Abstract
In this review we focus on the adult stem/progenitor cells that were initially isolated from bone marrow and first referred to as colony forming units-fibroblasticsee 1, then as marrow stromal cells2, and subsequently as either mesenchymal stem cells3 or multipotent mesenchymal stromal cells (MSCs)4. The current interest in MSCs and similar cells from other tissues is reflected in over 10,000 citations in PubMed as of this writing with 5 to 10 new publications per day. It is also reflected in over 100 registered clinical trials with MSCs or related cells (http//www.clinicaltrials.gov). As a guide to the vast literature, this review will attempt to summarize many of the publications in terms of three paradigms that have directed much of the work (Figure 1): an initial paradigm that the primary role of the cells was to form niches for hematopoietic stem cells (Paradigm I); a second paradigm that the cells repaired tissues by engraftment and differentiation to replace injured cells (Paradigm II); and the more recent paradigm that MSCs engage in cross-talk with injured tissues and thereby generate microenvironments or “quasi-niches” that enhance the repair tissues (Paradigm III).
Paradigm I: The Hematopoietic Niche
Early attempts to culture bone marrow revealed that a small fraction of the cells that adhered to culture dishes were not hematopoietic precursors. Some investigators were struck by the morphological similarity of the non-hematopoietic cells to the spindle-shaped cells that formed the stroma of marrow2. Therefore, they developed the paradigm that the cells formed niches for the propagation of hematopoietic stem cells. The paradigm proved extremely useful in that the confluent cultures of MSCs were found to be effective feeder layers for the culture of hematopoietic stem cells5,6. The niche role of MSCs was directly demonstrated by the observation that islands of hematopoiesis were formed within ceramic cubes that were seeded with human MSCs and then inserted under the skin of immunodeficient mice7. Also, the niche role of MSCs was indirectly supported by clinical trials in which the cells were shown to hasten the recovery of the hematopoietic system after bone marrow transplants8.
Paradigm II: Engraftment/Differentiation
Early investigators studying cultures of bone marrow were impressed with the facility with which the adherent, spindle-shaped cells differentiated into distinct cellular phenotypes. In particular, Friedenstein and others1 demonstrated that the cells readily became mineralizing cells or chondrocytes both in culture and after implantation in diffusion chambers in vivo. These observations suggested the paradigm that MSCs might repair injured tissues by engraftment and differentiation. The paradigm had broad implications for medical therapies in part because of the ease with which the cells could be isolated from a small sample of human bone marrow and then rapidly expanded in culture through 30 or more population doublings9–11.
Early Observations on Engraftment and Differentiation
Repair by Paradigm II was supported by early observations that local administrations of MSCs improved bone repair12. The potential therapeutic implications of the paradigm were expanded by the observation that after systemic infusions of MSCs containing a mutated human gene into irradiated young mice, the mutated gene was detected in multiple tissues of the mice13. Also, further support for the therapeutic potentials was provided by the observation that infusions of MSCs from wild-type mice produced small but significant improvements in the bones of a transgenic mouse model for osteogenesis imperfecta14. The potential therapeutic implications were expanded still further by the observation that, after BrdU-labeled MSCs were injected into the cerebral ventricles of newborn mice, the cells migrated throughout the brain, and a few of the cells became astrocytes15.
These early observations prompted a clinical trial in which children with severe osteogenesis imperfecta first received bone marrow transplants from a haplotype-matched normal donor and then were treated, several years later, with intravenous infusions of a large number of MSCs from the same donors16. The therapy produced a transient but significant improvement in the clinical course of the children. Most importantly, there was only one adverse event: one of the children developed a mild allergic reaction to fetal calf serum in which the MSCs were expanded. The results were followed by a clinical trial in which administration of MSCs produced encouraging results in children with severe lysosomal storage diseases17. These initial observations raised the possibility that Paradigm II might provide new therapies for a broad spectrum of human diseases.
Technical Challenges in Testing Paradigm II
The early efforts to test the paradigm encountered a series of technical challenges: (a) No endogenous markers for MSCs were available that could be used to track the cells in vivo18. Exogenous markers such as dyes or transduced genes were employed instead, but most produced unexpected artifacts19–21. (b) Only a small number of antibodies and other markers were available to follow differentiation of the cells in vivo. Also, the microscopes and algorithms to overcome some of the artifacts of immunohistochemistry were not commonly available. (c) Species differences in MSCs created a significant experimental barrier. Cultures of human MSCs were relatively easy to purify from hematopoietic precursors by simply replating the cells. Cultures of mouse MSCs remained contaminated by hematopoietic precursors through several passages. Also, as was observed much earlier with mouse fibroblasts22, cultures of mouse MSCs expanded slowly until they underwent “crisis” during which a few cells were transformed and then expanded rapidly 23. Rat MSCs initially resembled human MSCs but at a later stage also underwent crisis and transformation24,25. (d) MSCs were not readily transplanted into marrow ablated mice and therefore presented a further limitation in the use of transgenic mice. (e) Most importantly, tissue repair is a highly complex biological process that varies with the type of injury and the tissue injured26. Also, there are marked species differences in inflammatory and immune responses27 and as a result many experimental animals, especially rodents, repair tissues much more efficiently than humans. In effect, there were several serious barriers to definitive experiments to test Paradigm II.
The Impetus to Test the Paradigm II in Clinical Trials
Despite these technical challenges, there continues to be great interest in testing the medical implications inherent in Paradigm II. The paradigm has been pursued against the history that discoveries of new therapies in medicine have rarely been linear processes. Initial tests of a potential therapy in vitro are rarely as convincing as one would like, because of the limitations of experiments with purified molecular components and the artifacts inherent in culturing cells. The data from animal experiments are usually even more limited because of the difficulty of mimicking human diseases. The history of medicine is replete with examples of therapies that failed in the patients in spite of extensive basic and preclinical research. However, the history of medicine also includes examples of therapies that were not fully developed or whose beneficial effects were not understood until after they were first tested in patients28. The examples include discovery of the anti-thrombotic effects of aspirin29,30, the need of HLA typing in bone marrow transplants31, the revised rationale and design of bisphosphonates for therapy of bone diseases32, and the failure of sildenafil (Viagra) as a therapy for angina in spite of the Nobel prize research that led to its development33,34 (See Supplemental Materials).
