Abstract
Synaptic dysfunction is widely thought to be a pathogenic precursor to neurodegeneration in Alzheimer’s disease (AD), and the extent of synaptic loss provides the best correlate for the severity of dementia in AD patients. Presenilins 1 and 2 are the major causative genes of early-onset familial AD. Conditional inactivation of presenilins in the adult cerebral cortex results in synaptic dysfunction and memory impairment, followed by age-dependent neurodegeneration. To characterize further the consequence of presenilin inactivation in the synapse, we evaluated the temporal development of presynaptic and postsynaptic deficits in the Schaeffer-collateral pathway of presenilin conditional double knockout (PS cDKO) mice prior to onset of neurodegeneration. Following presenilin inactivation at 4 weeks, synaptic facilitation and probability of neurotransmitter release are impaired in PS cDKO mice at 5 weeks of age, whereas postsynaptic NMDA receptor-mediated responses are normal at 5 weeks but impaired at 6 weeks of age. Long-term potentiation induced by theta burst stimulation is also reduced in PS cDKO mice at 6 weeks of age. These results show that loss of presenilins results in presynaptic deficits in short-term plasticity and probability of neurotransmitter release prior to postsynaptic NMDA receptor dysfunction, raising the possibility that presenilins may regulate postsynaptic NMDA receptor function in part via a trans-synaptic mechanism.
Keywords: Synaptic dysfunction, neurodegeneration, Alzheimer’s disease, conditional knockout, synaptic facilitation, NMDA receptor, LTP, neurotransmitter release
Introduction
Alzheimer’s disease (AD) is characterized clinically by progressive memory loss and other cognitive disabilities, and neuropathologically by synaptic and neuronal loss as well as accumulation of extracellular amyloid plaques and intraneuronal fibrillary tangles. The presenilin genes harbor the majority of the mutations linked to familial forms of early-onset AD (Sherrington et al. 1995, Levy-Lahad et al. 1995, Rogaev et al. 1995). Presenilin, along with Aph1, Pen2 and Nicastrin, are integral components of a multiprotein protease complex, termed γ-secretase, which is responsible for the intramembranous cleavage of type I transmembrane proteins, such as the amyloid precursor protein and Notch receptors (De Strooper et al. 1999, De Strooper et al. 1998, Fortini 2002). During development, presenilin plays a major role in the maintenance of neural progenitor population through the regulation of the Notch signaling pathway (Shen et al. 1997, Handler et al. 2000, Wines-Samuelson et al. 2005, Wines-Samuelson & Shen 2005, Kim & Shen 2008). In the adult brain, presenilin is expressed highly in excitatory neurons of the cerebral cortex, and is required for memory formation and age-dependent neuronal survival in a γ-secretase-dependent manner (Yu et al. 2001, Saura et al. 2004, Feng et al. 2004, Wines-Samuelson et al. 2010, Tabuchi et al. 2009).
Synaptic dysfunction is widely thought to be a key pathogenic event before frank neuronal death in AD pathogenesis (Hsia et al. 1999, Saura et al. 2004). Inactivation of presenilin in conditional double knockout (PS cDKO) results in striking pre- and post-synaptic impairments prior to progressive neurodegeneration that resemble key neuropathological features of AD, raising the possibility that partial loss of presenilin function may contribute to synaptic dysfunction and neurodegeneration in AD (Shen & Kelleher 2007, Saura et al. 2004, Zhang et al. 2009, Wines-Samuelson et al. 2010). Further dissection of synaptic dysfunction caused by loss of presenilin may provide additional insight into the mechanisms underlying age-related synaptic loss and neuronal degeneration in AD.
