Abstract
Background and Objective
The “attack rate” of asthma following viral LRTI is about 3 to 4 fold higher than that of the general population, however, the majority of children who develop viral LRTI during infancy do not develop asthma, and asthma incidence has been observed to continuously decrease with age. Thus, we do not understand how viral LRTI either predispose or serve as a marker of children to develop asthma. The Tennessee Children’s Respiratory Initiative (TCRI) has been established as a longitudinal prospective investigation of infants and their biological mothers. The primary goals are to investigate both the acute and the long-term health consequences of varying severity and etiology of clinically significant viral respiratory tract infections on early childhood outcomes.
Methods
Over four respiratory viral seasons, 2004–2008, term, non-low birth weight previously healthy infants and their biological mothers were enrolled during an infant’s acute viral respiratory illness. Longitudinal follow-up to age 6 years is ongoing.
Results
This report describes the study objectives, design, and recruitment results of the over 650 families enrolled in this longitudinal investigation. The TCRI is additionally unique because it is designed in parallel with a large retrospective birth cohort of over 95,000 mother-infant dyads with similar objectives to investigate the role of respiratory viral infection severity and etiology in the development of asthma.
Conclusions
Future reports from this cohort will help to clarify the complex relationship between infant respiratory viral infection severity, etiology, atopic predisposition, and the subsequent development of early childhood asthma and atopic diseases.
Keywords: asthma, allergic rhinitis, IgE, bronchiolitis, respiratory virus
INTRODUCTION
The Tennessee Children’s Respiratory Initiative (TCRI) has been established as a longitudinal prospective investigation of term, non-low birth weight otherwise healthy infants and their biological mothers. The primary goals of the study are: (1) to investigate both the acute and the long-term health consequences of varying severity and etiology of clinically significant viral respiratory tract infections on the outcomes of allergic rhinitis and early childhood asthma, and (2) to identify the potentially modifiable factors that define children who are at greatest risk of developing asthma following infant respiratory viral infection. This study is unique, in that it was designed in parallel with our Tennessee Asthma Bronchiolitis Study (TABS) which is a retrospective birth cohort study of over 95,000 infants and their biological mothers similarly designed to elucidate the factors predisposing to childhood asthma and allergic diseases, but lacking biospecimens. Thus, we designed the prospective Tennessee Children’s Respiratory Initiative to establish a base for the evaluation of both the risks and benefits of documented significant infant viral respiratory infection of varying severity and etiology and other environmental exposures on childhood atopy outcomes and to establish a biospecimen repository for analyses including biomarker testing and genotyping. The prospective cohort has the longitudinal design properties that may overcome potential limitations intrinsic to retrospective studies, such as our TABS cohort.1–5 It is our eventual goal that the findings from these investigations, in conjunction with other investigations that have helped to elucidate genetic and environmental factors associated with asthma development, will help in the identification of primary and secondary prevention strategies for asthma. This report describes the study objectives, design, and recruitment results of this study cohort.
METHODS
Study objectives and design
The TCRI is a prospective cohort of mother-infant dyads enrolled in a longitudinal investigation of the relationship of infant viral respiratory infection severity and etiology and the interaction of other risk factors on the development of childhood asthma and allergic diseases. The study was approved by the Vanderbilt Institutional Review Board, and parents provided written informed consent for both their and their child’s study participation.
Subject recruitment and study population
Term, non-low birth weight, otherwise healthy infants were enrolled along with their biological mothers, at a single academic institution, Vanderbilt Children’s Hospital, at the time of an acute visit (hospitalization, emergency department or unscheduled outpatient visit) for presumed viral bronchiolitis or upper respiratory tract infection during respiratory viral seasons September through May 2004–2008. Inclusion and exclusion criteria are outlined in Table 1. Because of the grant funding start date, the first study season did not begin until November 2004. Recruitment was solely hospital and clinic based, and was performed 7 days/week during the first two years of cohort accrual, and 5 days per week for the two subsequent years. Screening and recruitment were done by experienced research nurses using computerized medical charts to screen infants with presumed respiratory viral illness.
Table 1.