Tests of the Paradigm II with local administrations
Engraftment and differentiation of MSCs, as predicted by Paradigm II, were seen in several settings. In models for bone and cartilage defects, a series of reports demonstrated that direct implantation of MSCs themselves or MSCs embedded in scaffolds enhanced repair35–38. There is a consensus that some of the administered cells differentiated into osteoblasts or chondrocytes. However, most reports indicated the MSCs disappeared in several weeks36,39, and most of the differentiated cells seen in long term grafts are host cells, at least in part because of the normal turnover of the tissues.
In models of cardiac defects, several reports indicated that locally implanted MSCs engrafted and differentiated into cardiomyocytes40,41. However, it has not been conclusively established that locally administered MSCs provide a sufficient number of fully integrated cardiomyocytes to account for the improvements in ventricular function observed in many experiments42.
In the central nervous system, some experiments indicated that MSCs injected into the ventricles of embryos or of newborn pups migrated throughout the brain and differentiated as the organ developed15,43,44. In one series of experiments, quantitative PCR assays indicated that the number of MSCs or MSC-derived cells increased as much as 30-fold in a few days after male MSCs were injected into the ventricles of newborn female mice43. The possibility of neural differentiation was supported by the observation that some preparations of MSCs differentiated in culture into dopaminergic-like neurons with the appropriate electrophysiological properties45. However, it was difficult to establish differentiation of MSCs into functional neural cells in vivo46,47.
In contrast to transplants into embryonic brains, very few MSCs injected into the brains of adult rodents survived more than 1 or 2 weeks21,48,49. Surprisingly, the rate of disappearance was about the same with human MSCs injected into the hippocampi of both immunodeficient and wild-type mice49.
In models for spinal cord injury, local administration of MSCs produced improved motor function but few, if any, of the cells engrafted for prolonged periods or differentiated into neural cells50,51. One initial impression was that the cells formed a scaffold for regeneration of nerve tracts in the cord51. A recent study suggested that the therapeutic benefits were explained by anti-inflammatory effects of the cells52.
Tests of Paradigm II with Systemic Infusion
Tests of Paradigm II with systemic infusions of the cells proved problematic. Numerous reports described functional improvements after systemic infusions of MSCs in models for human disease that included osteogenensis imperfecta53; stroke54; myocardial infarction55; acute kidney injury56; and diabetes57,58. The initial interpretations of the data were based on Paradigm II and assumed that the cells had homed to injured tissues, engrafted and differentiated to replace injured cells. However, it was difficult to demonstrate extensive engraftment of the cells. Also, the interpretations were not intuitively consistent with several reports about the fate of systemically infused MSCs: Observations with whole body imaging techniques indicated that most MSCs were trapped in the lungs after intravenous infusions into rodents, the route used in most of the experiments59–61. Therefore, the functional improvement of distal organs after intravenous infusions of the cells was paradoxical.
To explore the paradox, we recently employed quantitative PCR assays for human MSCs infused into mice62, a strategy introduced earlier by Phinney and associates for tracking MSCs infused into the brain43. (Previous data developed from gel-based PCR assays probably overestimated engraftment of MSCs after systemic infusion13.) An improved protocol for quantitative PCR assay of human Alu sequences demonstrated that after IV infusion of the human MSCs, essentially all of the cells were cleared from the circulation within 5 minutes62. Most of the human cells were recovered in the lungs. The cells in the lungs disappeared with a half life of about 24 hours but only trace amounts were recovered in the 6 other tissues that were assayed. Therefore, the results questioned whether Paradigm II could account for the functional improvement observed after intravenous infusions of MSCs in animal models for diseases of distal organs. In addition, Paradigm II could not account for reports that conditioned medium from cultures of MSCs were as effective in some disease models as the cells themselves63–65.
Paradigm III: Transient “quasi-niches”
The accumulating evidence that MSCs could repair injured tissues without significant engraftment and differentiation called for a new paradigm that required re-examination of some of the early observations on cultures of the cells and more detailed examination of their effects in vivo.
Unusual features of MSCs in culture
The early observations that confluent and non-propagating MSCs provided effective feeder layers for cultures of hematopoietic cells were explained in part by the cells secreting paracrine factors5,6,66,67. However, the effectiveness of MSCs as feeder layers was not entirely explained by secretion of soluble factors; cell to cell contact was also required for reasons that were not apparent5,6.
Unusual features of MSCs in culture were also apparent from observing the cells after they were plated at clonal densities. The cells expanded as single-cell derived colonies but the properties of the cells changed as the colonies expanded. In the many of the colonies that formed, distinct inner and outer regions were apparent. The outer regions consisted of rapidly self-renewing cells (RS-MSCs) and the inner regions consisted of slowly replicating cells (SRMSCs) that were partially differentiated68. Moreover, the cells displayed a remarkable plasticity in that the cells from both the inner and outer regions generated single-cell derived colonies with the same characteristics if they were lifted and re-plated at low density. Therefore, the MSCs expanded at clonal densities appeared to reversibly create their own microenvironments or “quasi-niches” in culture in a manner that paralleled their ability to provide niches for hematopoietic stem cells.
Cross-talk with injured tissues
Although MSCs in culture secreted many cytokines66,67, it was not initially apparent that MSCs responded to injured tissues by being activated to express high levels of additional therapeutic proteins. In effect, there was cross-talk in which signals from injured cells activated MSCs to alter expression of large families of genes. At the same time signals from the activated MSCs both up-regulated and down-regulated large families of genes in the injured cells.
One of the first examples of cross-talk was observed between MSCs and multiple myeloma cells69. Co-culture experiments demonstrated that signals from the myeloma cells stimulated the MSCs to increase secretion of IL-6 and this IL-6 in turn, increased the proliferation of the myeloma cells. At the same time, the myeloma cells secreted high levels of Dkk-1, an inhibitor of Wnt signaling, that kept the MSCs in cell cycle and inhibited them from differentiating into osteoblasts. The cross-talk provided an explanation for why patients with multiple myeloma develop osteolytic lesions in which the cancer cells proliferate but osteoblasts are not recruited to fill the lesions69.
