Abstract
This study describes the in vivo biocompatibility of intra-articular poly(DL-lactic-co-glycolic acid) (PLGA) microparticle (MP) formulations in the rat temporomandibular joint (TMJ). To our knowledge, this is the first intra-articular microparticle-based drug delivery system for the TMJ. The impact of PLGA MP concentration on rat TMJ function was quantified via computerized meal pattern analysis; in this non-invasive technique, previously validated markers of TMJ pain (specifically, meal duration and food intake) were recorded using computer-monitored pellet feeders. Bilateral intra-articular injection of 15, 30, or 50 mg/mL PLGA MPs had no impact on meal duration or food intake over six days, compared to controls that did not receive injections. Histological analysis showed that the MPs were retained within the synovial lining. These findings indicate that the PLGA MPs described herein are biocompatible and suitable for intra-articular delivery to the rat TMJ, a finding that has significant implications for the improvement of TMJ therapeutics.
Keywords: Temporomandibular Joint, Temporomandibular Disorder, Intra-articular, Drug delivery, Microparticle
INTRODUCTION
Temporomandibular joint (TMJ) disorders are a heterogeneous group of diseases that cause pain (Tanaka et al., 2008), resulting in an estimated $4 billion per year in treatment costs in the United States. Unlike other joint diseases, TMJ disorders have a higher incidence in adolescents and young adults (National Institute of Dental and Craniofacial Research (NIDCR) Data & Statistics, 2008). Effective pain reduction and restoration of TMJ function remain unmet challenges (Mountziaris et al., 2009; Tanaka et al., 2008). In cases of TMJ degeneration, intra-articular injections of corticosteroids and hyaluronic acid are used to treat pain. However, these intra-articular formulations are complicated by rapid clearance of injected agents, so that frequent injections are needed, increasing the risk of iatrogenic injury (Mountziaris et al., 2009; Tanaka et al., 2008). There is a need for sustained release formulations to effectively alleviate TMJ pain.
Over the past two decades, microcarrier-based drug delivery systems, particularly polymeric microparticles, have been evaluated as novel strategies for intra-articular controlled release in animal models of knee joint disorders [recent reviews, (Butoescu et al., 2009a; Mountziaris et al., 2009)]. Poly(DL-lactic-co-glycolic acid) (PLGA) microparticles (MPs) have been successfully applied for intra-articular controlled release in the knee joints of rabbits, rats, and mice (Butoescu et al., 2009b; Horisawa et al., 2002; Liggins et al., 2004; Tuncay et al., 2000). However, to our knowledge, no intra-articular microparticle-based system has yet been evaluated in the TMJ (Mountziaris et al., 2009).
In this study, we used an established double emulsion solvent extraction technique to generate PLGA MPs previously shown to be capable of controlled release of bioactive molecules including proteins (Cleek et al., 1997; Hedberg et al., 2004; Patel et al., 2008). We evaluated the in vivo biocompatibility of these PLGA MPs following bilateral intra-articular injection into rat TMJs using a non-invasive method, computerized meal pattern analysis. This technique, whose development was inspired by the painful, impaired eating in human patients with TMJ disorders, has been used to evaluate the effect of various analgesic agents on TMJ pain (Bellinger et al., 2007; Harper et al., 2000; Kerins et al., 2003; 2004; Kerins et al., 2005). Increased meal duration has been shown to be a specific marker of TMJ pain (Kerins et al., 2005), and reduced food intake is often associated with this trend, e.g., due to exacerbation of pain by chewing (Harper et al., 2000).
This study addresses the following questions: (i) Are PLGA MPs biocompatible in vivo following intra-articular injection into the TMJ, and (ii) what is the impact of intra-articular PLGA MP concentration on TMJ function? We hypothesized that all concentrations of intra-articular PLGA MPs would be well tolerated in healthy rat TMJs. Our ultimate goal was to determine the suitability of PLGA MPs for intra-articular sustained release in the rat TMJ.
