Abstract
Long-term weight management by dieting has a high failure rate. Pharmacological targets have focused on appetite reduction, while less is understood as to the potential contributions of the stress state during dieting in long-term behavioral modification. In a mouse model of moderate caloric restriction in which a 10–15% weight loss similar to human dieting is produced, we examined physiological and behavioral stress measures. Following three weeks of restriction, mice showed significant increases in immobile time in a tail suspension test and stress-induced corticosterone levels. Increased stress was associated with brain region specific alterations of corticotropin-releasing factor (CRF) expression and promoter methylation, changes that were not normalized with re-feeding. Similar outcomes were produced by high fat diet withdrawal, an additional component of human dieting. In examination of long-term behavioral consequences, previously restricted mice showed a significant increase in binge-eating of a palatable high fat food during stress exposure. Orexegenic hormones, melanin concentrating hormone (MCH) and orexin, were significantly elevated in response to the high fat diet only in previously restricted mice. Further, administration of the MCH receptor-1 antagonist GSK-856464 significantly reduced total caloric intake in these mice during high fat access. These results reveal reprogramming of key central pathways involved in regulating stress responsivity and orexegenic drives by moderate caloric restriction experience. In humans, such changes would be expected to reduce treatment success by promoting behaviors resulting in weight re-gain, and suggest that management of stress during dieting may be beneficial in long-term maintenance.
Keywords: dieting, stress, reward, epigenetic
Introduction
Dieting as a mode of behavioral modification in weight management has proven to have little long-term success with a greater than 80% failure rate (Wing and Phelan, 2005). The elevated weight regain and increased risk for diabetes and related metabolic disease following dieting make identification of novel therapeutic targets critical (Lissner et al., 1991; Brownell and Rodin, 1994; Guagnano et al., 2000). Current treatments predominantly focus on appetite reduction, while less is known regarding the central mechanisms contributing to treatment resistance and failure, especially that of the involvement of stress pathways.
As stress and the stress hormone corticotropin-releasing factor (CRF) are known promoters of increased consumption of calorically-dense foods, reprogramming of stress circuitry may be a central mechanism driving repeated dieting, or the ‘yo-yo’ effect, resulting in an increased risk for greater weight gain and later obesity (Epel et al., 2001; Dallman et al., 2006; Amigo and Fernandez, 2007; Teegarden and Bale, 2008). Stress-induced activation of the hypothalamic-pituitary-adrenal (HPA) stress axis and release of CRF increases reward-seeking behaviors (Dallman et al., 2005; Ghitza et al., 2006). CRF antagonism in the bed nucleus of the stria terminalis (BNST) decreases stress-induced drug reinstatement, while injection of CRF promotes drug seeking, demonstrating an intersection of stress and reward pathways (Erb and Stewart, 1999; Koob, 2009). Evidence from these and other studies suggests that stress plays a critical role in increasing consummatory behavior of rewarding substances, including calorically-dense foods (Shaham et al., 2000; Sinha, 2001; Wang et al., 2005; Dallman et al., 2006; Teegarden and Bale, 2008).
We hypothesized that previous restriction experience would produce changes in stress neurocircuitry leading to a subsequent increased stress sensitivity and tendency to over-consume high fat food. To examine this hypothesis, we compared physiological and behavioral stress responses in a mouse model of caloric restriction which produces a 10–15% reduction in body weight, levels typical of human weight loss during dieting (Redman et al., 2007; Sarwer et al., 2009). The long-term impact and programming effect of this moderate restriction on behavioral measures and stress responsivity were determined. Mechanistically, CRF expression and promoter methylation was examined. In addition to reduced calories, a major component of human dieting is a decrease in fat content and diet palatability. We have previously shown in mice that withdrawal from a high fat diet increases the relative stress state, and promotes reinstatement behaviors in which mice select an aversive environment to gain access to the preferred high fat diet (Teegarden and Bale, 2007). Therefore, we similarly compared CRF expression and methylation patterns up to eight weeks following withdrawal from a high fat diet, demonstrating the long-term programming that occurs.
To explore the behavioral impact of epigenetic modifications in stress circuitry on food intake, we examined binge-eating of a high fat diet following chronic stress in previously restricted mice. In addition, sensitivity to a melanin-concentrating hormone (MCH) receptor-1 antagonist on this behavior was assessed. Together, these results may provide novel therapeutic directions by which more effective and lasting weight management treatments are designed.
Methods
Animals
All mice in these studies were males (C57Bl/6J; 7–8 weeks of age) obtained from Jackson Laboratories (Bar Harbor, Maine) and individually housed on a 12-hour light/dark schedule with food and water available ad libitum, except where otherwise noted. House chow contained 28% protein, 60% carbohydrates, and 12% fat by calories and 4.00 kcal/g (Purina Lab Diet, St. Louis, Missouri). High fat diet contained 20% protein, 35% carbohydrates, and 45% fat by calories and 4.73 kcal/g (Research Diets, New Brunswick, NJ). This diet is highly preferred to house chow in C57Bl/6J mice (Teegarden and Bale, 2007). All studies were done according to University of Pennsylvania University of Laboratory Animal Resources and the Institutional Animal Care and Use Committee standards and guidelines.
Caloric Restriction
Average daily food intake was established over 10 days. Mice were randomly assigned to caloric restriction (Rstr), 75% of average caloric intake, or ad libitum treatment groups (Rstr, n = 41; Ad lib, n = 37). This reduction was selected to produce 10–15% body weight loss to mimic typical diet regimens in humans. For restricted groups, pre-weighed house chow pellets were placed in cages 2.5 hrs prior to lights out for 21 days. This schedule was utilized to ensure that mice had access to food before and after lights out when the majority of food consumption occurs and to avoid food entrained oscillators by allowing consumption during the dark phase (Mendoza, 2007). Body weights were recorded every 2–3 days.
Leptin
To determine the impact of caloric restriction and chow or high fat diet re-feeding on leptin levels, a blood sample was obtained at the time of sacrifice in a subset of mice (Ad lib, n = 11; Rstr, n = 12). Samples were centrifuged, plasma collected and frozen at −80°C until analysis. Plasma levels were measured by radioimmunoassay kit for leptin (LINCO Research, Inc., St. Louis, MO). The protocol was modified to use 50 μl of plasma for each assay, and each sample was assessed in duplicate. The intra-assay coefficient of variation was less than 6%.
Stress sensitivity
TST
The tail suspension test was administered according to standard procedure between 1100 hr and 1300 hr on day 19 of caloric restriction (Ad lib, n = 11; Rstr, n = 12) (McEuen et al., 2009). Briefly, each mouse was suspended by the tail at a height of 40 cm for 6 min, during which immobility was measured by an experimenter blind to treatment using AnyMaze software (Stoelting, Wood Dale, IL). An animal was considered to be immobile when it did not show any movement and hanged passively.
