Abstract
We recently reported that concentrated conditioned medium from human CD133-derived bone marrow progenitor cells (CD133 CdM) was neuroprotective after stroke. Here we identify stromal-derived factor 1 alpha (SDF-1) as a potential neuroprotective candidate in CD133 CdM by interrogating the transcriptional responses of CD133-derived MSCs (CD133dMSCs) after cell injection into the ischemic brain. Human SDF-1 mRNA was upregulated 79-fold by CD133dMSCs when injected into the stroke peri-infarct area compared with cells injected into the uninjured parenchyma of sham-operated animals. In cell protection assays, we replaced typical growth medium in mouse neural progenitor cell (mNPC) cultures with serum-free CD133 CdM immediately before exposure to hypoxia (1% oxygen) for 48 hours. CD133 CdM significantly increased the survival of mNPCs during hypoxia exposure and growth factor withdrawal. To determine whether MSC-secreted SDF-1 influenced mNPC survival, we used lentiviral short hairpin RNA (shRNA) to knockdown SDF-1 expression in CD133dMSCs. The CdM generated from shSDF-1-treated cells had a 94% decrease in secreted SDF-1 and was significantly less protective for mNPCs when compared with control CdM from MSCs transduced with scrambled shRNA. Pharmacological inhibition of the two known SDF-1 receptors, CXCR4 and CXCR7, revealed that only CXCR7 activity was functionally linked to survival signaling in mNPCs during hypoxia exposure. Treatment of mNPCs with CD133 CdM and CXCR7 inhibitor decreased mNPC viability by 36.5 ± 12.8% compared with DMSO control and decreased cell number by 21 ± 6.7% compared with DMSO control. These data indicate that SDF-1 is a key neuroprotective cytokine secreted by CD133dMSCs that protects mNPCs through CXCR7.
INTRODUCTION
Administration of multipotent stromal cells (MSCs) from bone marrow provides enhanced functional recovery in several animal models of neurological disease and injury, including stroke. Recent reports indicate that the tissue rescue/repair and functional benefits provided by MSCs are mediated, in part, through paracrine action of MSC-secreted factors [1–3]. Further support for this paracrine hypothesis comes from studies that used conditioned medium from MSCs to treat injury models, but excluded the cells [4–7]. Constituents of the MSC secretome include growth factors, cytokines, peptide hormones, and neurotrophic factors that can influence the survival of injured tissues either directly, by reducing apoptosis/necrosis, and/or indirectly by altering the inflammatory response, increasing angiogenesis, or through the mobilization of endogenous reparative cells [8–11]. Many of the active factors secreted by MSCs remain unidentified.
Changes in MSC secretion following tissue injury may indicate a response for repair by paracrine action [12–14]. Thus, proteins/peptides that increase in expression and secretion when MSCs are exposed to ischemic environments may represent neuroprotective factors that could reduce injury from stroke. Here we demonstrate that adult human bone marrow-derived multipotent stromal cells enriched by the CD133 epitope (CD133-derived MSCs, CD133dMSCs) robustly increased SDF-1 mRNA levels in response to the ischemic environment of stroke. CD133dMSCs secreted physiologically-relevant levels of SDF-1 ex vivo that protected mNPCs from hypoxia-mediated death. Of therapeutic interest, SDF-1 survival signaling was mediated through CXCR7 and not CXCR4.
METHODS
Isolation and preparation of MSCs
Isolation and preparation of CD133dMSCs was performed as previously described [5]. CD133dMSCs were cultured in Nunclon Delta–coated 150 cm2 dishes (Nunc; Thermo Fisher Scientific, Rochester, NY) with complete culture medium (CCM) containing alpha Minimum Essential Medium (alpha MEM; Invitrogen, Carlsbad, CA), 20% fetal bovine serum (lot selected for rapid growth of hMSCs; Atlanta Biologicals, Lawrenceville, GA), 100 units/ml penicillin, 100 μg/ml streptomycin, and 2 mmol/l l-glutamine (Mediatech, Herndon, VA).
Lentiviral transduction of CD133dMSCs
For cell tracking in vivo, CD133dMSCs were transduced with lentivirus to express GFP as previously reported [15]. For shRNA knockdowns, CD133dMSCs were transduced with puromycin-selectable lentivectors expressing GFP (SHC005V MISSION eGFP shRNA Control Transduction Particles), scrambled (non-targeting) shRNA (SHC002V) or sequence-specific shRNA complementary to SDF-1 (MISSION shRNA Transduction particles TRCN0000003311 and TRCN0000003312) (Sigma-Aldrich, St. Louis, MO). Transduced CD133dMSCs were selected by growth in CCM containing 2 μg/ml puromycin for 3 days, followed by expansion in CCM with 1 μg/ml puromycin for 2 weeks.
hMSC conditioned media production (CdM)
CdMs for ELISAs and mNPC bioassays were produced and concentrated as previously described [5]. 1x and 10x CdMs were thawed once at 37°C immediately prior to application to neural cultures to minimize deactivation of bioactive proteins. Protein concentrations of CdMs were measured by Bradford dye-binding assay (Bio-Rad, Hercules, CA). The protein concentration of shRNA knockdown CdM and control CdM did not differ (1× shSDF, 101.6 ± 14.1 μg/ml; 1x shScram, 92.3 ± 19.2 μg/ml).