MATERIALS & METHODS
PLGA microparticle preparation and characterization
PLGA microparticles consisting of a physical blend of 5% w/w poly(ethylene glycol) (PEG; nominal molecular weight of 4600; Aldrich, Milwaukee, WI) in PLGA with a copolymer ratio of 50:50 (Lakeshore Biomaterials, Birmingham, AL) were synthesized using a previously described double emulsion solvent extraction technique (Cleek et al., 1997; Hedberg et al., 2004; Patel et al., 2008). For fluorescent MPs, 27 μL of Vybrant DiI (Molecular Probes, Carlsbad, CA) were added prior to generating the first emulsion. To prevent quenching of the fluorescent dye, all vials were wrapped with aluminum foil and indirect lighting was used. The number average molecular weight (Mn) of the PLGA 50:50 was 42500 ± 1600 Da and its polydispersity index was 1.53 ± 0.03, determined via gel permeation chromatography (n = 3 samples; Phenogel Linear Column, Phenomenex, Torrance, CA; Differential Refractometer 410, Waters, Milford, MA) using a polystyrene standard curve (Fluka, Switzerland). MP diameter was determined using a Multisizer3 Coulter Counter (Beckman Coulter, Fullerton, CA). MP morphology was observed via scanning electron microscopy (SEM; FEI Quanta 400 Environmental, Hillsboro, OR) at 15 kV accelerating voltage, after coating the MPs with a thin layer of gold.
In vivo microparticle localization
All rat studies were approved by the Institutional Animal Care and Use Committee and adhered to the National Institutes of Health guidelines. Before assessing the impact of intra-articular MPs on TMJ function, a pilot study was conducted to localize the MPs immediately after injection. Pre-weighed fluorescent PLGA MPs were sterilized via exposure to ethylene oxide gas for 14h, aired out for 24h, and then suspended at 50 mg/mL in Microfil (Flowtech, Carver, MA), which was prepared in a 4:5 Microfil:diluent volume ratio with 5% v/v curing agent. Two adult male Sprague-Dawley rats (Harlan Industries, Houston, TX) (250–300g) were anesthetized with isoflurane (5% v/v in oxygen) and received bilateral 50 μL TMJ injections into the superior joint space according as previously described (Bellinger et al., 2007; Harper et al., 2000; Kerins et al., 2005). All rats were euthanized immediately afterward with a carbon dioxide overdose. The Microfil was then allowed to cure for 3–4 h, so that it would encase the fluorescent dye-loaded PLGA MPs. Each TMJ was then removed en bloc, fixed in 10% v/v formalin for two days, and embedded in acrylic. Tissue sections (500 μm) were not stained to prevent quenching of the fluorescent dye. Phase contrast and fluorescence (excitation: 515–575 nm; emission: 560–680 nm) images were captured using a Nikon Eclipse 80i microscope (Melville, NY) with a Photometrics CoolSNAP K4 camera (Tucson, AZ) and Metamorph software (Sunnyvale, CA).
Meal pattern analysis
35 healthy adult male Sprague-Dawley rats (Harlan Industries) (250–300g) were housed in previously described computer-activated pellet feeder cages (Harper et al., 2000; Kerins et al., 2005). After 2 days to acclimate, 32 rats received bilateral 50 μL TMJ injections as described above. The injected material consisted of 15, 30, or 50 mg sterile PLGA MPs (n = 8 rats/group) per mL of sterile 10% v/v Tween 80 (Sigma, St. Louis, MO) in normal (0.9% w/v) saline. Tween 80 was necessary to prevent MP aggregation, which enabled aspiration and injection with a fine bore (25 gauge) needle that minimized iatrogenic TMJ damage. The remaining rats received carrier solution only (n = 8) or (n = 3) had no injections.