HPA axis stress response
Corticosterone levels were measured in response to a 15 min restraint stress starting at 900 hr on day 21 of caloric restriction (Ad lib, n = 11; Rstr, n = 12). Mice were placed in a 50 ml conical tube for 15 min and tail blood samples obtained at 0, 15, 45, and 75 min. All samples were stored on ice until centrifuged at 5000 rpm at 4°C. Plasma was removed and stored at −80°C until radioimmunoassay was performed. The corticosterone assay was performed according to manufacturer’s instructions (ICN Biomedicals, Irvine, CA). Each sample was assessed in duplicate. The minimum detection limit of the assay was 7.7ng/ml, and the intra-assay coefficient of variation was 7.3%.
Examination of stress pathways
Re-feeding
Following 21 days of caloric restriction, a subset of mice were re-fed either a high fat or chow diet ad libitum for 1 wk (Ad lib, n = 4; Rstr, n = 5; Rstr-Chow (Refed), n = 5; Ad lib-HF, n = 5; Rstr-HF, n = 6). In addition, a subset of mice that had previously been fed chow ad libitum was given high fat diet ad libitum for 1 wk. These mice served as a dietary control for mice previously restricted and then placed on the high fat diet. All mice were sacrificed on the same day following 1 wk of re-feeding.
High Fat Withdrawal
A separate cohort of mice was allowed to habituate to the facility for two weeks. Mice were then divided into two groups, 4 wk high fat diet exposure (n = 60) and chow (n = 12). To examine the impact of varying lengths of high fat withdrawal, the high fat diet group was further subdivided into high fat diet exposed (HF, n = 12), or withdrawn (returned to chow) for 2, 4, 6 or 8 weeks (n = 12/group). The start of high fat exposure was staggered such that all mice were sacrificed simultaneously at the end of the withdrawal period.
Taqman Real-time PCR
Expression of individual genes following caloric restriction, re-feeding, and high fat withdrawal was assessed using Taqman gene expression assays. Tissue punches were taken from 300 μm sections using a 1 mm circular punch (Ted Pella, Redding, CA). For the CeA and BNST, punches from one hemisphere were utilized for RNA isolation for gene expression analyses, while punches from the opposing hemisphere were taken for genomic DNA isolation for bisulfite sequencing. Lateral hypothamalus (LH) tissue punches from both hemispheres were isolated for RNA. For both the CeA and BNST three punches were isolated from each side while a total of 8 punches were isolated for the LH. The areas punched correspond to areas diagramed in the Paxinos and Franklin mouse atlas (Paxinos and Franklin, 2001). Tissue was added directly to TRIzol reagent (Invitrogen, Carlsbad, CA) and total RNA isolation was performed with chloroform followed by isopropanol precipitation. cDNA was synthesized from 500 ng of total RNA using SuperScript First-Strand Synthesis System (Invitrogen). Changes in corticotropin releasing factor (CRF; Mm01293920_m1), melanin-concentrating hormone (MCH; Mm01242886_m1), and orexin (Hcrt; Mm01964030_m1) were measured using quantitative real-time PCR and TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA). β-actin (Actb;4352933E) served as an endogenous control for the caloric restriction studies and GAPDH (4352932E) for the high fat withdrawal studies. The cycle threshold for the control was subtracted from the target threshold value. All samples were run in duplicate for both target gene and endogenous control. Samples were analyzed using the Applied Biosystems 7500 Fast Real-Time PCR System. The amplification profile consisted of 20s denaturation at 95°C, and 40 cycles of 3s at 95°C and 30s at 60°C. The cycle number at threshold (CT value) was used for calculations of relative mRNA levels. The CT value of each target gene was normalized by subtracting the endogenous control CT value [raw ΔCT value]. The ΔCT value was converted to fold change by calculating 2(−ΔCT) for each sample and normalizing each value to the average for the control chow.
Analysis of DNA Methylation
The impact of caloric restriction, chow re-feeding, or high fat withdrawal, on methylation status of specific CpG dinucleotides within the promoter region of mouse CRF were analyzed (McGill et al., 2006). Genomic DNA from the central amygdala (CeA) and bed nucleus of the stria terminalis (BNST) was isolated from micropunches. Briefly, tissue was added directly to high molecular weight DNA extraction buffer (1M Tris-HCl, EDTA, SDS, and water) plus RNase A and incubated for 1 hr at 37°C followed by proteinase K addition and incubation overnight at 55°C. Next a choloform/isoamyl alcohol extraction was performed before DNA was precipitated with sodium acetate and glycogen at −80°C for 1 hr followed by centrifugation (10 min, 12,000rpm, RT). The pellet was saved and resuspended in TE buffer. Pyrosequencing was performed by EpigenDx (Worcester, MA) as previously described (Kim et al., 2007; Liu et al., 2007). Briefly, bisulfate modification was performed converting unmethylated cytosine to uracil. The promoter sequence for the CRF gene was PCR amplified and the biotinylated product purified for pyrosequencing. Site specific primers were designed to determine the CpG dinucleotide methylation status.
Stress induced binge-eating
Limited Access
To determine the potential impact of neurocircuitry reprogramming that occurs as a result of previous caloric restriction on binging of a palatable high fat diet during chronic stress, we exposed mice to a limited access procedure (Teegarden and Bale, 2008). Following 3 wks of caloric restriction, mice were re-fed house chow ad libitum until restricted groups reached the same weight as controls (10 days). Limited access consisted of providing a single pellet of pre-weighed high fat diet in the home cage for 1 hr (1500 hr–1600hr) along with pre-weighted house chow. Mice were allowed 3 days to habituate to the high fat diet and feeding schedule prior to 10 days of testing. On Day 1 of testing, a subset of mice was exposed to chronic variable stress (CVS) throughout testing (Ad lib-Ctrl, n = 8; Ad lib-CVS, n = 9; Rstr-Ctrl, n = 9; Rstr-CVS, n = 9). Chronic variable stress consisted of one stressor per day for 10 days in an unpredictable pattern and included: 15 min restraint, 30 min of total darkness (3X) during the light cycle, exposure to novel objects overnight, damp bedding overnight, cage changes (3X) during the light cycle, novel noise overnight, and 15 min exposure to a predator odor (McEuen et al., 2009). These mild stressors were designed to not induce pain and be resistant to habituation. The amount of high fat diet consumed within 1 hr, and the following 24 hrs of house chow was measured.
Reversal of high fat diet withdrawal induced binge-eating
As we hypothesized that previously restricted mice based on their dysregulation of stress and orexegenic pathways would show a greater sensitivity to an acute treatment with the MCHr1 antagonist GSK-856464, we examined caloric intake and binge-eating following high fat diet exposure and withdrawal stress. To determine if serotonergic pathways may also be involved in the stress dysregulation in these mice, we included treatment with the SSRI citalopram for comparison. Immediately following caloric restriction, all mice were given ad libitum access to the high fat diet for 9 wks and then withdrawn (returned to chow) for 10 days. Mice were provided 3 days access to a pre-weighed high fat pellet for 1 h (1500 h 1600 h) and ad libitum access to chow (Ad lib, n = 23; Rstr, n = 24). On the fourth day of this limited access, mice received an i.p. injection of drug (GSK-856464 (30 mg/kg); Citalopram (20 mg/kg)) or vehicle 1 hr prior to the start of limited access to the high fat pellet (Ad lib-VEH, n = 7; Ad lib-GSK, n = 8; Ad lib-CIT, n = 8; Rstr-VEH, n = 8; Rstr -GSK, n = 8; Rstr-CIT, n =8). High fat diet consumption within 1 h was measured, in addition to 24 hrs of chow and total calories calculated.
