Abstract
Gαi/o protein-coupled dopamine D3 receptors (D3Rs) are preferentially expressed in the limbic system, including the nucleus accumbens. This situates the receptor well in the regulation of limbic function and in the pathogenesis of various neuropsychiatric and neurodegenerative disorders. The intracellular domains of the receptor, mainly the large third intracellular loop and the intracellular C-terminal tail, interact with multiple submembranous proteins. These interactions are critical for the control of surface expression of the receptor and the efficacy of receptor signaling. Recently, a synapse-enriched protein kinase, Ca2+/calmodulin-dependent protein kinase II (CaMKII), has been found to interact with D3R in the above mentioned interaction model. CaMKII directly binds to the N-terminal of the third loop of D3R. This binding is Ca2+-dependent and is sustained by the autophosphorylation of the kinase. In rat accumbal neurons, the increase in Ca2+ level induces the recruitment of CaMKII to D3R, and CaMKII phosphorylates the receptor at a specific serine site. The CaMKII-induced phosphorylation could inhibit the receptor function and further regulate the behavioral response to the psychostimulant cocaine. These findings reveal a prototypic protein association model between a G protein-coupled receptor and CaMKII. Through the dynamic protein-protein interactions, the abundance, turnover cycle, and function of D3R can be regulated by multiple signals and enzymatic proteins.
Keywords: striatum, caudate, phosphorylation, cocaine, addiction, cAMP, CaMKII
1 Introduction
Dopamine receptors are a family of G protein-coupled receptors. Through Gαs (D1 and D5) and Gαi/o (D2, D3, and D4) proteins, these receptors response to the neurotransmitter dopamine stimulation and regulate a variety of neuronal and synaptic activities[1]. Among the 5 dopamine receptor subtypes, the dopamine D3 receptor (D3R) is unique in several aspects. Firstly, D3R is preferentially expressed in the mesolimbic dopaminergic projection areas, including the nucleus accumbens[2,3], while D1 and D2 receptors are much more broadly expressed in the entire brain. Such a distinct distribution pattern suggests a significant role of D3R in the multiple limbic functions related to reward, motivation, cognition, emotion, and extrapyramidal motor control[4,5]. Secondly, the third intracellular loop of D3R is large, which provides an intrinsic structural domain for its dynamic interactions with many membrane-bound or submembranous cytosol proteins. Indeed, several proteins have been identified to directly bind to the third intracellular loop. Such binding is critical for the control of the expression and/or function of the receptor. Finally, D3R is less investigated compared to other subtypes of dopamine receptors, especially D1 and D2 receptors. Studies are further needed to elucidate its roles in the regulation of limbic functions and in the pathogenesis of various neuropsychiatric and motor disorders, such as schizophrenia, depression, substance addiction, and Parkinson’s disease.
2 Interactions involving non-phosphorylation of D3R
Several proteins have been found to interact with D3R, although they have no direct effects on the phosphorylation status of the receptor. Filamin A, a protein ubiquitously expressed in neurons, has been revealed to directly interact with the intracellular loop of D3R[6]. It cross-links with the structural protein actin, which promotes orthogonal branching of actin filaments and links membrane glycoproteins to the actin cytoskeleton[7]. Using the yeast two-hybrid approach, Lin et al. have identified that filamin A interacts with the intracellular third loop of D3R[6], although the biological significance is unclear. However, based on the fact that the interaction between filamin A and D2 receptors stabilizes D2 receptors at the plasma membrane[6], it is speculated that the interaction between filamin A and D3R may control the receptor expression in the cell surface.
Another cytoskeletal protein, protein 4.1N, has also been demonstrated to interact with D3R[8]. Protein 4.1N is a member of the 4.1 family of cytoskeletal-associated proteins and is specifically enriched in neurons of mammalian brain[9]. As a critical component of the spectrin-actin cytoskeleton, it offers attachment between the cytoskeleton and the cell plasma membrane. Data from yeast two-hybrid screen, pulldown assays, and co-immunoprecipitation reveal that the C-terminal region of protein 4.1N interacts with the N-terminal region of the third intracellular domain of D3R. In transfected HEK293 cells and mouse neuroblastoma Neuro2A cells, protein 4.1N and D3R are co-expressed at the plasma membrane. The mutation in protein 4.1N which causes the lack of membrane-binding domain can reduce the surface expression of D3R. Thus, the interaction between protein 4.1N and D3R is critical for the stability of D3R at the plasma membrane.