Meal duration and food intake were recorded for 6 days after injection using the computer-activated pellet feeders, as previously described (Bellinger et al., 2007; Harper et al., 2000). After 6 days, all rats were deeply anesthetized (9 mg ketamine and 1.5 mg Rompun per 100 g body weight) and perfused with 4% w/v paraformaldehyde in PBS (pH 7.0). The TMJs were removed en bloc and demineralized using an established microwave-accelerated technique (Ekuni et al., 2006). Sagittal cryosections (20μm) were imaged using a Zeiss Axioplan microscope (Thornwood, NY) with a Diagnostic Instrument Inc., Spot CCD camera (Sterling Heights, MI).
Statistical Analysis
Meal pattern data are reported as mean ± standard error of the mean, and represent a percentage of the average pre-injection value. Data were analyzed using two-way analysis of variance (p < 0.05), followed by Bonferroni post-hoc analysis (p < 0.05).
RESULTS
PLGA MP morphology
The MPs had a diameter of 22 ± 7 μm (mean ± standard deviation; n = 1500 MPs). SEM imaging (Fig. 1d) confirmed that they had spherical morphology and size consistent with Coulter counter measurements.
Figure 1.
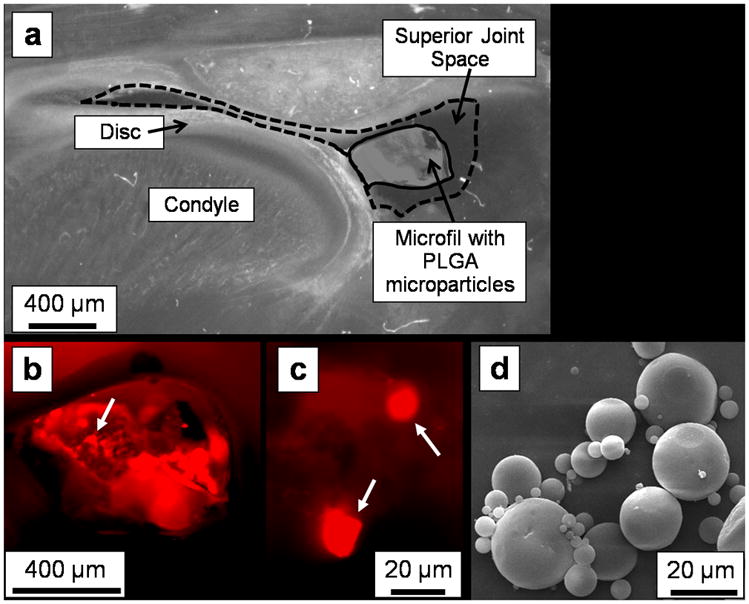
Phase contrast and fluorescence microscopy images indicated successful injection of fluorescent-dye-loaded PLGA microparticles (MPs) into the superior joint space. In this representative unstained histological section from a rat euthanized immediately after injection (2.5× magnification), (a) the Microfil (solid outline) containing the PLGA MPs is visible within the superior joint space (dashed outline) via phase contrast imaging. A higher magnification (4×) fluorescent image of this same section confirms that (b) the fluorescent-dye-loaded MPs are contained within the Microfil (arrow points to an area with MPs), with (c) individual MPs (white arrows) visible within the Microfil at 40× magnification. A scanning electron micrograph (d) of the spherical PLGA microparticles is included for comparison.
In vivo microparticle localization
Injections of the fluorescent-dye loaded PLGA MPs were successfully made into the superior joint space of the TMJ. The hardened Microfil (Fig. 1a, solid outline) held the MPs in place within the superior joint space (Fig. 1a, dashed outline) during tissue processing. By fluorescence microscopy, the dye-loaded microparticles were visible within the pores of the Microfil (Fig. 1b), and individual MPs could be resolved at higher magnification (Fig. 1c).
