Abstract
Multiple sclerosis (MS) is a disease characterized by inflammatory demyelination and a strong neurodegenerative component. Axonal damage is characteristically detected in MS brains, although the pathogenic mechanisms are not clearly understood. Here, we discuss the importance of HDAC1 localization as one of the potential mechanisms initiating damage in demyelinating conditions. We suggest the occurrence of a two-stage mechanism of damage. The first event is a calcium-dependent HDAC1 nuclear export in a CRM1-dependent manner and the second event is the interruption of mitochondrial transport resulting from the cytoplasmic localization of HDAC1. In the cytosol of neurons challenged by cytokines and excitatory aminoacids, HDAC1 formed complexes with motor-protein and microtubules and this resulted in blockade of axonal transport and release of cargo from motor proteins. We suggest that these findings might be the framework for future studies and for the development of novel therapeutic targets for axonal damage in demyelinating conditions.
Keywords: neurodegeneration, histone deacetylase, multiple sclerosis, demyelination, nuclear export
Axonal Damage in Demyelinating Disease, Multiple Sclerosis
Although Multiple sclerosis (MS) is well-known as an inflammatory and demyelinating disorder affecting more than 2.5 million adults in North America and Europe,1 previous studies have also suggested MS as a neurodegenerative disorder. Magnetic resonance imaging (MRI) studies have reported brain atrophy in the early disease course of remitting relapsing MS (RRMS),2 secondary progressive MS (SPMS)3 and in primary progressive MS (PPMS) patients4 as a sign of axonal damage. Proton MR spectroscopy studies which is a useful method to assess neurodegeneration in patients also revealed significantly reduced N-acetyl aspartate (NAA) levels in MS patients.5 NAA is synthesized from L-aspartate and acetyl-CoA by L-aspartate-N-acetyltransferase in neuronal mitochondria.6 Therefore, decreased level of NAA can be a marker of axonal damage and reflects mitochondrial dysfunction within axons. Clinical studies supported the existence of a correlation between brain atrophy, decreased levels of NAA and neurological disability score, as measured by the expanded disability status scale (EDSS) scores thereby suggesting an association between axonal damage and disease progression.7–9 Histological studies in post-mortem samples of MS patients reported morphological changes of axons, such as swollen axons and amyloid precursor protein (APP) positive end bulb structures in acute inflammatory lesions and at the margins of chronic lesions.10 Since APP is a protein which is normally transported by fast axonal transport, its accumulation was suggestive of altered axonal transport and related to the presence of axonal enlargements. Together, these studies provided evidences of axonal damage in MS and also suggested that impaired axonal transport could be one of the underlying mechanisms of axonal damage in MS.
Axonal Transport and Impaired Axonal Transport in Neurons
Regulation of axonal transport
Neurons are polarized cells composed of dendrites, which are extensions with many branches and receive majority of signal and a single axon, which is a long and thin structure that carries nerve signals away from the cell body. Since most neuronal proteins are synthesized in the cell body, the communication between cell body, dendrites and axon is critically important for neuronal function and survival. The main mechanism for this communication is microtubule-based axonal transport. Axonal transport is a complex bidirectional process: anterograde and retrograde transports, and is divided into fast and slow axonal transport. Vesicles and mitochondria move by fast axonal transport at the speed of ~1 μm/s, whereas cytoskeletal components move by slow axonal transport at the speed of ~1 mm/day.11,12 Axonal transport is mediated by two major components: microtubules are the tracks and motor proteins (i.e., kinesin and dynein) are the vehicles that transfer cargos to the destination.
Transport is regulated by several steps including regulation of motor proteins, cargo binding and release from motor proteins and microtubule dynamics. Although the mechanism of regulation of motor protein activity remains poorly understood, the best known molecular mechanism is the conformational change of protein structure.13 Recent biochemical and structural studies have shown that one of the motor proteins, kinesin-1 (also known as KIF5), self-regulates its motile activities by conformational changes. Deletion or point mutation in the central hinge region of kinesin-1 prevents the folding corresponding to the active state for transport. In other words, since the folded conformation of kinesin-1 blocks the interaction between motor domain and microtubules as well as ATPase activity, conformational changes of kinesin-1 can regulate axonal transport.13
The post-translational modifications (PTMs) of motor proteins regulate axonal transport through the control of motor-cargo binding and release. Recent studies have demonstrated a role of distinct signaling pathways in regulating the interaction of motor proteins with their cargos. The phosphorylation of c-Jun N-terminal kinase interacting protein (JIP) by the mitogen-activated protein cascade results in dissociation of the JIP cargo proteins from the kinesin-1 motor.14 The protein kinase glycogen synthase kinase 3β (GSK3β) phosphorylates the kinesin light chain (KLC) subunit and decreases its association with membrane-bound organelles.15 Another signaling pathway through Ca2+/calmodulin-dependent proteins Kinase II (CamKII) also plays an important part in regulating the dissociation of the cargo from motors in neurons.16 Together, recent work has elucidated the role of PTMs of motor proteins in regulating the interaction of motors with cargos.
