Abstract
Amphibians such as frogs can restore lost organs during development, including the lens and tail. In order to design biomedical therapies for organ repair, it is necessary to develop a detailed understanding of natural regeneration. Recently, ion transport has been implicated as a functional regulator of regeneration. While voltage-gated sodium channels play a well-known and important role in propagating action potentials in excitable cells, we have identified a novel role in regeneration for the ion transport function mediated by the voltage-gated sodium channel, NaV1.2. A local, early increase in intracellular sodium is required for initiating regeneration following Xenopus tail amputation, while molecular and pharmacological inhibition of sodium transport causes regenerative failure. NaV1.2 is absent under non-regenerative conditions, but misexpression of human NaV1.5 can rescue regeneration during these states. Remarkably, pharmacological induction of a transient sodium current is capable of restoring regeneration even after the formation of a non-regenerative wound epithelium, confirming that it is the regulation of sodium transport which is critical for regeneration. Our studies reveal a previously undetected competency window in which cells retain their intrinsic regenerative program, identify a novel endogenous role for NaV in regeneration, and show that modulation of sodium transport represents an exciting new approach to organ repair.
Keywords: NaV channel, NaV, sodium, regeneration, bioelectric, Xenopus, tail
Introduction
Humans have limited ability to repair injured or damaged organs. Interestingly, most mammalian organs contain resident progenitor cells (Zupanc, 2006), but it is not known why these cells are not mobilized for repair, suggesting that there may be an absence of instructive signals. Recently, it has become clear that biophysical signals such as ion currents and patterned voltage gradients (Reid et al., 2005; Adams et al., 2007) are key components for the induction of regeneration and patterning of new tissue in structures such as amphibian limbs and mammalian corneas (Zhao et al., 2006; Adams et al., 2007). To identify the molecular basis for regenerative currents in regeneration, we used Xenopus laevis--a powerful model that fully restores its larval appendages upon injury, utilizes pathways conserved to mammalian regeneration, and regenerates similar to mammals through tissue renewal and not transdifferentiation (Gargioli and Slack, 2004; Slack et al., 2004; Chen et al., 2006).
The Xenopus larval tail is a complex organ containing multiple cell types: muscle, peripheral nerves, spinal cord, notochord, and vasculature. After tail amputation, wound healing occurs within 6–8 hours post amputation (hpa). By 24 hpa, an initial swelling containing progenitor cells, called the regeneration bud, is formed at the injury site. Subsequently, tissue outgrowth and patterning begin as the tail is rebuilt over approximately seven days (Beck et al., 2009).
Several molecular components regulating tail regeneration have been identified. TGF-β signaling is required for proper wound healing and is detected at the wound as early as 15 minutes after amputation (Ho and Whitman, 2008). The proton (H+) pump, V-ATPase, is active by 6 hours post amputation, and its modulation of the transmembrane potential in regeneration bud cells is required during the first 24 hpa (Adams et al., 2007). Apoptosis in the regeneration bud during the first 24 hpa is also required for regeneration as in other systems (Tseng et al., 2007; Chera et al., 2009; Li et al., 2010). Components of signaling pathways such as BMP, Notch, Wnt, and FGF are expressed later and are involved in driving regenerative outgrowth and patterning (Beck et al., 2006; Mochii et al., 2007), recapitulating their well-characterized roles during axial development. Several of these pathways have been targeted to induce regeneration of spinal cord and other tissues in non-regenerative states. To date, all of these functional interventions were applied prior to the actual injury. However, in order for significant advances in regenerative biomedicine to occur, it is necessary to identify new pathways that can be targeted by therapies to induce appendage regeneration after injury and non-permissive wound healing.
Here we identify a new mechanism controlling vertebrate regeneration by modulating in vivo sodium (Na+) transport, endogenously mediated by the voltage-gated sodium channel, NaV1.2. Crucially, direct modulation of sodium transport is sufficient to induce vertebrate regeneration even after a non-regenerative wound epithelium has formed. Our data reveal a novel bioelectrical regulator of regeneration, and suggest a new therapeutic approach independent of transgenesis that can promote the regeneration of a complex appendage.
Materials and Methods
Tail regeneration assay
Xenopus laevis larvae were cultured via approved protocols (IACUC #M2008-08). Tails at stages 40–41 (regenerative), or stages 45–47 (refractory period) (Nieuwkoop and Faber, 1967) were amputated at the midpoint between the anus and the tip. Tadpoles were cultured in 0.1X MMR ± reagent at 22°C for 7 days and scored for tail regeneration. Unless indicated, 250µM MS222 (Sigma) was used for assays. To quantify and compare regeneration in groups of tadpoles treated with different reagents, we determined a composite “Regeneration Index” (RI), ranging from 0 (no regeneration) to 300 (complete regeneration) as previously described in (Adams et al., 2007). This index, and the specific definitions of regeneration phenotype categories (Full, Good, Weak, None) are given in Figure S1. For comparison, a group of tails in which >80% were fully regenerated would have an RI ranging from 240–300; if full regeneration occurred in < 10% of the animals within the group, its RI would range from 0–30.
RNA interference and embryo injections
DNA oligos encoding short RNA hairpins targeting Xenopus NaV1.2 (AY121368), dsRED (AY679106), or SIK (1319975) were cloned downstream of a U6 RNA Pol III promoter; the vector also contained CMV-driven GFP (Miskevich et al., 2006). RNAi target sequences are: NaV1.2, 5’-GCCATGGAGCATTATCCAATG-3’; dsRED, 5’-GTTCAAGTCCATCTACATGGC-3’; and SIK, 5’- GCCAGTTCTCTACTCACAAAC-3’. These constructs were micro-injected into 1 or 2-cell stage embryos. The presence of the shRNA in st. 40 tail tissues was identified by GFP fluorescence, indicating that the plasmid was expressed in the cells. For overexpression, full-length β-galactosidase or human NaV1.5 (GI:184038) RNA were transcribed using mMessage Machine kit (Ambion). Approximately 5ng of each target was mixed with 500pg of GFP mRNA and was injected into 2-cell embryos. Tails with good GFP expression were selected prior to amputation for experiments.
Modulation of sodium flux and imaging of reporter dyes
At 23 hpa, st. 40 tadpoles were incubated in 90µM of CoroNa Green indicator dye (Invitrogen) in 0.1X MMR for 45 min and washed twice in 0.1X MMR and 30µM N-benzyl-p-toluene sulphonamide (BTS, Tocris) to immobilize tadpole movement. At 24 hpa, the CoroNa Green signal was excited at 488nm and fluorescence emission data was collected at 516nm. Data was analyzed using IPLab software (BD Biosciences). For induction of Na+ current, 0.1X MMR was supplemented with sodium gluconate (Sigma) to increase the Na+ concentration to 90mM. For refractory period analysis, tails were amputated at st. 46–47, and at 18 hpa animals were treated with or without 90mM sodium and 20µM monensin (Sigma) in 0.1X MMR with 90µM CoroNa Green for 45 min and washed twice in 0.1X MMR and 50µM BTS. Imaging of transmembrane potential using DiBAC4(3) (Invitrogen) with controls was performed exactly as described extensively in (Adams et al., 2007).
In situ hybridization and immunohistochemistry
In situ hybridization was carried out according to standard protocols (Harland, 1991) with probes to: NaV1.2 (AY121368), NaV1.5 (Armisen et al., 2002), Notch1 (Coffman et al., 1990), Msx1 (Feledy et al., 1999), and SIK (1319975). Xenopus embryos were fixed overnight in MEMFA (Sive et al., 2000), heated for 2 hrs at 65°C in 50% formamide (to inactivate endogenous alkaline phosphatases), permeabilized in PBS and 0.1% Triton-X100 for 30 min, and processed for immunohistochemistry using alkaline phosphatase secondary antibody (Levin, 2004) until signal was optimal and background minimal. The expression profiles represent consensus patterns obtained from the analysis of 8–12 tails at each stage. Anti-NaV1.2 antibody (Millipore), anti-acetylated α-tubulin (Sigma), and anti-phospho-H3 (Upstate) antibodies were used at 1:1000. Quantification of phospho-H3-positive cells were performed as described (Adams et al., 2007).