Impact of intra-articular microparticles on meal patterns
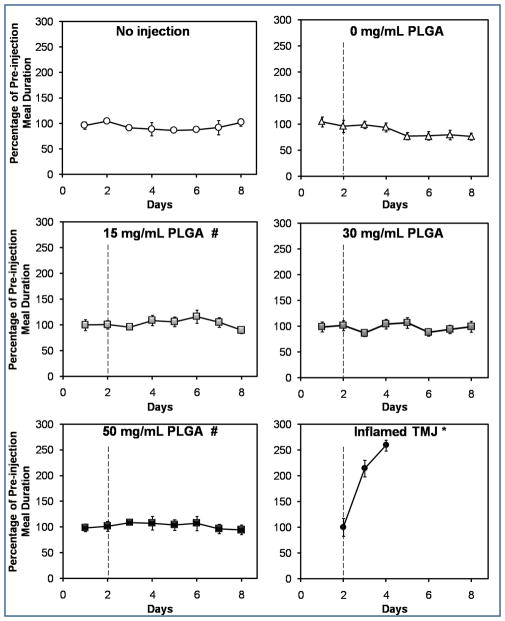
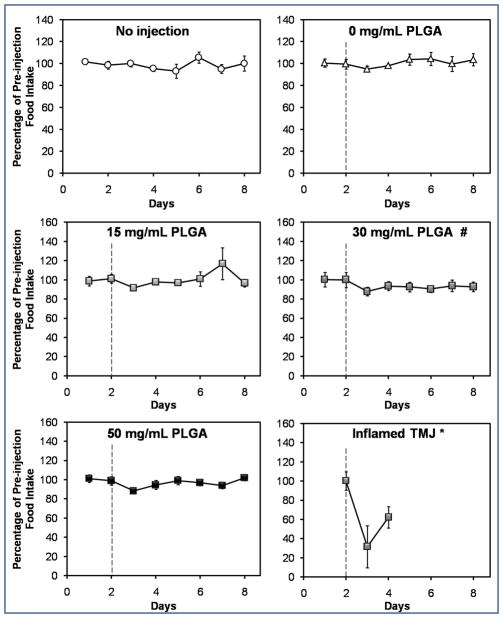
Intra-articular injection of PLGA MPs did not significantly increase (p > 0.05) meal duration (Fig. 2a) or decrease (p > 0.05) food intake (Fig. 2b) compared to baseline. These findings are particularly striking when compared to the “Inflamed TMJ *” values (Fig. 2a,b), which represent the previously reported response following bilateral injection of complete Freund’s adjuvant (CFA). The 0 mg/mL PLGA control differed significantly (p < 0.05) from the 15 and 50 mg/mL PLGA groups in terms of meal duration (Fig. 2a), and from the 30 mg/mL PLGA group in terms of food intake (Fig. 2b). However, none of the groups (Fig. 2a,b) differed significantly from the “No injection” control (p > 0.05).
Figure 2.
