Abstract
Diabetes is an epidemic of worldwide proportions caused by β-cell failure. Nutrient fluctuations and insulin resistance drive β–cells to synthesize insulin beyond their capacity for protein folding and secretion and thereby activate the unfolded protein response (UPR), an adaptive signaling pathway to promote cell survival upon accumulation of unfolded protein in the endoplasmic reticulum (ER). PERK signals one component of the UPR through phosphorylation of eukaryotic initiation factor 2 on the alpha subunit (eIF2α) to attenuate protein synthesis, thereby reducing the biosynthetic burden. B–cells uniquely require PERK-mediated phosphorylation of eIF2α to preserve cell function. Unabated protein synthesis in β–cells is sufficient to initiate a cascade of events, including oxidative stress, that are characteristic of β–cell failure observed in type 2 diabetes. In contrast to acute adaptive UPR activation, chronic activation increases expression of the proapoptotic transcription factor CAAT-enhancer binding protein homologous protein (CHOP). Chop deletion in insulin-resistant mice profoundly increases β–cell mass and prevents β–cell failure to forestall the progression of diabetes. The findings suggest an unprecedented link by which protein synthesis and/or misfolding in the ER cause oxidative stress and should encourage the development of novel strategies to treat diabetes.
Keywords: eukaryotic initiation factor 2, PERK, CHOP, antioxidant, apoptosis, translation, mitochondria, protein folding
Intoduction
Type 2 diabetes is an epidemic in which tissues, such as muscle, fat, and liver, become resistant to insulin and the pancreas fails to compensate adequately [1]. Insulin deficiency or resistance causes hyperglycemia and hyperlipidemia characteristic of the diabetic state. Unfortunately, little is known about how the β–cell responds to elevated blood glucose to increase insulin transcription, translation, and secretion. When the β–cell compensates for insulin resistance, it must restructure the secretory apparatus to support high-level insulin production. Recent studies suggest that the ability for β–cells to compensate for hyperglycemia-driven increases in insulin production requires an intracellular signaling pathway, the unfolded protein response (UPR) that emanates from the ER.
The Endoplasmic Reticulum: Protein Folding, Quality Control, and ERAD
The ER is the site where proteins destined for the cell surface and endomembrane system enter the secretory pathway. Approximately one-third of all proteins translocate across the ER membrane where they fold into their proper three-dimensional structures and are subject to glycosylation, hydroxylation, lipidation, and disulfide bond formation [2–4]. The ER contains an extremely high Ca+2 concentration and is occupied by chaperone proteins, catalysts of protein folding and enzymatic machinery for post-translational modifications [5]. Only properly folded proteins exit the ER to the Golgi compartment for further processing prior to trafficking to their final destination. The mechanisms for this exquisitely sensitive and specific quality control are under active investigation, but are not yet well defined. Retention of unfolded proteins in the ER lumen occurs through interaction with ER resident peptide-binding proteins, such as BiP and GRP94. ER-associated protein degradation (ERAD) is the process whereby misfolded proteins are recognized in the ER, targeted to a channel within the ER membrane, extracted from the ER membrane, and delivered to the proteasome [6]. At a minimum, quality control is mediated through the processing of N-linked glycans that are primarily recognized by lectin-binding proteins in the ER lumen [7]. These lectins direct refolding (eg. CNX, CRT), anterograde transport (eg. LMAN1), or ERAD (eg. EDEMs, OS-9, XPT3-B) [8,9].
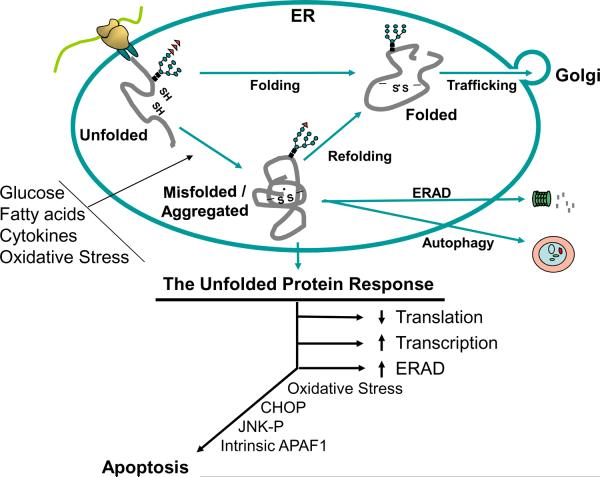
Protein misfolding occurs in the ER as a consequence of a number of insults, including pharmacological perturbation, alterations in calcium, redox status, or nutrient availability, mutations in ER chaperones or their client proteins, viral infection, as well as differentiation of cells that secrete large amounts of proteins. The accumulation of unfolded proteins in the ER lumen activates the UPR [5,10]. The UPR is an adaptive response designed to resolve the protein folding defect by: 1) reducing protein influx into the ER; 2) increasing the capacity to promote productive protein folding; and 3) increasing clearance of misfolded proteins in the ER lumen through ERAD and autophagy (Fig. 1). These strategies may exhibit temporally separate phases of the UPR. Reduced influx occurs rapidly and transiently, and prevents further generation of misfolded proteins, while upregulation of protein maturation and degradation machinery occurs after UPR-dependent gene induction. Over time, homeostasis may be re-established in the ER to resolve the protein-folding defect [11]. If homeostasis is not restored, the UPR is chronically activated and leads to apoptosis. The mechanisms that decipher the protein folding status and orchestrate a coordinated downstream response, either adaptation or apoptosis, are fundamentally significant, unknown, and require further investigation.
Figure 1. Pathways of protein misfolding that lead to cell death.
Nascent unfolded polypeptides enter the ER and interact with chaperones and catalysts of protein folding to mature into compact, thermodynamically favorable structures. Failure of this process results in persistence of misfolded polypeptide-chaperone complexes, or extraction of soluble, misfolded protein from the ER and degradation through ERAD. Formation of insoluble protein aggregates requires clearance by autophagy. ER stress stimuli impair polypeptide folding and induce adaptive increases in chaperones and catalysts within the ER lumen through UPR sensor activation. Chronic or overwhelming stimuli elicit a number of apoptotic signals including oxidative stress, JNK activation, CHOP expression, cleavage of caspase 12, and activation of the intrinsic mitochondrial-dependent cell death pathway. Physiological stimuli that can activate the UPR in the β-cell include expression of misfolded proinsulin or IAPP, oxidative stress (ROS), and increases in the extracellular concentrations of glucose, fatty acids, or cytokines.
