Abstract
During enamel maturation, hydroxyapatite crystallites expand in volume releasing protons which acidifies the developing enamel. This acidity is neutralized by buffering activity of carbonic anhydrases and ion transporters. Less hydroxyapatite forms in matrix metalloproteinase-20 null (Mmp20−/−) mouse incisors because enamel thickness is reduced by approximately 50%. We therefore asked if ion regulation was altered in Mmp20−/− mouse enamel. Staining of wild-type and Mmp20−/− incisors with pH indicators demonstrated that wild-type mice had pronounced changes in enamel pH as development progressed. These pH changes were greatly attenuated in Mmp20−/− mice. Expression of four ion regulatory genes (Atp2b4, Slc4a2, Car6, Cftr) was significantly decreased in enamel organs from Mmp20−/− mice. Notably, expression of secreted carbonic anhydrase (Car6) was reduced to almost undetectable levels in the null enamel organ. In contrast, Odam and Klk4 expression were unaffected. We conclude that a feedback mechanism regulates ion responsive gene expression during enamel development.
INTRODUCTION
Enamel development is stage specific. The two predominant stages are the secretory and maturation stages. During the secretory stage, a protein scaffold is formed and the mineral phase appears as long thin ribbons that grow out to form the full thickness of the enamel layer. During the maturation stage, the protein scaffold is removed while the enamel ribbons grow in width and thickness as the enamel matures into its final hardened form. It is during the maturation stage that the vast majority of hydroxyapatite mineral precipitates and this precipitation reaction releases between 8–14 mol of H+ ions (depending on the phosphate precursor) for every mol of hydroxyapatite produced (Simmer and Fincham, 1995). Thus, the newly formed enamel becomes acidic and this acidity is neutralized by the combined activities of carbonic anhydrases and ion transporters. Five of these genes are known to be expressed by ameloblasts during enamel formation. These are carbonic anhydrase II (Car2, CAII; intracellular pH regulation), carbonic anhydrase VI (Car6, CAVI; extracellular pH regulation), cystic fibrosis transmembrane conductance regulator (Cftr; membrane associated Cl− channel), solute carrier family 4 anion exchanger member 2 (Slc4a2, AE2; Cl−-HCO3−exchanger), and solute carrier family 4 sodium bicarbonate cotransporter member 4 (Scla4a4, NBCe1; Na+--coupled HCO3 transporter) (Gawenis et al., 2001; Lin et al., 1994; Lyaruu et al., 2008; Paine et al., 2008; Smith et al., 2006; Sui et al., 2003). Models for how these ion transporter genes work cooperatively to regulate pH have been proposed (Lacruz et al., 2010).
Additionally, the process of mineralization requires a large volume of Ca2+ to be transported by the ameloblasts into the forming enamel matrix. Genes encoding four enamel organ calcium transporters have been implicated in this process. They include plasma membrane Ca2+ ATPase-1 (Atp2b1; PMCA-1), plasma membrane Ca2+ ATPase-4 (Atp2b4; PMCA-4), and the newly identified solute carrier family 8 member 1 (Slc8a1; NCX1; Na+-Ca2+ exchanger) and solute carrier family 8 member 3 (Slc8a3; NCX3; Na+-Ca2+ exchanger) (Borke et al., 1995; Okumura et al., 2010)
Four proteins are secreted into the enamel matrix during the secretory stage of enamel formation. These are: amelogenin, ameloblastin, enamelin, and matrix metalloproteinase-20 (MMP20, enamelysin). Disruption of any one of the genes encoding these proteins in mice causes severe dental enamel defects and mutations in all but ameloblastin have been demonstrated to cause enamel defects (amelogenesis imperfecta) in humans [reviewed in (Hu et al., 2007)]. The full-length proteins are present for only a short time near the mineralizing front of the most recently formed enamel. MMP20 cleaves the secreted enamel proteins and these cleavage products may form an organic mold that supports the growth of the elongating ribbons. Interestingly, the enamel from Mmp20 null mice is severely affected during the maturation stage of development when MMP20 is no longer expressed (Bartlett et al., 2004; Caterina et al., 2002). Perhaps this can be attributed to the formation of a defective scaffold during the secretory stage that interferes with the normal growth in width and thickness of the enamel ribbons during the maturation stage. In any case, the enamel from Mmp20 null incisors have 7–16% higher-than-normal levels of water and protein per unit weight than wild-type animals and the enamel mineral content is reduced by approximately 50% (Bartlett et al., 2004). Therefore, the quantity of protons released by the precipitating enamel should also be reduced by approximately 50%. This prompted us to ask if expression of ion responsive genes were reduced in the poorly mineralized enamel.