Intra-articular microparticles did not induce TMJ pain, based on (a) meal duration, and (b) food intake measurements for two days prior and six days following intra-articular injection of 15, 30, or 50 mg/mL PLGA microparticle formulations (abbreviated “PLGA”). A dashed line indicates the time of injection. The upper panels of both (a) and (b) depict the two control groups, consisting of rats that did not receive any injections (“No injection”), and rats where only the carrier fluid was injected (“0 mg/mL PLGA”). (a) Meal duration differed significantly between the 0 mg/mL PLGA group and both the 15 mg/mL PLGA and 50 mg/mL PLGA groups (p < 0.05; indicated by #). However, none of the groups differed from the “No injection” negative control (p > 0.05). Meal duration data from a previous study where rat TMJ inflammation was induced via bilateral injection of a pro-inflammatory agent (15 μg CFA/joint; “Inflamed TMJ *” group) are included in (a) for comparison. In that study, a significant increase (p < 0.05) in meal duration was observed compared to the baseline pre-injection value (Bellinger et al., 2007). None of the groups in this study showed a significant increase in meal duration compared to baseline (p > 0.05). (b) Food intake differed significantly (p < 0.05) between the 30 mg/mL PLGA group and both the 15 mg/mL PLGA experimental group and the 0 mg/mL PLGA control group. However, none of the groups differed from the “No injection” negative control (p > 0.05). Food intake results from a previous study where rat TMJ inflammation was induced via bilateral injection of a pro-inflammatory agent (300 μg CFA/joint; “Inflamed TMJ *” group) are included in (b) for comparison. In that study, a significant decrease (p < 0.05) in food intake was observed compared to the baseline pre-injection value (Harper et al., 2000). None of the groups in the present study showed a significant decrease in food intake compared to the baseline values (p > 0.05). Values for each group are expressed as a percentage of the average pre-injection value. Data points represent the mean ± standard error of the mean for n = 8 rats (or n = 3 rats in the “No injection” group). Error bars are included for all groups, though they are too small to resolve in some cases. Groups that differ significantly (p < 0.05) from “0 mg/mL PLGA” are indicated by #.
In vivo biocompatibility of intra-articular microparticles
Injection of the carrier solution induced a mild inflammatory infiltrate, and no additional tissue reaction was observed in the 15, 30, or 50 mg/mL PLGA groups. The MPs were embedded within the posterior synovial tissue (arrows, Fig. 3) and were surrounded by leukocytes (hematoxylin-stained blue nuclei, Fig. 3b,c) that often had the nuclear morphology of macrophages.
Figure 3.
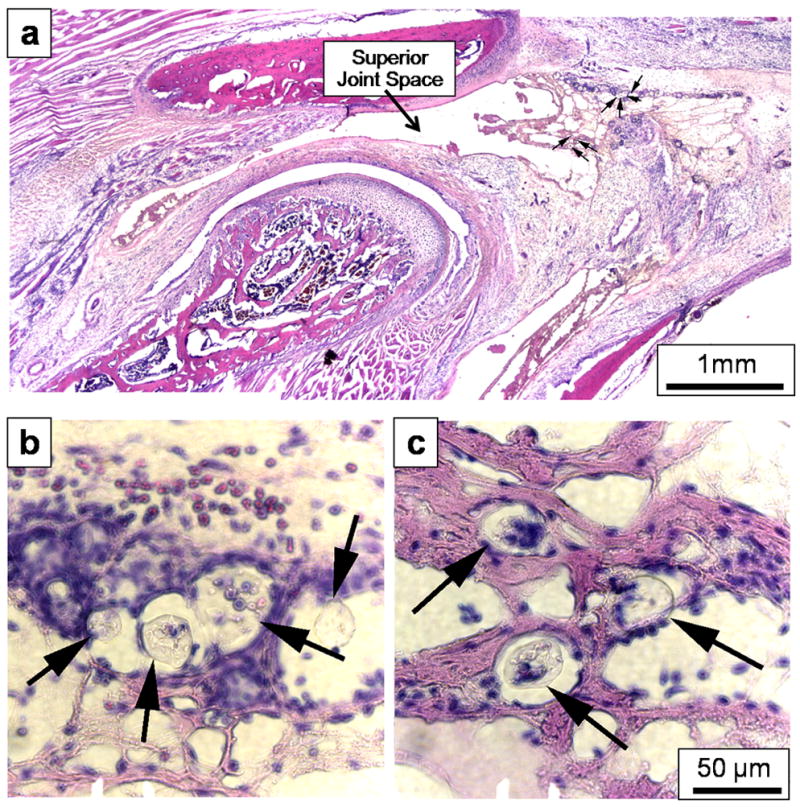
Representative images from the 30 mg/mL PLGA group indicating (a) the location of the PLGA MPs (arrows), which were primarily embedded within the synovial tissue lining the posterior surface of the superior joint space, adjacent to the TMJ vasculature. The specific MPs identified in (a) are shown at higher (40×) resolution in (b) and (c), again indicated by arrows. The MPs were surrounded by inflammatory cells (blue nuclei), but were too large to be engulfed by these cells. All histological sections shown were stained with hematoxylin and eosin. The 50 μm error bar in (c) also applies to (b). The original magnification of (a) was 2.5×.