Statistical analysis
Differences in weight and plasma leptin levels were analyzed by either Student’s t-test or one-way ANOVA. Corticosterone data were analyzed using two-way repeated measures ANOVA for restriction and time. For TST, data were analyzed using Student’s t-test. For CRF gene expression following caloric restriction or high fat withdrawal, data were analyzed using a one-way ANOVA with Fisher’s PLSD post hoc tests. Methylation data were analyzed using a one-way ANOVA to determine difference between groups within each GpC site and overall methylation across the promoter. For stress-induced 10 day binge-eating, data were analyzed using a one-way repeated measures ANOVA with control and CVS groups analyzed separately. Total caloric intake data during binge-eating were analyzed using a Student’s t-test. For gene expression analysis of orexegenic hormones, fold changes were computed relative to ad lib chow controls and a two-way ANOVA were used with Fisher’s PLSD post hoc tests. For reversal of high fat withdrawal induced binge-eating, data from the first three days were analyzed using a one-way repeated measures ANOVA and data from the fourth day were analyzed using a two-way ANOVA with Fisher’s PLSD post hoc tests. All statistical analysis was performed using SigmaStat (Systat Software, Inc.).
Results
Caloric restriction induced stress-sensitivity
Body weights and Leptin
Male mice calorically restricted to 75% of their normal chow intake show significant weight loss at the end of 3 wks of restriction. Over the 3-wk period, the control mice gained weight [t(20) = −4.294, p ≤ 0.001] while restricted mice lost weight [t(22) = 8.214, p ≤ 0.001] thus at the end of restriction the weights were significantly different [t(21) = 11.815, p ≤ 0.001]. Plasma leptin levels were measured from a subset of mice sacrificed at the end of the 3-wk restriction period. Calorically restricted mice had significantly lower levels of leptin [t(8) = 2.715, p = 0.03]. (Table 1)
Table 1.
Body weights (BW) and leptin levels following caloric restriction
| Start BW(g) | End BW(g) | Leptin(ng/ml) | |
|---|---|---|---|
| Ad lib | 25.7 ± 0.2 | 27.3 ± 0.3 | 10.4 ± 1.1 |
| Rstr | 26.3 ± 0.3 | 22.7 ± 0.3* | 7.0 ± 0.5* |
Effect of caloric restriction (Weight: p ≤ 0.001; Leptin: p < 0.05)
HPA axis stress response
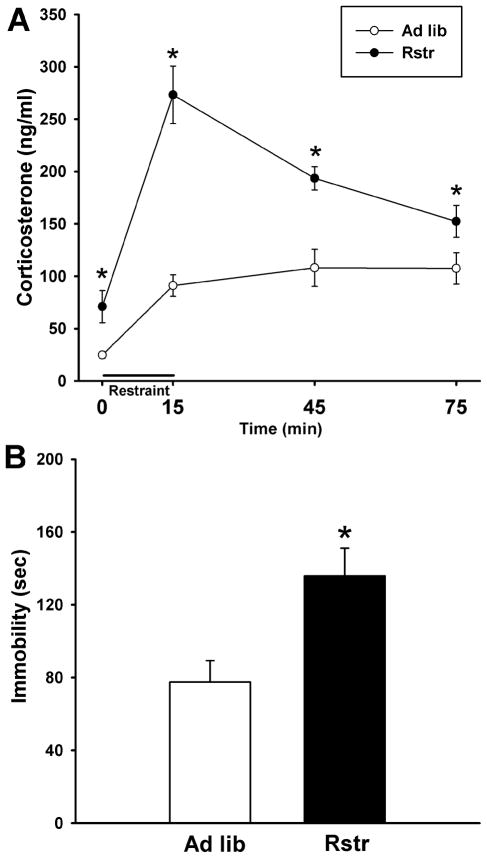
To evaluate the physiological stress response following caloric restriction, mice were restraint stressed and a time course of corticosterone levels was measured. Calorically restricted mice showed a significantly increased stress response [F(1,21)=26.649, p < 0.001] with higher basal corticosterone levels as found by post-hoc tests (p < 0.05). In addition, this group had a higher maximal rise in response to the 15 min restraint (p < 0.001) and a delayed stress recovery (45 min, p < 0.001; 75 min, p = 0.05) (Fig. 1A).
Figure 1. Moderate calorie restriction promotes an increased stress state.
(A) Restraint stress induced HPA axis corticosterone response was significantly elevated in calorically restricted (Rstr) mice (Ad lib, n = 11; Rstr, n = 12) (*, p < 0.05). (B) Maladaptive behavioral responses were detected in a tail suspension test where restricted mice showed a significant increase in time spent immobile compared to ad libitum (Ad lib) fed mice (n = 11–12). Data are mean +/− SEM.
Tail suspension test
To assess stress-induced behavioral responses, the tail suspension test was administered following 19 days of caloric restriction. There was a significant effect on time spent immobile [t(21) = −2.989, p < 0.01] with the calorically restricted group spending more time immobile than controls (Fig. 1B).
CRF Expression
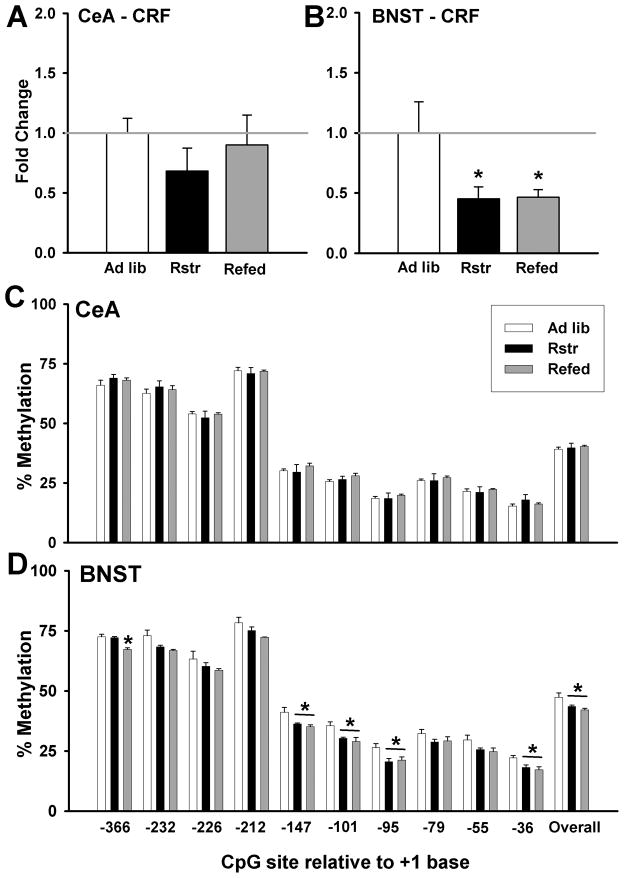
The impact of previous caloric restriction on gene expression changes in stress pathways was assessed using quantitative TaqMan RT-PCR. Corticotropin-releasing factor (CRF) levels in the central nucleus of the amygdala (CeA) were unchanged with caloric restriction or chow re-feeding following restriction (Fig. 2A). CRF levels in the bed nucleus of the stria terminalis (BNST) were significantly different between groups [F(2,13) = 8.335, p < 0.01] (Fig. 2B). Caloric restriction significantly decreased BNST CRF (p < 0.01), and CRF remained reduced following re-feeding (p < 0.01).