The post-translational modifications can differentially regulate cargo and vesicle transport. PTMs of tubulin, which is the main component of microtubules, also have an important role in regulation of axonal transport by modulating microtubule stability.17 Acetylation on lysine 40 of α-tubulin is unique among the known tubulin modifications and is found in stable microtubules.18 Also acetylated α-tubulin can recruit more motor proteins into microtubules because of the higher affinity of kinesin-1 binding to acetylated α-tubulin in vitro.19 The acetylation enzyme of α-tubulin has not been identified. However, two enzymes, histone deacetylase 6 (HDAC6) and SIRT2 have been reported to deacetylate α-tubulin in vitro and in vivo.20,21 HDAC6 and SIRT2 are HDAC family members, traditionally classified into four classes based on their primary structures: class I (HDAC 1, 2, 3 and 8), class II (HDAC 4, 5, 6, 7, 9 and 10), class III (SIRT1-7) and class IV (HDAC 11).22–24 Small molecule inhibitors of HDAC6 or silencing either HDAC6 or SIRT2 enhance acetylation of α-tubulin and rescue the anterograde and retrograde transport of brain-derived neurotrophic factor (BDNF) vesicles in neurons expressing mutant huntingtin (htt) proteins.25 Together, these studies provide important evidence that post-translational modifications of proteins regulate organelle and vesicle transports.
Impaired axonal transport
The accumulations of organelles and proteins in the cell body and axon are hallmarks of axonopathies for many human neurodegenerative diseases including MS.26–28 Aggregates of mutant Huntingtin protein (htt) are detected in Huntington’s disease (HD), accumulation of tau proteins is detected in Alzheimer’s disease (AD), α-synuclein positive Lewy bodies are observed in Parkinson’s disease (PD) and neurofilament accumulations are seen in amyotrophic lateral sclerosis (ALS).26–29 These pathologies suggest that impaired axonal transport may underlie the pathogenic accumulations of proteins in neurodegenerative diseases.
As we mentioned above, impaired axonal transport may result from damage to motor proteins, impaired interaction between motor proteins and either cargo or microtubules, or damage to the microtubules. In addition altered mitochondrial function can also induce impaired axonal transport due to reduction of ATP supply.30 Motor-driven mitochondrial transport is required to produce ATP at appropriate sites along the axons. Therefore, impaired mitochondria transport and dysfunction can disrupt axonal transport by decreasing ATP supply. At the same time, disruptions of axonal transport can interfere with mitochondrial transport, impair transport and induce morphological changes. Together, these results suggest that impaired transport contributes to axonal damage and may underlie the pathological accumulation of organelles or cytoskeletal components.
The Molecular Mechanisms of Axonal Damage in Demyelinating Conditions
As discussed above, previous studies have provided evidence of axonal swellings and impaired axonal transport in MS. However, the molecular mechanisms of axonal damage in MS have not been well characterized. Therefore, we addressed the molecular mechanisms of axonal damage in demyelinating conditions using autoptic brain samples from MS patients, from mice with cuprizone-induced demyelination and also using ex vivo models of demyelination or cultured neurons exposed to glutamate and TNFα to mimic the inflammatory environment. Our results showed that HDAC1 nuclear export was induced by pathological stimuli prior to the impaired mitochondrial transport and the onset of morphological changes. Silencing of HDAC1, but not other HDAC isoforms and treatment with pharmacological inhibitors of HDAC1, but not of HDAC6 prevented the alterations in mitochondrial transport and the associated morphological changes.31 Therefore, we suggested HDAC1 nuclear export as a critical event that impairs mitochondrial transport and induces morphological changes.
This HDAC1-dependent mechanism of axonal impairment can be divided into nuclear and cytoplasmic events. We shall first discuss HDAC1 nuclear export induced by pathological conditions and then address the function of cytoplasmic HDAC1.