In the current study, we performed a detailed electrophysiological analysis in the hippocampal Schaeffer-collateral pathway to establish temporal development of the pre- and post-synaptic defects caused by loss of presenilin in PS cDKO mice. We found that the presynaptic deficits, such as frequency facilitation and Ca2+ dependency of facilitation, occur prior to postsynaptic NMDAR deficits. Furthermore, we show that the presynaptic deficits are associated with a reduction in release probability. Our study suggests that presynaptic deficit in neurotransmitter release is the initial impairment prior to postsynaptic NMDAR-mediated dysfunction in PS cDKO mice.
Materials and methods
Mice
The generation of PS cDKO mice has been previously described (Saura et al. 2004). Briefly, fPS1/PS1;PS2−/−;CamKII-Cre mice were bred with fPS1/fPS1;PS2−/− mice to obtain PS cDKO mice (fPS1/fPS1;PS2−/−;Cre) and fPS1/fPS1;Cre were bred with fPS1/fPS1 to obtain control mice (fPS1/fPS1). The genetic background of the PS cDKO and control mice was similar in the C57BL6/129 hybrid background.
Western analysis
Quantitative western blots were carried out as previously described (Ho et al. 2006). Briefly, equal amount of protein were resolved on SDS-PAGE and radioisotope 125I-labeled secondary antibodies were used for quantitative analyses followed by PhosphorImager (Molecular Dynamics).
Electrophysiological analysis
Electrophysiology experiments were performed on male PS cDKO mice at various ages. Acute hippocampal slices at 400 μm were prepared using a vibratome 2000 as previously described (Saura et al. 2004). Slices were maintained in a storage chamber containing oxygenized artificial cerebrospinal fluid (aCSF in mM: 124 NaCl, 5 KCl, 1.25 NaH2PO4, 1.3 MgCl2, 2.6 CaCl2, 26 NaHCO3, 10 glucose, pH 7.4, 310 – 315 mOsm) at room temperature after one hour’s recovery at 30°C. Synaptic responses were evoked at the Schaeffer-collateral pathway by a bipolar concentric metal electrode (500 μs biphasic pulses), and recorded with aCSF-filled glass microelectrodes (1 to 2 MΩ) extracellularly in CA1 stratum radiatum. The recording chamber and aCSF perfusion solution was kept at 30°C. LTP was induced by five episodes of TBS delivered at 0.1 Hz. Each episode contains ten stimulus trains (5 pulses at 100 Hz) delivered at 5 Hz. Average responses (mean ± s.e.m.) are expressed as percentage of pre-TBS baseline responses, which were evoked by half-maximum stimulation intensity with 0.2 ms pulses at 0.033 Hz. NMDAR responses were recorded in Mg2+-free aCSF containing 10 μM CNQX and 100 μM picrotoxin, which block AMPA receptor- and GABA type A receptor-mediated responses, respectively. Whole-cell recordings were performed in CA1 pyramidal neurons visually identified with an IR-DIC BX51 Olympus microscope. Patch pipettes (2–4 MΩ) were filled with internal solution consisting of (in mM): 130 CsCl, 8 KCl, 10 EGTA, 10 HEPES, 5 QX-314, 3 ATP, 0.3 Na2GTP, pH 7.2, 280–285 mOsm. Chemicals were from Sigma unless otherwise noted. Experimenters were blind to the genotypes of the mice.
Statistics
All data shown are mean ± SEM. Statistical analysis was performed with Student’s t-test, *=p<0.05; **=p<0.01; ***=p<0.001.
Results
Impaired frequency facilitation in PS cDKO mice at 5 weeks of age
Our previous Western analysis showed that PS1 expression begins to be reduced in the cerebral cortex of PS cDKO mice between postnatal days 18 and 22 (Yu et al. 2001). At 2 months of age, in addition to spatial and associative memory impairments, marked pre- and post-synaptic defects, including paired-pulse facilitation and NMDA receptor- (NMDAR-) mediated responses, are observed in the hippocampal Schaeffer-collateral pathway of PS cDKO mice (Saura et al. 2004). To establish the time course of temporal development of these presynaptic and postsynaptic defects, we evaluated presynaptic and postsynaptic function in PS cDKO mice before the age of 2 months. Our Western analysis showed ~50% reduction of PS1 in the cerebral cortex of PS cDKO mice at 4 weeks of age (Fig. 1a). The residual PS1 in cortical lysates prepared from PS cDKO mice is due to expression of PS1 in glia, interneurons and perhaps a small percentage of excitatory neurons that lack Cre expression (Yu et al. 2001).