Isolation and treatment of mNPCs
Isolation of GFP mNPCs was performed as previously described [5]. mNPCs were grown in Neural Stem Cell (NSC) growth medium containing Neurobasal A (Invitrogen), 10 ng/ml EGF, 10 ng/ml bFGF, 2 μg/ml heparin, 1 × B27 supplement (Invitrogen), 2 mM L-glutamine, and 100 units/ml Penicillin/100μg/ml Streptomycin. For bioassays, mNPCs were seeded at 20,000 cells per well on 24 well NunclonΔ plates (Nunc, Rochester, NY) that were previously coated overnight with 5 μg/ml mouse laminin and 5 μg/ml poly-D-lysine (BD Biosciences, Bedford, MA). Cells were grown for 48 hours prior to replacement with experimental mediums. For survival assays, we determined the number of viable mNPCs via cellular dehydrogenase metabolic activity (conversion of MTS to formazan) (CellTiter96™ AQueous Assay, Promega, Madison, WI). In some cases, mNPC cell number was quantified by dye-labeling of nucleic acids (CyQUANT, Invitrogen, Carlsbad, CA). Two pharmacological inhibitors were used: AMD3100 (1ug/ml in DMSO) (Sigma-Aldrich, St. Louis, MO) and CCX771 (2.5 uM in DMSO) (ChemoCentryx Inc., Mountain View, CA). Experimental medium and control treatments were performed in triplicate or quadruplicate in 24-well plates. Multi-lineage differentiation of mNPCs was performed by plating neurospheres (passage 0–2), followed by exposure the next day to differentiation medium (Neurobasal A containing 1% FBS, 100 units/ml Penicillin/100μg/ml Streptomycin, B27 supplement, 2mM L-Glutamine) for 7 days.
Mouse Middle Cerebral Artery ligation (MCAL) model
All animal work was approved by the University of Vermont College of Medicines’ Office of Animal Care in accordance with American Association for Accreditation of Laboratory Animal Care and National Institutes of Health guidelines. Twenty-four C57/Bl6 mice were anesthetized with isoflurane (1–5%, to effect) and body temperature was maintained by heating pad. Permanent distal middle cerebral artery ligation was performed as previously reported [5]. For twelve mice, the MCA was encircled with 10-0 monofilament nylon suture using a surgical needle and ligated. For the twelve mice that underwent sham surgery, the MCA was encircled with nylon suture as above, but not ligated. GFP-positive CD133dMSCs (125,000 cells resuspended in 5 μl of α-MEM medium) were injected 1 day after MCA ligation with a 30 gauge floating needle to the inferior aspect of the peri-infarct area. Sham-operated control animals were injected with the same number of GFP CD133dMSCs from the same cultures and into the analogous brain region. Animals were euthanized at 48 hours post cell injection and brains were removed for total RNA extraction.
Total RNA isolation and Real-time PCR for human-specific transcripts
Brains from sham-operated mice and those with stroke were cut into coronal sections with a scalpel and tissue block (Zivic Miller, Portersville, PA). The upper cerebral quadrants containing the infarct zone and injected cells (or analogous area in shams) were cut from the coronal sections and homogenized in a guanidine-based RNA extraction buffer (PowerGen 125, Fisher Scientific, Pittsburg, PA). Approximately 100–200 mg of tissue was homogenized in 1 ml of buffer. The extraction buffer consisted of: 4M guanidine thiocyanate (47.26 g), sodium citrate (0.30 g), anhydrous citric acid (0.35 g), N-lauryl sarcosine (0.5 g), and 1% beta-mercaptoethanol, dissolved into 100 ml (final volume) of de-ionized water. 2M sodium acetate, pH 4.0 was then added to the homogenates (1/10th of total volume). Total RNA was separated from DNA and protein by standard phenol/chloroform/isoamyl alcohol extraction (pH 4.3). The resulting RNA was DNase-treated (TurboDNase, Ambion Inc., Austin, TX).
RNA was reverse transcribed into cDNA (Superscript III, Invitrogen, Carlsbad, CA). The resulting cDNA (300 ng per sample) was used for real-time PCR reactions (7500 Fast Cycler Applied Biosystems, Foster City, CA). Because RNA samples contained both mouse brain RNA and human RNA from the injected CD133dMSCs, we used commercially validated human-specific primers and Fam-Tam probes (Taqman® Gene Expression Assays, Applied Biosystems). In addition, mouse cDNA generated from control brain samples (not containing human cells) was used to confirm the human specificity of primer sets. These controls were performed for all real-time PCR reactions along with no template controls (water). Cycle thresholds (CTs) were calculated for each transcript (auto baseline function, ABI 7500 fast PRISM software), and then normalized to human-specific GAPDH levels determined from the same samples. Relative quantifications for transcript levels were calculated by the ΔΔCT method and statistical significance was determined by student t-test (2-tailed, type 3) that compared ΔCT values between sham and stroke animals [16]. All real-time PCR reactions were performed in duplicate.