Cloning and Gene Expresion
A tBLASTn search using mammlian SIK sequences identified a homologous Xenopus laevis cDNA clone #6641975 (accession #BU915306) in the Xenopus Gene Collection library. This clone was purchased and the sequence of this clone was submitted to Genebank (accession #1319975). 15–20 st. 40 24hpa regeneration buds were collected and RNA isolated by Trizol (Invitrogen). A cDNA library was generated using the SMART cDNA synthesis kit (Clontech). Primers were designed to the sequence (Forward: 5’TCCAGTCAGTTTCCGAGAAGGCAGACG-3’, Reverse 5’- GCACCAGGTTCTGCATCTGCTGGGAATG’-3’) were used to identify the presence of the Xenopus homolog in the cDNA library. To detect gene expression by RT-PCR, 15–20 regeneration buds from amputated tail were collected and RNA isolated by Trizol (Invitrogen). RNA was reverse transcribed using the Quantitect RT kit (Qiagen) to generate cDNA for real-time PCR analysis. Primers used were: NaV1.2 (forward: 5’-GCAGCCACTGCTACCCCCAC-3’, reverse: 5’-GCACTGCCACCATTCCCGGT-3’) and EF1 (forward: 5’-CAGGCCAGATTGGTGCTGGATATGC-3’, reverse: 5’-GCTCTCCACGCACATTGGCTTTCCT-3’). Target expression was normalized to EF1 expression.
Statistical analysis
To compare tail regeneration experiments, raw data from scoring was used. Comparison of two treatments was analyzed with Mann-Whitney U test for ordinal data with tied ranks, using normal approximation for large sample sizes. Multiple treatments were compared using a Kruskal-Wallis test, with Dunn’s Q corrected for tied ranks. All other experiments were analyzed using a student’s T-test.
Results
Requirement for Sodium Transport in Regeneration
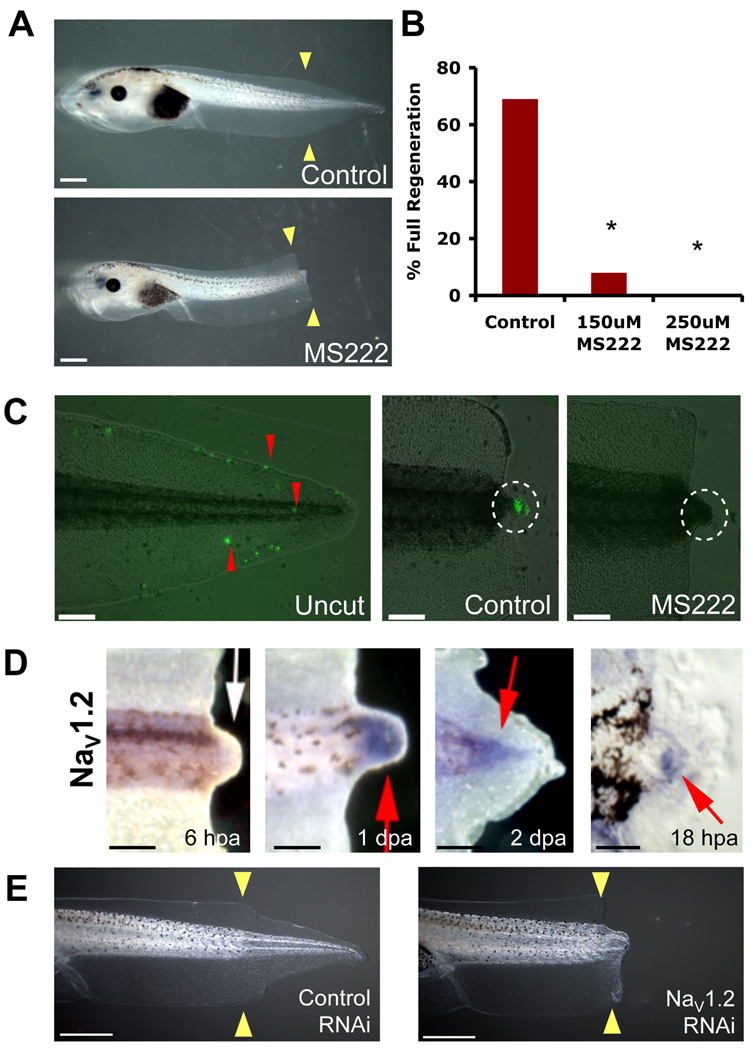
We identified a requirement for sodium transport in the course of a chemical genetics screen for bioelectric regulators specifically controlling regeneration (Adams et al., 2007). MS222 (tricaine) is a well-known inhibitor of all voltage-gated sodium channels (VGSCs or NaVs) (Hedrick and Winmill, 2003) that blocks inward sodium currents (Frazier and Narahashi, 1975). Treatment of animals with either 150 or 250µM MS222 immediately after tail amputation for the duration of the 7-day assay significantly inhibited regenerative ability (as calculated using the composite Regeneration Index (RI), see Figure S1 for full explanation of regenerative classes and calculation methods, RI=114, n=65, and RI=44, n=63, respectively) in a dose-dependent manner when compared to control siblings (RI=265, n=68, p<0.01) (Figure 1A–B). MS222 treatment did not induce significant apoptosis in the bud and overall development of the animals (including growth of the primary tail) was normal, suggesting that this is a regeneration-specific effect. Importantly, the concentrations used were approximately ten-fold lower than those commonly used to induce tadpole paralysis (mM range), and the phenotypes we describe occur in tadpoles with normal behavior and mobility. To demonstrate the effect of MS222-mediated NaV inhibition on sodium transport, we visualized sodium flux using CoroNa Green (Figure 1C), a fluorescent sodium indicator dye that selectively interacts with sodium ions and exhibits an increase in fluorescent emission upon binding (Meier et al., 2006). In intact tails, a few cells appear positive for the CoroNa Green signal relative to the majority of the tail population. At 24 hpa, a strong CoroNa Green signal is seen in the regeneration bud region but not in the rest of the tail and trunk, suggesting a significant increase in sodium transport into the cells of the bud during regeneration. When the amputated tails were treated with MS222, the CoroNa Green signal was abolished (n=8 per treatment, p<0.005), confirming that inhibition of NaV abrogates sodium influx into the bud. Therefore, we conclude that modulation of NaV-dependent sodium flux in bud cells is an important regulator of regeneration.
Figure 1. Sodium Transport is Required For Tail Regeneration.
(A) Control tails amputated at st. 40 regenerate fully while siblings treated with 250µM MS222 fail to regenerate. (B) Effects of NaV chemical inhibition on regeneration showing dose-dependent inhibition. (C) Fluorescent indicator dye (CoroNa Green) of sodium flux (red arrows). Uncut tail has scattered fluorescence. 24 hpa cut tail shows strong sodium influx into the regeneration bud (white circle) in contrast to MS2222-treated buds. (D) Wholemount in situ hybridization for NaV1.2 in amputated tails. 6 hpa regeneration bud lacks NaV1.2 expression. By 24 hpa, NaV1.2 is expressed in the regeneration bud and persists until 48 hpa. A section through an 18 hpa regeneration bud reveals NaV1.2 in mesenchymal cells but not the wound epidermis. (E) Control tails amputated at st. 40 regenerate fully but animals expressing NaV RNAi construct do not. Scale bars: (A, E) 1mm, (C–D) 500µm, except for section=100µm. Red arrows indicate expression; white arrow, lack of expression; yellow arrows, amputation plane. *=p<0.001.
