Abstract
Recent studies in Drosophila melanogaster retina indicate that absorption of light causes the translocation of signaling molecules and actin from the photoreceptor’s signaling membrane to the cytosol, but the underlying mechanisms are not fully understood. As ezrin-radixin-moesin (ERM) proteins are known to regulate actin–membrane interactions in a signal-dependent manner, we analyzed the role of Dmoesin, the unique D. melanogaster ERM, in response to light. We report that the illumination of dark-raised flies triggers the dissociation of Dmoesin from the light-sensitive transient receptor potential (TRP) and TRP-like channels, followed by the migration of Dmoesin from the membrane to the cytoplasm. Furthermore, we show that light-activated migration of Dmoesin results from the dephosphorylation of a conserved threonine in Dmoesin. The expression of a Dmoesin mutant form that impairs this phosphorylation inhibits Dmoesin movement and leads to light-induced retinal degeneration. Thus, our data strongly suggest that the light- and phosphorylation-dependent dynamic association of Dmoesin to membrane channels is involved in maintenance of the photoreceptor cells.
Introduction
The Drosophila melanogaster eye is composed of ~800 repeat units, referred to as ommatidia. Each ommatidium is composed of six elongated peripheral photoreceptor cells (R1–6), which extend across the length of the ommatidium, and two shorter central photoreceptors (R7 and -8; Ready et al., 1976). Photoreceptors are highly polarized cells composed of two well defined compartments: a cell body and a signaling compartment called the rhabdomere. The rhabdomere contains tightly packed, actin-rich microvilli that harbor the signaling proteins required to generate the photoreceptor potential upon illumination. The D. melanogaster transient receptor potential (TRP) protein is a light-sensitive cation channel that provides a major component of the light-induced current (Hardie and Minke, 1992). TRP is also required for anchoring a supramolecular signaling complex that includes the inactivation-no-afterpotential D (INAD) PSD95/DlgA/ZO-1 homology (PDZ) scaffold protein, PLCβ, and the eye-specific PKC (eyePKC) to the plasma membrane (Huber et al., 1996; Shieh et al., 1997; Tsunoda et al., 1997; Montell, 1998). TRP-like (TRPL) is a second light-activated channel (Phillips et al., 1992) that, together with TRP, participates in the production of the light-induced current (Hardie and Minke, 1995). Genetic elimination of both TRP and TRPL channels completely eliminates the photoreceptor potential (Niemeyer et al., 1996; Scott et al., 1997).
The photoreceptor potential is only one of several responses to light. Another light-induced response is the translocation of signaling proteins (Gqα and TRPL) and actin between the rhabdomeric membrane and the cell body (Bähner et al., 2002; Kosloff et al., 2003). The molecular mechanisms underlying translocation of these proteins from the microvilli to the cell body remain largely unknown (Minke and Agam, 2003).
In several tissues, microvillar organization depends on protein members of the ezrin-radixin-moesin (ERM) family, which form a bridge between the actin cytoskeleton and the plasma membrane (for review see Bretscher et al., 2002). ERM proteins bind to integral membrane proteins either directly or through PDZ scaffold proteins, such as ezrin binding phosphoprotein 50 (EBP50)/Na+/H+ exchanger regulatory factor and NHE3 kinase A regulatory protein. This binding is a dynamic process, which takes place upon the binding of phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphorylation of the ERM protein (Hirao et al., 1996; Bretscher et al., 2002). Dmoesin, the unique member of the ERM family in D. melanogaster (Polesello and Payre, 2004), is required for the specific organization of different actin-rich structures during development. Furthermore, Dmoesin plays an essential structural role in D. melanogaster photoreceptor morphogenesis (Karagiosis and Ready, 2004). Dmoesin mutations disrupt the polarized localization of posterior determinants in oocytes (Jankovics et al., 2002; Polesello et al., 2002). Mutations that disrupt the dynamics of Dmoesin phosphorylation produce severe defects in actin reorganization and cell shape (Polesello et al., 2002; Speck et al., 2003; Karagiosis and Ready, 2004). Although Dmoesin has been shown to accumulate in rhabdomeres (Karagiosis and Ready, 2004), its physiological function in mature photoreceptors and its relationship to light reception is not known.
In this study we used wild-type (WT) and mutant D. melanogaster strains to show that Dmoesin only interacts with the TRP and TRPL channels in dark-raised flies. Furthermore, we show that illumination induces dephosphorylation of the conserved COOH-terminal threonine 559 (T559) of Dmoesin, which subsequently dissociates from the channel proteins and moves from the rhabdomeric membrane to the cytosol. Consistent with this conclusion, our results show that mutations that impair phosphorylation of Dmoesin (Polesello et al., 2002; Speck et al., 2003) abolish the movement of Dmoesin upon illumination and result in light-activated degeneration of the photoreceptor cells.
Results
Light induces subcellular redistribution of Dmoesin
We recently showed that actin moves from the rhabdomere to the photoreceptor cell body after the illumination of dark-raised flies (Kosloff et al., 2003). To investigate a possible mechanism that underlies light-activated actin movement in photoreceptors, we examined the role of Dmoesin, a known regulator of the dynamic reorganization of actin-rich cell structures (Polesello and Payre, 2004). Activation of Dmoesin involves a redistribution of the protein from the cytoplasm (dormant form) to the plasma membrane (active form; Polesello et al., 2002). Thus, modification of Dmoesin intracellular localization might be related to the light-dependent reorganization of actin filaments.
To test this possibility, we examined the distribution of Dmoesin between the membrane and cytosolic fractions in head extracts of dark-raised and illuminated D. melanogaster. Although Dmoesin was detected in both the soluble and membrane fractions, the majority of Dmoesin was associated with the membrane fraction in dark-raised flies (Fig. 1 A). In contrast, Dmoesin was predominantly in the soluble fraction in illuminated WT flies (Fig. 1 A). Quantification of the Dmoesin membrane/cytosolic ratio further supports the conclusion that illumination induces a substantial movement of Dmoesin from the membrane to the soluble fraction (Fig. 1 B). Upon illumination, nearly 50% of the membrane-associated Dmoesin of the head moved to the cytosol. By using mutants lacking eyes (Fig. S1, available online at http://www.jcb.org/cgi/content/full/jcb.200503014/DC1) we found that the Dmoesin protein present in heads, outside the photoreceptors, remained associated with membranes upon illumination and therefore was not affected by light.
Figure 1. Protein blot showing light-dependent movement of Dmoesin from the membrane to the cytosol.
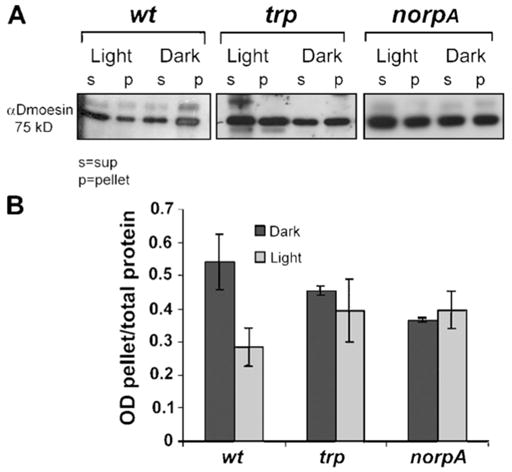
(A) Membrane-associated (pellet) and soluble (sup) protein fractions were separated by high-speed centrifugation and processed for Western blotting with αDmoesin antibodies. Head extracts were prepared from dark-raised (Dark) or illuminated (Light) flies of the following genotypes: WT, trpP343 (trp), and norpAP24 (norpA). Although illumination induces redistribution of Dmoesin from membranes to the cytosol in WT flies, inactivation of either TRP or NORPA blocks the light-dependent movement of Dmoesin. (B) The histogram plots Dmoesin levels in the pellet divided by the total amount of Dmoesin present in extracts from WT, trp, and norpA heads, as revealed from repeated experiments done in similar conditions to those in A. P < 0.01; n = 5. The error bars are SEM.
To further test whether the observed intracellular movement of Dmoesin depends on the activation of the visual signaling cascade, we analyzed Dmoesin distribution in mutants that inactivated phototransduction. The norpA and trp genes encode the PLCβ isoform and the major light-activated channel TRP, respectively, and both are required for D. melanogaster phototransduction. In contrast to WT flies, no significant changes in the intracellular distribution of Dmoesin were observed upon illumination in head extracts from trpP343 or norpAP24 mutants (Fig. 1, A and B). In both mutants, similar amounts of Dmoesin were detected in the membrane and soluble fractions, regardless of the illumination regime. Therefore, the light-induced intracellular movement of Dmoesin is dependent on a functional phototransduction cascade in photoreceptors.