RT-PCR
mNPCs were collected with trypsin (0.25%/1mM EDTA; Mediatech, Herndon, VA) from laminin/poly-D-lysine (5 μg/ml)-coated 150 cm2 Nunclon plates, pelleted by centrifugation at 1000 × g for 8 minutes, and RNA was isolated (RNaqueous kit, Ambion Inc. Austin, TX). RNA was further purified by a second column due to the high genomic DNA content of mNPCs. Primers were designed using MIT primer design software (Primer3, http://frodo.wi.mit.edu/primer3/). CXCR7 primers were generated against mouse mRNA (C57BL/6J) sequence accession no. AK031100.1; Sense: 5′-AGT TGT GCT GCC CAC TGT CT-3′ and antisense: 5′-CTG GCC TCA ACC TCA CAG AG-3′, product size 221 bp. CXCR4 primers were generated against mouse mRNA (C57BL/6J) sequence accession no. AB000803.1; sense: 5′-AAA CCT CTG AGG CGT TTG GT-3′ and antisense: 5′-CGG TAC TTG TCC GTC ATG CT-3′, product size 294 bp. Amplification was performed by an initial denaturing step at 94°C for 5 minutes, followed by 40 cycles of 94°C for 45 seconds, 60°C for 45 seconds, 72°C for 45 seconds, and extension at 72°C for 7 minutes. PCR products were visualized by 2% agarose gel electrophoresis.
Immunocytochemistry
After differentiation, neurospheres were fixed with 4% paraformaldehyde for 15 min at room temperature and washed with PBS 3 times. Cells were blocked with blocking buffer, and incubated with primary antibodies: Beta tubulin III (1:500, PRB-435P, Covance), GFAP (1:1000, Z0334, DAKO, Glostrup, Denmark), and O4 (1:40, O7139, Sigma). After 3 PBS washes, cells were incubated in secondary antisera (Alexa Fluor 488 Alexa Fluor 350 or Alexa Fluor 594, 1:500) for 1 hour at room temperature. After 3 PBS washes, the coverglasses were counter-stained and mounted (Citifluor Antifadent Mounting Medium AF1, Electron Microscopy Sciences, Hatfield, PA).
GFP mNPCs were grown in the absence of extracellular matrix and triturated in trypsin EDTA (Mediatech, Herndon, VA) to dissociate neurospheres into single cell suspensions. mNPCs were plated at 20,000 cells/well in to 24 well plates (Nunc; Thermo Fisher Scientific, Rochester, NY) containing laminin/poly D-lysine-coated 12mm2 round glass inserts (Electron Microscopy Sciences, Hatfield, PA) and grown for 2 days in mNSC medium. These inserts were stained, inverted, and mounted onto glass slides for microscopic analyses. For staining, cells were washed with PBS and fixed in 4% paraformaldehyde for 15 minutes. Cells were incubated in blocking agent (5% goat serum, 0.4% triton X-100 in PBS) for 1.5 hours at room temperature prior to incubation with primary antibodies: CXCR7 mouse monoclonal ChemoCentryx (11G8, 10 μg/ml); CXCR4 rabbit antibody fusin (H-118) Santa Cruz SC-9046 (2 μg/ml). After 3 PBS washes, goat anti-mouse Alexa-594 and goat anti-rabbit Alexa-594 (1:800) were incubated with the cells for 1 hour to visualize CXCR7 and CXCR4, respectively. After an additional 3 PBS washes, slides were mounted with vectashield containing DAPI (Vector Laboratories, Burlingame, CA).
ELISAs
Sandwich ELISAs for SDF-1, IL-6, and HGF were performed according to the manufacturer’s instructions (R and D systems, Minneapolis, MN). Poly-HRP-streptavidin (Pierce Endogen, 1:2,000, Rockford, IL) was used in all assays for biotinylated antibody detection (1-Step™ ABTS, Pierce). Absorbance measurements for all samples and standards were performed in triplicate at 450 nm wavelength (Synergy HT, BioTek Instruments, Inc., Winooski, VT).
RESULTS
CD133dMSCs increase SDF-1 mRNA levels at the stroke penumbra
We sought to determine how MSCs react with regard to growth factor and cytokine gene expression when placed in the ischemic environment of the brain after stroke. We performed stroke surgeries in C57/Bl6 mice and injected CD133dMSCs into the inferior aspect of the peri-infarct area one day later. By epifluorescence microscopy, GFP-labeled CD133dMSCs were observed throughout the needle track just inferior to the autofluorescent stroke infarct core 48 hrs after cell injection (Fig. 1A). Brain tissue encompassing the human cells was removed and analyzed by human-specific real-time PCR assays. We examined gene expression for proteins we had previously established to be secreted from CD133dMSCs following nutrient deprivation and hypoxia exposure (simulated ischemia) for 48 hrs [5]. In the present study, gene expression for cells injected into mice with stroke was compared with that of cells injected into the non-stroke brain parenchyma of sham-operated mice (no ligation of the MCA) at 48 hrs following transplantation.