MATERIALS & METHODS
All animals used in this study were housed in an Association for Assessment and Accreditation of Laboratory Animal Care accredited facilities. Wild-type and Mmp20 null C57BL/6 strain mice were previously described (Caterina et al., 2002).
Quantitative Real-time PCR
Expression of odontogenic ameloblast-associated protein precursor (Odam; Apin) and kallikrein-4 (Klk4) was examined in wild-type and Mmp20 null enamel organ by qPCR. Five ion responsive genes (Car2, Car6, Cftr, Slc4a2 and Slc4a4) and two calcium exchange genes (Atp2b1, Atp2b4) were also examined. Mouse first molars were harvested from wild-type and Mmp20−/− mice at post-natal day 11 (mid-maturation stage, n = 6). The dental papilla was carefully removed and total RNA was extracted from the enamel organ according to the manufacturer’s protocol using TRIzol (Invitrogen). cDNA was transcribed using the SuperScript III First-Strand Synthesis system (Invitrogen). The enamel organ was subjected to qPCR analysis by iQ SYBR green (Bio-Rad). Gene specific primers (Table 1) were from PrimerBank (Spandidos et al., 2008) or designed by analysis of annealing sites that flank intron-exon boundaries (DNAStar Lasergene, madison WI, USA). Standard curves were generated with each primer set using control cDNA preparations and a 10-fold dilution series ranging from 100 ng/μl to 100 pg/μl. PCR efficiencies and relative expression levels of as a function of multiple housekeeping genes [Eef1α1 (eukaryotic translation elongation factor 1α1 is responsible for the enzymatic delivery of aminoacyl tRNAs to the ribosome), Casc3 (cancer susceptibility candidate 3 functions in nonsense-mediated mRNA decay), Actb (β-actin), and Gapdh (glyceraldehyde-3-phosphate dehydrogenase)] were calculated as previously described (Vandesompele et al., 2002). Statistical significance was determined by t-test (GraphPad Prism 5).
TABLE 1. Gene specific primers for quantitative PCR.
Primers were designed by analysis of annealing sites by DNAStar Lasergene software and designed to flank intron-exon boundaries or were taken from PrimerBank (Spandidos et al., 2008; Wang and Seed, 2003). n/a not applicable. °C = annealing temperature.
| GenBank ID # | 5′ Primer | 3′ Primer | PrimerBank ID | °C | |
|---|---|---|---|---|---|
| Klk4 | NM_019928 | CTGGCAGCCGGATGTTAGAGG | AGGAGTGGGGCATTGGGTAGC | n/a | 64 |
| Odam | NM_027128 | GCTTTTGACAGCTTTGTAGGC | AAGCAGGCTTCCTTCTACTGG | n/a | 60 |
| Atp2b1 | NM_026482 | GCCATCTTCTGCACCATTG | CCGCCAAACTGCACAATTA | n/a | 64 |
| Atp2b4 | NM_213616 | AGATGTCGGGTTTGCTATGG | ATGATGTCTGACGCCTCCTT | n/a | 63 |
| Car2 | NM_009801 | ACTGGAACACCAAATATGGGGA | GCAAGGGTCGAAGTTAGCAAAG | 31981657a3 | 63 |
| Car6 | NM_009802 | CTAACAACGGACACACAGTATCG | GCCTTTGAGATGAACTCAGTGC | 28461317a2 | 63 |
| Cftr | NM_021050 | CCGGTGACAACATGGAACACATAC | CCAGTACGCACCAAATCAGCACTA | n/a | 63 |
| Slc4a2 | NM_009207 | AACTTCGTACCTTAGGTGTGGA | GGCGGTGGTATTCAAAGTCTT | 6678021a2 | 63 |
| Slc4a4 | NM_018760 | GAAGGTCACCACACGATCTACA | TCCACATCAGATTTGTCGGAGT | 9055346a1 | 64 |
| Ef1α1 | NM_010106 | ATTCCGGCAAGTCCACCACAA | CATCTCAGCAGCCTCCTTCTCAAAC | n/a | 62 |
| Actb | NM_007393 | TGACGGCCAGGTCATCACTATT | ACCCAAGAAGGAAGGCTGGAAA | n/a | 65 |
| Casc3 | NM_138660 | GGATCGGAAAAACCCAGCCTACAT | TGTTCCCAGAGACCCTCATCTT | n/a | 65 |
| Gapdh | NM_008084 | GCAAAGTGGAGATTGTTGCCAT | CCTTGACTGTGCCGTTGAATTT | n/a | 64 |
Staining of Incisors with pH Indicators
Hemimandibles and hemimaxillae from adult wild-type and Mmp20 null mice were removed, immersed in liquid nitrogen and freeze-dried at −55°C for 48 hours. The bone and enamel organs covering the incisors were removed, and the exposed enamel surfaces were gently wiped clean of cellular debris. Incisors were then dipped into their respective pH indicator (methyl red, bromophenol red or resazruin) and photographed (Sasaki et al., 1991).