UPR Translational and Transcriptional Control
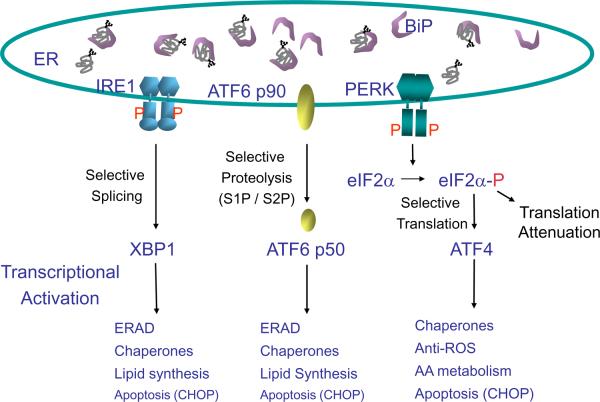
The UPR is signaled through activation of the protein kinases IRE1 and PERK and cleavage of the transcription factor ATF6 (Fig. 2)[11,12]. The three UPR sensors are maintained in an inactive state through interaction with the protein chaperone BiP. It is proposed that as unfolded proteins accumulate, bind and sequester BiP, they promote BiP dissociation from PERK, IRE1 and ATF6 [13,14]. BiP release from IRE1 and PERK permit their homodimerization, trans-autophosphorylation, and activation. Activated PERK phosphorylates the alpha subunit of the translation initiation factor 2 (eIF2α) leading to rapid and transient inhibition of protein synthesis [15]. eIF2 is a heterotrimeric GTPase required to bring initiator methionyl tRNA to the ribosome for AUG initiation codon selection [16]. Phosphorylation of eIF2α inhibits the GDP/GTP exchange reaction on eIF2, thereby preventing eIF2 recycling and the initial step of polypeptide synthesis [15,17,18]. Paradoxically, eIF2α phosphorylation is required for the translation of several mRNAs(Gadd34, Atf5, Cat1), including the bZiP transcription factor ATF4 [19]. This mechanism apparently involves ribosomes scanning through upstream open reading frames in the 5' end of the mRNA due to limiting amounts of GTP-bound eIF2-tRNAmet.
Figure 2. Signaling the unfolded protein response.
The UPR sensors PERK, IRE1, and ATF6 control mRNA translation and transcriptional induction of UPR-regulated genes. Interaction of BiP with each UPR sensor prevents UPR signaling. Upon accumulation of unfolded protein, BiP is released from each sensor, leading to its activation. The ER protein kinase PERK is activated by homodimerization and autophosphorylation to phosphorylate eIF2α, thereby reducing the rate of mRNA translation and the biosynthetic protein-folding load on the ER. eIF2α phosphorylation paradoxically increases translation of Atf4 mRNA to produce a transcription factor that activates expression of genes encoding protein chaperones, ERAD machinery, enzymes that reduce oxidative stress, and functions in amino acid biosynthesis and transport. Dimerization of the ER protein kinase IRE1 triggers its endoribonuclease activity to induce cleavage of Xbp1 mRNA. Xbp1 mRNA is then ligated by an uncharacterized RNA ligase and translated to produce XBP1s. Concurrently, ATF6 released from BiP transits to the Golgi where it is cleaved to release a transcriptionally active fragment. Cleaved ATF6 acts in concert with XBP1s to induce expression of genes encoding protein chaperones and ERAD machinery. The RNase activity of IRE1 also degrades selective cellular mRNAs to reduce the client protein load upon the ER.
Although there are alpha and beta alleles of both IRE1 and ATF6 in the mammalian genome, only IRE1α is expressed in all tissues and only ATF6α signals the UPR. IRE1β is selectively expressed in intestinal epithelial cells and it is not known what genes are regulated by ATF6β. IRE1 activation elicits an endoribonuclease function that induces non-conventional splicing of Xbp1 mRNA. Splicing of Xbp1 mRNA, the only known splicing substrate of IRE1, removes a 26 base intron that alters the translation reading frame to produce a highly active bZiP transcription factor that activates genes encoding ER protein chaperones, lipid biosynthetic enzymes, and ERAD functions [20–22]. Upon release from BiP, ATF6 traffics to the Golgi complex where it is cleaved by the S1P and S2P processing enzymes to produce a cytosolic fragment that activates transcription of genes providing complementary and overlapping functions with those activated by XBP1 that restore productive ER protein folding and increase ERAD [23–25]. Indeed, cells deleted in either Ire1α, Xbp1, or Atf6α are defective in ERAD [21,25,26,27].
Signals Downstream of eIF2α Phosphorylation – Regulation of Transcription by ATF4 and CHOP
During periods of ER stress, the selective translation of Atf4 mRNA produces a factor that binds to the amino acid response element (AARE) in target genes [28,29] such as Atf3, Chop/Gadd153, Atf3 and Gadd34/MyD116/Ppp1r15a [30,31]. Recent studies, as well as our own, indicate chronic UPR activation causes induction of CHOP that is essential for the apoptotic response to chronic protein misfolding in the ER [32–34]. CHOP was originally isolated as a gene induced in response to DNA damage and it consists of a transcriptional activation/repression domain followed by a basic leucine zipper domain at its C terminus [35]. Although it was thought that CHOP functions as a negative regulator to sequester binding partners [36], subsequent studies demonstrated that a CHOP-C/EBP heterodimer could bind DNA and function as a transcriptional activator [37–39]. It is proposed that CHOP mediates induction of the apoptotic genes Gadd34, Dr5, Bim, and Trb3, and repression of anti-apoptotic Bcl2 expression [33,39,40].