To directly visualize the movement of Dmoesin after illumination in the retina, we analyzed the immunocytochemical localization of Dmoesin using anti-Dmoesin antibodies (αDmoesin; Fig. 2). In dark-raised flies Dmoesin was mainly localized to the base of the rhabdomeres and cortical actin region (Karagiosis and Ready, 2004). After illumination, Dmoesin level of peripheral R1–6 rhabdomeres was much reduced, with a concomitant increase in nonrhabdomeric regions in WT flies (Fig. 2, A–D) that is consistent with the Western blot analysis of Fig. 1. The failure of Dmoesin to move upon illumination in the central R7 cell can be attributed to the inefficient absorption of visible light by the UV rhodopsin of R7 cells (Chou et al., 1996), which constitutes a negative control (Fig. 2, A–D). In agreement with the biochemical data, the light-triggered redistribution of Dmoesin observed by immunocytochemistry required the activation of the phototransduction cascade because it was abolished in the mutant norpAP24 (Fig. 2, E–H).
Figure 2. Light-dependent movement of Dmoesin in the photoreceptor cell of WT D. melanogaster retina.
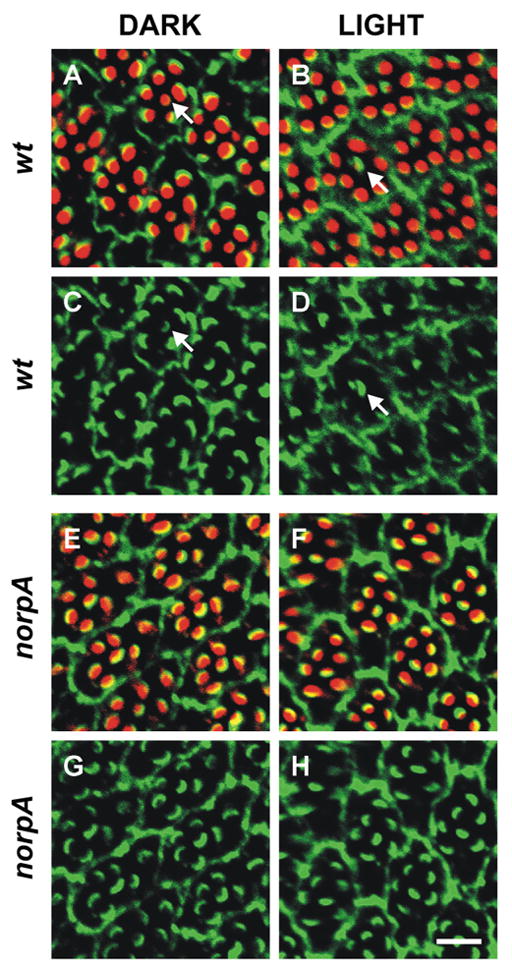
(A–D) Cross sections through dark-raised and illuminated wt flies (Schott BG28 blue light for 1 h). Flies were double labeled with rhodamin-coupled phalloidin (red) and αDmoesin (1:2,000 dilution). Primary Dmoesin antibody was detected by a Cy5-coupled secondary antibody (green; C and D). The overlay of both markers appears yellow in some ommatidia (A and B). Arrows indicate R7 cells. (E and F) The same experiment as in A–D was performed with norpAP24 mutant. Bar, 8 μm.
Altogether, these results support the conclusion that retinal Dmoesin is predominantly associated with rhabdomeric membranes in dark-raised flies and that activation of the phototransduction cascade induces Dmoesin movement to the cytosol in the photoreceptor cells.
Dmoesin binds TRP and TRPL channels in dark-raised flies, whereas illumination dissociates Dmoesin from the channel proteins
Results from our cell fractionations and immunocytochemistry suggest that illumination regulates Dmoesin dissociation from the membrane proteins of the signaling compartment of photoreceptors. Several studies have reported that ERM/EBP50 proteins interact with members of the TRP family of proteins, such as TRPC4 and TRPC5 (Tang et al., 2000; Mery et al., 2002; Obukhov and Nowycky, 2004). Therefore, we examined whether Dmoesin interacts with TRP and TRPL, the two major channel proteins of the microvilli in D. melanogaster photoreceptors.
To test this hypothesis, we analyzed WT and mutant head extracts by immunoprecipitation with monospecific anti-TRP antibodies (αTRP). Protein complexes precipitated with αTRP were fractionated by SDS-PAGE and probed in Western blots with αDmoesin. In dark-raised WT flies, a strong Dmoesin signal was observed, indicating that Dmoesin and TRP formed a protein complex in vivo (Fig. 3). In control experiments, no Dmoesin staining was detected in head extracts of dark-raised trpP343 null mutant or in WT extracts immunoprecipitated with nonimmune serum (NIS; Fig. 3), thus demonstrating the specificity of the Dmoesin–TRP interaction. We then examined the effect of illumination on the Dmoesin–TRP interaction. Interestingly, illumination caused a strong reduction of Dmoesin staining in complexes immunoprecipitated with αTRP, although similar amounts of TRP were immunoprecipitated from dark-raised and illuminated flies (Fig. 3, bottom). Therefore, TRP and Dmoesin interact in vivo only in dark-raised flies, and illumination dissociates the Dmoesin–TRP interaction.
Figure 3. TRP interacts with Dmoesin only in dark-raised flies.
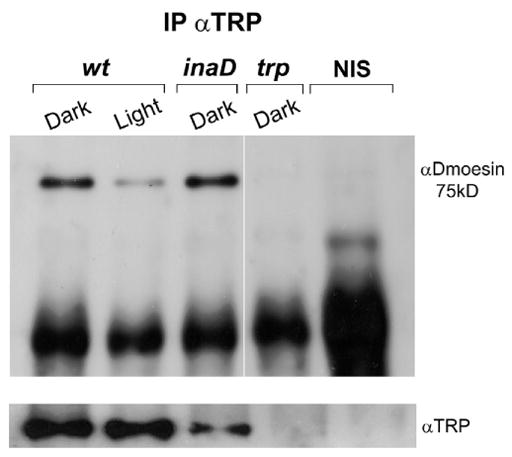
Coimmuno-precipitation of TRP and Dmoesin. Protein extracts from heads of dark-raised and illuminated fly strains of WT, inaD1, and trpP343 mutant flies were immunoprecipitated using αTRP or NIS and fractionated by SDS-PAGE. Western blot was probed with αDmoesin. (bottom) Western blot of the same head extracts probed with antibodies against αTRP. Bradford analysis indicated that roughly equal amounts of proteins were loaded on each lane (n = 12).
Because TRP is known to bind INAD, which is a multi PDZ-domain scaffold protein (Shieh and Zhu, 1996; Chevesich et al., 1997; Tsunoda et al., 1997), we examined whether INAD is required for Dmoesin–TRP interaction. Head extracts of dark-raised young (<2-d-old) inaD1 null mutants were immunoprecipitated with αTRP. Western blot analysis showed that Dmoesin immunoprecipitation was unaffected in the inaD1 mutant flies (Fig. 3), indicating that Dmoesin interacts with TRP independently from INAD. The observed reduction in TRP level in the inaD1 mutant (Fig. 3) is consistent with the known slow degradation of TRP in inaD1 mutants (Tsunoda et al., 1997). As an additional control, αTRP-precipitated complexes were probed with antibodies against another major membrane protein of the microvilli, Chaoptin (Van Vactor et al., 1988). Western blot analysis did not reveal any Chaoptin signal (unpublished data), providing additional evidence of the specificity of the Dmoesin–TRP interaction.
To address whether activation of the phototransduction cascade is required for the observed dissociation of the Dmoesin–TRP complex, we examined the coimmunoprecipitation of Dmoesin in a visual transduction mutant. In the PLCβ mutant (norpAP24), a strong Dmoesin signal was observed in protein complexes precipitated with αTRP, regardless of illumination (Fig. 4). Western blot analysis of the same head extracts probed with αINAD revealed that roughly equal amounts of signaling proteins were coprecipitated in each lane (Fig. 4, bottom). These data show that in norpAP24 eyes, TRP interacts with Dmoesin equally in both dark- and light-raised flies, thus indicating that the dissociation of the Dmoesin–TRP complex depends on activation of the phototransduction cascade.