Voltage-gated sodium channels (NaVs), the target of MS222 action, are plasma membrane proteins that regulate sodium influx into cells (Yu and Catterall, 2003). In Xenopus, NaV1.2 and NaV1.5 have been identified, and we examined their expression in the regeneration bud. NaV1.5 was not expressed (Figure S2). Consistent with a role for NaV1.2 in the early processes of tail regeneration, NaV1.2 RNA was expressed by 18 hpa in the mesenchymal cells of the regeneration bud and persisted as late as 2 days post amputation (dpa) (Figure 1D). NaV1.2 protein was similarly expressed (Figure S2).
To confirm the molecular basis for the regeneration-relevant sodium signaling in tail regeneration, we next ablated NaV1.2 activity by RNA interference (RNAi) using a plasmid encoding a short RNA hairpin construct specifically targeting the Xenopus NaV1.2 gene. The targeting vector carries a GFP marker (Miskevich et al., 2006) on the same plasmid, enabling the accurate selection of animals that expressed the RNAi construct in the distal tail. Real-time PCR quantification of NaV1.2 mRNA in the regeneration bud at 24 hpa revealed an overall decrease of 40% when compared to controls, showing the effectiveness of NaV1.2 RNAi knockdown in vivo. Despite the necessarily mosaic uptake of NaV1.2 RNAi construct, expression of the NaV1.2 short RNAi hairpin in the tail region at the site of amputation was able to significantly inhibit tail regeneration (RI=198, n=100, p<0.01) as compared with control RNAi targeting dsRED, a fluorescent protein that is not endogenous to Xenopus (RI=261, n=72), confirming our pharmacological loss-of-function data (Figure 1E). Although we cannot rule out additional contributions from as-yet unidentified NaV isoforms, these data demonstrate that NaV1.2 is specifically required for tail regeneration, reveal an important component of endogenous regenerative response in Xenopus, and identify a new role for voltage-gated sodium channels.
Early Control of Regenerative Processes by Sodium Influx
To examine the temporal requirement for NaV-mediated sodium transport during regeneration, we amputated tails of tadpoles, treated them with 250µM MS222 for specific durations, and assayed their regenerative ability. Exposure to NaV blocker MS222 for the first 24 hpa prevented tail regeneration in 45% of the tadpoles (n=106), indicating that sodium transport plays a role in regeneration bud establishment. When the duration of MS222 treatment was expanded to include the first 48 hpa, 78% of the tails failed to regenerate (n=105), fully recapitulating the severity of phenotype seen when NaV inhibition occurs throughout the entire 7 days required for regeneration (n=103). We then examined the effect of NaV inhibition after amputation. MS222 treatment targeting NaV activity at 24 hpa resulted in 26% regenerative failure (n=107), demonstrating that the initial expression of NaV1.2 at 18 hpa is critical to initiating regeneration. However, addition of MS222 at 48 hpa (n=63), after regenerative outgrowth has begun, had no effect. Together, these results are consistent with our data for NaV1.2 (Figure 1D) and strongly suggest that sodium transport is principally required during the establishment and early outgrowth phases of regeneration. Given this early role, what is the relationship between sodium influx and the known later steps in tail regenerative outgrowth?
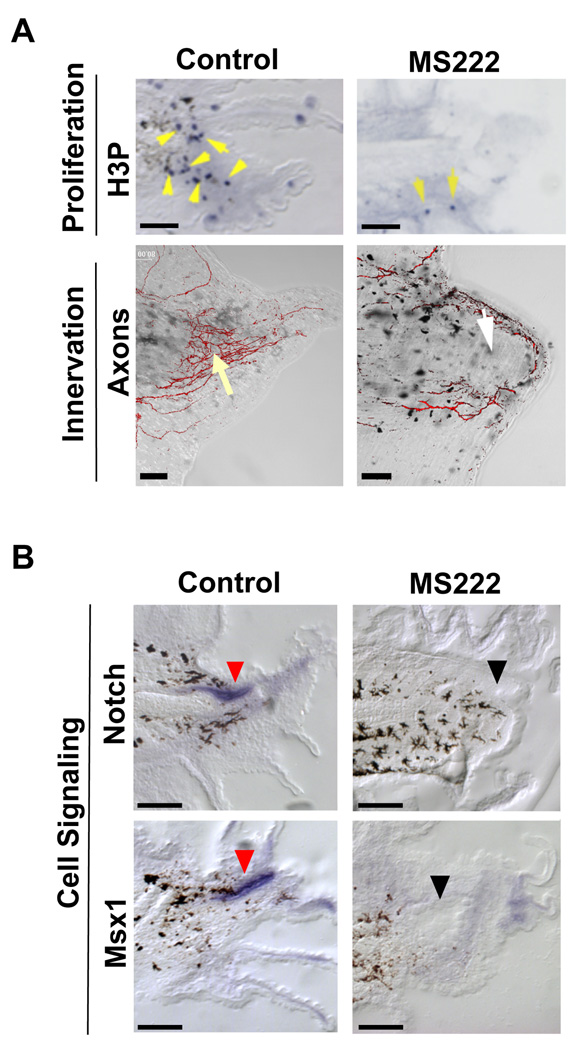
Rapidly rebuilding a tail requires the increase of proliferating cells observed at the regeneration bud by 48 hpa (Adams et al., 2007). To understand the cellular basis for the regenerative failure caused by block of sodium transport, we examined proliferation in sodium flux-inhibited animals. During development, mitotic cells are observed to be randomly located in the growing tail (Adams et al., 2007). We quantified the number and distribution of proliferating cells in regenerating tails at 48 hpa using an antibody to phosphorylated Histone 3B (H3P), a marker of the G2/M transition of the cell cycle that identifies mitotic cells in Xenopus and many other systems (Saka and Smith, 2001; Adams et al., 2006). NaV inhibition using MS222 treatment caused a 90% decrease in the number of mitotic cells in the regeneration bud region (1.3 ± 1.5, n=4) as compared to control siblings (12.5 ± 4.8, n=4, p<0.005). Many H3P-positive cells were seen in the wild-type regeneration bud but very few are detected in MS222-treated buds (Figure 2A, top panels). Importantly, no significant change in proliferation was seen in the central tail “flank” region (71 ± 27 H3P-positive cells for controls as compared to 66.3 ± 10 for MS222 treatment, n=8, p=0.38), showing that NaV-mediated sodium flux is not a general requirement for normal cell division. Together, these data demonstrate that sodium transport is necessary for the specific up-regulation of proliferation in the regenerative growth region.
Figure 2. NaV-Mediated Sodium Transport Acts Early During Regeneration.
(A) Effect of NaV inhibition (MS222) on proliferation and innervation. (Top) Immunohistochemistry of 48 hpa tails using an anti-H3P antibody (blue) in sagittal sections (yellow arrows are mitotic cells, melanocytes are black). (Bottom) 72 hpa tails stained with acetylated α-tubulin antibody to identify axons. Control axon bundles run parallel to the A–P axis and concentrate at the tip (yellow arrow). MS222 treatment reduces axons (white arrow) that trace along the edge. (B) Effect of NaV inhibition on genes that regulate regenerative outgrowth (as shown by RNA in situ hybridization in sagittal sections at 48 hpa). Notch RNA (top) is expressed in the neural ampulla (red arrows) and in the regeneration bud mesenchyme, whereas Msx1 (bottom) is expressed solely in the neural ampulla. Gene expression is abolished after NaV1.2 inhibition (black arrows). Scale bar =250µm.
Another important requirement for regenerative growth is proper innervation (Singer, 1952, 1965). Thus we examined the neuronal pattern in the amputated tail stumps of tadpoles treated with MS222 (Figure 2A, bottom panels). In normal 3-day old tail regenerates, axons appear in bundles that grow and concentrate toward the end of the regenerate, in a direction parallel to the anterior-posterior axis. In contrast, MS222 treatment caused axons to extend circumferentially along the edge of the regeneration bud, perpendicular to the main axis of tail growth. Notably, the overall quantity of neurons appeared to be reduced as compared to control siblings. These results suggest that sodium flux is required for proper innervation of the regenerate.