Figure 2. Moderate caloric restriction induces changes in stress pathways.
(A) There were no significant changes in CRF gene expression levels in the CeA compared to controls. (B) CRF levels in the BNST were significantly decreased following 3 wks of caloric restriction and remained reduced after 1 wk of re-feeding with chow (Refed) (*, p < 0.01). (C) There were no changes in methylation of the CRF promoter at individual cytosines in the CeA. (D) In the BNST, there were significant decreases in methylation at multiple cytosines in calorically restricted mice that remained down following 1 wk chow re-feeding (Refed) (* post-hoc: p < 0.05) (Ad lib, n = 4; Rstr, n = 4; Rstr-Chow (Refed), n = 4). In addition, there was an overall effect of caloric restriction on methylation (p < 0.05). Data are mean +/− SEM.
DNA Methylation of CRF Promoter
As re-feeding did not normalize CRF expression in the BNST, we examined epigenetic changes in promoter methylation. Similar to CRF expression no differences in methylation were detected in the CeA (Fig. 2C). Within the BNST, there was a significant reduction in methylation during caloric restriction that was not normalized during re-feeding at 6 of the 10 cytosines within the reported CpG island [−366 bp: F(1,11) = 17.066, p < 0.001 (p < 0.001, post-hoc for Ad lib vs. Refed and Rstr vs. Refed); −147 bp:: F(1,11) = 6.126, p < 0.05 (p < 0.05, post-hoc for Ad lib vs. both Rstr and Refed); −101 bp:: F(1,11) = 6.509, p < 0.05 (p < 0.05, post-hoc for Ad lib vs. both Rstr and Refed); −95 bp:: F(1,11) = 4.839, p < 0.05 (p < 0.05, post-hoc for Ad lib vs. both Rstr and Refed); −36 bp:: F(1,11) = 5.399, p < 0.05 (p < 0.05, post-hoc for Ad lib vs. both Rstr and Refed)]. In addition, there was an overall effect of caloric restriction without normalization of the CRF promoter [F(2,11) = 5.992, p < 0.05] (Fig. 2D).
High fat withdrawal reprogramming of stress pathways
CRF Expression
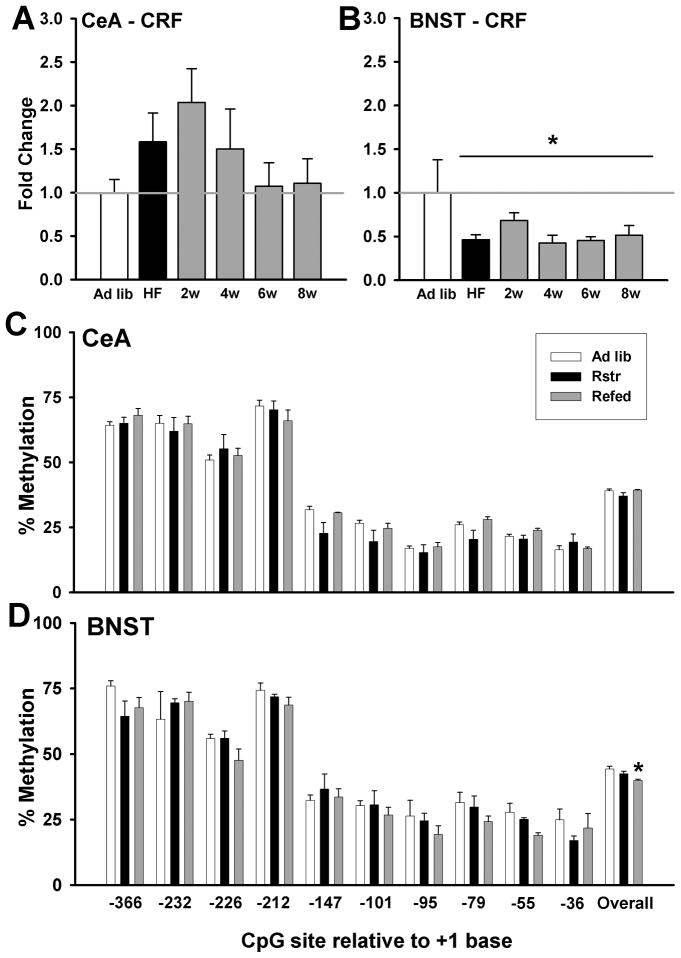
The impact of high fat diet exposure and varying lengths of withdrawal on gene expression changes in stress pathways was assessed using quantitative TaqMan RT-PCR. Corticotropin-releasing factor (CRF) levels in the central nucleus of the amygdala (CeA) showed a non-significant peak of gene expression at 2 wks post-withdrawal and returned to baseline by 6 wks (Fig. 3A). CRF levels in the bed nucleus of the stria terminalis (BNST) were significantly decreased by high fat diet exposure and remained reduced for at least 8 wks post-withdrawal [F(5,24) = 4.4, p < 0.01] (Fig. 3B).
Figure 3. High fat withdrawal induces changes in stress pathways.
(A) There were no significant changes in CRF gene expression leves in the CeA compared to controls following high fat exposure and subsequent withdrawal (n = 12). (B) CRF levels in the bed nucleus of the stria terminalis (BNST) were significantly decreased by high fat diet exposure and remained reduced for at least 8 wks post-withdrawal (n = 12) (*, p < 0.01). (C) In the CeA, there were no changes in methylation of the CRF promoter at individual cytosines or overall (n = 5). (D) There was an overall significant decrease in methylation of the CRF promoter in the BNST (n = 5) following high fat withdrawal (*, p < 0.05). Data are mean +/− SEM.
DNA Methylation of CRF Promoter
As CRF expression in the BNST remained reduced for at least 8 wks following the end of high fat diet exposure, we examined epigenetic changes in promoter methylation. Similar to CRF gene expression no differences in methylation were detected in the CeA (Fig. 3C). In contrast, in the BNST, we observed an overall effect of diet treatment on methylation of the CRF promoter [F(2,12) = 4.4, p < 0.05], which post-hoc testing revealed to be driven by a reduction in methylation in the withdrawal group relative to controls (p < 0.01) (Fig. 3D).
Stress-induced binge-eating
Body weights
Following caloric restriction control mice weighed significantly more than the calorically restricted mice [t(34) = 6.971, p ≤ 0.001]. After 3-wk of restriction, mice were placed back on ad libitum chow (Re-feeding) until the significant weight difference between control and restricted mice were no longer present prior to the start of limited access to ensure that intake differences were not due to differences in weight. Weights were continually monitored during limited access as well and there were no significant differences during or at the end of these trials. (Table 2).