Figure 4. Dissociation of the Dmoesin–TRP complex depends on activation of the phototransduction cascade by light.
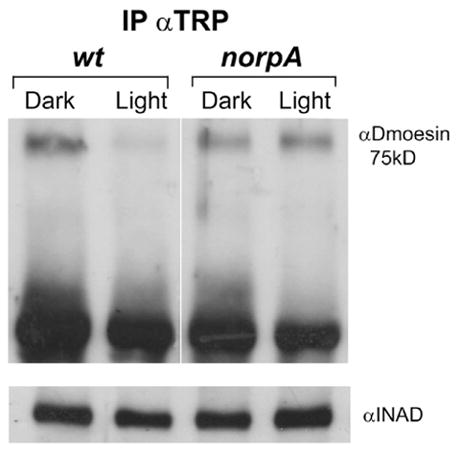
Coimmunoprecipitation of TRP and Dmoesin from D. melanogaster photoreceptors cells. Protein extracts from heads of WT and norpAP24 mutant flies were immunoprecipitated using αTRP as indicated, and analyzed in Western blot with αDmoesin. The norpA mutation prevented dissociation of the Dmoesin–TRP complex by light. (bottom) Western blot of the same head extracts probed with αINAD revealed that equivalent amounts of INAD, which interacts with TRP, are present in all samples (n = 4).
We then addressed whether Dmoesin also interacts in vivo with TRPL, the second light-activated channel of the microvilli. Analysis of protein complexes immunoprecipitated with anti-TRPL antibodies revealed that Dmoesin specifically interacts with TRPL in WT dark-raised flies (Fig. 5). Consistent with this conclusion, Dmoesin is undetectable in similar conditions with extracts from trpl null mutants, or in WT extracts when using NIS (Fig. 5). As observed with TRP, the Dmoesin–TRPL interaction occurs specifically in dark-raised flies, whereas illumination dissociates the Dmoesin–TRPL complex (Fig. 5).
Figure 5. TRPL interacts with Dmoesin only in dark-raised flies.
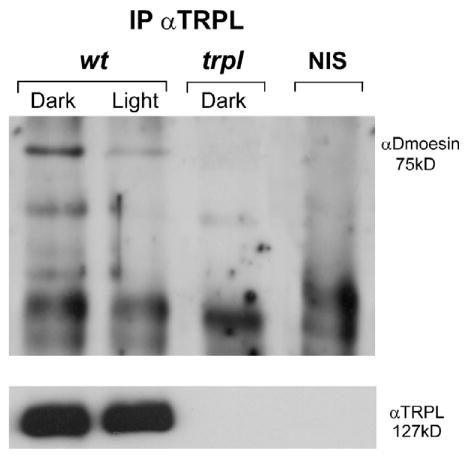
Coimmuno-precipitation of TRPL and Dmoesin from D. melanogaster photoreceptor cells. Immune complexes obtained from heads of dark-raised and illuminated fly strains including WT and trpl302 null mutant (trpl) flies using αTRPL or NIS were fractionated by SDS-PAGE, and the Western blot was probed with αDmoesin. (bottom) Western blot of the same head extracts probed with αTRPL (n = 5).
Altogether, these results show that Dmoesin interacts specifically with both TRP and TRPL. Moreover, activation of the light-induced signaling cascade disrupts the Dmoesin–TRP interaction, leading to movement of a portion of Dmoesin to the soluble fraction.
Light-dependent movement of Dmoesin from the membrane to the cytosol is regulated by T559 phosphorylation
Phosphorylation of a conserved threonine residue located in the actin-binding domain of ERM proteins has been shown to regulate both mammalian ERM (Bretscher et al., 2002) and D. melanogaster Dmoesin activity and subcellular localization (Polesello and Payre, 2004). Therefore, we examined if the light-sensitive interaction of Dmoesin with the photoreceptor channel depends on Dmoesin phosphorylation.
To address the influence of Dmoesin phosphorylation, extracts were immunoprecipitated with an antibody directed against an evolutionarily conserved COOH-terminal peptide of ERM (Polesello et al., 2002) that specifically recognizes the phosphorylated T559 of Dmoesin (designated hereafter as α-phospho-ERM). Protein complexes precipitated with α-phospho-ERM were then analyzed on Western blots probed with αDmoesin. Although Dmoesin was readily detected in extracts of dark-raised flies, Dmoesin staining was strongly reduced in extracts from illuminated WT flies treated under identical conditions (Fig. 6 A, left). In addition, the TRP channel and the INAD scaffold protein that binds to TRP were also detected only in head extracts of dark-raised flies immunoprecipitated with α-phospho-ERM (Fig. 6 A, right). These results are consistent with our previous findings, which demonstrated that Dmoesin only interacts with TRP in dark-raised flies. To support this notion, extracts were immunoprecipitated with αTRP and analyzed on Western blots probed with α-phospho-ERM (Fig. 6 B). Although α-phospho-ERM staining was detected in the protein complexes of dark-raised head extracts precipitated with αTRP, α-phospho-ERM staining was strongly reduced in the protein complexes of illuminated flies (Fig. 6 B, left). In control experiments, no α-phospho-ERM staining was detected in head extracts of dark-raised trpP343 null mutant or in NIS-precipitated WT membranes (Fig. 6 B, middle and right).
Figure 6. Light- and phosphorylation-dependent interactions between Dmoesin and the TRP channel.
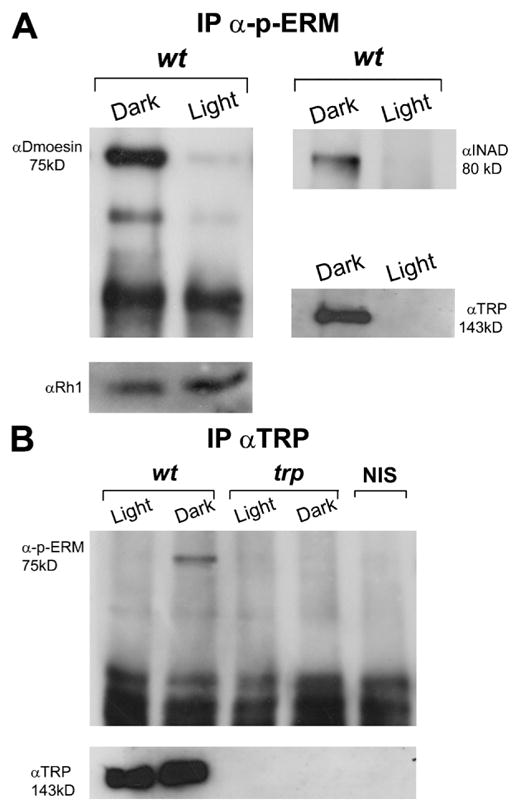
(A) Immunoprecipitation of D. melanogaster head extracts using α-phospho-ERM. Extracts were prepared from fly heads of dark-raised and illuminated WT flies and protein complexes were probed with αDmoesin (left) and with αINAD or αTRP (right) in a separate experiment. (bottom) Western blot analysis of the same head extracts probed with the major rhodopsin, αRh1. To detect TRP and INAD proteins in the immune complex, a threefold larger amount of head extracts were used (n = 5). (B) The experiments in A were repeated exactly, except that αTRP was used for the immunoprecipitation from head extracts of WT and trpP343 mutant, and protein complexes were probed with α-phospho-ERM. (bottom) Western blot of the same head extracts probed with αTRP. The two right lanes are Western blots from WT and trpP343 head extract probed with α-phospho-ERM (n = 4).
Together, the results suggest that light-activated dephosphorylation of channel-bound Dmoesin triggers its dissociation and movement from the membrane to the cytosol.