RESULTS
Loss of MMP20 Expression Does Not Effect the Expression of ODAM or Kallikrein-4
Enamel from Mmp20 null incisors has higher-than-normal levels of protein than do wild-type animals. We therefore sought to determine if loss of MMP20 expression caused a compensatory upregulation of Klk4 in an attempt to clear the excess protein. At the mRNA level, we found no difference in Klk4 expression between wild-type and Mmp20 null mouse enamel organ (Figure 1). Similarly the expression of the maturation stage specific gene Odam was not altered (Figure 1).
Figure 1. Expression of Odam and Klk4 in maturation stage mouse enamel organ.
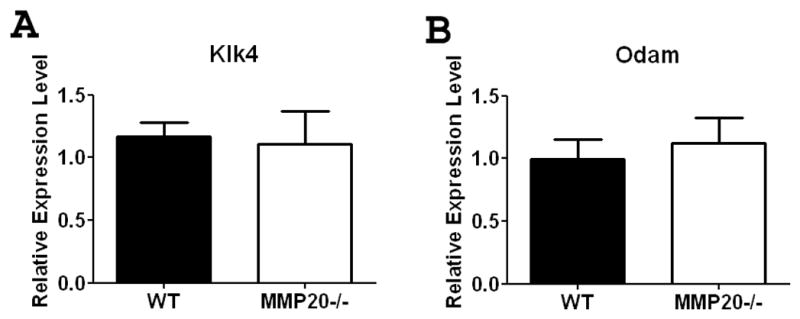
Expression of Klk4 (A) and Odam (B) were not significantly altered in the Mmp20 null mouse. Data are presented as mean ± SEM and represent measurements of six individual mice with duplicate measurements for each mouse (n=6). Results are presented as relative gene expression normalized to the geometric mean of Eef1α1, Gapdh, β-actin and Casc3 mRNA expression. Statistical analysis was determined by t-test.
Staining of Mouse Incisors with pH Indicators Demonstrates that Mmp20 Null Enamel has an Altered Banding Pattern Indicative of Altered Ion Control
In Mmp20 null incisors, the enamel mineral content is reduced by approximately 50% which reduces the quantity of protons released by hydroxyapatite growth. We therefore examined the pH of Mmp20 null enamel during development. A comparison of wild-type rat and mouse incisor banding pattern was made by use of methyl red staining (Fig. 2, Top Panels). Methyl red stains areas of acidity red and neutral areas remain unstained. Note that for mandibular incisors, the rat has at least one more band of acidity than does the mouse. The four bottom panels of Figure 2 show incisors from wild-type and Mmp20 null mice. For each of these panels the top incisor is stained with methyl red, the middle incisor is stained with bromophenol red (neutral areas stain a shade of pink and acid areas stain brownish-red), and the bottom incisor is stained with resazurin (neutral areas stain light blue and acid areas stain a shade of brown). The staining pattern of the Mmp20 null incisors is distinctly different from the wild-type control. The areas of acidity are greatly reduced in the null mouse enamel when compared to controls. This prompted us to ask if ion exchange or bicarbonate regulation was altered in the null mouse enamel organ.