Protein Misfolding in the ER and Oxidative Stress
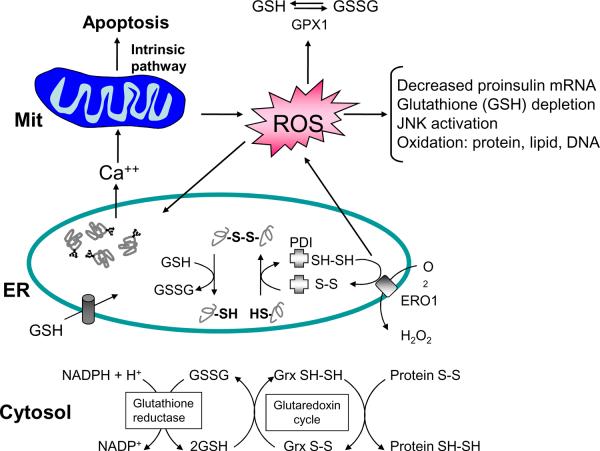
There is accumulating evidence to suggest that protein misfolding in the ER and production of reactive oxygen species (ROS) are closely linked, however, this area of ER stress is not well explored. In eukaryotes, oxidative protein folding occurs in the ER. A growing family of ER oxidoreductases, including PDI (protein disulphide isomerase), ERp57, ERp72, PDIR, PDIp and P5, catalyze these protein-folding reactions in mammalian cells. PDI catalyzes the formation, isomerization and reduction of disulfide bonds in vitro. When disulfide bond formation occurs, cysteine residues within the PDI active site [-C-X-X-C-] accept two electrons from thiol residues in the polypeptide chain substrate. This electron transfer results in the oxidation of the substrate and the reduction of the PDI active site. It is now recognized that reduced PDI transfers its electrons through ERO1 to molecular oxygen as the final electron acceptor [41].
During formation of disulfide bonds, hydrogen peroxide is formed as a byproduct from the sequential action of PDI and ERO1 in transferring electrons from thiol groups in proteins to molecular oxygen. It has been estimated that approximately 25% of the ROS generated in a cell may result from formation of disulfide bonds in the ER during oxidative protein folding [41]. In addition, ROS may be formed as a consequence of the GSH depletion that occurs as GSH reduces unstable and improper disulfide bonds. The consumption of GSH would return thiols involved in non-native disulfide bonds to their reduced form so they may again interact with ERO1/PDI to be reoxidized. This would generate a futile cycle of disulfide bond formation and breakage in which each cycle would generate ROS and consume GSH (Fig. 3). As a consequence, it is expected that proteins that have multiple disulfide bonds may be more prone to generating oxidative stress. Our recent findings demonstrate that excessive protein synthesis and unfolded protein accumulation in the ER lumen can cause oxidative stress [42]. The ROS generated by these processes appear to be a critical second signal to initiate apoptosis (Fig. 3). In addition, our findings suggest ROS can further disrupt protein misfolding in the ER. Intriguingly, antioxidants can improve protein folding and reduce apoptosis under conditions of ER stress.
Figure 3. ER stress, protein misfolding, and oxidative stress are intimately interrelated.
Protein folding within the ER lumen is ushered by a family of oxidoreductases that catalyze disulfide bond formation and isomerization. ER stress causes an increase in the formation of incorrect intermolecular and/or intramolecular disulfide bonds that require breakage and reformation for proteins to attain the appropriate folded conformation. PDI catalyzes disulfide bond formation and isomerization, whereas glutathione transported into the ER reduces improperly paired disulfide bonds. Reoxidation of PDI is mediated by ERO1; however, ROS are produced in the process. Cellular ROS can deplete glutathione and increase the misfolded protein load in the ER. In turn, ROS can also cause ER stress through modification of proteins and lipids that are necessary to maintain ER homeostasis. Consumption of excessive cellular glutathione due to ER stress could inhibit glutaredoxin reduction and cause accumulation of oxidized cytosolic proteins. ER stress also causes calcium leak from the ER for accumulation in the inner mitochondrial matrix. This calcium loading in the mitochondria can generate additional ROS through disruption of electron transport and opening of the mitochondrial permeability pore. Thus, accumulation of misfolded protein in the ER increases ROS production that can further amplify ER stress, disrupt insulin production, and cause cell death.
UPR Induction of ATF4 and CHOP and Apoptosis
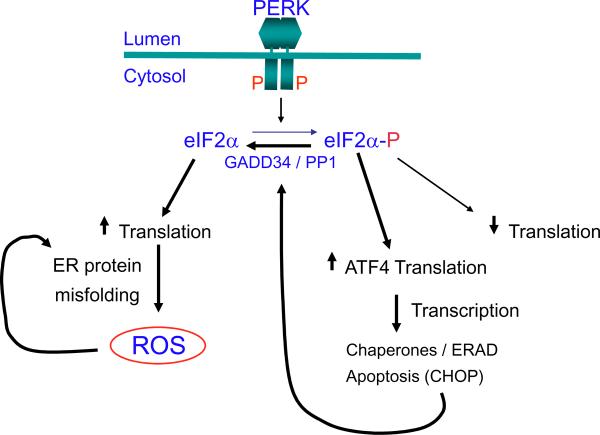
How do cells decide between the fates of survival and death in response to activation of the UPR? Our recent studies, as well as others, indicate that CHOP is a crucial factor that signals apoptosis [43]. Surprisingly, cells in which the UPR is chronically activated at a low level can propagate indefinitely because the stress from the initial insult is resolved. The resolution correlates with up-regulation of ER chaperones and ERAD machinery. Survival under these conditions is associated with attenuated expression of CHOP, and GADD34, a downstream target of CHOP [43]. In the presence of perpetual CHOP expression, cells succumb to apoptotic death. We demonstrated that the half-lives for mRNAs and proteins encoding adaptive functions of the UPR (i.e. BiP, GRP94, p58IPK, etc) are long-lived, whereas those encoding apoptotic functions, ATF4, CHOP, and GADD34 are short-lived. Under adaptive conditions, the steady-state levels of proteins that promote protein folding and ERAD are increased, which feeds back to shut off UPR signaling. In contrast, a perpetual strong misfolded protein signal is required to activate the CHOP-mediated death program. We have shown that Chop deletion improves both β–cell survival and proinsulin secretion. Our studies suggest that CHOP mediates apoptosis through induction of oxidative stress. However, the relationship between signaling through the PERK/eIF2α pathway to production of ROS is unknown. A model was proposed that ATF4-mediated induction of CHOP causes transcriptional induction of GADD34 [31]. GADD34 encodes a regulatory subunit of protein phosphatase 1 (PP1) that targets PP1 to dephosphorylate eIF2α, thereby reversing the translational attenuation. We propose that under conditions where protein folding is challenged, an increase in protein synthesis would generate an increased amount of misfolded protein (Fig. 4). Although the most significant pathway that leads to CHOP induction is mediated through PERK, both the IRE1/XBP1 and ATF6 pathways contribute.