Tennessee Children’s Respiratory Initiative Enrolment Inclusion and Exclusion Criteria
| Enrolment Inclusion Criteria | Operational Definition |
|---|---|
| Gestational age | ≥ 37 weeks |
| Birth weight | ≥ 2275 grams |
| Previously healthy infant | See exclusions |
| Birth – 12 months of age | Birth through 12 months of age during the acute respiratory illness enrolment visit |
| Biological mother available | Biological mother available to complete skin testing and questionnaire |
| Presumed viral bronchiolitis, LRTI, or URI | Based on both admitting physician diagnosis AND documentation of symptoms with duration < 11 days (including any two of the following: cough, nasal congestion, rhinorrhea, wheezing, dyspnea, fever) and requirement for either hospitalization, including 23 hour stay, ED visit, or acute care clinic visit |
| Enrolment Exclusion Criteria | |
| Adopted or foster child | |
| Unable to determine maternal history | |
| Significant co-morbidities or cardio-pulmonary disease | Congenital or acquired chronic heart or lung disease, prior requirement for mechanical ventilation for cardiac or pulmonary disease, immunodeficiency, neurologic disease with possible aspiration, significant gastroesophageal reflux disease felt to contribute to pulmonary disease, tracheomalacia |
| Ever received one or more doses of RSV-IVIG or palivizumab | |
| Prior study inclusion | |
| Fever and neutropenia | |
| Children whose parents or guardians were not able to understand the consent process, or a language barrier* |
Spanish speaking families were enrolled during the first two years of the study, and not thereafter because of lack of trained bilingual research personnel.
Examination components of the enrolment visit
The components and timeline of the subject visits are outlined in Table 2. The trained research nurses administered a structured questionnaire during an in-person interview. The questionnaire was used to obtain information on demographics, acute medical symptoms, previous medical history, infant feeding method, development, co-morbid conditions, detailed family history of asthma and atopic diseases using a family tree form routinely used in genetic epidemiology studies, responses to the International Study of Asthma and Allergies in Children (ISAAC) questionnaire, home exposures including pets and detailed smoking exposure, ill contacts, number of siblings, and daycare attendance.6–8 A pilot sample of mothers of hospitalized infants also completed the Block 2000-Brief food frequency questionnaire (FFQ), Nutritionquest, Berkeley, CA.9–11 Infant biospecimen collection included nasal and throat swabs for viral detection, and bag urine samples for biomarker determination. Mothers underwent prick skin testing to 8 common aeroallergens and a blood specimen was obtained by venepuncture for serum IgE and DNA. A structured abstraction form was used to obtain information from the medical record regarding the index enrolment visit: current infant weight, confirmation of birth weight, room air pulse oximetry, requirement for supplemental oxygen, medication administration, prior wheezing episodes, and detailed information on the current illness and hospital course. Following discharge from the hospital or outpatient setting, the final discharge diagnosis and results of culture data were obtained through chart review.
Table 2.
Components of the Tennessee Children’s Respiratory Initiative enrolment visit, and each follow-up contact.
| Enrolment | 1 to 2 Year Old Well child follow-up visit |
Year 1 – Year 3 | Year 4 – Year 5 | Year 6 follow-up | |
|---|---|---|---|---|---|
| 0–12 month old term, non low birth weight otherwise healthy infant enrolled at the time of a respiratory viral illness* | 2 year well-child visit conducted in the CRC† or during home visit | Phone contact | Phone contact | Phone contact | |
| Time line | Sept-May 2004–2008 | 2005–2009 | 2005–2011 | 2008–2012 | 2010–2014 |
| Maternal skin testing‡ | ✓ | ||||
| Questionnaire and chart review | ✓ | ||||
| Administration of structured phone questionnaire | ✓ | ✓ | |||
| Infant nasal and throat swab for viral identification | ✓ | ||||
| Infant urine specimen | ✓ | ||||
| Family history, including detailed atopic history | ✓ | ||||
| Infant nasal epithelium (for cell culture repository) | ✓ | ||||
| Child serum IgE and allergen specific IgE determination | ✓ | ||||
| Infant DNA | ✓ (or) | ✓ | |||
| Maternal DNA | ✓ (or) | ✓ | |||
| Routine phone/mailing contact | ✓ | ✓ | ✓ | ✓ | |
| Every 12–18 months | |||||
These infant-mother dyads were recruited and enrolled at Vanderbilt Children’s Hospital (VCH), VCH pediatric emergency department, and VCH pediatric acute care clinic.
Clinical Research Center
Allergen specific IgE (Phadiatop) is performed if unable to perform prick skin testing or negative histamine/positive control
Cohort follow-up
There are three phases of cohort follow-up. (1) Mothers and children undergo an in-person well-child follow-up visit during the child’s second year of life conducted in the Vanderbilt Clinical Research Center (CRC), or during a home visit. (2) Families are re-contacted every 12–18 months by phone and/or mailings for purposes of cohort retention, and to provide reminders about the remaining study components. (3) Mothers, or the current guardians, undergo a phone interview during the fourth and sixth years of life to identify children with asthma, transient wheezing, allergic rhinitis and eczema.
Examination components of the second year well child follow-up visit
The 2-year in-person well-child visit is conducted in the Vanderbilt Clinical Research Center (CRC), or during a home visit offered to those unable to return to the study institution. During the visit, the ISAAC questionnaire is administered, blood samples are obtained from children and mothers (if not previously collected), and a buccal swab is collected for DNA if blood cannot be obtained.