Figure 2. Staining of Incisors with pH Indicators.
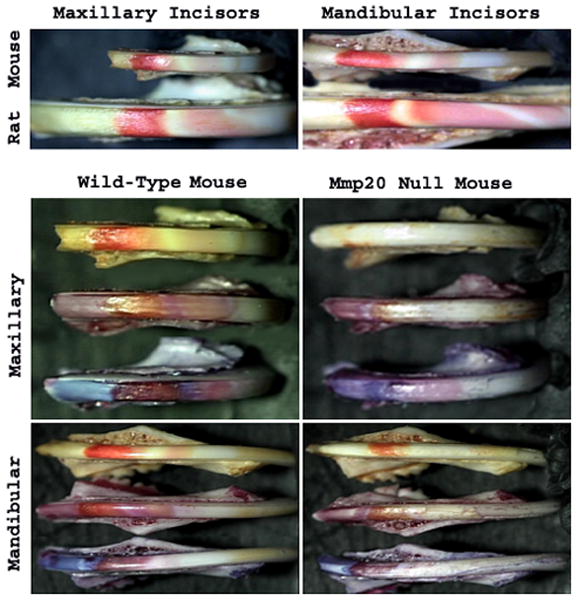
A comparison of wild-type rat and mouse incisor banding pattern was made by use of methyl red staining (Top Panels). Note that for mandibular incisors, the rat has at least one more band of acidity than does the mouse. The four bottom panels show incisors from wild-type and Mmp20 null mice. For each of these panels the top incisor is stained with methyl red, the middle incisor is stained with bromophenol red and the bottom incisor is stained with resazurin. The staining pattern of the Mmp20 null incisors is distinctly different from the wild-type control and the areas of acidity are greatly reduced in the null mouse enamel.
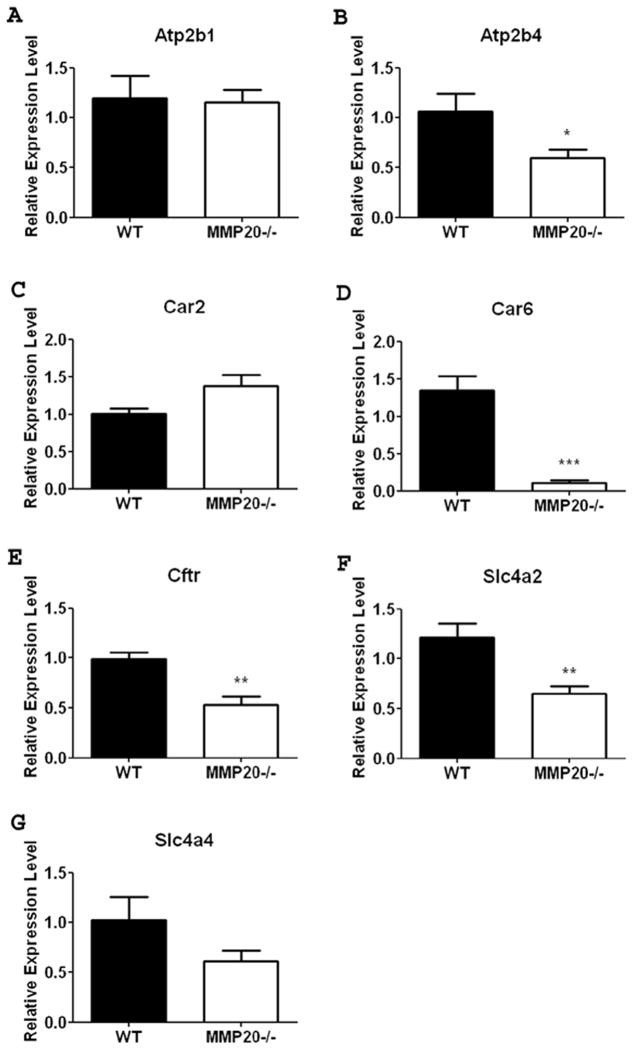
Expression of Selected Ion Responsive Genes are Significantly Reduced in Mmp20 Null Mouse First Molar Enamel Organs Compared to Enamel Organs From Wild-Type Controls
Unerupted first molars from day 11 mice will contain predominately mid-maturation stage ameloblasts. Compared to wild-type enamel organ, the Mmp20−/− mouse enamel organ had significant reductions in maturation stage gene expression for Atp2b4, Cftr and solute carrier Slc4a2 (Figs. 3b, 3e-f). No significant change in expression was seen for Atp2b1, Car2 or Slc4a4 (Figs. 3a, 3c, 3g). Car6 showed the most dramatic change in gene expression between wild-type and null enamel organs as it was decreased to almost undetectable expression levels in the Mmp20 null mouse (Fig. 3d). This suggests that while enamel matrix genes are not sensitive to feedback inhibition, ion responsive genes do sense and adjust to external ion concentrations.