Figure 4. B–cells require exquisite regulation of protein synthesis by eIF2α phosphorylation.
Our studies have shown that PERK activation initiates a cascade of events that include eIF2α phosphoryation, increased ATF4 mRNA translation and transcriptional activation of CHOP. Increased CHOP expression is associated with oxidative stress. However, we have also shown that a defect in eIF2α phosphorylation results in increased protein synthesis that also leads to oxidative stress. We propose that increased CHOP expression induces expression of GADD34 to direct PP1-mediated dephosphorylation of eIF2α, thereby increasing protein synthesis. If protein misfolding is not resolved, the increased cargo would further increase misfolded protein accumulation, leading ROS. The heavy black arrows depict the signaling pathway that occurs upon eIF2α phosphorylation.
UPR Activation in β–cells Associated with Type II Diabetes
If ER stress and UPR signaling are important for β–cell function/survival, then 1) UPR gene induction should be detectable in the islets of diabetic mice and human patients; 2) accumulation of unfolded protein in β–cells should cause β–cell failure and diabetes; and 3) genetic deletion of critical UPR signal transduction components should cause overwhelming ER stress and diabetes. There is now evidence to support each of these predictions.
A. UPR Activation in Islets from Diabetic Mice and Men
Perhaps the most difficult test of UPR association with β–cell failure is the detection of ER stress or defective ER stress signaling in islet samples from human patients. However, CHOP nuclear localization was reported in pancreata of human obese diabetic individuals, but it was rarely found in perinuclear or nuclear localization in pancreata from control or type 1 diabetic patients [44]. The classical UPR-induced proteins p58IPK, BiP, and CHOP were significantly elevated in islets in tissue sections from patients with type 2 diabetes patients [45]. Increased levels of eIF2α phosphorylation, increased splicing of Xbp1 mRNA, and increased CHOP and BiP protein were detected in the islets of db/db mice, a common model of insulin resistance and β–cell failure [45,46]. The detection of UPR-induced signals in these samples does not prove that ER stress was a causative event in the disease process; however, it does provide the first evidence that UPR markers are elevated specifically in the islets of diabetic men and mice. Further substantiating that excessive ER stress or defective stress signaling are pathogenic determinants of human diabetes will require more advanced knowledge of the stimuli and function of the UPR in β–cells and development of sensitive methods to detect markers of the UPR in human samples. One outstanding question is whether insulin resistance causes an increase in proinsulin synthesis that generates greater amounts of misfolded protein in β–cells. The discovery of drugs that improve ER protein folding and/or modulate ER stress signaling that can be evaluated in diabetes animal models and in human clinical trials will greatly advance our understanding of the importance of ER stress in development and progression of diabetes.
B. β–Cell Failure: Mutant Proinsulin
Studies of Akita and Munich mice reveal that mutations at cysteine residues that interfere with proper disulfide bond formation within proinsulin induce ER stress and severe β–cell destruction [47,48]. Deletion of the UPR-induced proapoptotic gene Chop delayed onset of hyperglycemia and β–cell failure in the Akita mouse [48]. Human proinsulin with the analogous Akita C96Y mutation was analyzed and compared with wild-type proinsulin through the development of expression constructs that fuse green fluorescence protein (GFP) with the C peptide [49]. In these studies it was possible to elucidate that processing of hProC96YCpepGFP to insulin was completely impaired in INS-1 cells and expression was “proteotoxic” in comparison to control hProCpepGFP. In humans, neonatal dominantly inherited diabetes in 16 families was associated with missense mutations in the Ins gene [50]. The mutations are predicted to impair proinsulin disulfide bond formation and activate ER stress. The missense mutations affect residues directly involved in disulfide bond formation, crucial residues adjacent to disulfide bridges, and also introduce new cysteine residues that could interfere with correct pairing of cysteine residues as nascent proinsulin molecules undergo oxidative folding. Thus, disruption of disulfide bond pairing in proinsulin, a crucial determinant of secondary structure and protein folding, is sufficient to induce diabetes in both humans and mice.
C. β–Cell failure: Deletion of ER Co-chaperone p58IPK
p58IPK was first described as an inhibitor of the double-stranded RNA-activated eIF2α protein kinase PKR. It was subsequently shown to inhibit activation of the eIF2α kinase PERK [51,52]. The subcellular localization and function of this protein has been a subject of debate; however, recent evidence supports the notion that the majority of p58IPK is imported into the ER lumen [53]. p58IPK is a member of the DnaJ co-chaperone family that functions to stimulate the ATPase activity of members of the Hsp70 family. Therefore, it was proposed that p58IPK may act in the ER lumen as a co-chaperone for the Hsp70 family member BiP [53]. Mice with null mutation of p58IPK develop spontaneous diabetes due to destruction of the islet mass, and p58IPK-null mutation worsens the outcome of diabetes due to the Akita Ins2 C96Y mutation [54,55]. These intriguing findings merit further study on the role of p58IPK co-chaperone function in proinsulin folding and maturation and in diabetes. The observations suggest that there may be a number of protein-folding chaperones that play highly significant roles in preservation of ER function in the β–cell to prevent diabetes.
D. β–Cell failure: Wolfram Syndrome and ER dysfunction
Wolfram syndrome is a rare autosomal-recessive neurodegenerative disorder that is characterized by juvenile-onset diabetes mellitus, optic atrophy, and hearing impairment [56]. This syndrome is caused by loss-of-function mutations in the Wfs1 gene that encodes the protein Wolframin [57,58]. Although WFS1 is not a direct sensor of the UPR, analysis of Wfs1−/− mice indicates that WFS1 function is closely linked with ER homeostasis. Wfs1-null mutation reduces intracellular calcium signaling upon glucose stimulation, induces UPR-regulated genes, and disrupts cell cycle control, leading to apoptosis [59–61]. Recently, a physical interaction between WFS1 and the Na(+)/K(+)ATPase β1 subunit was discovered, and it was discerned that WFS1 was required for trafficking of the subunit to the cell surface. Reduced levels of this ATPase subunit were detected in the plasma membrane fraction of Wfs1 mutant fibroblasts and of Wfs1 knockdown MIN6 β–cells [62]. Wolframin may serve a general function to assist in the assembly of subunits of oligomeric proteins before exit from the ER. Consistent with these observations, loss of function in the chaperone WFS1 causes ER stress and β–cell failure.