Paralemmin is a lipid-anchoring protein enriched in neuronal plasma membranes at synaptic sites. It regulates plasma membrane dynamics and neuronal plasticity[10]. Recently, it has been indicated to interact with D3R[11]. However, unlike aforementioned filamin A and protein 4.1N, paralemmin decreases the D3R level at the plasma membrane in HEK293 cells, which indicates that paralemmin serves to limit surface D3R expression, either through accelerating internalization of surface-expressed D3R, or through diminishing the externalization of intracellular D3R to the plasma membrane.
The intracellular C-terminal region of D3R also harbors binding sites for other proteins. Being a member of the chloride intracellular channel (CLIC) family, the rat CLIC6 specifically interacts with D2-like receptors, including D3R[12]. Anther protein that has been identified to interact with the C-terminal of D3R is GAIP interacting protein, C terminus (GIPC)[13]. Functionally, as a PDZ (PSD-95/Dig/ZO-1) domain-containing protein, GIPC may uncouple D3R from its signaling cascade and subsequently link it to the cytoskeleton, leading to receptor sequestration in vesicles and protecting against degradation[13]. Finally, at the receptor level, D3 and D1 receptors may form a receptor heteromer in striatal neurons[14]. This receptor complex represents a synergistic interaction between the 2 receptors.
3 Interactions involving phosphorylation of D3R
As a member of G protein-coupled receptor family, D3R may be subject to the modulation by protein kinase-mediated phosphorylation of its cytoplasmic domains. Indeed, G protein-coupled receptor kinase 4 (GRK4) interacts with D3R to phosphorylate D3R and regulate the efficacy of D3R signaling[15]. In addition, Liu et al. have identified a novel synaptic model for regulation of D3R through a direct protein association mechanism involving protein kinase-mediated phosphorylation[16]. Given that D3R contains a large third intracellular domain, a glutathione-S-transferase (GST)-fusion protein containing the third loop was used as a bait to screen for new binding partners in rat accumbal lysates. They find that the third loop of D3R specifically pulls down a 50 kD protein, which is subsequently identified as Ca2+/calmodulin-dependent protein kinase II (CaMKII). Further in vitro binding assays concerning the purified third loop protein and CaMKIIα show that it is through its catalytic domain that CaMKIIα directly binds to an N-terminal region of the D3R third loop containing a 14-residue motif (RILTRQNSQCISIR).
CaMKII is activated by Ca2+/calmodulin. The binding of Ca2+/calmodulin to the regulatory domain of the kinase can release the autoinhibition, leading to activation of the kinase. The activated CaMKII exhibits a markedly higher affinity for D3R[16], which establishes a Ca2+-dependent property of the CaMKII-D3R binding modulation. The Ca2+-dependency is noteworthy as it might imply an activity-dependent interaction between the two proteins in live neurons in vivo. Activated CaMKII triggers autophosphorylation at threonine 286 (Thr286) in its own regulatory domain, and phosphorylates its various substrates. Such autophosphorylation can keep the enzyme in a catalytically active conformation, an autonomous (Ca2+/calmodulin-independent) activity, even after attenuation of the initial Ca2+/calmodulin signal[17]. Interestingly, autophosphorylation can also transform the CaMKII-D3R binding to a prolonged autonomous state.
The amino acid sequence of D3R CaMKII-binding site is remarkably homologous with those of other known substrates of CaMKII, including NR2B, autocamtide-2, and syntide-2. This suggests that D3R may serve as a phosphorylation substrate of CaMKII. Indeed, CaMKII can phosphorylate the third loop of D3R[16]. Further phosphorylation site mapping of the third loop with active CaMKII has identified a serine residue (Ser229) as the site of phosphorylation. This residue lies within the CaMKII-binding motif in the N-terminal of the third loop and aligns with a phospho-S/T site predicted from the consensus CaMKII phosphorylation sequence, (I/L)XRXX(S/T)[18].