Several pathways have been shown to be required for driving regenerative outgrowth and patterning in the tail, including Notch, Msx1, and BMP (Beck et al., 2003; Sugiura et al., 2004; Beck et al., 2006). To determine whether these pathways are controlled by sodium influx, we performed in situ hybridization using gene-specific RNA probes to examine the expression patterns of Notch and Msx1 in the regeneration bud after NaV inhibition (Figure 2B). At 48 hpa, Notch1 is normally expressed in the neural ampulla and in the mesenchymal region of the regeneration bud, and Msx1 is expressed in the neural ampulla and at the epithelial edge of the regenerating tail tip. In contrast, MS222 treatment largely abolished expression of Notch1 and Msx1. Likewise, levels of BMP2, BMP4, and Delta were greatly reduced in the presence of MS222 (Figure S2). Although not evidence for direct regulation, these results demonstrate that sodium flux acts upstream to induce the later expression of several key genes known to control downstream regenerative outgrowth and patterning.
Induction of Regeneration by a Transient Sodium Current
Mammals exhibit an age-dependent decrease in regenerative potential (Illingworth, 1974). Similarly, Xenopus tadpoles also show a loss of regenerative potential during the refractory period, an endogenous period (st. 45–47) during development in which tadpoles are unable to regenerate tails (Beck et al., 2003). Our results are consistent with NaV1.2 expression being predictive of regenerative competency; it is absent in non-regenerating tail stumps, including those amputated during the refractory period (Figure S2). Thus induction of sodium current could be sufficient to promote regeneration during the non-regenerative refractory state.
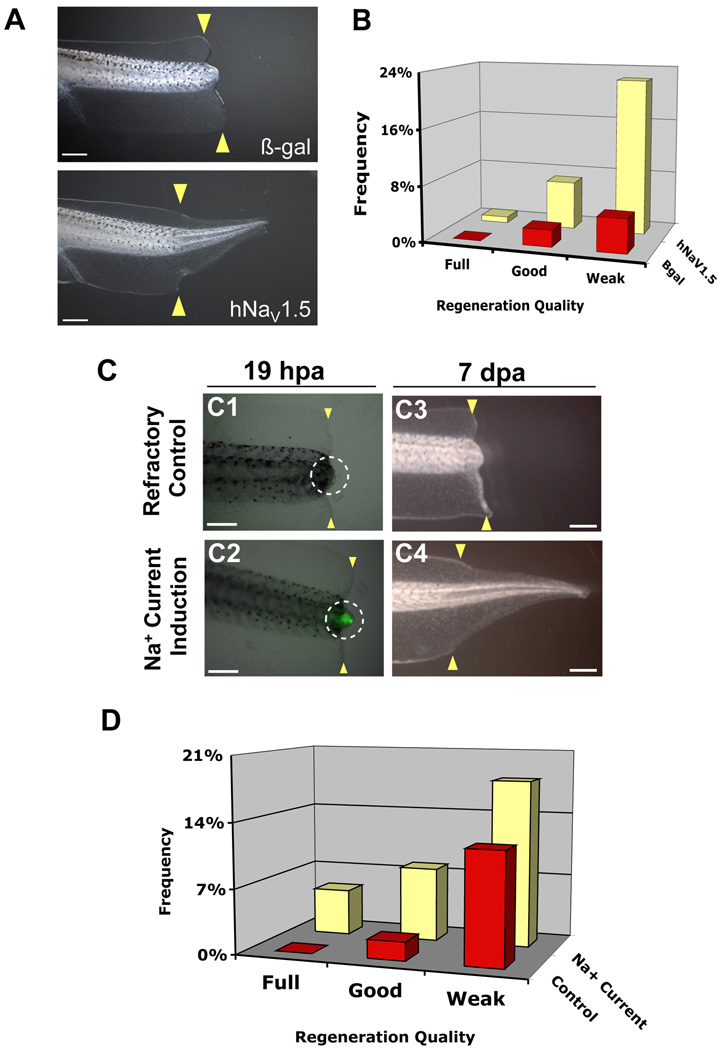
Crucially, a primary role for sodium influx in the induction of reparative growth suggests that any functional NaV could fulfill this requirement. We therefore expressed the human cardiac sodium channel, NaV1.5 (Fraser et al., 2005), ubiquitously by early embryo injection and assessed its ability to rescue regeneration. As expected, ectopic expression of hNaV1.5 in refractory cut tails (where endogenous NaV1.2 is absent, Figure S2) did indeed rescue regeneration (Figure 3A–B; β-gal control: RI=10, n=82, as compared to hNaV1.5: RI=39, n=116, p<0.001). Supporting this observation, expression of hNaV1.5 was also able to rescue regenerative failure due to V-ATPase inhibition by concanamycin (Adams et al., 2007) (β-gal control: RI=120, n=145, as compared to hNaV1.5: RI=231, n=132, p<0.0001). These results confirm that sodium conductance, not a specific native NaV protein, is required for driving regenerative outgrowth.
Figure 3. Transient Induction of Sodium Current Drives Regeneration.
(A) Regeneration rescue by human NaV1.5. Control tail stumps (β-gal-injected) cut during the refractory period regenerate poorly, which is rescued by hNaV1.5 expression. (B) Effects of regeneration rescue by hNaV1.5 during refractory period block. (C) CoroNa Green analysis of Sodium current induction. (C1) Control (vehicle only), non-induced refractory stage bud has little CoroNa Green signal. (C2) Induction with 90mM sodium and 20µM monensin for 1 hour (18–19 hpa) significantly increases intracellular sodium (green). Images are merged brightfield and fluorescence of the same exposure time. white circle = refractory bud. (C3) Most refractory stage amputations fail to regenerate. (C4) Stimulation with sodium current restores full regeneration. (D) Transient sodium current rescues non-regenerative wound epidermis. Stimulation of sodium current increased regeneration >2-fold and improved regeneration quality as compared to control siblings (treated with vehicle only: 0.01% ethanol). Treatment with either monensin or 90mM sodium alone showed no effect. Scale bars: (A) 1mm, (C) 500µm.
We then asked whether directly modulating sodium transport without genetic manipulation promotes regeneration. Monensin is an ionophore that selectively transports sodium ions into cells (Mollenhauer et al., 1990). Tails of animals in the refractory period were amputated, and at 18 hpa (recapitulating the timing of NaV1.2 expression) treated with 20µM monensin in a medium containing 90mM sodium (normal culture medium contains 10mM sodium). To confirm that monensin induces an increase in intracellular sodium levels, we used the CoroNa Green indicator dye to visualize sodium content in the amputated caudal stump. At 19 hpa, normal refractory tail buds showed very weak CoroNa Green signal (Figure 3C1, white circle). In contrast, monensin-treated tails after a one-hour current induction in high sodium medium showed a strong CoroNa Green fluorescence at the amputation site (Figure 3C2, white circle), demonstrating that monensin treatment does increase intracellular sodium content in the regeneration bud.
We then assessed the consequence of monensin treatment on regeneration. During the refractory period, the regenerative ability of Xenopus tadpoles was extremely poor (RI=16.8, n=179). Most animals failed to regenerate any tissue in the amputated tails (Figure 3C3). Strikingly, treatment at 18 hpa with 20µM monensin in a medium containing 90mM sodium for just one hour induced a significant increase in both the quality and quantity of regeneration (RI=48.5, n=101, p<0.001) compared to refractory controls (Figure 3C4 and 3D). The animals were developmentally normal and did not grow ectopic tissues. Importantly, the same treatment with either monensin alone (RI=10.8, n=117) or high extracellular sodium alone (RI=20.8, n=125) did not improve regenerative ability (p>0.05), showing that neither monensin nor high extracellular sodium alone is capable of this effect and ruling out effects of osmolarity changes as an important factor in this treatment. It is the induced sodium influx that promotes regeneration. Consistent with our hypothesis, the same monensin induction method was observed to rescue another non-regenerative condition--that caused by a pharmacological block of apoptosis (Figure S3). Thus the sufficiency of a transient pulse of sodium current at 18 hpa to restore regeneration demonstrates that control of the intracellular sodium level in the regeneration bud can be utilized as a brief treatment to initiate regeneration of a vertebrate neuromuscular appendage. This observation suggests that, surprisingly, this instructive signal does not have to be present at the time of injury and is not required long-term to drive regeneration.