Figure 3. Ion Transporter gene expression in maturation stage mouse enamel organ.
Expression of Atp2b4 (B), Car6 (D), Cftr (E) and, Slc4a2 (F) all showed a significant decrease in mRNA expression in the absence of MMP20. Expression of Atp2b1 (A) and Car2 (C) were not significantly altered in the Mmp20 null mouse. Although a trend existed, the data for Slc4a4 (G) was not significant due to variability within the data sets. Data are presented as mean + SEM and represent measurements of six individual mice with duplicate measurements for each mouse (n=6). Results are presented as relative gene expression normalized to the geometric mean of Eef1α1, Gapdh, β-actin and Casc3 mRNA expression. Statistical analysis was determined by T-test (*, p<0.05, ** p <0.01, *** p<0.001).
DISCUSSION
Here we show that loss of MMP20 causes decreased expression of several ion responsive genes in mouse enamel organ. We attribute this to a feedback mechanism that senses altered pH during enamel development. During the maturation stage of enamel formation the mass precipitation of hydroxyapatite normally causes the extracellular matrix to become mildly acidic with pH as low as 6.2 (Smith et al., 1996). Mice lacking Mmp20 expression contain approximately 50% less bulk mineral so less H+ ions are produced during the maturation of Mmp20−/− enamel resulting in less acidity. Examination of incisors dipped in various pH indicators clearly demonstrate that the pH of developing enamel from Mmp20−/− mice is different from wild-type mice. The Mmp20 null mouse enamel is less acidic.
The regulation of ion concentration and pH during enamel development is vital for proper enamel formation. Acidotic and alkalotic rats and dogs have disturbed enamel mineralization (Angmar-Mansson and Whitford, 1990; Whitford and Angmar-Mansson, 1995). Individuals with mutations and mice that are null for Slc4a2, Slc4a4 or Cftr have malformed dental enamel amongst other abnormalities thus highlighting the critical role of these genes in enamel formation (Demirci et al., 2006; Gawenis et al., 2007; Lyaruu et al., 2008; Sui et al., 2003; Wright et al., 1996a; Wright et al., 1996b). Additionally, two enzymes generating bicarbonate (CAII and CAVI) are expressed in enamel organ. Our results show that the expression of these genes, with the exception of Car2 (CAII), was decreased during enamel formation in the Mmp20−/− mouse enamel organ. The most dramatic change was the decrease to almost undetectable Car6 (CAVI) expression in the Mmp20 null mouse.
CAVI is a secreted zinc metalloenzyme that catalyzes the reversible hydration reaction of carbon dioxide with water to produce carbonic acid [CO2 + H2O→H+ + HCO3−]. CAVI has been identified in numerous fluids and tissues including serum, saliva, milk, salivary and mammary glands and liver (Nishita et al., 2007). It is implicated in the protection of surface epithelial cells from gastric ulcers (Parkkila et al., 1997) and decreased secretion of CAVI is associated with distortion and loss of taste and smell (Henkin et al., 1999). There is no null mouse for Car6 to elucidate the function of CAVI in enamel formation. However it was postulated that CAVI may aid with local buffering by providing bicarbonate ions or recycle excess carbonic acid (Smith et al., 2006).