A more important question is whether and how CaMKII regulates D3R function through binding and phosphorylation. D3R may preferentially inhibit the adenylyl cyclase activity, thereby reducing cAMP formation. In rat accumbal slices, selective activation of D3R with a D3R selective agonist PD128907 could reduce forskolin (an adenylyl cyclase activator)-induced production of cAMP. The effect of PD128907 could be antagonized by a Ca2+ ionophore, ionomycin. This antagonism is believed to be mediated by the upregulated CaMKII-D3R interaction, since this effect can be reversed by the CaMK inhibitor KN93 and a membrane-permeable Tat-peptide that selectively interferes with the CaMKII-D3R interaction. Thus, at the receptor level, the rise in Ca2+ level recruits CaMKII to the third loop of D3R and D3R is phosphorylated at a selective serine site, thus inhibiting the D3R function in reducing cAMP formation. In a population of neurons co-expressing D3R and Gs-coupled D1 receptors, the inhibition of D3R signaling by CaMKII is necessary for the positive response of D1 receptors to dopamine stimulation.
Moreover, it is of great interest whether the neurochemical model also functions in behavioral regulation. It seems that D3R can suppress the motor responses to cocaine[19]. Thus, it is speculated that cocaine would promote the interactions between CaMKII and D3R to attenuate D3R function in inhibiting behavioral responses to cocaine, which serves to enhance behavioral sensitivity to cocaine stimulation. Data from behavioral experiments conducted by Liu et al.[16] are consistent with this speculation. Besides, acute systemic injection of cocaine can increase the formation of CaMKII-D3R complex in rat nucleus accumbens. And this increase is blocked by pretreatment with a Tat-peptide that can selectively interfere with the CaMKII-D3R interaction. Importantly, the Tat-peptide can reduce the motor response to cocaine.
4 Conclusion
D3R is an important dopamine receptor. Due to its preferential expression in the limbic system, the receptor plays a critical role in the regulation of normal neuronal and synaptic activities. The dysfunction of this receptor has also been linked to various mental and neurodegenerative disorders[20]. Accumulative evidence has demonstrated that the receptor in its subcellular expression, trafficking, and signaling efficacy, is subject to the dynamic modulation by a molecular mechanism involving protein association. Available data show that several cytoskeletal proteins interact with the third loop or the C-terminal of D3R, and these interactions control surface expression of D3R or the receptor signaling. A recent study uncovers a previously unrecognized Ca2+- and phosphorylation-dependent route of D3R regulation. As illustrated in Fig. 1, a rise in intracellular Ca2+ level can promote the binding of cytosolic CaMKIIα to the N-terminal region of the third intracellular domain of D3R. This leads to a site-specific phosphorylation, thereby attenuating the D3R-mediated inhibition of adenylyl cyclase. While this study and others provide initial interesting findings, many questions still remain to be answered. Firstly, it is unclear whether the phosphorylation-based regulation by CaMKII can extend to other dopamine receptor subtypes or neurotransmitter receptors. It is possible that the CaMKII-mediated negative or positive feedback also happens to other G protein-coupled receptors. Secondly, intracellular domains of dopamine receptors may interact with other structural, signaling, or enzymatic proteins. Using a GST-fusion intracellular domain to screen potential new interacting proteins is an efficient way to identify novel interacting proteins. Thirdly, while CaMKII phosphorylates and regulates the D3R signaling to cAMP, detailed mechanisms underlying this process are unknown. CaMKII may regulate the affinity of ligand-receptor binding, surface receptor expression, or G protein signaling to alter the readout (cAMP) of D3R activity. Finally, plastic changes and implications of the association between D3R and CaMKII or other proteins in various neuropsychiatric and neurodegenerative diseases remain elusive. Alterations in interactions between D3R and these proteins may play a significant role in the pathogenesis of these disorders. In sum, studies on the relations between protein-protein interaction and regulation of D3R are still at an initial stage. Future studies are required to identify new partners and define the functional roles of known and new interacting proteins in regulating expression and function of the receptor.
Fig. 1.