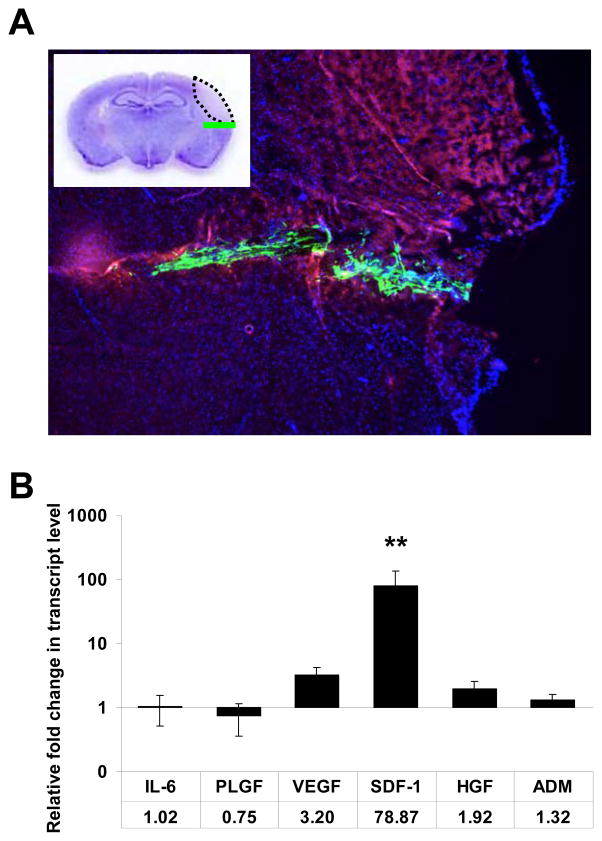
Figure 1. CD133dMSCs increase SDF-1 mRNA levels at the stroke peri-infarct area.
A) Epifluorescence image of coronal section from a representative mouse brain that underwent MCAL and was injected with 1.25 × 105 GFP CD133dMSCs the following day. Red autofluorescence indicates the stroke area and GFP CD133dhMSCs localize to the needle track in the peri-infarct area 48 hrs post-injection. Inset: Cresyl violet stain and schematic of stroke area (dotted black line) and injected cells (green line). B) Human-specific quantitative RT-PCR for mRNAs of CD133dMSC secreted-factors was performed on RNA isolated from brain tissues of mice that underwent MCAL or sham surgery three days prior. Numerical values indicate the fold change in mRNA levels for each factor in MCAL versus sham-operated mice. The mRNA level for each sample was normalized to its corresponding human GAPDH mRNA level. mRNA levels were quantified by the ΔΔCt method. Animal numbers for each factor: IL-6: 4 sham, 4 stroke; ADM: 8 sham, 8 stroke; VEGF: 5 sham, 8 stroke; SDF-1: 10 sham, 12 stroke; PLGF: 4 sham, 4 stroke; HGF: 7 sham, 6 stroke. ** P ≤ 0.01. Error bars indicate standard error of mean.
Of the genes tested, only mRNA for SDF-1 was significantly upregulated by CD133dMSCs (78.9-fold, stroke n=12, sham n=10, P=0.008; Fig. 1B). Although the mRNA levels for VEGF increased by 3.2-fold, this response was not statistically significant (P = 0.068). The mRNA levels for the other secreted factors did not change significantly. As a result, we continued to investigate the role of SDF-1 as a stem cell-derived neuroprotective agent ex vivo. CD133dMSCs that were serum-starved and exposed to hypoxic culture conditions for 48 hrs also increased SDF-1 mRNA levels compared with control cultures (2.15-fold, P = 0.0014) (data not shown).
Multipotent mNPCs express both SDF-1 receptors
mNPCs were grown as spheres and then differentiated for 7 days (Fig. 2A). Triple staining immunocytochemistry was performed to indicate the formation of neurons (Beta-III tubulin), astrocytes (GFAP), and oligodendrocytes (O4) (Fig. 2A). Staining clearly demonstrated the presence of the three committed lineages derived from the mNSCs.
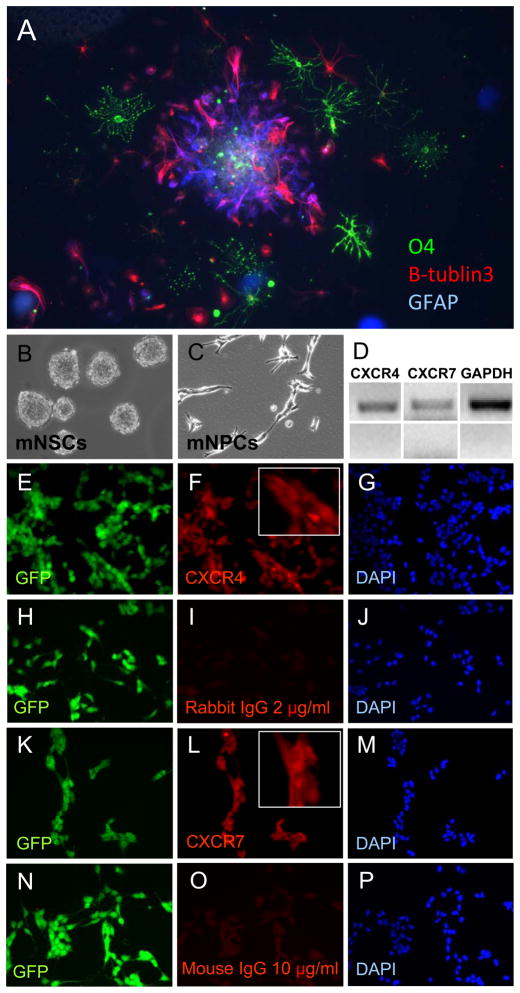
Figure 2. Expression of CXCR4 and CXCR7 by mNPCs.