To directly visualize intracellular movements of Dmoesin in photoreceptors upon illumination, we made use of transgenic lines that allow the expression of functional Dmoesin fused to GFP (Polesello et al., 2002). Dmoesin-GFP fusions were expressed under the control of the Rh1-Gal4 driver, which is specific to mature peripheral R1–6 photoreceptors. Upon application of long wavelength excitation light that elicits a strong autofluorescence of the rhabdomeres (without excitation of the GFP), locations and dimensions of the rhabdomeres in each ommatidium are readily observed in the living retina. The typical structure of the ommatidium is visible as seven red circles, representing the R1–6 peripheral rhabdomeres and the smaller R7 rhabdomere, at the center. Live retinae were dissected under dim red light, and the sub-cellular localization of Dmoesin-GFP was examined with confocal microscopy. Fig. 7 A shows a representative image of ommatidia from dark-raised flies expressing Dmoesin-WT-GFP, which localizes to the rhabdomeres and cortical actin of R1–6 photoreceptors. Because of variability in the expression levels of Dmoesin-GFP in the various ommatidia, some photoreceptor cells did not express Dmoesin (Fig. 7 A). In the photoreceptor cells that did express Dmoesin-GFP, the intense fluorescence of the GFP masked the weaker red autofluorescence and the merged images appeared green. When the level of Dmoesin-GFP in the rhabdomeres was reduced (Fig. 7 F) a yellow color appeared in the merged images. The lack of green fluorescence from the central R7 rhabdomere (which does not express Dmoesin-GFP) provides an internal negative control. After illumination a marked redistribution of Dmoesin-GFP is observed, with the green fluorescence moving from the rhabdomeres to the cell body region (Fig. 7 B). Although there are some differences in the detailed localization and movement of the native Dmoesin (Fig. 2) and Dmoesin-WT-GFP, the results of Fig. 7 confirm our interpretation and clearly indicate that illumination induces a redistribution of the Dmoesin protein from the rhabdomere to the cytoplasm of the cell body.
Figure 7. Light- and phosphorylation-dependent movement of Dmoesin from the rhabdomere to the cell body.
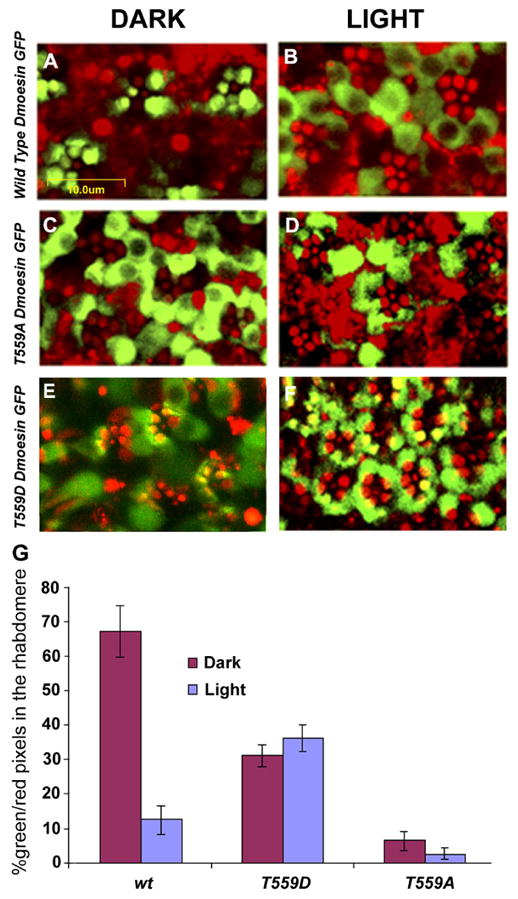
(A–F) Intracellular distribution of Dmoesin-GFP protein fusions, as observed in confocal micrograph cross sections of living D. melanogaster retinae of the transgenic lines: UAS Dmoesin-WT-GFP (A and B), UAS Dmoesin-T559A -GFP (C and D), and UAS Dmoesin-T559D-GFP (E and F). Green indicates GFP fluorescence. The strong autofluorescence of the rhabdomeres (red) allows localizing Dmoesin distribution with respect to photoreceptor compartments. Dark-raised flies were kept in obscurity (A, C, and E) or submitted to blue light illumination (B, D, and F). In flies expressing Dmoesin-WT-GFP (A and B), the fluorescent protein moves from the rhabdomere and cortical actin regions to the cell body of photoreceptors in response to light. Almost all Dmoesin-T559A-GFP (C and D) proteins accumulate outside of the rhabdomeres, independently of the illumination regime, and a significant fraction of Dmoesin-T559D-GFP (E and F) was observed in the rhabdomeres and cortical actin regions regardless of illumination regime. (G) The histogram plots the ratio of the number of green (GFP) to red (autofluorescence) pixels in the rhabdomere and cortical actin regions, as defined by the area that displays autofluorescence. P < 0.01; n = 20 for each fly strain. The error bars are SEM.
To address the influence of T559 phosphorylation on Dmoesin light-induced movement as observed in vivo, we expressed variant Dmoesin proteins with point mutations. The T559A mutation prevents phosphorylation of Dmoesin to keep it in the dormant state, whereas the T559D mutation is a phosphomimetic mutation that is expected to prevent phosphorylation but to keep the molecule in the “open” (presumably active) state (Polesello et al., 2002). When compared with Dmoesin-WT-GFP, distribution of Dmoesin-T559A-GFP in dark-raised flies showed a marked difference, with the major fraction of the GFP fluorescence in the cell body (Fig. 7 C), which is reminiscent of illuminated Dmoesin-WT-GFP flies (Fig. 7 B). In addition, illumination did not induce a significant change in the subcellular distribution of the Dmoesin phosphomutant T559A (Fig. 7 D). Dmoesin-T559D-GFP of dark-raised mutants showed intermediate distribution between rhabdomeres and the cell body. Although GFP fluorescence in the rhabdomere and cortical actin regions was significantly reduced relative to WT retina, it was much higher relative to the Dmoesin-T559A mutant (Fig. 7 E). Quantification of GFP colocalization with the rhabdomere autofluorescence confirmed that the Dmoesin-WT present in rhabdomeres of dark-raised flies was drastically reduced (greater than fivefold) upon illumination (Fig. 7 G). In contrast, most of Dmoesin-T559A fluorescence was not confined to the rhabdomeres in dark-raised retinae, and there was no significant movement after light exposure. Also, similar levels of Dmoesin-T559D were observed in both dark- and light-raised retinae. Thus, these data support the conclusion that phosphorylation of T559 controls the rhabdomeric localization of Dmoesin and that dephosphorylation controls its light-activated movement to the cell body.
To support this interpretation using a different approach, we analyzed the impact of T559 mutations on Dmoesin movement through biochemical characterization. The membrane and soluble fractions of head extracts from flies expressing Dmoesin-WT-GFP, Dmoesin-T559A-GFP, and Dmoesin-T559D-GFP were fractionated by SDS-PAGE and analyzed by Western blots using anti-GFP antibodies (Fig. 8 A). Although illumination reduced the Dmoesin level in the membrane fraction and concomitantly increased Dmoesin levels in the cytosol of Dmoesin-WT-GFP, the distribution of phosphorylation-defective Dmoesin mutants was unmodified by light (Fig. 8, A and B). As expected, the major fraction of Dmoesin-T559A-GFP was restricted to the soluble fraction, whereas the Dmoesin-T559D-GFP appeared in both the membrane-associated and the cytosol fractions (Fig. 8).
Figure 8. The light-dependent movement of Dmoesin from the membrane to the cytosol is blocked in the Dmoesin-T559A and Dmoesin-T559D mutants.
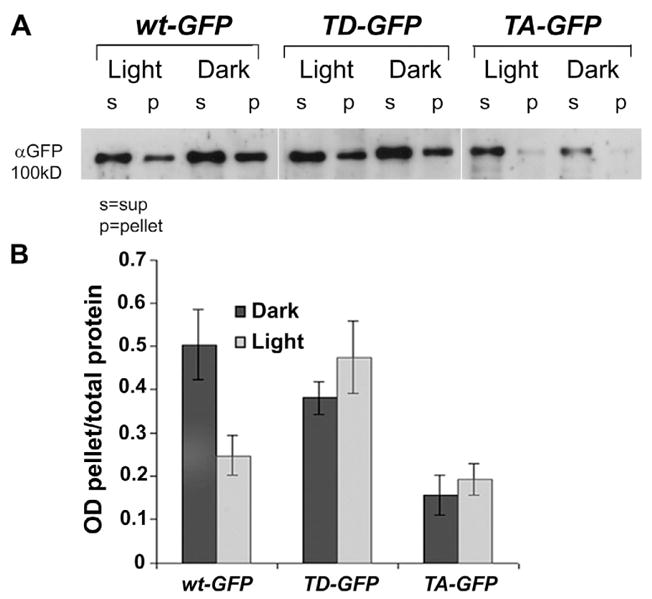
(A) Western blot analysis of Dmoesin distribution in membrane-bound or -soluble fractions of D. melanogaster head protein extracts. Membrane and soluble proteins extracted from dark-raised and illuminated Dmoesin-GFP transgenic lines were Western blotted using αGFP. Extracts were prepared from the same fly strains as Fig. 7, as indicated. (B) The histogram plots the ratio of membrane-bound to total Dmoesin signals from replicate experiments similar to that shown in A. Although illumination halves levels of WT Dmoesin in association with membranes (P < 0.01; n = 3), no significant modification of Dmoesin distribution is provoked by illumination of Dmoesin-T559A-GFP and Dmoesin-T559D-GFP. The error bars are SEM.