Regenerative Control Through Modulation of Intracellular Sodium Levels
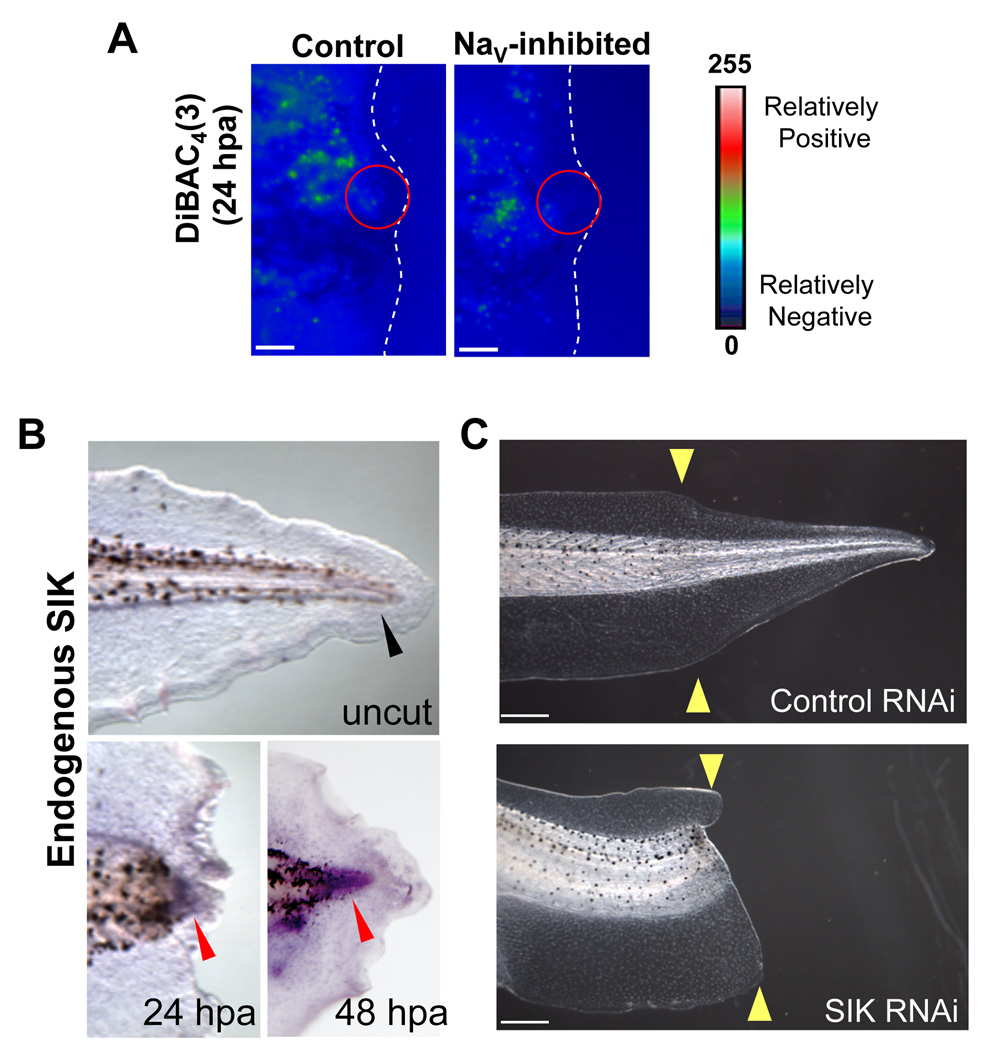
To gain a detailed mechanistic understanding of NaV activity, we examined the consequences of modulating sodium ion transport in the regenerating tail. NaV activity is also a well-known major determinant of some cells’ membrane potential (Vmem), thus sodium flux could regulate regeneration by modulating the membrane voltage state of the regeneration bud. Using DiBAC4(3), we did not observe any changes in membrane voltage in the regeneration bud cells of MS-222 treated tails as compared to controls (Figure 4A, regeneration bud area outlined in red), suggesting that, rather than changes in transmembrane potential, it is the sodium ion concentration per se that is the mechanism by which NaV function controls regenerative behavior.
Figure 4. Salt-Inducible Kinase is Required for Tail Regeneration.
(A) Comparison of the relative voltage patterns of tail regeneration buds at 24 hpa using the voltage dye, DiBAC4(3). Green is more depolarized than blue. Distal tail end (amputation site) is outlined in white. Scale bar = 100µm. The regeneration bud (red circle) of controls was polarized (blue color). MS-222 treated buds show a similar pattern. (B) RNA in situ hybridization for endogenous SIK in wholemount cut tails. SIK is expressed in the regeneration bud at 24 and 48 hpa (red arrows) but not in uncut tails (black arrow). (C) Effect of SIK RNAi on regeneration. SIK RNAi-expressing tadpoles fail to regenerate but develop normally.
One known effector of sodium ion-based signaling is the salt-inducible kinase (SIK), a member of the AMP-activated protein kinase (AMPK) family that responds to changes in intracellular sodium levels (Sanz, 2003). Because SIK is a potential candidate to act as a molecular sensor of NaV activity during regeneration, we searched and were able to identify a single Xenopus SIK clone from a regeneration bud-specific cDNA library, which a BLAST search showed is most homologous to the SIK2 isoform. X-SIK is expressed in the regeneration bud at 24 and 48 hpa but not in the uncut tail (Figure 4B). We found that when a SIK RNAi construct was expressed in the tail, overall development was normal but regeneration was significantly reduced compared to control dsRED RNAi (Figure 4C, Control RI=234, SIK RI=151, n=207, p<0.001), demonstrating that SIK is indeed required for this process. Furthermore, pharmacological inhibition of SIK using Staurosporine (STS) after sodium current induction during the refractory period also successfully blocked regeneration (no STS RI=54.4, 10nM STS RI=1.8, n=225, p<0.001). Together, these results suggest that, as occurs in rats (Stenstrom et al., 2009), Xenopus SIK may transduce physiological sodium transport activity into second messenger cascades that control important cell functions necessary for tail regeneration, highlighting an important role for SIK during regeneration.
Discussion
Voltage-gated sodium channels have mainly been studied for their role in mediating the conduction of rapid electrical signaling in excitable cells of the nervous system and muscle (Diss et al., 2005), although roles in directing cell migration are beginning to be dissected (Brackenbury et al., 2008). The importance of bioelectric cues in regenerative events has been suggested for a very long time (Mathews, 1903; Lund, 1947). Both classical and recent work has indicated a requirement for sodium and electric fields in natural vertebrate appendage regeneration (Borgens et al., 1979; Robinson, 1983; Reid et al., 2009). However, the molecular source of regeneration-relevant currents in non-excitable cells, and the cell-biological consequences of their modulation, remain largely unknown. Here, we reveal a novel and unexpected role for NaV-mediated sodium transport regulation of regeneration in a complex vertebrate structure. We show that sodium transport is required during the initiating stage of tail regeneration in Xenopus. NaV1.2 but not NaV1.5 is specifically expressed during endogenous regeneration, and its expression is a predictor of regenerative ability—although additional unidentified NaVs may also play a role in this process. It should be kept in mind that while our quantitative analyses clearly indicate the efficacy of loss- and gain-of-function approaches targeting NaV1.2, the penetrance of the inhibition and rescue had to be artificially suppressed by tittering the treatments to low levels. This was made necessary by the importance of NaV1.2 channels in the nervous system and heart; thus, overly strong modulation of sodium currents results in toxicity that would have made regeneration phenotypes impossible to detect. Along these lines for example, our experiments with the NaV1.2 blocker MS-222 were conducted at low levels that did not impair nerve function (did not cause paralysis or change in behavior).