E. β–cell failure: Mutations in PERK/eIF2α
Wolcott-Rallison syndrome was first reported in the early 1970s as a human disease characterized by infantile diabetes, multiple epiphyseal dysplasia, and growth retardation [63,64]. Pancreas atrophy and endocrine and exocrine insufficiency were observed [65,66]. Wolcott-Rallison syndrome has been associated with multiple other pathologies including osteopenia, hepatic and renal complications, cardiovascular disease, and mental retardation. Remarkably, it was learned nearly 30 years later that this syndrome results from loss of protein kinase function mutations in the eIF2α kinase PERK (EIF2AK3) [67,68]. Furthermore, polymorphisms at the PERK locus were linked to type 1 diabetes in South Indian populations [69].
Perk- deletion in the mouse recapitulates many of the defects of the human syndrome including diabetes due to degeneration of β–cell mass after birth and failure of the exocrine pancreas [70,71]. In these studies, ER distention, a characteristic of ER dysfunction, was observed in pancreatic β–cells. In addition, the rate of glucose-stimulated proinsulin synthesis was enhanced, consistent with a defect in the ability to properly attenuate proinsulin mRNA translation. The findings suggested that the β–cells of these mice were susceptible to ER overload and unresolved ER stress leading to apoptosis. Conditional deletion of Perk at varying times in development suggested that development of β–cell mass, but not maintenance of a population of adult β–cells, is dependent upon this kinase [72]. It is possible that one or more additional eIF2α kinases, general amino acid control 2 (GCN2), heme-regulated inhibitor (HRI), or ds-RNA-activated protein kinase (PKR), are capable of supporting the minimal requirement for eIF2α phosphorylation and translational control in response to in vivo stimuli.
Concurrent with studies on Perk-null mice, mice that harbor a homozygous knock-in mutation at the PERK phosphorylation site in eIF2α (Ser51Ala) were shown to have defects in embryonic β–cell survival, liver glycogen storage, post-natal induction of gluconeogenesis, inhibition of translation under conditions of ER stress, and transcriptional induction of UPR genes [17]. The Ser51AlaeIF2α mutation prevents any compensatory phosphorylation due to activation of other eIF2α kinases and therefore, very effectively blocks stress-induced translation attenuation and ATF4-dependent transcriptional induction. Mice with homozygous Ser51AlaeIF2α mutation die from post-natal hypoglycemia. This observation was the first indication that the UPR has a broader role than maintaining functional ER protein folding, but is also intimately connected with metabolic homeostasis [17]. It is now known that all UPR signaling pathways contribute to glucose and lipid homeostasis [22,73,74]. Because the homozygous Ser51AlaeIF2α mutation was a neonatal lethal phenotype with a severe β–cell deficiency, further studies were performed by analysis of β–cell function in heterozygous Ser51AlaeIF2α mice [75]. The heterozygous animals did not spontaneously manifest β–cell failure due to reduced ER stress signaling. However, upon feeding a 45% high-fat diet, these mice developed elevated fasting blood glucose, glucose intolerance, and a β–cell secretion defect. It was demonstrated that the insulin secretion defect was due to an increased rate of glucose-stimulated translation that overwhelmed the protein folding machinery of the ER and led to 1) distention of the ER compartment, 2) prolonged association of misfolded proinsulin with the ER chaperone BiP, and 3) reduced secretory granule content. Thus, regulation of translational initiation through eIF2α phosphorylation is required for ER stress signaling to prevent β–cell dysfunction when insulin demand is increased due to a high-fat diet and insulin resistance. These findings demonstrated that translational control through eIF2α phosphorylation is essential to maintain the functional integrity of the ER.
As ER distention and β–cell death in homozygous Ser51AlaeIF2α islets was apparent embryonically in the absence of any exogenous pressure to drive β–cell failure, it is likely that there are physiological stimuli that cause eIF2α phosphorylation early in development that is crucial for β–cell survival [17]. It is unlikely the β–cell requirement for eIF2α phosphorylation is mediated through increased translation of Atf4 mRNA because Atf4−/− mice do not display β–cell defects [76]. However, the embryonic β–cell apoptosis observed in homozygous Ser51AlaeIF2α mice was likely, at least in part, signaled through CHOP. Although disruption of the Chop gene did not rescue the post-natal hypoglycemia-induced lethality of Ser51AlaeIF2α mice, the β–cells in islets from E18.5 Ser51AlaeIF2α;Chop−/− mice were significantly increased in number and insulin content, and displayed reduced apoptosis compared to islets from Ser51AlaeIF2α;Chop+/+ mice [77]. These findings support the hypothesis that a significant portion of the β–cell apoptosis in Ser51AlaeIF2α mutant mice is caused by CHOP, and that CHOP induction is not solely dependent on eIF2α phosphorylation. However, importantly, these findings show that CHOP is not the only pathway leading to β–cell death in the absence of eIF2α phosphorylation. In the absence of CHOP, other death pathways involving both mitochondrial- dependent and independent pathways are invoked. The sum of these findings indicate that genetic defects in the PERK/eIF2α signal transduction pathway are sufficient to disrupt regulated mRNA translation and interfere with ER function in the β–cell, thereby causing reduced insulin secretion, β–cell death, and diabetes in mice and humans.