As we do not detect any differences in expression of Odam or Klk4 in the knockout mouse, we assume the decreased expression of the ion responsive genes is directly correlated with altered pH. Although the mechanisms of regulation of these ion transporter genes are still being elucidated, it is evident that their expression is affected by pH and that they regulate one another. Car2 expression is upregulated in fish exposed to acidic conditions (Hirata et al., 2003) and Paine et al. found Slc4a2 and Slc4a4 transcripts increased when the pH fell below 7.0 in LS8 ameloblast-like cells (Paine et al., 2008). In contrast, Slc4a2 is upregulated in the kidney cortical collecting duct under alkaline load and is decreased during metabolic acidosis (Fejes-Toth et al., 1998). Furthermore Slc4a2 is activated by alkaline pH in transiently transfected CHOP and 293 cells (reviewed in (Alper et al., 2002)). Slc4a2 may be regulated by Car2 (Alper et al., 2002) and Cftr is regulated by Slc26a6 which encodes a chloride, oxalate, sulfate and bicarbonate transporter (Wang et al., 2006). CFTR in turn is an ion channel regulator which through feedback mechanisms controls the activity of SLC26a6 and other transporters including the Na+/H+ exchanger (NHE), HCO3−/Cl− exchanger and cotransporters, intermediate conductance outwardly rectifying (ICOR) Cl− channels, and Ca2+- and volume-activated Cl−channels (reviewed in (Linsdell, 2006; Steward et al., 2005)). Therefore, although the ion-responsive genes influence each others activity, it is not known specifically how this occurs or which gene is the master regulator.
It has been suggested that ions will likely affect calcium transport. External Na+ concentration would be expected to affect Na+-Ca2+ exchangers whereas external H+ would likely affect plasma membrane Ca2+−ATPase expression (Hubbard, 2000). We found the expression of two Na+-Ca2+ exchangers, Slc8a1 (NCX1) and Slc8a3 (NCX3), to be at very low levels in mouse enamel organ and were therefore not included in our present analysis. We were able to examine the effect of loss of MMP20 on Atp2b4 (PMCA-4) and Atp2b1 (PMCA-1) expression. Expression of Atp2b4 was significantly decreased in null mice whereas Atp2b1 expression was unaltered. These two ubiquitously expressed Ca2+-ATPases were originally proposed to serve as housekeeping genes but recent studies suggest that they are functionally independent. PMCA-1 is proposed to be required for maintenance of intracellular Ca2+ and PMCA-4 may play a primary role in Ca2+ transport and efflux (Borke et al., 1995; Magosci et al., 1992). We propose that the decreased H+ production in the null mouse downregulates Atp2b4 expression thereby reducing Ca2+ extrusion and causing further reduction of hydroxyapatite formation. Interestingly, 2 of the 3 ion regulating genes whose expression was not altered are the proposed intracellular-regulating genes Car2 and Atp2b1.
We conclude that a feedback mechanism regulates ion responsive gene expression during enamel development. Current models depict CAII, CAVI, CFTR, AE2 and NBCe1 working cooperatively to regulate ion concentrations and pH in ameloblasts and the enamel matrix (Lacruz et al., 2010). pH regulation is critical for proper enamel formation and involves an integrated network of cabonic anhydrases and ion transporters.
Acknowledgments
This investigation was supported by research grant DE016276 from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892.
References
- Alper SL, Chernova MN, Stewart AK. How pH regulates a pH regulator: a regulatory hot spot in the N-terminal cytoplasmic domain of the AE2 anion exchanger. Cell Biochem Biophys. 2002;36(2–3):123–36. doi: 10.1385/CBB:36:2-3:123. [DOI] [PubMed] [Google Scholar]