Together, these results demonstrate that the phosphorylation/dephosphorylation reactions of T559 regulate light-induced subcellular movement of Dmoesin in photoreceptors. In dark-raised flies, Dmoesin binds to the channels in the rhabdomere and illumination induces both Dmoesin dephosphorylation and relocation to the photoreceptor cytoplasm. Consistent with this interpretation, mutations impairing T559 phosphorylation either prevent (T559A) or reduce (T559D) association of Dmoesin with the rhabdomere. These mutations totally block intracellular redistribution of the protein in response to light.
Estimation of Dmoesin dynamics was obtained from Western blot analysis in which the reduction of Dmoesin levels in the membrane fraction and increase in the soluble fraction were measured after increasing durations of illumination (Fig. S2, available online at http://www.jcb.org/cgi/content/full/jcb.200503014/DC1). After 5 min of illumination, a significant fraction of Dmoesin was still detectable in the membranes. After 10, 30, and 60 min of light exposure, the level of Dmoesin further decreased in the membranes and increased in the soluble fraction (Fig. S2). Thus, the time scale of Dmoesin movement from the rhabdomere is similar to the time scale of TRPL translocation (Bähner et al., 2002).
Impaired phosphorylation of Dmoesin leads to degeneration of photoreceptor cells during prolonged illumination
Together with the redistribution of signaling molecules, we examined if the light-induced movement of Dmoesin was important for the physiology of photoreceptors. We examined the effects of Dmoesin mutations that impair T559 phosphorylation on the retinal structure after extended exposure to light. As prolonged illumination produces toxic effects in photoreceptors expressing GFP (unpublished data), we used transgenic lines carrying the same mutations, with the exception of Dmoesin, which was tagged with the myc epitope instead of GFP (Speck et al., 2003).
Newly eclosed flies expressing WT, T559A, or T559D Dmoesin proteins were subjected to constant light for 2, 4, and 7 d, and the ultrastructure of the ommatidia was analyzed by transmission electron microscopy. Ommatidia of flies expressing Dmoesin-WT-myc were indistinguishable from WT controls and presented a well organized ommatidium after 7-d illumination (Fig. 9, A and B). In contrast, the retinae of flies expressing T559A Dmoesin-myc were abnormal and revealed the initial stages of degeneration (Rubinstein et al., 1989) after only 2-d illumination (Fig. 9, E and F). Although the ommatidia of the illuminated T559D mutants appeared normal after 2-d illumination, slight degeneration was visible after 4-d illumination and a significant degeneration appeared after 7-d illumination (Fig. 9, C and D). Control experiments in which flies that expressed either WT or mutant Dmoesin forms were kept in the dark and did not show any sign of retinal degeneration (unpublished data).
Figure 9. Impaired phosphorylation of Dmoesin leads to degeneration of photoreceptor cells during prolonged illumination.
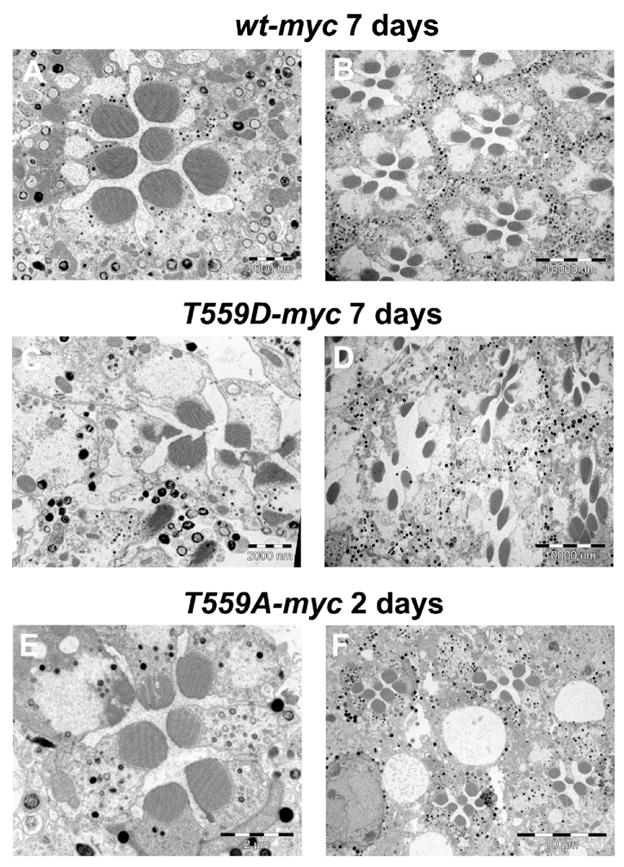
Electron micrographs showing the ultrastructural organization of ommatidia after 2- or 7-d-long white illumination. Eye sections from D. melanogaster expressing Dmoesin-WT-myc (A and B), Dmoesin-T559D-myc (C and D), and Dmoesin-T559A-myc (E and F).
These data suggest that the dynamic regulation of Dmoesin phosphorylation is critical for photoreceptor viability upon illumination. The slower degeneration of T559D mutants suggests that the presence of “active” Dmoesin in the rhabdomere is probably necessary, but not sufficient, to prevent degeneration during prolonged illumination and that dynamic phosphorylation/dephosphorylation reactions are required to prevent degeneration.
Discussion
Since their initial discovery in the 1980s, the ERM family of proteins has been implicated in numerous aspects of the control of actin organization (for review see Bretscher et al., 2002). Interestingly, ERM proteins are activated by signaling pathways to bridge actin filaments to membrane receptors and channels, thereby constituting a signal-dependent regulation of the cytoskeleton–membrane interface. However, functional analysis of ERM proteins during vertebrate development has been hampered by the functional redundancy of the three ERM paralogs. As their genome contains a unique ERM gene, Caenorhabditis elegans and D. melanogaster have become alternative model systems that recently revealed the important developmental roles of ERM proteins (Polesello and Payre, 2004). Several lines of evidence implicate Dmoesin in the regulation of the actin cytoskeleton throughout the stages of fly development (Jankovics et al., 2002; Polesello et al., 2002; Speck et al., 2003), including eye development (Karagiosis and Ready, 2004). This study extends the functional analysis of ERM proteins to investigate their role in the physiology of mature eyes. Our results provide novel data on the mechanism by which illumination may initiate reorganization of the cytoskeleton and suggest that the light-induced regulation of Dmoesin distribution is required to protect illuminated photoreceptors from degeneration.
Light- and phosphorylation-dependent interaction of Dmoesin with the light-activated channels TRP and TRPL
Illumination of D. melanogaster photoreceptor cells induces multiple molecular responses, which are initiated in the rhabdomere. Actin has been reported to undergo light-induced reorganization in both squid (Tsukita and Matsumoto, 1988) and D. melanogaster photoreceptors (Kosloff et al., 2003), thus showing that light-sensitive cytoskeletal rearrangements are a common phenomenon. However, it remains unclear how illumination can modify the intracellular distribution of both signaling and cytoskeletal molecules. As a step toward understanding the molecular mechanisms that underlie these aspects of the light response in D. melanogaster photoreceptors, we analyzed the potential role of Dmoesin in this process.