A second example of cross-talk was encountered in experiments in which human MSCs were injected into the hippocampi of mice following transient cerebral ischemia. The human MSCs reduced neuronal death and improved the neurological deficits49. Assays of RNA from the hippocampus with human-specific mRNA/cDNA microarrays demonstrated that in the ischemia injured brain, the human MSCs increased expression of genes that modulated immune and inflammatory responses. Assays of the same RNA on mouse-specific microarrays demonstrated that the presence of the human MSCs modulated expression of mouse genes involved in immune responses to the ischemic environment.
A similar example of cross-talk was obtained by using species-specific mRNA/cDNA microarrays to survey the lungs of mice a few hours after intravenous infusions of human MSCs62. By producing microemboli, the human cells altered expression of hundreds of mouse genes in the lung. At the same time, signals from the mouse cells altered expression of hundreds of genes in the human MSCs. In parallel with these observations, reports from several laboratories demonstrated that the expression of potentially therapeutic cytokines was markedly increased by exposing MSCs to cytokines typically released by injured tissues70,71.
Modulation of Inflammation in Paradigm III
The experiments in which human MSCs were infused intravenously into mice with myocardial infarcts provided a clue to how they enhanced tissue repair. One of the most interesting genes up-regulated in human MSCs that were trapped in the lung after intravenous infusion62 was TNFα stimulated gene/protein-6 (TSG-6)72,73. Extensive previous research demonstrated that TSG-6 had remarkable anti-inflammatory properties in a number of experimental settings, including in both wild-type and transgenic mice72,73. Experiments with recombinant TSG-6 and siRNAs demonstrated that the secretion of TSG-6 by MSCs trapped in the lung largely accounted for previous reports that intravenously administered MSCs improved mice with myocardial infarcts55,74–76. TSG-6 decreased activation of the inflammatory network of proteases in the heart and decreased monocyte and granulocyte infiltration. The TSG-6 thereby decreased the damage to cardiomyocytes and the size of the myocardial scar that subsequently formed (Figure 2).
Figure 2. Effects of human MSCs and recombinant TSG-6 in mice (NOD/scid) with myocardial infarcts (MI).
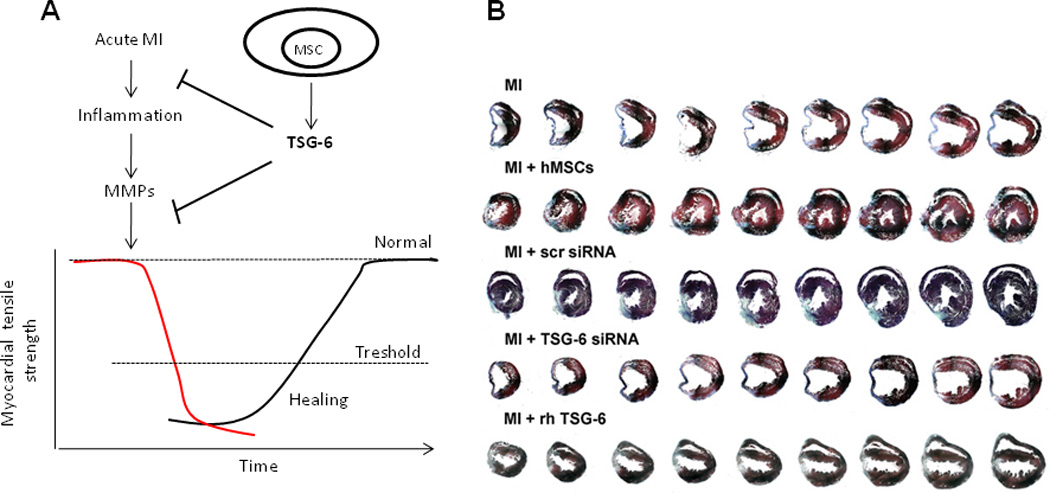
A. Schematic illustrating the progressive damage to the myocardium following myocardial infarction (MI). The ischemia triggers invasion by inflammatory cells. The inflammatory cells and the matrix metalloproteinases (MMP) they release accentuate damage to the myocardium. TSG-6 synthesized by MSCs or recombinant TSG-6 limits the injury and thereby enhances repair. Reproduced with permission and modified from100. B. Protective/reparative properties of MSCs and TSG-6 in MI. Three weeks after permanent ligation of the anterior descending coronary artery in mice to produce myocardial infarcts (MI), each heart was cut from the apex through base into over 400 sequential 5 µm sections and stained with Masson Trichrome. Every 20th section is shown. Symbols: Normal, naïve mice; –, MI only; hMSCs, 2 × 106 hMSCs infused intravenously (IV) 1 hr after MI; scr siRNA, 2 × 106 hMSCs transduced with scrambled siRNA infused IV 1 hr after MI; TSG-6 siRNA, 2 × 106 hMSCs transduced with TSG-6 siRNA infused IV 1 hr after MI; rhTSG-6, 30 µg recombinant TSG-6 protein infused IV 1 hr and again 24 hr after MI. Reproduced with permission from62.
Modulation of Apoptosis in Paradigm III
Several reports indicated that one of the potential therapeutic effects of MSCs was to decrease apoptosis77,78. Co-culture experiments demonstrated that MSCs decreased apoptosis in two model systems in part by being activated to express stanniocalcin-1(STC-1), a calcium regulatory protein79. The effects of STC-1 on apoptosis were apparently explained by its uncoupling of oxidative phosphorylation and suppression of reactive oxygen species80. Suppression reactive oxygen species also explains the recent observation the STC-1 has anti inflammatory properties81.
Modulation of immune reactions
Preliminary observations made in clinical trials to improve bone marrow transplants with MSCs provided an unexpected observation: In a few patients, the MSCs improved the effects of graft versus host disease (GVHD)82. These and related observations led to experiments that demonstrated intravenous infusions of MSCs reduced neurological deficits in the experimental autoimmune encephalitis (EAE) model for multiple sclerosissee 83. The findings spurred extensive efforts to define the mechanisms whereby MSCs modulated the immune system. The results have provided several different scenarios. Here we will focus on four recent accounts. (For more complete reviews, see 83,84.)