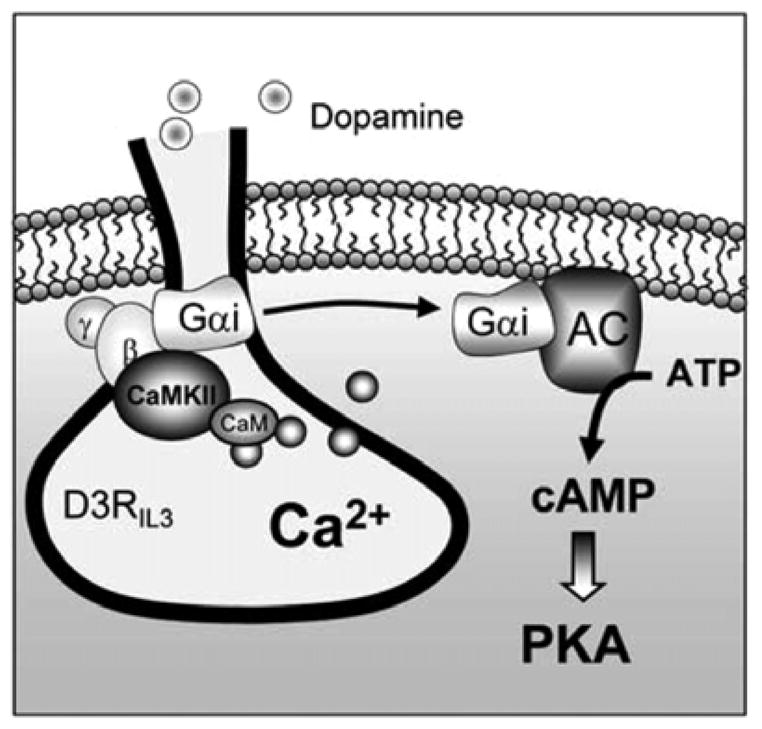
A schematic illustration of the CaMKII-D3R interaction in regulating D3R signaling. The third intracellular domain of D3R and the associated heterotrimeric G protein that is made up of αi, β, and γ subunits are shown. Under basal conditions, the CaMKII-mediated phosphorylation and inhibition of D3R remain at a low level due to the limited amount of CaMKII bound to D3R. This allows D3R to respond to the baseline level of dopamine to inhibit adenylyl cyclase (AC). Of note, D3R is the only subtype of dopamine receptors with an affinity (Ki = 30 nmol/L[20]) within the nanomolar range of basal dopamine concentrations at synapses. When transformed to a stimulated state in which the synaptic dopamine level is increased to the micromolar range, CaMKII is recruited to D3R by the increased Ca2+ level, thereby depressing D3R. This, in concert with the simultaneously activated D1 efficacy, induces a net upregulation of the cAMP-PKA pathway. AC: adenylyl cyclase; ATP: adenosine triphosphate; CaM: calmodulin; IL3: intracellular loop 3; PKA: protein kinase A.
Acknowledgments
This work was supported by the grant from the Saint Luke’s Hospital Foundation (Kansas City, MO, USA) and grants (No. R01-DA010355, R01-MH061469) from the National Institutes of Health (Bethesda, MD, USA).