Fig. 1.
Impaired frequency facilitation in PS cDKO mice. (a) Reduction of PS1 in cortical lysates of PS cDKO mice at 4 weeks of age. (b–c) Representative traces from 5-week-old control and PS cDKO mice at 1 and 20 Hz stimulation frequency, respectively. Averaged responses to stimulus trains of 1 and 20 Hz stimulations recorded from control and PS cDKO mice. Values of EPSC amplitude were normalized to the amplitude of first EPSC in stimulation trains. (d) Summary graph comparing frequency dependence of synaptic facilitation in 5-week-old PS cDKO mice. (e) Summary graph comparing frequency dependence of synaptic facilitation in 4-week-old PS cDKO mice. The values in parentheses indicate the number of hippocampal neurons (left) and the number of mice (right) used in each experiment.
To establish the temporal development of presynaptic deficits in PS cDKO mice, we analyzed frequency-dependent synaptic facilitation, one form of short-term plasticity that is dependent primarily on presynaptic release probability (Pr) at CA3-CA1 hippocampal synapses (Zucker & Regehr 2002). Excitatory postsynaptic currents (EPSCs) were evoked by extracellular stimulation of Schaeffer-collateral commissural fibers. We recorded EPSCs evoked by stimulus trains of 20 action potentials at frequencies ranging from 1 to 20 Hz in CA1 pyramidal neurons of acute hippocampal slices. At 4 weeks of age, the EPSC amplitude of synaptic facilitation at all four frequencies was normal in PS cDKO mice (Fig. 1e). By 5 week of age, we found that synaptic facilitation of EPSC amplitude was significantly reduced at stimulation frequencies higher than 5 Hz in PS cDKO mice, whereas synaptic facilitation elicited at 1 Hz stimulus was not changed (Fig. 1b–d). Similar results were obtained by measurement of frequency-dependent facilitation of excitatory postsynaptic potential (EPSP) slope using extracellular recording of the Schaeffer-collateral pathway of PS cDKO mice (data not shown). These results show that frequency facilitation impairments begin at 5 weeks of age in PS cDKO mice.
Calcium-dependency of synaptic facilitation
To examine whether the deficit in synaptic facilitation in PS cDKO mice at 5 weeks of age is calcium-dependent and can be rescued by higher external Ca2+ concentration, we measured calcium-dependence of synaptic facilitation by changing external calcium concentration. Synaptic responses were elicited by stimulus trains containing 300 pulses at 14 Hz. In PS cDKO mice, frequency facilitation deficits were calcium-dependent. Synaptic facilitation was significantly reduced at low external calcium concentration ([Ca2+]e = 0.5 mM) in PS cDKO mice (Fig. 2a and 2c). When [Ca2+]e was increased to 2.5 mM, synaptic facilitation was still reduced; however, the difference is much smaller (Fig. 2c). Conversely, synaptic facilitation is indistinguishable at 5 and 7.5 mM external calcium concentration (Fig. 2b and 2c). We also measured calcium dependency in 4-week-old PS cDKO mice, and found no difference compared with control mice (data not shown), which is consistent with the observation that frequency facilitation underlying physiological calcium concentration is normal at 4 weeks of age, but impaired at 5 weeks of age in PS cDKO mice (Fig. 1). Interestingly, at 2.5 mM external calcium concentration, the decrease of synaptic responses underlying 14 Hz stimulus trains (300 pulses), which is thought to reflect the rate of depletion of releasable vesicles pool, were not affected in 5 week old PS cDKO mice (averages of last 20 EPSCs in 300-pulse trains were compared; control = 28.8 ± 5.3%; PS cDKO = 31.1 ± 4.4%), arguing against an impairment of synaptic vesicle recycling as a cause of the decreased synaptic facilitation. These results demonstrate that the frequency facilitation deficit caused by presenilin inactivation is calcium-dependent.