Shi and associates70 demonstrated that the immunosuppressive effects of murine MSCs were triggered by the cells being stimulated by IFNγ together with any one of three other proinflammatory cytokines (TNFα, IL-1α, or IL-1β). The stimulated MSCs expressed several cytokines and inducible nitric oxide synthase (iNOS). The chemokines attracted T cells to the MSCs and then the T cells were suppressed by nitric oxide (NO) from the MSCs. They subsequently found a marked species difference in that human and monkey MSCs did not synthesize NO under similar conditions. Instead, the MSCs suppressed T cells by secreting indoleamine 2,3-dioxygenase (IDO) that depleted tryptophan in the medium or generated toxic concentrations of kynurenine and other metabolites to suppress T cells85.
Galipeau and associates86 examined the effects of MSCs on activated CD4(+) T cells in the EAE model for multiple sclerosis). They found that the MSCs inhibited activation of the T cells by secreting both CCL2 (monocyte chemotactic protein-1 or MCP-1) and MMP-9 that cleaved the CCL2 into an antagonistic derivative. The role of the soluble factors was confirmed by the demonstration that conditioned medium from MSCs inhibited activation of CD4(+) T cells from EAE mice and that the effects of MSCs were not observed in CCL2(−/−) EAE mice. The same laboratory also demonstrated that MSCs can stimulate immune and inflammatory responses. They found that MSCs can cross-present exogenous antigen and induce an effective CD8(+) T-cell immune response87. They can also be activated through Toll-like receptors to recruit inflammatory and immune cells71.
Mahon and associates88 suggested that MSCs might exert their immune regulatory effects by enhancing T regulatory cells. They demonstrated that allogeneic MSC induced expression in CD4(+) T cells of forkhead box P3 (FoxP3+) and CD25(+), both markers of T regulatory cells. Their results supported a sequential process in which a first step required direct contact between MSCs and CD4(+) T cells followed by secretion of TGF-β1 and prostaglandin E2 by the MSCs to drive differentiation of T cells to T regulator cells.
Uccelli et al.83 offered a more pleiotrophic account of the effects of MSCs on the immune system. They suggested that MSCs produced a variety of effects such as (a) decreased proliferation, cytotoxicity and cytokine production by NK cells; (b) impaired maturation and antigen presentation by dendritic cells; (c) decreased proliferation of T cells and impaired T helper cells; and (d) decreased proliferation and antibody production by B cells.
At the moment, it is not clear which of the proposals best accounts for the immune modulatory effects of MSCs in vivo.
Paradigm III and the similarities to Paradigm I
The experiments in which MSCs enhance tissue repair without significant engraftment suggest that there is a complex series of interactions between the MSCs and the injured tissues. One of the key interactions is a sequence in which TNFα and other signals from injured tissues activate the MSCs to secrete TSG-6, STC-1 and probably other soluble factors that decrease the production of TNFα and other inflammatory signals from the injured tissues (Figure 3). In effect, the MSCs introduce a negative feedback loop into excessive responses by tissues that frequently occur in injuries not accompanied by invading organisms. Such excessive inflammatory and immune responses are now recognized to contribute to the pathoetiology of many diseases, including diabetes and artherosclerosis89,90. Secretion of soluble factors probably explains the therapeutic effects of intravenous infusion of MSCs or conditioned medium from MSC cultures in some animal models. However, some of the therapeutic effects, such as in models for immune diseases, may require direct cell-to-cell contact between MSCs and target cells. Also, in addition to modulating inflammatory/immune reactions, MSCs may enhance repair of tissues by stimulating the proliferation and differentiation of tissue endogenous stem/progenitor cells as was observed with infusion of MSCs into the hippocampus of mice48. Many of the effects of MSCs on tissue repair are transient “hit and run” events (Paradigm III) but they have some similarities to the ability of the cells to provide a niche for hematopoietic cells (Paradigm I).
Figure 3. Schematic for MSCs providing a niche for hematopoietic stem cells (HSCs) as in Paradigm I and modulating excessive inflammatory and immune responses as in Paradigm III.
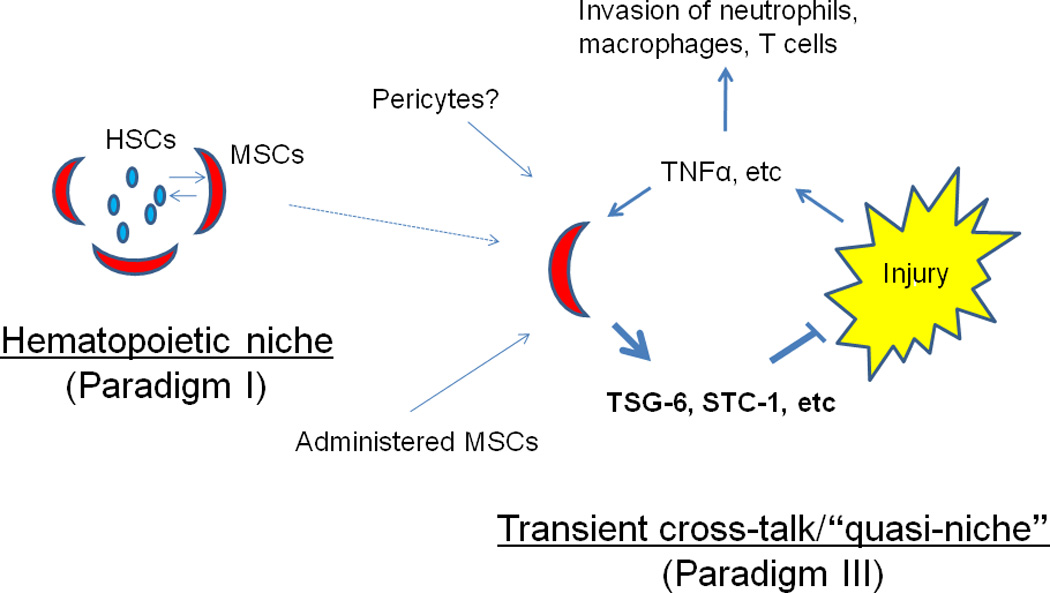
One effect of administered MSCs is to introduce a negative feedback into the excessive responses of tissues to sterile injury. They may also enhance repair by increasing propagation and differentiation of tissue endogenous stem/progenitor cells (not shown). Some of the therapeutic effects of MSCs may require direct cell-to-cell contact and transfer of components such as mitochondria.