A) Mouse NPCs are multipotent. Triple staining is shown for astrocytes (GFAP, blue), neurons (Beta3 tubulin, red), and oligodendrocytes (O4, green). B) Floating neural spheres prior to trituation to single cell suspension. C) After trituation, the single cell suspension was plated and expanded as neural progenitor cells (NPCs). D) RT-PCR for mRNAs of CXCR4, CXCR7, and GAPDH. Bottom panels show no reverse transcriptase controls for each primer set. E-G) Immunocytochemistry for CXCR4. H-J) Isotype controls for CXCR4 (rabbit isotype). K-M) Immunocytochemistry for CXCR7. N-P) Isotype controls for CXCR7 (mouse isotype).
To determine the expression of SDF-1 receptors in the mNPCs, we performed RT-PCR assays and immunocytochemistry for CXCR4 and CXCR7. Both mRNAs were detected and mNPCs grown as a monolayer in NSC growth medium stained positive for both receptors (Fig. 2B-P).
Lentiviral shRNA expression to deplete SDF-1 secretion from CD133dMSCs
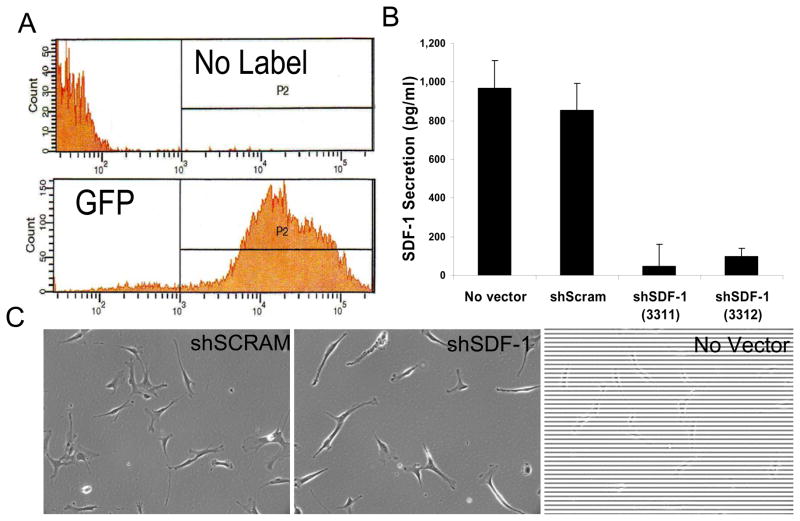
Lentiviral transduction of CD133dMSCs with shRNA specific to SDF-1 reduced the levels of secreted SDF-1 by 94% (shSDF-1 CdM) compared with secretion from cells transduced with non-targeting shRNA (scrambled shRNA, shScram CdM) or no lentivector (ELISAs, P < 0.001, Fig. 3). To control for possible off-target effects of shRNA expression on other secreted proteins, we performed ELISAs for IL-6 and HGF. The levels of IL-6 and HGF contained in shSDF-1 CdM and shScram CdM did not differ (IL-6: shSDF-1, 365 ± 31 pg/ml vs. shScram 202 ± 81 pg/ml, P = 0.183; HGF: shSDF-1, 1,287 ± 99 pg/ml vs. shScram 1,672 ± 163 pg/ml, P = 0.128). Subsequently, CdMs generated from the transduced cells were used in ex vivo bioassays.
Figure 3. Knockdown of SDF-1 secretion in CD133dMSCs by lentiviral shRNA.
CD133dMSCs were transduced with puromycin-selectable lentivectors expressing GFP (transduction control), scrambled (non-specific) shRNA or sequence-specific shRNA (against SDF-1). A) FACS of control cells (no label) and those transduced with GFP lentivector and selected by puromycin to determine cell purity after selection regime. B) ELISA for SDF-1 showed a 94% and 89% reduction in level of secreted SDF-1 from CD133dMSCs after lentiviral transduction of 2 different short hairpin RNA sequences for the SDF-1 transcript compared with a scrambled vector. SDF-1 secretion levels were not different from cells that received the scrambled vector and cells that were not transduced (no vector control). All measurements were performed in triplicate. C) Phase contrast microscopy images of CD133dMSCs that received lentivectors and no vector control cells (magnification 10x).
SDF-1 knockdown diminished the neuroprotective effect of CD133 CdM
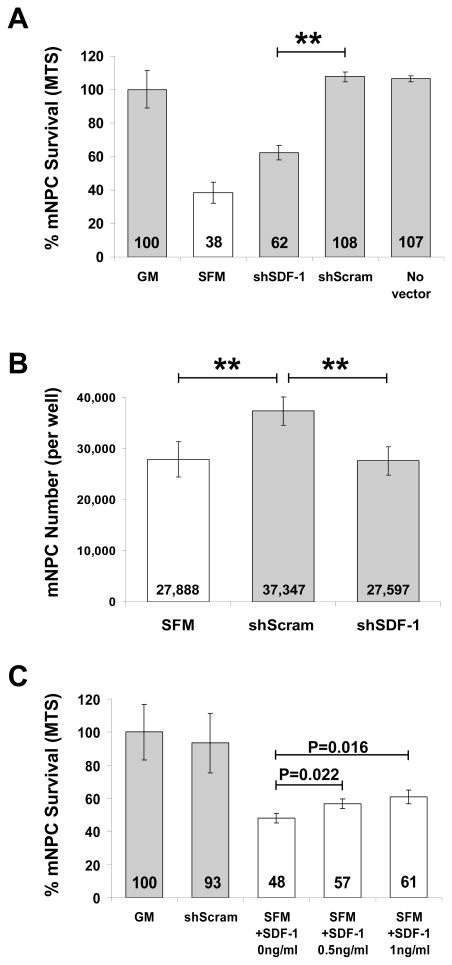
mNPCs were challenged by hypoxia exposure (1% O2) and growth factor withdrawal by replacement of NSC growth medium (GM) with serum-free α-MEM (SFM), shSDF-1 CdM (10x), or shScram CdM (10x), and cultured for 48 hours. shSDF-1 CdM had a significantly reduced capacity for neural cell protection compared with shScram CdM (62.3 ± 7.4% vs. 107.8 ± 5.1%, P=0.0016, GM standardized to 100%, Fig. 4A). The level of mNPC protection conferred by shSDF-1 CdM was still greater from that provided by SFM (62.3 ± 7.5% versus 38.3 ± 10.7%, P = 0.039).