Table 2.
Body weights (BW) following high fat binge-eating
| End Restriction(g) | Re-fed BW(g) | End Binge BW(g) | |
|---|---|---|---|
| Ad lib | 24.5 ± 0.3 | 26.9 ± 0.4 | 27.4 ± 0.3 |
| Rstr | 23.0 ± 0.3* | 26.6 ± 0.4 | 27.1 ± 0.4 |
Effect of caloric restriction (p ≤ 0.001)
Limited Access
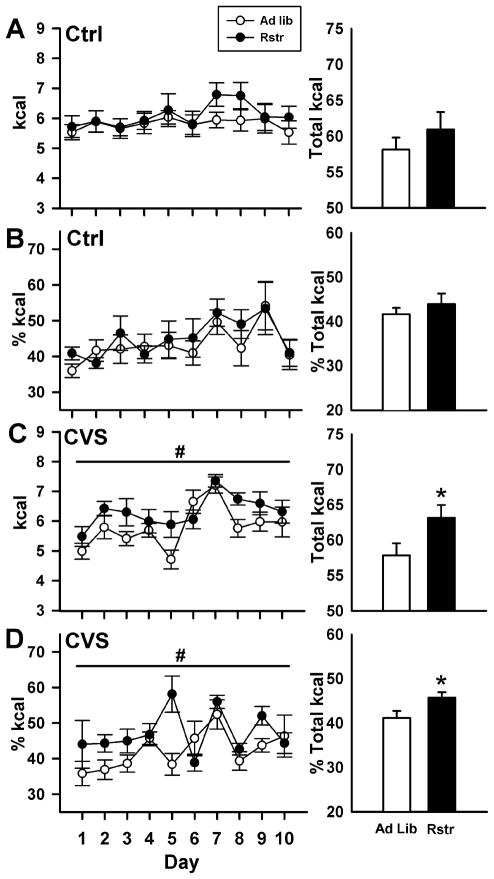
To determine if the increased stress responsivity and changes in CRF epigenetic programming in the BNST following calorie reduction would result in increased stress-induced binge-eating, mice were provided limited access to a high fat diet during chronic variable stress (CVS). Under basal non-stress conditions (Ctrl), there were no significant differences between groups in the calories from high fat diet consumed over the course of testing [F(1,15) = 0.881, p = 0.363] (Fig. 4A, left panel), or as a percentage of total calories consumed (Fig. 4B, left panel). All mice displayed binge-eating, consuming approximately 50% of their total 24 hr caloric intake during the one hour of access, demonstrating the overall preference mice have for the high fat food (Teegarden and Bale, 2008). During chronic variable stress, mice that had undergone previous caloric restriction consumed significantly more calories than control mice over the 10 days of testing [F(1,16) = 4.479, p < 0.05] (Fig. 4C, left panel). There was also a main effect of day [F(1,9) = 7.144, p < 0.001], with intake increasing over the course of testing. Over the entire 10 days of testing previously calorically restricted mice consumed significantly greater total amounts of high fat diet than control mice [t(16) = −2.133, p < 0.05] (Fig. 4C, right panel). Overall, there were no significant differences in the total amount of calories consumed, high fat and chow diet, by all groups (data not shown). Under chronic variable stress, the percentage of calories from the high fat diet is significantly greater for calorically restricted mice compared to controls [F(1,16) = 5.581, p < 0.05] (Fig. 4D, left panel). The percentage of calories from high fat food significantly changed over the 10 days of testing [F(1,9) = 3.538, p < 0.001], and a significant interaction between restriction and day [F(1,143) = 2.305, p = 0.02]. Over the 10 days of testing, the total percentage of high fat calories consumed was significantly greater in the calorically restricted mice [t(16) = −2.312, p < 0.05] (Fig. 4D, right panel).
Figure 4. Increased stress induced binge-like eating of a high fat diet following 3-wks of caloric.
restriction. (A, B) Under control conditions, there were no differences in calories from high fat diet over the course of the 10 days of testing or as a percentage of calories. (C, left panel) During chronic variable stress (CVS), calorically restricted mice consumed significantly more calories of high fat diet compared to controls (Ad lib) over the 10 days of testing (#, p < 0.05). (C, right panel) Calorically restricted mice consumed significantly greater total amounts of high fat diet (*, p < 0.05). (D) In addition, the total percentage of calories from high fat was significantly greater in the restricted mice (#,*, p < 0.05). (Ad lib-Ctrl, n = 8; Ad lib-CVS, n = 9; Rstr-Ctrl, n = 9; Rstr-CVS, n = 9) Data are mean +/− SEM.
Orexegenic gene expression in response to high fat exposure
Body weights
At the end of caloric restriction control mice weighed significantly more than the restricted mice [t(25) = 2.434, p < 0.05]. Re-feeding for 1 wk on high fat diet did not significantly increase weights. (Table 3).
Table 3.
Body weights (BW) following 1 wk ad lib high fat
| Start BW(g) | End BW(g) | |
|---|---|---|
| Ad lib | 24.9 ± 0.4 | 26.7 ± 1.1 |
| Rstr | 23.8 ± 0.2* | 27.5 ± 0.5 |
Effect of caloric restriction (p < 0.05)
MCH and Orexin Expression
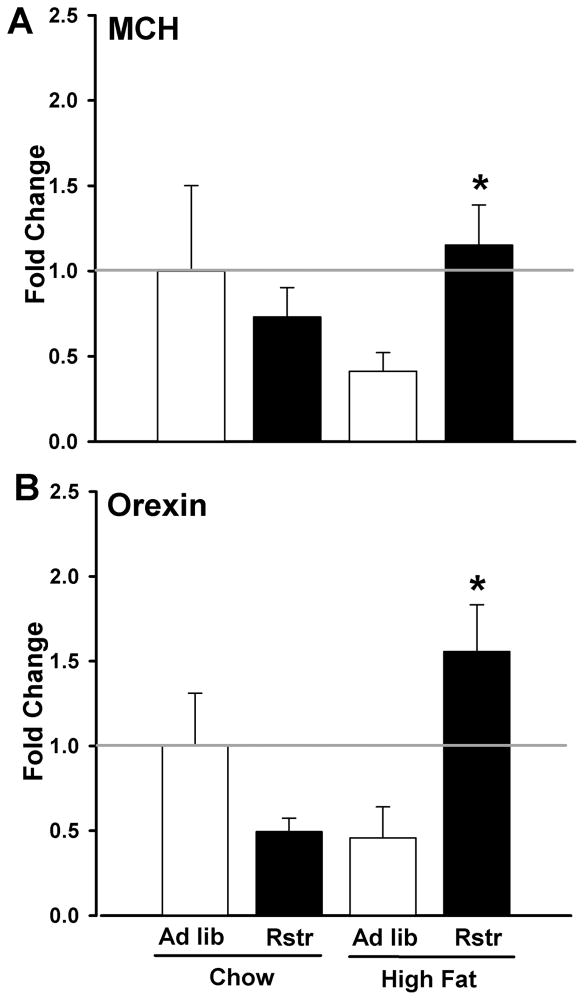
In order to determine molecular mechanisms that may be downstream of stress pathways driving increased high fat intake following restriction, we examined expression of the orexegenic hormones MCH and orexin in the lateral hypothalamus in calorically restricted mice re-fed on a chow or high fat diet. Gene expression levels of MCH revealed an interaction of previous caloric restriction and diet [F(1,12) = 6.378, p < 0.05], where MCH expression was significantly greater in restricted mice on a high fat diet compared to ad lib mice re-fed on high fat (post-hoc; p < 0.05) (Fig. 5A). Analysis of orexin gene expression revealed an interaction between restriction and diet [F(1,12) = 16.577, p < 0.01] where orexin expression was significantly greater in restricted mice on a high fat diet compared to ad lib mice re-fed on high fat (p < 0.05) (Fig. 5B).