Conclusions/Perspectives
Our knowledge of MSCs has evolved largely by serendipity, beginning with the first efforts to culture cells from bone marrow. Although our knowledge continues to expand at a rapid pace, a number of important questions still need to be addressed. Some examples include:
Why is administration of MSCs beneficial?
Bone marrow, fat, and many other tissues contain MSCs or MSC-like cells. Therefore, it is not apparent why adequate numbers are not normally mobilized in response to tissue injury. One possibility is that the isolation of the cells from tissues or their expansion in culture may activate therapeutic properties of the cells that are otherwise latent. Another is that the mechanisms for mobilizing MSCs are simply not adequate to modulate the excessive inflammatory and immune responses to sterile tissue injuries.
Better assays for the potency of MSCs?
A major barrier to progress in the field is lack of an in vivo potency assay for MSCs. What is needed is an assay equivalent to the marrow ablated mouse that was key to essentially all the progress in the study of hematopoietic stem cells. Data on the transcriptomes or proteomes of cultured MSC are not adequate since they are simply snap-shot pictures of the cells. Instead, what is needed is an assay of the potential of MSCs to respond to environmental factors such as signals from injured tissues. Unfortunately, current in vitro assays of differentiation or clonogenicity continue to disappoint. Given the multiple modes of action of MSCs, a battery of in vivo potency assays may be required.
Are MSCs pericytes?
Recent reports have provided convincing data for earlier suggestions see 9 that MSCs share many of the features of pericytes91–93; cells that have fascinated investigators since they were first described by Rouget in1873see 94. The similarities between MSCs and pericytes are impressive, including the sharing of several epitopes and the ability of pericytes to differentiate into multiple cellular phenotypes such as fibroblasts, osteoblasts, adipocytes, chondrocytes, and endothelial cells. However, the overlap in properties is not complete. For example, pericytes from different vessels vary but most display contractility and myogenic properties not observed with isolated MSCs. Also, pericytes propagate much more slowly than MSCs, i.e., initial population doubling rates as slow as 162 hours92 versus 12 to 20 hours for MSCs. Therefore, pericytes and MSCs clearly have similar but perhaps not identical properties.
Therapies with recombinant proteins?
Recent observations suggest that therapies with some of the proteins produced by MSCs could replace therapies with the cells themselves. Use of the proteins has many attractions, but MSCs may provide major advantages in many situations by their responsiveness to the particular injury and their ability to deliver factors in high local concentrations. Also, as suggested by Paradigm III, some of the therapeutic benefits of MSCs may require cell-to-cell contact for transfer of vesicles or other components such as mitochondria95 that have not yet been defined.
Additional questions in developing therapies with MSCs
A number of additional questions need to be resolved to develop therapies with MSCs. Although no significant adverse events have been reported from clinical trials to date, all interventional therapies have some inherent risks and questions about the potential risks of therapies with MSCs must be carefully weighed against the potential benefits to patients. One question about the potential risks is whether MSCs, like embryonic stem cells or induced pluripotent stem cells, can cause tumors and malignancies96. The risk cannot be ignored, particularly since MSCs were observed to enhance the growth of some tumors97. However, MSCs in culture differ from embryonic stem cells and induced pluripotent cells in that they are not immortal cells and undergo senescence when expanded in culture. (A recent report indicated that a previous observation of malignant transformation of human MSCs during expansion in culture was explained by contamination of the cultures by small numbers of malignant cells98). Another question still under debate is whether autologous MSCs should be used and whether therapies with heterologous MSCs from “universal donors” can be employed, a strategy currently embraced by several biotech companies. We all await the data from carefully conducted clinical trials and from additional basic research to resolve these and other remaining questions about MSCs.
Supplementary Material
Figure 1. Schematic summarizing three evolving paradigms for the repair of tissues by MSCs.
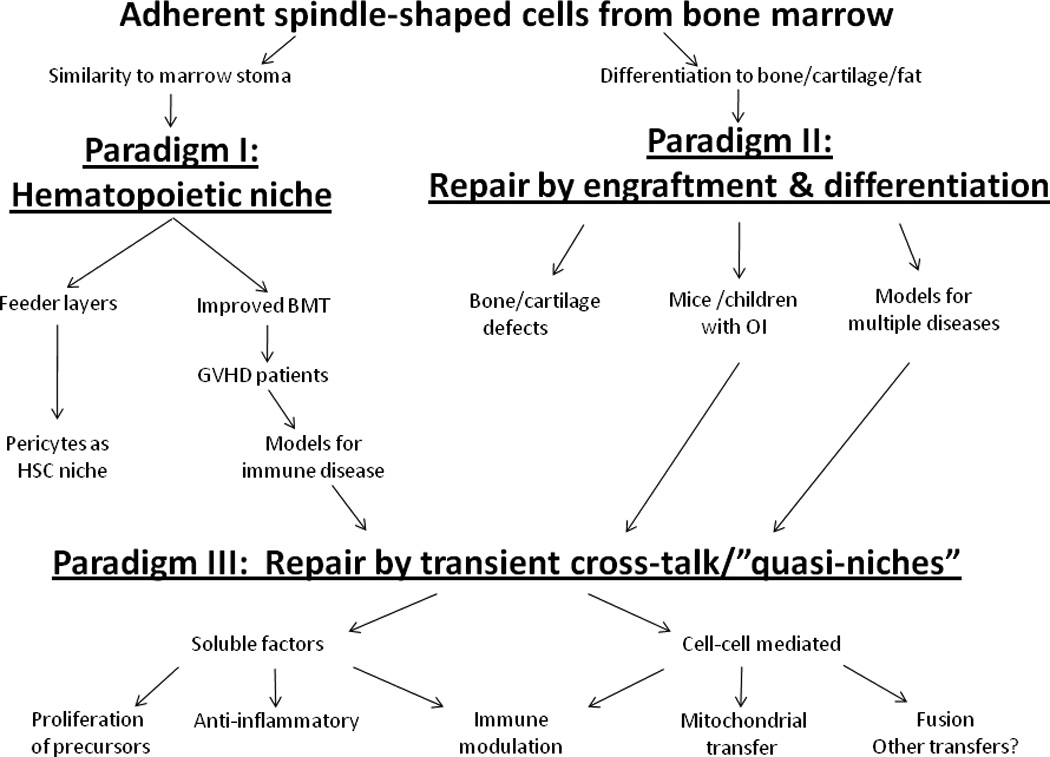
The morphology of a small number of adherent cells from bone marrow suggested the paradigm that the cells served as a niche for hematopoietic cells (Paradigm I). The ready differentiation of the cells in culture suggested that the cells could repair tissues by engrafting and differentiating (Paradigm II). Clinical trials using the cells to improve bone marrow transplants unexpectedly demonstrated that they improved graft-versus-host diseases in a few patients and thereby drew attention to their immune modulatory properties. Functional improvement without significant engraftment in animal models and a few patients suggested that MSCs enhanced repair by forming microenvironments or “quasi-niches” (Paradigm III).