Examination components of the fourth and sixth year of life questionnaire
A structured telephone questionnaire is administered to the mother/parent when their child is 4 and again at 6 years. Trained interviewers employed in the Vanderbilt Survey Research Shared Resource, use a web-based computer system to conduct structured telephone interviews which capture detailed information on asthma and atopy diagnoses and symptoms, extensive environmental exposure history, physical activity, and co-morbidities. Asthma, allergic rhinitis, and atopic dermatitis outcomes are determined using the ISAAC questionnaire. For children with report of asthma and/or asthma symptoms in the previous 12 months, the Asthma Therapy Assessment Questionnaire (ATAQ) is administered, and information on asthma medications, and asthma-related health care visits are sought.12,13
Biospecimen collection and laboratory analyses
Table 3 outlines the details of cohort biospecimen collection, repository, and testing, which includes infant nasopharyngeal, urine, blood and nasal epithelial cell sample collection, and maternal prick skin testing and blood samples.
Table 3.
Tennessee Children’s Respiratory Initiative biospecimen collection and laboratory analyses
| Biospecimen type |
Collection timepoint |
Maternal or child bio- specimen |
Collection and Analyses | |
|---|---|---|---|---|
| Nasopharyngeal swab for identification of viral pathogens | Two nasal and throat swabs | Enrolment | Infant | Two nasal and throat swabs are obtained from infants at enrolment during the acute illness using Dacron swabs placed in both Hank’s viral transport media and lysis buffer. The biospecimens are processed, aliquoted, and stored at −80°C for en batch for RSV A and B, human RV, adenovirus, hMPV, coronaviruses, influenza A and B, and parainfluenza types 1, 2 and 3, using the described molecular techniques. First nucleic acid extraction is performed with a Roche MagNApure LC automated instrument that is capable of high-throughput specimen processing. The laboratory has developed highly sensitive and specific qRT-PCR assays for many common respiratory viruses, including hMPV, HCoV, RSV, influenza A and B, parainfluenzaviruses 1–3, and rhinovirus. Realtime RT-PCR is performed using the Cepheid Smart Cycler II. All specimens are first tested for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to confirm integrity of RNA and monitor for potential PCR inhibitors. Negative and positive controls are included with each run, including RNA runoff transcripts to generate a quantitation curve.19–26 If rapid antigen and/or culture for RSV or Influenza were performed at the discretion of the admitting physicians during the index visit, these results are also captured and entered in the database |
| Maternal prick skin testing or specific IgE determination | Skin test or blood sample | Enrolment | Maternal | Maternal prick skin testing was performed by trained research nurses. Women were first screened for pregnancy, and those who were not pregnant or who had no other contraindication to skin testing underwent prick skin testing to saline, histamine and eight aeroallergens: cat pelt, Alternaria, Grass Mix #7, Ragweed mix, Oak mix, Tricophytan, Mite mix, cockroach mix (Quintest Extract Tray, HollisterStier, Spokane, WA). Phadiatop was also performed on maternal blood samples for women who could not undergo prick skin testing, or who had an inadequate skin test. |
| Specific and total IgE | Blood | Follow-up well-child visit | Infant | The total serum IgE and multi-allergen screen (Phadiatop, Phadia, Kalamazoo, MI) were measured on the ImmunoCAP250. Data were reported in kIU/L (total IgE) where 1 IU = 2.42 ng and kUa/L for the multi-allergen screen. The Johns Hopkins DACI laboratory will perform these tests and is a Federally licensed (CLIA-88 certified) laboratory.27,28 A positive Phadiatop is defined as ≥ 0.35 KUA/L. |
| Nasal epithelial cells | Nasal turbinate swab | Follow-up well-child visit | Child | Primary cultures of nasal respiratory epithelia are established by methods developed for the cultivation of epidermal keratinocytes with modifications and collected to examine innate immune response and phenotypic differences to in vitro infection among cells from infants with LRTI v URI.29 The cells are collected using Mid Turbinate Peds Nylon Flocked Swabs (MicroRheologics, Brescia, Italy), and placed into collection and digestion media, followed by processing and plating onto collagen IV coated tissue culture and grown at 37°C in a 5% CO2 incubator. To develop techniques for isolation, short-term culture, and in vivo modeling of epithelial and stromal cells, a xenograph model has been developed. Following short-term growth in culture, cells are transferred to denuded rat tracheas and implanted subcutaneously in nude mice. |
| DNA | Blood or buccal swab | Follow-up well-child visit | Mother and child | DNA is collected from both the mother and child during the blood collection, or using a buccal swab if blood can not be obtained. DNA is extracted by the Vanderbilt Center for Human Genetics Research Core Laboratory and stored following extraction for future studies. |
| Urine | Bagged infant urine collection | Enrolment | Infant | Urine is collected from hospitalized infants during the acute infant illness at study enrolment, and from a convenience sample of outpatient subjects. Urinary measurements including, leukotrienes C4/D4/E4 (LTC4/D4/E4), and urinary metabolite of the isoprostane, 15-F2t-IsoP (8-iso-PGF2α) will be measured by a gas chromatographic, and other biomarkers and the remainder of the urinary biospecimens will be maintained in the repository.30,31 |
Ascertainment of ARI, childhood asthma and allergic diseases
Acute respiratory illness (ARI) type and severity
The discharge diagnosis and supporting clinical parameters of the infant ARI visit were reviewed to confirm whether each child had LRTI, URI (N = 628) or another diagnosis (N = 46). LRTI and URI were defined using both the physician discharge diagnosis, as well as post-discharge chart review, and those cases which were not clearly identified as either LRTI or URI were reviewed by a panel of pediatricians who determined whether the illness represented an LRTI, URI, croup or other, which included those which could not be categorized with the available clinical information.