Fig. 2.
Calcium dependency of synaptic facilitation in PS cDKO mice. (a–b) Representative traces of synaptic facilitation from 5 week old control and PS cDKO mice at external calcium concentration ([Ca2+]e = 0.5 mM and 7.5 mM, respectively). (c) Synaptic responses were elicited by high frequency stimulation at different extracellular calcium concentration ([Ca2+]e = 0.5, 2.5, 5.0 and 7.5 mM) between control and PS cDKO mice. Values of EPSP slope were normalized to the first stimulus in a stimulation train. The values in parentheses indicate the number of hippocampal slices (left) and the number of mice (right) used in each experiment.
Reduced release probability in PS cDKO mice
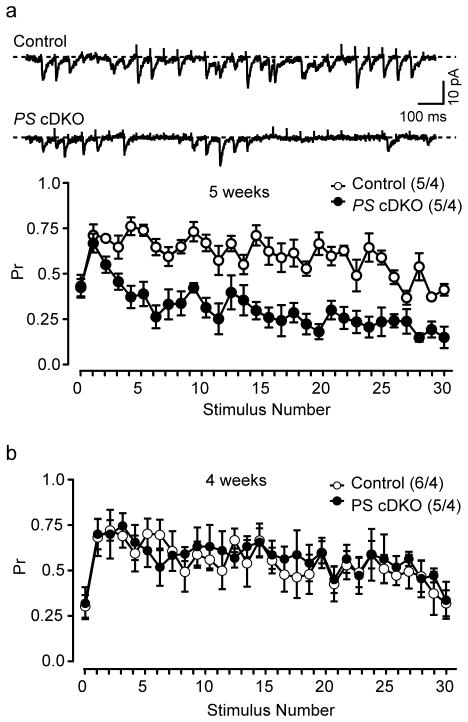
The calcium-dependence frequency facilitation deficit caused by presenilin inactivation at 5 weeks of age suggests that probability of release (Pr) during repetitive stimulation is reduced in PS cDKO mice. To test this directly, we examined Pr by recording trains of unitary synaptic responses evoked by low intensity stimulation. The plot of occurrence of unitary responses directly reflects the release probability at the single recording synapse. Probability of release during 20 Hz stimulus trains was normal in PS cDKO mice at 4 weeks of age (Fig. 3b), but it was significantly reduced at 5 weeks of age (Fig. 3a). These results show that probability of neurotransmitter release is impaired in PS cDKO mice at 5 weeks of age.
Fig. 3.
Reduced release probability (Pr) in PS cDKO mice. (a) Representative traces of unitary EPSCs recorded in response to short trains from control and PS cDKO mice at 5 weeks of age. Plot of Pr during repetitive stimulation showing reduced Pr at unitary synapses from control and PS cDKO mice. (b) Normal Pr during repetitive stimulation in PS cDKO mice at 4 weeks of age. The values in parentheses indicate the number of hippocampal neurons (left) and the number of mice (right) used in each experiment.