Figure 4. SDF-1 knockdown diminishes the protective effect of CdM.
A, B) mNPC survival was significantly reduced after 48 hrs of hypoxic culture when treated with shSDF-1 CdM compared with shScram CdM. C) Addition of recombinant SDF-1 to alpha MEM containing 20ug/ml heparin increased mNPC survival in a dose-response manner: 0.5 ng/ml SDF-1: 18.3 ± 5.9% increase, P = 0.022; 1 ng/ml SDF-1: 26.7 ± 8.8% increase, P = 0.016. N.S., not significant; *P ≤ 0.05; **P ≤ 0.01; †P ≤ 0.01. GM, NSC growth medium. SFM, serum-free alpha MEM (base medium).
By phase microscopy, the cells incubated in shScram CdM were less numerous, larger and appeared to be “more differentiated”; they had more cellular processes than did mNPCs cultured in GM (data not shown). The MTS assay we used to assess cell survival measured cell metabolic viability by dehydrogenase enzyme activity, which may be altered by the state of mNPC differentiation. To more thoroughly ascertain the effects of the different CdMs, in parallel assays we determined mNPC number by dye-labeling of nucleic acid content (CyQUANT assay). Similar to the results obtained with MTS, mNPC number was greater after incubation in shScram CdM compared with culture in shSDF-1 CdM or SFM (shScram: 37,347 vs. shSDF-1: 27,597, P = 0.0024; and shScram: 37,347 vs. SFM: 27,888, P = 0.0056). Cell numbers did not differ between cultures that received shSDF-1 CdM and SFM (P = 0.899) (Fig. 4B).
We next tested whether addition of recombinant SDF-1 to shSDF-1 CdM could restore its ability to protect mNPCs. The amount of SDF-1 added to shSDF-1 CdM made its SDF-1 concentration equivalent to that of shScram CdM (8 ng/ml, as assayed by ELISA, see Fig. 3B). After the addition of recombinant SDF-1, the level of protection conferred by shSDF-1 CdM was no longer statistically different from that of shScram CdM (93.7 ± 7.9% vs. 100 ± 7.6%, P= 0.381, data not shown). Furthermore, addition of recombinant SDF-1 to SFM containing 20 μg/ml heparin increased mNPC viability in a dose-responsive manner (0.5 ng/ml, P = 0.022, 1 ng/ml, P = 0.016; Fig. 4C). Interestingly, shSDF-1 CdMand shScram CdM provided similar levels of neuroprotection during growth factor withdrawal in normoxic conditions (21% O2), suggesting that secreted SDF-1 in CD133 CdM was important for mNPC survival signaling specifically in the context of hypoxia (data not shown).
CXCR7 provides the neuroprotection conferred by SDF-1 in CD133 CdM
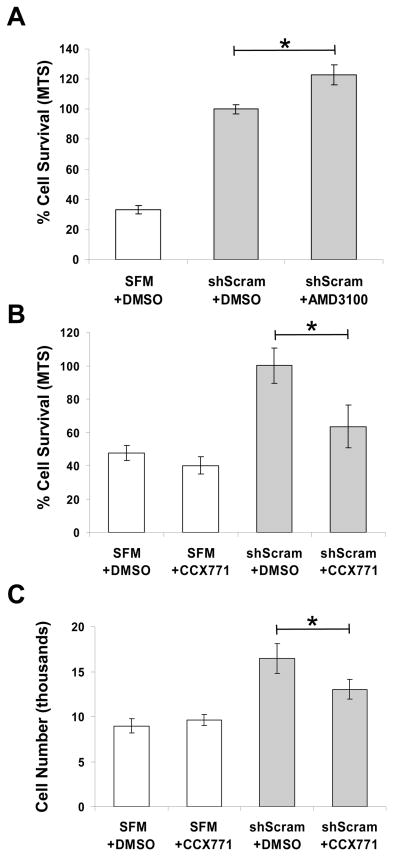
To determine the relative importance of CXCR4 and CXCR7 in mediating mNPC survival, we tested inhibitors of CXCR4 (AMD3100) and CXCR7 (CCX771) in our cell protection assay. mNSC GM was replaced with shScram CdM that contained either inhibitor or an equal amount of DMSO (vehicle control). To control for toxicity, CCX771 was added to mNPCs incubated in SFM alone. Contrary to our expectation that addition of AMD3100 to shScram CdM would decrease cell survival, by MTS assay the inhibitor instead increased cell survival by 22.7 ± 6.7% (P = 0.0157) (Fig. 5A). In contrast, addition of the CXCR7 inhibitor CCX771 to shScram CdM decreased mNPC viability by 36.5 ± 12.8% compared with shScram control (P = 0.021) and decreased cell number by 21 ± 6.7% (P = 0.049) (Fig. 5B and C). In toxicity controls, CCX771 did not significantly decrease mNPC viability or cell number when added to SFM (Fig. 5B and C).