DISCUSSION
In this study, we report the development and in vivo biocompatibility of a PLGA MP formulation for intra-articular drug delivery in the rat TMJ. Although microparticle-based controlled release systems exist for various applications, to our knowledge, this is the first such system for intra-articular delivery in the TMJ. Due to the lack of previous data regarding the TMJ tissue response to such systems (Mountziaris et al., 2009), this study utilized blank PLGA MPs injected into healthy rat TMJs. Another reason for this cautious approach was the terrible history of early alloplastic (Proplast-Teflon) TMJ implants, whose susceptibility to mechanical failure gave rise to Teflon microparticles that triggered severe immune reactions and catastrophic TMJ damage (Mercuri and Giobbie-Hurder, 2004).
PLGA MPs were selected based on their successful intra-articular application in rodent and rabbit knees (Butoescu et al., 2009b; Horisawa et al., 2002; Liggins et al., 2004; Tuncay et al., 2000). Although knee joint results cannot be directly applied to the TMJ due to significant differences amongst the two joints (Herring, 2003), we hypothesized that the tissue response would be similar in both cases. MPs (22 ± 7 μm diameter; Fig. 1d) were selected over smaller particles based on a previous report that similar PLGA MPs (27 ± 9 μm diameter) were retained in rat knee joint synovial tissue, while PLGA nanoparticles (265 ± 15 nm) were not. The nanoparticles were phagocytosed by macrophages responding to the injury associated with injection. Within a week, these macrophages migrated away from the synovial lining, taking the nanoparticles along (Horisawa et al., 2002). We chose MPs in order to maximize retention within the TMJ. At the same time, PLGA MP diameter was kept small (~20 μm) to facilitate delivery with the thin needle necessary to minimize iatrogenic injury.
The ability to deliver a high concentration of microparticles is important for establishing a depot capable of delivering a sufficient drug dose. The highest dose of MPs (50 mg/mL) was the maximum achievable suspension that could be reproducibly aspirated and delivered via the 25 gauge needle necessary for rat TMJ injections. This concentration exceeds values of intra-articular PLGA MPs delivered in saline to rodent knees (Butoescu et al., 2009b; Horisawa et al., 2002), and is similar to PLGA MP doses delivered to much larger joints, e.g., rabbit knees (Liggins et al., 2004). The high PLGA MP concentrations achieved in our study may be due to the addition of Tween 80, which reduced MP aggregation and facilitated intra-articular delivery. This agent is already approved by the United States Food and Drug Administration (FDA) as an inactive component of intra-articular injection formulations (Polysorbate 80, 2009). Injection of 50 μL of the 50 mg/mL formulation resulted in intra-articular delivery of 2.5 mg of PLGA MPs per TMJ. This amount, together with drug loading efficiency, is an important design parameter for future studies with drug-loaded MPs, as it will determine the maximum achievable drug dose.