Figure 5. Previous calorie reduction significantly enhances orexegenic hormone responses to high fat diet.
(A) MCH levels in the lateral hypothalamus (LH) were significantly increased in calorically restricted mice re-fed on a high fat diet compared to ad lib mice re-fed on high fat (n = 4–5) (*, p < 0.05). (B) Orexin levels in the LH were significantly increased in restricted mice re-fed on a high fat diet compared to restricted mice re-fed on chow or ad lib mice re-fed on high fat (n = 4–5) (*, p < 0.05). Data are mean +/− SEM.
Reversal of high fat diet withdrawal induced binge-eating
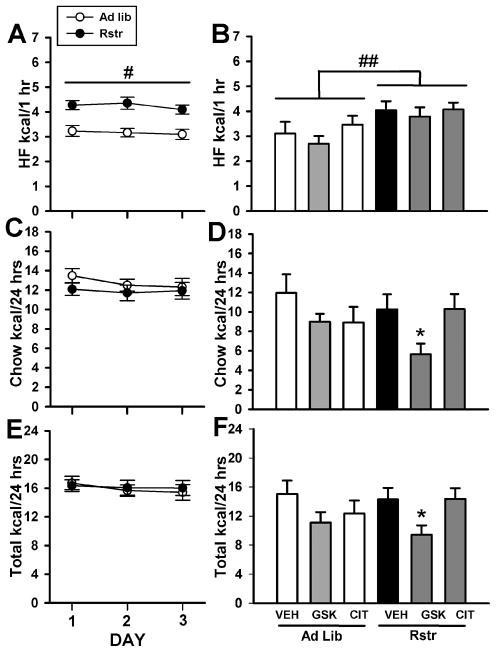
As we hypothesized that previously restricted mice would show a greater sensitivity to an acute treatment with the MCHr1 antagonist GSK-856464, we examined caloric intake and binge-eating following high fat diet exposure and withdrawal stress. As expected, previous restriction increased high fat binge-eating over three days of limited access [F(1,185) = 24.652, p < 0.001] (Fig. 6). Previous caloric restriction did not increase chow intake (Fig. 6C) or total caloric intake (Fig. 6E). There was no effect of any drug on high fat caloric intake on the 4th day of limited access, however, the effect of restriction remained with previously calorically restricted mice having a greater caloric intake of the high fat pellet compared to controls [F(1,41) = 9.072, p < 0.01] (Fig. 6B). 24 hr chow intake was significantly changed by drug treatment [F(2,41) = 3.678, p < 0.05] with post-hoc tests revealing a significant decrease in chow caloric intake in previously restricted mice treated with the MCHr1 antagonist, GSK-856464 (P < 0.05) (Fig. 6D). 24 hr total caloric intake was significantly changed by drug treatment [F(2,41) = 4.128, p < 0.05] with post-hoc tests revealing a significant decrease in chow caloric intake in previously restricted mice treated with GSK-856464 (P < 0.05) (Fig. 6F).
Figure 6. MCHr1 antagonist reduced caloric intake in calorically restricted mice following high fat diet withdrawal.
(A) During 3 days limited access to a high fat pellet, mice previously calorically restricted (Rstr) show increased binge-like consumption of high fat diet compared to controls (Ad lib) (#, p < 0.001). (B) Increased caloric intake of the high fat pellet continued in the restricted mice during the fourth day of testing (##, p < 0.01), however, there was no impact of drug on high fat caloric intake. (D) The MCHr1 antagonist, GSK-856464 reduced chow caloric intake only in CR mice compared to vehicle and citalopram treated calorically restricted mice (*, p < 0.05). (F) The MCHr1 antagonist, GSK-856464 reduced overall caloric intake only in CR mice compared to vehicle and citalopram treated restricted mice (*, p < 0.05). (Ad lib-VEH, n = 7; Ad lib-GSK, n = 8; Ad lib-CIT, n = 8; Rstr-VEH, n = 8; Rstr-GSK, n = 8; Rstr-CIT, n =8). Data are mean +/− SEM.
Discussion
Dieting failure and consequent weight regain result in an elevated risk for obesity and metabolic disease (Guagnano et al., 2000; Amigo and Fernandez, 2007). Determining potential long-term effects of caloric restriction on programming of stress pathways may identify mechanisms contributing to treatment resistance. We have examined the behavioral and physiological stress state, changes in CRF gene expression and DNA methylation and the consequent vulnerability to stress-induced binge-eating produced following moderate calorie reduction in mice. While a majority of studies examining outcomes of caloric restriction have focused on its beneficial effects on longevity in organisms ranging from drosophila to rodents and nonhuman primates, examination of the stress sensitivity during caloric restriction has been neglected (Bishop and Guarente, 2007; Levenson and Rich, 2007). Unlike experimental animals, humans do not usually live in a restricted environment, thereby making examination of stress state important in determining the long-term treatment success and potential factors that may contribute to dieting failure.
Caloric restriction reprograms stress pathways
In our study, calorically restricted mice lost 10–15% of their body weight over 3 weeks compared to ad lib control mice, weight loss typical of that obtained during behavioral modification and dieting in humans (Sarwer et al., 2009). In order to examine the stress state produced during caloric restriction, physiological and behavioral stress responses were measured. In a tail suspension test, calorically restricted mice showed a significant increase in time spent immobile compared to control mice, supportive of an increase depressive-like beahvior (Holmes et al., 2002). Studies that have employed a more severe restriction that produced a greater and more rapid weight loss have reported increased swimming behavior in a forced swim test in mice, suggesting that there may be underlying mechanistic differences in central responses between moderate and more severe weight loss (Lutter et al., 2008a; Lutter et al., 2008b).