Our results reveal both the upstream molecular pathways responsible for NaV1.2-dependent induction of sodium flow (V-ATPase activity, Figure S2) and those downstream events controlled by sodium influx (Notch and Msx1 induction). The data characterized the effects of functional inhibition of sodium transport at several levels: physiologically (decrease of sodium influx), molecularly (lack of induction of regeneration-specific late genes, an effect likely mediated by SIK), and cellularly (changes in cell proliferation and axonal patterning). Taken together, these data suggest a model (Figure 5) that integrates the biochemical, genetic, and physiological data into a stepwise pathway leading to regeneration of the tail. Importantly, our model shows that the bioelectric signals produced by V-ATPase and NaV form a physiological module; this module is both induced by, and in turn influences, gene expression while participating in the orchestration of multiple aspects of epimorphic regeneration.
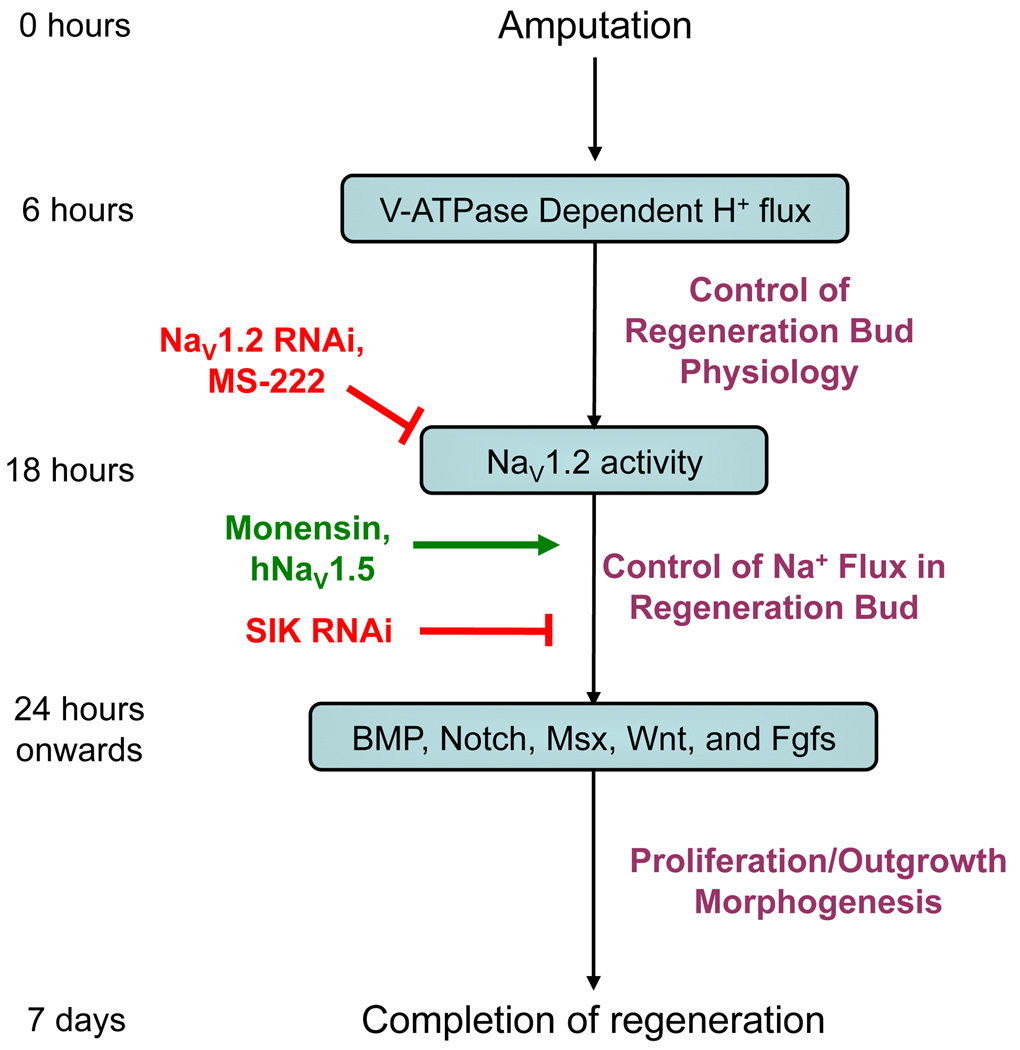
Figure 5. A Model Integrating NaV in Regeneration.
By 6 hpa, the H+ pump V-ATPase is expressed in the regeneration bud where it regulates the membrane voltage of the bud. V-ATPase activation results in the up-regulation of NaV1.2 by 18 hpa. Ablation of NaV1.2 expression (RNAi) or NaV function (pharmacological treatment) inhibits regeneration. NaV activity enables sodium ions to enter regeneration bud cells and, potentially through SIK, to activate downstream pathways (such as BMP and Notch) by 24 hpa, driving regenerative outgrowth and patterning. By 7 days after injury, the rebuilding of the tail is largely complete. Importantly, monensin-mediated induction of a transient sodium flux into non-regenerative buds is sufficient to restore full tail regeneration, demonstrating that intracellular sodium signaling is a key regulator of regeneration able to initiate repair even after a non-regenerative wound epithelium has formed.
A mechanism by which NaV might be expected to function is through the control of membrane voltage. We found no evidence of this (Figure 4), suggesting that regeneration bud cells regulate other transport events to maintain voltage despite changing levels of sodium ions. Furthermore, the results of the monensin experiments show that direct sodium increases are functionally instructive for regeneration during non-regenerative states that occurred naturally (refractory period, Figure 3) or induced chemically (apoptosis inhibition, Figure S3). These currents are endogenously provided by NaV (and in Xenopus by NaV1.2) but can be strikingly recapitulated by a very rapid, transient external induction of sodium flux. Although extracellular sodium currents are known to be a major component of transepithelial potentials (TEP) during repair in both Xenopus (Rajnicek et al., 1988; Jenkins et al., 1996) and humans (Shapiro et al., 2005), the mechanism we identified is different because it does not rely on modulation of the TEP. Our data most clearly support a role for intracellular sodium ions intrinsically guiding regenerative outgrowth through cell proliferation and gene expression in the bud--a novel mechanism for regeneration.
How then, does sodium flow control downstream events? Unlike calcium, relatively few effectors of sodium influx have been identified. Chemical treatments using inhibitors of either the sodium-activated potassium channel, Slo2.2 (Slo Inibitor treatment: RI=270, n=74 as compared to Control: RI=258, n=33, p>0.05) or sodium/calcium exchangers (Adams et al., 2007) did not affect tail regeneration, suggesting that the effect of sodium is unlikely to be mediated by secondary effects on calcium. A likely candidate for transducing changes of intracellular sodium level into second messenger cascades is SIK, salt-inducible kinase, which has been implicated in stress response (Wang et al., 2008), global regulation of gene expression (Verdin et al., 2003) and may potentially regulate regenerative pathway genes such as BMPs (Shaked et al., 2008). The identification of an increase of intracellular sodium as a key regulator of regenerative response is a crucial new area for future work integrating the roles of bioelectric signaling and the more canonical biochemical pathways (Blackiston et al., 2009; McCaig et al., 2009).
NaV regulates regenerative growth in part by its influence on downstream signaling genes including Notch1, BMP and Msx1. These genes are also important for regeneration in other systems such as the tadpole limb, zebrafish fin and heart, and mammalian digit tips (Poss et al., 2000; Han et al., 2003). Consistent with the conserved roles of ion currents in regulating global patterning and morphogenetic cues from fungi to mammals (Nuccitelli et al., 1986; Levin, 2009), it is likely that the early mechanisms of ion transporter signaling in regeneration are also utilized in other species and other structures besides the tail.