In dark-raised flies, Dmoesin interacts with both the TRP and TRPL channels, as evidenced by reciprocal coimmunoprecipitation experiments. In contrast, virtually no Dmoesin–TRP and –TRPL complexes are coimmunoprecipitated from illuminated eyes, thus providing strong evidence for Dmoesin binding to the photoreceptor-specific channels primarily in the dark. Furthermore, our results show that light induces dissociation of Dmoesin from TRP and TRPL channels followed by movement of Dmoesin from the rhabdomere membranes to the cytoplasm. As there is increasing evidence to suggest that functions of invertebrate TRPs are conserved in their mammalian counterparts, our findings might provide new insights for characterizing vertebrate TRP functions. Interestingly, TRPC3 is part of a multimolecular signaling complex containing Ezrin, PLCβ1, and Gαq/11 that is involved in Ca2+-mediated regulation of channel activity and cytoskeletal reorganization (Lockwich et al., 2001). In addition, it has been shown that the ERM adaptor EBP50/Na+/H+ exchanger regulatory factor associates with PLCβ, TRPC4, and TRPC5 and regulates channel activity and sub-cellular localization (Tang et al., 2000; Mery et al., 2002; Obukhov and Nowycky, 2004). Altogether, these data strongly suggest that TRP–ERM interactions are an evolutionarily conserved mechanism with important functional properties. Our ability to modify Dmoesin binding to TRPs in vivo using illumination should constitute an invaluable tool for investigating the molecular mechanisms regulating this interaction.
In this study, the critical role of T559 phosphorylation on Dmoesin activation (Polesello et al., 2002; Speck et al., 2003) was extended through the demonstration that dissociation of the Dmoesin from the channel proteins upon illumination depends on T559 dephosphorylation. Accordingly, specific antibodies for the phosphorylated T559 form of Dmoesin immunoprecipitated the TRP channel of dark-raised flies, but not of illuminated flies. Moreover, monospecific TRP antibody immuno-precipitated the phosphorylated form of Dmoesin only in dark-raised flies. These results strongly suggest that only the phosphorylated form of Dmoesin binds TRP (Fig. 10). This finding further suggests that light induces dephosphorylation of Dmoesin, leading to dissociation of Dmoesin from the channel proteins, followed by its movement to the cell body.
Figure 10. A scheme that summarizes the subcellular organization of Dmoesin and the major signaling proteins in the rhabdomeric membrane.
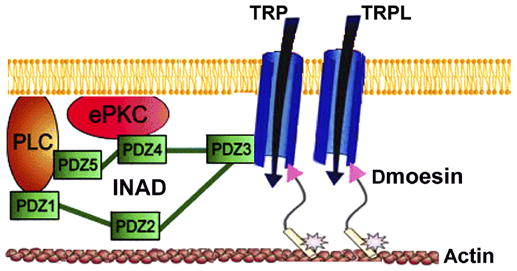
TRP anchors the INAD signaling complex, which includes PLC and eyePKC (ePKC), to the plasma membrane via the PDZ3 domain of INAD. The NH2-terminal region of Dmoesin molecules (arrowheads) bind either directly or through a PDZ adaptor protein to the TRP and TRPL channels, whereas the COOH-terminal region is bound to the actin cytoskeleton. The phosphorylated site of T559 is indicated by an asterisk.
Using WT and transgenic flies that express Dmoesin-GFP fusion proteins, we directly visualized the light-induced movement of Dmoesin from the rhabdomere to the cell body, through confocal imaging of fixed and living retinae. The critical role of T559 phosphorylation on light-induced Dmoesin movement in vivo was further demonstrated through the use of two mutant forms of Dmoesin, in which T559 was replaced by alanine or aspartate residues. The fact that light-activated movement of Dmoesin was blocked in the T559A mutant that remains localized primarily to the soluble fraction of the cell body strongly supports the conclusion that phosphorylation of T559 is crucial for binding of Dmoesin to the channel proteins. Although the T559A mutation kept Dmoesin in its inactive cytosolic state, the T559D phosphomimetic mutation was expected to keep Dmoesin constitutively active. Although some T559D Dmoesin was also found in the cytosol, we indeed found a significant fraction of T559D Dmoesin in the membrane fraction that remains associated with the rhabdomeres after illumination. In addition, we found that both T559A and T559D mutations blocked the light-dependent movement of Dmoesin.
How could nontrafficking forms of Dmoesin (Dmoesin T559A and T559D) lead to light-induced retinal degeneration when expressed in an otherwise WT background? T559 phosphomutants of the Dmoesin protein have been shown to perturb the role of endogenous Dmoesin in actin organization and Oskar localization during oogenesis (Polesello et al., 2002). We found consistently that expression of DmoesinT559A-GFP and DmoesinT559D-GFP impairs the ability of endogenous Dmoesin to move upon illumination (unpublished data). As ERM proteins are capable of homotypic interaction (usually as dimers; Bretscher et al., 2002), Dmoesin T559A and Dmoesin T559D can titrate either WT Dmoesin or other functional partners. Therefore, the light-induced degeneration observed upon Dmoesin T559A and Dmoesin T559D expression can be explained by this reduction of endogenous Dmoesin traffic.
Altogether, these findings indicate that the rhabdomeric localization of Dmoesin requires its open (active) state, which is achieved either by phosphorylation or by the T559D phosphomimetic mutation. Our results, further, support that light-induced dephosphorylation triggers the movement of Dmoesin to the cytosol, and when this reaction is impaired by mutations, the light dependent movement of Dmoesin is blocked.
The implications of light-induced Dmoesin movement on translocation of signaling molecules, cytoarchitectural changes, and maintenance of the photoreceptor cells
Recent studies have demonstrated reversible light-induced reorganization of the actin cytoskeleton of the microvilli (Kosloff et al., 2003) and translocation of the TRPL channel (Bähner et al., 2002) from the rhabdomere to the cell body in time scales comparable to that of light-induced Dmoesin movement. Therefore, the light-induced movement of Dmoesin is likely involved in the control of the aforementioned processes.
Interestingly, genetic elimination of either signaling protein PLCβ (norpA) or TRP prevents the light-induced movement of Dmoesin. These mutations are known to either block (norpA), or to strongly reduce (trp), the light-induced Ca2+ entry into the photoreceptor cells (Hardie and Minke, 1992; Peretz et al., 1994a). The effect of light on Dmoesin movement could thus be mediated via Ca2+-induced dephosphorylation of Dmoesin; e.g., by activation of a Ca2+-dependent phosphatase. PLCβ-mediated hydrolysis of PIP2 (which is highly enriched in rhabdomere membranes) might also participate in the release of Dmoesin into the cytoplasm upon illumination, as several works have shown the positive effect of PIP2 binding on ERM protein activation, membrane localization, and binding to their partners (Niggli et al., 1995; Barret et al., 2000; Fievet et al., 2004). The data we accumulated in this study indicate the existence of a tight link between light reception and the Dmoesin-mediated reorganization of the rhabdomere cytoarchitecture.
Although the elucidation of the full spectrum of the physiological functions of the light-induced Dmoesin movement now awaits further works, we would like to suggest that these light-induced changes are necessary for the functional maintenance of photoreceptor cells. Photoreceptors are vulnerable cells because of their prolonged interaction with light (Kirschfeld and Franceschini, 1977). The peculiar organization of the rhabdomere in the form of very long (and tightly packed) microvilli makes it difficult for housekeeping mechanisms to operate in the rhabdomere. Light-activated reorganization of actin, along with cytoarchitectural changes, may allow the housekeeping function to operate and/or to participate in the down-regulation of signaling mechanisms triggered by light reception.
Materials and methods
Fly strains and genetics
D. melanogaster of the following strains were used: WT Oregon-R w; trpP343 (Scott et al., 1997) and norpAP24 (Pearn et al., 1996) null mutants for the TRP channel and eye-specific PLCβ, respectively (both obtained from W.L. Pak, Purdue University, West Lafayette, IN); trpl302 (Niemeyer et al., 1996) and inaD1 (obtained from C.S. Zuker, University of California, San Diego, San Diego, CA); and UAS Dmoesin-WT-GFP, UAS Dmoesin-T559A-GFP, UAS Dmoesin-T559D-GFP (Polesello et al., 2002), UAS Dmoesin-WT-myc, UAS Dmoesin-T559A-myc, and UAS Dmoesin-T559D-myc (Speck et al., 2003). Dmoesin variants encoded in the transgenic lines were expressed using the Gal4/UAS targeted expression (Brand and Perrimon, 1993) using the Rh1-Gal4 driver line (obtained from C. Desplan, New York University, New York, NY).