Figure 5. Selective inhibition of CXCR7 but not CXCR4 diminishes the neuroprotection conferred by shScram CdM.
A) Addition of CXCR4 pharmacological inhibitor AMD3100 to shScram CdM increased mNPC survival by 22.7± 6.7% compared with DMSO control after 48 hrs in hypoxia (P = 0.0157). B, C) Addition of CXCR7 pharmacological inhibitor CCX771 to shScram CdM decreased mNPC survival by 36.5± 12.8% (B: P = 0.021) and mNPC number by 21 ± 6.7% (C: P = 0.049). mNPC viability and number was not different for cultures exposed to SFM with CCX771 or DMSO control. *P = 0.05.
DISCUSSION
We found that CD133dMSCs significantly increased expression of SDF-1 mRNA when transplanted into brain tissue injured by stroke. To our knowledge, this study is the first to analyze the transcriptional behavior of human MSCs within the stroke environment. CD133dMSCs increased SDF-1 mRNA levels ~80-fold in ischemic vs. control brains after stroke. In contrast, cultured CD133dMSC SDF-1 transcript levels increased only 2.15-fold after growth factor withdrawal and an equal amount of time in hypoxia (1% O2), indicating that other variables in vivo affect SDF-1 expression by CD133dMSCs. Importantly, the inflammation and/or injury caused by the needle during cell injection into the brain occurred in both sham-operated mice and those with stroke. Therefore, the changes in SDF-1 gene expression we detected were specific to the environment of cerebral ischemia.
Through cell protection assays in culture we showed that SDF-1 is one of the neuroprotective factors in CD133 CdM. SDF-1/CXCL12 is a member of the cytokine family of chemokines with attractant effects on cells that harbor the fusin/CXCR4 receptor, notably hematopoietic cells and uncommitted neural cells. The SDF-1/CXCR4 axis has been shown to induce hematopoietic cell mobilization and migration [17], as well as adhesion [18], engraftment [19], trans-endothelial migration [20], retention in the bone marrow [21], and the modulation of hematopoiesis [22]. Since many leukocytes commonly possess CXCR4, SDF-1 also displays pro-inflammatory properties, mobilizing cells of the innate immune response to the ischemic brain [23]. The presence of CXCR4 on embryonic and fetal neural cells is critical for the anatomical development of the mammalian central nervous system [24]. Following cerebral ischemia in adults, CXCR4 has been shown to mediate neural stem/progenitor cell migration to SDF-1-rich sites in the brain [25–27].
SDF-1 signaling has anti-apoptotic effects in stem/progenitor cells from the bone marrow [28], vascular endothelium [29], kidney [30], pancreas [31], and the inner cell mass of embryos [32]. SDF-1-based survival signaling was recently shown for primary cortical neurons [27], and its ability to protect primary NPCs from hypoxic injury was demonstrated here. CXCR7 is a second and recently deorphanized SDF-1 receptor that appears to mediate anti-apoptotic SDF-1 signaling [30].
We report here that CXCR7 and not CXCR4 promoted the survival of mNPCs. Furthermore, instead of reducing mNPC survival during hypoxia exposure, CXCR4 antagonism (AMD3100) increased it. Of note, AMD3100 was recently reported to act as an agonist of CXCR7 [33]. CXCR7 expression in the brain has been shown to increase after stroke [34]. It will be of particular interest to determine whether a selective agonist of CXCR7 can provide neuroprotection after stroke.
Acknowledgments
We thank Thomas Buttolph, University of Vermont, Burlington, VT for real-time PCR instrument support. We thank Dr. Thomas J. Schall of ChemoCentryx, Inc. Mountain View, CA for providing the CXCR7 inhibitor and antibody used in this study. This work was supported by grants from the National Institutes of Health: NHLBI HL085210 (JLS) and NIH/NCRR P20 RR016435 (Parsons R, COBRE PI, JLS, PI project 3).
Footnotes
AUTHOR DISCLOSURE STATEMENT
No competing financial interests exist.
Contributor Information
Benjamin Bakondi, Email: bbakondi@uvm.edu.
Issei S. Shimada, Email: ishimada@uvm.edu.
Brittni M. Peterson, Email: BMPETERSON4@stthomas.edu.
Jeffrey L. Spees, Email: jspees@uvm.edu.