ARI severity was determined using the ordinal bronchiolitis score which incorporates admission information on respiratory rate, flaring or retractions, room air oxygen saturation, and wheezing, into a score ranging from 0–12 (12 being most severe).14,15
Familial, maternal and child atopic status
(1) The family history of atopy was obtained using a family tree. (2) Maternal atopy will be categorized as evidence of atopy by skin testing or specific IgE, and/or or clinical symptoms of an atopic disease as assessed by the ISAAC questionnaire. (3) Atopic status of the child will be determined by laboratory evidence of specific IgE during the second year of life, and by clinical evidence based on the above definitions.
Childhood asthma
The diagnosis of asthma will be determined at age 6 years based on responses to the ISAAC questionnaire.6–8 The following criteria will define asthma during the sixth year of life: (1) 12-month prevalence of symptoms of asthma (current wheeze) or the presence of exercise-induced wheeze or dry cough at night not due to a cold or chest infection, and (2) physician diagnosis as determined by the ISAAC questionnaire using either parental reported physician diagnosis of asthma or chronic use/prescription of asthma-specific medications. Probable asthma will be defined as physician diagnosis only and analyzed separately.
Transient early wheezing will be defined as wheezing episodes present in the first four years of life, but not meeting the definition for childhood asthma at age 4 and 6 years.16
Allergic rhinitis (AR) will be determined through the ISAAC core questions on AR.7,8 Children will be considered to have definite AR if each of three conditions is present between age 5 and 6 years: (1) a history of nasal congestion, runny nose, itchy watery eyes, sinus pain or pressure or headaches, sneezing, blocked nose, loss of sense of smell; and (2) substantial variability in symptoms over time or seasonality; and (3) diagnosed as having allergic rhinitis by a physician or on medications for AR. Probable allergic rhinitis will be defined as meeting two of the three criteria, or only criteria 3.
Atopic dermatitis will be determined through the ISAAC core questions on atopic dermatitis which are based on a list of major and minor criteria widely applied in clinical studies.8,17,18 As eczema is probably more readily confirmed by objective tests than either asthma or rhinitis, patients will be considered to have definite atopic dermatitis if between age 5 and 6 years they report ever having an itchy rash that comes and goes for at least 6 months, and being diagnosed with eczema by a physician.17,18 Probable atopic dermatitis will be defined as one of the two above criteria.
Quality-Control Procedures
In order to standardize and monitor the quality of data collection and processing, all study personnel received training and were certified for all the study procedures. Information is recorded on paper case report forms, data is entered and then checked by a second reviewer. Logical data checks are programmed and additionally performed by our systems analyst, investigators and again by our biostatisticians. For laboratory analyses, blind quality control samples are included in each biospecimen run. Telephone interviewers complete classroom training, orientation to the study population, computer modules, role play interviewing, and training on study-specific protocols, and are formally evaluated at the end of training. A verbatim-recording of the interviewer and participant responses, and 10% participant re-contact allows quality control staff to verify responses.
Statistical analyses
The outcome variables of interest are the incidence of asthma and allergic rhinitis. The primary exposure variables of interest are the severity and etiology of the infant viral illness and maternal and familial atopic status and other environmental exposures. Cumulative asthma incidence over time, taking into account loss to follow-up, will be used for illustrating incidence data. Incidence of asthma and allergic diseases among the enrolled infant population with viral respiratory illness will be calculated by dividing the number of incident asthma cases by the person time of follow-up. A Kaplan-Meier plot of cumulative incidence over time, taking into account loss to follow-up, will be used for illustrating incidence data. Incidence rates will be calculated by dividing the patient population into quartiles/quintiles of bronchiolitis severity scores. The adjusted risk of asthma and allergic rhinitis with bronchiolitis severity will be evaluated using the Cox proportional hazard regression model.