- Angmar-Mansson B, Whitford GM. Environmental and physiological factors affecting dental fluorosis. J Dent Res. 1990;69(Spec No):706–13. doi: 10.1177/00220345900690S137. discussion 721. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Beniash E, Lee DH, Smith CE. Decreased mineral content in MMP-20 null mouse enamel is prominent during the maturation stage. J Dent Res. 2004;83(12):909–913. doi: 10.1177/154405910408301204. [DOI] [PubMed] [Google Scholar]
- Borke JL, Zaki Ae-M, Eisenmann DR, Mednieks MI. Localization of plasma membrane Ca2+ pump mRNA and protein in human ameloblasts by in situ hybridization and immunohistochemistry. Connect Tissue Res. 1995;33(1–3):139–44. doi: 10.3109/03008209509016993. [DOI] [PubMed] [Google Scholar]
- Caterina JJ, Skobe Z, Shi J, Ding Y, Simmer JP, Birkedal-Hansen H, et al. Enamelysin (matrix metalloproteinase 20)-deficient mice display an amelogenesis imperfecta phenotype. J Biol Chem. 2002;277(51):49598–49604. doi: 10.1074/jbc.M209100200. [DOI] [PubMed] [Google Scholar]
- Demirci FY, Chang MH, Mah TS, Romero MF, Gorin MB. Proximal renal tubular acidosis and ocular pathology: a novel missense mutation in the gene (SLC4A4) for sodium bicarbonate cotransporter protein (NBCe1) Mol Vis. 2006;12:324–30. [PubMed] [Google Scholar]
- Fejes-Toth G, Rusvai E, Cleaveland ES, Naray-Fejes-Toth A. Regulation of AE2 mRNA expression in the cortical collecting duct by acid/base balance. Am J Physiol. 1998;274(3 Pt 2):F596–601. doi: 10.1152/ajprenal.1998.274.3.F596. [DOI] [PubMed] [Google Scholar]
- Gawenis LR, Spencer P, Hillman LS, Harline MC, Morris JS, Clarke LL. Mineral content of calcified tissues in cystic fibrosis mice. Biol Trace Elem Res. 2001;83(1):69–81. doi: 10.1385/BTER:83:1:69. [DOI] [PubMed] [Google Scholar]
- Gawenis LR, Bradford EM, Prasad V, Lorenz JN, Simpson JE, Clarke LL, et al. Colonic anion secretory defects and metabolic acidosis in mice lacking the NBC1 Na+/HCO3-cotransporter. J Biol Chem. 2007;282(12):9042–52. doi: 10.1074/jbc.M607041200. [DOI] [PubMed] [Google Scholar]
- Henkin RI, Martin BM, Agarwal RP. Decreased parotid saliva gustin/carbonic anhydrase VI secretion: an enzyme disorder manifested by gustatory and olfactory dysfunction. Am J Med Sci. 1999;318(6):380–91. doi: 10.1097/00000441-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Hirata T, Kaneko T, Ono T, Nakazato T, Furukawa N, Hasegawa S, et al. Mechanism of acid adaptation of a fish living in a pH 3.5 lake. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1199–212. doi: 10.1152/ajpregu.00267.2002. [DOI] [PubMed] [Google Scholar]
- Hu JC, Chun YH, Al Hazzazzi T, Simmer JP. Enamel formation and amelogenesis imperfecta. Cells Tissues Organs. 2007;186(1):78–85. doi: 10.1159/000102683. [DOI] [PubMed] [Google Scholar]
- Hubbard MJ. Calcium transport across the dental enamel epithelium. Crit Rev Oral Biol Med. 2000;11(4):437–66. doi: 10.1177/10454411000110040401. [DOI] [PubMed] [Google Scholar]
- Lacruz RS, Nanci A, Kurtz I, Wright JT, Paine ML. Regulation of pH During Amelogenesis. Calcif Tissue Int. 2010;86(2):91–103. doi: 10.1007/s00223-009-9326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HM, Nakamura H, Noda T, Ozawa H. Localization of H(+)-ATPase and carbonic anhydrase II in ameloblasts at maturation. Calcif Tissue Int. 1994;55(1):38–45. doi: 10.1007/BF00310167. [DOI] [PubMed] [Google Scholar]
- Linsdell P. Mechanism of chloride permeation in the cystic fibrosis transmembrane conductance regulator chloride channel. Exp Physiol. 2006;91(1):123–9. doi: 10.1113/expphysiol.2005.031757. [DOI] [PubMed] [Google Scholar]
- Lyaruu DM, Bronckers AL, Mulder L, Mardones P, Medina JF, Kellokumpu S, et al. The anion exchanger Ae2 is required for enamel maturation in mouse teeth. Matrix Biol. 2008;27(2):119–27. doi: 10.1016/j.matbio.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magosci M, Yamaki M, Penniston JT, Dousa TP. Localization of mRNAs coding for isozymes of plasma membrane Ca(2+)-ATPase pump in rat kidney. Am J Physiol. 1992;263(1 Pt 2 ):F7–14. doi: 10.1152/ajprenal.1992.263.1.F7. [DOI] [PubMed] [Google Scholar]