Light-dependent localization of Dmoesin
Flies were raised in complete darkness from the first instar larval stage to eclosion. For illumination experiments, live flies were placed in a transparent dish with reflective aluminum foil at the bottom and subjected to illumination with blue light (18 W fluorescent lamp with a wide band filter [1 mm; model BG 28; Schott]) for various durations at 22°C. Illumination with white light of the same intensity produced similar results. After illumination, the flies were moved to 4°C in the dark and the fly heads were promptly dissected. Three to five flies were used for each lane of the Western blots. Fly heads were homogenized in a hypotonic buffer (50 mM Hepes, pH 7, 300 mM NaCl, 3 mM MgCl, 10% vol/vol glycerol, protease inhibitor [Sigma-Aldrich], phosphatase inhibitor [Sigma-Aldrich], and 1 μM caliculin A [Calbiochem]). Membrane and cytosolic fractions were separated by centrifugation at 15,800 g for 15 min, at 4°C. The pellet was washed and centrifuged again, and the supernatants were combined. Ultracentrifugation at 150,000 g for 30 min did not substantially change the distribution of Dmoesin between the fractions. Protein samples ran on 10% SDS-PAGE and were subjected to Western blotting using anti-Dmoesin or anti-GFP polyclonal antibodies. Quantification of the gels was performed using the BAS-1000 system (Fujifilm Worldwide) with TINA version 2.0 software.
Immunoprecipitation
Frozen heads (500–2,000) of dark-raised or illuminated flies were homogenized in 200 μl of buffer containing 50 mM Hepes, pH 7.6, 150 mM NaCl, 3 mM MgCl2, 10% glycerol, and protease and phosphatase inhibitors (Sigma-Aldrich). The homogenate was centrifuged at 100 g for 5 min to remove chitin materials. Membranes were isolated by centrifugation at 20,000 g for 30 min at 4°C and resuspended to a final equivalent of 200 μl. Membrane proteins were extracted by incubating the membranes with 1% Triton X-100 and 500 mM NaCl for 1 h and centrifuging at 20,000 g for 30 min. For immunoprecipitations, protein A beads were incubated first with the relevant antibody (αTRP, αTRPL, αGFP, αINAD, or αDmoesin) overnight at 4°C. The membrane extracts (of WT, inaD, trp, norpA, and trpl mutants) were incubated with the relevant antibody crosslinked to protein A beads (vol 20–100 μl) in 200 μl of total volume overnight at 4°C. The beads were washed in the Triton X-100 washing buffer (0.1% Triton X-100, 100 mM NaCl, and 50 mM Tris-HCl, pH 8.0). Immunoprecipitated proteins were eluted from protein A–agarose beads with 50 μl of 1× SDS-PAGE loading buffer and subsequently analyzed by Western blot.
Immunoblots were incubated with primary antibodies in blocking buffer at the following dilutions: monoclonal mouse αTRP (Pollock et al., 1995) and mAb83F36 (obtained from S. Benzer, California Institute of Technology, Pasadena, CA), 1:2,000; rabbit αDmoesin (obtained from D. Keihart, Duke University, Durham, NC), 1:2,000; rabbit αINAD, 1:1,000; rabbit α-Phospho-ERM (Cell Signaling Technology), 1:2,000; mouse αTRPL, αRh1, and αChaoptin (Hybridoma Bank), 1:1,000. Immunoreactive bands were visualized by chemiluminescence reaction (obtained from Biological Industries), using HRP-conjugated goat anti–rabbit and anti–mouse as secondary antibodies (Jackson ImmunoResearch Laboratories).
EM
Fly heads were separated, bisected longitudinally, and fixed for 12 h in a solution of 1.5% PFA and 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. The heads were washed three times in the same phosphate buffer and dehydrated in graded aqueous ethanol concentrations of up to 90%. After fixation, eyes were post-fixed with 1% osmium tetroxide for 4 h, dehydrated in ethanol and propylene oxide, and embedded in Epon. Thin sections were cut and stained with saturated aqueous uranyl acetate and lead citrate. Sections were observed with a Tecnai-12 transmission electron microscope (FEI) and photographed with a Mega-view II charge-coupled device camera (Philips).
Fluorescent confocal microscopy
Live retinae from dark-raised or illuminated transgenic flies were isolated from the cornea and brain and kept in Ringer’s solution as described previously (Peretz et al., 1994b). Optical sections of single ommatidia were visualized using the Fluoview confocal microscope (model 200 IX70; Olympus) using a LUM Plano Fl 60×, 0.9 NA, water objective. Optical sections were recorded from the upper region of the ommatidia, at a depth of 6–10 μm from the tip of the ommatidium. Autofluorescence and GFP fluorescence were recorded sequentially using laser excitation wavelengths of 568 and 488 nm, respectively. Pictures are merged sequential images obtained by 568- followed by 488-nm excitation lights, on separate channels.
Immunocytochemistry
Dissected eyes of yw D. melanogaster or norpAP24 mutant in w background were fixed in 2% PFA in PBS (175 mM NaCl, 8 mM Na2HPO4, and 1.8 mM NaH2PO4, pH 7.2) for 1 h at RT, and then washed two times in 0.1 M phosphate buffer (0.1 M Na2HPO4 and 0.1 M NaH2PO4). This was followed by three washes in 10% sucrose and two washes in 25% sucrose for 15 min each. Eyes were then infiltrated with 50% sucrose overnight at 4°C, cryofixed in melting pentane, and sectioned at 10 μm thickness in a cryostat (Mikrom Laborgeräte GmbH) at −25°C. The cryosections were incubated in 2% PFA in PBS for 10 min, washed two times in PBS, and then blocked in 1% BSA and 0.3% Triton X-100 in PBS (PBS-T) for 2 h at RT. The sections were incubated with α-Dmoesin diluted 1:2,000 or α-phospho-ERM diluted1:50 in PBS-T overnight at 4°C. The sections were subsequently washed three times in PBS and were incubated with a Cy5-coupled secondary goat anti–rabbit antibody (Dianova) and rhodamin-coupled phalloidin (Sigma-Aldrich) in 0.5% fish gelatine and 0.1% ovalbumin in PBS for at least 4 h at RT. The sections were finally washed three times in PBS, mounted in Mowiol 4.88 (Polyscience), and examined with a confocal laser-scanning microscope (LSM-SP).
Acknowledgments
We thank D. Kiehart, R. Montague, and S. Benzer for their generous gift of antibodies. We also gratefully thank C.S. Zuker, W.L. Pak, C. Desplan, and R.G. Fehon for providing us fly strains. We thank R. Timberg for the skilful EM pictures and D. Hyde and Z. Selinger for stimulating discussions and critical reading of the manuscript.
Abbreviations used in this paper
- EBP50
ezrin binding phosphoprotein 50
- ERM
ezrin-radixin-moesin
- INAD
inactivation-no-afterpotential D
- NIS
non-immune serum
- PDZ
PSD95/DlgA/ZO-1 homology
- PIP2
phosphatidylinositol 4,5-bisphosphate
- T559
threonine 559
- TRP
transient receptor potential
- TRPL
TRP-like
- WT
wild-type
Footnotes
Publisher's Disclaimer: This article is an un-copyedited author manuscript that has been accepted for publication in The Journal of Neuroscience, copyright 2006 Society for Neuroscience. The Society for Neuroscience disclaims any responsibility or liability for errors or omissions in this version of the manuscript or any version derived from it by NIH or other parties.
Online supplemental material
Fig. S1 shows that Dmoesin of eyeless D. melanogaster heads does not display light-dependent movement from the membrane to the cytosol. Fig. S2 depicts the kinetics of light-dependent movement of Dmoesin from the rhabdomere to the cell body and time-course analysis of Dmoesin redistribution upon illumination, as observed in Western blot analysis. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200503014/DC1.
The online version of this article contains supplemental material.
This research was supported by grants from the National Institutes of Health (EY 03529), the Israel Science Foundation, the Minerva Foundation, the US–Israel Binational Science Foundation, the Israel–Korea Cooperation Program, the Ministère de la Recherche (ACI n° 030023), the German Israeli Foundation, and the Deutsche Forschungsgemeinschaft (Hu 839/2–4).