To assess the relationship between biomarker concentrations and increased risk of infant bronchiolitis, and early childhood asthma outcomes, we will conduct a nested case-control study. Geometric means of urinary biomarker concentrations for those who develop and do not develop asthma will be calculated separately and compared using the paired T-test. The association between these biomarkers and the risk of asthma and allergic rhinitis will be assessed using odds ratios and their corresponding 95% confidence intervals from adjusted logistic regression models. The potential confounders that will be considered in multivariable analyses will include demographic and exposure characteristics.
RESULTS
Subject recruitment for the TCRI Study occurred over four years, and was completed in May 2008. Overall, 9,329 visits were screened, representing 7,632 unique infants, and 2,986 of these infants met study eligibility requirements. From the 2,986 eligible infants, 674 infants and their biological mothers were enrolled (figure 1). Among the 2,312 subjects who were available during the recruitment periods, the major reasons for non-response were refusal (22%), insufficient time/unwilling to stay for the visit (outpatients) (39%), conflict with or already enrolled in another study (20%), and other (18%) (includes language barrier, mother/guardian not present, previously enrolled, and other miscellaneous). In 99.9% of the cohort, the nasal / throat swab was obtained, and in 79% of the hospitalized infants one spot urine sample was obtained at hospital admission. Weekly enrolment into the cohort is depicted in figure 2.
Figure 1.
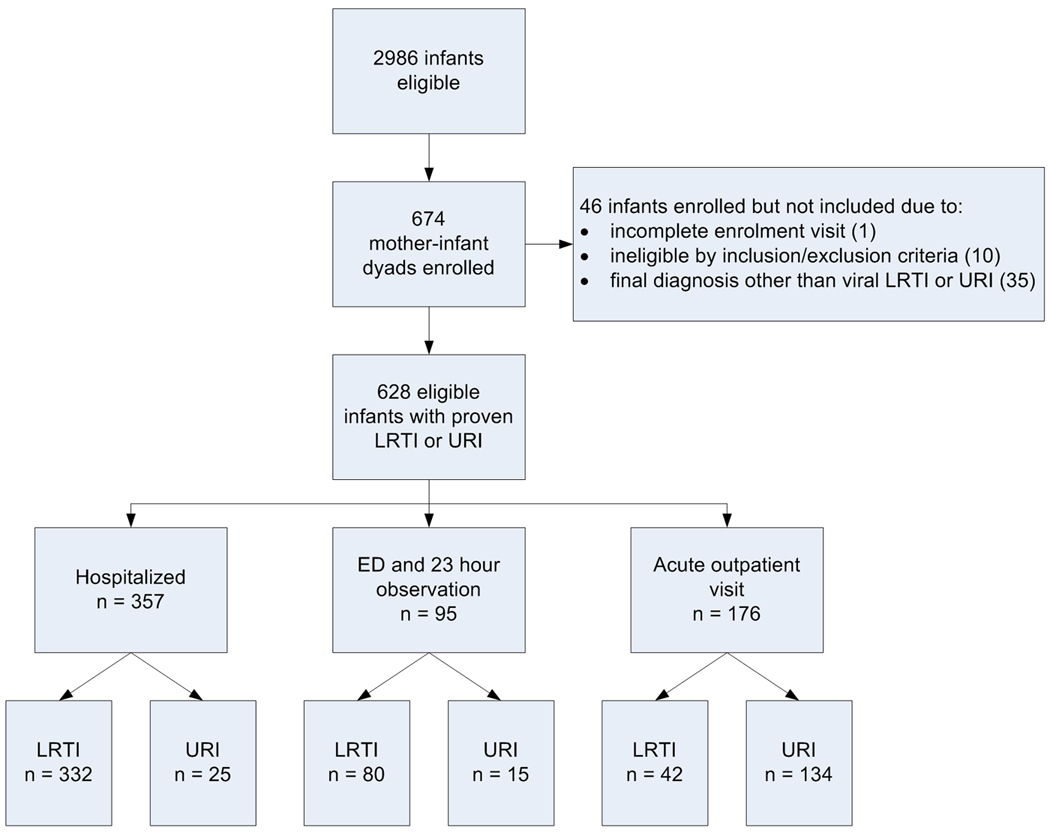
Flow diagram of participants through the screening, enrolment, and initial analysis phases of the Tennessee Children’s Respiratory Initiative.
Figure 2.