Reduced NMDAR-mediated responses in PS cDKO mice
We next examined the temporal development of postsynaptic deficits in PS cDKO mice by measuring NMDAR-mediated synaptic responses at 5 weeks of age, when presynaptic changes in short-term plasticity were observed (Fig. 1–3). NMDAR-mediated synaptic responses were measured by pharmacologically applying blockade of AMPA and GABAA receptors (10 μM CNQX and 100 μM picrotoxin, respectively). We found that input-output curve of NMDAR-mediated synaptic responses, is normal in PS cDKO mice at 5 weeks of age (Fig. 4a). However, at 6 weeks of age, we observed a small but significant reduction in NMDAR-dependent responses in PS cDKO (Fig. 4b). Furthermore, we quantified the ratio of NMDAR- to AMPA receptor (AMPAR)-mediated responses recorded from CA1 pyramidal neurons, a more precise measurement in monitoring postsynaptic NMDARs. AMPAR-mediated response was recorded at −70 mV holding potential in the presence of Mg2+ to block NMDAR-mediated component. NMDAR-mediated response was measured 75 ms after the peak of synaptic responses recorded at +40 mV holding potential. Consistently, we found similar temporal patterns in NMDA/AMPA ratio: normal at 5 weeks and impaired at 6 weeks of age in PS cDKO mice (Fig. 4c).
Fig. 4.
Decreased NMDAR-mediated responses in PS cDKO mice. (a) Normal input/output (I/O) curve for NMDAR-mediated responses in PS cDKO mice at 5 weeks of age. The initial slope of NMDAR-mediated synaptic responses was plotted as a function of fiber volley (FV). (b) Reduced NMDAR-mediated I/O curve in PS cDKO mice at 6 weeks of age. (c) Reduced NMDAR/AMPAR ratio in PS cDKO mice at 6 weeks of age but not at 5 weeks of age. (d) Reduced TBS-induced LTP in PS cDKO mice at 6 weeks of age but not at 5 weeks of age. The values in parentheses indicate the number of hippocampal slices (left) and the number of mice (right) used in each experiment.
Next, we measured long-term potentiation (LTP) in hippocampal Schaffer-collateral pathway of PS cDKO mice. LTP was induced by 5 trains of theta burst stimulation (TBS) and the magnitude of LTP measured 60 min after induction was indistinguishable in PS cDKO and control mice at 5 weeks of age (Fig. 4d). However, LTP was significantly impaired in PS cDKO mice at 6 weeks of age (Fig. 4d). Together, these results show that PS cDKO mice exhibit deficits in synaptic transmission (both short and long-term plasticity) and presynaptic changes in neurotransmitter release occur prior to the postsynaptic receptor changes in PS cDKO mice.
Discussion
It is well known that the extent of synaptic loss provides the best correlates to the severity of cognitive impairments during the early and late stages of AD (Hanks & Flood 1991, Braak & Braak 1997, Terry et al. 1991). Loss of presenilin function and accumulation of β-amyloid peptides can induce synaptic impairment in the absence of neuronal degeneration (Hsia et al. 1999, Saura et al. 2004, Zhang et al. 2009), suggesting that synaptic dysfunction fmay be a pathogenic precursor before frank neurodegeneration in AD. Our prior analysis of PS cDKO mice at 2 months of age, before the onset of neurodegeneration, revealed that loss of presenilin function in the adult cerebral cortex causes both presynaptic and postsynaptic deficits, such as paired pulse facilitation, NMDAR-mediated responses and LTP (Saura et al. 2004). In the current study, we pursued further the role of presenilins in the synapse by exploring the temporal development of presynaptic and postsynaptic deficits in the CA3-CA1 synapse of PS cDKO mice, in which presenilins are inactivated in both CA3 and CA1 hippocampal neurons at 4 weeks of age. We found that impaired synaptic function in PS cDKO mice is age-dependent and the earliest synaptic changes occur at 5 weeks of age, when presynaptic deficit in neurotransmitter release is the initial impairment prior to postsynaptic dysfunction. We found that frequency facilitations are selectively impaired in a calcium-dependent manner, whereas use-dependent depression is normal in PS cDKO mice. Furthermore, probability of evoked glutamate release during repetitive stimulus trains is also substantially reduced, providing further evidence for presynaptic deficits in short-term plasticity. In contrast, postsynaptic NMDAR-mediated responses are normal at 5 weeks of age in PS cDKO mice. It is not until 6 weeks of age, we observed reduced postsynaptic NMDAR-mediated responses in PS cDKO mice. Our studies suggest that the presynaptic deficits occur prior to postsynaptic NMDAR dysfunction in the absence of presenilins at mature synapses, raising the possibility that postsynaptic NMDAR alteration might be caused in part via a trans-synaptic mechanism.