References
- Bähner M, Frechter S, Da Silva N, Minke B, Paulsen R, Huber A. Light-regulated subcellular translocation of Drosophila TRPL channels induces long-term adaptation and modifies the light-induced current. Neuron. 2002;34:83–93. doi: 10.1016/s0896-6273(02)00630-x. [DOI] [PubMed] [Google Scholar]
- Barret C, Roy C, Montcourrier P, Mangeat P, Niggli V. Mutagenesis of the phosphatidylinositol 4,5-bisphosphate (PIP2) binding site in the NH2-terminal domain of ezrin correlates with its altered cellular distribution. J Cell Biol. 2000;151:1067–1080. doi: 10.1083/jcb.151.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- Chou WH, Hall KJ, Wilson DB, Wideman CL, Townson SM, Chadwell LV, Britt SG. Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron. 1996;17:1101–1115. doi: 10.1016/s0896-6273(00)80243-3. [DOI] [PubMed] [Google Scholar]
- Chevesich J, Kreuz AJ, Montell C. Requirement for the PDZ domain protein, INAD, for localization of the TRP store-operated channel to a signaling complex. Neuron. 1997;18:95–105. doi: 10.1016/s0896-6273(01)80049-0. [DOI] [PubMed] [Google Scholar]
- Fievet BT, Gautreau A, Roy C, Del Maestro L, Mangeat P, Louvard D, Arpin M. Phosphoinositide binding and phosphorylation act sequentially in the activation mechanism of ezrin. J Cell Biol. 2004;164:653–659. doi: 10.1083/jcb.200307032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC, Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992;8:643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Hardie RC, Minke B. Phosphoinositide-mediated phototransduction in Drosophila photoreceptors: the role of Ca2+ and trp. Cell Calcium. 1995;18:256–274. doi: 10.1016/0143-4160(95)90023-3. [DOI] [PubMed] [Google Scholar]
- Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita S. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber A, Sander P, Gobert A, Bähner M, Hermann R, Paulsen R. The transient receptor potential protein (Trp), a putative store-operated Ca2+ channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC and InaD. EMBO J. 1996;15:7036–7045. [PMC free article] [PubMed] [Google Scholar]
- Jankovics F, Sinka R, Lukacsovich T, Erdelyi M. MOESIN crosslinks actin and cell membrane in Drosophila oocytes and is required for OSKAR anchoring. Curr Biol. 2002;12:2060–2065. doi: 10.1016/s0960-9822(02)01256-3. [DOI] [PubMed] [Google Scholar]
- Karagiosis SA, Ready DF. Moesin contributes an essential structural role in Drosophila photoreceptor morphogenesis. Development. 2004;131:725–732. doi: 10.1242/dev.00976. [DOI] [PubMed] [Google Scholar]
- Kirschfeld K, Franceschini N. Photostable pigments within the membrane of photoreceptors and their possible role. Biophys Struct Mech. 1977;3:191–194. doi: 10.1007/BF00535818. [DOI] [PubMed] [Google Scholar]
- Kosloff M, Elia N, Joel-Almagor T, Timberg R, Zars TD, Hyde DR, Minke B, Selinger Z. Regulation of light-dependent Gqalpha translocation and morphological changes in fly photoreceptors. EMBO J. 2003;22:459–468. doi: 10.1093/emboj/cdg054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwich T, Singh BB, Liu X, Ambudkar IS. Stabilization of cortical actin induces internalization of transient receptor potential 3 (Trp3)-associated caveolar Ca2+ signaling complex and loss of Ca2+ influx without disruption of Trp3-inositol trisphosphate receptor association. J Biol Chem. 2001;276:42401–42408. doi: 10.1074/jbc.M106956200. [DOI] [PubMed] [Google Scholar]
- Mery L, Strauss B, Dufour JF, Krause KH, Hoth M. The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci. 2002;115:3497–3508. doi: 10.1242/jcs.115.17.3497. [DOI] [PubMed] [Google Scholar]
- Minke B, Agam K. TRP gating is linked to the metabolic state and maintenance of the Drosophila photoreceptor cells. Cell Calcium. 2003;33:395–408. doi: 10.1016/s0143-4160(03)00052-6. [DOI] [PubMed] [Google Scholar]
- Montell C. TRP trapped in fly signaling web. Curr Opin Neurobiol. 1998;8:389–397. doi: 10.1016/s0959-4388(98)80066-4. [DOI] [PubMed] [Google Scholar]
- Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- Niggli V, Andreoli C, Roy C, Mangeat P. Identification of a phosphatidylinositol-4,5-bisphosphate-binding domain in the N-terminal region of ezrin. FEBS Lett. 1995;376:172–176. doi: 10.1016/0014-5793(95)01270-1. [DOI] [PubMed] [Google Scholar]
- Obukhov AG, Nowycky MC. TRPC5 activation kinetics are modulated by the scaffolding protein ezrin/radixin/moesin-binding phosphoprotein-50 (EBP50) J Cell Physiol. 2004;201:227–235. doi: 10.1002/jcp.20057. [DOI] [PubMed] [Google Scholar]
- Pearn MT, Randall LL, Shortridge RD, Burg MG, Pak WL. Molecular, biochemical, and electrophysiological characterization of Drosophila norpA mutants. J Biol Chem. 1996;271:4937–4945. doi: 10.1074/jbc.271.9.4937. [DOI] [PubMed] [Google Scholar]
- Peretz A, Sandler C, Kirschfeld K, Hardie RC, Minke B. Genetic dissection of light-induced Ca2+ influx into Drosophila photoreceptors. J Gen Physiol. 1994a;104:1057–1077. doi: 10.1085/jgp.104.6.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A, Suss-Toby E, Rom-Glas A, Arnon A, Payne R, Minke B. The light response of Drosophila photoreceptors is accompanied by an increase in cellular calcium: effects of specific mutations. Neuron. 1994b;12:1257–1267. doi: 10.1016/0896-6273(94)90442-1. [DOI] [PubMed] [Google Scholar]
- Phillips AM, Bull A, Kelly LE. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992;8:631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- Polesello C, Payre F. Small is beautiful: what flies tell us about ERM protein function in development. Trends Cell Biol. 2004;14:294–302. doi: 10.1016/j.tcb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Polesello C, Delon I, Valenti P, Ferrer P, Payre F. Dmoesin controls actin-based cell shape and polarity during Drosophila melanogaster oogenesis. Nat Cell Biol. 2002;4:782–789. doi: 10.1038/ncb856. [DOI] [PubMed] [Google Scholar]
- Pollock JA, Assaf A, Peretz A, Nichols CD, Mojet MH, Hardie RC, Minke B. TRP, a protein essential for inositide-mediated Ca2+ influx is localized adjacent to the calcium stores in Drosophila photoreceptors. J Neurosci. 1995;15:3747–3760. doi: 10.1523/JNEUROSCI.15-05-03747.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready DF, Hanson TE, Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976;53:217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Rubinstein CT, Bar-Nachum S, Selinger Z, Minke B. Chemically induced retinal degeneration in the rdgB (retinal degeneration B) mutant of Drosophila. Vis Neurosci. 1989;2:541–551. doi: 10.1017/s0952523800003485. [DOI] [PubMed] [Google Scholar]
- Scott K, Sun Y, Beckingham K, Zuker CS. Calmodulin regulation of Drosophila light-activated channels and receptor function mediates termination of the light response in vivo. Cell. 1997;91:375–383. doi: 10.1016/s0092-8674(00)80421-3. [DOI] [PubMed] [Google Scholar]
- Shieh BH, Zhu MY. Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron. 1996;16:991–998. doi: 10.1016/s0896-6273(00)80122-1. [DOI] [PubMed] [Google Scholar]
- Shieh BH, Zhu MY, Lee JK, Kelly IM, Bahiraei F. Association of INAD with NORPA is essential for controlled activation and deactivation of Drosophila phototransduction in vivo. Proc Natl Acad Sci USA. 1997;94:12682–12687. doi: 10.1073/pnas.94.23.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck O, Hughes SC, Noren NK, Kulikauskas RM, Fehon RG. Moesin functions antagonistically to the Rho pathway to maintain epithelial integrity. Nature. 2003;421:83–87. doi: 10.1038/nature01295. [DOI] [PubMed] [Google Scholar]
- Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, Ramesh V, Zhu MX. Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J Biol Chem. 2000;275:37559–37564. doi: 10.1074/jbc.M006635200. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Matsumoto G. Light-induced structural changes of cytoskeleton in squid photoreceptor microvilli detected by rapid-freeze method. J Cell Biol. 1988;106:1151–1160. doi: 10.1083/jcb.106.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, Socolich M, Zuker CS. A multivalent PDZ-domain protein assembles signalling complexes in a G-protein-coupled cascade. Nature. 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- Van Vactor D, Jr, Krantz DE, Reinke R, Zipursky SL. Analysis of mutants in chaoptin, a photoreceptor cell-specific glycoprotein in Drosophila, reveals its role in cellular morphogenesis. Cell. 1988;52:281–290. doi: 10.1016/0092-8674(88)90517-x. [DOI] [PubMed] [Google Scholar]