The Role of Protein Misfolding and Oxidative Stress in β–cell Failure
To determine whether CHOP contributes to the β–cell failure associated with type 2 diabetes, we analyzed the effect of Chop deletion in three different models of murine type 2 diabetes: 1) heterozygous Ser51AlaeIF2α mutant mice fed a high-fat diet; 2) high-fat diet and streptozotocin (STZ)-treated mice; and 3) db/db leptin receptor-null mice [77]. In all models, Chop deletion increased β–cell mass, improved β–cell function, reduced β–cell apoptosis, and prevented glucose intolerance. Surprisingly, Chop deletion not only reduced apoptosis, but also preserved β–cell function as monitored by the integrity of the secretory pathway, insulin expression, and glucose-stimulated insulin secretion. Analysis of gene expression suggested that Chop deletion may improve the capacity of the β–cell to tolerate oxidative stress. Indeed, islets from Chop−/− mice displayed no significant difference in oxidative damage compared to wild-type mice. However, upon incubation in the presence of tunicamycin to inhibit N-linked glycosylation and cause misfolded protein accumulation in the ER, there was a significant increase in protein oxidation and lipid peroxidation in wild-type islets, where the damage was significantly attenuated in Chop−/− islets [77]. In contrast, upon treatment with the oxidant H2O2, similar amounts of oxidative damage were detected in islets from both strains of mice. Therefore, Chop deletion reduced the oxidative damage that occurs in response to protein misfolding in the ER, but not in response to general oxidative stress. We conclude that Chop deletion not only promoted islet hyperplasia, but surprisingly also improves the function of the β–cells to maintain insulin production, possibly through reduction of oxidative stress.
eIF2α Phosphorylation Prevents Oxidative Stress in β–cells
To elucidate why eIF2α phosphorylation is required to preserve β–cell function, we generated mice with conditional homozygous Ser51AlaeIF2α mutation in β–cells. Ubiquitous transgenic expression of wild-type eIF2α cDNA rescued the post-natal lethality associated with the homozygous Ser51AlaeIF2α mutation. The rescued mice were viable, fertile, and displayed normal glucose tolerance and homeostasis. LoxP sites flanked the wild-type eIF2α cDNA within the transgene so that when deleted, green fluorescence protein (GFP) was expressed. Upon breeding these mice to transgenic mice harboring the rat insulin II promoter-tamoxifen(Tam)-regulated Cre recombinase [78], the wild-type eIF2α cDNA was efficiently deleted in over 90% of the β–cells. Three weeks after administration of Tam, nearly all of the β–cells had deleted the wild-type eIF2α cDNA, although there was no significant change in islet mass or insulin content, although the mice were glucose intolerant [76]. At 3 weeks after Tam injection there were TUNEL-positive β–cells, prior to detectable hyperglycemia, suggesting that β–cell failure and apoptosis was not a consequence of lipid- or glucose-toxicity[76]. In addition, ultrastructural analysis identified significantly distended ER and swollen mitochondria in β–cells at 3 weeks after deletion of the transgene. Finally, oxidative damage was detected coincident with the appearance of TUNEL-positive β–cells, suggesting that blockade of ER stress signaling by preventing eIF2α phosphorylation overloads the ER compartment causing accumulation of unfolded protein, oxidative stress, and subsequent irreversible commitment to cell death.
We explored the causal relationship between ROS and disruption of glucose homeostasis in this in vivo mouse model of diabetes due to translation-induced overload. We found that diet supplemented with anti-oxidant butylated hydroxyanisole (BHA) could reduce β–cell apoptosis, increase insulin content, and improve glucose homeostasis in mice with homozygous Ser51AlaeIF2α mutation in β–cells[76]. These findings suggest that reducing ROS in β–cells deficient in eIF2α phosphorylation could improve their function and that ROS may be a cause for the β–cell failure and apoptosis.
The Role of IRE1α and ATF6α UPR Signaling in β–cells
A fundamental question regarding β-cell function and survival is which UPR subpathways are required for β-cell function and what elements of these responses are protective or detrimental to β-cell survival upon acute or unresolved ER stress. We have recently demonstrated that β-cell-specific deletion of Ire1α cause hyperglycemia due to β-cell failure (unpublished). Therefore, signaling through IRE1α is apparently also required to preserve β-cell function. In contrast, mice with homozygous deletion of Atf6α displayed normal glucose homeostasis on a standard chow diet. Future studies should investigate whether high fat diet may uncover a requirement for ATF6α for β-cell compensation when challenged with insulin resistance. Evidence that defects in the IRE1α and ATF6α subpathways of the UPR are akin to null mutation of PERK in causing human diabetes has yet to be presented. However, further analysis of mouse models with UPR gene deletions should solidify the concept that the UPR sensors act in concert with each pathway supporting a unique and indispensable role in preservation of β-cell function and/or survival.
Conclusion
Our results show that the absence of eIF2α phosphorylation, as well as excessive eIF2α phosphorylation (though CHOP induction), leads to oxidative stress. We propose that both events lead to increases in protein synthesis that contributes to the oxidative stress (Fig. 4). Presently, most data support the idea that CHOP is induced by the PERK/eIF2α/ATF4 as well as the IRE1/XBP1 and ATF6 UPR subpathways to activate proapoptotic gene expression, restore translation initiation, and increase the oxidizing potential in the ER lumen [31, 79]. CHOP induces expression of GADD34, a subunit of type 1 protein phosphatase that directs eIF2α dephosphorylation to increase mRNA translation as homeostasis in the ER is restored [80] [31]. CHOP is also implicated in the induction of ERO1α, a molecule that oxidizes protein disulfide isomerase (PDI) so it can function to rearrange improperly formed disulfide bonds within unfolded proteins. Disulfide bond formation during oxidative protein folding in the ER generates oxidative stress as a consequence of electron transfer from cysteine residues through PDI and ERO1 to molecular oxygen to form hydrogen peroxide [41]. Future studies should elucidate whether Chop deletion protects β cells from oxidative damage through the reduced expression of GADD34 and/or ERO1.