References
- 1.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109:1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 2.Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, Semprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iso Y, Spees JL, Serrano C, Bakondi B, Pochampally R, Song YH, Sobel BE, Delafontaine P, Prockop DJ. Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun. 2007;354:700–6. doi: 10.1016/j.bbrc.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakondi B, Shimada IS, Perry A, Munoz JR, Ylostalo J, Howard AB, Gregory CA, Spees JL. CD133 identifies a human bone marrow stem/progenitor cell sub-population with a repertoire of secreted factors that protect against stroke. Mol Ther. 2009;17:1938–47. doi: 10.1038/mt.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 7.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20:661–9. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 8.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–84. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 9.Kinnaird T, Stabile E, Burnett MS, Epstein SE. Bone-marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ Res. 2004;95:354–63. doi: 10.1161/01.RES.0000137878.26174.66. [DOI] [PubMed] [Google Scholar]
- 10.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 11.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isele NB, Lee HS, Landshamer S, Straube A, Padovan CS, Plesnila N, Culmsee C. Bone marrow stromal cells mediate protection through stimulation of PI3-K/Akt and MAPK signaling in neurons. Neurochem Int. 2007;50:243–50. doi: 10.1016/j.neuint.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Katakowski M, Li Y, Lu D, Wang L, Zhang L, Chen J, Xu Y, Gautam S, Mahmood A, Chopp M. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: growth factor production. J Neurosci Res. 2002;69:687–91. doi: 10.1002/jnr.10334. [DOI] [PubMed] [Google Scholar]
- 14.Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, Chen X, Chopp M. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27:355–63. doi: 10.1111/j.1440-1789.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–8. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–20. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759–69. doi: 10.1016/s1074-7613(00)00074-1. [DOI] [PubMed] [Google Scholar]
- 19.Peled A, Petit I, Kollet O, Magid M, Ponomaryov T, Byk T, Nagler A, Ben-Hur H, Many A, Shultz L, Lider O, Alon R, Zipori D, Lapidot T. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–8. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 20.Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–30. [PubMed] [Google Scholar]
- 21.Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, Hardan I, Deutsch V, Gazit Z, Gazit D, Karlsson S, Lapidot T. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–9. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 23.Stumm RK, Rummel J, Junker V, Culmsee C, Pfeiffer M, Krieglstein J, Hollt V, Schulz S. A dual role for the SDF-1/CXCR4 chemokine receptor system in adult brain: isoform-selective regulation of SDF-1 expression modulates CXCR4-dependent neuronal plasticity and cerebral leukocyte recruitment after focal ischemia. J Neurosci. 2002;22:5865–78. doi: 10.1523/JNEUROSCI.22-14-05865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–48. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- 25.Imitola J, Raddassi K, Park KI, Mueller FJ, Nieto M, Teng YD, Frenkel D, Li J, Sidman RL, Walsh CA, Snyder EY, Khoury SJ. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–22. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O. Persistent production of neurons from adult brain stem cells during recovery after stroke. Stem Cells. 2006;24:739–47. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- 27.Shyu WC, Lin SZ, Yen PS, Su CY, Chen DC, Wang HJ, Li H. Stromal cell-derived factor-1 alpha promotes neuroprotection, angiogenesis, and mobilization/homing of bone marrow-derived cells in stroke rats. J Pharmacol Exp Ther. 2008;324:834–49. doi: 10.1124/jpet.107.127746. [DOI] [PubMed] [Google Scholar]
- 28.Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105:3793–801. doi: 10.1182/blood-2004-11-4349. [DOI] [PubMed] [Google Scholar]
- 29.Zheng H, Dai T, Zhou B, Zhu J, Huang H, Wang M, Fu G. SDF-1alpha/CXCR4 decreases endothelial progenitor cells apoptosis under serum deprivation by PI3K/Akt/eNOS pathway. Atherosclerosis. 2008;201:36–42. doi: 10.1016/j.atherosclerosis.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, Parente E, Mancina R, Netti GS, Becherucci F, Gacci M, Carini M, Gesualdo L, Rotondi M, Maggi E, Lasagni L, Serio M, Romagnani S, Romagnani P. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205:479–90. doi: 10.1084/jem.20071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kayali AG, Van Gunst K, Campbell IL, Stotland A, Kritzik M, Liu G, Flodstrom-Tullberg M, Zhang YQ, Sarvetnick N. The stromal cell-derived factor-1alpha/CXCR4 ligand-receptor axis is critical for progenitor survival and migration in the pancreas. J Cell Biol. 2003;163:859–69. doi: 10.1083/jcb.200304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo Y, Hangoc G, Bian H, Pelus LM, Broxmeyer HE. SDF-1/CXCL12 enhances survival and chemotaxis of murine embryonic stem cells and production of primitive and definitive hematopoietic progenitor cells. Stem Cells. 2005;23:1324–32. doi: 10.1634/stemcells.2005-0085. [DOI] [PubMed] [Google Scholar]
- 33.Kalatskaya I, Berchiche YA, Gravel S, Limberg BJ, Rosenbaum JS, Heveker N. AMD3100 is a CXCR7 ligand with allosteric agonist properties. Mol Pharmacol. 2009;75:1240–7. doi: 10.1124/mol.108.053389. [DOI] [PubMed] [Google Scholar]
- 34.Schonemeier B, Kolodziej A, Schulz S, Jacobs S, Hoellt V, Stumm R. Regional and cellular localization of the CXCl12/SDF-1 chemokine receptor CXCR7 in the developing and adult rat brain. J Comp Neurol. 2008;510:207–20. doi: 10.1002/cne.21780. [DOI] [PubMed] [Google Scholar]