Footnotes
Conflict of Interest Statement:
The Authors confirm that there are no conflicts of interest.
References
- 1.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 2.Allen TD, Dexter TM. Long term bone marrow cultures: an ultrastructural review. Scan Electron Microsc. 1983;Pt 4:1851–1866. [PubMed] [Google Scholar]
- 3.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9(5):641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 4.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 5.Eaves CJ, Cashman JD, Sutherland HJ, Otsuka T, Humphries RK, Hogge DE, Lansdorp PL, Eaves AC. Molecular analysis of primitive hematopoietic cell proliferation control mechanisms. Ann N Y Acad Sci. 1991;628:298–306. doi: 10.1111/j.1749-6632.1991.tb17260.x. [DOI] [PubMed] [Google Scholar]
- 6.Whetton AD, Dexter TM. Influence of growth factors and substrates on differentiation of haemopoietic stem cells. Curr Opin Cell Biol. 1993;5(6):1044–1049. doi: 10.1016/0955-0674(93)90090-d. [DOI] [PubMed] [Google Scholar]
- 7.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2008;131(2):324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 9. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissue. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. A review of the early work in the field.
- 10.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 11.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97(7):3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruder SP, Fink DJ, Caplan AI. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J Cell Biochem. 1994;56(3):283–294. doi: 10.1002/jcb.240560809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira RF, Halford KW, O'Hara MD, Leeper DB, Sokolov BP, Pollard MD, Bagasra O, Prockop DJ. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci U S A. 1995;92(11):4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998;95(3):1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96(19):10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Horwitz EM, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. The first clinical trial with MSCs.
- 17.Koç ON, et al. Bone marrow-derived mesenchymal stem cells remain host-derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp Hematol. 2000;27(11):1675–1681. doi: 10.1016/s0301-472x(99)00101-0. [DOI] [PubMed] [Google Scholar]
- 18.Lee RH, Hsu SC, Munoz J, Jung JS, Lee NR, Pochampally R, Prockop DJ. A subset of human rapidly self-renewing marrow stromal cells preferentially engraft in mice. Blood. 2006;107(5):2153–2161. doi: 10.1182/blood-2005-07-2701. [DOI] [PubMed] [Google Scholar]
- 19.Krause DS. Bone marrow-derived lung epithelial cells. Proc Am Thorac Soc. 2008;5(6):699–702. doi: 10.1513/pats.200803-031AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhn HG, Cooper-Kuhn CM. Bromodeoxyuridine and the detection of neurogenesis. Curr Pharm Biotechnol. 2007;8(3):127–131. doi: 10.2174/138920107780906531. [DOI] [PubMed] [Google Scholar]
- 21.Coyne TM, Marcus AJ, Woodbury D, Black IB. Marrow stromal cells transplanted to the adult brain are rejected by an inflammatory response and transfer donor labels to host neurons and glia. Stem Cells. 2006;24(11):2483–2492. doi: 10.1634/stemcells.2006-0174. [DOI] [PubMed] [Google Scholar]
- 22. Rubin H. Multistage carcinogenesis in cell culture. Dev Biol (Basel) 2001;106:61–66. discussion 67, 143–60. This is a classical description of the spontaneous transformation of mouse fibroblasts as they are expanded in culture.
- 23.Tolar J, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25(2):371–379. doi: 10.1634/stemcells.2005-0620. [DOI] [PubMed] [Google Scholar]
- 24.Foudah D, Redaelli S, Donzelli E, Bentivegna A, Miloso M, Dalprà L, Tredici G. Monitoring the genomic stability of in vitro cultured rat bone-marrow-derived mesenchymal stem cells. Chromosome Res. 2009;17(8):1025–1039. doi: 10.1007/s10577-009-9090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furlani D, et al. A transformed cell population derived from cultured mesenchymal stem cells has no functional effect after transplantation into the injured heart. Cell Transplant. 2008;18(3):319–331. doi: 10.3727/096368909788534906. [DOI] [PubMed] [Google Scholar]
- 26.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2008;172(5):2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 28.Ban TA. The role of serendipity in drug discovery. Dialogues Clin Neurosci. 2006;8(3):335–344. doi: 10.31887/DCNS.2006.8.3/tban. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris CDW. Acetylsalicyclic acid and platelet sickness. Lancet. 1967;i:279–280. [Google Scholar]
- 30.Patrono C, Rocca B. Aspirin, 110 years later. J Thromb Haemost. 2009;7(Suppl 1):258–261. doi: 10.1111/j.1538-7836.2009.03391.x. [DOI] [PubMed] [Google Scholar]
- 31.Thomas ED, Blume KG. Historical markers in the development of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 1999;5(6):341–346. doi: 10.1016/s1083-8791(99)70010-8. [DOI] [PubMed] [Google Scholar]
- 32.Fleisch H. Development of bisphosphonates. Breast Cancer Res. 2002;4(1):30–34. doi: 10.1186/bcr414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bryan NS, Bian K, Murad F. Discovery of the nitric oxide signaling pathway and targets for drug development. Front Biosci. 2009;14:1–18. doi: 10.2741/3228. [DOI] [PubMed] [Google Scholar]
- 34.Ghofrani HA, Osterloh IH, Grimminger F. Sildenafil: from angina to erectile dysfunction to pulmonary hypertension and beyond. Nat Rev Drug Discov. 2006;5(8):689–702. doi: 10.1038/nrd2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mankani MH, Kuznetsov SA, Wolfe RM, Marshall GW, Robey PG. In vivo bone formation by human bone marrow stromal cells: reconstruction of the mouse calvarium and mandible. Stem Cells. 2006;24(9):2140–2149. doi: 10.1634/stemcells.2005-0567. [DOI] [PubMed] [Google Scholar]
- 36.Horie M, Sekiya I, Muneta T, Ichinose S, Matsumoto K, Saito H, Murakami T, Kobayashi E. Intra-articular injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells. 2009;27(4):878–887. doi: 10.1634/stemcells.2008-0616. [DOI] [PubMed] [Google Scholar]
