Sanford LD. Differential effects of two types of environmental novelty on activity and sleep in BALB/cJ and C57BL/6J mice. Physiol Behav. 2005;85:419–429. doi: 10.1016/j.physbeh.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Van Dort CJ, Baghdoyan HA, Lydic R. Adenosine A1 and A2A receptors in mouse prefrontal cortex modulate acetylcholine release and behavioral arousal. J Neurosci. 2009;29:871–881. doi: 10.1523/JNEUROSCI.4111-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanini G, Lydic R, Baghdoyan HA. GABAergic modulation of REM sleep. In: Mallick BN, Pandi-Perumal R, McCarley RW, Morrison AR, editors. Rapid Eye Movement Sleep – Regulation and Function. Cambridge University Press; 2010. p in press. [Google Scholar]
- Vanini G, Watson CJ, Lydic R, Baghdoyan HA. Gamma-aminobutyric acid-mediated neurotransmission in the pontine reticular formation modulates hypnosis, immobility, and breathing during isoflurane anesthesia. Anesthesiology. 2008;109:978–988. doi: 10.1097/ALN.0b013e31818e3b1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanini G, Wathen BL, Lydic R, Baghdoyan HA. GABA levels in cat pontine reticular formation (PRF) are lower during rapid eye movement (REM) sleep and the neostigmine-induced REM sleep-like state (REM-Neo) than during wakefulness. Sleep. 2009;32 (Abstr Suppl):0011. [Google Scholar]
- Vazquez J, Baghdoyan HA. GABAA receptors inhibit acetylcholine release in cat pontine reticular formation: implications for REM sleep regulation. J Neurophysiol. 2004;92:2198–2206. doi: 10.1152/jn.00099.2004. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Tobler I, Winsky-Sommerer R. Alteration of behavior in mice by muscimol is associated with regional electroencephalogram synchronization. Neuroscience. 2007;147:833–841. doi: 10.1016/j.neuroscience.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Watson CJ, Venton BJ, Kennedy RT. In vivo measurements of neurotransmitters by microdialysis sampling. Anal Chem. 2006;78:1391–1399. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- Watson CJ, Lydic R, Baghdoyan HA. Sleep and GABA levels in the oral part of rat pontine reticular formation are decreased by local and systemic administration of morphine. Neuroscience. 2007;144:375–386. doi: 10.1016/j.neuroscience.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CJ, Soto-Calderon H, Lydic R, Baghdoyan HA. Pontine reticular formation (PnO) administration of hypocretin-1 increases PnO GABA levels and wakefulness. Sleep. 2008;31:453–464. doi: 10.1093/sleep/31.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur J Neurosci. 2009;29:1779–1794. doi: 10.1111/j.1460-9568.2009.06716.x. [DOI] [PubMed] [Google Scholar]
- Xi MC, Morales FR, Chase MH. Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism. J Neurophysiol. 1999;82:2015–2019. doi: 10.1152/jn.1999.82.4.2015. [DOI] [PubMed] [Google Scholar]