We show that several non-regenerative states (e.g., age-dependent decline or “refractory period,” and an apoptosis-inhibited failure of regeneration) can be rescued by an 1-hour pharmacological treatment that recapitulated the NaV-dependent influx of sodium in caudal bud cells and restored regeneration. Remarkably, this treatment only induced tail structures and did not generate ectopic growths or neoplasia during development, suggesting that sodium flow could act as a “master control point” to initiate tightly-coordinated, self-limiting downstream morphogenetic cascades (Figure S4). This result has several important implications for regenerative medicine. The ability to restore regeneration using a temporally controllable pharmacological approach not requiring gene therapy is extremely exciting. Moreover, that a short-term induction of sodium current was sufficient to restore regeneration suggests the possibility that the correct initiating signal is not required long-term to drive completion of the process—a highly desirable property for regenerative therapies.
Most importantly, our finding that the amputated refractory tail can be induced to regenerate as late as 18 hours after amputation reveals that tissues normally fated for regenerative failure still maintain their intrinsic ability and can be reprogrammed to reactivate regeneration. Furthermore, this observation challenges the view that differences in wound healing decisively determine regenerative ability (Tasava and Olsen, 1982; Campbell and Crews, 2008). In Xenopus tail regeneration, regenerative wound healing is completed by 8 hpa (Ho and Whitman, 2008). In contrast, refractory tail amputation results in a thickened, non-regenerative wound epidermis (Beck et al., 2003) which can be observed by 18 hpa (Figure S5). Thus the induction of a regenerative signal at 18 hpa is not predicted to impact wound healing. Our results demonstrate that non-regenerative wound healing is not a permanent block to the later induction of regeneration. That the instructive capacity of the wound epithelium can be overcome by biophysical signals such as that mediated by NaV reveals the existence of a competency window within which cells retain their capability to initiate the regenerative program--if the appropriate signals are provided. It also suggests that potential therapeutic treatments need not be administered immediately after acute injury (or indeed prior to injury, as is done in many studies of regenerative induction in this system). Further studies of the NaV-mediated sodium transport regenerative signaling pathway will provide a detailed understanding of the requirements for initiating regeneration during different non-regenerative conditions and a more complete understanding of the molecular physiology of regeneration. Capitalizing upon such bioelectrical cues will be a rewarding and exciting area for regenerative medicine and developmental biology.
Supplementary Material
Acknowledgements
This work was supported by NIH (R01-GM078484 and F32-GM083547 (WSB)), NHTSA (DTNH22-06-G-00001), DOD (W81XWH-10-2-0058) and DARPA (W911NF-07-1-0572). We thank D. Adams for assistance with dye imaging and confocal microscopy, P. Koustubhan and A. Currier for their assistance with Xenopus husbandry, D. Qiu and J. Boltax for technical assistance and histology and F. Miskevich for advice for generating RNAi targeting constructs. Clones for XNaV1.2 and XNaV1.5 were gifts of M. Kukuljan. Clone for hNaV1.5 was a gift of M. Djamgoz. The cloning vector for RNAi was a gift of F. Miskevich. We are grateful to the advice and support of P. Smith at the BioCurrents Resource Center.
References
- Adams D, Masi A, Levin M. Xenopus tadpole tail regeneration requires the activity of the proton pump V-ATPase, and proton pumping is sufficient to partially rescue the loss-of-function phenotype. Developmental Biology. 2006;295:355–356. [Google Scholar]
- Adams DS, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- Armisen R, Fuentes R, Olguin P, Cabrejos ME, Kukuljan M. Repressor element-1 silencing transcription/neuron-restrictive silencer factor is required for neural sodium channel expression during development of Xenopus. Journal of Neuroscience. 2002;22:8347–8351. doi: 10.1523/JNEUROSCI.22-19-08347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck CW, Christen B, Slack JM. Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Dev Cell. 2003;5:429–439. doi: 10.1016/s1534-5807(03)00233-8. [DOI] [PubMed] [Google Scholar]
- Beck CW, Izpisua Belmonte JC, Christen B. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev Dyn. 2009;238:1226–1248. doi: 10.1002/dvdy.21890. [DOI] [PubMed] [Google Scholar]
- Beck CW, Christen B, Barker D, Slack JM. Temporal requirement for bone morphogenetic proteins in regeneration of the tail and limb of Xenopus tadpoles. Mech Dev. 2006;123:674–688. doi: 10.1016/j.mod.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009;8:3519–3528. doi: 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgens RB, Vanable JW, Jr, Jaffe LF. Reduction of sodium dependent stump currents disturbs urodele limb regeneration. Journal of Experimental Zoology. 1979;209:377–386. doi: 10.1002/jez.1402090304. [DOI] [PubMed] [Google Scholar]
- Brackenbury WJ, Djamgoz MB, Isom LL. An emerging role for voltage-gated Na+ channels in cellular migration: regulation of central nervous system development and potentiation of invasive cancers. Neuroscientist. 2008;14:571–583. doi: 10.1177/1073858408320293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell LJ, Crews CM. Wound epidermis formation and function in urodele amphibian limb regeneration. Cell Mol Life Sci. 2008;65:73–79. doi: 10.1007/s00018-007-7433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lin G, Slack JM. Control of muscle regeneration in the Xenopus tadpole tail by Pax7. Development. 2006;133:2303–2313. doi: 10.1242/dev.02397. [DOI] [PubMed] [Google Scholar]
- Chera S, Ghila L, Dobretz K, Wenger Y, Bauer C, Buzgariu W, Martinou JC, Galliot B. Apoptotic cells provide an unexpected source of Wnt3 signaling to drive hydra head regeneration. Dev Cell. 2009;17:279–289. doi: 10.1016/j.devcel.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Coffman C, Harris W, Kintner C. Xotch, the Xenopus homolog of Drosophila notch. Science. 1990;249:1438–1441. doi: 10.1126/science.2402639. [DOI] [PubMed] [Google Scholar]
- Diss JK, Stewart D, Pani F, Foster CS, Walker MM, Patel A, Djamgoz MB. A potential novel marker for human prostate cancer: voltage-gated sodium channel expression in vivo. Prostate Cancer Prostatic Dis. 2005;8:266–273. doi: 10.1038/sj.pcan.4500796. [DOI] [PubMed] [Google Scholar]
- Feledy JA, Beanan MJ, Sandoval JJ, Goodrich JS, Lim JH, Matsuo-Takasaki M, Sato SM, Sargent TD. Inhibitory patterning of the anterior neural plate in Xenopus by homeodomain factors Dlx3 and Msx1. Dev Biol. 1999;212:455–464. doi: 10.1006/dbio.1999.9374. [DOI] [PubMed] [Google Scholar]
- Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, Brackenbury WJ, Theodorou D, Koyuturk M, Kaya H, Battaloglu E, De Bella MT, Slade MJ, Tolhurst R, Palmieri C, Jiang J, Latchman DS, Coombes RC, Djamgoz MB. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–5389. doi: 10.1158/1078-0432.CCR-05-0327. [DOI] [PubMed] [Google Scholar]
- Frazier DT, Narahashi T. Tricaine (MS-222): effects on ionic conductances of squid axon membranes. Eur J Pharmacol. 1975;33:313–317. doi: 10.1016/0014-2999(75)90175-2. [DOI] [PubMed] [Google Scholar]
- Gargioli C, Slack JM. Cell lineage tracing during Xenopus tail regeneration. Development. 2004;131:2669–2679. doi: 10.1242/dev.01155. [DOI] [PubMed] [Google Scholar]
- Han M, Yang X, Farrington JE, Muneoka K. Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development. 2003;130:5123–5132. doi: 10.1242/dev.00710. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole mount method for Xenopus embryos. In: Kay BK, Peng HB, editors. Xenopus laevis: Practical uses in cell and molecular biology. San Diego: Academic Press; 1991. pp. 685–695. [DOI] [PubMed] [Google Scholar]