In the nucleus
Since HDAC1 is a nuclear enzyme, it is important to understand how it is exported from the nucleus in response to pathological stimuli (i.e., glutamate and TNFα). In the case of class II HDACs, nucleus export signals (NESs) allow binding to the CRM1 nuclear export receptor and favor exit through the nuclear pore.32 To study whether HDAC1 adopts a similar strategy, we searched the protein sequence of HDAC1 for NESs. We detected three potential lucine-rich NESs motifs: LXXXLXXL or LXXLXL which resembled the originally described NESs in viral proteins.33 We then tested the interaction between HDAC1 and CRM1 in primary neurons in response to pathological stimuli. HDAC1 bound with CRM1 only during the first 5 min of treatment, a time point when the majority of HDAC1 was still detected in nuclear or peri-nuclear locations. Moreover, point mutations in the potential NES sequences reduced the interaction between HDAC1 and CRM1 and suggested that HDAC1 binding to CRM1 occurred through these sequences. Pretreatment with the pharmacological inhibitor of CRM1-dependent export, leptomycin B (LMB) confirmed that HDAC1 used a CRM1-dependent mechanism of nuclear export. In the presence of LMB, HDAC1 stayed nuclear and no alterations of mitochondrial movement or morphological changes were observed. For this reason, we suggested CRM1-mediated nuclear export of HDAC1 as the early event modulating mitochondrial transport and the onset of swellings in pathological conditions.
However, we cannot exclude the possibility that, similar to class II HDACs, also HDAC1 shuttles between nucleus and cytoplasm and accumulation may be consequent to decreased import. Since HDAC1 has also a nuclear localization sequences (NLS), once synthesized in the cytoplasm, it is imported into the nucleus. In time course experiments using immunocytochemistry of primary cultures in response to glutamate and TNFα, we detected peri-nuclear HDAC1 after 5 minutes of treatment and evenly distributed HDAC1 along neurites after 20 minutes of treatment. These data suggest that either HDAC nuclear export is a very early event or is result of impaired nuclear import of newly synthesized HDAC1, both resulting in a rapid accumulation of HDAC1 in the cytoplasm. Further studies are needed to determine whether pathological stimuli also block HDAC1 nuclear import.
It remains unanswered why HDAC1 binds with CRM1 only in pathological, but not in physiological conditions. In other words, although HDAC1 and CRM1 are in the nucleus in physiological conditions, they do not bind to each other. Only after exposure to pathological stimuli, the two molecules interact. We have shown that HDAC1 nuclear export was calcium-dependent because pre-treatment with the calcium chelator EDTA prevented export and the onset of neurite swellings in a dose-dependent manner. However additional studies are needed to define the calcium-dependent signaling events responsible for HDAC1 export in a CRM1-dependent manner. We would like to suggest that post-translational modifications of HDAC1 may modulate this interaction since HDAC1 itself is subject to post-translational modifications that modulate its stability, localization, activity and protein-protein interactions.34 Recent studies have shown that phosphorylation of HDAC1 by casein kinase 2 (CK2) and protein kinase A (PKA) is required for its interaction with other co-repressors.13 In addition to phosphorylation, acetylation of HDAC1 was found to be important for the stability of HDAC1 complex35 and enzymatic activity.36 HDAC1 is also a substrate for sumoylation, which affects its activity,37 protein localization and transport to sub-cellular compartments.38 Therefore, it will be important to determine whether pathological stimuli induce post-translational modifications of HDAC1 that alter its binding partners or localization in pathological conditions.
In the cytoplasm
To define the role of HDAC1 in the cytoplasm, we identified the potential binding partners of this molecule using MALDI-TOF analysis of proteins extracted from the brain of animal models of demyelination and from primary neurons treated with glutamate and TNFα. In pathological conditions, we detected binding of cytoplasmic HDAC1 and selected motor and cytoskeletal proteins. Time course experiments in primary cultured neurons showed binding with KIF2A, KIF5 motors and with α-tubulin after treatment with pathological stimuli for 20 minutes and prior to the onset of neuritic swellings. At this time point, immunoprecipitation experiments with anti-dynamin antibody showed the dissociation of cargo-protein from the motor protein-microtubule complex. Treatment with the HDAC1 inhibitor, MS-275 and silencing of HDAC1 using shRNA lentiviral particles decreased the interaction of HDAC1 with motor proteins and α-tubulin and prevented alteration of mitochondrial transport and the morphological changes induced by glutamate and TNFα treatment. Therefore, MS-275, but not Tubacin, rescued impaired mitochondrial transport induced by pathological stimuli. Taken together, these results suggest that cytoplasmic HDAC1 binds to cargo-motor protein-microtubule complexes and releases cargos from motor proteins thereby blocking axonal transport and possibly allowing accumulation of proteins along neurites and following the onset of morphological changes.