Notably, our data showed that synaptic facilitation is impaired in a calcium-dependent manner in PS cDKO mice, which support the notion that inactivation of presenilins affects calcium homeostasis and induces presynaptic deficits. Our recent report using two independent lines of more restricted PS cDKO mice, in which presenilins are inactivated in either CA3 or CA1 neurons, demonstrated that loss of presynaptic presenilins alone is sufficient to cause defects in glutamate release and LTP via alterations in intracellular calcium signaling (Zhang et al. 2009). However, inactivation of presenilins in either presynaptic CA3 or postsynaptic CA1 neurons alone is insufficient to cause NMDAR-mediated responses, suggesting that both cell-autonomous and trans-synaptic mechanisms are at work in presenilin-dependent NMDAR functions (Figure 5). The findings from the current study showing NMDAR impairment follows presynaptic deficits in PS cDKO mice lacking presenilins in both CA3 and CA1 neurons provide further support for this trans-synaptic mechanism. Thus, LTP deficits in the absence of presenilins are likely caused by both the neurotransmitter release impairment due to loss of presynaptic presenilins and the NMDAR dysfunction due to loss of presynatpic and postsynaptic presenilins (Figure 5). These findings raised the possibility that impaired presynaptic glutamate release and NMDAR function may both play roles in AD pathophysiology.
Fig. 5.

A model depicts how presenilins regulate synaptic function and memory. Presenilins can promote LTP induction and memory via two independent mechanisms: presynaptic PS regulates probability of glutamate release through its control of calcium release from the ER, whereas both presynaptic and postsynaptic PS are involved in the regulation of NMDAR functions.
Impairment in neurotransmitter release may be one of the earliest pathogenic changes prior to neurodegeneration in AD. Recent studies show that PINK1, DJ-1 and Parkin, proteins associated with familial Parkinson’s disease (PD), are crucial for action-potential induced dopamine release from nigrostriatal terminals and striatal synaptic plasticity without detectable neurodegeneration (Goldberg et al. 2005, Kitada et al. 2007, Kitada et al. 2009). Together with our observations, it seems possible that reduced neurotransmitter release may represent a convergent mechanism leading to neurodegeneration in affected circuits in AD and PD, and perhaps other neurodegenerative diseases.
Acknowledgments
This work was supported by the National Institute of Health NS041783 (to J.S.). We would like to thank Xiaoyan Zou and Huailong Zhao for technical assistance
References
- Braak E, Braak H. Alzheimer’s disease: transiently developing dendritic changes in pyramidal cells of sector CA1 of the Ammon’s horn. Acta Neuropathol. 1997;93:323–325. doi: 10.1007/s004010050622. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, et al. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- Feng R, Wang H, Wang J, Shrom D, Zeng X, Tsien JZ. Forebrain degeneration and ventricle enlargement caused by double knockout of Alzheimer’s presenilin-1 and presenilin-2. Proc Natl Acad Sci U S A. 2004;101:8162–8167. doi: 10.1073/pnas.0402733101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortini ME. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol. 2002;3:673–684. doi: 10.1038/nrm910. [DOI] [PubMed] [Google Scholar]
- Goldberg MS, Pisani A, Haburcak M, et al. Nigrostriatal dopaminergic deficits and hypokinesia caused by inactivation of the familial Parkinsonism-linked gene DJ-1. Neuron. 2005;45:489–496. doi: 10.1016/j.neuron.2005.01.041. [DOI] [PubMed] [Google Scholar]