- Hedrick MS, Winmill RE. Excitatory and inhibitory effects of tricaine (MS-222) on fictive breathing in isolated bullfrog brain stem. Am J Physiol Regul Integr Comp Physiol. 2003;284:R405–R412. doi: 10.1152/ajpregu.00418.2002. [DOI] [PubMed] [Google Scholar]
- Ho DM, Whitman M. TGF-beta signaling is required for multiple processes during Xenopus tail regeneration. Dev Biol. 2008;315:203–216. doi: 10.1016/j.ydbio.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth CM. Trapped fingers and amputated finger tips in children. J Pediatr Surg. 1974;9:853–858. doi: 10.1016/s0022-3468(74)80220-4. [DOI] [PubMed] [Google Scholar]
- Jenkins LS, Duerstock BS, Borgens RB. Reduction of the current of injury leaving the amputation inhibits limb regeneration in the red spotted newt. Developmental Biology. 1996;178:251–262. doi: 10.1006/dbio.1996.0216. [DOI] [PubMed] [Google Scholar]
- Levin M. A novel immunohistochemical method for evaluation of antibody specificity and detection of labile targets in biological tissue. Journal of Biochemical and Biophysical Methods. 2004;58:85–96. doi: 10.1016/s0165-022x(03)00149-0. [DOI] [PubMed] [Google Scholar]
- Levin M. Errors of geometry: regeneration in a broader perspective. Semin Cell Dev Biol. 2009;20:643–645. doi: 10.1016/j.semcdb.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, Li CY. Apoptotic cells activate the "phoenix rising" pathway to promote wound healing and tissue regeneration. Sci Signal. 2010;3:ra13. doi: 10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E. Bioelectric fields and growth. Austin: Univ. of Texas Press; 1947. [Google Scholar]
- Mathews AP. Electrical polarity in the hydroids. American Journal of Physiology. 1903;8:294–299. [Google Scholar]
- McCaig CD, Song B, Rajnicek AM. Electrical dimensions in cell science. J Cell Sci. 2009;122:4267–4276. doi: 10.1242/jcs.023564. [DOI] [PubMed] [Google Scholar]
- Meier SD, Kovalchuk Y, Rose CR. Properties of the new fluorescent Na+ indicator CoroNa Green: comparison with SBFI and confocal Na+ imaging. J Neurosci Methods. 2006;155:251–259. doi: 10.1016/j.jneumeth.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Miskevich F, Doench JG, Townsend MT, Sharp PA, Constantine-Paton M. RNA interference of Xenopus NMDAR NR1 in vitro and in vivo. J Neurosci Methods. 2006;152:65–73. doi: 10.1016/j.jneumeth.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Mochii M, Taniguchi Y, Shikata I. Tail regeneration in the Xenopus tadpole. Dev Growth Differ. 2007;49:155–161. doi: 10.1111/j.1440-169X.2007.00912.x. [DOI] [PubMed] [Google Scholar]
- Mollenhauer HH, Morre DJ, Rowe LD. Alteration of intracellular traffic by monensin; mechanism, specificity and relationship to toxicity. Biochim Biophys Acta. 1990;1031:225–246. doi: 10.1016/0304-4157(90)90008-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin) 2nd Edition. North-Holland Publishing Company: Amsterdam; 1967. [Google Scholar]
- Nuccitelli R, Robinson K, Jaffe L. On electrical currents in development. Bioessays. 1986;5:292–294. doi: 10.1002/bies.950050616. [DOI] [PubMed] [Google Scholar]
- Poss KD, Shen J, Nechiporuk A, McMahon G, Thisse B, Thisse C, Keating MT. Roles for Fgf signaling during zebrafish fin regeneration. Developmental Biology. 2000;222:347–358. doi: 10.1006/dbio.2000.9722. [DOI] [PubMed] [Google Scholar]
- Rajnicek AM, Stump RF, Robinson KR. An endogenous sodium current may mediate wound healing in Xenopus neurulae. Developmental Biology. 1988;128:290–299. doi: 10.1016/0012-1606(88)90292-8. [DOI] [PubMed] [Google Scholar]
- Reid B, Song B, Zhao M. Electric currents in Xenopus tadpole tail regeneration. Dev Biol. 2009;335:198–207. doi: 10.1016/j.ydbio.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Reid B, Song B, McCaig CD, Zhao M. Wound healing in rat cornea: the role of electric currents. Faseb J. 2005;19:379–386. doi: 10.1096/fj.04-2325com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KR. Endogenous electrical current leaves the limb and prelimb region of the Xenopus embryo. Developmental Biology. 1983;97:203–211. doi: 10.1016/0012-1606(83)90077-5. [DOI] [PubMed] [Google Scholar]
- Saka Y, Smith JC. Spatial and temporal patterns of cell division during early Xenopus embryogenesis. Dev Biol. 2001;229:307–318. doi: 10.1006/dbio.2000.0101. [DOI] [PubMed] [Google Scholar]
- Sanz P. Snf1 protein kinase: a key player in the response to cellular stress in yeast. Biochem Soc Trans. 2003;31:178–181. doi: 10.1042/bst0310178. [DOI] [PubMed] [Google Scholar]
- Shaked M, Weissmuller K, Svoboda H, Hortschansky P, Nishino N, Wolfl S, Tucker KL. Histone deacetylases control neurogenesis in embryonic brain by inhibition of BMP2/4 signaling. PLoS One. 2008;3:e2668. doi: 10.1371/journal.pone.0002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S, Borgens R, Pascuzzi R, Roos K, Groff M, Purvines S, Rodgers RB, Hagy S, Nelson P. Oscillating field stimulation for complete spinal cord injury in humans: a phase 1 trial. J Neurosurg Spine. 2005;2:3–10. doi: 10.3171/spi.2005.2.1.0003. [DOI] [PubMed] [Google Scholar]
- Singer M. The influence of the nerve in regeneration of the amphibian extremity. Q Rev Biol. 1952;27:169–200. doi: 10.1086/398873. [DOI] [PubMed] [Google Scholar]
- Singer M. A theory of the trophic nervous control of amphibian limb regeneration, including a re-evaluation of quantiative nerve requirements; Proceedings of Regeneration in Animals; 1965. [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus Laevis. New York: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- Slack JM, Beck CW, Gargioli C, Christen B. Cellular and molecular mechanisms of regeneration in Xenopus. Philos Trans R Soc Lond B Biol Sci. 2004;359:745–751. doi: 10.1098/rstb.2004.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenstrom K, Takemori H, Bianchi G, Katz AI, Bertorello AM. Blocking the salt-inducible kinase 1 network prevents the increases in cell sodium transport caused by a hypertension-linked mutation in human alpha-adducin. J Hypertens. 2009;27:2452–2457. doi: 10.1097/HJH.0b013e328330cf15. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Taniguchi Y, Tazaki A, Ueno N, Watanabe K, Mochii M. Differential gene expression between the embryonic tail bud and regenerating larval tail in Xenopus laevis. Dev Growth Differ. 2004;46:97–105. doi: 10.1111/j.1440-169x.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- Tasava R, Olsen C. Higher Vertebrates Do Not Regenerate Digits and Legs Because the Wound Epidermis Is Not Functional. Differentiation. 1982;22:151–155. doi: 10.1111/j.1432-0436.1982.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Tseng AS, Adams DS, Qiu D, Koustubhan P, Levin M. Apoptosis is required during early stages of tail regeneration in Xenopus laevis. Dev Biol. 2007;301:62–69. doi: 10.1016/j.ydbio.2006.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin E, Dequiedt F, Kasler HG. Class II histone deacetylases: versatile regulators. Trends Genet. 2003;19:286–293. doi: 10.1016/S0168-9525(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Wang B, Goode J, Best J, Meltzer J, Schilman PE, Chen J, Garza D, Thomas JB, Montminy M. The insulin-regulated CREB coactivator TORC promotes stress resistance in Drosophila. Cell Metab. 2008;7:434–444. doi: 10.1016/j.cmet.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biology. 2003;4:207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–460. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- Zupanc GK. Neurogenesis and neuronal regeneration in the adult fish brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006;192:649–670. doi: 10.1007/s00359-006-0104-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.