Since cytoplasmic HDAC1 bound with α-tubulin in demyelinating conditions and deacetylated α-tubulin releases motor proteins from microtubules, we tested whether cytoplasmic HDAC1 also decreased the acetylation of α-tubulin. Western blot analysis with protein extracts from demyelinating region of cuprizone-treated mice and cultured neurons exposed to glutamate and TNFα did not show altered level of acetylated α-tubulin. In other words, even though HDAC1 was exported from the nucleus to the cytoplasm and formed complexes with α-tubulin, this interaction did not result in deacetylation of α-tubulin. However, the interaction between HDAC1 and its binding partners were dependent on enzymatic activity because treatment with inhibitors prevented interaction. Therefore, although HDAC1 did not deacetylate α-tubulin, we cannot rule out alternative cytoplasmic substrates. The acetylation of motor proteins and cytoskeletal proteins has not been well studied since target proteins for acetylation may differ in distinct cell types, physiological conditions or cell cycle stages. In 2009, Choudhary and colleagues have published 1,750 proteins as the lysine acetylation targets in mammalian cell lines in response to HDAC inhibitors either MS-275 or SAHA using stable isotope labeling with amino acids in cell culture (SILAC) and high-resolution mass spectrometry. Interestingly, they identified acetylation targets as large macromolecular complexes involved in diverse cellular processes including nuclear transport and vesicular trafficking.39 In the case of nuclear transport, for instance acetylation itself did not regulate nuclear transport per se. However, deacetylated lysine residues in several components of the nuclear transport complex, such as the nuclear receptor coactivator 3 (NcoA3) and the RAN-guanosine triphosphatase (GTPase) (RANGAP1)40,41 were targets for sumoylation and this modulated-nuclear export. These results also supported the fact that PTMs of proteins are important for nuclear export. Additional targets for lysine acetylation in vesicle trafficking complex included several motor proteins and motor protein associated proteins. These results were exciting because the published lysine acetylation targets included several molecules that we identified as potential cytoplasmic HDAC1 binding partners in pathological conditions, such as kinesin light chain, myosin-9 and cytoplasmic dynein 1. These results suggest that acetylation of proteins may be involved in regulation of nuclear export and transport of proteins or organelles. However, it still remains to be determined whether those proteins are acetylated in neurons and deacetylated in pathological conditions by cytoplasmic HDAC1 to affect on their functions.
Conclusion
Impaired axonal transport and swelling along the axons have been considered as early events of axonal damage.42 However, it remains to be established whether decreased levels of nuclear HDAC1 also modulates the expressions of genes related to cell death or neurodegeneration. Future studies will need to address the relationship between gene expressions due to decreased HDAC1 levels in the nucleus and neuronal death in long-term cuprizone-treated mice or cultures treated with glutamate and TNFα for longer than 2 hours.
We used the HDAC1 inhibitor or silencing using shRNA to test the role of HDAC1 in axonal damage. However, in physiological conditions, neurons still need HDAC1 enzyme activity in the nucleus. So, targeting HDAC1 for treatment of impaired axonal transport can also inhibit HDAC1 activity in the nucleus of neurons as well as other cell types. From a translational standpoint, it will be critical to screen for small molecules able to block the interaction between HDAC1 and CRM1 in the nucleus thereby preventing HDAC1 nuclear export or inhibit only the cytoplasmic HDAC1 activity. We hope that future studies on the molecular mechanisms induced by cytoplasmic HDAC1 will lead to the diverse novel therapeutic targets of axonal damage in demyelinating disorders.
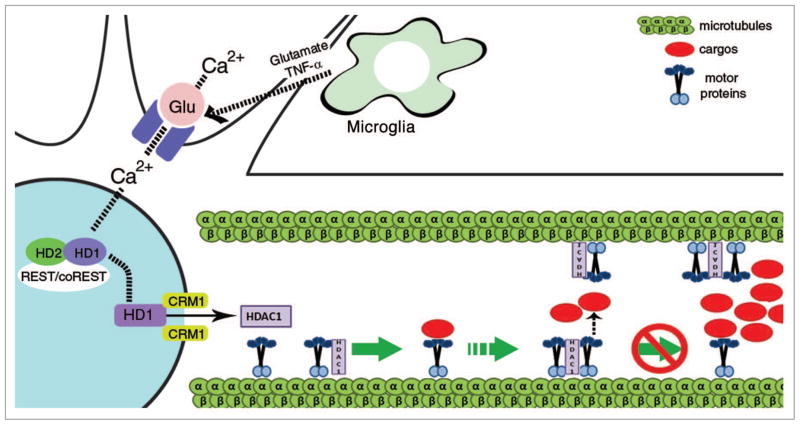
Figure 1.