Injection of 15, 30, or 50 mg/mL PLGA MPs caused no additional tissue reaction after one week, compared to the carrier solution alone. The mild inflammatory infiltrate observed (Fig. 3) is consistent with the rat and rabbit knee response one week after injection of 40–75 mg/mL PLGA MPs (Horisawa et al., 2002; Liggins et al., 2004). Future studies will probe the long-term biocompatibility of intra-articular PLGA MPs in the TMJ. The localization of the MPs within the adipocyte-rich posterior TMJ synovial tissue (Fig. 3) is consistent with reports for PLGA MPs in rodent knees (Butoescu et al., 2009b; Horisawa et al., 2002) and has been attributed to the hydrophobicity of the polymer (Nishide et al., 1999). The biocompatibility of the MPs is underscored when compared to rat TMJ response to CFA injections, which resulted in severe inflammation that was worst in the posterior synovial tissue adjacent to the TMJ vasculature (Harper et al., 2000), the same anatomical region where the PLGA MPs were localized in the present study. PLGA MPs within the TMJ synovium were surrounded by inflammatory cells (blue nuclei in Fig. 3b,c), as reported for rodent and rabbit knees, where PLGA MPs that were too large to be phagocytosed were instead enveloped by macrophages (Butoescu et al., 2009a; Butoescu et al., 2009b; Horisawa et al., 2002). Future studies will build upon these promising findings, to determine whether PLGA MPs are also biocompatible in the diseased TMJ.
Computerized meal pattern analysis has been extensively validated in rat TMJ disorder models (Bellinger et al., 2007; Harper et al., 2000; Kerins et al., 2003; 2004; Kerins et al., 2005). The use of this non-invasive method of quantifying TMJ pain enabled repeated measurements of each rat. Remarkably, the PLGA MP formulations had essentially no effect on meal duration (Fig. 2a) and food intake (Fig. 2b), both markers of TMJ pain. Although some groups differed from the 0 mg/mL PLGA (carrier solution only) control, these statistical differences are unlikely to be scientifically significant because the magnitude of the difference is extremely small when compared to the changes induced by inflammatory agents (“Inflamed TMJ *” group in Fig. 2a,b). Moreover, none of the groups differed from the “No injection” negative control (p > 0.05).
In this work, we report the in vivo biocompatibility of PLGA MPs for intra-articular sustained release in the rat TMJ. We found that bilateral intra-articular injection of 15, 30, or 50 mg/mL PLGA MPs had no impact on rat TMJ function over six days, as determined by histology and computerized meal pattern analysis. This highlights the exciting potential of these formulations to serve as the first intra-articular controlled release system for the TMJ, and thus greatly improve TMJ therapeutics.
Acknowledgments
We acknowledge support by the National Institutes of Health (R01 DE15164) (AGM). PMM is supported by a training fellowship from the Keck Center Nanobiology Training Program of the Gulf Coast Consortia (NIH Grant No. 5 T90 DK070121-04).
Footnotes
Indicate Prior Preliminary Publication: Preliminary report presented at the Society for Biomaterials 2009 Annual Meeting, San Antonio, TX
References
- Bellinger LL, Spears R, King CM, Dahm F, Hutchins B, Kerins CA, et al. Capsaicin sensitive neurons role in the inflamed TMJ acute nociceptive response of female and male rats. Physiol Behav. 2007;90:782–789. doi: 10.1016/j.physbeh.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Butoescu N, Jordan O, Doelker E. Intra-articular drug delivery systems for the treatment of rheumatic diseases: A review of the factors influencing their performance. Eur J Pharm Biopharm. 2009a;73:205–218. doi: 10.1016/j.ejpb.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Butoescu N, Seemayer C, Palmer G, Guerne P-A, Gabay C, Doelker E, et al. Magnetically retainable microparticles for drug delivery to the joint: efficacy studies in an antigen-induced arthritis model in mice. Arthritis Res Ther. 2009b;11:R72. doi: 10.1186/ar2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleek RL, Ting KC, Eskin SG, Mikos AG. Microparticles of poly(DL-lactic-co-glycolic acid)/poly(ethylene glycol) blends for controlled drug delivery. J Control Release. 1997;48:259–268. [Google Scholar]