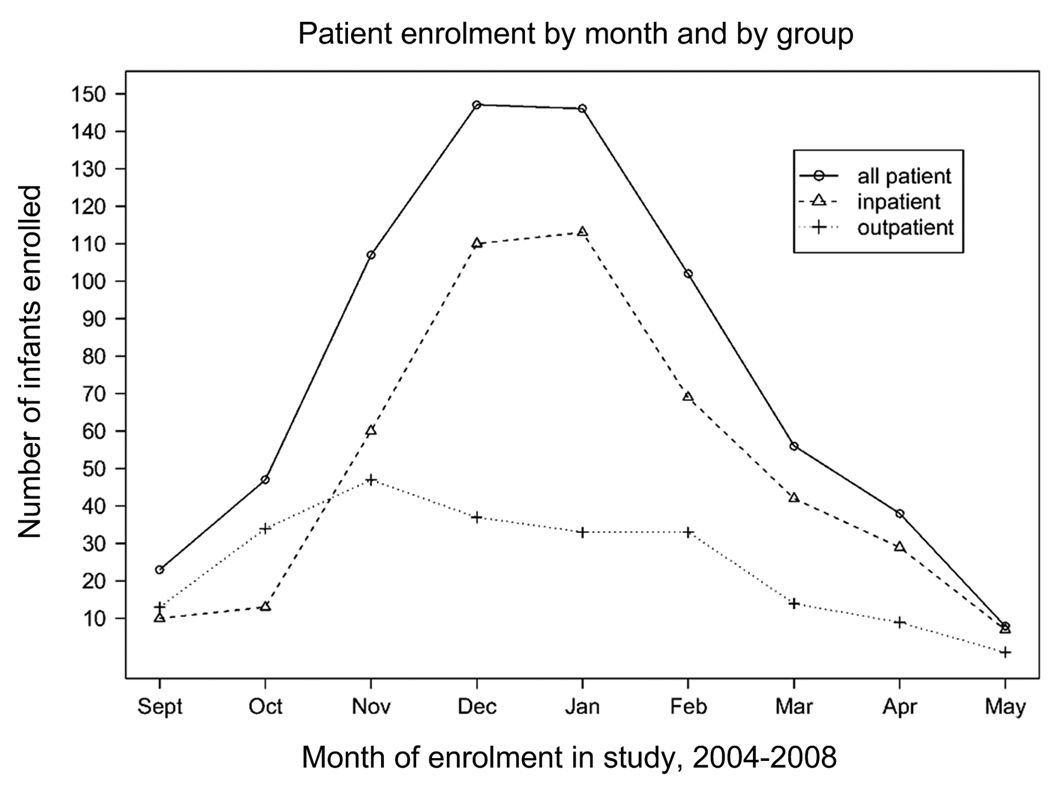
Weekly enrolment during the September through May enrolment periods of the Tennessee Children’s Respiratory Initiative, 2004 – 2008.
DISCUSSION
The TCRI is a large and comprehensive prospective epidemiologic study of mothers and their biologic children enrolled during infancy with a clinically significant LRTI or URI who are being followed through early childhood. This study will provide important information about the role of infant respiratory viral infection severity, etiology, biomarkers and predictors important in the development of early childhood asthma and allergic rhinitis. The TCRI is additionally unique because it is designed in parallel with a large retrospective birth cohort of over 95,000 mother-infant dyads with similar objectives to investigate the role of respiratory viral infection severity and etiology in the development of asthma.
As evidence suggests that the development of asthma may result in part from respiratory viral infection during infancy, which has a predilection for infecting, destroying, and/or in some way biologically altering lower airway epithelium, this study will help to delineate whether the severity of that infection, and other early life events impact the risk of asthma and allergic disease development in later childhood.3 Despite a high attack rate of developing asthma following viral bronchiolitis, the majority of children who have infant bronchiolitis do not develop childhood asthma. Thus while viral respiratory infections may alter lung physiology and target the inflammatory response to the lower airway, this may only occur during a vulnerable time-period during development of the immune system or lung, or in the presence of other risk factors. This developmental component may further reflect important gene–environment interactions that regulate both short- and long-term airway physiological alterations that manifest themselves clinically as childhood asthma. Efforts to determine and define the role of these factors, including disease severity, maternal atopy and other environmental exposures such as second hand smoke, to asthma pathogenesis are the focus and goal of the Tennessee Children’s Respiratory Initiative (TCRI).