Similar to the increased stress sensitivity detected in the tail suspension test, calorically restricted mice also showed significantly increased corticosterone levels following an acute 15-min restraint stress with a higher maximal rise and a delayed stress recovery. Similar results have been shown in rats that were food restricted to 90% of initial body weight (Marinelli et al., 1996). As these studies were conducted in C57Bl/6 mice, a low stress responding strain (as shown by the low rise in corticosterone in control mice), the magnitude of increased corticosterone levels produced following calorie reduction supports a profound impact of a moderate reduction in caloric availability that is translated into heightened physiological stress responsivity (Goel and Bale, 2007, 2008). Individuals with affective disorders typically present with increased stress sensitivity and HPA axis dysregulation supporting that dieting in humans may promote a period in which the acute and long-term stress state becomes a key factor in continued treatment outcome and maintenance (Pariante and Lightman, 2008; Gallagher et al., 2009).
As the key conductor in the orchestration of stress responsivity, CRF expression was analyzed following caloric restriction in the central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST), regions of the limbic system distinct in their efferent and afferent projections (Swanson et al., 1983). While CRF in the CeA was unchanged, CRF in the BNST was significantly decreased. To determine if these effects would be maintained during ad libitum re-feeding, CRF was again measured once mice had attained normalized body weights. Surprisingly, BNST CRF expression remained significantly reduced, suggesting a possible epigenetic programming in response to caloric restriction. Therefore, we examined the DNA methylation of the characterized CpG island in the CRF promoter (McGill et al., 2006; Mueller and Bale, 2008). Similar to the CRF expression, CRF methylation changes were brain region specific where caloric restriction produced a significant change in methylation in the BNST, but no effect was detected in the CeA. Further, methylation patterns were not ameliorated during re-feeding. In previous studies, we reported similar increases in stress responsivity in a model of high fat diet withdrawal (Teegarden and Bale, 2007). As reductions in diet palatability and fat content are additional components of dieting, we examined CRF expression and promoter methylation in mice following exposure and subsequent withdrawal from a high fat diet. To determine if the withdrawal effects persisted we analyzed expression at multiple points over 8 weeks. In the CeA, we observed peak expression of CRF at 2 wks post-withdrawal, and a return to baseline around 6 wks. Increased CRF levels in the CeA have been correlated with diet cycling and relapse to compulsive eating (Cottone et al., 2009). Interestingly, CRF expression in the BNST decreased with high fat diet exposure and remained unchanged following withdrawal. Examination of promoter methylation showed an overall decrease in methylation in the BNST, but not in the CeA. While high fat diet exposure reduced CRF expression in the BNST similar to that seen with withdrawal, only the withdrawal produced a significant change in promoter methylation, suggesting that distinct transcriptional mechanisms are involved in regulating CRF in the BNST. The brain region specific changes in CRF expression and promoter methylation were similar between caloric restriction and high fat withdrawal indicating that increased stress sensitivity may be a common component regardless of the method employed to lose weight.
Recent studies have identified novel roles for methyl binding proteins such as methyl CpG binding protein 2 (MeCP2) to recruit transcription machinery and activate rather than suppress transcription (Chahrour et al., 2008). As we detected reduced methylation within the CRF promoter, these results support long-term epigenetic regulation of CRF following caloric restriction and high fat withdrawal. CRF containing GABAergic neurons in the BNST project to and inhibit the paraventricular hypothalamic nucleus (PVN) (Cullinan et al., 1993). Therefore, a reduced inhibition may be contributing to the increased HPA axis responsivity detected in these calorically restricted mice.
Caloric restriction reprograms orexegenic pathways and promotes binge-eating
Stress pathways have a major influence on motivational behaviors via their intersection with brain reward centers (Self and Nestler, 1998; Koob, 2009). Stress experience increases drug-seeking behaviors as well as the motivation for natural rewards, including palatable calorically-dense foods (Kelley et al., 2005; Teegarden and Bale, 2007; Foster et al., 2009; Shalev et al., 2010). To examine a long-term behavioral consequence of previous restriction, we examined binge-eating of a high fat food during stress exposure in these mice (Teegarden and Bale, 2008). As predicted, stress-sensitive previously restricted mice consumed significantly more of the high fat food compared to stressed ad lib mice. During the limited access testing, all mice displayed binge-eating, consuming approximately 50% of their total 24 hr intake of calories during the one hour of access supporting the high preference mice have for the high fat diet.
To determine a potential underlying mechanism for the increased binge-eating of the high fat food during stress in previously restricted mice, expression of the orexegenic hormones melanin concentrating hormone (MCH) and orexin were examined. Intriguingly, while orexegenic hormones were reduced in control animals during exposure to high fat, previously restricted mice showed robust increases in both genes (Beck et al., 2006; Teegarden et al., 2008). MCH and orexin interact with the mesolimbic dopamine system and are thought to promote the motivational behaviors associated with increased consumption of calorically-dense and preferred foods (Georgescu et al., 2005; Pecina et al., 2006; Borgland et al., 2009; Zheng et al., 2009; Sears et al., 2010). Thus, atypical elevations in these orexegenic hormones in response to a high fat calorically-dense diet in previously restricted mice, combined with long-term changes in stress sensitivity likely contribute to the stress-induced binge-eating detected in these animals (Shalev et al., 2010).
Previous studies have demonstrated that stress-induced reinstatement of rewarding substances, including high fat food, can be blunted by administration of melanin concentrating hormone receptor-1 (MCHr1) antagonist (Chung et al., 2009). In addition, high fat food-reinforced operant responding was also reduced following MCHr1 antagonist treatment (Nair et al., 2009). Therefore, we hypothesized that previously restricted mice based on their dysregulation of stress and orexegenic pathways would show a greater sensitivity to an acute treatment with the MCHr1 antagonist GSK-856464. We examined caloric intake and binge-eating following high fat diet exposure and withdrawal stress. As expected, previously restricted mice showed an increase in high fat binge-eating compared to ad lib controls. Further, acute GSK-856464 treatment significantly reduced overall caloric intake in previously restricted mice, supporting our hypothesis that these mice would show a greater sensitivity to MCH antagonism. Acute treatment with the SSRI citalopram did not produce an effect on food intake in these studies suggesting that the central programming changes produced following caloric restriction may not involve serotonergic pathways.
Together, these results suggest that the stress associated with moderate caloric restriction promotes long-term alterations in genes critical in feeding and reward circuitry that influence food intake and stress-related behaviors. These changes may in part be driven by epigenetic mechanisms promoting increased sensitivity of stress pathways that project to and alter reward circuitry. Such epigenetic mechanisms likely hold an evolutionary advantage in times of famine, but in our current environment of high caloric availability would function against our health and contribute to difficulty in weight management. It seems appropriate that the brain developed strategies to guard against loss of calories by increasing the likelihood that a prior restriction experience would promote future behaviors to increase consumption of calorically-dense foods.
Acknowledgments
These studies were supported by funding from the University of Pennsylvania Diabetes Center NIH DK019525, HRFF Funds, and AstraZeneca, Wilmington, DE. We thank Kendall Carlin for technical assistance.