- Nishita T, Tanaka Y, Wada Y, Murakami M, Kasuya T, Ichihara N, et al. Measurement of carbonic anhydrase isozyme VI (CA-VI) in bovine sera, saliva, milk and tissues. Vet Res Commun. 2007;31(1):83–92. doi: 10.1007/s11259-006-3423-0. [DOI] [PubMed] [Google Scholar]
- Okumura R, Shibukawa Y, Muramatsu T, Hashimoto S, Nakagawa K, Tazaki M, et al. Sodium-calcium exchangers in rat ameloblasts. J Pharmacol Sci. 2010;112(2):223–30. doi: 10.1254/jphs.09267fp. [DOI] [PubMed] [Google Scholar]
- Paine ML, Snead ML, Wang HJ, Abuladze N, Pushkin A, Liu W, et al. Role of NBCe1 and AE2 in secretory ameloblasts. J Dent Res. 2008;87(4):391–5. doi: 10.1177/154405910808700415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkila S, Parkkila AK, Lehtola J, Reinila A, Sodervik HJ, Rannisto M, et al. Salivary carbonic anhydrase protects gastroesophageal mucosa from acid injury. Dig Dis Sci. 1997;42(5):1013–9. doi: 10.1023/a:1018889120034. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Takagi T, Suzuki M. Cyclical changes in pH in bovine developing enamel as sequential bands. Arch Oral Biol. 1991;36(3):227–31. doi: 10.1016/0003-9969(91)90090-h. [DOI] [PubMed] [Google Scholar]
- Simmer JP, Fincham AG. Molecular mechanisms of dental enamel formation. Crit Rev Oral Biol Med. 1995;6(2):84–108. doi: 10.1177/10454411950060020701. [DOI] [PubMed] [Google Scholar]
- Smith CE, Issid M, Margolis HC, Moreno EC. Developmental changes in the pH of enamel fluid and its effects on matrix-resident proteinases. Adv Dent Res. 1996;10(2):159–169. doi: 10.1177/08959374960100020701. [DOI] [PubMed] [Google Scholar]
- Smith CE, Nanci A, Moffatt P. Evidence by signal peptide trap technology for the expression of carbonic anhydrase 6 in rat incisor enamel organs. Eur J Oral Sci. 2006;114(Suppl 1):147–153. doi: 10.1111/j.1600-0722.2006.00273.x. [DOI] [PubMed] [Google Scholar]
- Spandidos A, Wang X, Wang H, Dragnev S, Thurber T, Seed B. A comprehensive collection of experimentally validated primers for Polymerase Chain Reaction quantitation of murine transcript abundance. BMC Genomics. 2008;9:633. doi: 10.1186/1471-2164-9-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward MC, Ishiguro H, Case RM. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu Rev Physiol. 2005;67:377–409. doi: 10.1146/annurev.physiol.67.031103.153247. [DOI] [PubMed] [Google Scholar]
- Sui W, Boyd C, Wright JT. Altered pH regulation during enamel development in the cystic fibrosis mouse incisor. J Dent Res. 2003;82(5):388–92. doi: 10.1177/154405910308200512. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 2003;31(24):e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Soyombo AA, Shcheynikov N, Zeng W, Dorwart M, Marino CR, et al. Slc26a6 regulates CFTR activity in vivo to determine pancreatic duct HCO3- secretion: relevance to cystic fibrosis. EMBO J. 2006;25(21):5049–57. doi: 10.1038/sj.emboj.7601387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford GM, Angmar-Mansson B. Fluorosis-like effects of acidosis, but not NH+4, on rat incisor enamel. Caries Res. 1995;29(1):20–5. doi: 10.1159/000262035. [DOI] [PubMed] [Google Scholar]
- Wright JT, Hall KI, Grubb BR. Enamel mineral composition of normal and cystic fibrosis transgenic mice. Adv Dent Res. 1996a;10(2):270–4. doi: 10.1177/08959374960100022501. discussion 275. [DOI] [PubMed] [Google Scholar]
- Wright JT, Kiefer CL, Hall KI, Grubb BR. Abnormal enamel development in a cystic fibrosis transgenic mouse model. J Dent Res. 1996b;75(4):966–73. doi: 10.1177/00220345960750041101. [DOI] [PubMed] [Google Scholar]