Several limitations of this study should be noted. First, the study sample was not randomly selected from the general population, but instead was recruited from a single hospital and clinic-based setting, the Vanderbilt Children’s Hospital. While Vanderbilt hospitalizations represent greater than 90% of Davidson County/Nashville infant hospitalizations, it represents a smaller proportion of emergency department visits (51%), and likely even fewer pediatric acute care visits.2 The relatively low participation rate among eligible subjects is multifactorial and the result of the long-term nature of the study, the lack of study personnel to enroll all eligible subjects, as well as lower willingness among outpatients to extend their visit in order to participate in the study. While this impacts the demographics and exposures of the study population, and thus generalizibility of our study results, it should not impact the findings of the role of infant viral infection on the outcomes of interest. Next, while airway hyperreactivity is not assessed in making the diagnosis of childhood asthma, the identification of incident asthma cases will take into consideration the positive response to a validated written questionnaire that has been compared with bronchial provocation testing.6,8 While such an ascertainment strategy might result in the under-diagnosis of asthma, it is unlikely to result in false positive diagnoses during the sixth year of life. Lastly, as with many studies, where all eligible participants were approached for participation, difficulty was encountered in follow-up of those currently age-eligible for follow-up. Strategies to address this include study personnel doing a significant number of follow-up visits at the subject’s home, and shipping follow-up materials and requests to pediatricians of subjects who have moved from the region.
Future reports from this cohort will help to clarify the complex relationship between infant respiratory viral infection severity, etiology, atopic predisposition, and the development of early childhood asthma and other atopic diseases. Ultimately, this study, along with the companion TABS cohort, has the potential to provide new approaches to identify infants at high risk of developing early childhood asthma and allergic diseases, as well as provide important information that may contribute to the development of prevention strategies.
Acknowledgements
We would like to thank the many families, pediatricians and their office staff, who have participated in this study, as without them, this work would not have been possible.
The Vanderbilt Center for Asthma and Environmental Health Research investigators and collaborators include: Paul Moore, John V. Williams, Marie Griffin, Kathryn Edwards, William Dupont, Kathryn Miller, Asad Ali, Toddra Liddell, Rachel Armstrong-Richardson, Rachel Hayes, Pingsheng Wu, Robert Valet, Jennifer Ker, Megan Lemke, Sara Van Driest, Zhouwen Liu, Janice Brooks, Carol Meisch, James Gern, R. Stokes Peebles, L. Jackson Roberts II, Wendall Yarbrough, Adriana Bialozosky.
This work was supported by Thrasher Research Fund Clinical Research Grant (to TVH), NIH mid-career investigator award K24 AI 077930 (to TVH), UL1 RR024975 (Vanderbilt CTSA), and NIH K01 AI070808 mentored clinical scientist award (to KNC). TVH is also supported by NIH U01 HL 072471 [which supports the Tennessee Asthma Bronchiolitis Study (TABS) cohort], R01 AI 05884, and NIH K12 ES 015855.
Abbreviations
- TCRI
Tennessee Children’s Respiratory Initiative
- TABS
Tennessee Asthma Bronchiolitis Study
- TN
Tennessee
- LRTI
Lower respiratory tract infection
- URI
Upper respiratory tract infection
- ARI
acute respiratory illness
- IgE
Immunoglobulin E
Footnotes
Summary at a Glance
This report describes the study objectives, design, and recruitment results of the Tennessee Children’s Respiratory Initiative, designed to investigate the role of respiratory viral infection severity and etiology in asthma development. Future reports will address the complex relationship between infant respiratory viral infection and asthma and atopic diseases.
REFERENCES
- 1.Carroll KN, Gebretsadik T, Griffin MR, et al. Increasing burden and risk factors for bronchiolitis-related medical visits in infants enrolled in a state health care insurance plan. Pediatrics. 2008;122:58–64. doi: 10.1542/peds.2007-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 3.Wu P, Dupont WD, Griffin MR, et al. Evidence of a Causal Role of Winter Virus Infection During Infancy on Early Childhood Asthma. Am J Respir Crit Care Med. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carroll KN, Wu P, Gebretsadik T, et al. The Severity-Dependent Relationship of Infant Bronchiolitis on the Risk and Morbidity of Early Childhood Asthma. J Allergy Clin Immunol. 2009;123:1055–1061. doi: 10.1016/j.jaci.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll KN, Wu P, Gebretsadik T, et al. Season of infant bronchiolitis and estimates of subsequent risk and burden of early childhood asthma. J Allergy Clin Immunol. 2009;123:964–966. doi: 10.1016/j.jaci.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sole D, Vanna AT, Yamada E, et al. International Study of Asthma and Allergies in Childhood (ISAAC) written questionnaire: validation of the asthma component among Brazilian children. J Investig Allergol Clin Immunol. 1998;8:376–382. [PubMed] [Google Scholar]