- 37.Deschaseaux F, Pontikoglou C, Sensébé L. Bone regeneration: the stem/progenitor cells point of view. J Cell Mol Med. 2010;14(1–2):103–115. doi: 10.1111/j.1582-4934.2009.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krause U, Harris S, Green A, Ylostalo J, Zeitouni S, Lee N, Gregory CA. Pharmaceutical Modulation of Canonical Wnt Signaling in Multipotent Stromal Cells for Improved Osteoinductive Therapy. Proc Natl Acad Sci USA. 2010;107(9):4147–4152. doi: 10.1073/pnas.0914360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oshima Y, Harwood FL, Coutts RD, Kubo T, Amiel D. Variation of mesenchymal cells in polylactic acid scaffold in an osteochondral repair model. Tissue Eng Part C Methods. 2009;15(4):595–604. doi: 10.1089/ten.tec.2008.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukuda K. Regeneration of cardiomyocytes from bone marrow: Use of mesenchymal stem cell for cardiovascular tissue engineering. Cytotechnology. 2003;41(2–3):165–175. doi: 10.1023/A:1024882908173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quevedo HC, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc Natl Acad Sci U S A. 2009;106(33):14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stamm C, Nasseri B, Choi YH, Hetzer R. Cell therapy for heart disease: great expectations, as yet unmet. Heart Lung Circ. 2009;18(4):245–256. doi: 10.1016/j.hlc.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 43.McBride C, Gaupp D, Phinney DG. Quantifying levels of transplanted murine and human mesenchymal stem cells in vivo by real-time PCR. Cytotherapy. 2003;5(1):7–18. doi: 10.1080/14653240310000038. [DOI] [PubMed] [Google Scholar]
- 44.Muñoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB. Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci. 2004;24(19):4585–4595. doi: 10.1523/JNEUROSCI.5060-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tatard VM, D'Ippolito G, Diabira S, Valeyev A, Hackman J, McCarthy M, Bouckenooghe T, Menei P, Montero-Menei CN, Schiller PC. Neurotrophin-directed differentiation of human adult marrow stromal cells to dopaminergic-like neurons. Bone. 2007;40(2):360–373. doi: 10.1016/j.bone.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25(11):2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 47.Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40(7):609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- 48.Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A. 2005;102(50):18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105(38):14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, Lu M, Rosenblum M. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11(13):3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 51.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci U S A. 2002;99(4):2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abrams MB, Dominguez C, Pernold K, Reger R, Wiesenfeld-Hallin Z, Olson L, Prockop D. Multipotent mesenchymal stromal cells attenuate chronic inflammation and injury-induced sensitivity to mechanical stimuli in experimental spinal cord injury. Restor Neurol Neurosci. 2007;27(4):307–321. doi: 10.3233/RNN-2009-0480. [DOI] [PubMed] [Google Scholar]
- 53.Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998;95(3):1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chopp M, Li Y, Zhang ZG. Mechanisms underlying improved recovery of neurological function after stroke in the rodent after treatment with neurorestorative cell-based therapies. Stroke. 2009;40(3 Suppl):S143–S145. doi: 10.1161/STROKEAHA.108.533141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krause U, et al. Intravenous delivery of autologous mesenchymal stem cells limits infarct size and improves left ventricular function in the infarcted porcine heart. Stem Cells Dev. 2007;16(1):31–37. doi: 10.1089/scd.2006.0089. [DOI] [PubMed] [Google Scholar]
- 56.Tögel F, Westenfelder C. Stem cells in acute kidney injury repair. Minerva Urol Nefrol. 2009;61(3):205–213. [PubMed] [Google Scholar]
- 57.Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD, Prockop DJ. Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A. 2006;103(46):17438–17443. doi: 10.1073/pnas.0608249103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ezquer F, Ezquer M, Simon V, Pardo F, Yañez A, Carpio D, Conget P. Endovenous administration of bone-marrow-derived multipotent mesenchymal stromal cells prevents renal failure in diabetic mice. Biol Blood Marrow Transplant. 2009;15(11):1354–1365. doi: 10.1016/j.bbmt.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 59.Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169(1):12–20. doi: 10.1159/000047856. [DOI] [PubMed] [Google Scholar]
- 60.Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39(2):573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 61.Kidd S, Spaeth E, Dembinski JL, Dietrich M, Watson K, Klopp A, Battula VL, Weil M, Andreeff M, Marini FC. Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells. 2009;27(10):2614–2623. doi: 10.1002/stem.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106(38):16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26(4):1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 65.Timmers L, et al. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1(2):129–137. doi: 10.1016/j.scr.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 66.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166(3):585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 67.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 68.Ylöstalo J, Bazhanov N, Prockop DJ. Reversible commitment to differentiation by human multipotent stromal cells in single-cell-derived colonies. Exp Hematol. 2008;36(10):1390–1402. doi: 10.1016/j.exphem.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gunn WG, Conley A, Deininger L, Olson SD, Prockop DJ, Gregory CA. A crosstalk between myeloma cells and marrow stromal cells stimulates production of DKK1 and interleukin-6: a potential role in the development of lytic bone disease and tumor progression in multiple myeloma. Stem Cells. 2006;24(4):986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 70.Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–150. doi: 10.1016/j.stem.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 71.Romieu-Mourez R, François M, Boivin MN, Bouchentouf M, Spaner DE, Galipeau J. Cytokine modulation of TLR expression and activation in mesenchymal stromal cells leads to a proinflammatory phenotype. J Immunol. 2009;18212:963–973. doi: 10.4049/jimmunol.0803864. [DOI] [PubMed] [Google Scholar]
- 72. Wisniewski HG, Vilcek J. Cytokine-induced gene expression at the crossroads of innate immunity, inflammation and fertility: TSG-6 and PTX3/TSG-14. Cytokine Growth Factor Rev. 2004;15(2–3):129–146. doi: 10.1016/j.cytogfr.2004.01.005. This is a comprehensive review of the extensive research completed on the structure, function and biology of TSG-6 and related proteins.