Schematic model of the mechanism relating HDAC1 to impaired axonal transport in response to glutamate and cytokines. In physiological conditions, cargos are transported by motor proteins along microtubules. However when neurons are exposed to in_ammatory stimuli such as glutamate and TNFα, activated glutamate receptors uptake calcium into the neurons and induce HDAC1 export in a CRM1-dependent manner. In the cytoplasm, HDAC1 competes with the adaptor proteins for binding to the motor proteins. In these conditions, the cargo is unable to form functional interactions with the motors and therefore axonal transport is blocked.
Acknowledgments
This work was supported from ARRA Funds R01-NS 42925-S2, NMSS RG-4134 and a generous donation from the Slone Foundation.
References
- 1.Hauser SL, Oksenberg JR. The neurobiology multiple sclerosis: genes, inflammation and neurodegeneration. Neuron. 2006;52:7–9. doi: 10.1016/j.neuron.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Carswell R. Pathological anatomy: Illustrations of the elementary forms of disease. Longman, Orme, Brown, Green and Longman; 1838. [Google Scholar]
- 3.Minagar A, Toledo EG, Alexander JS, Kelley RE. Pathogenesis of brain and spinal cord atrophy in multiple sclerosis. J Neuroimaging. 2004;14:5–10. doi: 10.1177/1051228404266263. [DOI] [PubMed] [Google Scholar]
- 4.Zivadinov R, Bakshi R. Central nervous system atrophy and clinical status in multiple sclerosis. J Neuroimaging. 2004;14:27–35. doi: 10.1177/1051228404266266. [DOI] [PubMed] [Google Scholar]
- 5.Bjartmar C, Kidd G, Mork S, Rudick R, Trapp BD. Neurological disability correlates with spinal cord axonal loss and reduced N-acetyl aspartate in chronic multiple sclerosis patients. Ann Neurol. 2000;48:893–901. [PubMed] [Google Scholar]
- 6.Clark JB. N-acetyl aspartate: a marker for neuronal loss or mitochondrial dysfunction. Dev Neurosci. 1998;20:271–6. doi: 10.1159/000017321. [DOI] [PubMed] [Google Scholar]
- 7.Sastre-Garriga J, Ingle GT, Chard DT, Ramio-Torrenta L, McLean MA, Miller DH, et al. Metabolite changes in normal-appearing gray and white matter are linked with disability in early primary progressive multiple sclerosis. Arch Neurol. 2005;62:569–73. doi: 10.1001/archneur.62.4.569. [DOI] [PubMed] [Google Scholar]
- 8.Barkhof F. MRI in multiple sclerosis: correlation with expanded disability status scale (EDSS) Mult Scler. 1999;5:283–6. doi: 10.1177/135245859900500415. [DOI] [PubMed] [Google Scholar]
- 9.Fisher E, Rudick RA, Cutter G, Baier M, Miller D, Weinstock-Guttman B, et al. Relationship between brain atrophy and disability: an 8 year follow-up study of multiple sclerosis patients. Mult Scler. 2000;6:373–7. doi: 10.1177/135245850000600602. [DOI] [PubMed] [Google Scholar]
- 10.Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–9. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- 11.Roy S, Coffee P, Smith G, Liem RK, Brady ST, Black MM. Neurofilaments are transported rapidly but intermittently in axons: implications for slow axonal transport. J Neurosci. 2000;20:6849–61. doi: 10.1523/JNEUROSCI.20-18-06849.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasek RJ, Garner JA, Brady ST. Axonal transport of the cytoplasmic matrix. J Cell Biol. 1984;99:212–21. doi: 10.1083/jcb.99.1.212s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai D, Hoppe AD, Swanson JA, Verhey KJ. Kinesin-1 structural organization and conformational changes revealed by FRET stoichiometry in live cells. J Cell Biol. 2007;176:51–63. doi: 10.1083/jcb.200605097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horiuchi D, Collins CA, Bhat P, Barkus RV, Diantonio A, Saxton WM. Control of a kinesin-cargo linkage mechanism by JNK pathway kinases. Curr Biol. 2007;17:1313–7. doi: 10.1016/j.cub.2007.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST. Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 2002;21:281–93. doi: 10.1093/emboj/21.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillaud L, Wong R, Hirokawa N. Disruption of KIF17-Mint1 interaction by CaMKII-dependent phosphorylation: a molecular model of kinesin-cargo of release. Nat Cell Biol. 2008;10:19–29. doi: 10.1038/ncb1665. [DOI] [PubMed] [Google Scholar]