- Handler M, Yang X, Shen J. Presenilin-1 regulates neuronal differentiation during neurogenesis. Development. 2000;127:2593–2606. doi: 10.1242/dev.127.12.2593. [DOI] [PubMed] [Google Scholar]
- Hanks SD, Flood DG. Region-specific stability of dendritic extent in normal human aging and regression in Alzheimer’s disease. I. CA1 of hippocampus. Brain Res. 1991;540:63–82. doi: 10.1016/0006-8993(91)90493-f. [DOI] [PubMed] [Google Scholar]
- Ho A, Morishita W, Atasoy D, Liu X, Tabuchi K, Hammer RE, Malenka RC, Südhof TC. Genetic analysis of Mint/X11 proteins: essential presynaptic functions of a neuronal adaptor protein family. J Neurosci. 2006;26:13089–13101. doi: 10.1523/JNEUROSCI.2855-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, et al. Plaque-independent disruption of neural circuits in Alzheimer’s disease mouse models. Proc Natl Acad Sci U S A. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Shen J. Presenilins are required for maintenance of neural stem cells in the developing brain. Mol Neurodegener. 2008;3:2. doi: 10.1186/1750-1326-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Karouani M, et al. Impaired dopamine release and synaptic plasticity in the striatum of parkin−/− mice. J Neurochem. 2009;110:613–621. doi: 10.1111/j.1471-4159.2009.06152.x. [DOI] [PubMed] [Google Scholar]
- Kitada T, Pisani A, Porter DR, et al. Impaired dopamine release and synaptic plasticity in the striatum of PINK1-deficient mice. Proc Natl Acad Sci U S A. 2007;104:11441–11446. doi: 10.1073/pnas.0702717104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Lahad E, Wasco W, Poorkaj P, et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Rogaev EI, Sherrington R, Rogaeva EA, et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- Saura CA, Choi SY, Beglopoulos V, et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron. 2004;42:23–36. doi: 10.1016/s0896-6273(04)00182-5. [DOI] [PubMed] [Google Scholar]
- Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in Presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- Shen J, Kelleher RJ., 3rd The presenilin hypothesis of Alzheimer’s disease: evidence for a loss-of-function pathogenic mechanism. Proc Natl Acad Sci U S A. 2007;104:403–409. doi: 10.1073/pnas.0608332104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington R, Rogaev EI, Liang Y, et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Chen G, Südhof TC, Shen J. Conditional forebrain inactivation of nicastrin causes progressive memory impairment and age-related neurodegeneration. J Neurosci. 2009;29:7290–7301. doi: 10.1523/JNEUROSCI.1320-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Wines-Samuelson M, Handler M, Shen J. Role of presenilin-1 in cortical lamination and survival of Cajal-Retzius neurons. Dev Biol. 2005;277:332–346. doi: 10.1016/j.ydbio.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Wines-Samuelson M, Schulte EC, Smith MJ, Aoki C, Liu X, Kelleher RJ, 3rd, Shen J. Characterization of age-dependent and progressive cortical neuronal degeneration in presenilin conditional mutant mice. PLoS One. 2010;5:e10195. doi: 10.1371/journal.pone.0010195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wines-Samuelson M, Shen J. Presenilins in the developing, adult, and aging cerebral cortex. Neuroscientist. 2005;11:441–451. doi: 10.1177/1073858405278922. [DOI] [PubMed] [Google Scholar]
- Yu H, Saura CA, Choi SY, et al. APP processing and synaptic plasticity in presenilin-1 conditional knockout mice. Neuron. 2001;31:713–726. doi: 10.1016/s0896-6273(01)00417-2. [DOI] [PubMed] [Google Scholar]
- Zhang C, Wu B, Beglopoulos V, Wines-Samuelson M, Zhang D, Dragatsis I, Südhof TC, Shen J. Presenilins are essential for regulating neurotransmitter release. Nature. 2009;460:632–636. doi: 10.1038/nature08177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]