Acknowledgements
RJK is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Kahn BB. Type 2 diabetes: when insulin secretion fails to compensate for insulin resistance. Cell. 1998;92:593–596. doi: 10.1016/s0092-8674(00)81125-3. [DOI] [PubMed] [Google Scholar]
- 2.Ron D. Translational control in the endoplasmic reticulum stress response. J Clin Invest. 2002;110:1383–1388. doi: 10.1172/JCI16784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 5.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 6.Tsai B, Ye Y, Rapoport TA. Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat Rev Mol Cell Biol. 2002;3:246–255. doi: 10.1038/nrm780. [DOI] [PubMed] [Google Scholar]
- 7.Ruddock LW, Molinari M. N-glycan processing in ER quality control. J Cell Sci. 2006;119:4373–4380. doi: 10.1242/jcs.03225. [DOI] [PubMed] [Google Scholar]
- 8.McCracken AA, Brodsky JL. Recognition and delivery of ERAD substrates to the proteasome and alternative paths for cell survival. Curr Top Microbiol Immunol. 2005;300:17–40. doi: 10.1007/3-540-28007-3_2. [DOI] [PubMed] [Google Scholar]
- 9.Aebi M, Bernasconi R, Clerc S, Molinari M. N-glycan structures: recognition and processing in the ER. Trends Biochem Sci. 35:74–82. doi: 10.1016/j.tibs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 11.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Scheuner D, Kaufman RJ. The unfolded protein response: a pathway that links insulin demand with beta-cell failure and diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen J, Chen X, Hendershot L, Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell. 2002;3:99–111. doi: 10.1016/s1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 14.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 15.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 16.Sonenberg N, Dever TE. Eukaryotic translation initiation factors and regulators. Curr Opin Struct Biol. 2003;13:56–63. doi: 10.1016/s0959-440x(03)00009-5. [DOI] [PubMed] [Google Scholar]
- 17.Scheuner D, Song B, McEwen E, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 18.Prostko CR, Brostrom MA, Malara EM, Brostrom CO. Phosphorylation of eukaryotic initiation factor (eIF) 2 alpha and inhibition of eIF-2B in GH3 pituitary cells by perturbants of early protein processing that induce GRP78. J Biol Chem. 1992;267:16751–16754. [PubMed] [Google Scholar]
- 19.Harding HP, Novoa I, Zhang Y, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 20.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oda Y, Okada T, Yoshida H, Kaufman RJ, Nagata K, Mori K. Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation. J Cell Biol. 2006;172:383–393. doi: 10.1083/jcb.200507057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee K, Tirasophon W, Shen X, et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 2002;16:452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okada T, Yoshida H, Akazawa R, Negishi M, Mori K. Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J. 2002;366:585–594. doi: 10.1042/BJ20020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J, Rutkowski DT, Dubois M, et al. ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell. 2007;13:351–364. doi: 10.1016/j.devcel.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K. A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell. 2003;4:265–271. doi: 10.1016/s1534-5807(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto K, Sato T, Matsui T, et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell. 2007;13:365–376. doi: 10.1016/j.devcel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harding HP, Zhang Y, Zeng H, et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 30.Jiang HY, Wek SA, McGrath BC, et al. Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol. 2004;24:1365–1377. doi: 10.1128/MCB.24.3.1365-1377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marciniak SJ, Yun CY, Oyadomari S, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silva RM, Ries V, Oo TF, et al. CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J NeuroChem. 2005;95:974–986. doi: 10.1111/j.1471-4159.2005.03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–45502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 34.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 35.Fornace AJ, Jr., Alamo I, Jr., Hollander MC. DNA damage-inducible transcripts in mammalian cells. Proc Natl Acad Sci U S A. 1988;85:8800–8804. doi: 10.1073/pnas.85.23.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ron D, Habener JF. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev. 1992;6:439–453. doi: 10.1101/gad.6.3.439. [DOI] [PubMed] [Google Scholar]
- 37.Ubeda M, Wang XZ, Zinszner H, Wu I, Habener JF, Ron D. Stress-induced binding of the transcriptional factor CHOP to a novel DNA control element. Mol Cell Biol. 1996;16:1479–1489. doi: 10.1128/mcb.16.4.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang XZ, Kuroda M, Sok J, et al. Identification of novel stress-induced genes downstream of chop. EMBO J. 1998;17:3619–3630. doi: 10.1093/emboj/17.13.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol. 2001;21:1249–1259. doi: 10.1128/MCB.21.4.1249-1259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malhotra JD, Miao H, Zhang K, et al. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutkowski DT, Arnold SM, Miller CN, et al. Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol. 2006;4:e374. doi: 10.1371/journal.pbio.0040374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang CJ, Haataja L, Galasso R, et al. Induction of endoplasmic reticulum stress induced β-cell apoptosis and accumulation of polyubiquitinated proteins by human islet amyloid polypeptide. Am J Physiol Endocrinol Metab. 2007;293:E1656–E1662. doi: 10.1152/ajpendo.00318.2007. [DOI] [PubMed] [Google Scholar]
- 45.Laybutt DR, Preston AM, Akerfeldt MC, et al. Endoplasmic reticulum stress contributes to β–cell apoptosis in type 2 diabetes. Diabetologia. 2007;50:752–763. doi: 10.1007/s00125-006-0590-z. [DOI] [PubMed] [Google Scholar]
- 46.Yusta B, Baggio LL, Estall JL, et al. GLP-1 receptor activation improves β–cell function and survival following induction of endoplasmic reticulum stress. Cell Metab. 2006;4:391–406. doi: 10.1016/j.cmet.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Herbach N, Rathkolb B, Kemter E, et al. Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe beta-cell loss in Munich Ins2C95S mutant mice. Diabetes. 2007;56:1268–1276. doi: 10.2337/db06-0658. [DOI] [PubMed] [Google Scholar]