- 17.Verhey KJ, Gaertig J. The tubulin code. Cell Cycle. 2007;6:2152–60. doi: 10.4161/cc.6.17.4633. [DOI] [PubMed] [Google Scholar]
- 18.LeDizet M, Piperno G. Identification of an acetylation site of Chlamydomonas alpha-tubulin. Proc Natl Acad Sci USA. 1987;84:5720–4. doi: 10.1073/pnas.84.16.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–72. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, et al. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–8. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- 21.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–44. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 22.de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–49. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guardiola AR, Yao TP. Molecular cloning and characterization of a novel histone deacetylase HDAC10. J Biol Chem. 2002;277:3350–6. doi: 10.1074/jbc.M109861200. [DOI] [PubMed] [Google Scholar]
- 24.Gao L, Cueto MA, Asselbergs F, Atadja P. Cloning and functional characterization of HDAC11, a novel member of the human histone deacetylase family. J Biol Chem. 2002;277:25748–55. doi: 10.1074/jbc.M111871200. [DOI] [PubMed] [Google Scholar]
- 25.Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington’s disease by increasing tubulin acetylation. J Neurosci. 2007;27:3571–83. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–72. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 27.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 28.Xiao S, McLean J, Robertson J. Neuronal intermediate filaments and ALS: a new look at an old question. Biochim Biophys Acta. 2006;1762:1001–12. doi: 10.1016/j.bbadis.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Bates G. Huntingtin aggregation and toxicity in Huntington’s disease. Lancet. 2003;361:1642–4. doi: 10.1016/S0140-6736(03)13304-1. [DOI] [PubMed] [Google Scholar]
- 30.Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117:2791–804. doi: 10.1242/jcs.01130. [DOI] [PubMed] [Google Scholar]
- 31.Kim JY, Shen S, Dietz K, He Y, Howell O, Reynolds R, Casaccia P. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat Neurosci. 13:180–9. doi: 10.1038/nn.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKinsey TA, Zhang CL, Olson EN. Identification of a signal-responsive nuclear export sequence in class II histone deacetylases. Mol Cell Biol. 2001;21:6312–21. doi: 10.1128/MCB.21.18.6312-6321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–73. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 34.Brandl A, Heinzel T, Kramer OH. Histone deacetylases: salesmen and customers in the post-translational modification market. Biol Cell. 2009;101:193–205. doi: 10.1042/BC20080158. [DOI] [PubMed] [Google Scholar]
- 35.Brush MH, Guardiola A, Connor JH, Yao TP, Shenolikar S. Deactylase inhibitors disrupt cellular complexes containing protein phosphatases and deacetylases. J Biol Chem. 2004;279:7685–91. doi: 10.1074/jbc.M310997200. [DOI] [PubMed] [Google Scholar]
- 36.Qiu Y, Zhao Y, Becker M, John S, Parekh BS, Huang S, et al. HDAC1 acetylation is linked to progressive modulation of steroid receptor-induced gene transcription. Mol Cell. 2006;22:669–79. doi: 10.1016/j.molcel.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 37.David G, Neptune MA, DePinho RA. SUMO-1 modification of histone deacetylase 1 (HDAC1) modulates its biological activities. J Biol Chem. 2002;277:23658–63. doi: 10.1074/jbc.M203690200. [DOI] [PubMed] [Google Scholar]
- 38.Melchior F. SUMO—nonclassical ubiquitin. Annu Rev Cell Dev Biol. 2000;16:591–626. doi: 10.1146/annurev.cellbio.16.1.591. [DOI] [PubMed] [Google Scholar]
- 39.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Lin RJ, Xie W, Wilpitz D, Evans RM. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase. Cell. 1999;98:675–86. doi: 10.1016/s0092-8674(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 41.Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- 42.Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat Rev Neurosci. 2005;6:889–98. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]