Footnotes
Supplementary Materials: None
References
- Amigo I, Fernandez C. Effects of diets and their role in weight control. Psychol Health Med. 2007;12:321–327. doi: 10.1080/13548500600621545. [DOI] [PubMed] [Google Scholar]
- Beck B, Kozak R, Moar KM, Mercer JG. Hypothalamic orexigenic peptides are overexpressed in young Long-Evans rats after early life exposure to fat-rich diets. Biochem Biophys Res Commun. 2006;342:452–458. doi: 10.1016/j.bbrc.2006.01.158. [DOI] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–844. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell KD, Rodin J. Medical, metabolic, and psychological effects of weight cycling. Arch Intern Med. 1994;154:1325–1330. [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Hopf FW, Nagasaki H, Li CY, Belluzzi JD, Bonci A, Civelli O. The melanin-concentrating hormone system modulates cocaine reward. Proc Natl Acad Sci U S A. 2009;106:6772–6777. doi: 10.1073/pnas.0811331106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan WE, Herman JP, Watson SJ. Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J Comp Neurol. 1993;332:1–20. doi: 10.1002/cne.903320102. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, La Fleur SE, Warne JP, Ginsberg AB, Akana SF, Laugero KC, Houshyar H, Strack AM, Bhatnagar S, Bell ME. Glucocorticoids, chronic stress, and obesity. Prog Brain Res. 2006;153:75–105. doi: 10.1016/S0079-6123(06)53004-3. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150:2325–2333. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher P, Reid KS, Ferrier IN. Neuropsychological functioning in health and mood disorder: Modulation by glucocorticoids and their receptors. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006;31:2188–2196. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel N, Bale TL. Identifying early behavioral and molecular markers of future stress sensitivity. Endocrinology. 2007;148:4585–4591. doi: 10.1210/en.2007-0479. [DOI] [PubMed] [Google Scholar]
- Goel N, Bale TL. Organizational and activational effects of testosterone on masculinization of female physiological and behavioral stress responses. Endocrinology. 2008;149:6399–6405. doi: 10.1210/en.2008-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guagnano MT, Ballone E, Pace-Palitti V, Vecchia RD, D’Orazio N, Manigrasso MR, Merlitti D, Sensi S. Risk factors for hypertension in obese women. The role of weight cycling. Eur J Clin Nutr. 2000;54:356–360. doi: 10.1038/sj.ejcn.1600963. [DOI] [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Baldo BA, Pratt WE. A proposed hypothalamic-thalamic-striatal axis for the integration of energy balance, arousal, and food reward. J Comp Neurol. 2005;493:72–85. doi: 10.1002/cne.20769. [DOI] [PubMed] [Google Scholar]
- Kim HR, Hwang KA, Kim KC, Kang I. Down-regulation of IL-7Ralpha expression in human T cells via DNA methylation. J Immunol. 2007;178:5473–5479. doi: 10.4049/jimmunol.178.9.5473. [DOI] [PubMed] [Google Scholar]
- Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson CW, Rich NJ. Eat less, live longer? New insights into the role of caloric restriction in the brain. Nutr Rev. 2007;65:412–415. doi: 10.1111/j.1753-4887.2007.tb00319.x. [DOI] [PubMed] [Google Scholar]
- Lissner L, Odell PM, D’Agostino RB, Stokes J, 3rd, Kreger BE, Belanger AJ, Brownell KD. Variability of body weight and health outcomes in the Framingham population. N Engl J Med. 1991;324:1839–1844. doi: 10.1056/NEJM199106273242602. [DOI] [PubMed] [Google Scholar]
- Liu T, Zhang X, So CK, Wang S, Wang P, Yan L, Myers R, Chen Z, Patterson AP, Yang CS, Chen X. Regulation of Cdx2 expression by promoter methylation, and effects of Cdx2 transfection on morphology and gene expression of human esophageal epithelial cells. Carcinogenesis. 2007;28:488–496. doi: 10.1093/carcin/bgl176. [DOI] [PubMed] [Google Scholar]
- Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008a;28:3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Sakata I, Osborne-Lawrence S, Rovinsky SA, Anderson JG, Jung S, Birnbaum S, Yanagisawa M, Elmquist JK, Nestler EJ, Zigman JM. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat Neurosci. 2008b;11:752–753. doi: 10.1038/nn.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli M, Le Moal M, Piazza PV. Acute pharmacological blockade of corticosterone secretion reverses food restriction-induced sensitization of the locomotor response to cocaine. Brain Res. 1996;724:251–255. doi: 10.1016/0006-8993(96)00309-5. [DOI] [PubMed] [Google Scholar]
- McEuen JG, Semsar KA, Lim MA, Bale TL. Influence of sex and corticotropin-releasing factor pathways as determinants in serotonin sensitivity. Endocrinology. 2009;150:3709–3716. doi: 10.1210/en.2008-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill BE, Bundle SF, Yaylaoglu MB, Carson JP, Thaller C, Zoghbi HY. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc Natl Acad Sci U S A. 2006;103:18267–18272. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J. Circadian clocks: setting time by food. J Neuroendocrinol. 2007;19:127–137. doi: 10.1111/j.1365-2826.2006.01510.x. [DOI] [PubMed] [Google Scholar]
- Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair SG, Adams-Deutsch T, Pickens CL, Smith DG, Shaham Y. Effects of the MCH1 receptor antagonist SNAP 94847 on high-fat food-reinforced operant responding and reinstatement of food seeking in rats. Psychopharmacology (Berl) 2009;205:129–140. doi: 10.1007/s00213-009-1523-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, Lightman SL. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 2008;31:464–468. doi: 10.1016/j.tins.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. San Diego: Academic Press; 2001. [Google Scholar]
- Pecina S, Schulkin J, Berridge KC. Nucleus accumbens corticotropin-releasing factor increases cue-triggered motivation for sucrose reward: paradoxical positive incentive effects in stress? BMC Biol. 2006;4:8. doi: 10.1186/1741-7007-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–872. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwer DB, von Sydow Green A, Vetter ML, Wadden TA. Behavior therapy for obesity: where are we now? Curr Opin Endocrinol Diabetes Obes. 2009;16:347–352. doi: 10.1097/MED.0b013e32832f5a79. [DOI] [PubMed] [Google Scholar]
- Sears RM, Liu RJ, Narayanan NS, Sharf R, Yeckel MF, Laubach M, Aghajanian GK, DiLeone RJ. Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J Neurosci. 2010;30:8263–8273. doi: 10.1523/JNEUROSCI.5858-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl) 2001;158:343–359. doi: 10.1007/s002130100917. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Rivier J, Vale WW. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology. 1983;36:165–186. doi: 10.1159/000123454. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Bale TL. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol Psychiatry. 2007;61:1021–1029. doi: 10.1016/j.biopsych.2006.09.032. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Bale TL. Effects of stress on dietary preference and intake are dependent on access and stress sensitivity. Physiol Behav. 2008;93:713–723. doi: 10.1016/j.physbeh.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teegarden SL, Nestler EJ, Bale TL. Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol Psychiatry. 2008;64:941–950. doi: 10.1016/j.biopsych.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]
- Zheng H, Lenard NR, Shin AC, Berthoud HR. Appetite control and energy balance regulation in the modern world: reward-driven brain overrides repletion signals. Int J Obes (Lond) 2009;33(Suppl 2):S8–13. doi: 10.1038/ijo.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]