- Ekuni D, Firth JD, Putnins EE. RNA integrity and in situ RT-PCR in dento-alveolar tissues after microwave accelerated demineralisation. Arch Oral Biol. 2006;51:164–169. doi: 10.1016/j.archoralbio.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Harper RP, Kerins CA, Talwar R, Spears R, Hutchins B, Carlson DS, et al. Meal pattern analysis in response to temporomandibular joint inflammation in the rat. J Dent Res. 2000;79:1704–1711. doi: 10.1177/00220345000790091101. [DOI] [PubMed] [Google Scholar]
- Hedberg EL, Shih CK, Solchaga LA, Caplan AI, Mikos AG. Controlled release of hyaluronan oligomers from biodegradable polymeric microparticle carriers. J Control Release. 2004;100:257–266. doi: 10.1016/j.jconrel.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Herring SW. TMJ anatomy and animal models. J Musculoskelet Neuronal Interact. 2003;3:391–394. [PMC free article] [PubMed] [Google Scholar]
- Horisawa E, Kubota K, Tuboi I, Sato K, Yamamoto H, Takeuchi H, et al. Size-dependency of DL-lactide/glycolide copolymer particulates for intra-articular delivery system on phagocytosis in rat synovium. Pharm Res. 2002;19:132–139. doi: 10.1023/a:1014260513728. [DOI] [PubMed] [Google Scholar]
- Kerins CA, Carlson DS, McIntosh JE, Bellinger LL. Meal pattern changes associated with temporomandibular joint inflammation/pain in rats; analgesic effects. Pharmacol Biochem Behav. 2003;75:181–189. doi: 10.1016/s0091-3057(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Kerins CA, Carlson DS, McIntosh JE, Bellinger LL. A role for cyclooxygenase II inhibitors in modulating temporomandibular joint inflammation from a meal pattern analysis perspective. J Oral Maxillofac Surg. 2004;62:989–995. doi: 10.1016/j.joms.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Kerins CA, Carlson DS, Hinton RJ, Hutchins B, Grogan DM, Marr K, et al. Specificity of meal pattern analysis as an animal model of determining temporomandibular joint inflammation/pain. Int J Oral Maxillofac Surg. 2005;34:425–431. doi: 10.1016/j.ijom.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Liggins RT, Cruz T, Min W, Liang L, Hunter WL, Burt HM. Intra-articular treatment of arthritis with microsphere formulations of paclitaxel: biocompatibility and efficacy determinations in rabbits. Inflamm Res. 2004;53:363–372. doi: 10.1007/s00011-004-1273-1. [DOI] [PubMed] [Google Scholar]
- Mercuri LG, Giobbie-Hurder A. Long-term outcomes after total alloplastic temporomandibular joint reconstruction following exposure to failed materials. J Oral Maxillofac Surg. 2004;62:1088–1096. doi: 10.1016/j.joms.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Mountziaris PM, Kramer PR, Mikos AG. Emerging intra-articular drug delivery systems for the temporomandibular joint. Methods. 2009;47:134–140. doi: 10.1016/j.ymeth.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Dental and Craniofacial Research (NIDCR) Data & Statistics. Bethesda: National Institutes of Health; 2008. [Google Scholar]
- Nishide M, Kamei S, Takakura Y, Tamai S, Tabata Y, Ikada Y. Fate of biodegradable DL-lactic acid oligomer microspheres in the articulus. J Bioact Compat Polym. 1999;14:385–398. [Google Scholar]
- Patel ZS, Yamamoto M, Ueda H, Tabata Y, Mikos AG. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater. 2008;4:1126–1138. doi: 10.1016/j.actbio.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polysorbate 80. Inactive ingredient search for approved drug products. Rockville: United States Food and Drug Administration; 2009. [Google Scholar]
- Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. J Dent Res. 2008;87:296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- Tuncay M, Calis S, Kas HS, Ercan MT, Peksoy I, Hincal AA. Diclofenac sodium incorporated PLGA (50:50) microspheres: formulation considerations and in vitro/in vivo evaluation. Int J Pharm. 2000;195:179–188. doi: 10.1016/s0378-5173(99)00394-4. [DOI] [PubMed] [Google Scholar]