References
- 1.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res. 2004;24:165–205. doi: 10.1081/rrs-200029981. [DOI] [PubMed] [Google Scholar]
- 2.Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature. 1990;347:146–151. doi: 10.1038/347146a0. [DOI] [PubMed] [Google Scholar]
- 3.Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- 4.Heidbreder CA, Gardner EL, Xi ZX, Thanos PK, Mugnaini M, Hagan JJ, et al. The role of central dopamine D3 receptors in drug addiction: a review of pharmacological evidence. Brain Res Rev. 2005;49:77–105. doi: 10.1016/j.brainresrev.2004.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokoloff P, Diaz J, Le Foll B, Guillin O, Leriche L, Bezard E, et al. The dopamine D3 receptor: a therapeutic target for the treatment of neuorpsychiatric disorders. CNS Neurol Disord Drug Targets. 2006;5:25–43. doi: 10.2174/187152706784111551. [DOI] [PubMed] [Google Scholar]
- 6.Lin R, Karpa K, Kabbani N, Goldman-Rakic P, Levenson R. Dopamine D2 and D3 receptors are linked to the actin cytoskeleton via interaction with filamin A. Proc Natl Acad Sci U S A. 2001;98:5258–5263. doi: 10.1073/pnas.011538198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorlin JB, Yamin R, Egan S, Stewart M, Stossel TP, Kwiatkowski D, et al. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J Cell Biol. 1990;111:1089–1105. doi: 10.1083/jcb.111.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binda AV, Kabbani N, Lin R, Levenson R. D2 and D3 dopamine receptor cell surface localization mediated by interaction with protein 4.1N. Mol Pharmacol. 2002;62:507–513. doi: 10.1124/mol.62.3.507. [DOI] [PubMed] [Google Scholar]
- 9.Walensky LD, Blackshaw S, Laio D, Watkins CC, Weier HU, Parra M, et al. A novel neuron-enriched homolog of the erythrocyte membrane cytoskeletal protein 4.1. J Neurosci. 1999;19:6457–6467. doi: 10.1523/JNEUROSCI.19-15-06457.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutzleb C, Sanders G, Yamamoto R, Wang X, Lichte B, Petrasch-Parwez E, et al. Paralemmin, a prenyl-palmitoyl-anchored phosphoprotein abundant in neurons and implicated in plasma membrane dynamics and cell process formation. J Cell Biol. 1998;143:795–813. doi: 10.1083/jcb.143.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basile M, Lin R, Kabbani N, Karpa K, Kilimann M, Simpson I, et al. Paralemmin interacts with D3 dopamine receptors: implications for membrane localization and cAMP signaling. Arch Biochem Biophy. 2006;446:60–68. doi: 10.1016/j.abb.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Griffon N, Jeanneteau F, Prieur F, Diaz J, Sokoloff P. CLIC6, a member of the intracellular chloride channel family, interacts with dopamine D2-like receptors. Mol Brain Res. 2003;117:47–57. doi: 10.1016/s0169-328x(03)00283-3. [DOI] [PubMed] [Google Scholar]
- 13.Jeanneteau F, Diaz J, Sokoloff P, Griffon N. Interactions of GIPC with dopamine D2, D3 but not D4 receptors define a novel mode of regulation of G protein-coupled receptors. Mol Biol Cell. 2004;15:696–705. doi: 10.1091/mbc.E03-05-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorentini C, Busi C, Spano P, Missale C. Dimerization of dopamine D1 and D3 receptors in the regulation of striatal function. Curr Opin Pharmacol. 2009;10:1–6. doi: 10.1016/j.coph.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Villar VA, Jones JE, Armando I, Palmes-Saloma C, Yu P, Pascua AM, et al. G protein-coupled receptor kinase 4 (GRK4) regulates the phosphorylation and function of the dopamine D3 receptor. J Biol Chem. 2009;284:21425–21434. doi: 10.1074/jbc.M109.003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu XY, Mao LM, Zhang GC, Papasian CJ, Fibuch EE, Lan HX, et al. Activity-dependent modulation of limbic dopamine D3 receptors by CaMKII. Neuron. 2009;61:425–438. doi: 10.1016/j.neuron.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudmon A, Schulman H. Neuronal Ca2+/calmodulin-dependent protein kinase II: the role of structure and autoregulation in cellular function. Annu Rev Biochem. 2002;71:473–510. doi: 10.1146/annurev.biochem.71.110601.135410. [DOI] [PubMed] [Google Scholar]
- 18.White RR, Kwon YG, Taing M, Lawrence DS, Edelman AM. Definition of optimal substrate recognition motifs of Ca2+-calmodulin-dependent protein kinases IV and II reveals shared and distinctive features. J Biol Chem. 1998;273:3166–3179. doi: 10.1074/jbc.273.6.3166. [DOI] [PubMed] [Google Scholar]
- 19.Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, et al. Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron. 1997;19:837–848. doi: 10.1016/s0896-6273(00)80965-4. [DOI] [PubMed] [Google Scholar]
- 20.Sokoloff P, Martres MP, Giros B, Bouthenet ML, Schwartz JC. The third dopamine receptor (D3) as a novel target for antipsychotics. Biochem Pharmacol. 1992;43:659–666. doi: 10.1016/0006-2952(92)90227-a. [DOI] [PubMed] [Google Scholar]