- 48.Oyadomari S, Koizumi A, Takeda K, et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–532. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu M, Hodish I, Rhodes CJ, Arvan P. Proinsulin maturation, misfolding, and proteotoxicity. Proc Natl Acad Sci U S A. 2007;104:15841–15846. doi: 10.1073/pnas.0702697104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoy J, Edghill EL, Flanagan SE, et al. Insulin gene mutations as a cause of permanent neonatal diabetes. Proc Natl Acad Sci U S A. 2007;104:15040–15044. doi: 10.1073/pnas.0707291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee TG, Tang N, Thompson S, Miller J, Katze MG. The 58,000-dalton cellular inhibitor of the interferon-induced double-stranded RNA-activated protein kinase (PKR) is a member of the tetratricopeptide repeat family of proteins. Mol Cell Biol. 1994;14:2331–2342. doi: 10.1128/mcb.14.4.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yan W, Frank CL, Korth MJ, et al. Control of PERK eIF2alpha kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci U S A. 2002;99:15920–15925. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rutkowski DT, Kang SW, Goodman AG, et al. The role of p58IPK in protecting the stressed endoplasmic reticulum. Mol Biol Cell. 2007;18:3681–3691. doi: 10.1091/mbc.E07-03-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ladiges WC, Knoblaugh SE, Morton JF, et al. Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes. 2005;54:1074–1081. doi: 10.2337/diabetes.54.4.1074. [DOI] [PubMed] [Google Scholar]
- 55.Oyadomari S, Yun C, Fisher EA, et al. Cotranslocational degradation protects the stressed endoplasmic reticulum from protein overload. Cell. 2006;126:727–739. doi: 10.1016/j.cell.2006.06.051. [DOI] [PubMed] [Google Scholar]
- 56.Barrett TG, Bundey SE. Wolfram (DIDMOAD) syndrome. J Med Genet. 1997;34:838–841. doi: 10.1136/jmg.34.10.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khanim F, Kirk J, Latif F, Barrett TG. WFS1/wolframin mutations, Wolfram syndrome, and associated diseases. Hum Mutat. 2001;17:357–367. doi: 10.1002/humu.1110. [DOI] [PubMed] [Google Scholar]
- 58.Cryns K, Sivakumaran TA, Van den Ouweland JM, et al. Mutational spectrum of the WFS1 gene in Wolfram syndrome, nonsyndromic hearing impairment, diabetes mellitus, and psychiatric disease. Hum Mutat. 2003;22:275–287. doi: 10.1002/humu.10258. [DOI] [PubMed] [Google Scholar]
- 59.Ishihara H, Takeda S, Tamura A, et al. Disruption of the WFS1 gene in mice causes progressive beta-cell loss and impaired stimulus-secretion coupling in insulin secretion. Hum Mol Genet. 2004;13:1159–1170. doi: 10.1093/hmg/ddh125. [DOI] [PubMed] [Google Scholar]
- 60.Riggs AC, Bernal-Mizrachi E, Ohsugi M, et al. Mice conditionally lacking the Wolfram gene in pancreatic islet β–cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia. 2005;48:2313–2321. doi: 10.1007/s00125-005-1947-4. [DOI] [PubMed] [Google Scholar]
- 61.Yamada T, Ishihara H, Tamura A, et al. WFS1-deficiency increases endoplasmic reticulum stress, impairs cell cycle progression and triggers the apoptotic pathway specifically in pancreatic beta-cells. Hum Mol Genet. 2006;15:1600–1609. doi: 10.1093/hmg/ddl081. [DOI] [PubMed] [Google Scholar]
- 62.Zatyka M, Ricketts C, da Silva Xavier G, et al. Sodium-potassium ATPase 1 subunit is a molecular partner of Wolframin, an endoplasmic reticulum protein involved in ER stress. Hum Mol Genet. 2008;17:190–200. doi: 10.1093/hmg/ddm296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wolcott CD, Rallison ML. Infancy-onset diabetes mellitus and multiple epiphyseal dysplasia. J Pediatr. 1972;80:292–297. doi: 10.1016/s0022-3476(72)80596-1. [DOI] [PubMed] [Google Scholar]
- 64.Stoss H, Pesch HJ, Pontz B, Otten A, Spranger J. Wolcott-Rallison syndrome: diabetes mellitus and spondyloepiphyseal dysplasia. Eur J Pediatr. 1982;138:120–129. doi: 10.1007/BF00441137. [DOI] [PubMed] [Google Scholar]
- 65.Thornton CM, Carson DJ, Stewart FJ. Autopsy findings in the Wolcott-Rallison syndrome. Pediatr Pathol Lab Med. 1997;17:487–496. [PubMed] [Google Scholar]
- 66.Castelnau P, Le Merrer M, Diatloff-Zito C, Marquis E, Tete MJ, Robert JJ. Wolcott-Rallison syndrome: a case with endocrine and exocrine pancreatic deficiency and pancreatic hypotrophy. Eur J Pediatr. 2000;159:631–633. doi: 10.1007/pl00008394. [DOI] [PubMed] [Google Scholar]
- 67.Delepine M, Nicolino M, Barrett T, Golamaully M, Lathrop GM, Julier C. EIF2AK3, encoding translation initiation factor 2-alpha kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nat Genet. 2000;25:406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 68.Senee V, Vattem KM, Delepine M, et al. Wolcott-Rallison Syndrome: clinical, genetic, and functional study of EIF2AK3 mutations and suggestion of genetic heterogeneity. Diabetes. 2004;53:1876–1883. doi: 10.2337/diabetes.53.7.1876. [DOI] [PubMed] [Google Scholar]
- 69.Allotey RA, Mohan V, McDermott MF, et al. The EIF2AK3 gene region and type I diabetes in subjects from South India. Genes Immun. 2004;5:648–652. doi: 10.1038/sj.gene.6364139. [DOI] [PubMed] [Google Scholar]
- 70.Harding HP, Zeng H, Zhang Y, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk−/−mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 71.Zhang P, McGrath B, Li S, et al. The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol Cell Biol. 2002;22:3864–3874. doi: 10.1128/MCB.22.11.3864-3874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR. PERK EIF2AK3 control of pancreatic β–cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 2006;4:491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 73.Rutkowski DT, Wu J, Back SH, et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Dev Cell. 2008;15:829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scheuner D, Mierde DV, Song B, et al. Control of mRNA translation preserves endoplasmic reticulum function in β–cells and maintains glucose homeostasis. Nat Med. 2005;11:757–764. doi: 10.1038/nm1259. [DOI] [PubMed] [Google Scholar]
- 76.Back SH, Scheuner D, Han J, et al. Translation attenuation through eIF2α phosphorylation preserves ER integrity, prevents oxidative stress,and maintains insulin production in β–cells. Cell Metabolism. 2009;10:1–2. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ. Chop deletion reduces oxidative stress, improves β–cell function, and promotes cell survival in multiple mouse models of Diabetes. J Clin Invest. 2008;118:3378–3389. doi: 10.1172/JCI34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 79.Ma Y, Brewer JW, Diehl JA, Hendershot LM. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol. 2002;318:1351–1365. doi: 10.1016/s0022-2836(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 80.Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S. Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol. 2001;21:6841–6850. doi: 10.1128/MCB.21.20.6841-6850.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]