- 73.Mahoney DJ, Mikecz K, Ali T, Mabilleau G, Benayahu D, Plaas A, Milner CM, Day AJ, Sabokbar A. TSG-6 regulates bone remodeling through inhibition of osteoblastogenesis and osteoclast activation. J Biol Chem. 2008;283(38):25952–25962. doi: 10.1074/jbc.M802138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Halkos ME, et al. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008;103(6):525–536. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 75.Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354(3):700–706. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolf D, Reinhard A, Seckinger A, Gross L, Katus HA, Hansen A. Regenerative capacity of intravenous autologous, allogeneic and human mesenchymal stem cells in the infarcted pig myocardium-complicated by myocardial tumor formation. Scand Cardiovasc J. 2009;43(1):39–45. doi: 10.1080/14017430802100280. [DOI] [PubMed] [Google Scholar]
- 77.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, Mu H, Pachori A, Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci U S A. 2007;104(5):1643–1648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hung SC, Pochampally RR, Chen SC, Hsu SC, Prockop DJ. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;25(9):2363–2370. doi: 10.1634/stemcells.2006-0686. [DOI] [PubMed] [Google Scholar]
- 79.Block GJ, Ohkouchi S, Fung F, Frenkel J, Gregory C, Pochampally R, Dimattia G, Sullivan DE, Prockop DJ. Multipotent Stromal Cells (MSCs) are Activated to Reduce Apoptosis in Part by Upregulation and Secretion of Stanniocalcin-1 (STC-1) Stem Cells. 2009;27(3):670–681. doi: 10.1002/stem.20080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang Y, Huang L, Abdelrahim M, Cai Q, Truong A, Bick R, Poindexter B, Sheikh-Hamad D. Stanniocalcin-1 suppresses superoxide generation in macrophages through induction of mitochondrial UCP2. J Leukoc Biol. 2009;86(4):981–988. doi: 10.1189/jlb.0708454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang L, Garcia G, Lou Y, Zhou Q, Truong LD, DiMattia G, Lan XR, Lan HY, Wang Y, Sheikh-Hamad D. Anti-inflammatory and renal protective actions of stanniocalcin-1 in a model of anti-glomerular basement membrane glomerulonephritis. Am J Pathol. 2009;174(4):1368–1378. doi: 10.2353/ajpath.2009.080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Le Blanc K, Rasmusson I, Sundberg B, Götherström C, Hassan M, Uzunel M, Ringdén O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363(9419):1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 83. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–736. doi: 10.1038/nri2395. A comprehensive review of immune modulatory effects of MSCs.
- 84.Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101–117. doi: 10.1111/j.1749-6632.2009.04607.x. [DOI] [PubMed] [Google Scholar]
- 85.Ren G, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27(8):1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 86.Rafei M, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182(10):5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 87.François M, Romieu-Mourez R, Stock-Martineau S, Boivin MN, Bramson JL, Galipeau J. Mesenchymal stromal cells cross-present soluble exogenous antigens as part of their antigen-presenting cell properties. Blood. 2009;114(13):2632–2638. doi: 10.1182/blood-2009-02-207795. [DOI] [PubMed] [Google Scholar]
- 88.English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156(1):149–160. doi: 10.1111/j.1365-2249.2009.03874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132(6):2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 90.Theuma P, Fonseca VA. Inflammation, insulin resistance, and atherosclerosis. Metab Syndr Relat Disord. 2004;2(2):105–113. doi: 10.1089/met.2004.2.105. [DOI] [PubMed] [Google Scholar]
- 91.Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res. 2003;18(4):696–704. doi: 10.1359/jbmr.2003.18.4.696. [DOI] [PubMed] [Google Scholar]
- 92.Crisan M, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 93.Díaz-Flores L, Gutiérrez R, Madrid JF, Varela H, Valladares F, Acosta E, Martín-Vasallo P, Díaz-Flores L Jr. Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol Histopathol. 2009;24(7):909–969. doi: 10.14670/HH-24.909. [DOI] [PubMed] [Google Scholar]
- 94.Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58(1):1–10. doi: 10.1002/glia.20898. [DOI] [PubMed] [Google Scholar]
- 95.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103(5):1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park IH, Daley GQ. Human iPS cell derivation/reprogramming. Chapter 4. Curr Protoc Stem Cell Biol. 2009;(Unit 4A.1) doi: 10.1002/9780470151808.sc04a01s8. [DOI] [PubMed] [Google Scholar]
- 97.Kidd S, Spaeth E, Klopp A, Andreeff M, Hall B, Marini FC. The (in) auspicious role of mesenchymal stromal cells in cancer: be it friend or foe. Cytotherapy. 2008;10(7):657–667. doi: 10.1080/14653240802486517. [DOI] [PubMed] [Google Scholar]
- 98. Garcia S, Martín MC, de la Fuente R, Cigudosa JC, Garcia-Castro J, Bernad A. Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp Cell Res. 2010;316(9):1648–1650. doi: 10.1016/j.yexcr.2010.02.016. The authors demonstrate that their previous observations on the spontaneous transformation of human MSCs in culture were explained by contamination with a malignant cell line.
- 99.Fang L, Gao XM, Moore XL, Kiriazis H, Su Y, Ming Z, Lim YL, Dart AM, Du XJ. Differences in inflammation, MMP activation and collagen damage account for gender difference in murine cardiac rupture following myocardial infarction. J Mol Cell Cardiol. 2007;43(5):535–544. doi: 10.1016/j.yjmcc.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 100.Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17(6):939–946. doi: 10.1038/mt.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