- 7.Braun-Fahrlander C, Wuthrich B, Gassner M, et al. Validation of a rhinitis symptom questionnaire (ISAAC core questions) in a population of Swiss school children visiting the school health services. SCARPOL-team. Swiss Study on Childhood Allergy and Respiratory Symptom with respect to Air Pollution and Climate. International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 1997;8:75–82. doi: 10.1111/j.1399-3038.1997.tb00147.x. [DOI] [PubMed] [Google Scholar]
- 8.Asher MI, Keil U, Anderson HR, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J. 1995;8:483–491. doi: 10.1183/09031936.95.08030483. [DOI] [PubMed] [Google Scholar]
- 9.Block G, Sinha R, Gridley G. Collection of dietary-supplement data and implications for analysis. Am J Clin Nutr. 1994;59:232S–239S. doi: 10.1093/ajcn/59.1.232S. [DOI] [PubMed] [Google Scholar]
- 10.Block G, Hartman AM, Dresser CM, et al. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 11.Block G, Block T, Block CH. Nutrition Quest, Assessment Tools for Health Researchers. 1993. http://www.nutritionquest.com/index.htm. Ref Type: Electronic Citation. [Google Scholar]
- 12.Schatz M, Sorkness CA, Li JT, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 13.Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Goebel J, Estrada B, Quinonez J, et al. Prednisolone plus albuterol versus albuterol alone in mild to moderate bronchiolitis. Clin Pediatr (Phila) 2000;39:213–220. doi: 10.1177/000992280003900404. [DOI] [PubMed] [Google Scholar]
- 15.Tal A, Bavilski C, Yohai D, et al. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics. 1983;71:13–18. [PubMed] [Google Scholar]
- 16.Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 17.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44–47. [Google Scholar]
- 18.Diepgen TL, Fartasch M, Hornstein OP. Evaluation and relevance of atopic basic and minor features in patients with atopic dermatitis and in the general population. Acta Derm Venereol Suppl (Stockh) 1989;144:50–54. doi: 10.2340/000155551445054. [DOI] [PubMed] [Google Scholar]
- 19.Williams JV, Crowe JE, Enriquez R, et al. Human metapneumovirus plays an etiologic role in acute asthma hospitalization in adults. J Infect Dis. 2005;192:1149–1153. doi: 10.1086/444392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee WM, Grindle KA, Pappas TE, et al. High-throughput, sensitive, and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. J Clin Microbiol. 2007;45:2626–2634. doi: 10.1128/JCM.02501-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams JV, Tollefson SJ, Heymann PW, et al. Human metapneumovirus infection in children hospitalized for wheezing. J Allergy Clin Immunol. 2005;115:1311–1312. doi: 10.1016/j.jaci.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams JV, Martino R, Rabella N, et al. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis. 2005;192:1061–1065. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams JV, Tollefson SJ, Nair S, Chonmaitree T. Association of human metapneumovirus with acute otitis media. Int J Pediatr Otorhinolaryngol. 2006;70:1189–1193. doi: 10.1016/j.ijporl.2005.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams JV, Wang CK, Yang CF, et al. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehlmann M, Bonner AB, Williams JV, et al. Comparison of the MChip to viral culture, reverse transcription-PCR, and the QuickVue influenza A+B test for rapid diagnosis of influenza. J Clin Microbiol. 2007;45:1234–1237. doi: 10.1128/JCM.02202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee WM, Kiesner C, Pappas T, et al. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One. 2007;2:e966. doi: 10.1371/journal.pone.0000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton RG, Franklin AN., Jr In vitro assays for the diagnosis of IgE-mediated disorders. J Allergy Clin Immunol. 2004;114:213–225. doi: 10.1016/j.jaci.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton RG, Adkinson NF., Jr 23. Clinical laboratory assessment of IgE-dependent hypersensitivity. J Allergy Clin Immunol. 2003;111:S687–S701. doi: 10.1067/mai.2003.123. [DOI] [PubMed] [Google Scholar]
- 29.Rheinwald, Green . Methods in Molecular Medicine: Human Cell Culture Protocols. 2nd ed. Totowa, New Jersey: Humana Press Inc; 1975. [Google Scholar]
- 30.Gentile DA, Fireman P, Skoner DP. Elevations of local leukotriene C4 levels during viral upper respiratory tract infections. Ann Allergy Asthma Immunol. 2003;91:270–274. doi: 10.1016/S1081-1206(10)63529-6. [DOI] [PubMed] [Google Scholar]
- 31.Morrow JD, Zackert WE, Yang JP, et al. Quantification of the major urinary metabolite of 15-F2t-isoprostane (8-iso-PGF2alpha) by a stable isotope dilution mass spectrometric assay. Anal Biochem. 1999;269:326–331. doi: 10.1006/abio.1999.4008. [DOI] [PubMed] [Google Scholar]


